Abstract
Background
There is mounting evidence for a connection between the gut and Parkinson’s disease (PD). Dysbiosis of gut microbiota could explain several features of PD.
Objective
To determine if PD involves dysbiosis of gut microbiome, disentangle effects of confounders, and identify candidate taxa and functional pathways to guide research.
Methods
197 PD cases and 130 controls were studied. Microbial composition was determined by 16S rRNA gene sequencing of DNA extracted from stool. Metadata were collected on 39 potential confounders including medications, diet, gastrointestinal symptoms, and demographics. Statistical analyses were conducted while controlling for potential confounders and correcting for multiple testing. We tested differences in the overall microbial composition, taxa abundance, and functional pathways.
Results
Independent microbial signatures were detected for PD (P=4E-5), subjects’ region of residence within the United States (P=3E-3), age (P=0.03), sex (P=1E-3) and dietary fruits/vegetables (P=0.01). Among patients, independent signals were detected for catechol-O-methyltransferase-inhibitors (P=4E-4), anticholinergics (P=5E-3), and possibly carbidopa/levodopa (P=0.05). We found significantly altered abundance of Bifidobacteriaceae, Christensenellaceae, [Tissierellaceae], Lachnospiraceae, Lactobacillaceae, Pasteurellaceae and Verrucomicrobiaceae families. Functional predictions revealed changes in numerous pathways including metabolism of plant-derived compounds and xenobiotics degradation.
Conclusion
PD is accompanied by dysbiosis of gut microbiome. Results coalesce divergent findings of prior studies, reveal altered abundance of several taxa, nominate functional pathways, and demonstrate independent effects of PD medications on the microbiome. The findings provide new leads and testable hypotheses on the pathophysiology and treatment of PD.
Keywords: Parkinson’s disease, Medications, Confounding, Gut microbiome, Functional Pathways
Evidence linking PD to the gut precedes our recent appreciation of the microbiome. Gastrointestinal (GI) symptoms, including constipation, often precede the motor signs of PD.1 Lewy bodies and α-synuclein, which are the neuropathological hallmarks of PD, may appear in the gut before they appear in the brain.2 Colonic inflammation has also been documented in PD.3 These observations have led to the hypothesis that PD starts in the gut and spreads to the brain. Increased intestinal permeability in conjunction with presence of α-synuclein in the gut at early stages of disease4 suggests that a leaky gut membrane may contribute to the spread of the disease. Decreased incidence of PD among individuals who underwent vagotomy5 adds to the evidence that PD might start in the gut and spread to the brain via the enteric nervous system.
The human gut hosts tens of trillions of microorganisms including more than 1000 species of bacteria.6, 7 The collective genomes of the microorganisms in the gut (the microbiome) is over 100 times larger than the number of genes in the human genome. A well-balanced gut microbiota is critical for maintaining general health. Alterations in the composition of gut microbiota have been linked to a range of disorders including inflammatory, metabolic, neurologic, and oncologic (reviewed in 8). Research on human disease and the gut microbiota is a relatively new field, and so far, most studies have treated the disease as a single predictor, disregarding the wide range of variables that could also affect the microbiome and obscure the disease signature. The need to disentangle the gut microbiota signature of disease from that of medication and other confounders is becoming increasingly evident.9
Studies linking the gut microbiome to PD include one conducted in mice, which showed colonization with microbiota from PD patients enhanced neuro-inflammation and motor symptoms in animals overexpressing α-synuclein,10 and four conducted in humans which reached divergent conclusions.11–14 A direct comparison of the results is difficult because they had relatively small sample sizes (68 to 144 cases and controls combined), and differed in subject inclusion/exclusion criteria, sequencing techniques, statistical methods, and the treatment of confounders. Here we report a case-control study which included 327 subjects and a systematic analysis of 39 variables as potential confounders. We applied different techniques when available to assure results were robust to methodological differences, and examined the gut microbiome at global, taxonomic, and functional levels. The results help coalesce a seemingly inconsistent literature.
Patients and Methods
Subject recruitment and data collection
Institutional Review Boards and Human Subject Committees at participating institutions approved the study. Written informed consent was obtained. 212 PD cases and 136 control subjects were enrolled from among the participants of the NeuroGenetics Research Consortium (NGRC) in Seattle, WA; Atlanta, GA; and Albany, NY. The methods, and the clinical and genetic characteristics of NGRC dataset have been described in detail.15 Briefly, PD subjects were diagnosed by a movement disorder specialist according to the modified UK Brain Bank criteria.16 Controls were self-reported as being free of neurodegenerative disease. None of the patients and controls was genetically related to any other patient or control. Fifty-four case-control pairs were spouses; 143 cases and 76 controls were not connected.
Medication data were extracted from the medical records by the treating neurologists and included only the medications that the patient was prescribed for the treatment of PD at the time of this study. Spousal relationships were collected at each study site. Hoehn & Yahr (H&Y) and Movement Disorder Society (MDS) UPDRS III scores were assessed on the “on” state, as were in prior studies, and were used only to replicate prior reports. Disease duration was the difference between age-at-study and age-at-onset. All other metadata were collected using questionnaires that were completed by the subject on the day of stool sample collection.
Stool samples were collected at home using DNA/RNA-free sterile swabs (BD BBL CultureSwab Sterile, Media-free Swabs kit from Fisher Scientific) and shipped immediately via standard US postal service at ambient temperature.
Two subjects were excluded for having unreliable metadata and 19 were excluded based on sequencing metrics (see below). The final sample size for analysis was 197 PD cases and 130 controls (Table S1).
16S rRNA amplicon analysis
DNA extraction from stool and 16S rRNA amplicon sequencing were performed according to the Earth Microbiome Project Protocols, as previously described.17, 18 Sequencing was done using an Illumina MiSeq (La Jolla, CA). All samples were sequenced at once and at one laboratory.
Operational taxonomic units (OTUs) were picked using a closed reference in Quantitative Insights Into Microbial Ecology (QIIME) 1.9.119 using SortMeRNA 2.020 against the August 2013 release of the Greengenes 16S rRNA gene sequence database21 at 97% similarity. To ensure consistency, we also used de-novo OTU calling with HITdb as reference22 and the RDP classifier,23 which yielded similar results as Greengenes. A total of 4567 OTUs were called. Rarefaction at 5,000 sequences/sample resulted in the exclusion of 19 samples.
