Abstract
Enteropathogenic Escherichia coli (EPEC) is an important cause of diarrhea in humans. EPEC infection of cultured intestinal epithelial cells induces attaching and effacing (A/E) lesions, alters intestinal ion transport, increases paracellular permeability, and stimulates inflammation. The lack of a small-animal model has restricted in vivo studies examining EPEC-host interactions. The aim of this study was to characterize the C57BL/6J mouse as a model of EPEC infection. We have shown that EPEC can adhere to and colonize the intestinal epithelium of C57BL/6J mice. Animal weight and water intake were not altered during 10 days of EPEC infection. The proximal colon of infected mice contained semisolid stool, with stool pellets forming only in the distal colon. In contrast, the entire colon of control mice contained formed stool. Microvillous effacement and actin rearrangement, characteristic of A/E lesions, were seen in the intestine of infected mice but not control mice. Histological assessment revealed increased numbers of lamina propria neutrophils with occasional crypt abscesses, intraepithelial lymphocytes, and goblet cells in the intestine of EPEC-infected mice. Altogether, these data suggest that the C57BL/6J mouse is susceptible to infection by EPEC and will provide a suitable in vivo model for studying the consequences of EPEC infection.
Enteropathogenic Escherichia coli (EPEC) is a human enteric pathogen that attaches to the surface of intestinal epithelial cells and causes watery diarrhea (41). The first stage of EPEC attachment to the human host is called localized adherence, where EPEC forms dense microcolonies on the surface of intestinal epithelial cells (44). This initial attachment of EPEC to host cells occurs through a plasmid-encoded bundle-forming pilus (BFP) (18, 22). After initial adherence, EPEC induces localized effacement of microvilli and intimately attaches to the host cell surface, forming characteristic attaching and effacing (A/E) lesions (22, 70). Beneath the adherent bacteria, cytoskeleton-rich pedestal-like structures form and contain predominantly filamentous actin but also α-actinin, ezrin, talin, and other cytoskeletal proteins (41, 53).
The EPEC genes that confer virulence are located on a 35-kb pathogenicity island called the locus of enterocyte effacement (LEE) (20). The LEE encodes a type III secretion apparatus, secreted proteins, chaperones, and an outer membrane protein called intimin (20). One of the secreted proteins, EspA, forms hollow filaments through which EPEC delivers effector molecules directly into host cells (32, 33). One effector, Tir (translocated intimin receptor), is inserted into the host plasma membrane, where it serves as a receptor for the outer bacterial protein intimin (30, 52). The LEE-encoded proteins EspF, EspG, EspH, and Map (mitochondrion-associated protein) are believed to be effector molecules that are translocated into host cells and contribute to pathogenesis (11, 19, 31, 40, 66).
EPEC infection triggers physiological changes in the intestinal epithelium, including altered ion transport (25, 26), increased paracellular permeability (60), and initiation of inflammatory responses (54, 55). These physiological perturbations are likely due to the subversion by EPEC of host signaling pathways, including myosin light-chain kinase (74, 76), tyrosine kinases (51), inositol phosphate fluxes (6), protein kinase C (12, 56), mitogen-activated protein kinases (14, 15, 57), and NF-κB (54). While the activation of some of these pathways has been related to physiological alterations induced by EPEC, the activation of most has not.
Despite progress made during the last decade regarding the study of EPEC pathogenesis, relatively little is known about EPEC-induced physiological changes. In order to adequately define these changes, an animal model is needed. Animal models have been used to study host responses to EPEC homologues; these models include rabbits infected with rabbit EPEC (REPEC) (1, 63) and mice infected with Citrobacter rodentium (17, 23, 27, 36, 45). Although REPEC-induced disease in rabbits is similar to EPEC-induced disease (1, 63, 70), there are limitations to the use of this model, such as a paucity of genetic and immunological resources (1). Mouse models have the advantage of allowing the use of genetically modified animals for further studies. Murine models of C. rodentium infection have been used to study host responses to this related A/E lesion-forming enteric pathogen (23, 27, 36, 45). However, despite genetic similarities between EPEC and the mouse pathogen C. rodentium, there are significant pathophysiological differences between these two organisms (16, 36). For example, unlike EPEC, C. rodentium appears to possess a primary adhesin, rather than relying on A/E lesion formation to mediate host cell attachment (29). In addition, C. rodentium-infected mice develop mucosal hyperplasia, which is not seen in EPEC-infected hosts (36). Previously published data revealed that EPEC infection of the C57BL/6J mouse induced physiological alterations (26, 39). The aims of this study therefore were to assess the ability of EPEC to colonize the intestine of the C57BL/6J mouse and to determine the resulting clinical and histological changes.
(This work was presented in preliminary form at the annual meeting of the American Gastroenterological Association Digestive Disease Week in New Orleans, La., on 18 May 2004.)
MATERIALS AND METHODS
Cell cultures.
Mouse intestinal epithelial CMT-93 cells (passages 14 to 34) and human intestinal epithelial T84 cells (passages 35 to 55) were used in these studies. CMT-93 cells (American Type Culture Collection, Manassas, Va.) were propagated in Dulbecco-Vogt modified Eagle's medium (DMEM) (Gibco-BRL, Grand Island, N.Y.) with 10% fetal bovine calf serum according to the manufacturer's protocol. Human intestinal epithelial T84 cells, a generous gift from Kim Barrett (University of California, San Diego), were grown in a 1:1 (vol/vol) mixture of DMEM and Ham's F-12 medium (Gibco-BRL) as described previously (37).
Bacterial strains and infection of intestinal epithelial cells in tissue cultures.
EPEC wild-type strain E2348/69 and a bfp deletion (Δbfp) mutant were obtained from James Kaper (University of Maryland, Baltimore). Overnight bacterial cultures grown in Luria-Bertani broth were diluted (1:33) in serum- and antibiotic-free tissue culture medium containing 0.5% mannose and grown at 37°C to the mid-log growth phase (optical density at 600 nm, ∼0.4). Confluent human and mouse intestinal epithelial cell monolayers were infected with 4 × 107 organisms per well in 24-well plates for various times.
In vitro adherence assay.
The method used for the adherence assay was previously described by Cue and Cleary (13). Monolayers infected with EPEC or the Δbfp mutant for 1 and 3 h were washed three times with phosphate-buffered saline (PBS) and trypsinized with 0.25% trypsin for 15 min. Trypsinized cells were resuspended in distilled water and placed on ice for 15 min after vigorous pipetting and vortexing in order to lyse the epithelial cells and disperse the attached organisms. Aliquots were diluted (1:104 and 1:105) and plated on Luria-Bertani agar plates. All plates were incubated overnight at 37°C, and bacterial colonies were counted the next day.
Immunofluorescence staining of host actin and bacterial LPS.
Immunofluorescence staining was performed on monolayers of CMT-93 cells infected for 3 h with wild-type EPEC and the Δbfp mutant. Monolayers were fixed in 3.7% paraformaldehyde (pH 7.4) in PBS for 15 min, rinsed with PBS, permeabilized with 0.2% Triton X-100 for 15 min, and blocked with 1% bovine serum albumin in PBS. Monolayers were incubated with fluorescein isothiocyanate-phalloidin (Molecular Probes, Eugene, Oreg.) and antibody against E. coli lipopolysaccharide (LPS) (Sigma, St. Louis, Mo.) for 1 h. Detection of the anti-LPS primary antibody was performed by use of an anti-rabbit secondary antibody conjugated with Alexa 568 (Molecular Probes). After monolayers were washed, they were mounted with Vectashield (Molecular Probes) and assessed by use of a Nikon Opti-Phot microscope. Images were captured by use of a Zeiss-RT digital imaging system.
