Abstract
Endogenous neurosteroids such as allopregnanolone, allotetrahydrodeoxycorticosterone, and androstanediol are synthesized either de novo in the brain from cholesterol or are generated from the local metabolism of peripherally derived progesterone or corticosterone. Fluctuations in neurosteroid concentrations are important in the regulation of a number of physiological responses including anxiety and stress, reproductive, and sexual behaviors. These effects are mediated in part by the direct binding of neurosteroids to γ-aminobutyric acid type-A receptors (GABAARs), resulting in the potentiation of GABAAR-mediated currents. Extrasynaptic GABAA Rs containing the δ subunit, which contribute to the tonic conductance, are particularly sensitive to low nanomolar concentrations of neurosteroids and are likely their preferential target. Considering the large charge transfer generated by these persistently open channels, even subtle changes in neurosteroid concentrations can have a major impact on neuronal excitability. Consequently, aberrant levels of neurosteroids have been implicated in numerous disorders, including, but not limited to, anxiety, neurodegenerative diseases, alcohol abuse, epilepsy, and depression. Here we review the modulation of GABAA R by neurosteroids and the consequences for health and disease.
Keywords: allopregnanolone, γ-aminobutyric acid (GABA), neurosteroids, allotetrahydrodeoxycorticosterone (THDOC)
Introduction
The term neurosteroids was first introduced in the 1980s by Baulieu to describe steroids produced de novo in the brain from cholesterol; it was later expanded to include those derived from the local metabolism of peripherally derived steroid precursors such as, progesterone, corticosterone, or testosterone (1–3). Neurosteroids are modulators of aminobutyric acid type A receptors (GABAARs) and can induce analgesic, anxiolytic, sedative, anesthetic, and anticonvulsant effects (4, 5). The ability of neurosteroids to modulate GABAAR function was first shown in 1984 by Harrison and Simmonds who demonstrated that alpha-xalone, a synthetic neuroactive steroid with anesthetic properties, potently potentiated GABAAR currents (6). This result was repeated shortly afterward with the endogenous neurosteroids 5α-pregnane-3α-ol-20-one (allopregnanolone) and 5α-pregnane-3α,21-diol-20-one (THDOC) (7). Fluctuations in the concentration of endogenous neurosteroids and changes in GABAergic signaling have been implicated in a variety of physiological and pathophysiological conditions including stress, pregnancy, reproductive/sexual behaviors, depression, and epilepsy (8–15). Here we review the neurosteroid-mediated regulation of GABAergic transmission, the effects on neuronal excitability, and the implications for health and disease.
Neurosteroidogenesis
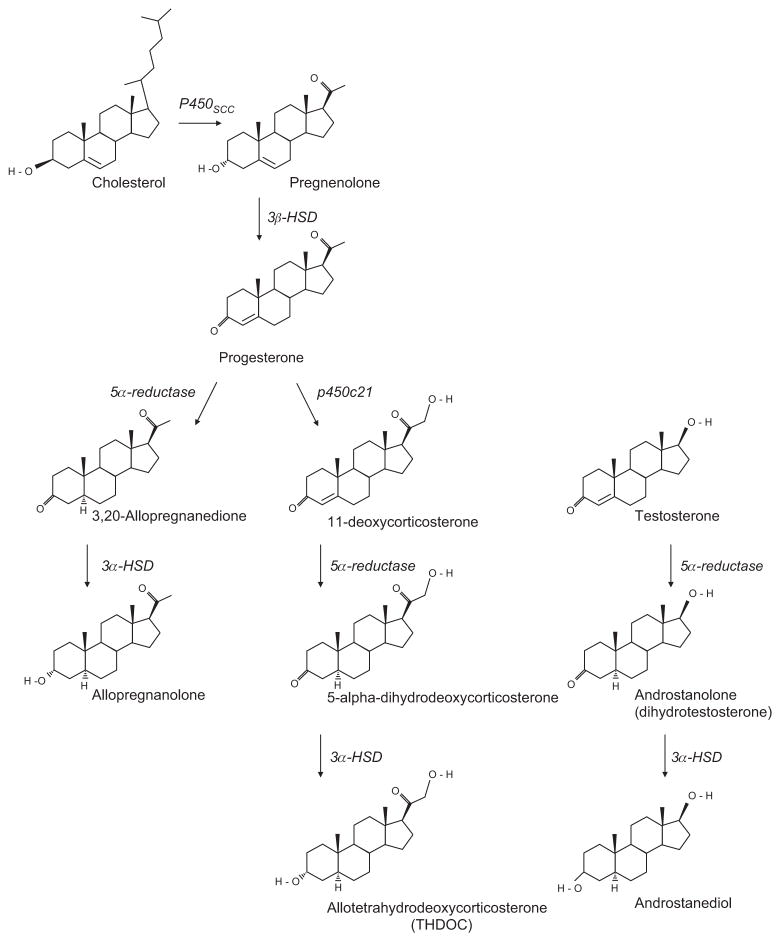
There are three main classes of neurosteroids: the pregnane (e.g., allopregnanolone), the sulfated (e.g., dehydroepiandrosterone sulfate, or DHEAS), and the androstane (e.g., androstanediol), which are classified according to their structural homology (9) (Figure 1). The 3-α hydroxy ring A-reduced pregnane steroids, such as allopregnanolone and THDOC, are the most potent positive modulators of GABAARs and will be the focus of this review whereas; the sulfated neurosteroids are often inhibitory and act as noncompetitive antagonists at GABAARs (16). Allopregnanolone and THDOC can be synthesized from cholesterol by a series of steroidogenic enzymes [for reviews, see (2, 5, 17, 18)] (Figure 1). Briefly, the key pathways are as follows: cholesterol is transported into the inner mitochondrial membrane via the steroidogenic acute regulatory protein (StAR) and translocator protein 18 kDa (TSPO), also known as the peripheral benzodiazepine receptor (19). Here, mitochondrial cholesterol side-chain cleavage enzyme (cytochrome P450scc) catalyzes a side chain cleavage to convert cholesterol into pregnenolone, an important rate-limiting step for the production of allopregnanolone and THDOC. Pregnenolone is then converted by 3β-hydroxysteroid dehydrogenase (3β-HSD) into progesterone with further metabolism of progesterone by 21 hydroxylase (p450c21), yielding deoxycorticosterone. Finally, progesterone and deoxycorticosterone are metabolized by 5α-reductase followed by 3α-hydroxysteroid dehydrogenase (3α-HSD), to yield allopregnanolone and THDOC, respectively. In addition, androstanediol, another potent positive modulator of GABAARs, also utilizes the 5α-reductase/3α-HSD metabolic pathway to catalyze its synthesis from testosterone (3, 9) (Figure 1).
Figure 1.
The major biosynthetic pathways in the synthesis of allopregnanolone (3α,5α-tetrahydroprogesterone, 5α-pregnan-3α-ol-20-one, 3α-hydroxy-5α-pregnan-20-one, or 5α3α-THPROG), THDOC (allotetrahydrodeoxycorticosterone, 5α-pregnane-3α,21-diol-20-one, or 5α3α-THDOC), and androstanediol (5α-androstane-3α,17β-diol or 3α-diol).
The corresponding neurosteroidogenic enzymes are shown in italics adjacent to each reaction.
The steroidogenic enzymes are not uniformly distributed throughout the brain but are localized in specific brain regions and cell types (20). Cytochrome p450scc, for example, is expressed in both principal neurons and glial cells in various brain regions including the amygdala, hypothalamus, thalamus, cortex, and hippocampus (21). Furthermore, both 5α-reductase protein and 3α-HSD mRNA have been shown to colocalize in principal neurons in the thalamus, striatum, cerebellum, cortex, amygdala, and hippocampus, indicating that these are likely sites of neurosteroidogenesis (22). However, there is limited or no expression in interneurons with weak 5α-reductase/3α-HSD expression found only in the granule cells of the cerebellum and olfactory bulb (22). As neurosteroids are produced in the same neurons that express GABAARs, they may act in an autocrine as well as a paracrine fashion to alter neuronal excitability. Interestingly, p450c21 mRNA has so far only been found in the brain stem and at very low levels in the cerebellum, suggesting that local metabolism of steroid hormone precursors from the periphery might be the prominent pathway for neuronal THDOC synthesis, which coincides with the observation that THDOC is not detectable in the brains of adrenalectomized animals (20, 23). Indeed, because steroid hormones are small and lipophilic, peripherally derived hormones from the adrenal cortex, placenta, or gonads can readily cross the blood-brain barrier and plasma membrane, where they can be locally metabolized into neurosteroids (24). It has also been observed that some steroidogenic enzymes are found in more than one subcellular compartment. For instance, cytochrome p450c17, an important enzyme in the pathway that mediates the conversion of pregnanolone into DHEAS and androstenediol, is found in the cell body, axon, and dendrites of embryonic basal ganglia and cerebellum neurons (21, 25). Therefore, neurosteroids may be synthesized at some distance away from the cell body, and thus, it can by hypothesized that distantly synthesized or trafficked neurosteroids could mediate effects in brain regions apparently devoid of the necessary enzymes for neurosteroid synthesis (21). However, due to technical difficulties in the quantification of neurosteroids, it is difficult to directly measure local neurosteroid production.
Baseline circulating plasma neurosteroid levels and levels in the brain are generally low, but they increase in response to certain physiological triggers such as stress, the ovarian cycle, and pregnancy. The basal THDOC concentration in the plasma of rats (26, 27) and humans (28, 29) is approximately ≤5 nM at rest. However, a stressful episode activates the hypothalamic-pituitary-adrenal axis, resulting in the release from the adrenal gland of corticosterone in rats and cortisol in humans (30). Plasma levels of THDOC increase approximately threefold to fourfold in rats subjected to an acute swim stress (26) and in humans responding to panic induction with cholecystokinin-tetrapeptide (29), which parallels changes in corticosterone/cortisol levels. The peak THDOC response occurs 10–30 min after the cessation of the stress and can be prevented by the 5α-reductase inhibitor, finasteride (23, 26, 27, 31). Allopregnanolone is also found at low nanomolar concentrations in the plasma of both humans (32, 33) and rats (34–36) and fluctuates in response to stress (23, 36, 37) stage of menstrual/estrous cycle (32, 38) and pregnancy (33, 37, 39–41), reflecting changes in peripheral progesterone levels. During pregnancy, plasma allopregnanolone levels have been shown to reach concentrations ranging from 40 nM to >100 nM in both rats (35) and humans (33, 37, 39–41). Similarly, allopregnanolone levels have been shown to increase during pregnancy in the rat cerebral cortex, peaking by day 19 and returning to control levels upon parturition (day 21) (35). It is important to note that although basal and peak neurosteroids levels have been detected at nanomolar concentrations under normal physiological circumstances, these concentrations are sufficient to positively modulate GABAARs. Further, neurosteroid concentrations may be significantly higher at specific neuronal locations reflecting local synthesis, diffusion barriers, and metabolism.