Confounders
Thirty-nine variables were interrogated as potential confounders (Table S1). PD medications, disease duration, spousal relationship and geographic site were automatically tagged as potential confounders. The remaining variables were tested to determine if they differed between cases and controls, using Fisher’s exact test for dichotomous variables and Mann-Whitney U test for quantitative variables. Since the purpose of this test was to protect against potential confounding, we used a cautious uncorrected P<0.1 to tag the variables as a potential confounder. In all, 20 of the 39 variables were chosen as potential confounders (Table S1). The variables were tested for collinearity with PD using variance inflation factor (VIF) in the R package HH. Twelve of the 20 variables had no evidence for collinearity with PD (VIF<2) and were treated as covariates. The remaining 8 variables (6 PD medications, disease duration and Caesarean section (C-section)) were seen exclusively in patients and were treated individually, as described below.
Analysis of overall composition of gut microbiome
We calculated the dissimilarities (distance) between the microbiomes of the 197 PD and 130 control samples. To ensure that the choice of the metric did not affect the results, we calculated the distances using three metrics: Unweighted UniFrac,24 Weighted UniFrac,24 and Canberra distance.25, 26 The rarefied OTU table was used for all three metrics. UniFrac distances were calculated in QIIME 1.9.1 and Canberra distances in the R package vegan_2.4-0. The differences between cases and controls were then tested, for each metric in turn, using Permutational Multivariate Analysis of Variance (PERMANOVA).27 Significance was determined using the adonis2 function in vegan in R with 99,999 permutations, and if significance reached its maximum possible at P=1E-5, permutations were increased to 9,999,999 for added precision down to P=1E-7.
To test for confounding, we conducted adjusted PERMANOVA with PD and 12 covariates in the model and tested the marginal effects:
where age (years), transit-time (days), and BMI were continuous variables, and the other variables were categorical (see Table S1). To test confounding by C-section, we excluded the subjects born by C-section and repeated PERMANOVA of PD vs. controls. To test effect of PD-medications on the microbiome, PEMANOVA was used in patients only (PD-medications were not collinear (VIF<2)):
PD-medications that were significant were re-tested while adjusting for covariates:
Fraction of the total variance explained by each variable was calculated in the PERMANOVA model.
Testing differences in the abundance of taxa in PD vs. controls
Differences were tested at OTU, genus and family level. Taxa present in <10% of samples were removed, resulting in 709 OTUs, 103 genera and 55 families. We tested the abundance of each taxon in cases vs. controls, using the Analysis of Composition of Microbiomes (ANCOM)28 and Kruskal-Wallis rank sum test.29 Kruskal-Wallis tests the null hypothesis that the taxon abundance in a random specimen taken from two or more ecosystems are equal in distribution, whereas ANCOM tests the null hypothesis that the taxon abundance (per unit volume) in two or more ecosystems are equal on average. Thus, ANCOM makes comparisons at the ecosystem level whereas the Kruskal-Wallis test makes comparisons at the specimen level. If results differed, we cautiously proceeded with the subset of findings that were significant by both methods. ANCOM was conducted using default parameters in the python implementation of ANCOM in scikit-bio 0.4.2. Kruskal-Wallis test was run using kruskal.test in R. Both analyses incorporate false-discovery rate (FDR) correction for multiple testing (FDR<0.05).
To test for potential confounding, taxa that were significant with ANCOM and Kruskal-Wallis were retested adjusting for covariates, using generalized linear model (GLM) with negative binomial distribution and controlling for zero-inflation as appropriate, in the R package glmmADMB:
To test if the associations of PD with taxon were driven by PD-medications, we excluded patients who were on COMT-inhibitors or anticholinergics and repeated the GLM. To test if altered taxa abundance was a consequence of disease duration, we used GLM:
If taxa abundance varied by disease duration, patients were stratified by disease duration and taxa abundance was tested for each stratum against controls.
Functional analysis of predicted metagenomes
We used Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt)30 to infer metagenome composition in the samples, following the recommended pipeline of normalizing OTUs by copy number (to account for differences in number of copies of 16S rRNA between taxa), predicting functions using Kyoto Encyclopedia of Genes and Genomes (KEGG)31 orthologs, and grouping predicted pathways by KEGG hierarchical level 3. We also calculated nearest sequenced taxon index (NSTI) values, yielding a mean±SD per sample of 0.07±0.03, closer to the well-characterized ecology of Human Microbiome Project (0.03±0.02) than more diverse sample sets (up to 0.23±0.07), listed in Langille et al. 2013,30 who noted that lower NSTI scores are more likely to have accurate PICRUSt results. We tested case-control differences for all metabolism pathways present in at least 10% of our samples (N=136 pathways) using the Statistical Analysis of Metagenomic (and other) Profiles (STAMP) software.32 We compared cases vs controls using Welch’s t-test, using Storey FDR<0.05 as a cutoff for significance.
Results
Overall composition of the gut microbiome
Testing the PD vs. control samples, without controlling for potential confounders, revealed a statistically significant difference (Table 1A), regardless of the metric used (P<1E-7 for Canberra distance, P=3E-7 for unweighted UniFrac, P=2E-3 for weighted UniFrac, using PERMANOVA with 9,999,999 permutations).
Table 1.
Testing the association of PD and PD medications with the microbiome, adjusted for potential confounders.
