Abstract
Anthocyanins are secondary metabolites that contribute to red, blue, and purple colors in plants and are affected by light, but the effects of low light on the physiological responses of purple pak-choi plant leaves are still unclear. In this study, purple pak-choi seedlings were exposed to low light by shading with white gauze and black shading in a phytotron. The responses in terms of photosynthetic properties, carbohydrate metabolism, antioxidant enzyme activity, anthocyanin biosynthetic enzyme activity, and the relative chlorophyll and anthocyanin content of leaves were measured. The results showed that chlorophyll b, intracellular CO2 content, stomatal conductance and antioxidant activities of guaiacol peroxidase, catalase and superoxide dismutase transiently increased in the shade treatments at 5 d. The malondialdehyde content also increased under low light stress, which damages plant cells. With the extension of shading time (at 15 d), the relative chlorophyll a, anthocyanin and soluble protein contents, net photosynthetic rate, transpiration rate, stomata conductance, antioxidant enzyme activities, and activities of four anthocyanin biosynthetic enzymes decreased significantly. Thus, at the early stage of low light treatment, the chlorophyll b content increased to improve photosynthesis. When the low light treatment was extended, antioxidant enzyme activity and the activity of anthocyanin biosynthesis enzymes were inhibited, causing the purple pak-choi seedlings to fade from purple to green. This study provides valuable information for further deciphering genetic mechanisms and improving agronomic traits in purple pak-choi under optimal light requirements.
Introduction
Light is one of the most important environmental factors and plays a critical function in plant development and metabolism [1,2]. Additionally, light is indispensable for photosynthesis and photomorphogenesis. Low light is a pervasive abiotic stress in plant breeding and cultivation due to light block from horticulture facilities, clouds and snow. Low light was shown to substantially affect the agronomic traits of plants and inhibit physiological metabolic processes, including photosynthesis and antioxidant characteristics, as well as carbon and nitrogen fixation [3–6]. It causes slow growth, decrease of leaf weight and flower bud number. Furthermore, this stressor reduces sugar and starch contents in eggplant, grape and rice [7–9], and changes the coloration and extends the maturity time in cherry [10].
Chlorophyll is an important pigment involved in absorbing, transmitting and converting solar energy into electrochemical energy [11]. It was reported [12] that low light-tolerant hybrid rice -exhibited a higher content of chlorophyll b following exposure to low light. Low light negatively affects stomata conductance and results in enhanced concentration of intercellular CO2 in rice leaves [13,14]. Moreover, stomata conductance and photosynthetic efficiency under low light decreases by the number of 24.31% and 79.84%, respectively compared to that of natural light [15].
Antioxidant metabolism plays an important role in protecting plants from a wide variety of environmental stresses, such as drought, extreme temperatures, pollutants, ultraviolet radiation and high levels of light [16,17]. Enhancement of antioxidant defense in plants can thus increase tolerance to different stresses. Antioxidants include the enzymes peroxidase (POD), catalase (CAT), ascorbate peroxidase (APX) and superoxide dismutase (SOD) [18]. Analyses of membrane lipid peroxidation in peach fruit showed that decreasing the light intensity decreased CAT, G-POD and APX activity but increased malondialdehyde (MDA) content with more cell membrane damage [6,19].
Pak-choi (Brassica campestris ssp. Chinensis Makino L.) originated from China is one of the most important vegetables worldwide in terms of its planting areas and annual yields. Purple pak-choi contains high levels of light-dependent anthocyanin in its leaves. This plant is very popular in China, Japan and surrounding countries. Anthocyanins, a class of secondary metabolites, contribute to the red, blue, and purple colors in flowers, fruits, and leaves [20]. They also act as antioxidants and protect DNA and the photosynthetic apparatus from damage due to high radiation fluxes [21]. Additionally anthocyanin protects plants against cold and drought stress [22]. Anthocyanin is accumulated in response to light in the seedlings of mustard and tomato. Phytochrome is an important photoreceptor controlling the accumulation of anthocyanins [23–26]. However, how phytochrome regulates anthocyanin and other key enzymes under low light remains unclear.