Infection of mice.
Six- to 8-week-old C57BL/6J mice were purchased from Jackson Laboratories and allowed to acclimate for 3 days in the Biological Resources Laboratory at the University of Illinois, Chicago. All experiments involving mice were approved by the University of Illinois, Chicago, Animal Care Committee in accordance with National Institutes of Health standards. Approximately 2 × 108 EPEC organisms resuspended in 200 μl of PBS were introduced into animals by gavage with a 4-cm-long curved needle with a steel ball at the tip. Control animals received 200 μl of sterile PBS. Over the course of infection, animals were observed daily for activity level and water intake, and weight was measured. At various times following infection, animals were sacrificed, and intestinal tissues were processed for further analysis.
Determination of in vivo adherence.
To determine the numbers of EPEC organisms in mouse stool and adherent to the intestinal epithelium, C57BL/6J mice were infected with EPEC and sacrificed after 3 days. The small and large intestines were resected, and the stool was removed and diluted in sterile PBS. The small intestine, cecum, and colon were separated, washed vigorously with PBS, and homogenized in PBS containing 1% Triton X-100. Aliquots of diluted stool and homogenized intestinal tissues were plated on agar plates containing ampicillin, to which wild-type EPEC is resistant (Ampr) because of the ampicillin gene inserted in plasmid pMAR. Bacterial colonies from diluted stool represented nonadherent EPEC, while colonies grown from plated homogenized tissues represented adherent EPEC.
PCR analysis of selected bacterial colonies.
Bacterial colonies grown on ampicillin-containing agar plates were further assessed to confirm that they were EPEC. Samples from randomly selected Ampr bacterial colonies were added to a PCR mixture, which was prepared according to the manufacturer's protocol (Invitrogen, Carlsbad, Calif.) to yield final concentrations of 1× PCR buffer, 200 μM each nucleotide, 400 μM dUTP, 5.5 mM MgCl2, 1.25 U of AmpliTaq DNA polymerase, and 200 nM each primer for EPEC gene espB (5′-TATTATGAATACTATCGA and 3′-AATTACCCAGCTAAGCGAG). E. coli laboratory strain HB101 was used as a negative control, and wild-type EPEC was used as a positive control. The terminal cycling conditions included 95°C for 10 min and 40 cycles of amplification consisting of denaturation at 95°C for 15 s and annealing-extension at 60°C for 1 min. These primers generated a 960-bp product on a 1% agarose gel.
TEM.
C57BL/6J mice infected with EPEC were sacrificed, and the distal small intestine and the proximal colon were cut into ∼2-mm-long pieces and fixed in 4% glutaraldehyde. Further routine processing for transmission electron microscopy (TEM) was performed in the Electron Microscopy Facility at the University of Illinois, Chicago.
Immunofluorescence staining of actin and EPEC protein intimin in intestinal tissues.
Mice infected with EPEC for 5 days were sacrificed, and tissues were rinsed with PBS, fixed in 10% formalin, processed with a Tissue-Tek VIP5 processor (Sakura Finetek, Torrance, Calif.), and embedded in paraffin. Tissue sections 5 μm thick were fixed in ice-cold acetone for 10 min, blocked with 1% bovine serum albumin, and incubated with a rabbit anti-intimin primary antibody (a gift from Jorge Giron, University of Arizona, Tucson) for 60 min. Following extensive washing with PBS, tissue sections were incubated with the anti-rabbit secondary antibody conjugated with Alexa 568. Host cell filamentous actin was stained with Alexa 488-conjugated phallodin (Molecular Probes). Samples were mounted with ProLong (Molecular Probes) and assessed by use of a Nikon Opti-Phot microscope. Images were captured by use of a Zeiss-RT digital imaging system.
Histological analysis.
For histological analysis, colon tissues of control and EPEC-infected mice were washed with PBS, fixed in 10% neutral buffered formalin, processed by use of a Tissue-Tek VIP 5 processor (Sakura Finetek, Torrance, Calif.), and embedded in paraffin. Tissue sections 5 μm thick were cut by use of a microtome (Sakura Finetek) and stained with hematoxylin and eosin (H&E) or periodic acid-Schiff (PAS) solutions. Images were acquired by use of a DMLB microscope equipped with Fluotar objectives (Leica Microsystems Inc., Bannockburn, Ill.) and a Micropublisher 5.0 camera (Q Imaging, Burnaby, British Columbia, Canada). Images were collected by use of QCapture software 2.6. Images were processed postacquisition by use of Photoshop 7.0 (Adobe Systems Inc., San Jose, Calif.).
Statistical analysis.
All data are presented as the mean and standard error of the mean. Data comparisons were made with Student's t test. Differences were considered significant when the P value was ≤0.05.
RESULTS
EPEC attaches to mouse intestinal epithelial CMT-93 cells.
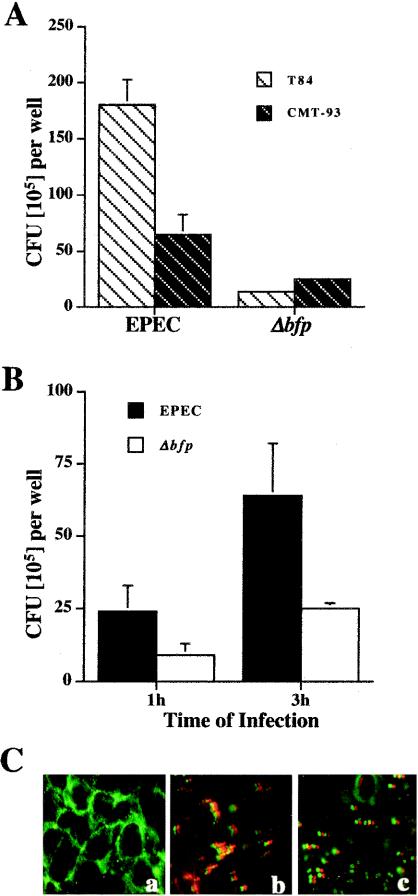
Our initial hypothesis was that if the human enteric pathogen EPEC could adhere to cultured mouse intestinal epithelial cells, then it likely would adhere to native mouse intestinal epithelium. Therefore, we first determined the rate of adherence of EPEC to mouse intestinal epithelial CMT-93 cells. It was previously reported that deletion of the EPEC bfp gene increased EPEC attachment to mouse CMT-93 cells (64). We therefore compared the attachment of the Δbfp mutant and the attachment of wild-type EPEC to CMT-93 cells. Figure 1A shows that in contrast to wild-type EPEC, the Δbfp mutant adhered to mouse CMT-93 cells more efficiently than to human T84 cells. However, the adherence of wild-type EPEC to both CMT-93 and T84 cells was greater than the adherence of the Δbfp mutant (Fig. 1B). Immunofluorescence staining of control and infected CMT-93 monolayers for host actin and bacterial LPS revealed that actin rearrangement corresponds to attached bacterial organisms (Fig. 1C). Additionally, the adherence of wild-type EPEC microcolonies was greater than the adherence of Δbfp as single organisms. Overall, these studies demonstrated that EPEC adheres to mouse intestinal epithelial cells in vitro and that, in contrast to previously published data, deletion of the bfp gene did not increase adherence. We therefore focused on wild-type EPEC in the in vivo studies.