| N Case | N control | Canberra | Unweighted Unifrac | Weighted Unifrac | |
|---|---|---|---|---|---|
| (A) PD (yes vs. no) | 197 | 130 | <1E-7 | 3E-7 | 2E-3 |
|
| |||||
| (B) PD and covariates | 164 | 107 | |||
| PD (yes vs. no) | 4E-5 | 1E-4 | 0.01 | ||
| Sex (male vs. female) | 1E-3 | 5E-3 | 0.32 | ||
| Site (Seattle, Atlanta, Albany) | 3E-3 | 4E-3 | 0.06 | ||
| Eats fruits or vegetables daily (yes vs. no) | 0.01 | 0.03 | 3E-3 | ||
| Age (continuous) | 0.03 | 0.02 | 0.11 | ||
| BMI (continuous) | 0.07 | 0.03 | 0.31 | ||
| GI discomfort on day of stool collection (yes vs. no) | 0.14 | 0.13 | 0.23 | ||
| Lost ≥10 pounds in past year (yes vs. no) | 0.32 | 0.60 | 0.57 | ||
| Drinks alcohol (yes vs. no) | 0.38 | 0.23 | 0.59 | ||
| Constipation in past 3 months (yes vs. no) | 0.44 | 0.33 | 0.20 | ||
| Spouse (yes vs. no) | 0.46 | 0.70 | 0.24 | ||
| Transit time (continuous) | 0.58 | 0.62 | 4E-4 | ||
| Takes digestive medication (yes vs. no) | 0.97 | 0.99 | 0.94 | ||
|
| |||||
| (C) PD excluding C-section | 186 | 130 | <1E-7 | 5E-7 | 3E-3 |
|
| |||||
| (D) PD drugs | 185 | _ | |||
| COMT inhibitor | 4E-4 | 1E-3 | 0.20 | ||
| Anticholinergic | 5E-3 | 0.03 | 0.04 | ||
| Carbidopa/levodopa | 0.05 | 0.06 | 0.19 | ||
| Amantadine | 0.15 | 0.09 | 0.07 | ||
| Dopamine agonist | 0.30 | 0.13 | 0.21 | ||
| MAO-B inhibitor | 0.82 | 0.64 | 0.29 | ||
|
| |||||
| (E) PD drugs and covariates | 182 | _ | |||
| COMT inhibitor | 4E-3 | 0.02 | 0.27 | ||
| Anticholinergic | 3E-3 | 0.01 | 0.04 | ||
| Carbidopa/levodopa | 0.22 | 0.09 | 0.43 | ||
| Sex | 1E-3 | 5E-3 | 0.54 | ||
| Age | 9E-3 | 8E-3 | 0.07 | ||
| Site | 0.06 | 0.07 | 0.04 | ||
| Eats fruits or vegetables daily | 0.45 | 0.72 | 2E-3 | ||
| Transit time | 0.25 | 0.36 | 7E-3 | ||
Permutational multivariate analysis of variance (PERMANOVA) was conducted with and without confounder adjustment. Confounders were selected in Table S1. Analyses were repeated with three distance measures (Canberra, Unweighted Unifrac, Weighted Unifrac). (A) Testing PD irrespective of other variables. (B) Testing PD and 12 other non-collinear variables in the model, including individuals with complete data on all variables. The P values are the significance for each variable when adjusted for all other variables in the model. The overall model explained between 6% (Canberra) and 11% (weighted Unifrac) of the total variation in the microbiome, with PD and geographic site each explaining ~1% of the total variation, and sex and age each explaining ~0.5% of the variation. (C) PD vs. controls excluding the 11 PD patients who were born by C-section. (D) Testing association of 6 classes of PD medications with microbiome. Only patients were used. The P values are the significance of the association of each drug with the microbiome adjusted for other drugs in the model. Each drug explained ≤ 1% of the total variation in the microbiome among patients. (E) Re-testing the significant drugs (those with P<0.05 in Part D) while adjusting for significant covariates (those with P<0.05 in Part B).
Numerous factors can potentially affect the microbiome. If the distribution of such a variable differs between cases and controls, then it is possible to find a significant difference in microbiota between cases and controls that is solely an artifact of the associated variable. To that end, we had collected data on 39 variables, 20 of which presented as potential confounders (Table S1). The six classes of PD medications, disease duration, geographic site, and spousal relationship were chosen without testing. For the remainder of the variables, cases were compared to controls, and if the difference was even remotely significant (uncorrected P<0.1), the variable was considered a potential confounder. Constipation (P=6E-16), GI discomfort (P=2E-9), and current use of digestive medication (P=5E-3) were more prevalent in PD. Patients had lower BMI (P=9E-3), reported more weight-loss (P=0.02), ate fruits and vegetables less often (P=0.02), and drank less alcohol (P=0.03) than controls. The sex difference (P=9E-7) reflected the higher prevalence of PD in men and greater participation of women as volunteers. Patients were on average 2 years younger (P=0.04), and were more likely to have been born by C-section (P=8E-3). Samples from patients spent on average 14 hours longer in transit (P=1E-3).
To determine if the difference between cases and controls might have been skewed or completely driven by confounders, we repeated the PERMANOVA with all 13 predictors in the model (PD, geography, spousal relation, constipation, GI discomfort, digestive medication, BMI, weight loss, fruits-vegetables, alcohol, sex, age, and stool travel time) where the effect of each variable was tested against the microbiome while adjusting for all other variables. PD status was significant, regardless of the distance matrix used (Table 1B). In addition, sex, age, geography, and fruits-vegetables were significant using Canberra or unweighted UniFrac; whereas fruits-vegetables and transit-time were significant using weighted UniFrac. The overall model explained between 6% (Canberra) and 11% (weighted UniFrac) of the total variation in the microbiome, PD and geographic site each explained ~1%, and sex and age each explained ~0.5% which is in line with population estimates6,7. Confounding by C-section was ruled out by removing subjects who were born by C-section (Table 1C).
We investigated PD-medications by using patients only, including all six classes of PD medication in the PERMANOVA model, and testing association of each PD-medications with the microbiome adjusted for other PD-medications. We found significant signals for COMT-inhibitors (P=4E-4), anticholinergics (P=5E-3) and a borderline signal for carbidopa/levodopa (P=0.05) (Table 1D). COMT-inhibitors and anticholinergics retained significance when adjusted for covariates (Table 1E).
Identification of taxa that differed between PD and control samples
The commonly used method, the Kruskal-Wallis test, yielded 100 OTUs, 48 genera and 19 families, whereas ANCOM, the newer method with lower false positive rate, identified only 13 OTUs, 8 genera and 7 families as having significantly different abundance in cases and controls (Table S2, Fig. S1). The taxa identified by ANCOM were among the most significant signals detected by Kruskal-Wallis (Table S2). To be rigorous, we continued the study with the taxa that both methods identified as being significantly associated with PD (Table 2A, Fig. 1). All of these associations retained significance when adjusted for covariates (Table 2B). Excluding subjects born by C-section did not alter the results.
Table 2.