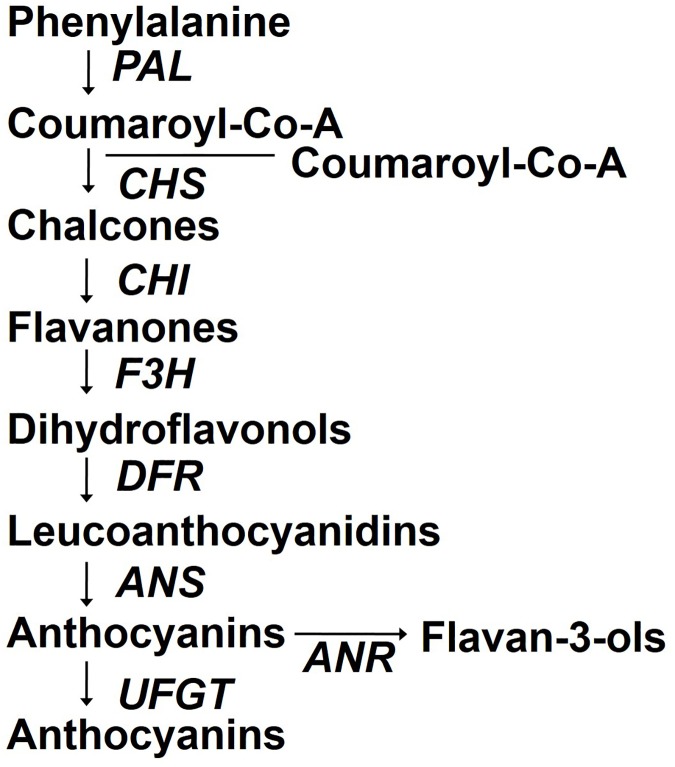
Anthocyanin is synthesized via a branch of the phenylpropanoid pathway, i.e., the flavonoid pathway (Fig 1) [27]. Anthocyanin biosynthesis consists of sequential reactions leading to the production of different anthocyanins. The gene structure of 73 anthocyanin biosynthetic genes was identified in B. rapa [28]. These gene expression analyses showed that almost all late biosynthetic genes of anthocyanin were highly up-regulated in all purple leaves of Brassica [29]. The key enzymes in the anthocyanin biosynthetic pathway include chalcone synthase (CHS), chalconeisomerase (CHI), flavanone 3-hydroxylase (F3H), dihydroflavonol-4-reductase (DFR), leucoanthocyanidin oxygenase (LDOX), anthocyanidin synthase (ANS), and anthocyanidin reductase (ANR). CHI is the first identified key enzyme in the flavonoid metabolic pathway [30], while CHS is the first enzyme in the pathway [31].
Fig 1. Diagram of the flavonoid pathway.
The enzymes for each step are italicized, with the following enzymes required for anthocyanin biosynthesis: chalcone synthase (CHS), chalcone isomerase (CHI), flavanone-3-hydroxylase (F3H), dihydroflavonol-4-reductase (DFR), anthocyanidin synthase (ANS), anthocyanidin reductase (ANR) and UDP-glucose: flavonoid-3-O-glycosyltranferase (UFGT).
Cominelli et al. [32] investigated different light treatments and found that the activities of ANS and ANR in Arabidopsis were related to their gene expression level. The regulation of anthocyanin accumulation under different light levels was shown to be due to transcriptional control or transcription factors [33–37].
The lowest light levels caused death and decreased anthocyanin content in Anacampseros rufescens, maize and perilla [38–40]. The shading of stems and leaves of Eustoma grandiflorum resulted in a significant anthocyanin reduction in petal color [41], while incubation in complete darkness was beneficial to the nutritional quality of the brassica sprouts [42]. When B. rapa was exposed to low light, the levels of phenolics and shoot biomass were reduced [43]. Still, a comprehensive study of physiological change after low light treatment is lacking. Thus, we investigated the responses of various plant parameters, such as photosynthesis, chlorophyll, and the activities of anthocyanin biosynthetic and antioxidant enzymes, in purple pak-choi under low light stress by shading the plants in a phytotron. Moreover, the examination of anthocyanin accumulation in this purple plant under different low light intensities provides invaluable guidance for artificially supplementing light intensity in agricultural facilities.
Materials and methods
Ethics statement
This study was carried out in a phytotron. No specific permissions were required. The study did not involve any endangered or protected species.
Plant material and treatments
The variety of purple pak-choi (Brassica campestris ssp. Chinensis Makino L. "ziyi") was selected by the Horticultural Research Institute of Shanghai Academy of Agricultural Sciences, China. Initially 192 purple pak-choi seeds were sown in 12 plastic plates growing at a temperature of 28/15°C day/night in a greenhouse on October 20, 2015. Plants were watered and fertilized daily with a half-strength Hoagland nutrient solution. The low light treatments were started when the plant had three expanded leaves (four weeks after sowing on November 17). They were transferred and maintained in a phytotron with the temperature of 28/15°C day/night and 60% humidity. They were divided into four groups and were exposed to low light treatment as follows (Table 1): (1) normal light (NL, 1000 μmol m–2 s–1), (2) low light 1 (TL1, 750 μmol m–2 s–1), (3) low light 2 (TL2, 500 μmol m–2 s–1), and (4) low light 3 (TL3, 250 μmol m–2 s–1). The light intensity was measured by a Specbos 4001 (JETI Company, Germany). The experiment was carried out in triplicate, and approximately 100 plants were used in each replicate.