Although neurosteroid concentration measurements have been made in the central nervous system (CNS) of both rats (23, 35, 36, 42, 43) and humans (44–46), accurately measuring neurosteroid concentrations is difficult and reflected in the range of neurosteroid concentrations reported in the literature. Radioimmunoassays are commonly used to measure neurosteroid levels and are highly sensitive. However, sample contamination, antibody cross-reactivity, and different sample extraction, and purification procedures likely underscore some of the variability in the literature. Alternative approaches include separation of cross-reacting steroids followed by enzyme-linked immunosorbent assays (47) and liquid or gas chromatography coupled with mass spectrometry, which have provided lower estimates of brain-derived neurosteroids [for reviews, see (48, 49)]. However, despite the difficulties in accurately measuring neurosteroid levels in both plasma and the CNS, the relative changes in neurosteroid concentration during different physiological states are likely to be accurate (48) and will have important implications for neuronal and network excitability.
Neurosteroid modulation of GABARs
GABAA Rs are assembled from a combination of 19 sub-units (α1–6, β1–3, γ1–3, δ, ε, θ, π, ρ1–3) to form a heteropentameric structure around a central ion channel pore, which fluxes chloride (50 –52). The exact receptor subunit combination determines not only its pharmacological and biophysical properties but also its subcellular localization. For instance, receptor combinations containing the γ2 subunit are found predominantly at the synapse where they mediate rapid synaptic (phasic) transmission (53, 54). Meanwhile, assemblies containing the δ subunit have a high affinity for GABA and are found either perisynaptically or extrasynaptically (54 – 57). These properties make them ideally suited to sense the nanomolar concentrations of ambient GABA predicted to be found in the extra-cellular space with persistent receptor activation resulting in the generation of a tonic chloride conductance (54, 58, 59).
Positive neurosteroids such as allopregnanolone and THDOC are potent modulators of GABAARs and act by increasing the open probability of the channel without changing the single channel conductance (60, 61). At low nanomolar concentrations, neurosteroids act as positive allosteric modulators. Indeed, in recombinant expression systems, neurosteroids have been shown to potentiate the peak current generated by the majority of GABAAR sub-types in response to subsaturating GABA concentrations (62). Yet, at higher micromolar concentrations, neurosteroids directly activate the receptor in the absence of GABA (63). However, not all neurosteroids are positive modulators of GABAA-Rs. Adding to the diversity of neurosteroid mediated regulation, two members of the sulfated neurosteroid family, pregnanolone sulfate and DHEAS, inhibit GABAA Rs (9). The actions of these negative modulators of GABAARs are thought to be mediated by a binding site different from the one that mediates the actions of allopregnanolone and THDOC (9). Although pregnane neurosteroids can potentiate synaptic GABAergic responses as demonstrated by a prolongation of IPSC decay time, low physiological concentrations of neurosteroids preferentially potentiate the extrasynaptic δ-subunit-containing receptors enhancing the tonic component of GABAergic inhibition (64). For instance, in both dentate gyrus and cerebellar granule cells, 10 nM THDOC selectively potentiates the tonic conductance with little effect on the phasic response (64). Consistent with the action of neurosteroids on extrasynaptic GABAARs, neurosteroid sensitivity is greatly reduced in mice deficient in the GABAAR δ subunit (Gabrd−/− mice) (65). Furthermore, the neurosteroid sensitivity of receptors containing the δ subunit has also been confirmed in recombinant expression systems (62, 66, 67). GABA binds to δ-subunit-containing receptors with high affinity but relatively low efficacy; therefore, GABA is inefficient at promoting the open state. As neurosteroids increase the efficacy of the receptors by encouraging more frequent and longer open times, they are more effective at potentiating the effects of GABA at δ-subunit-containing receptors compared with other isoforms where GABA is already a potent agonist (68–71).
Although more efficacious at δ-subunit-containing receptors, neurosteroids can potentiate the effects of GABA at receptors containing most isoforms. In fact, the binding site for neurosteroids does not involve the δ subunit. Using a combination of site-directed mutagenesis, electrophysiology, and homology modeling, two neurosteroid-binding sites have been identified on GABAARs composed of α1β2γ2 subunits (63). First, threonine 236 on the α subunit, which lies close to the α/β interface, and tyrosine 284 on the β subunit are essential for the direct activation of the receptor by allopregnanolone. Second, the α-subunit residue glutamine 241 located on transmembrane 1 is crucial for mediating both the allosteric potentiation and direct neurosteroid activation of the receptor (63, 72–74), although neighboring residues are also likely to be important for forming the steroid-binding site (75, 76). Recently, photoaffinity labeling using (3α,5β)-6-azi-pregnanolone identified phenylalanine 301 in the β3 subunit as a unique residue for neurosteroid binding, which likely forms part of the direct activation site (77). It will be of interest to modify this residue and examine both neurosteroid potentiation and direct activation of α1β3γ2-GABAAR subtypes using electrophysiology. In addition, photoaffinity labeling of native receptors subtypes could be used to distinguish those residues that are involved in the direct activation vs. allosteric modulation by neuro-steroids (75, 78).
Despite being shown to potentiate the majority of GABAAR subtypes, the actions of positive neurosteroids at GABAAR subtypes containing the ε subunit ( ε-GABAARs) are less clear. Compared with other GABAAR subtypes, ε-GABAARs are relatively insensitive to the potentiating effects of a number of intravenous anesthetics including the neurosteroid allopregnanolone (62, 79, 80) [but see (81) ]. However, pregnane neurosteroids have been shown to directly activate ε-GABAARs in the absence of endogenous agonist (62, 82 – 84). As inclusion of the ε subunit has been shown to confer constitutive activity to the GABAAR in recombinant expression systems (81, 84, 85), it is difficult to determine whether neurosteroid action is mediated by allosteric potentiation of spontaneous openings or via steroid binding to the direct activation site (86). Furthermore, understanding the actions of neurosteroids at ε-GABAARs is complicated because neurosteroid actions may be influenced by receptor stoichiometry (83). Therefore, further studies using native receptor populations such as in vitro slice models are required for the actions of neurosteroids at ε-GABAARs to be fully understood. For example, recent evidence from brain stem respiratory neurons of the ventral respiratory column showed an increased in ε-GABAARs subunit expression during pregnancy and reduced sensitivity to intravenous anesthetics. These data suggest that increased expression of ε-GABAARs during pregnancy might protect against respiratory depression despite elevated neurosteroid levels (87).
Regulation of GABAA Rs and changes in neuronal excitability
The presence of low concentrations (i.e., 10 – 30 nm) of neurosteroids results in the potentiation of extrasynaptic GABAARs. Although the magnitude of potentiation will depend on receptor subtype, local GABA concentration, and steroid metabolism, the large charge transfer generated by these persistently open channels means that even a small increase in the tonic conductance will have a major impact on excitability. Generally, an increase in the tonic conductance will reduce the input resistance narrowing the temporal and spatial integration of synaptic events and increasing the amount of excitatory input required to generate an action potential (54, 88). In addition, changes in tonic inhibition can impact the sensitivity of a neuron to changes in inputs (the neuronal gain) by shunting the background synaptic noise (54, 88, 89) [but see (90) ]. Larger increases in neurosteroid concentration (i.e., ≥100 nm) will reduce neuronal excitability further by potentiating the phasic component of GABAergic inhibition by prolonging IPSPs as well as enhancing tonic GABAergic inhibition (64). Therefore, as neurosteroid concentrations vary under both physiological and pathological conditions, GABAergic signaling requires dynamic regulation to maintain optimal levels of inhibition [for a review, see (91) ].
Fluctuations in steroid hormones, such as those that occur during stress, the ovarian cycle, and pregnancy, have been shown to correspond to changes in GABAergic inhibition and subunit expression (8, 10, 35, 92 – 97). For example, the δ subunit has been shown to increase while the γ2 subunit decreases in mouse hippocampus at times of the ovarian cycle when progesterone levels are high, resulting in an increase in tonic inhibition and decreased levels of anxiety and seizure susceptibility (95). Similar changes have been observed in the periaqueductal gray matter (98) and the CA1 region of the hippocampus in response to elevated steroid levels (97). These changes in subunit expression can be prevented by blocking neurosteroid synthesis with finasteride and can be mimicked in males by progesterone administration (11). Similar changes have also been demonstrated in response to elevations in neurosteroids following acute stress (11). However, no changes in GABAAR mRNA expression levels were found in gonadotropin-releasing hormone neurons in the medial preoptic area in cycling mice (99), suggesting that steroid-mediated modulation of GABAAR expression is likely cell type-specific.
The conditions in which there are prolonged changes in neurosteroid levels, such as during pregnancy, has been shown to induce alterations in the cerebrocortical and hippocampal expression of the GABAAR γ2 subunit (35, 94, 100, 101) and the hippocampal GABAAR δ subunit (94), which can be prevented by blocking the neurosteroid synthesis with finasteride (35, 100, 101). These changes in GABAAR subunit expression during pregnancy are correlated with alterations in network excitability (10). Further, hippocampal expression of the α 4 subunit has also been shown to fluctuate in response to changes in progesterone concentration (8, 96, 102, 103). Therefore, neurosteroids can alter GABAergic inhibition via the direct modulation of GABAergic inhibition as well as by altering GABAAR subunit expression, which exerts dramatic effects on neuronal excitability. Thus, the neurosteroid regulation of GABAergic inhibition has significant implications for neuronal excitability in health and disease.
Role of neurosteroids in disease
Neurosteroids have been implicated in numerous disorders, including, but not limited to, depression, anxiety, alcohol abuse, epilepsy, and neurodegenerative diseases (104 – 111). The evidence of altered neurosteroid levels associated with several neuropsychiatric and neurological disorders has generated a great deal of enthusiasm for targeting neurosteroids or their site of action for treatment [for a review, see (9) ]. Furthermore, the actions of neurosteroids on specific GABAAR subtypes have further increased enthusiasm for the therapeutic potential of these compounds. The following section will review the role of neurosteroids in disease as well as the therapeutic potential of targeting neurosteroids, focusing specifically on neurosteroids that exhibit positive modulation of GABAARs.
