Reduced aerobic capacity: a central feature of normative aging and heart failure
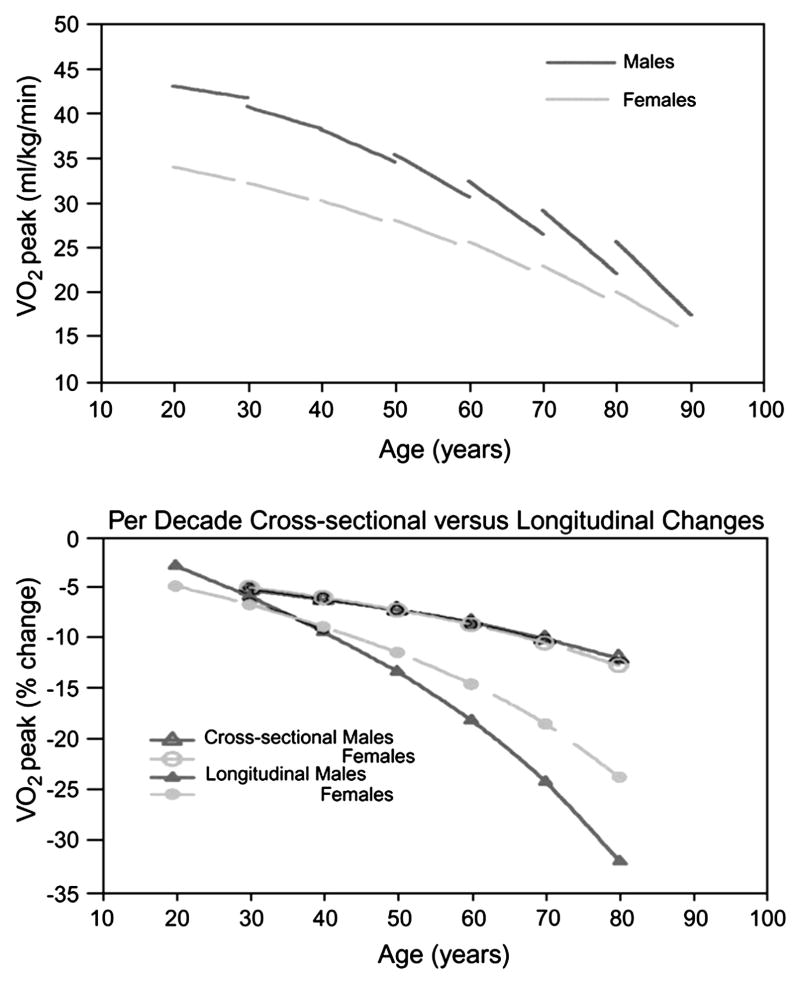
Reduction in aerobic capacity, best quantified by peak oxygen consumption (VO2), is a central feature of both normative aging and chronic heart failure (HF). Numerous observational studies over the past half-century have documented declines in peak VO2 of nearly 50% across the adult age span in apparently healthy populations (Fig. 1) [1–4]. In men, peak VO2 decreases from approximately 45 mL/kg/min in a healthy 25-year-old to ~25 mL/kg/min in a 75-year-old (see Fig. 1). Comparable numbers in women are about 20% lower because of their smaller proportion of muscle mass and lower hemoglobin levels. A healthy 80-year-old woman typically has a peak VO2 of 15–20 mL/kg/min, a range characteristic of mild HF. Even lower peak VO2 is common in older adults with significant comorbidities such as pulmonary disease, and coronary or peripheral arterial disease, arthritis, orthopedic or neurologic disorders that further impair aerobic capacity. Furthermore, recent data suggest that longitudinal age-associated declines in peak VO2 in healthy volunteers accelerate with age, exceeding 20% per decade in the 8th and 9th decades (see Fig. 1) [5].
Figure 1.
Cross-sectional and longitudinal changes in peak VO2 per kg weight in healthy adults by age decade and gender. (Top panel) The per-decade longitudinal change in peak VO2 for age decades from the 20s through the 70s, predicted from a mixed-effects regression model. Peak VO2 declines more steeply with successive age decades, especially in men. (Bottom panel) Per-decade percent cross-sectional and longitudinal changes in peak VO2 by age decade and gender, derived from the mixed-effects model. From the 50s onward, longitudinal declines in peak VO2 substantially exceed cross-sectional declines.
From Fleg JL, Morrell CH, Bos AG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation 2005;12:674–82; with permission.
Peak VO2 is the product of cardiac output (CO) and arteriovenous oxygen (AVO2) difference. In healthy Baltimore Longitudinal Study of Aging volunteers, declines in peak heart rate (HR) and AVO2 difference make similar contributions to the decline in peak VO2 with aging [6]. In contrast, exercise stroke volume (SV) is not age related among individuals screened for the absence of coronary heart disease by clinical criteria and exercise thallium scintigraphy. In these individuals, enhanced use of the Frank–Starling mechanism augments left ventricular end-diastolic volume (LVEDV), compensating for modest blunting of systolic emptying with age [7]. Plasma catecholamines are increased with age at peak exercise [8]. This exercise hemodynamic profile of normal aging resembles that of β-adrenergic blockade of a young adult [9].
Heart failure, like aging, is characterized by a major reduction in peak VO2, which provides powerful prognostic information regarding risk for hospitalization, mortality, and need for ventricular assist devices or or cardiac transplantation [10–12]. The impairment in peak VO2 in these patients is attributable to both cardiac and peripheral factors. In patients with systolic HF, also known as HF with reduced ejection fraction (HFrEF), peak HR and SV are reduced about 20% and 45%, respectively, compared with normal individuals [13,14]. Peripheral factors contributing to reduced AVO2 difference, and thence peak VO2, include reduced muscle mass, decreased mitochondrial density in exercising muscle, and peripheral vasoconstriction because of intrinsic abnormalities of smooth muscle vasodilation and neurohormonal factors [15,16]. A similar constellation of peripheral abnormalities contributes to the age-associated decrease in AVO2 difference at peak exercise (Table 1) [17]. In patients with HF and preserved systolic function (HFpEF), peak VO2 is reduced to nearly the same degree as in patients who have systolic HF [18]. The reduction in peak exercise SV in these individuals is explained primarily by a lower LVEDV [19].
Table 1.