Table 1. Low light treatment of purple pak-choi seedlings in a phytotron.
| Category | Rate of light transmittance (%) | Treatment | Illumination intensity (μmol m–2 s–1) |
|---|---|---|---|
| NL | 100 | Normal illumination | 1000 |
| TL1 | 75 | A layer of white gauze | 750 |
| TL2 | 50 | A layer of black shading with 50% transmittance | 500 |
| TL3 | 25 | A layer of black shading with 25% transmittance | 250 |
Relative pigment levels
Twenty-milligram samples of purple pak-choi leaves were incubated with 10 ml of 95% ethanol in the dark for 24 h and mixed by vortexing for 30 s after 12 h. The relative chlorophyll and carotenoid levels were measured with a spectrophotometer (DU 730, Beckman Coulter, Inc., Brea, CA, USA) at 649, 665, and 470 nm, and then the amount of chlorophyll a, chlorophyll b, and carotenoid was calculated using formulas 2.1, 2.2 and 2.3 [44]:
| (2.1) |
| (2.2) |
| (2.3) |
The total anthocyanin content (TAC) of purple pak-choi was quantified with a modified pH differential method (AOAC official method 2005.2) [45,46]. The TAC was derived using cyanidin-3-glucoside, which has a molar extinction coefficient of 26,900 L cm-1 mol-1 and a molecular weight of 449.2 g mol-1. The results are expressed as milligrams of cyanidin-3-glucoside equivalent per gram of fresh weight sample. Twenty-milligram leaf samples were incubated with 10 ml buffer (95% ethanol and 1.5 mol l-1 HCl (v/v) 85:15) at room temperature in the dark for 24 h. Then, 1 ml of leaf supernatant was mixed separately with 2 ml of 0.025 M KCl buffer at pH 1.0 and 0.4 M sodium acetate (NaAc) buffer at pH 4.5. Absorbance was read by a nucleic acid/protein analyzer (Beckman Coulter, Inc., USA) at 536 nm and at 700 nm in the pH 1.0 and pH 4.5 buffers, respectively. TAC was calculated with the following equation (2.4):
| (2.4) |
Leaf gas exchange
Leaf gas exchange was measured on a fully developed leaf from the middle of each seedling at 9:30 AM after 5 d, 10 d and 15 d of low light treatment by a Li-6400 Portable Photosynthesis System (Li-Cor Inc., Lincoln, NE, USA). The CO2 assimilation rate or net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular carbon dioxide (Ci) and transpiration rate (Tr) of purple pak-choi leaves were analyzed. After measurement, the largest leaves from each group in the same position were harvested. Three biological replicates were frozen immediately in liquid nitrogen and stored at -80°C for further analysis.
Quantification of MDA and soluble protein
The frozen leaf samples were ground to determine the MDA and soluble protein content. As described by Jiang and Zhang [47], the amount of MDA, which represents lipid peroxidation was calculated by its molar extinction coefficient (155 mM−1 cm−1) in the thiobarbituric acid reaction. Total soluble protein content was measured using the Bradford reagent [48].
Antioxidant enzyme activity assay
For the enzyme assays, 0.2 g of leaf samples were ground in 3 ml of ice-cold 25 mM HEPES buffer (0.2 mM EDTA, 2 mM ASA, and 2% PVP, pH 7.8). The homogenates were centrifuged at 4°C for 20 min at 12,000 g, and the supernatants were used to determine the enzymatic activities. The G-POD activity was measured by the modified method of Cakmak [49]. The reaction mixture had 25 mM phosphate buffer (pH 7.0), 0.05% guaiacol, 1.0 mM H2O2 and 100 μl of enzyme extract. The increase in absorbance at 470 nm caused by guaiacol oxidation (E = 26.6 mM cm-1) was used to determine the G-POD activity. CAT was assayed as described by Durner and Klessing [50], and the activity was determined as a decrease in the absorbance at 240 nm for 1 min following the decomposition of H2O2. APX was measured by monitoring the rate of ascorbate oxidation at 290 nm as described by Nakano and Asada [51]. SOD activity was measured in a mixture of 50 mM phosphate buffer (pH 7.8), 0.1 mM EDTA, 13 mM methionine, 75 μM nitroblue tetrazolium (NBT), 2 μM riboflavin, and 50 μl of enzyme [52]. One unit of SOD activity was defined as the amount of enzyme required to inhibit 50% of the p-nitro blue tetrazolium chloride reduction at 560 nm.