Depression
Neurosteroid levels are abnormal in patients with major depression [for a review, see (112) ]. For example, allopregnanolone levels are decreased in patients with major depression compared with healthy controls [for a review, see (112) ]. Conversely, the levels of the stress-derived neurosteroid, THDOC, are elevated in patients with major depression [for a review, see (112) ]. Antidepressant treatment normalizes the neurosteroid levels in depressed patients (106, 107, 112 – 114), which is thought to mediate the antidepressant effects of these drugs (107, 113, 114). These data implicate altered neurosteroid levels in the pathophysiology of depression as well as a role in the effectiveness of antidepressant treatment. Selective serotonin reuptake inhibitors (SSRIs) enhance the antidepressant effects of neurosteroids via increasing GABAergic tone (115), which are independent of effects on serotonergic transmission (113 – 115), suggesting that the antidepressant effects of SSRIs and allopregnanolone are mediated via the GABAergic system rather than the serotonergic system. Consistent with the role of neurosteroids in depression, exogenous administration of allopregnanolone exerts antidepressant effects in animal models (115, 116). Further, mice with deficits in the primary target for neurosteroid action in the brain, the δ-subunit-containing GABAARs ( Gabrd−/− mice), exhibit depression-like behavior during the postpartum period (10, 94).
Neurosteroids have also been implicated in mood disorders associated with the ovarian cycle. Allopregnanolone levels during the luteal phase are associated with symptom severity in patients with premenstrual dysphoric disorder (PMDD) (117) [for a review, see (118) ], and increased levels are correlated with symptom improvement (119) [for reviews, see (120, 121) ]. However, there are conflicting results regarding alterations in neurosteroid levels in patients with PMDD. Many studies suggest that there is no significant difference in allopregnanolone levels in patients with PMDD compared with controls, whereas other studies suggest that allopregnanolone levels are decreased or increased in patients with PMDD [for a review, see (118) ]. Given that there are no clear differences in neurosteroid levels in patients with PMDD, it has been proposed that these patients have altered responses to neurosteroids or the site of action of neurosteroids (95). Although the exact nature of the relationship remains unclear, these data demonstrate a role for neurosteroids and their site of action in the pathophysiology of depression.
Anxiety
Patients with generalized anxiety disorders have altered neurosteroid levels. Allopregnanolone levels are significantly decreased in patients with posttraumatic stress disorder (122) and in patients with panic disorder (123). Following experimentally induced panic attacks, allopregnanolone levels are decreased in patients with a history of panic disorders compared with healthy controls (124, 125), suggesting that there are deficits in neurosteroid signaling in patients with anxiety disorders. Together, these findings suggest that neurosteroids play a role in the pathophysiology of anxiety and panic disorders (126). However, the most convincing evidence for neurosteroid involvement in anxiety disorders is the potent anxiolytic actions of neurosteroids (127 – 131). Allopregnanolone (129, 132 – 134) and THDOC (127, 134) have been shown to exhibit anxiolytic properties in many different behavioral paradigms. However, the anxiolytic effects of neurosteroids appear to be state-dependent because neurosteroids do not exhibit anxiolytic properties following stress (135).
Epilepsy
Neurosteroids exhibit robust anticonvulsant actions in the pentylenetetrazol (PTZ), pilocarpine, kindling, bicuculline, and maximal electroshock models of epilepsy [for reviews, see (9, 38) ]. In addition to their ability to decrease seizure susceptibility, neurosteroids also delay the progression of epileptogenesis (136, 137) and are neuroprotective against seizure-induced cell death (138). Furthermore, alterations in the expression of δ-subunit-containing GABAARs, the primary target of neurosteroids, have been observed in the pilocarpine model of temporal lobe epilepsy (139) and have been proposed to play a role in the process of epileptogenesis. Consistent with the anticonvulsant role of neurosteroids, neurosteroid withdrawal has been demonstrated to increase seizure frequency and decrease the anticonvulsant effects of GABA agonists (140 – 142). These data implicate alterations in neurosteroid levels and/or their site of action in epileptogenesis and seizure susceptibility.
It has been proposed that neurosteroids are particularly therapeutically relevant for the treatment of catamenial epilepsy. Catamenial epilepsy is thought to result from changes in hormone levels during the menstrual cycle, resulting in increased seizure frequency at certain stages of the cycle (143). Progesterone has been used as an add-on therapy for the treatment of catamenial epilepsy (144, 145), with some success. Interestingly, simultaneous treatment with finasteride blocks the anticonvulsant actions of progesterone (146), demonstrating that the anticonvulsant effects of progesterone are mediated by neurosteroids. Progesterone withdrawal (147) and neurosteroid withdrawal (148) increases seizure susceptibility, which is thought to represent an animal model of catamenial epilepsy. Interestingly, following neurosteroid withdrawal, the anticonvulsant actions of the synthetic neuroactive steroid ganaxolone are enhanced (149), which may be due to alterations in the expression of neurosteroid-sensitive GABAARs (150). Animal models have demonstrated alterations in GABAARs associated with changes in hormone levels, which are thought to underlie the changes in neuronal excitability related to the estrous cycle (95, 96). Therefore, the evidence supports a role for altered neurosteroid levels and/or their site of action in the pathophysiology of epilepsy, particularly catamenial epilepsy.
Alcohol
Both neurosteroids and ethanol have a shared pharmacological target, GABAARs (7, 151, 152). A neurosteroid-binding site has been identified on the α/β interface of GABAARs (72), demonstrating the direct modulation of GABAARs by neurosteroids. Further, GABAAR δ-subunit-containing receptors confer sensitivity to neurosteroids and are thought to mediate the majority of their effects on GABAergic inhibition (62, 64, 65, 67) (see Neurosteroid Modulation of GABAARs). Because ethanol does not interfere with neurosteroid actions, it is thought to exert its actions on GABAARs via a site independent of the neurosteroid-binding site [for a review, see (153) ]. However, the direct actions of ethanol on specific GABAAR subtypes have been more controversial. Studies have demonstrated that ethanol enhances tonic GABAergic inhibition (154 – 156) likely via actions on GABAAR δ-subunit-containing receptors (157 – 159). However, as stated, these findings remain controversial and have not been able to be replicated by other investigators [for reviews, see (160, 161) ].
Ethanol has been shown to increase circulating concentrations of neurosteroids (162 – 166), which plays a role in modulating the sensitivity to ethanol [for reviews, see (167 – 169) ]. For example, ethanol-induced elevations in neurosteroid levels mediate the sedative properties of ethanol (170), ethanol-induced impairments in memory (171, 172), the anxiolytic and antidepressant properties of ethanol (173, 174), as well as the anticonvulsant effects (165). However, neurosteroids do not mediate the ethanol-induced motor impairments (175). These data demonstrate that ethanol induces elevations in neurosteroid levels, which, in part, mediate the behavioral effects of alcohol.
Neurodegeneration
Decreased levels of neurosteroids have been observed in patients with neurodegenerative diseases [for a review, see (176) ]. Allopregnanolone levels are decreased in patients with Alzheimer disease (AD), Parkinson disease (PD), multiple sclerosis (MS), and Niemann-Pick type C disease [for reviews, see (176, 177) ]. The expression of StAR, (178) one of the major neurosteroidogenic enzymes, is elevated in patients with AD. Similarly, there are changes in the expression of neurosteroidogenic enzymes in PD, MS, and Niemann-Pick type C disease [for a review, see (177) ]. Increased expression of the enzymes involved in neurosteroidogenesis has been proposed to reflect compensatory changes due to the decreased levels of neurosteroids related to neurodegeneration (176). Consistent with the involvement of neurosteroid deficits in neurodegenerative diseases, neurosteroids have been shown to have neuroprotective properties in numerous different animal models [for a review, see (179) ]. For instance, in a rodent model of Niemann-Pick type C disease, a lysosomal storage disorder with neuronal loss and a reduction in neurosteroidogenesis, administration of a single dose of allopregnanolone in the neonatal period significantly prevented neuronal cell death and a delay in the development in neurological symptoms. Although the exact mechanisms underlying the protective effects of allopregnanolone are unclear, these studies demonstrate the therapeutic potential of neurosteroids for some neurodegenerative disorders (180, 181) [for a review, see (182) ]. Thus, several studies implicate neurosteroids in the pathophysiology of several neurodegenerative disorders, including AD, PD, MS, and Niemann-Pick type C disease.
Therapeutic potential of neurosteroids
Neurosteroids have been demonstrated to have a therapeutic potential, particularly in patients with epilepsy (144, 145). However, naturally occurring neurosteroids have several limitations, which minimize their therapeutic potential. First, neurosteroids are rapidly metabolized and thus have low bioavailability [for a review, see (9) ]. In addition, neurosteroids can be converted to compounds that can act on steroid hormone receptors (183), thus mediating unwanted actions that may offset the desired effects of these compounds. Due to these limitations, synthetic neurosteroids have been designed that exhibit a better pharmacological profile than endogenous neurosteroids. For example, ganaxolone is a synthetic analogue of allopregnanolone developed as a potential therapeutic agent [for reviews, see (184, 185) ]. Ganaxolone has been shown to be effective in animal models of, infantile spasms (186), catamenial epilepsy (149), PTZ-induced seizures (187, 188), and kindling (140). In clinical trials, ganaxolone has shown to significantly improve seizure frequency in epileptic adults and infants/children (184, 186, 189) and was explored as a sleep aide [for reviews, see (184, 185) ]. However, the enthusiasm for the therapeutic potential of ganaxolone has diminished due to the adverse side effects, the most common of which were somnolence and nausea [for reviews, see (69, 184) ].
Acknowledgments
J.M. is supported by NS073574.
Biographies
 Georgina MacKenzie received her bachelor’s degree in Biochemistry from the University of Bath (Bath, UK) in 2006. She then moved to Imperial College London (London, UK) where she completed a Master’s degree in Biochemical Research in 2007 before pursuing a PhD in Neuroscience under the supervision of Dr Stephen Brickley. She was awarded her PhD in 2011 and is now undertaking her postdoctoral training with Dr Jamie Maguire in the Department of Neuroscience at Tufts University School of Medicine (Boston, MA, USA).
Georgina MacKenzie received her bachelor’s degree in Biochemistry from the University of Bath (Bath, UK) in 2006. She then moved to Imperial College London (London, UK) where she completed a Master’s degree in Biochemical Research in 2007 before pursuing a PhD in Neuroscience under the supervision of Dr Stephen Brickley. She was awarded her PhD in 2011 and is now undertaking her postdoctoral training with Dr Jamie Maguire in the Department of Neuroscience at Tufts University School of Medicine (Boston, MA, USA).