PD-Associated taxa
| (A) Taxa identified as significant by Kruskal-Wallis and ANCOM | (B) Adjusted for covariates | (C) Excluding COMT & AC users, adjusted for covariates | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abundance | Ratio b | P c | Abundance | Ratio b | P c | ||||||||
| Phylum | Class | Order | Family | Genus | OTU a | Cases | Controls | Cases | Controls | ||||
| N=197 | N=130 | N=141 | N=130 | ||||||||||
| OTU | |||||||||||||
| Actinobacteria | Actinobacteria | Bifidobacteriales | Bifidobacteriaceae | Bifidobacterium | 4347159 | 0.0119 | 0.0071 | 1.66 | 3E-5 | 0.0073 | 0.0071 | 1.03 | 0.02 |
| Bacteroidetes | Bacteroidia | Bacteroidales | Porphyromonadaceae | Parabacteroides | 4365130 | 0.0203 | 0.0145 | 1.40 | 0.05 | 0.0207 | 0.0145 | 1.43 | 0.04 |
| Bacteroidetes | Bacteroidia | Bacteroidales | Prevotellaceae | Prevotella | 1077373 | 0.0029 | 0.0004 | 7.29 | 1E-3 | 0.0034 | 0.0004 | 8.50 | 5E-3 |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Blautia | 4465907 | 0.0051 | 0.0084 | 0.61 | 2E-3 | 0.0060 | 0.0084 | 0.71 | 0.03 |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Coprococcus | 173969 | 0.0011 | 0.0024 | 0.44 | 2E-4 | 0.0011 | 0.0024 | 0.44 | 8E-4 |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Roseburia | 4392188 | 0.0031 | 0.0060 | 0.53 | 3E-3 | 0.0038 | 0.0060 | 0.64 | 0.04 |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Roseburia | 4481427 | 0.0020 | 0.0044 | 0.45 | 3E-3 | 0.0022 | 0.0044 | 0.50 | 0.01 |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | unclassified | 180999 | 0.0005 | 0.0013 | 0.39 | 1E-5 | 0.0006 | 0.0013 | 0.44 | 2E-4 |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | unclassified | 4325096 | 0.0008 | 0.0023 | 0.37 | 1E-3 | 0.0010 | 0.0023 | 0.46 | 0.04 |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | unclassified | 4457438 | 0.0149 | 0.0275 | 0.54 | 3E-3 | 0.0141 | 0.0275 | 0.51 | 6E-4 |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Faecalibacterium | 4481131 | 0.0155 | 0.0241 | 0.64 | 0.02 | 0.0177 | 0.0241 | 0.73 | 0.05 |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Faecalibacterium | 4381430 | 0.0059 | 0.0125 | 0.47 | 0.03 | 0.0064 | 0.0125 | 0.51 | 0.02 |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | unclassified | 4439469 d | 0.0047 | 0.0023 | 1.99 | 3E-3 | 0.0040 | 0.0023 | 1.71 | 8E-3 |
| GENUS | |||||||||||||
| Actinobacteria | Actinobacteria | Bifidobacteriales | Bifidobacteriaceae | Bifidobacterium | 0.0140 | 0.0076 | 1.85 | 3E-6 | 0.0089 | 0.0076 | 1.18 | 5E-3 | |
| Firmicutes | Bacilli | Lactobacillales | Lactobacillaceae | Lactobacillus | 0.0026 | 0.0004 | 7.27 | 4E-8 | 0.0017 | 0.0004 | 4.84 | 4E-7 | |
| Firmicutes | Clostridia | Clostridiales | Christensenellaceae | unclassified | 0.0073 | 0.0020 | 3.63 | 6E-9 | 0.0073 | 0.0020 | 3.59 | 1E-5 | |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Blautia | 0.0140 | 0.0194 | 0.72 | 0.01 | 0.0158 | 0.0194 | 0.81 | 0.52 | |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | Roseburia | 0.0065 | 0.0125 | 0.52 | 5E-4 | 0.0073 | 0.0125 | 0.59 | 0.01 | |
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | unclassified | 0.0487 | 0.0725 | 0.67 | 8E-5 | 0.0503 | 0.0725 | 0.69 | 2E-4 | |
| Firmicutes | Clostridia | Clostridiales | Ruminococcaceae | Faecalibacterium | 0.0317 | 0.0480 | 0.66 | 0.01 | 0.0363 | 0.0480 | 0.75 | 0.05 | |
| Verrucomicrobia | Verrucomicrobiae | Verrucomicrobiales | Verrucomicrobiaceae | Akkermansia | 0.0485 | 0.0185 | 2.63 | 1E-4 | 0.0476 | 0.0185 | 2.57 | 2E-4 | |
| FAMILY | |||||||||||||
| Actinobacteria | Actinobacteria | Bifidobacteriales | Bifidobacteriaceae | 0.0140 | 0.0076 | 1.85 | 2E-6 | 0.0090 | 0.0076 | 1.19 | 3E-3 | ||
| Firmicutes | Bacilli | Lactobacillales | Lactobacillaceae | 0.0029 | 0.0004 | 7.24 | 2E-7 | 0.0019 | 0.0004 | 4.84 | 1E-6 | ||
| Firmicutes | Clostridia | Clostridiales | [Tissierellaceae] | 0.0122 | 0.0040 | 3.08 | 8E-8 | 0.0120 | 0.0040 | 3.02 | 3E-7 | ||
| Firmicutes | Clostridia | Clostridiales | Christensenellaceae | 0.0073 | 0.0020 | 3.62 | 4E-9 | 0.0072 | 0.0020 | 3.59 | 1E-5 | ||
| Firmicutes | Clostridia | Clostridiales | Lachnospiraceae | 0.1099 | 0.1447 | 0.76 | 3E-3 | 0.1108 | 0.1447 | 0.77 | 2E-3 | ||
| Proteobacteria | Gammaproteobacteria | Pasteurellales | Pasteurellaceae | 0.0003 | 0.0012 | 0.21 | 3E-5 | 0.0002 | 0.0012 | 0.14 | 2E-4 | ||
| Verrucomicrobia | Verrucomicrobiae | Verrucomicrobiales | Verrucomicrobiaceae | 0.0481 | 0.0184 | 2.61 | 1E-4 | 0.0472 | 0.0184 | 2.56 | 2E-4 | ||
OTU: 13 operational taxonomic units (OTUs) had significantly altered abundances in PD vs. control samples, both using ANCOM (FDR<0.05) and Kruskal-Wallis tests (FDR<0.05).
GENUS: OTUs were assigned to genera and tested for association with PD. 8 genera differed in PD vs. controls using ANCOM (FDR<0.05) and Kruskal-Wallis (FDR<0.05).
FAMILY: OTUs were assigned to families and tested for association with PD. 7 families differed in PD vs. controls using ANCOM (FDR<0.05) and Kruskal-Wallis (FDR<0.05).
(A) Taxa whose abundance was significantly (FDR<0.05) altered in PD vs. controls according to two methods (ANCOM and Kuskal-Wallis)
(B) All PD vs. controls adjusted for sex, age, geographic site, dietary fruit/vegetable, and transit time, tested using GLM
(C) Patients who were not on COMT inhibitors or anticholinergic drugs (AC) vs. controls, adjusted for sex, age, geographic site, dietary fruit/vegetable, and transit time, tested using GLM
Taxon ID and name are from Greengenes database.
Ratio= Relative abundance in cases / Relative abundance in controls (there may be imprecision due to rounding).