Physiologic similarities between normative aging and heart failure
| Aginga | Heart failure | |
|---|---|---|
| Peak VO2 | ↓ ↓ | ↓ ↓ ↓ |
| Maximal stroke volume | – or ↓ | ↓ ↓ |
| Maximal heart rate | ↓ ↓ | ↓ |
| Maximal AV oxygen difference | ↓↓ | ↓↓ |
| Skeletal muscle mass | ↓ ↓ | ↓ ↓ |
| Mitochondrial oxidative enzymes | ↓ | ↓↓↓ |
Between the third and ninth decades.
From Fleg JL. Can exercise conditioning be effective in older heart failure patients? Heart Failure Rev 2002;7(1):99–103; with permission.
Do older people without heart failure respond to aerobic training like younger adults?
Although early studies suggested that aerobic capacity could not be augmented by exercise training (ET) in healthy older adults, multiple subsequent investigations have documented 10%–25% increases in peak VO2 in previously sedentary adults through the ninth decade, comparable to those seen in young adults [20–23]. In a meta-analysis of 41 trials in 2,102 individuals aged 60 and older, aerobic training elicited a 16.3% mean increase in peak VO2 [24]. These improvements in peak VO2 are mediated by enhanced AVO2 difference and augmented SV secondary to a larger LVEDV; maximal HR is unaffected by ET in healthy older adults.
Aerobic ET has also shown convincing benefits in older patients with coronary heart disease. In clinical studies of patients participating in traditional cardiac rehabilitation programs after a coronary event, individuals older than 70 years have derived relative improvements in exercise capacity similar to those in younger patients [25–27]. For example, Ades and colleagues [26] reported a 16% increase in peak VO2 in 60 patients aged 65 ± 5 years who underwent 3 months of training beginning 8 weeks after myocardial infarction or coronary revascularization. The increased peak VO2 was entirely attributable to a widened AVO2 difference, similar to the training response in younger coronary patients.
ET in patients with heart failure: state of the evidence
It may be useful to review the effects of ET in the general HF population before focusing specifically on its role in older HF patients. Multiple studies over the past decade have demonstrated that aerobic ET is effective and safe in patients with HF. Among such patients who were receiving diuretics, converting enzyme inhibitors, and digitalis at baseline, randomized trials have demonstrated increases in peak VO2 of 12%–33% [28,29]. Patients on β-blockers appear to derive similar training-induced improvements in aerobic capacity [30]. Increased AVO2 difference is the primary contributor to the training-induced augmentation of peak VO2 in HF patients, with modest increases in cardiac output also observed in some studies [31]. Parallel improvement in ventilatory or lactate threshold is generally observed, as well as lower HR and lactate levels at fixed submaximal workloads. These physiologic changes translate into improved exercise tolerance with fewer symptoms, both highly relevant clinical outcomes. Despite early concerns that aerobic training might exacerbate adverse LV remodeling, especially in coronary patients with pre-existing wall motion abnormalities [32], numerous subsequent investigations have shown no deleterious effects on LV structure or resting function after training.
Augmentation of peripheral blood flow and improved skeletal muscle morphology and function mediate much of the benefit from aerobic ET in HF patients. Increases in peak leg blood flow and oxygen delivery and reduced leg vascular resistance have been observed [33]. Training also augments the blunted endothelium-mediated flow-dependent vasodilation seen in patients who have HFrEF [34,35]. Several studies have shown less muscle acidosis and phosphocreatine depletion and accelerated resynthesis of adenosine triphosphate during recovery from localized limb exercise post-training [36–38). Increases of ~20% in mitochondrial volume density and 41% in cytochrome C oxidase-positive mitochondria were seen after 6 months of aerobic training; these increases correlated with improvements in exercise capacity [39].
Aerobic training also elicits favorable changes in autonomic function and neurohormonal profile in HF. The characteristic elevations of resting plasma levels of vasopressin, atrial natriuretic peptides, angiotensin, and aldosterone are reduced after training [40]. Decreased norepinephrine spillover is accompanied by a parallel reduction in low-frequency heart rate variability and reciprocal increases in high-frequency peaks, consistent with augmented vagal tone [41].
Although earlier studies of ET in HF were not adequately powered to detect an effect on clinical events or survival, Belardinelli and colleagues [42] observed reduced rates of hospital admissions and cardiac mortality in patients randomized to 14 months of supervised aerobic training compared with controls. Some of the reduction in events by training in this primarily ischemic sample may have been mediated by improved myocardial perfusion, observed in 75% of trained patients but only 2% of controls. The multicenter 2331 patient HF-ACTION trial was designed specifically to determine if aerobic ET could reduce all-cause hospitalization and mortality in patients with HFrEF [43]. Adults with stable HFrEF who were receiving optimal medical and device therapy were randomized to 36 sessions of supervised ET followed by home training on a treadmill or cycle ergometer for an additional 2 or more years or to usual care. A modest but significant 11% decrease in the primary endpoint was found on an prespecified analysis that adjusted for prognostic baseline covariates [43]. Similar findings were seen in the subset of 435 patients ≥70 years as in younger patients. A major limitation of this study was that only 30% of participants achieved the target weekly goals for exercise minutes/week during the home exercise phase, resulting in only a modest increase of 0.6 ml/kg/min in peak VO2 in the group randomized to ET.
Aerobic exercise training in older heart failure patients: clinical trial evidence in HFrEF
Despite the demonstrated favorable effects of aerobic ET in HF, most such trials have enrolled predominantly younger patients, similar to the age bias observed in non-exercise HF trials [44]. Given the advanced age typical of HF patients in the general community, it is imperative to examine the literature of HF training studies specific to older patients. A review of 29 such trials in 2004 revealed only 4 studies with a mean age greater than 65 years [45]; 11 of these 29 trials included only men, and another 11 enrolled fewer than 25% women. However, several more recent trials have provided additional data on training effects in older HF patients, including higher proportions of women.