FIG. 1.
EPEC adheres to mouse intestinal epithelial cells in vitro. (A) Monolayers of human intestinal T84 cells and mouse intestinal CMT-93 cells were infected for 3 h with wild-type EPEC and a Δbfp mutant. Adherence assays showed that while wild-type EPEC and the Δbfp mutant adhered to both cell lines, the adherence of wild-type EPEC was greater. (B) EPEC adherence to mouse CMT-93 cells progresses during infection. Mouse intestinal CMT-93 cell monolayers were infected for 1 and 3 h with wild-type EPEC and the Δbfp mutant. The adherence of both organisms increased over time, with EPEC adherence being greater than that of the Δbfp mutant. Data in panels A and B represent the mean and standard error of the mean for one of four experiments (n = 4); the P value was <0.05 for all comparisons between T84 and CMT-93 cells as well as between wild-type EPEC and the Δbfp mutant. (C) Immunofluorescence staining of host actin and bacterial LPS in mouse intestinal CMT-93 cell monolayers that were uninfected (a) or infected with wild-type EPEC (b) or Δbfp (c) for 3 h. Wild-type EPEC attached to mouse intestinal epithelial cells as microcolonies, whereas the Δbfp mutant attached as single organisms.
EPEC adheres to and colonizes the intestinal epithelium of C57BL/6J mice.
To determine whether EPEC can adhere to and colonize the intestine of C57BL/6J mice, the number of nonadherent EPEC organisms in stool as well as the number of adherent EPEC organisms were quantified at 3 days postinfection. The results are summarized in Table 1 and show that EPEC colonized both the small and the large intestines, with the highest level of adherence in the cecum. No colonies grew from stool or tissues obtained from control animals and cultured on ampicillin-containing agar plates. To confirm that the Ampr colonies were indeed EPEC, randomly selected colonies were examined for the presence of EPEC gene espB from the LEE locus by PCR. All colonies tested positive for EPEC gene espB, while nonpathogenic HB101 yielded no PCR product (data not shown). These data show that the human enteric pathogen EPEC can adhere to and colonize the small and large intestines of C57BL/6J mice.
TABLE 1.
EPEC colonizes and adheres to the intestinal epithelium of C57BL/6J micea
| EPEC | Mean ± SEM EPEC CFU (103) in:
|
||
|---|---|---|---|
| Small intestine | Cecum | Colon | |
| Nonadherent | 33.5 ± 23 | 36.3 ± 23.7 | 243.5 ± 56.5 |
| Adherent | 6.0 ± 1.5 | 16.3 ± 3.2 | 6.5 ± 3.0 |
C57BL/6J mice infected with EPEC were sacrificed 3 days later, and aliquots of feces (nonadherent EPEC) and homogenized intestinal tissues (adherent EPEC) were plated on ampicillin-containing agar plates. The experiments were performed twice with three animals; data from one representative experiment are shown. There were no significant differences between groups.
Clinical parameters for C57BL/6J mice infected with EPEC.
To assess the systemic effect of EPEC on infected mice, both body weight and water intake were monitored. No changes in either parameter were observed over the 10-day period of infection. Animals infected with the Δbfp mutant and C. rodentium were also monitored for comparison. Although infection with C. rodentium induced retardation in growth, no changes in body weight were detected in C57BL/6J mice after 10 days of infection. Also, reduced activity and ruffled fur were observed in EPEC-infected animals at the end of this period.
Effect of EPEC infection on the mouse intestine.
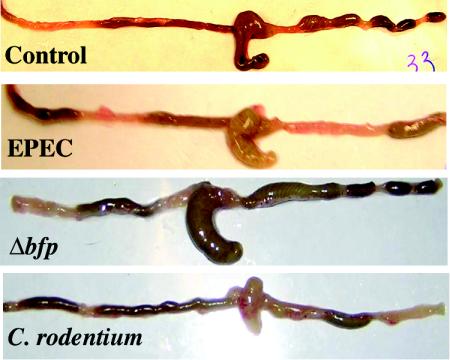
To define host responses to EPEC infection, the mouse intestine was examined. The colon of uninfected mice contained formed pellets of stool beginning just distal to the cecum. However, the proximal colon of animals infected with EPEC for 10 days contained semisolid stool, and formed stool pellets were not seen until the distal colon (Fig. 2). Also, the cecum appeared to be slightly engorged in EPEC- and Δbfp mutant-infected animals. In order to quantitate the size of the cecum, samples of resected intestine were placed on a flat surface with centimeter markers, and digital images were obtained. The perimeters of ceca of control animals and EPEC-, Δbfp mutant-, and C. rodentium-infected animals were measured and expressed as lengths in centimeters. There was a significant difference between the cecum of control mice and the cecum of EPEC-infected mice (5.2 ± 0.3 and 6.7 ± 0.4 cm, respectively; n = 4; P = 0.02). The cecum of Δbfp mutant-infected mice was also significantly larger than the cecum of control mice (6.3 ± 0.7 cm; n = 3; P = 0.6) but was not different from the cecum of EPEC-infected mice. In contrast, while the colon of mice infected with C. rodentium showed a similar distribution of stool pellets, the cecum was contracted and empty, as described previously (36).
FIG. 2.
Whole intestines of control mice and mice infected with EPEC for 10 days. The intestine of control mice showed semisolid stool in the distal small intestine and fully formed pellets of stool in the proximal colon immediately adjacent to the cecum. In contrast, in EPEC-infected mice, semisolid stool was present in the proximal half of the colon, with stool pellets forming only in the distal colon. Additionally, the cecum appeared to be significantly engorged in EPEC- and Δbfp-infected animals. In mice infected with C. rodentium, the colon showed similar changes, except that the cecum was contracted and empty.
C. rodentium has been reported to increase colon weight in infected mice at 10 days postinfection and to induce epithelial hyperplasia and mucosal inflammation (36). Therefore, we assessed colon weight (Table 2) and found that the colon of mice infected with EPEC showed a trend toward increased weight, but the weight at 10 days postinfection was not significantly different from that in control mice. A similar trend toward increased weight was noted for the colon of mice infected with C. rodentium, while the colon weight in Δbfp mutant-infected mice was no different from that in control mice.
TABLE 2.
Colon weights in control and infected micea
| Infecting organism | Colon wt (mean ± SEM [n = 4] mg/cm) | Pb |
|---|---|---|
| None (control) | 35.3 ± 2.2 | |
| EPEC | 43.4 ± 2.1 | 0.056 |
| Δbfp mutant | 32.7 ± 2.2 | 0.445 |
| C. rodentium | 44.9 ± 3.1 | 0.072 |
Colons were dissected from C57BL/6J mice at 10 days postinfection, stool was removed, and weights were determined.
For comparisons with control mice.
EPEC induces morphological changes in mouse intestinal epithelial cells.