P=significance from adjusted GLM
Increased abundance of OTU #4439469 is a consequence of disease duration (P=5E-4). 4439469 was not associated with PD in the first 10 years of disease, abundance ratio case/control=1.02, P=0.54. For duration >10 years, abundance ratio case/control=2.51, P=8E-5.
Excluding individuals born by C-section yielded consistently significant results.
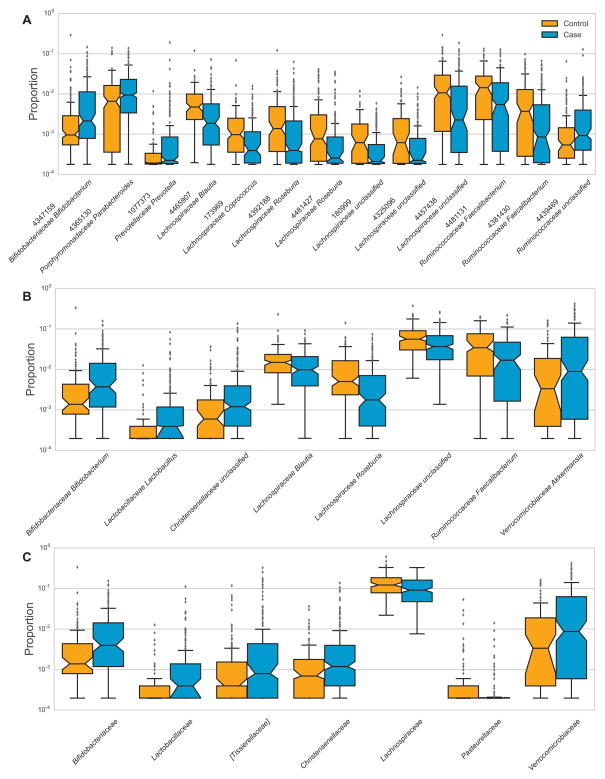
Figure 1. The relative abundances of PD-associated taxa.
Boxplots show the abundance of A) 13 OTUs, B) 8 genera, and C) 7 families that differed significantly between controls (orange) and PD cases (blue).
Taxa are identified by A) taxon ID and family and genus names, B) family and genus names, or C) family names. The relative abundance (proportion) is plotted as log10 scale on the y axis. The notch in each box indicates the confidence interval of the median. The bottom, middle, and top boundaries of each box represent the first, second (median), and third quartiles of the abundance. The whiskers (lines extending from the top and bottom of the box) extend to points within 1.5 times the interquartile range. The points extending above the whiskers are outliers. Note that the relative position of confidence intervals of the median is only a visual proxy for the difference between groups. Statistical testing was performed on the means.
To see if PD medications were driving the associations, we excluded patients who were on COMT-inhibitors or anticholinergics, and found notable reduction in the association signal for Bifidobacterium at the OTU level (1.66-fold increased abundance in patients reduced to 1.03-fold), and for Lachnospiraceae Blautia at the genus level (dropped in significance from P=0.01 to P=0.52). In sum, the majority of associations at the OTU and genus level, and all of the associations at the family level, were robust to the best that could be determined.
We tested taxa abundance as a function of disease duration and were able to determine that the increased abundance of OTU #4439469 Ruminococcaceae (ratio case/control=1.99) was a consequence of disease. The abundance of this taxon was associated with disease duration (P=5E-4), and when stratified by disease duration and compared to controls, the abundance was not higher in the first ten years of disease (ratio case/control=1.02, P=0.54), but was highly elevated in patients who had the disease for >10 years (ratio case/control=2.51, P=8E-5).
Functional prediction
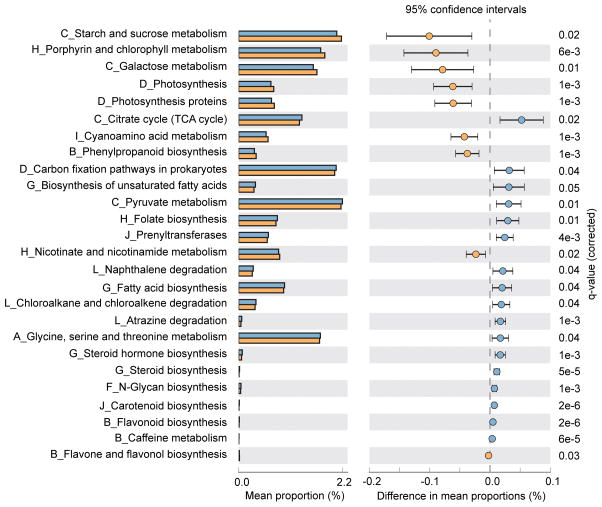
To evaluate functional differences in the microbiomes of PD vs. controls we used PICRUSt30, a computational tool that allows using 16S rRNA amplicon data to predict the genes that are present, calculate their abundance, assign them to metabolic pathways using KEGG,31 and then test the difference between cases and controls. Among 136 metabolic pathways tested, 26 were significantly different between cases and controls (Fig. 2). According to KEGG hierarchical level 2 classification, the 26 pathways are involved in carbohydrate metabolism, energy metabolism, lipid metabolism, metabolism of cofactors and vitamins, and xenobiotics biodegradation and metabolism.
Figure 2. Predicted functional differences between PD and control microbiomes.
Twenty-six metabolic pathways differed significantly between cases and controls. Pathways that were more abundant in cases are on the positive side (blue circle with 95% CI). Pathways that were more abundant in controls are on the negative side (orange circle).
q-value: the Storey FDR-corrected P-value. Mean proportions are shown in stacks for cases (blue) and controls (orange). Difference in mean proportions = mean proportion in cases minus mean proportion in controls. Only metabolic pathways at KEGG hierarchical level 1 were investigated to limit inclusion of nonbacterial pathways. Tests were conducted at KEGG hierarchical level 3, which included 136 pathways present in ≥10% of samples. The letter in front of each pathway name indicates the KEGG hierarchical level 2 for that pathway (A=Amino acid metabolism, B=Biosynthesis of other secondary metabolites, C=Carbohydrate metabolism, D=Energy metabolism, E=Enzyme families (none detected at FDR<0.05), F=Glycan biosynthesis and metabolism, G=Lipid metabolism, H=Metabolism of cofactors and vitamins, I=Metabolism of other amino acids, J=Metabolism of terpenoids and polyketides, K=Nucleotide metabolism (none detected at FDR<0.05), L=Xenobiotics biodegradation and metabolism.)