Results have been generally favorable in those ET trials that have included meaningful numbers of older HF patients, (Table 2). Willenheimer and coworkers [46] randomized 54 patients of mean age 64 years to 4 months of supervised cycle ergometry or a control group; an improved quality of life but no significant changes in peak VO2 or the dyspnea–fatigue index were found in those who trained. In 67 men with New York Heart Association (NYHA) class 2 or 3 HF and LVEF <40% who underwent 12 weeks of aerobic training, Wielenga and colleagues [47] observed similar increases in peak VO2 and exercise duration in patients younger versus those older than 65 years; however, the change in peak VO2 was not statistically significant in either group. In a study of 33 older HF patients, Gottlieb and coworkers [48] observed that 6 of 17 patients randomized to a 6-month aerobic training program did not tolerate ET; in the remaining 11 patients, both peak VO2 and 6-minute walk distance (6MWD) increased significantly, but neither daily energy expenditure nor perceived quality of life improved. In 22 HF patients aged 75–90 years old, a 12-week program of once weekly exercise sessions resulted in an 11% increase in 6MWD, but no significant improvement was found in quality of life as assessed by the Minnesota Living with Heart Failure Questionnaire (MLHFQ) [49].
Table 2.
Randomized controlled trials of ET in older patients with heart failure and reduced ejection fraction
| Authors | n | Mean age | Women (%) | Duration | Mode | Benefits |
|---|---|---|---|---|---|---|
| Austin, et al [51] | 200 | 72 | 34 | 24 weeks | Aerobic resistance | ↑6MWD 16% ↓NYHA Class; 16% [QOL |
| Antonicelli, et al [52]* | 138 | 77 | 43 | 6 months | Cycle | ↑6MWD 33%, ↑QOL, ↓BNP |
| Witham, et al [53] | 107 | 80 | 35 | 24 weeks | Walk/resistance | No Δ 6MWD or QOL |
| Gottlieb, et al [48] | 33 | 65 | 12 | 6 months | Cycle/treadmill | ↑Peak VO2 – 13% ↑6-min walk – 11% No Δ – QOL |
| McKelvie, et al [50] | 181 | 65 | 19 | 12 months | Cycle/resistance | No Δ 6MWD ↑Peak VO2 – 14% NoΔ – QOL |
| Owen, et al [49] | 22 | 81 | 25 | 12 weeks | Aerobic/resistance | ↑6-min walk – 11% No Δ – QOL |
| Pu, et al [71] | 16 | 77 | 100 | 10 weeks | Resistance | ↑Strength – 43% ↑6 min walk – 13% No Δ – Peak VO2 |
| Selig, et al [70] | 39 | 65 | 15 | 3 months | Resistance | ↑Peak VO2 – 11% ↑FBF 20% ↑HRV |
| Wielenga, et al [47] | 67 | 64 | None | 12 weeks | Walk/cycle | No Δ– Peak VO2 |
| Willenheimer, et al [46] | 54 | 64 | 28 | 16 weeks | Cycle | ↑QOL No Δ – Peak VO2, |
Abbreviations: FBF, forearm blood flow; HRV, heart rate variability; QOL, quality of life; 6MWD, 6-minute walk distance; Δ, change.
37% of the 373 patients had LV ejection fraction <40%
Four larger trials of aerobic ET have targeted the older HFrEF population. McKelvie and coworkers [50] randomized 181 NYHA class 2 to 3 HF patients of mean age 65 years (19% women) with LV ejection fraction <40% to 3 months of supervised aerobic and resistance training followed by 9 months of home training or to a control group. Most patients received diuretics, converting enzyme inhibitors, and digitalis, and approximately 20% received β-blockers. Peak VO2 in the training group increased 10% after 3 months and 14% after 12 months, whereas minimal changes occurred in controls. Modest increases in 6MWD were observed at 3 and 12 months in both groups, without significant intergroup differences. No significant changes from baseline in radionuclide cardiac function or quality of life occurred in either group.
Austin and colleagues [51] randomized 200 patients 60–89 years old (mean 72 years, 34% women) with NYHA class 2–3 HF and LVEF <40% to 24 weeks of ET or standard care. Training consisted of an 8-week twice weekly hospital-based cardiac rehabilitation program followed by 16 weeks of supervised community-based exercise sessions for 1 hour weekly. Throughout the 24-week program, patients performed aerobic training and low resistance/high repetition strength training and were encouraged to exercise an additional three times per week at home. Significant improvement occurred in health-related quality of life, NYHA class (from 2.4 to 2.0) and 6MWD (from 276 to 320 m) in exercisers, whereas no changes occurred in controls; peak VO2 was not measured. Furthermore, fewer patients in the exercise group (11%) than standard care patients (20%) were hospitalized by week 24 although mortality was similarly low in both groups. The low 12% dropout rate indicates that such a training program is feasible in most older patients with HF.
In the largest trial of ET in older HF patients, Antonicelli and colleagues [52] randomized 343 individuals >70 years old (mean 77 years, 43% women) with either HFrEF (37%) or HFpEF (63%) to 3 months of supervised ET 3 times/week on a cycle ergometer, followed by 3 additional months of home-telmonitored training or to usual care. The ET group showed substantial improvement in 6MWD (from 299 to 394 m), quality of life using the MLHFQ, and reduction in NT–pro-BNP level whereas no significant improvement in these variables occurred in usual care patients. In addition, all-cause hospitalization occurred in 37% of the latter group but only 15% of the ET group. No syncope, sustained arrhythmia or falls occurred during the exercise sessions. The changes after ET in HFrEF versus HFpEF patients were not reported.
In contrast to the prior 3 trials, Witham and colleagues [53] reported no improvement in 6MWD or MLHFQ and no decrease in healthcare costs in 107 patients with HFrEF ≥70 years old(mean 80 years, 35% women) after 8 weeks of twice weekly supervised ET followed by 16 weeks of home training. No significant difference in adverse events or hospitalization rate was found between the groups.
A meta-analysis of 7 ET trials comprising 530 HFrEF patients ≥70 years (including 4 of the trials reviewed above, but not the large Antonicelli trial) concluded that both 6MWD and general quality of life improved significantly after ET whereas no significant effects on peak VO2, disease-specific quality of life, or hospitalizations were seen [54]. However, peak VO2 was measured in only 3 of the smaller studies, and a non-significant reduction in hospitalizations from 28.5% in controls to 19.4% in the ET group was observed.