 Jamie Maguire received her bachelor’s degree in Neuroscience from the University of Pittsburgh (Pittsburgh, PA) in 1998 and then earned her PhD in Neuroscience in 2003 from The George Washington University (Washington, DC) under the mentorship of Dr. Margaret Sutherland. Jamie then trained as a postdoctoral fellow with Dr. Istvan Mody at the University of California, Los Angeles (Los Angeles, CA) prior to establishing her own laboratory in the Neuroscience Department at the Tufts University School of Medicine.
Jamie Maguire received her bachelor’s degree in Neuroscience from the University of Pittsburgh (Pittsburgh, PA) in 1998 and then earned her PhD in Neuroscience in 2003 from The George Washington University (Washington, DC) under the mentorship of Dr. Margaret Sutherland. Jamie then trained as a postdoctoral fellow with Dr. Istvan Mody at the University of California, Los Angeles (Los Angeles, CA) prior to establishing her own laboratory in the Neuroscience Department at the Tufts University School of Medicine.
Contributor Information
Georgina MacKenzie, Department of Neuroscience, School of Medicine, Tufts University, Boston, MA 02111, USA.
Jamie Maguire, Department of Neuroscience, School of Medicine, Tufts University, Boston, MA 02111, USA.
References
- 1.Baulieu EE, Robel P. Neurosteroids: a new brain function? J Steroid Biochem Mol Biol. 1990;37:395–403. doi: 10.1016/0960-0760(90)90490-c. [DOI] [PubMed] [Google Scholar]
- 2.Compagnone NA, Mellon SH. Neurosteroids: biosynthesis and function of these novel neuromodulators. Front Neuroendocrinol. 2000;21:1–56. doi: 10.1006/frne.1999.0188. [DOI] [PubMed] [Google Scholar]
- 3.Reddy DS, Jian K. The testosterone-derived neurosteroid androstanediol is a positive allosteric modulator of GABAA receptors. J Pharmacol Exp Ther. 2010;334:1031–41. doi: 10.1124/jpet.110.169854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic GABAA receptors: form, pharmacology, and function. J Neurosci. 2009;29:12757–63. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belelli D, Lambert JJ. Neurosteroids: endogenous regulators of the GABA(A) receptor. Nat Rev Neurosci. 2005;6:565–75. doi: 10.1038/nrn1703. [DOI] [PubMed] [Google Scholar]
- 6.Harrison NL, Simmonds MA. Alphaxalone selectively potentiates responses to GABA and muscimol in rat cuneate nucleus in vitro. J Physiol (Lond) 1984;346:42. [Google Scholar]
- 7.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid-hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–7. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 8.Smith SS, Shen H, Gong QH, Zhou X. Neurosteroid regulation of GABA(A) receptors: focus on the alpha4 and delta subunits. Pharmacol Ther. 2007;116:58–76. doi: 10.1016/j.pharmthera.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reddy DS. Neurosteroids: endogenous role in the human brain and therapeutic potentials. Prog Brain Res. 2010;186:113–37. doi: 10.1016/B978-0-444-53630-3.00008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maguire J, Ferando I, Simonsen C, Mody I. Excitability changes related to GABAA receptor plasticity during pregnancy. J Neurosci. 2009;29:9592–601. doi: 10.1523/JNEUROSCI.2162-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maguire J, Mody I. Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J Neurosci. 2007;27:2155–62. doi: 10.1523/JNEUROSCI.4945-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Erskine MS. Effects of an anti-androgen and 5 alpha-reductase inhibitors on estrus duration in the cycling female rat. Physiol Behav. 1983;30:519–24. doi: 10.1016/0031-9384(83)90214-7. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy MM, Felzenberg E, Robbins A, Pfaff DW, Schwartz-Giblin S. Infusions of diazepam and allopregnanolone into the midbrain central gray facilitate open-field behavior and sexual receptivity in female rats. Horm Behav. 1995;29:279–95. doi: 10.1006/hbeh.1995.1020. [DOI] [PubMed] [Google Scholar]
- 14.Frye CA. Novel substrates for, and sources of, progestogens for reproduction. J Neuroendocrinol. 2011;23:961–73. doi: 10.1111/j.1365-2826.2011.02180.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van BF, Verkes RJ. Neurosteroids in depression: a review. Psychopharmacology (Berl) 2003;165:97–110. doi: 10.1007/s00213-002-1257-1. [DOI] [PubMed] [Google Scholar]
- 16.Akk G, Covey DF, Evers AS, Steinbach JH, Zorumski CF, Mennerick S. The influence of the membrane on neurosteroid actions at GABA(A) receptors. Psychoneuroendocrinology. 2009;34(Suppl 1):S59–66. doi: 10.1016/j.psyneuen.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mellon SH, Griffin LD, Compagnone NA. Biosynthesis and action of neurosteroids. Brain Res Brain Res Rev. 2001;37:3–12. doi: 10.1016/s0165-0173(01)00109-6. [DOI] [PubMed] [Google Scholar]
- 18.Mensah-Nyagan AG, Do-Rego JL, Beaujean D, Luu-The V, Pelletier G, Vaudry H. Neurosteroids: expression of steroidogenic enzymes and regulation of steroid biosynthesis in the central nervous system. Pharmacol Rev. 1999;51:63–81. [PubMed] [Google Scholar]
- 19.Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, Norenberg MD, Nutt D, Weizman A, Zhang MR, Gavish M. Translocator protein (18 kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–9. doi: 10.1016/j.tips.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 20.Stromstedt M, Waterman MR. Messenger RNAs encoding steroidogenic enzymes are expressed in rodent brain. Brain Res Mol Brain Res. 1995;34:75–88. doi: 10.1016/0169-328x(95)00140-n. [DOI] [PubMed] [Google Scholar]
- 21.Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- 22.Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc Natl Acad Sci USA. 2006;103:14602–7. doi: 10.1073/pnas.0606544103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purdy RH, Morrow AL, Moore PH, Paul SM. Stress-induced elevations of gamma-aminobutyric-acid type-A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;88:4553–7. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Veliskova J, Desantis KA. Sex and hormonal influences on seizures and epilepsy. Horm Behav. 2012 doi: 10.1016/j.yhbeh.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Compagnone NA, Bulfone A, Rubenstein JL, Mellon SH. Steroidogenic enzyme P450c17 is expressed in the embryonic central nervous system. Endocrinology. 1995;136:5212–23. doi: 10.1210/endo.136.11.7588260. [DOI] [PubMed] [Google Scholar]
- 26.Reddy DS, Rogawski MA. Stress-induced deoxycorticosterone-derived neurosteroids modulate GABA(A) receptor function and seizure susceptibility. J Neurosci. 2002;22:3795–805. doi: 10.1523/JNEUROSCI.22-09-03795.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reddy DS. Is there a physiological role for the neurosteroid THDOC in stress-sensitive conditions? Trends Pharmacol Sci. 2003;24:103–6. doi: 10.1016/S0165-6147(03)00023-3. [DOI] [PubMed] [Google Scholar]
- 28.Tuveri A, Paoletti AM, Orrù M, Melis GB, Marotto MF, Zedda P, Marrosu F, Sogliano C, Marra C, Biggio G, Concas A. Reduced serum level of THDOC, an anticonvulsant steroid, in women with perimenstrual catamenial epilepsy. Epilepsia. 2008;49:1221–9. doi: 10.1111/j.1528-1167.2008.01555.x. [DOI] [PubMed] [Google Scholar]
- 29.Eser D, di Michele F, Zwanzger P, Pasini A, Baghai TC, Schüle C, Rupprecht R, Romeo E. Panic induction with cholecystokinin-tetrapeptide (CCK-4) increases plasma concentrations of the neuroactive steroid 3alpha, 5alpha tetrahydrodeoxycorticosterone (3alpha, 5alpha-THDOC) in healthy volunteers. Neuropsychopharmacology. 2005;30:192–5. doi: 10.1038/sj.npp.1300572. [DOI] [PubMed] [Google Scholar]
- 30.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barbaccia ML, Roscetti G, Bolacchi F, Concas A, Mostallino MC, Purdy RH, Biggio G. Stress-induced increase in brain neuroactive steroids: antagonism by abecarnil. Pharmacol Biochem Behav. 1996;54:205–10. doi: 10.1016/0091-3057(95)02133-7. [DOI] [PubMed] [Google Scholar]
- 32.Genazzani AR, Petraglia F, Bernardi F, Casarosa E, Salvestroni C, Tonetti A, Nappi RE, Luisi S, Palumbo M, Purdy RH, Luisi M. Circulating levels of allopregnanolone in humans: gender, age, and endocrine influences. J Clin Endocrinol Metab. 1998;83:2099–103. doi: 10.1210/jcem.83.6.4905. [DOI] [PubMed] [Google Scholar]
- 33.Gilbert Evans SE, Ross LE, Sellers EM, Purdy RH, Romach MK. 3alpha-reduced neuroactive steroids and their precursors during pregnancy and the postpartum period. Gynecol Endocrinol. 2005;21:268–79. doi: 10.1080/09513590500361747. [DOI] [PubMed] [Google Scholar]
- 34.Purdy RH, Moore PH, Morrow AL, Paul SM. Neurosteroids and GABA(A) receptor function. Adv Biochem Psychopharmacol. 1992;47:87–92. [PubMed] [Google Scholar]
- 35.Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci USA. 1998;95:13284–9. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vallee M, Rivera JD, Koob GF, Purdy RH, Fitzgerald RL. Quantification of neurosteroids in rat plasma and brain following swim stress and allopregnanolone administration using negative chemical ionization gas chromatography/mass spectrometry. Anal Biochem. 2000;287:153–66. doi: 10.1006/abio.2000.4841. [DOI] [PubMed] [Google Scholar]
- 37.Paul SM, Purdy RH. Neuroactive Steroids. FASEB J. 1992;6:2311–22. [PubMed] [Google Scholar]