Discussion
We investigated the relationship of the gut microbiome with PD using a large sample size and a systematic approach to controlling for potential confounders. We detected a significant effect for PD, and recovered the known effects of sex, dietary fruits/vegetables, and age.6, 7 We also detected an unexpected difference as a function of geographic site (i.e., region of residence of the subjects), which may reflect the environmental, life-style, and diet differences between the Northeast (Albany, NY), Northwest (Seattle, WA) and Southern (Atlanta, GA) USA.
In a case-only analysis we found a significant difference in the gut microbiome as a function of treatment with COMT-inhibitors, anticholinergics, and a borderline significance for carbidopa/levodopa. The data suggest the effects of COMT-inhibitors and anticholinergics are independent of the PD effect because (a) their impact on the overall microbiome was detected within patients (hence PD was controlled for), and (b) most of the PD-associated taxa were robustly associated with disease in patients who were not on either of these two drugs. We were unable to tease out the effects of carbidopa/levodopa and PD because 90% of patients were taking carbidopa/levodopa. The evidence for interaction between PD medications and the microbiome is not surprising, considering the growing literature on the role of the gut microbiome in the metabolism of prescription drugs, and the profound effects that the drugs can have in turn on the composition of the microbiome.6, 7 A prior study of PD has linked COMT-inhibitors to altered abundance of some taxa.11 Moreover, COMT-inhibitors33 and anticholinergics34 have gastrointestinal side effects, which may be related to a dysbiosis of the microbiome. The present findings lend support to the notion that the composition of the gut microbiome may hold new information for assessing efficacy and toxicity of PD medications. Additional studies are needed to assess the effect of carbidopa/levodopa, and other PD medications, with larger numbers of treated and untreated patients.
PD has many associated features that might affect the gut microbiome, including gastrointestinal symptoms, gender-imbalance, and the variety of medications that are used to treat PD. In this study we focused on such confounders and evaluated 39 variables that we suspected might skew a study of PD with the microbiome, narrowed them down to 20 potential confounders, and methodically assessed their potential effect on the observed associations with PD. We took an analytically conservative approach, requiring concordance across methods, adjusting for confounders, and setting stringent criteria for declaring significance. In the process, we might have missed important taxa that did not meet all the criteria, but in the end, we had higher confidence that the findings that were declared significant were in fact robust. We identified 13 taxa at the OTU level, 8 at the genus level, and 7 at the family level as being associated with PD. Only a few were potentially confounded, but most were robust.
Our data coalesce a seemingly inconsistent literature. There have been four prior studies of PD and the microbiome, which produced conflicting results with respect to the taxa involved.11–14 Considering the results side-by-side (Table S3 A–D), there is no overlap across the four studies. We questioned if this disparity was due to small sample sizes and the inconsistent classification of taxa (which our data may help resolve), or more deeply rooted in study-specific differences such as the populations that were studied. Our results (Table S3 E) confirmed many of the reported associations including elevated levels of Akkermansia,12Lactobacillus,13 and Bifidobacterium14 and reduced levels of Lachnospiraceae12 in PD. We did not, however, replicate the reported association with Prevotellaceae11 (case vs. control P=0.57, association with UPDRS III score P=0.24).
Several studies have implicated depletion of short chain fatty acids (SCFA) in the pathogenesis PD.10, 12, 14, 35–37 SCFA is made by bacteria in the gut, notably Lachnospiraceae. Our study shows reduced levels of Lachnospiraceae in PD, which is consistent with SCFA depletion. Moreover, although none of the SCFA metabolism pathways per se were significant, some of their key components were; e.g., butyrate kinase (KEGG_K00929) which catalyzes a reversible reaction between butyrate and butanoyl-phosphate38 was reduced in PD (FDR=0.04), and acetyl-CoA synthetase (KEGG_K01895) which converts acetate to acetyl-CoA39 was elevated (FDR=3E-3). SCFA deficiency is an attractive hypothesis for PD because it could potentially explain inflammation and microglial activation in the brain10, 40, 41, and gastrointestinal features of the disease (leaky gut,4, 42 constipation1, 43 and colonic inflammation3, 44, 45), but it is not the whole picture. There is sufficient evidence to speculate that, on one extreme, shortage of SCFA may be at the root of PD, and that replenishing the microbiome with SCFA-producing bacteria may prevent PD and reverse the disease in those who are affected. On the other hand, depletion of SCFA and SCFA-producing organisms has been observed in diverse disorders,46–50 which suggests SCFA deficiency may be a common consequence of illness rather than a specific cause or even a biomarker for PD. Our data revealed alterations in at least seven families of bacteria (Lachnospiraceae being the least significant), and numerous metabolic pathways, which indicate there is more to the microbiome dysbiosis in PD than SCFA deficiency.
A key question is what causes microbiome dysbiosis in PD. Our data suggest that there may be increased activity in PD of pathways that degrade xenobiotics; namely atrazine (herbicide), chloroalkane (flame retardant) and naphthalene (insect repellent). According to the US Environmental Protection Agency, atrazine is the most commonly detected pesticide/herbicide contaminant in stream and ground water in America. This may be relevant here because exposure to pesticides and herbicides in agricultural setting, including well water drinking, is known to increases the risk of developing PD,51, 52 and causes dopaminergic cell death and motor abnormalities in animal models.53 The evidence for increased xenobiotics degradation in the gut, therefore, raises testable hypotheses on the role of xenobiotics in initiating the dysbiosis of the microbiome, and whether recent or continued exposure to PD-associated xenobiotics may contribute to the progression of neurodegeneration.
This study has provided new leads and specific hypotheses that can be tested in experimental models and human studies. Cause and effect can be discerned in experimental models. Properly designed human studies can reveal how the microbiome changes from a healthy gut to early-stage PD and as disease progresses; how PD medications alter the microbiome and the side effects that may ensue; and conversely, how the composition of the microbiome (e.g., enterotypes54) affects the metabolism and hence the efficacy and toxicity of different treatments.
Supplementary Material
Acknowledgments
Funding sources: The study was supported by NINDS grant R01036960 to HP. Udall grant (P50 NS062684) provided additional support for sample collection at the Seattle site (CPZ).
We wish to thank Victoria Kusel, Jennifer Pate, Antonio Gonzalez, and Amnon Amir for their contributions to data collection and management.
Authors’ roles
EMH - Conception, organization, and execution of research project. Design and execution of statistical analysis. Writing of the first draft, review and critique of manuscript.
JWD – Organization and execution of research project. Review and critique of manuscript.
JTM – Review and critique of statistical analysis. Review and critique of manuscript.
WTW – Conception, organization and execution of research project. Review and critique of manuscript.
MRL – Execution of research project. Review and critique of manuscript.
ZDW - Execution of statistical analysis. Review and critique of manuscript.