Clinical trial evidence in older patients with HFpEF
Given that HFpEF is now recognized as the predominant form of HF in older adults, it is important to examine the evidence related to ET in this common condition. Over the past few years, several ET training trials have targeted these patients. Kitzman and coworkers [55] randomized 53 patients with HFpEF (mean age 70 years, 75% women) to 16 weeks of supervised aerobic ET or to attention control. Peak VO2 increased from 13.8 to 16.1 ml/kg/min in ET patients whereas there was no significant change in controls. Parallel increases were found in 6MWD and ventilatory anaerobic threshold and in the physical quality of life score in the ET group. A group of 64 HFpEF patients (mean age 65 years, 56% women) were randomized to 3 months (32 sessions) of combined endurance/resistance training or usual care. Peak VO2 increased from 16.1% to 18.7% ml/kg/min in the ET group and was accompanied by a decrease in LV volumes and diastolic E/e′ ratio and physical function quality of life whereas these were unchanged in controls [56]. A meta-analysis of 6 randomized controlled trials involving 276 patients (mean age 67 years, 69% women) demonstrated a mean 2.72 ml/kg/min in peak VO2 and a 4 point improvement in MLHFQ score compared to controls (Figure 2), but no change was seen in LV systolic or diastolic function [57].
Figure 2.
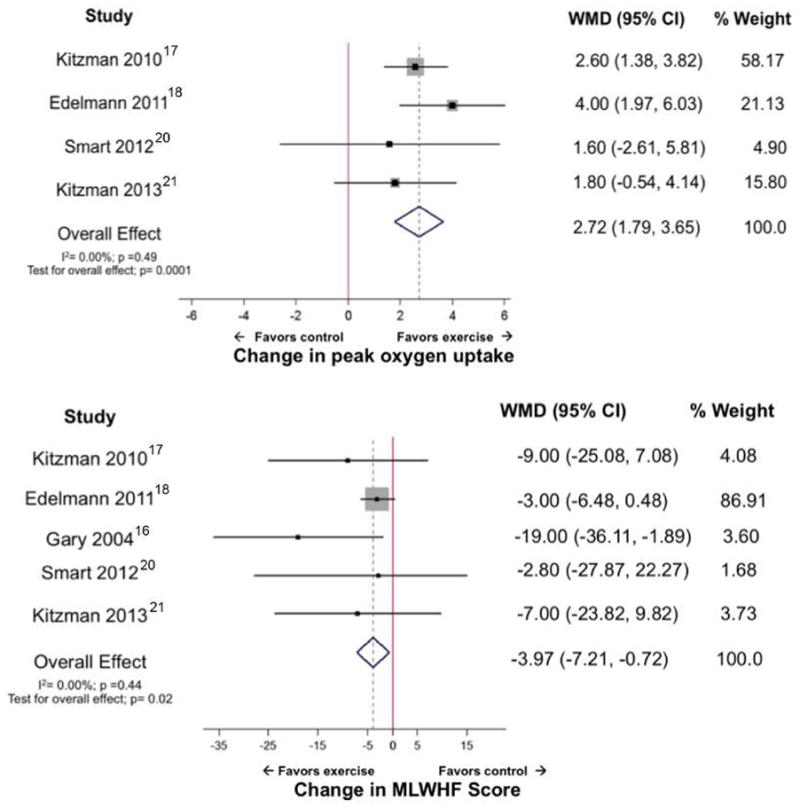
Forest plot showing effect of ET on cardiorespiratory fitness, measured as peak oxygen uptake (mL/kg per minute) and quality of life, estimated using Minnesota Living with Heart Failure (MLWHF) score, in participants with heart failure and preserved ejection fraction. CI indicates confidence interval; and WMD, weighted mean difference.
From Pandey A, Parashar A, Kumbhani DJ, et al. ET in patients with heart failure and preserved ejection fraction. Meta-analysis of randomized controlled trials. Circ Heart Fail 2015;8:33–40; with permission.
The mechanism for improvement in peak VO2 from aerobic ET in patients with HFpEF was investigated by Haykowsky and colleagues [58]. After 4 months of ET, peak HR increased from 131 to 139 beats/min, but no significant change occurred in LVEDV, stroke volume, or cardiac output; 84% of the increase in peak VO2 was mediated by an increase in AVO2 difference. A further analysis from this laboratory found no improvement in either brachial artery flow-mediated dilation or carotid artery distensibility, suggesting that other mechanisms such as increased skeletal muscle perfusion or oxygen utilization mediate training-induced increases in peak VO2 [59]. An interesting 20 week trial compared ET to caloric restriction or the combination in 100 obese patients with HFpEF (mean age 67 years, 81% women) [60]. Peak VO2 increased similarly in the ET (1.2 ml/kg/min) and diet (1.3 ml/kg/min) groups, and the increase was additive in the group receiving both interventions (2.5 ml/kg/min). However, the MLHFQ score did not improve significantly in any group.
Resistance training studies in older patients with heart failure
Although most ET trials in HF patients have focused on aerobic exercise to enhance the reduced aerobic capacity, another prominent characteristic of the HF syndrome is skeletal muscle atrophy [61–63]. Muscle atrophy is most pronounced in highly oxidative, fatigue-resistant Type I fibers, causing a shift toward glycolytic, more fatigue-prone Type II fibers [62]. Normative aging also is accompanied by significant loss of muscle mass [64,65], which accelerates after the sixth decade and is a major contributor to disability in the elderly. Older patients who have HF are therefore at especially high risk for skeletal muscle wasting.
High-intensity resistance training has produced dramatic increases in strength and sizeable increases in muscle mass in frail elderly nursing home residents in their 80s and 90s who did not have overt HF [66,67]. These improvements were accompanied by increases in gait speed; in some patients the need for walkers or canes was eliminated. Since these landmark studies, a growing literature has documented beneficial effects of resistance training in patients with HF.
Cider and colleagues [68] observed increased ventilator anaerobic threshold but no improvement in peak VO2, muscle strength, or quality of life in 24 NYHA class 2 to 3 HF patients (mean age 63 years, 33% women) randomized to 5 months of circuit weight training twice per week. In another study of 24 HF patients aged 63 ± 9 years (46% women), Tyni-Lenne and colleagues [69] documented increases in peak VO2, 6-minute walk distance, and quality of life, and reduced resting and submaximal plasma norepinephrine in those randomized to 8 weeks of resistance exercises. After 3 months of resistance training, Selig and coworkers [70] observed an increase in peak VO2, skeletal muscle strength, forearm blood flow, and heart rate variability in a randomized trial of resistance training in 39 Class 2 to 3 patients aged 65 ± 11 years (15% women) who had HF.