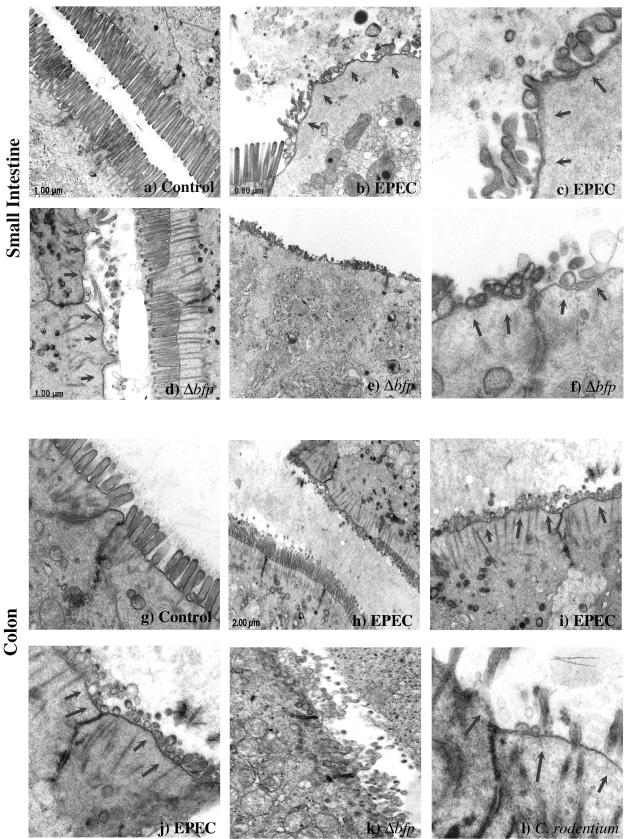
EPEC induces localized effacement of microvilli in human intestinal epithelial cells, forming characteristic A/E lesions (22, 70). To assess the effect of EPEC on mouse intestinal epithelial cells, TEM was performed on the small and large intestines. Figure 3 shows TEM of the distal small intestine and proximal colon of uninfected mice and those of mice infected for 5 days with EPEC. For uninfected mice, TEM of the small intestine and colon revealed well-defined microvilli. In contrast, infection with both wild-type EPEC and the Δbfp mutant induced distortion and loss of microvillous actin rootlets as well as microvillous fragmentation and effacement in the small intestine and colon. Infection with C. rodentium, as a positive control, induced similar changes in the microvillous architecture of the colon of infected mice. Although intimately attached organisms and classic A/E lesions, such as those demonstrated in cultured epithelial cells (18), were not readily found, the drastic degree of microvillous effacement was quite consistent with changes induced by EPEC and C. rodentium infections.
FIG. 3.
EPEC induces microvillous effacement in the intestinal epithelium of infected mice. C57BL/6J mice infected with EPEC were sacrificed after 5 days, and the distal small intestine and proximal colon were examined by TEM. Areas of effaced microvilli and disorganized actin rootlets are highlighted by arrows. The intestinal epithelium of the small intestine (a, ×20,000) and colon (g, ×30,000) in control mice displayed normal microvilli, while that in mice infected with wild-type EPEC (small intestine: b, ×15,000; and c, ×40,000; colon: h, ×1,200; i, ×10,000; and j, ×30,000) or the Δbfp mutant (small intestine: d, ×80,000; e, ×20,000; and f, ×80,000; colon: k, ×25,000) showed dramatic effacement of microvilli. Colon tissue infected with C. rodentium (l, ×60,000) was used as a positive control for microvillous effacement. In some areas, normal microvilli can be seen immediately adjacent to microvilli that have been effaced.
EPEC induces actin rearrangement in the mouse intestinal epithelium.
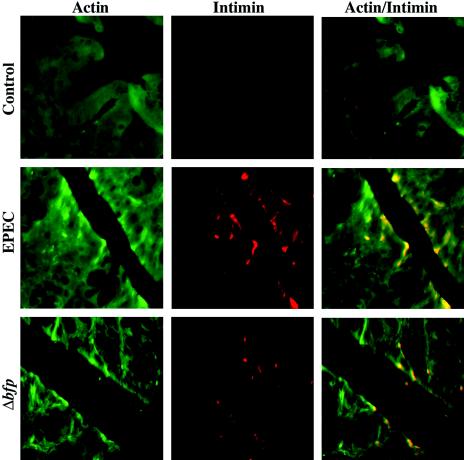
EPEC infection of human intestinal epithelial cells induces the accumulation of cytoskeleton-rich structures containing predominantly filamentous actin beneath adherent bacteria. In an attempt to identify attached EPEC in the intestine of infected mice, 5-μm-thick sections of colon tissues of control and infected mice were immunostained for actin and the EPEC outer membrane protein intimin. As shown in Fig. 4, intimin staining (red) was seen at focal areas of the epithelial surface in sections of infected tissues that were not present in sections of control tissues. Actin accumulation (green) was detected in association with intimin staining, suggesting the presence of adherent EPEC colonies. These data further support the conclusion that the human enteric pathogen EPEC attaches to and induces actin rearrangement in the mouse intestinal epithelium.
FIG. 4.
EPEC induces actin rearrangement in the mouse intestinal epithelium. Colon tissues of uninfected C57BL/6J mice and those infected with EPEC or Δbfp for 5 days were stained for host cell actin (green) and the EPEC outer membrane protein intimin (red) (original magnification, ×400). In control colonic tissue, actin staining was not accompanied by EPEC intimin staining, as expected. In contrast, in infected animals, attached bacteria represented by intimin staining (red) were seen on the surface of epithelial cells and colocalized with areas of actin aggregates.
EPEC induces histological changes in the mouse intestine.
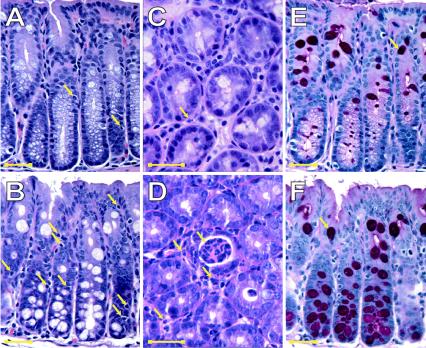
We next determined the effect of EPEC infection on the mouse intestine by examining H&E- and PAS-stained sections of colons of control and EPEC-infected mice. As shown in Fig. 5A and C, H&E staining of the colon of control animals revealed sparse intraepithelial lymphocytes (IELs) and lamina propria polymorphonuclear leukocytes (PMNs), consistent with the normal mucosal histology of conventionally housed mice. In contrast, the numbers of both IELs and lamina propria PMNs were significantly increased in the colon of EPEC-infected mice (Table 3 and Fig. 5B and D). Because intestinal inflammation has been linked to increased goblet cell differentiation (7, 10, 58, 61, 65, 72), the effect of EPEC infection on the number of goblet cells was assessed in PAS-stained sections. EPEC infection also caused a significant increase in the number of goblet cells (Table 3 and Fig. 5E and F). Increased numbers of IELs, lamina propria PMNs, and goblet cells were found throughout the proximal colon. In contrast, acute inflammation, as evident from intraepithelial PMNs and occasional crypt abscesses, occurred in a patchy distribution in the intestine of EPEC-infected mice and was not present in the intestine of control mice (Fig. 5C and D). Together, these data show that EPEC elicits an inflammatory response in the mouse intestine.
FIG. 5.