- 38.Reddy DS. The role of neurosteroids in the pathophysiology and treatment of catamenial epilepsy. Epilepsy Res. 2009;85:1–30. doi: 10.1016/j.eplepsyres.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pearson Murphy BE, Steinberg SI, Hu FY, Allison CM. Neuroactive ring A-reduced metabolites of progesterone in human plasma during pregnancy: elevated levels of 5 alpha-dihydroprogesterone in depressed patients during the latter half of pregnancy. J Clin Endocrinol Metab. 2001;86:5981–7. doi: 10.1210/jcem.86.12.8122. [DOI] [PubMed] [Google Scholar]
- 40.Luisi S, Petraglia F, Benedetto C, Nappi RE, Bernardi F, Fadalti M, Reis FM, Luisi M, Genazzani AR. Serum allopregnanolone levels in pregnant women: changes during pregnancy, at delivery, and in hypertensive patients. J Clin Endocrinol Metab. 2000;85:2429–33. doi: 10.1210/jcem.85.7.6675. [DOI] [PubMed] [Google Scholar]
- 41.Hill M, Cibula D, Havlíková H, Kancheva L, Fait T, Kancheva R, Parízek A, Stárka L. Circulating levels of pregnanolone isomers during the third trimester of human pregnancy. J Steroid Biochem Mol Biol. 2007;105:166–75. doi: 10.1016/j.jsbmb.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 42.Liu S, Sjovall J, Griffiths WJ. Neurosteroids in rat brain: extraction, isolation, and analysis by nanoscale liquid chromatography-electrospray mass spectrometry. Anal Chem. 2003;75:5835–46. doi: 10.1021/ac0346297. [DOI] [PubMed] [Google Scholar]
- 43.Liere P, Pianos A, Eychenne B, Cambourg A, Liu S, Griffiths W, Schumacher M, Sjövall J, Baulieu EE. Novel lipoidal derivatives of pregnenolone and dehydroepiandrosterone and absence of their sulfated counterparts in rodent brain. J Lipid Res. 2004;45:2287–302. doi: 10.1194/jlr.M400244-JLR200. [DOI] [PubMed] [Google Scholar]
- 44.Bixo M, Andersson A, Winblad B, Purdy RH, Backstrom T. Progesterone, 5 alpha-pregnane-3,20-dione and 3 alpha-hydroxy-5 alpha-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res. 1997;764:173–8. doi: 10.1016/s0006-8993(97)00455-1. [DOI] [PubMed] [Google Scholar]
- 45.Kancheva R, Hill M, Novak Z, Chrastina J, Kancheva L, Starka L. Neuroactive steroids in periphery and cerebrospinal fluid. Neuroscience. 2011;191:22–7. doi: 10.1016/j.neuroscience.2011.05.054. [DOI] [PubMed] [Google Scholar]
- 46.Lacroix C, Fiet J, Benais JP, Gueux B, Bonete R, Villette JM, Gourmel B, Dreux C. Simultaneous radioimmunoassay of progesterone, androst-4-enedione, pregnenolone, dehydroepiandrosterone and 17-hydroxyprogesterone in specific regions of human brain. J Steroid Biochem. 1987;28:317–25. doi: 10.1016/0022-4731(87)91025-9. [DOI] [PubMed] [Google Scholar]
- 47.Higashi T, Daifu Y, Ikeshima T, Yagi T, Shimada K. Studies on neurosteroids XV. Development of enzyme-linked immunosorbent assay for examining whether pregnenolone sulfate is a veritable neurosteroid. J Pharm Biomed Anal. 2003;30:1907–17. doi: 10.1016/s0731-7085(02)00534-4. [DOI] [PubMed] [Google Scholar]
- 48.Henderson LP. Steroid modulation of GABAA receptor-mediated transmission in the hypothalamus: effects on reproductive function. Neuropharmacology. 2007;52:1439–53. doi: 10.1016/j.neuropharm.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schumacher M, Liere P, Akwa Y, Rajkowski K, Griffiths W, Bodin K, Sjövall J, Baulieu EE. Pregnenolone sulfate in the brain: a controversial neurosteroid. Neurochem Int. 2008;52:522–40. doi: 10.1016/j.neuint.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 50.Simon J, Wakimoto H, Fujita N, Lalande M, Barnard EA. Analysis of the set of GABA(A) receptor genes in the human genome. J Biol Chem. 2004;279:41422–35. doi: 10.1074/jbc.M401354200. [DOI] [PubMed] [Google Scholar]
- 51.Chang Y, Wang R, Barot S, Weiss DS. Stoichiometry of a recombinant GABAA receptor. J Neurosci. 1996;16:5415–24. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tretter V, Ehya N, Fuchs K, Sieghart W. Stoichiometry and assembly of a recombinant GABAA receptor subtype. J Neurosci. 1997;17:2728–37. doi: 10.1523/JNEUROSCI.17-08-02728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mody I, Pearce RA. Diversity of inhibitory neurotransmission through GABAA receptors. Trends Neurosci. 2004;27:569–75. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 54.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–29. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 55.Nusser Z, Sieghart W, Somogyi P. Segregation of different GABA(A) receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wei WZ, Zhang NH, Peng ZC, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABA(A) receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–61. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saxena NC, Macdonald RL. Properties of putative cerebellar gamma-aminobutyric acid(A) receptor isoforms. Mol Pharmacol. 1996;49:567–79. [PubMed] [Google Scholar]
- 58.Brickley SG, CullCandy SG, Farrant M. Development of a tonic form of synaptic inhibition in rat cerebellar granule cells resulting from persistent activation of GABA(A) receptors. J Physiol Lond. 1996;497:753–9. doi: 10.1113/jphysiol.1996.sp021806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu Y, Wang W, Diez-Sampedro A, Richerson GB. Nonvesicular inhibitory neurotransmission via reversal of the GABA transporter GAT-1. Neuron. 2007;56:851–65. doi: 10.1016/j.neuron.2007.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lambert JJ, Belelli D, HillVenning C, Peters JA. Neurosteroids and GABA(A) receptor function. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- 61.Lambert JJ, Belelli D, Harney SC, Peters JA, Frenguelli BG. Modulation of native and recombinant GABA(A) receptors by endogenous and synthetic neuroactive steroids. Brain Res Rev. 2001;37:68–80. doi: 10.1016/s0165-0173(01)00124-2. [DOI] [PubMed] [Google Scholar]
- 62.Belelli D, Casula A, Ling A, Lambert JJ. The influence of subunit composition on the interaction of neurosteroids with GABA(A) receptors. Neuropharmacology. 2002;43:651–61. doi: 10.1016/s0028-3908(02)00172-7. [DOI] [PubMed] [Google Scholar]
- 63.Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate GABAA receptors through two discrete transmembrane sites. Nature. 2006;444:486–9. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 64.Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABA(A) receptors. Proc Natl Acad Sci USA. 2003;100:14439–44. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mihalek RM, Banerjee PK, Korpi ER, Quinlan JJ, Firestone LL, Mi ZP, Lagenaur C, Tretter V, Sieghart W, Anagnostaras SG, Sage JR, Fanselow MS, Guidotti A, Spigelman I, Li Z, DeLorey TM, Olsen RW, Homanics GE. Attenuated sensitivity to neuroactive steroids in gamma-aminobutyrate type A receptor delta subunit knockout mice. Proc Natl Acad Sci USA. 1999;96:12905–10. doi: 10.1073/pnas.96.22.12905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Houston CM, McGee TP, Mackenzie G, Troyano-Cuturi K, Rodriguez PM, Kutsarova E, Diamanti E, Hosie AM, Franks NP, Brickley SG. Are extrasynaptic GABAA receptors important targets for sedative/hypnotic drugs ? J Neurosci. 2012;32:3887– 97. doi: 10.1523/JNEUROSCI.5406-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary GABA(A) receptors containing the delta subunit. J Neurosci. 2002;22:1541–9. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bianchi MT, Macdonald RL. Neurosteroids shift partial agonist activation of GABA(A) receptor channels from low- to high-efficacy gating patterns. J Neurosci. 2003;23:10934–43. doi: 10.1523/JNEUROSCI.23-34-10934.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Brickley SG, Mody I. Extrasynaptic GABA(A) receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Twyman RE, Macdonald RL. Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. J Physiol. 1992;456:215–45. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chisari M, Eisenman LN, Covey DF, Mennerick S, Zorumski CF. The sticky issue of neurosteroids and GABA(A) receptors. Trends Neurosci. 2010;33:299–306. doi: 10.1016/j.tins.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hosie AM, Clarke L, da SH, Smart TG. Conserved site for neurosteroid modulation of GABA A receptors. Neuropharmacology. 2009;56:149–54. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- 73.Hosie AM, Wilkins ME, Smart TG. Neurosteroid binding sites on GABA(A) receptors. Pharmacol Ther. 2007;116:7–19. doi: 10.1016/j.pharmthera.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 74.Mitchell EA, Herd MB, Gunn BG, Lambert JJ, Belelli D. Neurosteroid modulation of GABAA receptors: molecular determinants and significance in health and disease. Neurochem Int. 2008;52:588–95. doi: 10.1016/j.neuint.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 75.Akk G, Li P, Bracamontes J, Reichert DE, Covey DF, Steinbach JH. Mutations of the GABA-A receptor alpha1 subunit M1 domain reveal unexpected complexity for modulation by neuroactive steroids. Mol Pharmacol. 2008;74:614–27. doi: 10.1124/mol.108.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bracamontes JR, Li P, Akk G, Steinbach JH. A neurosteroid potentiation site can be moved among GABAA receptor subunits. J Physiol. 2012 doi: 10.1113/jphysiol.2012.237255.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chen ZW, Manion B, Townsend RR, Reichert DE, Covey DF, Steinbach JH, Sieghart W, Fuchs K, Evers AS. Neurosteroid analogue photolabeling of a site in the TM3 domain of the beta3 subunit of the GABAA receptor. Mol Pharmacol. 2012;82:408–19. doi: 10.1124/mol.112.078410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li GD, Chiara DC, Cohen JB, Olsen RW. Neurosteroids allosterically modulate binding of the anesthetic etomidate to gamma-aminobutyric acid type A receptors. J Biol Chem. 2009;284:11771–5. doi: 10.1074/jbc.C900016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Davies PA, Hanna MC, Hales TG, Kirkness EF. Insensitivity to anaesthetic agents conferred by a class of GABA(A) receptor subunit. Nature. 1997;385:820–3. doi: 10.1038/385820a0. [DOI] [PubMed] [Google Scholar]