The relative “youth” and minimal numbers of women in the above studies of resistance training in HF patients were addressed by Pu and colleagues [71] in a study of 16 women with HF of mean age 77 years randomized to progressive resistance training for 10 weeks. At baseline these women had approximately 40% lower muscle strength than those of similar age who had other chronic diseases. Training was well tolerated and resulted in a 43% increase in strength and 13% increase in 6MWD but no increase in peak VO2. Increases in type I muscle fiber area (mean 10%) and citrate synthase activity (35%) were strong predictors of improved 6MWD. Thus, older patients who have HF thus seem to derive significant increases in muscle strength and endurance from resistance training. Additional studies combining aerobic and resistance training in older HF patients [49,50,72,73] have demonstrated similar benefits.
Is ET safe in older patients with HF?
An important concern for patients, their family, and healthcare providers is whether ET is safe in older HF patients. Although the older HF patients included in the published ET studies are typically in their late 60s or 70s and free of major disorders that would prevent them from exercising, the low rates of adverse events attributable to ET is encouraging. Most of the training studies included in this review reported no differences in adverse events between the ET and control groups. Nevertheless, the presence of common comorbidities such as obesity, arthritis, chronic lung disease, and orthopedic or neurological disorders mandate careful selection of the type of exercise intervention and the starting intensity. A general rule, as with medication titration, is to “start low and increase slowly” to avoid injury. Involvement of a physical therapist or exercise physiologist with experience in working with older patients should be encouraged.
Limitations of existing training studies in older heart failure patients
Despite the accumulating evidence that ET is beneficial in older patients with HF, several important limitations of existing studies must be recognized. As mentioned above, few of the studies to date have enrolled a sizeable number of patients older than 75 years, the group most representative of HF patients in the general community [74,75]. Similarly, although older women are well-represented in ET trials of HFpEF patients, they have been severely underrepresented in existing HFrEF training trials. These deficiencies in recruitment of older HFrEF patients, especially women, also are encountered in standard cardiac rehabilitation programs, representing both a failure of clinicians to refer such patients and logistic difficulties encountered by older patients in attending these programs [27]. The latter issue can be addressed successfully by home-based training, which elicits improvements in exercise capacity parallel to those seen in supervised programs. A further limitation of existing studies is the severe under-representation of patients who have atrial fibrillation, seen in approximately one quarter of HF patients in the community [43].
Challenges and unanswered questions
Given the huge burden imposed by HF on the health, functional status, and quality of life in older adults, ET in this population represents an underused therapeutic modality with enormous potential. To realize this potential, however, several obstacles must be surmounted. The multiple comorbidities in older HF patients, including arthritis, obstructive lung disease, peripheral arterial disease, and neuromuscular disorders, provide a challenge to ET. Ingenuity, great care, and patience are prerequisites to successful implementation of training programs in the elderly.
Older patients, especially women, often believe that they are too old to benefit from ET, despite the large body of data demonstrating relative improvements similar to those of younger individuals. In fact, debilitated elderly patients have the greatest potential for improvement in functional status and quality of life from such training.
Logistic factors, such as the need to care for a dependent spouse or lack of transportation, may prevent an otherwise willing older patient who has HF from attending a supervised rehabilitation program. Greater availability of home or community-based exercise programs may overcome such obstacles and may be more cost effective than traditional hospital-based training.
The greatest barrier to recruiting more elderly patients with HF into ET programs may lie within the medical community itself. Physicians and other health care providers must be educated in the benefits of ET in this age group so that they refer such patients to these programs.
Critical questions regarding the benefit of ET in the elderly remain to be answered. Perhaps the most important of these is whether such training prolongs survival or reduces morbidity in older patients who have HF, particularly the large subset with HFpEF. The large HF-ACTION trial, limited to patients with HFrEF, suggested that aerobic ET could elicit a modest reduction in the combined endpoint of all-cause mortality and hospitalizations as well as similar reduction in cardiovascular endpoints [43]. These positive results, though modest in effect size, resulted in Medicare extending its coverage of cardiac rehabilitation programs to include patients who have HFrEF. A parallel study of similar size is needed to investigate whether patients with HFpEF, who were excluded from HF-ACTION, will benefit from ET. Another important issue will be to determine whether a combination of resistance and aerobic training provides greater benefit than aerobic training alone on cardiovascular endpoints, functional measures, and quality of life.
Summary
Both the aging process and HF syndrome are characterized by a dramatic reduction of aerobic capacity caused by a combination of cardiac and peripheral factors. Significant decreases in muscle mass and strength are also common to both conditions. Although a growing literature has documented that aerobic ET elicits improvement in peak VO2, submaximal exercise measures, and quality of life in younger HF patients, relatively few HF training studies have included meaningful numbers of older individuals, especially those >80 years of age and older women with HFrEF. Nevertheless, the modest data available suggest similar benefits in older as in younger HF patients as well as excellent safety. Resistance training may provide additional benefit in older patients with HF, especially those with substantial muscle wasting. Whether ET can reduce mortality, hospitalizations, and overall health care costs in patients with HFpEF must await the outcome of adequately powered multicenter trials in this large subset of the HF population.
Key Points.
Both the aging process and HF syndrome are characterized by a dramatic reduction of aerobic capacity caused by a combination of cardiac and peripheral factors. Significant decreases in muscle mass and strength are also common to both conditions.
Although a growing literature has documented that aerobic ET elicits improvement in peak VO2, submaximal exercise measures, and quality of life in younger HF patients, relatively few HF training studies have included meaningful numbers of older individuals, especially those >80 years of age and older women with HFrEF. Nevertheless, the modest data available suggest similar benefits in older as in younger HF patients as well as excellent safety.
Resistance training may provide additional benefit in older patients with HF, especially those with substantial muscle wasting.
Whether ET can reduce mortality, hospitalizations, and overall health care costs in patients with HFpEF must await the outcome of adequately powered multicenter trials in this large subset of the HF population.