EPEC infection induces an inflammatory response in the colon of C57BL/6J mice. The proximal colon of C57BL/6J mice infected with EPEC for 10 days was examined following H&E staining and PAS staining. In control mice, H&E staining of the colon revealed normal mucosal morphology (magnifications: A, ×400; and C, ×1,000). Arrows in panels A and C indicate sparse IEL and lamina propria PMNs in control tissue. However, the colon of EPEC-infected mice showed increases in the numbers of intestinal IELs (B) (magnification, ×400) and lamina propria PMNs (arrows) with crypt abscesses (D) (magnification, ×1,000). PAS staining of goblet cell mucin revealed increased numbers of goblet cells in EPEC-infected colon (F) compared to controls (E) (magnification, ×400). The images shown in panels A and E and B and F were from the same fields. Scale bars in all images represented 25 μm.
TABLE 3.
Numbers of PMNs, IELs, and goblet cells are increased in the colon of EPEC-infected C57BL/6J micea
| Mice | No. of:
|
||
|---|---|---|---|
| PMNs/0.113 mm2 | IELs/crypt | Goblet cells/crypt | |
| Control | 1.5 ± 0.3 | 1.6 ± 0.1 | 5.9 ± 0.4 |
| EPEC infected | 8.4 ± 0.8b | 4.0 ± 0.1b | 8.9 ± 0.4b |
Well-oriented sections of colon tissues from control and EPEC-infected mice were assessed 10 days postinfection as shown in Fig. 5. The numbers of PMNs and IELs were determined from H&E-stained sections. The numbers of goblet cells were determined from PAS-stained sections.
The P value for comparisons with control mice was <0.001.
DISCUSSION
Earlier studies concerning the effect of enteric bacterial pathogens on the expression and function of the galanin receptor suggested that the C57BL/6J mouse was susceptible to infection by EPEC (26, 39). We therefore sought to characterize this potential model of EPEC infection in more detail. In this study, we showed that the human enteric pathogen EPEC adheres to the mouse intestinal epithelium and induces morphological and pathophysiological changes. Mouse models of enteric infections have been frequently used to investigate diseases caused by human pathogens, including Salmonella enterica serovar Typhimurium (38), Yersina enterocolitica (24), Helicobacter pylori (2), and enterohemorrhagic E. coli (EHEC) (35). However, some reports have suggested that the mouse does not develop intestinal inflammation in response to enteric pathogens and therefore is not a suitable host (28, 73). For example, Salmonella does not replicate efficiently in the mouse intestine; instead, it penetrates the epithelial barrier (8, 62, 71) and spreads to the liver and spleen, and the mice eventually surrender to systemic infection (5, 73) without the signs of intestinal inflammation seen in humans. However, Barthel et al. have reported that mice treated with antibiotics prior to infection with Salmonella develop epithelial ulceration accompanied by massive infiltration of PMN cells (3). Also, mice pretreated with antibiotics were used to study host intestinal responses to EHEC (34, 35). In contrast, others have reported that EHEC can colonize the intestines of conventional and non-antibiotic-treated mice (9, 43). Pretreatment with antibiotics eliminates commensal bacteria, thereby increasing the efficiency of adherence of the enteric pathogen to the mouse intestinal epithelium. However, commensal bacteria play an important role in gut homeostasis, and their elimination may cause altered intestinal epithelial responses to enteric pathogens. We speculate that although pretreatment with antibiotics may enhance intestinal responses to EPEC, our model more closely represents host intestinal responses to this pathogen.
We demonstrated in this study that EPEC adheres to both cultured and native mouse intestinal epithelia. The initial attachment of EPEC to the host cell occurs through the BFP (18, 22). We showed that EPEC with a deletion of the bfp gene adheres more efficiently to mouse CMT-93 cells than to human T84 cells, consistent with data reported previously by Tobe and Sasakawa (64). In contrast to that report, however, we found that wild-type EPEC adheres more efficiently than the Δbfp mutant to mouse CMT-93 cells. The importance of the BFP in the attachment of A/E lesion-forming pathogens to host cells is not clear in that EHEC, which lacks the BFP, still attaches to the human intestinal epithelium (44). Furthermore, the mouse pathogen C. rodentium possesses a type IV pilus gene cluster required for colonization (42) and does not rely on the BFP for initial attachment (29). The data presented here showed not only that wild-type EPEC adheres to cultured mouse intestinal epithelial cells but also that EPEC adheres to and colonizes intestinal epithelial cells of C57BL/6J mice as well. The relatively small number of EPEC colonies recovered from the intestine of C57BL/6J mice correlates with the CFU of C. rodentium found in several mouse strains at early time points after infection (68). Others have demonstrated that EPEC induces A/E lesions in other hosts, such as rabbits and piglets (41, 50, 67), and that REPEC colonizes the mouse intestinal epithelium as well (49). In contrast, Frankel et al. reported that no EPEC colonies could be cultured from the intestine of Swiss NIH mice at 15 days postinfection (21). It is possible that the use of different mouse strains, the age of the animals, and the period of time chosen to assay colonization account for these discrepancies.
The effect of EPEC infection on the clinical status of mice included reduced activity and ruffled fur but no change in water intake or growth. Similar signs of illness have been reported in mice infected with C. rodentium (36). Although retarded growth has been reported in mice infected with C. rodentium (69), this feature was not observed in our studies. Also, the increase in colon weight was not significant in our study, in contrast to a previous study (69). These discrepancies could be due to the fact that younger C57BL/6J mice (3 to 4 weeks old) infected for a longer period of time (14 days) were used in the previous studies, while we used older animals (6 to 8 weeks old) infected for only 10 days. Although EPEC-infected animals did not develop diarrhea, the proximal colon of EPEC-infected animals contained semisolid stool, and formed stool was present only in the distal colon. Also, there was a trend toward higher colon weight in EPEC-infected animals than in control animals, although this finding was not statistically significant. The changes that we observed in the intestine of mice infected with C. rodentium, such as a rigid distal colon devoid of formed stool and an often empty and contracted cecum, are consistent with a previous study (36).
Histological examination of the colon of EPEC-infected mice demonstrated mild diffuse inflammation, including increased numbers of lamina propria PMNs, IELs, and goblet cells. Occasional crypt abscesses were also present in EPEC-infected mice. All of these changes are consistent with the intestinal inflammation seen in humans infected with EPEC (44). We previously reported that infection of cultured human intestinal epithelial cells with EPEC induced the transepithelial migration of acute inflammatory PMNs (55), suggesting that inflammation is a common host response to this pathogen. Increased numbers of goblet cells may represent an intestinal epithelial response to the inflammation induced by EPEC infection. Increased numbers of goblet cells have been shown in other instances of injury (7, 10, 61, 65), leading some to propose that this adaptive response allows goblet cell-secreted mucin to form a viscous gel that traps microorganisms and irritants and limits their access to the epithelium (4). In chemically induced intestinal inflammation, the expression and secretion of mucin increased with disease progression and differed in the proximal colon and the distal colon (48). Also, in the same model of inflammation, a loss of crypts and surface epithelium and the subsequent loss of goblet cells in some areas (47) were partly compensated for by increases in the numbers of goblet cells in elongated crypts and surface epithelium in other areas (48). Furthermore, it has been reported that the overproduction and secretion of mucins are associated with inflammation caused by bacterial infection (59).