- 80.Ranna M, Sinkkonen ST, Moykkynen T, Uusi-Oukari M, Korpi ER. Impact of epsilon and theta subunits on pharmacological properties of alpha3beta1 GABAA receptors expressed in Xenopus oocytes. BMC Pharmacol. 2006;6:1. doi: 10.1186/1471-2210-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Whiting PJ, McAllister G, Vassilatis D, Bonnert TP, Heavens RP, Smith DW, Hewson L, O’Donnell R, Rigby MR, Sirinathsinghji DJ, Marshall G, Thompson SA, Wafford KA, Vasilatis D. Neuronally restricted RNA splicing regulates the expression of a novel GABAA receptor subunit conferring atypical functional properties [corrected; erratum to be published] J Neurosci. 1997;17:5027–37. doi: 10.1523/JNEUROSCI.17-13-05027.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thompson SA, Bonnert TP, Whiting PJ, Wafford KA. Functional characteristics of recombinant human GABA(A) receptors containing the epsilon-subunit. Toxicol Lett. 1998;100–1:233–8. doi: 10.1016/s0378-4274(98)00190-8. [DOI] [PubMed] [Google Scholar]
- 83.Thompson SA, Bonnert TP, Cagetti E, Whiting PJ, Wafford KA. Overexpression of the GABA(A) receptor epsilon subunit results in insensitivity to anaesthetics. Neuropharmacology. 2002;43:662–8. doi: 10.1016/s0028-3908(02)00162-4. [DOI] [PubMed] [Google Scholar]
- 84.Maksay G, Thompson SA, Wafford KA. The pharmacology of spontaneously open alpha 1 beta 3 epsilon GABA A receptor-ionophores. Neuropharmacology. 2003;44:994–1002. doi: 10.1016/s0028-3908(03)00116-3. [DOI] [PubMed] [Google Scholar]
- 85.Wagner DA, Goldschen-Ohm MP, Hales TG, Jones MV. Kinetics and spontaneous open probability conferred by the epsilon subunit of the GABAA receptor. J Neurosci. 2005;25:10462–8. doi: 10.1523/JNEUROSCI.1658-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lambert JJ, Belelli D, Peden DR, Vardy AW, Peters JA. Neurosteroid modulation of GABA(A) receptors. Prog Neurobiol. 2003;71:67–80. doi: 10.1016/j.pneurobio.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 87.Hengen KB, Nelson NR, Stang KM, Johnson SM, Crader SM, Watters JJ, Mitchell GS, Behan M. Increased GABA(A) receptor epsilon-subunit expression on ventral respiratory column neurons protects breathing during pregnancy. PLoS One. 2012;7:e30608. doi: 10.1371/journal.pone.0030608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABA(A) receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–9. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 89.Silver RA. Neuronal arithmetic. Nat Rev Neurosci. 2010;11:474–89. doi: 10.1038/nrn2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pavlov I, Savtchenko LP, Kullmann DM, Semyanov A, Walker MC. Outwardly rectifying tonically active GABAA receptors in pyramidal cells modulate neuronal offset, not gain. J Neurosci. 2009;29:15341–50. doi: 10.1523/JNEUROSCI.2747-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Maguire J, Mody I. Steroid hormone fluctuations and GABA(A)R plasticity. Psychoneuroendocrinology. 2009;34:S84–90. doi: 10.1016/j.psyneuen.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Follesa P, Biggio F, Caria S, Gorini G, Biggio G. Modulation of GABA(A) receptor gene expression by allopregnanolone and ethanol. Eur J Pharmacol. 2004;500:413–25. doi: 10.1016/j.ejphar.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 93.Majewska MD, Fordrice F, Falkay G. Pregnancy-induced alterations of GABAA receptor sensitivity in maternal brain – an antecedent of post-partum blues. Brain Res. 1989;482:397–401. doi: 10.1016/0006-8993(89)91208-0. [DOI] [PubMed] [Google Scholar]
- 94.Maguire J, Mody I. GABA(A)R plasticity during pregnancy: relevance to postpartum depression. Neuron. 2008;59:207–13. doi: 10.1016/j.neuron.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in GABA(A) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 96.Smith SS, Gong QH, Li XS, Moran MH, Bitran D, Frye CA, Hsu FC. Withdrawal from 3 alpha-OH-5 alpha-pregnan-20-one using a pseudopregnancy model alters the kinetics of hippocampal GABA(A)-gated current and increases the GABA(A), receptor alpha 4 subunit in association with increased anxiety. J Neurosci. 1998;18:5275–84. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shen H, Gong QH, Yuan M, Smith SS. Short-term steroid treatment increases delta GABA(A) receptor subunit expression in rat CA1 hippocampus: pharmacological and behavioral effects. Neuropharmacology. 2005;49:573–86. doi: 10.1016/j.neuropharm.2005.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Griffiths JL, Lovick TA. GABAergic neurones in the rat periaqueductal grey matter express alpha4, beta1 and delta GABAA receptor subunits: plasticity of expression during the estrous cycle. Neuroscience. 2005;136:457–66. doi: 10.1016/j.neuroscience.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 99.Penatti CA, Oberlander JG, Davis MC, Porter DM, Henderson LP. Chronic exposure to anabolic androgenic steroids alters activity and synaptic function in neuroendocrine control regions of the female mouse. Neuropharmacology. 2011;61:653–64. doi: 10.1016/j.neuropharm.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Concas A, Follesa P, Barbaccia ML, Purdy RH, Biggio G. Physiological modulation of GABA(A) receptor plasticity by progesterone metabolites. Eur J Pharmacol. 1999;375:225–35. doi: 10.1016/s0014-2999(99)00232-0. [DOI] [PubMed] [Google Scholar]
- 101.Follesa P, Floris S, Tuligi G, Mostallino MC, Concas A, Biggio G. Molecular and functional adaptation of the GABA(A) receptor complex during pregnancy and after delivery in the rat brain. Eur J Neurosci. 1998;10:2905–12. doi: 10.1111/j.1460-9568.1998.00300.x. [DOI] [PubMed] [Google Scholar]
- 102.Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases alpha 4 GABA(A) receptor subunit levels in association with increased anxiety in the famale rat. Brain Res. 2001;910:55–66. doi: 10.1016/s0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hsu FC, Waldeck R, Faber DS, Smith SS. Neurosteroid effects on GABAergic synaptic plasticity in hippocampus. J Neurophysiol. 2003;89:1929–40. doi: 10.1152/jn.00780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.di Michele F, Longone P, Romeo E, Lucchetti S, Brusa L, Pierantozzi M, Bassi A, Bernardi G, Stanzione P. Decreased plasma and cerebrospinal fluid content of neuroactive steroids in Parkinson’s disease. Neurol Sci. 2003;24:172–3. doi: 10.1007/s10072-003-0115-1. [DOI] [PubMed] [Google Scholar]
- 105.Guidotti A, Costa E. Can the antidysphoric and anxiolytic profiles of selective serotonin reuptake inhibitors be related to their ability to increase brain 3 alpha, 5 alpha-tetrahydroprogesterone (allopregnanolone) availability? Biol Psychiatry. 1998;44:865–73. doi: 10.1016/s0006-3223(98)00070-5. [DOI] [PubMed] [Google Scholar]
- 106.Romeo E, Ströhle A, Spalletta G, di Michele F, Hermann B, Holsboer F, Pasini A, Rupprecht R. Effects of antidepressant treatment on neuroactive steroids in major depression. Am J Psychiatry. 1998;155:910–3. doi: 10.1176/ajp.155.7.910. [DOI] [PubMed] [Google Scholar]
- 107.Uzunova V, Sheline Y, Davis JM, Rasmusson A, Uzunov DP, Costa E, Guidotti A. Increase in the cerebrospinal fluid content of neurosteroids in patients with unipolar major depression who are receiving fluoxetine or fluvoxamine. Proc Natl Acad Sci USA. 1998;95:3239–44. doi: 10.1073/pnas.95.6.3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schumacher M, Weill-Engerer S, Liere P, Robert F, Franklin RJ, Garcia-Segura LM, Lambert JJ, Mayo W, Melcangi RC, Parducz A, Suter U, Carelli C, Baulieu EE, Akwa Y. Steroid hormones and neurosteroids in normal and pathological aging of the nervous system. Prog Neurobiol. 2003;71:3–29. doi: 10.1016/j.pneurobio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 109.Strous RD, Maayan R, Lapidus R, Stryjer R, Lustig M, Kotler M, Weizman A. Dehydroepiandrosterone augmentation in the management of negative, depressive, and anxiety symptoms in schizophrenia. Arch Gen Psychiatry. 2003;60:133–41. doi: 10.1001/archpsyc.60.2.133. [DOI] [PubMed] [Google Scholar]
- 110.Brambilla F, Biggio G, Pisu MG, Purdy RH, Gerra G, Zaimovich A, Serra M. Plasma concentrations of anxiolytic neurosteroids in men with normal anxiety scores: a correlation analysis. Neuropsychobiology. 2004;50:6–9. doi: 10.1159/000077934. [DOI] [PubMed] [Google Scholar]
- 111.Marx CE, Trost WT, Shampine LJ, Stevens RD, Hulette CM, Steffens DC, Ervin JF, Butterfield MI, Blazer DG, Massing MW, Lieberman JA. The neurosteroid allopregnanolone is reduced in prefrontal cortex in Alzheimer’s disease. Biol Psychiatry. 2006;60:1287–94. doi: 10.1016/j.biopsych.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 112.van Broekhoven F, Verkes RJ. Neurosteroids in depression: a review. Psychopharmacology (Berl) 2003;165:97–110. doi: 10.1007/s00213-002-1257-1. [DOI] [PubMed] [Google Scholar]