Footnotes
This is an updated version of an article that appeared in Heart Failure Clinics, Volume 3, Issue 4.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buskirk ER, Hodgson JL. Age and aerobic power: the rate of change in men and women. Fed Proc. 1987;46:1824–9. [PubMed] [Google Scholar]
- 2.Jackson AS, Beard EF, Wier LT, et al. Changes in aerobic power of men ages 25–70 years. Med Sci Sports Exerc. 1995;27:113–20. [PubMed] [Google Scholar]
- 3.Fleg JL, Lakatta EF. Role of muscle loss in the age-associated reduction in VO2 max. J Appl Physiol. 1988;65:1147–51. doi: 10.1152/jappl.1988.65.3.1147. [DOI] [PubMed] [Google Scholar]
- 4.Ogawa T, Spina R, Martin WH, III, et al. Effects of aging, sex, and physical training on cardiovascular response to dynamic upright exercise. Circulation. 1992;86:404–503. doi: 10.1161/01.cir.86.2.494. [DOI] [PubMed] [Google Scholar]
- 5.Fleg JL, Morrell CH, Bos AG, et al. Accelerated longitudinal decline of aerobic capacity in healthy older adults. Circulation. 2005;112:674–82. doi: 10.1161/CIRCULATIONAHA.105.545459. [DOI] [PubMed] [Google Scholar]
- 6.Fleg JL, O’Connor FC, Becker LC, et al. Cardiac versus peripheral contributions to the age–associated decline in aerobic capacity. J Am Coll Cardiol. 1997;29:269A. [Google Scholar]
- 7.Fleg JL, O’Connor F, Gerstenblith G, et al. Impact of age on the cardiovascular response to dynamic upright exercise in healthy men and women. J Appl Physiol. 1995;78:890–900. doi: 10.1152/jappl.1995.78.3.890. [DOI] [PubMed] [Google Scholar]
- 8.Fleg JL, Tzankoff SP, Lakatta EG. Age–related augmentation of plasma catecholamines during dynamic exercise in healthy men. J Appl Physiol. 1985;59:1033–9. doi: 10.1152/jappl.1985.59.4.1033. [DOI] [PubMed] [Google Scholar]
- 9.Fleg JL, Schulman S, O’Connor F, et al. Effects of acute β-adrenergic receptor blockade on age-associated changes in cardiovascular performance during dynamic exercise. Circulation. 1994;90:2333–41. doi: 10.1161/01.cir.90.5.2333. [DOI] [PubMed] [Google Scholar]
- 10.Stelken AM, Younis LT, Jennison SH, et al. Prognostic value of cardiopulmonary exercise testing using percent achieved of predicted peak oxygen uptake for patients with ischemic and dilated cardio-myopathy. J Am Coll Cardiol. 1996;27:345–52. doi: 10.1016/0735-1097(95)00464-5. [DOI] [PubMed] [Google Scholar]
- 11.Francis DP, Shamin W, Davies LC, et al. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO2 slope and peak VO2. Eur Heart J. 2000;21:154–61. doi: 10.1053/euhj.1999.1863. [DOI] [PubMed] [Google Scholar]
- 12.Mancini DM, Eisen H, Kussmaul W, et al. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–86. doi: 10.1161/01.cir.83.3.778. [DOI] [PubMed] [Google Scholar]
- 13.Higginbotham MB, Morris KG, Conn EH, et al. Determinants of variable exercise performance among patients with severe left ventricular dysfunction. Am J Cardiol. 1983;51:52–60. doi: 10.1016/s0002-9149(83)80010-1. [DOI] [PubMed] [Google Scholar]
- 14.Colucci WS, Ribeiro JP, Rocco MB, et al. Impaired chronotropic response to exercise in patients with congestive heart failure. Role of post–synaptic beta-adrenergic desensitization. Circulation. 1989;80:314–23. doi: 10.1161/01.cir.80.2.314. [DOI] [PubMed] [Google Scholar]
- 15.Sullivan MJ, Hawthorne MH. Exercise intolerance in patients with chronic heart failure. Prog Cardiovasc Dis. 1995;38:1–22. doi: 10.1016/s0033-0620(05)80011-8. [DOI] [PubMed] [Google Scholar]
- 16.Pina IL, Apstein CS, Balady GJ, et al. Exercise and heart failure: A statement from the American Heart Association Committee on Exercise, Rehabilitation and Prevention. Circulation. 2003;107:1210–25. doi: 10.1161/01.cir.0000055013.92097.40. [DOI] [PubMed] [Google Scholar]
- 17.Fleg JL. Can exercise conditioning be effective in older heart failure patients? Heart Fail Rev. 2002;7:99–103. doi: 10.1023/a:1013758008044. [DOI] [PubMed] [Google Scholar]
- 18.Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiologic characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–50. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 19.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–74. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badenhop DJ, Cleary PA, Schoal SF, et al. Physiological adjustments to higher- and lower-intensity exercise in elders. Med Sci Sports Exerc. 1983;15:496–502. [PubMed] [Google Scholar]
- 21.Seals DR, Hagberg JM, Hurley BF, et al. Endurance training in older men and women. I. Cardiovascular response to exercise. J Appl Physiol. 1984;57:1024–9. doi: 10.1152/jappl.1984.57.4.1024. [DOI] [PubMed] [Google Scholar]
- 22.Hagberg JM, Graves JF, Limacher M, et al. Cardiovascular response of 70- to 79-year old men to ET. J Appl Physiol. 1989;66:2589–94. doi: 10.1152/jappl.1989.66.6.2589. [DOI] [PubMed] [Google Scholar]
- 23.Schulman SP, Fleg JL, Goldberg AP, et al. Continuum of cardiovascular performance across a broad range of fitness levels in healthy older men. Circulation. 1996;94:359–67. doi: 10.1161/01.cir.94.3.359. [DOI] [PubMed] [Google Scholar]
- 24.Huang G, Gibson CA, Tran ZV, et al. Controlled endurance ET and VO2max changes in older adults: a meta-analysis. Prev Cardiol. 2005;8:217–225. doi: 10.1111/j.0197-3118.2005.04324.x. [DOI] [PubMed] [Google Scholar]
- 25.Lavie CJ, Milani RV, Littman AB. Benefits of cardiac rehabilitation and ET in secondary coronary prevention in the elderly. J Am Coll Cardiol. 1993;22:678–83. doi: 10.1016/0735-1097(93)90176-2. [DOI] [PubMed] [Google Scholar]
- 26.Ades PA, Waldmann ML, Meyer WL, et al. Skeletal muscle and cardiovascular adaptations to exercise conditioning in older coronary patients. Circulation. 1996;94:323–30. doi: 10.1161/01.cir.94.3.323. [DOI] [PubMed] [Google Scholar]