In conclusion, our data provide evidence that EPEC adheres to mouse intestinal epithelial cells in vitro and in vivo and induces A/E lesions, crypt abscesses, increased numbers of lamina propria and intraepithelial inflammatory cells, and increased numbers of goblet cells. The data therefore suggest that the C57BL/6J mouse can be used as an animal model to study host responses, at least the inflammatory response, to the human enteric pathogen EPEC. This mouse model may also be useful for the study of other host intestinal epithelial responses to EPEC infection, including ion transport and barrier function. Additionally, this model will allow the contribution of each of these pathophysiological events to EPEC-induced diarrhea to be elucidated. The use of genetically modified mice as hosts will allow the involvement of specific intestinal epithelial factors in EPEC-mediated disease to be defined. This model will also provide a tool to assess the role of specific bacterial effectors in EPEC-induced disease.
Acknowledgments
This work was supported by a grant from the Crohn's and Colitis Foundation of America (career development award to S.D.S.), National Institutes of Health grant DK50694 (to G.H.), National Institutes of Health grant DK61931 (to J.R.T.), and merit review and research enhancement awards from the Department of Veterans Affairs (to G.H.).
Editor: J. T. Barbieri
REFERENCES
- 1.Abe, A., U. Heczko, R. G. Hegele, and B. B. Finlay. 1998. Two enteropathogenic Escherichia coli type III secreted proteins, EspA and EspB, are virulence factors. J. Exp. Med. 188:1907-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aleljung, P., H. O. Nilsson, X. Wang, P. Nyberg, T. Morner, I. Warsame, and T. Wadstrom. 1996. Gastrointestinal colonisation of BALB/cA mice by Helicobacter pylori monitored by heparin magnetic separation. FEMS Immunol. Med. Microbiol. 13:303-309. [DOI] [PubMed] [Google Scholar]
- 3.Barthel, M., S. Hapfelmeier, L. Quintanilla-Martinez, M. Kremer, M. Rohde, M. Hogardt, K. Pfeffer, H. Russmann, and W. D. Hardt. 2003. Pretreatment of mice with streptomycin provides a Salmonella enterica serovar Typhimurium colitis model that allows analysis of both pathogen and host. Infect. Immun. 71:2839-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belley, A., K. Keller, M. Gottke, K. Chadee, and M. Goettke. 1999. Intestinal mucins in colonization and host defense against pathogens. Am. J. Trop. Med. Hyg. 60:10-15. [DOI] [PubMed] [Google Scholar]
- 5.Carter, P. B., and F. M. Collins. 1974. The route of enteric infection in normal mice. J. Exp. Med. 139:1189-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Celli, J., M. Olivier, and B. B. Finlay. 2001. Enteropathogenic Escherichia coli mediates antiphagocytosis through the inhibition of PI 3-kinase-dependent pathways. EMBO J. 20:1245-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ciacci, C., D. Di Vizio, R. Seth, G. Insabato, G. Mazzacca, D. K. Podolsky, and Y. R. Mahida. 2002. Selective reduction of intestinal trefoil factor in untreated coeliac disease. Clin. Exp. Immunol. 130:526-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark, M. A., M. A. Jepson, N. L. Simmons, and B. H. Hirst. 1994. Preferential interaction of Salmonella typhimurium with mouse Peyer's patch M cells. Res. Microbiol. 145:543-552. [DOI] [PubMed] [Google Scholar]
- 9.Conlan, J. W., and M. B. Perry. 1998. Susceptibility of three strains of conventional adult mice to intestinal colonization by an isolate of Escherichia coli O157:H7. Can. J. Microbiol. 44:800-805. [DOI] [PubMed] [Google Scholar]
- 10.Conour, J. E., D. Ganessunker, K. A. Tappenden, S. M. Donovan, and H. R. Gaskins. 2002. Acidomucin goblet cell expansion induced by parenteral nutrition in the small intestine of piglets. Am. J. Physiol. 283:G1185-G1196. [DOI] [PubMed] [Google Scholar]
- 11.Crane, J. K., B. P. McNamara, and M. S. Donnenberg. 2001. Role of EspF in host cell death induced by enteropathogenic Escherichia coli. Cell. Microbiol. 3:197-211. [DOI] [PubMed] [Google Scholar]
- 12.Crane, J. K., and J. S. Oh. 1997. Activation of host cell protein kinase C by enteropathogenic Escherichia coli. Infect. Immun. 65:3277-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cue, D. R., and P. P. Cleary. 1997. High-frequency invasion of epithelial cells by Streptococcus pyogenes can be activated by fibrinogen and peptides containing the sequence RGD. Infect. Immun. 65:2759-2764. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Czerucka, D., S. Dahan, B. Mograbi, B. Rossi, and P. Rampal. 2001. Implication of mitogen-activated protein kinases in T84 cell responses to enteropathogenic Escherichia coli infection. Infect. Immun. 69:1298-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Grado, M., C. M. Rosenberger, A. Gauthier, B. A. Vallance, and B. B. Finlay. 2001. Enteropathogenic Escherichia coli infection induces expression of the early growth response factor by activating mitogen-activated protein kinase cascades in epithelial cells. Infect. Immun. 69:6217-6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng, W., Y. Li, B. A. Vallance, and B. B. Finlay. 2001. Locus of enterocyte effacement from Citrobacter rodentium: sequence analysis and evidence for horizontal transfer among attaching and effacing pathogens. Infect. Immun. 69:6323-6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng, W., B. A. Vallance, Y. Li, J. L. Puente, and B. B. Finlay. 2003. Citrobacter rodentium translocated intimin receptor (Tir) is an essential virulence factor needed for actin condensation, intestinal colonization and colonic hyperplasia in mice. Mol. Microbiol. 48:95-115. [DOI] [PubMed] [Google Scholar]
- 18.Donnenberg, M. S., J. B. Kaper, and B. B. Finlay. 1997. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 5:109-114. [DOI] [PubMed] [Google Scholar]
- 19.Elliott, S. J., E. O. Krejany, J. L. Mellies, R. M. Robins-Browne, C. Sasakawa, and J. B. Kaper. 2001. EspG, a novel type III system-secreted protein from enteropathogenic Escherichia coli with similarities to VirA of Shigella flexneri. Infect. Immun. 69:4027-4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elliott, S. J., L. A. Wainwright, T. K. McDaniel, K. G. Jarvis, Y. Deng, L.-C. Lai, B. P. McNamara, M. S. Donnenberg, and J. B. Kaper. 1998. The complete sequence of the locus of enterocyte effacement (LEE) from the enteropathogenic Escherichia coli E2348/69. Mol. Microbiol. 28:1-4. [DOI] [PubMed] [Google Scholar]
- 21.Frankel, G., A. D. Phillips, M. Novakova, H. Field, D. C. Candy, D. B. Schauer, G. Douce, and G. Dougan. 1996. Intimin from enteropathogenic Escherichia coli restores murine virulence to a Citrobacter rodentium eaeA mutant: induction of an immunoglobulin A response to intimin and EspB. Infect. Immun. 64:5315-5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Frankel, G., A. D. Phillips, I. Rosenshine, G. Dougan, J. B. Kaper, and S. Knutton. 1998. Enteropathogenic and enterohaemorrhagic Escherichia coli: more subversive elements. Mol. Microbiol. 30:911-921. [DOI] [PubMed] [Google Scholar]