Anthocyanin biosynthetic enzyme activity assay
For these assays, 0.2 g of leaf samples were ground in 2 ml of ice-cold 25 mM HEPES buffer (pH 7.4) containing 0.2 mM EDTA, 2 mM AsA, and 2% PVP. The homogenates were centrifuged at 4°C for 20 min at 12,000 g, and the supernatants were used to determine the enzymatic activities. The activity of 6 anthocyanin biosynthetic enzymes, CHS, CHI, F3H, DFR, ANS and ANR, were assayed using an ELISA Kit (U.S.A TSZ Biological Trade Co., Ltd.) according to the manufacturer’s instructions. This experimental method was based on a laboratory protocol deposited in protocols io, which was obtained from doi:dx.doi.org/10.17504/protocols.io.h2mb8c6.
Statistical analysis
Statistical Product and Service Solutions (SPSS, version 20, IBM Corporation, U.S.A) was used to performed analysis of variance (ANOVA). The physiological variables are presented as the mean ± standard deviation (SD), with a minimum of three replicates. Differences between the control and treatments were considered significant at p = 0.05. Significance between treatments was determined by Duncan’s t-test. The data were plotted using Origin 7.5 software (Origin Lab, Northampton, MA, USA).
Results
Analysis of relative pigment levels
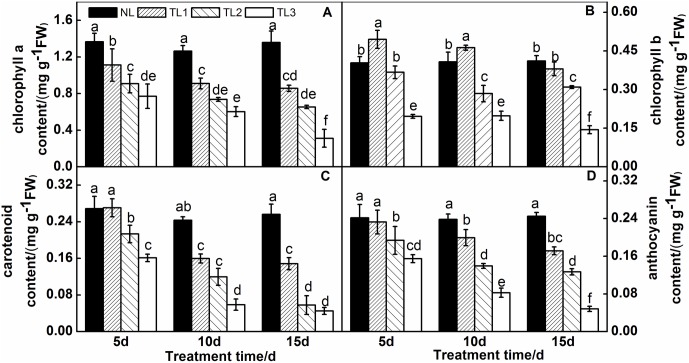
Low light stress had significant effects on the anthocyanin, carotenoid and relative chlorophyll contents of purple pak-choi (Fig 2). The chlorophyll a contents were significantly reduced after exposure to low light stress for 5 d, 10 d and 15 d (Fig 2A). The chlorophyll a content of TL1, TL2 and TL3 was significantly lower than that in the group exposed to NL by 18.59%, 33.45% and 51.19%, respectively, at 5 d; significantly decreased by 27.95%, 41.71% and 52.35%, respectively, at 10 d; and significantly declined by 40.36% 51.91% and 77.10%, respectively, at 15 d.
Fig 2.
Effects of low light treatment on chlorophyll a (A), chlorophyll b (B), carotenoid (C) and anthocyanin (D) content in leaves of purple pak-choi seedlings. The data are the mean of three replicates, and SDs are shown as vertical bars. The means marked with different letters indicate significant differences between treatments at p<0.05, as determined with Duncan’s multiple range test. Different lower-case letters at each time point indicate significant differences between treatments. FW is the abbreviation of fresh weight.
The chlorophyll b content in TL1 leaves was 22.53% and 13.39% higher than that in NL group at 5 d and 10 d, respectively (Fig 2B). However, when exposed to low light for 15 d, the chlorophyll b content of TL1 clearly decreased by 7.91%. The chlorophyll b content of TL2 and TL3 leaves greatly decreased at 10 d and 15 d by 9.11% and 51.51% respectively, compared with that of NL leaves at 5 d; the content was 30.30% and 51.42% lower, respectively, at 10 d; and 24.66% and 64.95% lower, respectively, at 15 d.
The carotenoid content in TL1 did not differ significantly from that in the NL group at 5 d (Fig 2C), but TL2 and TL3 were 20.03% and 40.08% lower than NL, respectively. The carotenoid content in TL1, TL2 and TL3 decreased by 34.31%, 50.81% and 75.82%, respectively, at 10 d; at 15 d, it decreased by 46.68%, 77.35% and 82.42%, respectively.