SDP - Review and critique of statistical analysis. Review and critique of manuscript.
SAF - Execution of research project. Review and critique of manuscript.
EM - Execution of research project. Review and critique of manuscript.
CPZ - Execution of research project. Review and critique of manuscript.
RK - Conception and execution of research project. Review of statistical analysis. Review and critique of manuscript.
HP - Conception, organization, and execution of research project. Design, review and critique of statistical analysis. Writing of the first draft, review and critique of manuscript. Acquired funding.
Footnotes
Financial disclosure/conflict of interest: None
Data accession
Sequence and metadata are in EBI as accession number ERP016332.
Full financial disclosure for the previous 12 months:
EMH – NIH grants NS036960 and NS067469 (to HP)
JWD – Robert Wood Johnson Foundation (to RK)
JTM – NSF GRFP DGE-1144086
WTW – NIH grants NS036960 and NS067469 (to HP)
MRL – NIH grant NS067469 (to HP)
ZDW – UAB graduate student funds and by NIH grant NS067469 (to HP).
SDP – NIEHS grant ZIA ES103066-05
SAF – Honoraria: Neurocrine, Lundbeck, Auspex/Teva, Avanir, Cynapsus, Adamas, UCB.
Grants: Ipsen, Allergan, Medtronics, Auspex, US World Meds, Pharm-Olam, Cynapsus Therapeutics, Solstice, CHDI Foundation, Michael J. Fox Foundation, NIH
Royalties: Demos, Blackwell Futura for textbooks, Uptodate
EM – Research support from: Merz Pharmaceuticals, CHDI, Kyowa Hakko Kirin Pharma, US World Meds, Auspex Pharmaceuticals, Acadia Pharmaceuticals, Pfizer, Civitas
Speakers Honoraria: US World Meds
Consulting Fees: US World Meds, Neurocrine Biosciences
CPZ – American Parkinson Disease Association, Department of Veterans Affairs, NIH, Dolsen Foundation
RK – Univ. of Wisconsin, Madison Moore Foundation, Binational Science Foundation, UC San Francisco National Multiple Sclerosis Society, Templeton, Harvard-Broad, NIH P01DK078669, Ludwig Maximilian Universitat Munchen, Alfred P. Sloan Foundation, Oak Crest Institute of Science, Department of Justice, Laureate Institute for Brain Research, Kenneth Rainin Foundation, Robert Wood Johnson Foundation, University of Colorado-Boulder /DOJ, University of Colorado-Boulder/ONR, USAMRAA, NSF, NIH U01AI24316 and P30MH062512.
HP – NIH grants NS036960 and NS067469, The John T and Juanelle D Strain Endowed Chair, Department of Neurology and School of Medicine of University of Alabama at Birmingham.
References
- 1.Cersosimo MG, Raina GB, Pecci C, et al. Gastrointestinal manifestations in Parkinson’s disease: prevalence and occurrence before motor symptoms. J Neurol. 2013;260(5):1332–1338. doi: 10.1007/s00415-012-6801-2. [DOI] [PubMed] [Google Scholar]
- 2.Adler CH, Beach TG. Neuropathological basis of nonmotor manifestations of Parkinson’s disease. Mov Disord. 2016;31(8):1114–1119. doi: 10.1002/mds.26605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devos D, Lebouvier T, Lardeux B, et al. Colonic inflammation in Parkinson’s disease. Neurobiol Dis. 2013;50:42–48. doi: 10.1016/j.nbd.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 4.Forsyth CB, Shannon KM, Kordower JH, et al. Increased intestinal permeability correlates with sigmoid mucosa alpha-synuclein staining and endotoxin exposure markers in early Parkinson’s disease. PLoS One. 2011;6(12):e28032. doi: 10.1371/journal.pone.0028032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Svensson E, Horvath-Puho E, Thomsen RW, et al. Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol. 2015;78(4):522–529. doi: 10.1002/ana.24448. [DOI] [PubMed] [Google Scholar]
- 6.Falony G, Joossens M, Vieira-Silva S, et al. Population-level analysis of gut microbiome variation. Science. 2016;352(6285):560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 7.Zhernakova A, Kurilshikov A, Bonder MJ, et al. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science. 2016;352(6285):565–569. doi: 10.1126/science.aad3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arnold JW, Roach J, Azcarate-Peril MA. Emerging Technologies for Gut Microbiome Research. Trends Microbiol. 2016 doi: 10.1016/j.tim.2016.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forslund K, Hildebrand F, Nielsen T, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528(7581):262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sampson TR, Debelius JW, Thron T, et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell. 2016;167(6):1469–1480. e1412. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scheperjans F, Aho V, Pereira PA, et al. Gut microbiota are related to Parkinson’s disease and clinical phenotype. Mov Disord. 2015;30(3):350–358. doi: 10.1002/mds.26069. [DOI] [PubMed] [Google Scholar]
- 12.Keshavarzian A, Green SJ, Engen PA, et al. Colonic bacterial composition in Parkinson’s disease. Mov Disord. 2015;30(10):1351–1360. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- 13.Hasegawa S, Goto S, Tsuji H, et al. Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson’s Disease. PLoS One. 2015;10(11):e0142164. doi: 10.1371/journal.pone.0142164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Unger MM, Spiegel J, Dillmann KU, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord. 2016 doi: 10.1016/j.parkreldis.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 15.Hamza TH, Zabetian CP, Tenesa A, et al. Common genetic variation in the HLA region is associated with late-onset sporadic Parkinson’s disease. Nat Genet. 2010;42(9):781–785. doi: 10.1038/ng.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Caporaso JG, Lauber CL, Walters WA, et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012;6(8):1621–1624. doi: 10.1038/ismej.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caporaso JG, Lauber CL, Walters WA, et al. Global patterns of 16S rRNA diversity at a depth of millions of sequences per sample. Proc Natl Acad Sci U S A. 2011;108(Suppl 1):4516–4522. doi: 10.1073/pnas.1000080107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuczynski J, Stombaugh J, Walters WA, Gonzalez A, Caporaso JG, Knight R. Using QIIME to analyze 16S rRNA gene sequences from microbial communities. Curr Protoc Bioinformatics. 2011;Chapter 10(Unit 10):17. doi: 10.1002/0471250953.bi1007s36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kopylova E, Noe L, Touzet H. SortMeRNA: fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. Bioinformatics. 2012;28(24):3211–3217. doi: 10.1093/bioinformatics/bts611. [DOI] [PubMed] [Google Scholar]