- 23.Goncalves, N. S., M. Ghaem-Maghami, G. Monteleone, G. Frankel, G. Dougan, D. J. Lewis, C. P. Simmons, and T. T. MacDonald. 2001. Critical role for tumor necrosis factor alpha in controlling the number of lumenal pathogenic bacteria and immunopathology in infectious colitis. Infect. Immun. 69:6651-6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hancock, G. E., R. W. Schaedler, and T. T. MacDonald. 1986. Yersinia enterocolitica infection in resistant and susceptible strains of mice. Infect. Immun. 53:26-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hecht, G., K. Hodges, R. K. Gill, F. Kear, S. Tyagi, J. Malakooti, K. Ramaswamy, and P. K. Dudeja. 2004. Differential regulation of Na+/H+ exchange isoform activities by enteropathogenic E. coli in human intestinal epithelial cells. Am. J. Physiol. Gastrointest. Liver Physiol. 287:G370-G378. [DOI] [PubMed] [Google Scholar]
- 26.Hecht, G., J. A. Marrero, A. Danilkovich, K. A. Matkowskyj, S. D. Savkovic, A. Koutsouris, and R. V. Benya. 1999. Pathogenic Escherichia coli increase Cl− secretion from intestinal epithelia by upregulating galanin-1 receptor expression. J. Clin. Investig. 104:253-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins, L. M., G. Frankel, G. Douce, G. Dougan, and T. T. MacDonald. 1999. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect. Immun. 67:3031-3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hurley, B. P., and B. A. McCormick. 2003. Translating tissue culture results into animal models: the case of Salmonella typhimurium. Trends Microbiol. 11:562-569. [DOI] [PubMed] [Google Scholar]
- 29.Johnson, E., and S. W. Barthold. 1979. The ultrastructure of transmissible murine colonic hyperplasia. Am. J. Pathol. 97:291-313. [PMC free article] [PubMed] [Google Scholar]
- 30.Kenny, B., and B. B. Finlay. 1997. Intimin-dependent binding of enteropathogenic Escherichia coli to host cells triggers novel signaling events, including tyrosine phosphorylation of phospholipase C-gamma1. Infect. Immun. 65:2528-2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kenny, B., and M. Jepson. 2000. Targeting of an enteropathogenic Escherichia coli (EPEC) effector protein to host mitochondria. Cell. Microbiol. 2:579-590. [DOI] [PubMed] [Google Scholar]
- 32.Kenny, B., L. C. Lai, B. B. Finlay, and M. S. Donnenberg. 1996. EspA, a protein secreted by enteropathogenic Escherichia coli, is required to induce signals in epithelial cells. Mol. Microbiol. 20:313-323. [DOI] [PubMed] [Google Scholar]
- 33.Knutton, S., I. Rosenshine, M. J. Pallen, I. Nisan, B. C. Neves, C. Bain, C. Wolff, G. Dougan, and G. Frankel. 1998. A novel EspA-associated surface organelle of enteropathogenic Escherichia coli involved in protein translocation into epithelial cells. EMBO J. 17:2166-2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurioka, T., Y. Yunou, and E. Kita. 1998. Enhancement of susceptibility to Shiga toxin-producing Escherichia coli O157:H7 by protein calorie malnutrition in mice. Infect. Immun. 66:1726-1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindgren, S. W., A. R. Melton, and A. D. O'Brien. 1993. Virulence of enterohemorrhagic Escherichia coli O91:H21 clinical isolates in an orally infected mouse model. Infect. Immun. 61:3832-3842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luperchio, S. A., and D. B. Schauer. 2001. Molecular pathogenesis of Citrobacter rodentium and transmissible murine colonic hyperplasia. Microbes Infect. 3:333-340. [DOI] [PubMed] [Google Scholar]
- 37.Madara, J. L., J. Stafford, K. Dharmsathaphorn, and S. Carlson. 1987. Structural analysis of a human intestinal epithelial cell line. Gastroenterology 92:1133-1145. [DOI] [PubMed] [Google Scholar]
- 38.Mahan, M. J., J. M. Slauch, and J. J. Mekalanos. 1993. Selection of bacterial virulence genes that are specifically induced in host tissues. Science 259:686-688. [DOI] [PubMed] [Google Scholar]
- 39.Matkowskyj, K. A., A. Danilkovich, J. Marrero, S. D. Savkovic, G. Hecht, and R. V. Benya. 2000. Galanin-1 receptor up-regulation mediates the excess colonic fluid production caused by infection with enteric pathogens. Nat. Med. 6:1048-1051. [DOI] [PubMed] [Google Scholar]
- 40.McNamara, B. P., A. Koutsouris, C. B. O'Connell, J. P. Nougayrede, M. S. Donnenberg, and G. Hecht. 2001. Translocated EspF protein from enteropathogenic Escherichia coli disrupts host intestinal barrier function. J. Clin. Investig. 107:621-629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moon, H. W., S. C. Whipp, R. A. Argenzio, M. M. Levine, and R. A. Giannella. 1983. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect. Immun. 41:1340-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mundy, R., D. Pickard, R. K. Wilson, C. P. Simmons, G. Dougan, and G. Frankel. 2003. Identification of a novel type IV pilus gene cluster required for gastrointestinal colonization of Citrobacter rodentium. Mol. Microbiol. 48:795-809. [DOI] [PubMed] [Google Scholar]
- 43.Nagano, K., K. Taguchi, T. Hara, S. Yokoyama, K. Kawada, and H. Mori. 2003. Adhesion and colonization of enterohemorrhagic Escherichia coli O157:H7 in cecum of mice. Microbiol. Immunol. 47:125-132. [DOI] [PubMed] [Google Scholar]
- 44.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newman, J. V., B. A. Zabel, S. S. Jha, and D. B. Schauer. 1999. Citrobacter rodentium espB is necessary for signal transduction and for infection of laboratory mice. Infect. Immun. 67:6019-6025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perna, N. T., G. F. Mayhew, G. Posfai, S. Elliott, M. S. Donnenberg, J. B. Kaper, and F. R. Blattner. 1998. Molecular evolution of a pathogenicity island from enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 66:3810-3817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Renes, I. B., J. A. Boshuizen, D. J. Van Nispen, N. P. Bulsing, H. A. Buller, J. Dekker, and A. W. Einerhand. 2002. Alterations in Muc2 biosynthesis and secretion during dextran sulfate sodium-induced colitis. Am. J. Physiol. 282:G382-G389. [DOI] [PubMed] [Google Scholar]
- 48.Renes, I. B., M. Verburg, D. J. Van Nispen, H. A. Buller, J. Dekker, and A. W. Einerhand. 2002. Distinct epithelial responses in experimental colitis: implications for ion uptake and mucosal protection. Am. J. Physiol. 283:G169-G179. [DOI] [PubMed] [Google Scholar]