- 113.Pinna G, Costa E, Guidotti A. SSRIs act as selective brain steroidogenic stimulants (SBSSs) at low doses that are inactive on 5-HT reuptake. Curr Opin Pharmacol. 2009;9:24–30. doi: 10.1016/j.coph.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pinna G, Costa E, Guidotti A. Fluoxetine and norfluoxetine stereospecifically and selectively increase brain neurosteroid content at doses that are inactive on 5-HT reuptake. Psychopharmacology (Berl) 2006;186:362–72. doi: 10.1007/s00213-005-0213-2. [DOI] [PubMed] [Google Scholar]
- 115.Khisti RT, Chopde CT. Serotonergic agents modulate antidepressant-like effect of the neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one in mice. Brain Res. 2000;865:291–300. doi: 10.1016/s0006-8993(00)02373-8. [DOI] [PubMed] [Google Scholar]
- 116.Khisti RT, Chopde CT, Jain SP. Antidepressant-like effect of the neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one in mice forced swim test. Pharmacol Biochem Behav. 2000;67:137–43. doi: 10.1016/s0091-3057(00)00300-2. [DOI] [PubMed] [Google Scholar]
- 117.Girdler SS, Straneva PA, Light KC, Pedersen CA, Morrow AL. Allopregnanolone levels and reactivity to mental stress in premenstrual dysphoric disorder. Biol Psychiatry. 2001;49:788–97. doi: 10.1016/s0006-3223(00)01044-1. [DOI] [PubMed] [Google Scholar]
- 118.Wihlback AC, Sundstrom-Poromaa I, Backstrom T. Action by and sensitivity to neuroactive steroids in menstrual cycle related CNS disorders. Psychopharmacology (Berl) 2006;186:388–401. doi: 10.1007/s00213-005-0185-2. [DOI] [PubMed] [Google Scholar]
- 119.Freeman EW, Frye CA, Rickels K, Martin PA, Smith SS. Allopregnanolone levels and symptom improvement in severe premenstrual syndrome. J Clin Psychopharmacol. 2002;22:516–20. doi: 10.1097/00004714-200210000-00013. [DOI] [PubMed] [Google Scholar]
- 120.Bäckström T, Andreen L, Birzniece V, Björn I, Johansson IM, Nordenstam-Haghjo M, Nyberg S, Sundström-Poromaa I, Wahlström G, Wang M, Zhu D. The role of hormones and hormonal treatments in premenstrual syndrome. CNS Drugs. 2003;17:325–42. doi: 10.2165/00023210-200317050-00003. [DOI] [PubMed] [Google Scholar]
- 121.Halbreich U, Borenstein J, Pearlstein T, Kahn LS. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD) Psychoneuroendocrinology. 2003;28:1–23. doi: 10.1016/s0306-4530(03)00098-2. [DOI] [PubMed] [Google Scholar]
- 122.Rasmusson AM, Pinna G, Paliwal P, Weisman D, Gottschalk C, Charney D, Krystal J, Guidotti A. Decreased cerebrospinal fluid allopregnanolone levels in women with posttraumatic stress disorder. Biol Psychiatry. 2006;60:704–13. doi: 10.1016/j.biopsych.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 123.Ströhle A, Romeo E, di Michele F, Pasini A, Yassouridis A, Holsboer F, Rupprecht R. GABA(A) receptor-modulating neuroactive steroid composition in patients with panic disorder before and during paroxetine treatment. Am J Psychiatry. 2002;159:145–7. doi: 10.1176/appi.ajp.159.1.145. [DOI] [PubMed] [Google Scholar]
- 124.Ströhle A, Romeo E, di Michele F, Pasini A, Yassouridis A, Holsboer F, Rupprecht R. Induced panic attacks shift gamma-aminobutyric acid type A receptor modulatory neuroactive steroid composition in patients with panic disorder: preliminary results. Arch Gen Psychiatry. 2003;60:161–8. doi: 10.1001/archpsyc.60.2.161. [DOI] [PubMed] [Google Scholar]
- 125.Zwanzger P, Eser D, Padberg F, Baghai TC, Schüle C, Rupprecht R, di Michele F, Romeo E, Pasini A, Ströhle A. Neuroactive steroids are not affected by panic induction with 50 microg cholecystokinin-tetrapeptide (CCK-4) in healthy volunteers. J Psychiatr Res. 2004;38:215–7. doi: 10.1016/s0022-3956(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 126.Rupprecht R. Neuroactive steroids: mechanisms of action and neuropsychopharmacological properties. Psychoneuroendocrinology. 2003;28:139–68. doi: 10.1016/s0306-4530(02)00064-1. [DOI] [PubMed] [Google Scholar]
- 127.Crawley JN, Glowa JR, Majewska MD, Paul SM. Anxiolytic activity of an endogenous adrenal steroid. Brain Res. 1986;398:382–5. doi: 10.1016/0006-8993(86)91500-3. [DOI] [PubMed] [Google Scholar]
- 128.Bitran D, Shiekh M, Mcleod M. Anxiolytic effect of progesterone is mediated by the neurosteroid allopregnanolone at brain GABA(A) receptors. J Neuroendocrinol. 1995;7:171–7. doi: 10.1111/j.1365-2826.1995.tb00744.x. [DOI] [PubMed] [Google Scholar]
- 129.Weiland NG, Orchinik M. Specific subunit mRNAs of the GABAA receptor are regulated by progesterone in subfields of the hippocampus. Brain Res Mol Brain Res. 1995;32:271–8. doi: 10.1016/0169-328x(95)00087-9. [DOI] [PubMed] [Google Scholar]
- 130.Reddy DS, Kulkarni SK. Development of neurosteroid-based novel psychotropic drugs. Prog Med Chem. 2000;37:135–75. doi: 10.1016/s0079-6468(08)70059-6. [DOI] [PubMed] [Google Scholar]
- 131.Finn DA, Roberts AJ, Long S, Tanchuck M, Phillips TJ. Neurosteroid consumption has anxiolytic effects in mice. Pharmacol Biochem Behav. 2003;76:451–62. doi: 10.1016/j.pbb.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 132.Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3A-hydroxy-5A[beta]-pregnan-20-one – endogenous metabolites of progesterone that are active at the GABA-A receptor. Brain Res. 1991;561:157–61. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- 133.Reddy DS, Kulkarni SK. Differential anxiolytic effects of neurosteroids in the mirrored chamber behavior test in mice. Brain Res. 1997;752:61–71. doi: 10.1016/s0006-8993(96)01447-3. [DOI] [PubMed] [Google Scholar]
- 134.Rodgers RJ, Johnson NJ. Behaviorally selective effects of neuroactive steroids on plus-maze anxiety in mice. Pharmacol Biochem Behav. 1998;59:221–32. doi: 10.1016/s0091-3057(97)00339-0. [DOI] [PubMed] [Google Scholar]
- 135.Sarkar J, Wakefield S, Mackenzie G, Moss SJ, Maguire J. Neurosteroidogenesis is required for the physiological response to stress: role of neurosteroid-sensitive GABAA receptors. J Neurosci. 2011;31:18198–210. doi: 10.1523/JNEUROSCI.2560-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Biagini G, Baldelli E, Longo D, Pradelli L, Zini I, Rogawski MA, Avoli M. Endogenous neurosteroids modulate epileptogenesis in a model of temporal lobe epilepsy. Exp Neurol. 2006;201:519–24. doi: 10.1016/j.expneurol.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 137.Biagini G, Longo D, Baldelli E, Zoli M, Rogawski MA, Bertazzoni G, Avoli M. Neurosteroids and epileptogenesis in the pilocarpine model: evidence for a relationship between P450scc induction and length of the latent period. Epilepsia. 2009;50(Suppl 1):53–8. doi: 10.1111/j.1528-1167.2008.01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Frye CA. The neurosteroid 3 alpha, 5 alpha-THP has antiseizure and possible neuroprotective effects in an animal model of epilepsy. Brain Res. 1995;696:113–20. doi: 10.1016/0006-8993(95)00793-p. [DOI] [PubMed] [Google Scholar]
- 139.Peng ZC, Huang CS, Stell BM, Mody I, Houser CR. Altered expression of the delta subunit of the GABA(A) receptor in a mouse model of temporal lobe epilepsy. J Neurosci. 2004;24:8629–39. doi: 10.1523/JNEUROSCI.2877-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Reddy DS, Rogawski MA. Ganaxolone suppression of behavioral and electrographic seizures in the mouse amygdala kindling model. Epilepsy Res. 2010;89:254–60. doi: 10.1016/j.eplepsyres.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Reddy DS, Rogawski MA. Chronic treatment with the neuroactive steroid ganaxolone in the rat induces anticonvulsant tolerance to diazepam but not to itself. J Pharmacol Exp Ther. 2000;295:1241–8. [PubMed] [Google Scholar]
- 142.Lawrence C, Martin BS, Sun C, Williamson J, Kapur J. Endogenous neurosteroid synthesis modulates seizure frequency. Ann Neurol. 2010;67:689–93. doi: 10.1002/ana.21989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–8. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- 144.Herzog AG. Progesterone Therapy in Women with Complex Partial and Secondary Generalized Seizures. Neurology. 1995;45:1660–2. doi: 10.1212/wnl.45.9.1660. [DOI] [PubMed] [Google Scholar]
- 145.Herzog AG. Progesterone therapy in women with epilepsy: a 3-year follow-up. Neurology. 1999;52:1917–8. doi: 10.1212/wnl.52.9.1917-a. [DOI] [PubMed] [Google Scholar]
- 146.Herzog AG, Frye CA. Seizure exacerbation associated with inhibition of progesterone metabolism. Ann Neurol. 2003;53:390–1. doi: 10.1002/ana.10508. [DOI] [PubMed] [Google Scholar]
- 147.Moran MH, Smith SS. Progesterone withdrawal I: pro-convulsant effects. Brain Res. 1998;807:84–90. doi: 10.1016/s0006-8993(98)00782-3. [DOI] [PubMed] [Google Scholar]
- 148.Reddy DS, Kim HY, Rogawski MA. Neurosteroid withdrawal model of perimenstrual catamenial epilepsy. Epilepsia. 2001;42:328–36. doi: 10.1046/j.1528-1157.2001.10100.x. [DOI] [PubMed] [Google Scholar]
- 149.Reddy DS, Rogawski MA. Enhanced anticonvulsant activity of ganaxolone after neurosteroid withdrawal in a rat model of catamenial epilepsy. J Pharmacol Exp Ther. 2000;294:909–15. [PubMed] [Google Scholar]
- 150.Gulinello M, Gong QH, Smith SS. Progesterone withdrawal increases the alpha 4 subunit of the GABA(A) receptor in male rats in association with anxiety and altered pharmacology – a comparison with female rats. Neuropharmacology. 2002;43:701–14. doi: 10.1016/s0028-3908(02)00171-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Allan AM, Harris RA. Gamma-aminobutyric acid and alcohol actions: neurochemical studies of long sleep and short sleep mice. Life Sci. 1986;39:2005–15. doi: 10.1016/0024-3205(86)90324-3. [DOI] [PubMed] [Google Scholar]