- 21.McDonald D, Price MN, Goodrich J, et al. An improved Greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012;6(3):610–618. doi: 10.1038/ismej.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ritari J, Salojarvi J, Lahti L, de Vos WM. Improved taxonomic assignment of human intestinal 16S rRNA sequences by a dedicated reference database. BMC Genomics. 2015;16:1056. doi: 10.1186/s12864-015-2265-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and environmental microbiology. 2007;73(16):5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lozupone C, Knight R. UniFrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol. 2005;71(12):8228–8235. doi: 10.1128/AEM.71.12.8228-8235.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lance GN, Williams WT. Computer programs for hierarchical polythetic classification (“similarity analyses”) The Computer Journal. 1966;9(1):60–64. [Google Scholar]
- 26.Cao Y, Williams WP, Bark AW. Similarity measure bias in river benthic Aufwuchs community analysis. Water Environment Research. 1997;69(1):95–106. [Google Scholar]
- 27.Anderson MJ. A new method for non-parametric multivariate analysis of variance. Austral ecology. 2001;26(1):32–46. [Google Scholar]
- 28.Mandal S, Van Treuren W, White RA, Eggesbo M, Knight R, Peddada SD. Analysis of composition of microbiomes: a novel method for studying microbial composition. Microb Ecol Health Dis. 2015;26:27663. doi: 10.3402/mehd.v26.27663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hollander M, Wolfe DA. Nonparametric Statistical Methods. New York: John Wiley & Sons; 1973. [Google Scholar]
- 30.Langille MG, Zaneveld J, Caporaso JG, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31(9):814–821. doi: 10.1038/nbt.2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M. The KEGG resource for deciphering the genome. Nucleic Acids Res. 2004;32(Database issue):D277–280. doi: 10.1093/nar/gkh063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parks DH, Tyson GW, Hugenholtz P, Beiko RG. STAMP: statistical analysis of taxonomic and functional profiles. Bioinformatics. 2014;30(21):3123–3124. doi: 10.1093/bioinformatics/btu494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaakkola S. Clinical pharmacology, therapeutic use and potential of COMT inhibitors in Parkinson’s disease. Drugs. 2000;59(6):1233–1250. doi: 10.2165/00003495-200059060-00004. [DOI] [PubMed] [Google Scholar]
- 34.Ness J, Hoth A, Barnett MJ, Shorr RI, Kaboli PJ. Anticholinergic medications in community-dwelling older veterans: prevalence of anticholinergic symptoms, symptom burden, and adverse drug events. Am J Geriatr Pharmacother. 2006;4(1):42–51. doi: 10.1016/j.amjopharm.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 35.Kidd SK, Schneider JS. Protection of dopaminergic cells from MPP+-mediated toxicity by histone deacetylase inhibition. Brain Res. 2010;1354:172–178. doi: 10.1016/j.brainres.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.St Laurent R, O’Brien LM, Ahmad ST. Sodium butyrate improves locomotor impairment and early mortality in a rotenone-induced Drosophila model of Parkinson’s disease. Neuroscience. 2013;246:382–390. doi: 10.1016/j.neuroscience.2013.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sharma S, Taliyan R, Singh S. Beneficial effects of sodium butyrate in 6-OHDA induced neurotoxicity and behavioral abnormalities: Modulation of histone deacetylase activity. Behav Brain Res. 2015;291:306–314. doi: 10.1016/j.bbr.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 38.Louis P, Duncan SH, McCrae SI, Millar J, Jackson MS, Flint HJ. Restricted distribution of the butyrate kinase pathway among butyrate-producing bacteria from the human colon. J Bacteriol. 2004;186(7):2099–2106. doi: 10.1128/JB.186.7.2099-2106.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kumari S, Tishel R, Eisenbach M, Wolfe AJ. Cloning, characterization, and functional expression of acs, the gene which encodes acetyl coenzyme A synthetase in Escherichia coli. J Bacteriol. 1995;177(10):2878–2886. doi: 10.1128/jb.177.10.2878-2886.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Erny D, Hrabe de Angelis AL, Jaitin D, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18(7):965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sanchez-Guajardo V, Tentillier N, Romero-Ramos M. The relation between alpha-synuclein and microglia in Parkinson’s disease: Recent developments. Neuroscience. 2015;302:47–58. doi: 10.1016/j.neuroscience.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 42.Donohoe DR, Garge N, Zhang X, et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011;13(5):517–526. doi: 10.1016/j.cmet.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pituch A, Walkowiak J, Banaszkiewicz A. Butyric acid in functional constipation. Prz Gastroenterol. 2013;8(5):295–298. doi: 10.5114/pg.2013.38731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Segain JP, Raingeard de la Bletiere D, Bourreille A, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut. 2000;47(3):397–403. doi: 10.1136/gut.47.3.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furusawa Y, Obata Y, Fukuda S, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504(7480):446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- 46.Pozuelo M, Panda S, Santiago A, et al. Reduction of butyrate- and methane-producing microorganisms in patients with Irritable Bowel Syndrome. Sci Rep. 2015;5:12693. doi: 10.1038/srep12693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490(7418):55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 48.Qin N, Yang F, Li A, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature. 2014;513(7516):59–64. doi: 10.1038/nature13568. [DOI] [PubMed] [Google Scholar]
- 49.Guo Z, Zhang J, Wang Z, et al. Intestinal Microbiota Distinguish Gout Patients from Healthy Humans. Sci Rep. 2016;6:20602. doi: 10.1038/srep20602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yamada T, Shimizu K, Ogura H, et al. Rapid and Sustained Long-Term Decrease of Fecal Short-Chain Fatty Acids in Critically Ill Patients With Systemic Inflammatory Response Syndrome. JPEN J Parenter Enteral Nutr. 2015;39(5):569–577. doi: 10.1177/0148607114529596. [DOI] [PubMed] [Google Scholar]
- 51.Gatto NM, Cockburn M, Bronstein J, Manthripragada AD, Ritz B. Well-water consumption and Parkinson’s disease in rural California. Environ Health Perspect. 2009;117(12):1912–1918. doi: 10.1289/ehp.0900852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Freire C, Koifman S. Pesticide exposure and Parkinson’s disease: epidemiological evidence of association. Neurotoxicology. 2012;33(5):947–971. doi: 10.1016/j.neuro.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 53.Blesa J, Phani S, Jackson-Lewis V, Przedborski S. Classic and new animal models of Parkinson’s disease. J Biomed Biotechnol. 2012;2012:845618. doi: 10.1155/2012/845618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arumugam M, Raes J, Pelletier E, et al. Enterotypes of the human gut microbiome. Nature. 2011;473(7346):174–180. doi: 10.1038/nature09944. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.