- 27.Ades PA. Cardiac rehabilitation in older coronary patients. J Am Geriatr Soc. 1999;47:98–105. doi: 10.1111/j.1532-5415.1999.tb01909.x. [DOI] [PubMed] [Google Scholar]
- 28.Afzal A, Brawner CA, Keteyian SJ. ET in heart failure. Prog Cardiovasc Dis. 1998;41:175–90. doi: 10.1016/s0033-0620(98)80054-6. [DOI] [PubMed] [Google Scholar]
- 29.Piepoli MT, Flather M, Coats AJS. Overview of studies of ET in chronic heart failure: the need for a prospective randomized multicentre European trial. Eur Heart J. 1998;19:830–41. doi: 10.1053/euhj.1998.1041. [DOI] [PubMed] [Google Scholar]
- 30.Curnier D, Galinier M, Pathak A, et al. Rehabilitation of patients with congestive heart failure with or without β-blockade therapy. J Card Fail. 2001;7:241–8. doi: 10.1054/jcaf.2001.26565. [DOI] [PubMed] [Google Scholar]
- 31.Hambrecht R, Gielen S, Linke A, et al. Effects of ET on left ventricular function and peripheral resistance in patients with chronic heart failure. JAMA. 2000;283:3095–101. doi: 10.1001/jama.283.23.3095. [DOI] [PubMed] [Google Scholar]
- 32.Jugdutt BI, Michorowski BL, Kappagoda CT. ET after anterior Q wave myocardial infarction: importance of regional left ventricular function and topography. J Am Coll Cardiol. 1988;12:362–72. doi: 10.1016/0735-1097(88)90407-x. [DOI] [PubMed] [Google Scholar]
- 33.Sullivan MJ, Higginbotham MB, Cobb FR. ET in patients with severe left ventricular dysfunction: hemodynamic and metabolic effects. Circulation. 1988;78:506–15. doi: 10.1161/01.cir.78.3.506. [DOI] [PubMed] [Google Scholar]
- 34.Hambrecht R, Fiehn E, Weigl C, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98:2709–15. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- 35.Katz SD, Yuen J, Bijou R. Training improves endothelium–dependent vasodilation in resistance vessels of patients with heart failure. J Appl Physiol. 1997;82:1488–92. doi: 10.1152/jappl.1997.82.5.1488. [DOI] [PubMed] [Google Scholar]
- 36.Minotti JR, Johnson EC, Hudson TC, et al. Skeletal muscle response to ET in congestive heart failure. J Clin Invest. 1990;86:751–8. doi: 10.1172/JCI114771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adamopoulos S, Coats AJ, Brunotte F. Physical training improves skeletal muscle metabolism in patients with heart failure. J Am Coll Cardiol. 1993;21:1101–6. doi: 10.1016/0735-1097(93)90231-o. [DOI] [PubMed] [Google Scholar]
- 38.Stratton JR, Dunn SF, Adamopoulos S, et al. Training partially reverses skeletal muscle metabolic abnormalities during exercise in heart failure. J Appl Physiol. 1994;76:1575–82. doi: 10.1152/jappl.1994.76.4.1575. [DOI] [PubMed] [Google Scholar]
- 39.Hambrecht R, Niebauer J, Fiehn E, et al. Physical training in patients with stable chronic heart failure. Effects on cardiorespiratory fitness and ultrastructural abnormalities of leg muscles. J Am Coll Cardiol. 1995;25:1239–45. doi: 10.1016/0735-1097(94)00568-B. [DOI] [PubMed] [Google Scholar]
- 40.Braith RW, Welsch MA, Feigenbaum MS, et al. Neuroendocrine activation in heart failure is modified by endurance exercise. J Am Coll Cardiol. 1999;34:1170–5. doi: 10.1016/s0735-1097(99)00339-3. [DOI] [PubMed] [Google Scholar]
- 41.Coats AJS, Adamopoulos S, Radaelli A. Controlled trial of physical training in chronic heart failure. Exercise performance, hemodynamics, ventilation, and autonomic function. Circulation. 1992;85:2119–31. doi: 10.1161/01.cir.85.6.2119. [DOI] [PubMed] [Google Scholar]
- 42.Belardinelli R, Georgiou D, Cianci G, et al. Randomized, controlled trial of long-term moderate ET in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation. 1999;99:1173–82. doi: 10.1161/01.cir.99.9.1173. [DOI] [PubMed] [Google Scholar]
- 43.O’Connor CM, Whellan DJ, Lee KL, et al. Efficacy and safety of ET in patients with chronic heart failure. HF-ACTION randomized controlled trial. JAMA. 2009;301:1439–50. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Heiat A, Gross CP, Krumholz HM. Representation of the elderly, women, and minorities in heart failure clinical trials. Arch Intern Med. 2002;162:1682–8. doi: 10.1001/archinte.162.15.1682. [DOI] [PubMed] [Google Scholar]
- 45.Rees K, Taylor RS, Singh S, et al. Exercise based rehabilitation for heart failure. The Cochrane Database Syst Rev. 2004;3:CD003331. doi: 10.1002/14651858.CD003331.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Willenheimer R, Erhardt L, Cline C, et al. ET in heart failure improves quality of life and exercise capacity. Eur Heart J. 1998;19:774–81. doi: 10.1053/euhj.1997.0853. [DOI] [PubMed] [Google Scholar]
- 47.Wielenga RP, Huisveld IA, Bol E, et al. ET in elderly patients with chronic heart failure. Coron Artery Dis. 1998;9:765–70. doi: 10.1097/00019501-199809110-00010. [DOI] [PubMed] [Google Scholar]
- 48.Gottlieb SS, Fisher ML, Freudenberger R, et al. Effect of ET on peak performance and quality of life in congestive heart failure patients. J Card Fail. 1999;3:188–94. doi: 10.1016/s1071-9164(99)90002-7. [DOI] [PubMed] [Google Scholar]
- 49.Owen A, Croucher L. Effect of an exercise programme for elderly patients with heart failure. Eur J Heart Fail. 2000;2:65–70. doi: 10.1016/s1388-9842(99)00067-7. [DOI] [PubMed] [Google Scholar]
- 50.McKelvie RS, Teo KK, Roberts R, et al. Effects of ET in patients with heart failure: The Exercise Rehabilitation Trial (EXERT) Am Heart J. 2002;144:23–30. doi: 10.1067/mhj.2002.123310. [DOI] [PubMed] [Google Scholar]