- 152.Suzdak PD, Schwartz RD, Skolnick P, Paul SM. Ethanol stimulates gamma-aminobutyric acid receptor-mediated chloride transport in rat brain synaptoneurosomes. Proc Natl Acad Sci USA. 1986;83:4071–5. doi: 10.1073/pnas.83.11.4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Helms CM, Rossi DJ, Grant KA. Neurosteroid influences on sensitivity to ethanol. Front Endocrinol (Lausanne) 2012;3:10. doi: 10.3389/fendo.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wei WZ, Faria LC, Mody I. Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABA(A) receptors in hippocampal neurons. J Neurosci. 2004;24:8379–82. doi: 10.1523/JNEUROSCI.2040-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Hanchar HJ, Dodson PD, Olsen RW, Otis TS, Wallner M. Alcohol-induced motor impairment caused by increased extrasynaptic GABA(A) receptor activity. Nat Neurosci. 2005;8:339–45. doi: 10.1038/nn1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Glykys J, Mody I. Activation of GABAA receptors: views from outside the synaptic cleft. Neuron. 2007;56:763–70. doi: 10.1016/j.neuron.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 157.Hanchar HJ, Wallner M, Olsen RW. Alcohol effects on gamma-aminobutyric acid type A receptors: are extrasynaptic receptors the answer? Life Sci. 2004;76:1–8. doi: 10.1016/j.lfs.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 158.Olsen RW, Hanchar HJ, Meera P, Wallner M. GABAA receptor subtypes: the “one glass of wine” receptors. Alcohol. 2007;41:201–9. doi: 10.1016/j.alcohol.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mody I. Extrasynaptic GABAA receptors in the crosshairs of hormones and ethanol. Neurochem Int. 2008;52:60–4. doi: 10.1016/j.neuint.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Borghese CM, Harris RA. Studies of ethanol actions on recombinant delta-containing gamma-aminobutyric acid type A receptors yield contradictory results. Alcohol. 2007;41:155–62. doi: 10.1016/j.alcohol.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Botta P, Radcliffe RA, Carta M, Mameli M, Daly E, Floyd KL, Deitrich RA, Valenzuela CF. Modulation of GABAA receptors in cerebellar granule neurons by ethanol: a review of genetic and electrophysiological studies. Alcohol. 2007;41:187–99. doi: 10.1016/j.alcohol.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Morrow AL, VanDoren MJ, Devaud LL. Effects of progesterone or neuroactive steroid? Nature. 1998;395:652–3. doi: 10.1038/27106. [DOI] [PubMed] [Google Scholar]
- 163.Barbaccia ML, Affricano D, Trabucchi M, Purdy RH, Colombo G, Agabio R, Gessa GL. Ethanol markedly increases “GABAergic” neurosteroids in alcohol-preferring rats. Eur J Pharmacol. 1999;384:R1–2. doi: 10.1016/s0014-2999(99)00678-0. [DOI] [PubMed] [Google Scholar]
- 164.Morrow AL, Janis GC, VanDoren MJ, Matthews DB, Samson HH, Janak PH, Grant KA. Neurosteroids mediate pharmacological effects of ethanol: a new mechanism of ethanol action? Alcohol Clin Exp Res. 1999;23:1933–40. doi: 10.1111/j.1530-0277.1999.tb04094.x. [DOI] [PubMed] [Google Scholar]
- 165.VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3alpha-hydroxy-5alpha-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–9. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.O’Dell LE, Alomary AA, Vallee M, Koob GF, Fitzgerald RL, Purdy RH. Ethanol-induced increases in neuroactive steroids in the rat brain and plasma are absent in adrenalectomized and gonadectomized rats. Eur J Pharmacol. 2004;484:241–7. doi: 10.1016/j.ejphar.2003.11.031. [DOI] [PubMed] [Google Scholar]
- 167.Morrow AL, Porcu P, Boyd KN, Grant KA. Hypothalamic-pituitary-adrenal axis modulation of GABAergic neuroactive steroids influences ethanol sensitivity and drinking behavior. Dialogues Clin Neurosci. 2006;8:463–77. doi: 10.31887/DCNS.2006.8.4/amorrow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Biggio G, Concas A, Follesa P, Sanna E, Serra M. Stress, ethanol, and neuroactive steroids. Pharmacol Ther. 2007;116:140–71. doi: 10.1016/j.pharmthera.2007.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Morrow AL, Biggio G, Serra M, Becker HC, Lopez MF, Porcu P, Alward SE, O’Buckley TK. The role of neuroactive steroids in ethanol/stress interactions: proceedings of symposium VII at the Volterra conference on alcohol and stress, May 2008. Alcohol. 2009;43:521–30. doi: 10.1016/j.alcohol.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Khisti RT, VanDoren MJ, O’Buckley T, Morrow AL. Neuroactive steroid 3 alpha-hydroxy-5 alpha-pregnan-20-one modulates ethanol-induced loss of righting reflex in rats. Brain Res. 2003;980:255–65. doi: 10.1016/s0006-8993(03)02978-0. [DOI] [PubMed] [Google Scholar]
- 171.Matthews DB, Morrow AL, Tokunaga S, McDaniel JR. Acute ethanol administration and acute allopregnanolone administration impair spatial memory in the Morris water task. Alcohol Clin Exp Res. 2002;26:1747–51. doi: 10.1097/01.ALC.0000037219.79257.17. [DOI] [PubMed] [Google Scholar]
- 172.Morrow AL, VanDoren MJ, Penland SN, Matthews DB. The role of GABAergic neuroactive steroids in ethanol action, tolerance and dependence. Brain Res Brain Res Rev. 2001;37:98–109. doi: 10.1016/s0165-0173(01)00127-8. [DOI] [PubMed] [Google Scholar]
- 173.Hirani K, Khisti RT, Chopde CT. Behavioral action of ethanol in Porsolt’s forced swim test: modulation by 3 alpha-hydroxy-5 alpha-pregnan-20-one. Neuropharmacology. 2002;43:1339–50. doi: 10.1016/s0028-3908(02)00330-1. [DOI] [PubMed] [Google Scholar]
- 174.Hirani K, Sharma AN, Jain NS, Ugale RR, Chopde CT. Evaluation of GABAergic neuroactive steroid 3alpha-hydroxy-5alpha-pregnane-20-one as a neurobiological substrate for the anti-anxiety effect of ethanol in rats. Psychopharmacology (Berl) 2005;180:267–78. doi: 10.1007/s00213-005-2169-7. [DOI] [PubMed] [Google Scholar]
- 175.Khisti RT, VanDoren MJ, Matthews DB, Morrow AL. Ethanol-induced elevation of 3 alpha-hydroxy-5 alpha-pregnan-20-one does not modulate motor incoordination in rats. Alcohol Clin Exp Res. 2004;28:1249–56. doi: 10.1097/01.alc.0000134232.44210.06. [DOI] [PubMed] [Google Scholar]
- 176.Luchetti S, Huitinga I, Swaab DF. Neurosteroid and GABA-A receptor alterations in Alzheimer’s disease, Parkinson’s disease and multiple sclerosis. Neuroscience. 2011;191:6–21. doi: 10.1016/j.neuroscience.2011.04.010. [DOI] [PubMed] [Google Scholar]
- 177.Mellon SH. Neurosteroid regulation of central nervous system development. Pharmacol Ther. 2007;116:107–24. doi: 10.1016/j.pharmthera.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Mackenzie SM, Dewar D, Stewart W, Fraser R, Connell JM, Davies E. The transcription of steroidogenic genes in the human cerebellum and hippocampus: a comparative survey of normal and Alzheimer’s tissue. J Endocrinol. 2008;196:123–30. doi: 10.1677/JOE-07-0427. [DOI] [PubMed] [Google Scholar]
- 179.Wojtal K, Trojnar MK, Czuczwar SJ. Endogenous neuroprotective factors: neurosteroids. Pharmacol Rep. 2006;58:335–40. [PubMed] [Google Scholar]
- 180.Griffin LD, Gong W, Verot L, Mellon SH. Niemann-Pick type C disease involves disrupted neurosteroidogenesis and responds to allopregnanolone. Nat Med. 2004;10:704–11. doi: 10.1038/nm1073. [DOI] [PubMed] [Google Scholar]
- 181.Liao G, Cheung S, Galeano J, Ji AX, Qin Q, Bi X. Allopregnanolone treatment delays cholesterol accumulation and reduces autophagic/lysosomal dysfunction and inflammation in Npc1−/− mouse brain. Brain Res. 2009;1270:140–51. doi: 10.1016/j.brainres.2009.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mellon SH, Gong W, Schonemann MD. Endogenous and synthetic neurosteroids in treatment of Niemann-Pick Type C disease. Brain Res Rev. 2008;57:410–20. doi: 10.1016/j.brainresrev.2007.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rupprecht R, Reul JM, Trapp T, van Steensel B, Wetzel C, Damm K, Zieglgänsberger W, Holsboer F. Progesterone receptor-mediated effects of neuroactive steroids. Neuron. 1993;11:523–30. doi: 10.1016/0896-6273(93)90156-l. [DOI] [PubMed] [Google Scholar]
- 184.Nohria V, Giller E. Ganaxolone. Neurotherapeutics. 2007;4:102–5. doi: 10.1016/j.nurt.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Reddy DS, Woodward R. Ganaxolone: a prospective overview. Drugs Future. 2004;29:227–42. [Google Scholar]
- 186.Kerrigan JF, Shields WD, Nelson TY, Bluestone DL, Dodson WE, Bourgeois BF, Pellock JM, Morton LD, Monaghan EP. Ganaxolone for treating intractable infantile spasms: a multicenter, open-label, add-on trial. Epilepsy Res. 2000;42:133–9. doi: 10.1016/s0920-1211(00)00170-4. [DOI] [PubMed] [Google Scholar]
- 187.Beekman M, Ungard JT, Gasior M, Carter RB, Dijkstra D, Goldberg SR, Witkin JM. Reversal of behavioral effects of pentylenetetrazol by the neuroactive steroid ganaxolone. J Pharmacol Exp Ther. 1998;284:868–77. [PubMed] [Google Scholar]
- 188.Gasior M, Ungard JT, Beekman M, Carter RB, Witkin JM. Acute and chronic effects of the synthetic neuroactive steroid, ganaxolone, against the convulsive and lethal effects of pentylenetetrazol in seizure-kindled mice: comparison with diazepam and valproate. Neuropharmacology. 2000;39:1184–96. doi: 10.1016/s0028-3908(99)00190-2. [DOI] [PubMed] [Google Scholar]
- 189.Laxer K, Blum D, Abou-Khalil BW, Morrell MJ, Lee DA, Data JL, Monaghan EP. Assessment of ganaxolone’s anticonvulsant activity using a randomized, double-blind, presurgical trial design. Ganaxolone Presurgical Study Group. Epilepsia. 2000;41:1187–94. doi: 10.1111/j.1528-1157.2000.tb00324.x. [DOI] [PubMed] [Google Scholar]