- 49.Robins-Browne, R. M., A. M. Tokhi, L. M. Adams, and V. Bennett-Wood. 1994. Host specificity of enteropathogenic Escherichia coli from rabbits: lack of correlation between adherence in vitro and pathogenicity for laboratory animals. Infect. Immun. 62:3329-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robins-Browne, R. M., W. C. Yam, L. E. O'Gorman, and K. A. Bettelheim. 1993. Examination of archetypal strains of enteropathogenic Escherichia coli for properties associated with bacterial virulence. J. Med. Microbiol. 38:222-226. [DOI] [PubMed] [Google Scholar]
- 51.Rosenshine, I., M. S. Donnenberg, J. B. Kaper, and B. B. Finlay. 1992. Signal transduction between enteropathogenic Escherichia coli (EPEC) and epithelial cells: EPEC induces tyrosine phosphorylation of host cell proteins to initiate cytoskeletal rearrangement and bacterial uptake. EMBO J. 11:3551-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenshine, I., S. Ruschkowski, M. Stein, D. J. Reinscheid, S. D. Mills, and B. B. Finlay. 1996. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J. 15:2613-2624. [PMC free article] [PubMed] [Google Scholar]
- 53.Sanger, J. M., R. Chang, F. Ashton, J. B. Kaper, and J. W. Sanger. 1996. Novel form of actin-based motility transports bacteria on the surfaces of infected cells. Cell. Motil. Cytoskeleton 34:279-287. [DOI] [PubMed] [Google Scholar]
- 54.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1997. Activation of NF-κB in intestinal epithelial cells by enteropathogenic Escherichia coli. Am. J. Physiol. 273:C1160-C1167. [DOI] [PubMed] [Google Scholar]
- 55.Savkovic, S. D., A. Koutsouris, and G. Hecht. 1996. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect. Immun. 64:4480-4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Savkovic, S. D., A. Koutsouris, and G. Hecht. 2003. PKC zeta participates in activation of inflammatory response induced by enteropathogenic E. coli. Am. J. Physiol. Cell. Physiol. 285:C512-C521. [DOI] [PubMed] [Google Scholar]
- 57.Savkovic, S. D., A. Ramaswamy, A. Koutsouris, and G. Hecht. 2001. EPEC-activated ERK1/2 participate in inflammatory response but not tight junction barrier disruption. Am. J. Physiol. 281:G890-G898. [DOI] [PubMed] [Google Scholar]
- 58.Seto, Y., H. Nakajima, A. Suto, K. Shimoda, Y. Saito, K. I. Nakayama, and I. Iwamoto. 2003. Enhanced Th2 cell-mediated allergic inflammation in Tyk2-deficient mice. J. Immunol. 170:1077-1083. [DOI] [PubMed] [Google Scholar]
- 59.Smirnova, M. G., L. Guo, J. P. Birchall, and J. P. Pearson. 2003. LPS up-regulates mucin and cytokine mRNA expression and stimulates mucin and cytokine secretion in goblet cells. Cell. Immunol. 221:42-49. [DOI] [PubMed] [Google Scholar]
- 60.Spitz, J., R. Yuhan, A. Koutsouris, C. Blatt, J. Alverdy, and G. Hecht. 1995. Enteropathogenic Escherichia coli adherence to intestinal epithelial monolayers diminishes barrier function. Am. J. Physiol. 268:G374-G379. [DOI] [PubMed] [Google Scholar]
- 61.Surawicz, C. M., R. C. Haggitt, M. Husseman, and L. V. McFarland. 1994. Mucosal biopsy diagnosis of colitis: acute self-limited colitis and idiopathic inflammatory bowel disease. Gastroenterology 107:755-763. [DOI] [PubMed] [Google Scholar]
- 62.Takeuchi, A. 1967. Electron microscope studies of experimental Salmonella infection. I. Penetration into the intestinal epithelium by Salmonella typhimurium. Am. J. Pathol. 50:109-136. [PMC free article] [PubMed] [Google Scholar]
- 63.Tauschek, M., R. A. Strugnell, and R. M. Robins-Browne. 2002. Characterization and evidence of mobilization of the LEE pathogenicity island of rabbit-specific strains of enteropathogenic Escherichia coli. Mol. Microbiol. 44:1533-1550. [DOI] [PubMed] [Google Scholar]
- 64.Tobe, T., and C. Sasakawa. 2002. Species-specific cell adhesion of enteropathogenic Escherichia coli is mediated by type IV bundle-forming pili. Cell. Microbiol. 4:29-42. [DOI] [PubMed] [Google Scholar]
- 65.Toward, T. J., and K. J. Broadley. 2002. Goblet cell hyperplasia, airway function, and leukocyte infiltration after chronic lipopolysaccharide exposure in conscious Guinea pigs: effects of rolipram and dexamethasone. J. Pharmacol. Exp. Ther. 302:814-821. [DOI] [PubMed] [Google Scholar]
- 66.Tu, X., I. Nisan, C. Yona, E. Hanski, and I. Rosenshine. 2003. EspH, a new cytoskeleton-modulating effector of enterohaemorrhagic and enteropathogenic Escherichia coli. Mol. Microbiol. 47:595-606. [DOI] [PubMed] [Google Scholar]
- 67.Tzipori, S., R. M. Robins-Browne, G. Gonis, J. Hayes, M. Withers, and E. McCartney. 1985. Enteropathogenic Escherichia coli enteritis: evaluation of the gnotobiotic piglet as a model of human infection. Gut 26:570-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vallance, B. A., W. Deng, K. Jacobson, and B. B. Finlay. 2003. Host susceptibility to the attaching and effacing bacterial pathogen Citrobacter rodentium. Infect. Immun. 71:3443-3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vallance, B. A., W. Deng, L. A. Knodler, and B. B. Finlay. 2002. Mice lacking T and B lymphocytes develop transient colitis and crypt hyperplasia yet suffer impaired bacterial clearance during Citrobacter rodentium infection. Infect. Immun. 70:2070-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vallance, B. A., and B. B. Finlay. 2000. Exploitation of host cells by enteropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 97:8799-8806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vazquez-Torres, A., J. Jones-Carson, A. J. Baumler, S. Falkow, R. Valdivia, W. Brown, M. Le, R. Berggren, W. T. Parks, and F. C. Fang. 1999. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401:804-808. [DOI] [PubMed] [Google Scholar]
- 72.Vermeer, P. D., R. Harson, L. A. Einwalter, T. Moninger, and J. Zabner. 2003. Interleukin-9 induces goblet cell hyperplasia during repair of human airway epithelia. Am. J. Respir. Cell Mol. Biol. 28:286-295. [DOI] [PubMed] [Google Scholar]
- 73.Wallis, T. S., and E. E. Galyov. 2000. Molecular basis of Salmonella-induced enteritis. Mol. Microbiol. 36:997-1005. [DOI] [PubMed] [Google Scholar]
- 74.Yuhan, R., A. Koutsouris, S. D. Savkovic, and G. Hecht. 1997. Enteropathogenic Escherichia coli-induced myosin light chain phosphorylation alters intestinal epithelial permeability. Gastroenterology 113:1873-1882. [DOI] [PubMed] [Google Scholar]
- 75.Zhu, C., T. S. Agin, S. J. Elliott, L. A. Johnson, T. E. Thate, J. B. Kaper, and E. C. Boedeker. 2001. Complete nucleotide sequence and analysis of the locus of enterocyte effacement from rabbit diarrheagenic Escherichia coli RDEC-1. Infect. Immun. 69:2107-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zolotarevsky, Y., G. Hecht, A. Koutsouris, D. E. Gonzalez, C. Quan, J. Tom, R. J. Mrsny, and J. R. Turner. 2002. A membrane-permeant peptide that inhibits MLC kinase restores barrier function in in vitro models of intestinal disease. Gastroenterology 123:163-172. [DOI] [PubMed] [Google Scholar]