- 51.Austin J, Williams R, Ross L, et al. Randomised controlled trial of cardiac rehabilitation in elderly patients with heart failure. Eur J Heart Fail. 2005;7:411–7. doi: 10.1016/j.ejheart.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 52.Antonicelli R, Spazzafumo L, Scalvini S, et al. Exercise: a “new drug” for elderly patients with chronic heart failure. Aging. 2016;8:860–9. doi: 10.18632/aging.100901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Witham MD, Fulton RL, Greig CA, et al. Efficacy and cost of an exercise program for functionally impaired older patients with heart failure: A randomized controlled trial. Circ Heart Fail. 2012;5:209–16. doi: 10.1161/CIRCHEARTFAILURE.111.963132. [DOI] [PubMed] [Google Scholar]
- 54.Chen YM, Li Y. Safety and efficacy of ET in elderly heart failure patients: a systematic review and metaanalysis. Int J Clin Pract. 2013;67:1192–8. doi: 10.1111/ijcp.12210. [DOI] [PubMed] [Google Scholar]
- 55.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. ET in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3:659–67. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Edelmann F, Gelbrich g, Dungen H-D, et al. ET improves exercise capacity and diastolic function in patients with heart failure with preserved ejection fraction. Results of the Ex-DHF (ET in Diastolic Heart Failure) pilot study. J Am Coll Cardiol. 2011;58:1780–91. doi: 10.1016/j.jacc.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 57.Pandey A, Parashar A, Kumbhani DJ, et al. ET in patients with heart failure and preserved ejection fraction. Meta-analysis of randomized controlled trials. Circ Heart Fail. 2015;8:33–40. doi: 10.1161/CIRCHEARTFAILURE.114.001615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Haykowsky MJ, Brubaker PH, Stewart KP, Morgan TM, Eggebeen J, Kitzman DW. Effect of endurance training on the determinants of peak oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. J Am Coll Cardiol. 2012;60:120–8. doi: 10.1016/j.jacc.2012.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kitzman DW, Brubaker PH, Herrington DM, et al. Effect of endurance ET on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. J Am Coll Cardiol. 2013;62:584–92. doi: 10.1016/j.jacc.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kitzman DW, Brubaker P, Morgan T, et al. Effect of caloric restriction or aerobic ET on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction. A randomized clinical trial. JAMA. 20116;315:36–46. doi: 10.1001/jama.2015.17346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mancini DM, Walter G, Reichek N, et al. Contribution of skeletal muscle atrophy to exercise intolerance and altered muscle metabolism in heart failure. Circulation. 1992;85:1364–73. doi: 10.1161/01.cir.85.4.1364. [DOI] [PubMed] [Google Scholar]
- 62.Drexler H, Reide V, Munzel T, et al. Alteration of skeletal muscle in chronic heart failure. Circulation. 1992;85:1751–9. doi: 10.1161/01.cir.85.5.1751. [DOI] [PubMed] [Google Scholar]
- 63.Toth MJ, Gottlieb SS, Fisher ML, et al. Skeletal muscle atrophy and peak oxygen consumption in heart failure. Am J Cardiol. 1997;79:1267–9. doi: 10.1016/s0002-9149(97)00098-2. [DOI] [PubMed] [Google Scholar]
- 64.Kallman DA, Plato CC, Tobin JD. The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J Gerontol. 1990;45:M82-8. doi: 10.1093/geronj/45.3.m82. [DOI] [PubMed] [Google Scholar]
- 65.Faulkner JA, Brooks SV, Zerba E. Muscle atrophy and weakness with aging: contraction-induced in jury as an underlying mechanism. J Gerontol. 1995;50A:124–9. doi: 10.1093/gerona/50a.special_issue.124. [DOI] [PubMed] [Google Scholar]
- 66.Fiataroni MA, Marks EC, Ryan ND, et al. High intensity strength training in nonagenarians: effects on skeletal muscle. JAMA. 1990;263:3029–34. [PubMed] [Google Scholar]
- 67.Fiataroni MA, O’Neill EF, Ryan ND, et al. ET and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–75. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 68.Cider A, Tygessen H, Hedberg M, et al. Peripheral muscle training in patients with clinical signs of heart failure. Scand J Rehabil Med. 1997;2:121–7. [PubMed] [Google Scholar]
- 69.Tyni-Lenne R, Dencker K, Gordon A, et al. Comprehensive local muscle training increases aerobic working capacity and quality of life and decreases neurohormonal activation in patients with chronic heart failure. Eur J Heart Fail. 2001;3:47–52. doi: 10.1016/s1388-9842(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 70.Selig SE, Carey MF, Menzies DG, et al. Moderate-intensity resistance training in patients with chronic heart failure improves strength, endurance, heart rate variability, and forearm blood flow. J Card Fail. 2004;10:21–30. doi: 10.1016/s1071-9164(03)00583-9. [DOI] [PubMed] [Google Scholar]
- 71.Pu CT, Johnson MT, Foreman DE, et al. Randomized trial of progressive resistance training to counteract the myopathy of chronic heart failure. J Appl Physiol. 2001;90:2341–50. doi: 10.1152/jappl.2001.90.6.2341. [DOI] [PubMed] [Google Scholar]
- 72.Maiorani A, O’Driscoll G, Cheetham C, et al. Combined aerobic and resistance ET improves functional capacity and strength in CHF. J Appl Physiol. 2000;88:1565–70. doi: 10.1152/jappl.2000.88.5.1565. [DOI] [PubMed] [Google Scholar]
- 73.Senden PJ, Sabelis LW, Zonderland ML, et al. The effect of physical training on workload, upper leg muscle function and muscle areas in patients with chronic heart failure. Int J Cardiol. 2005;100:293–300. doi: 10.1016/j.ijcard.2004.10.039. [DOI] [PubMed] [Google Scholar]
- 74.Kitzman DW, Gardin JM, Gottdiener JS, et al. for the Cardiovascular Health Study Research Group. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. Am J Cardiol. 2001;87:413–9. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 75.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]