The anthocyanin content decreased from 5 d to 15 d under low light stress (Fig 2D). The anthocyanin content in TL1, TL2 and TL3 was 3.69%, 19.81% and 35.93% lower than that in NL at 5 d, respectively; 16.24%, 41.51% and 65.34% lower than that in NL at 10 d, respectively; and 29.91%, 48.17% and 80.31% lower than that in NL at 15 d, respectively.
Leaf gas exchange analysis
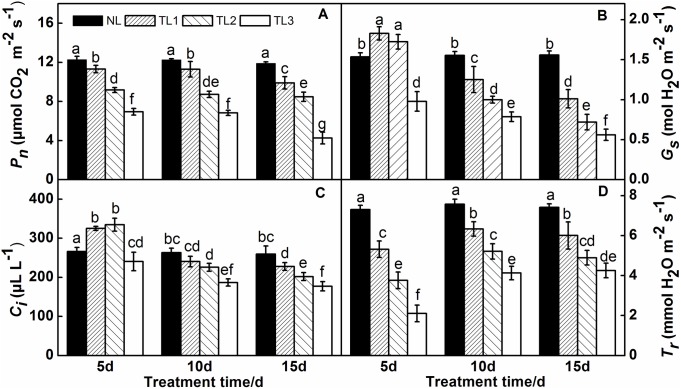
Pn, Gs, Ci and Tr were significantly influenced by low light treatment (Fig 3). The rate of CO2 assimilation (Pn) decreased sharply with low light treatment (Fig 3A). Five days after low light treatment, the Pn of TL1, TL2 and TL3 dramatically decreased by 7.90%, 25.33% and 43.47% relative to that of NL, respectively; at 10 d, the Pn decreased by 7.91%, 28.89% and 44.34% relative to that of NL, respectively; and the Pn was 16.84%, 28.79% and 64.23% lower than that in NL at 15 d, respectively. However, there were no significant differences between different treatment times for the same treatment.
Fig 3.
Effects of low light treatment on photosynthetic rate (Pn) (A), stomatal conductance (Gs) (B), intercellular CO2 concentration (Ci) (C), and transpiration rate (Tr) (D) of purple pak-choi seedlings. The data are the mean of three replicates, with SDs shown as vertical bars. The means marked with different letters indicate significant differences between treatments at p<0.05, as determined with Duncan’s multiple range test. Different lower-case letters at each time point indicate significant differences between treatments.
Gs in TL1 and TL2 was increased (Fig 3B) and the leaf stomata opened prominently when leaves were exposed to low light for 5 d, Gs was increased by 19.11% (TL1) and 18.83% (TL2) relative to that in NL. However, Gs in TL3 was 36.35% lower than that in NL. Gs in TL1, TL2 and TL3 was decreased by 12.94%, 35.60% and 49.38% at 10 d, respectively, and by 35.16%, 53.90% and 64.03% at 15 d, respectively. The changes in Ci were similar to those in Gs as the Ci value of TL1 and TL2 increased transiently after 5 d (Fig 3C); then, at 10 d and 15 d, the Ci values of all treatments were significantly decreased.
In terms of transpiration, the Tr of TL1, TL2 and TL3 showed a prominent decrease at all treatment times (5 d, 10 d, and 15 d) under low light stress (Fig 3D). The Tr was sharply lower than in NL, with 27.22%, 48.56% and 71.16% in TL1, TL2 and TL3 lower than NL at 5 d, respectively; 16.41%, 31.18% and 45.44% lower than NL at 10 d, respectively; and 19.00%, 34.00% and 42.65% lower than NL at 15 d, respectively.
MDA and soluble protein analysis
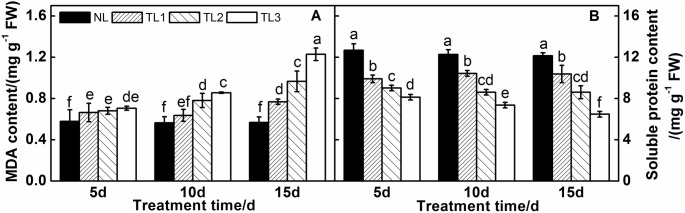
When purple pak-choi plants were exposed to low light stress, the MDA content increased in this experiment (Fig 4A). Under low light stress for 5 d, the MDA contents of TL1, TL2 and TL3 were higher than those in NL, but these differences were not statistically different. At 10 d and 15 d, the MDA content in these groups increased significantly, and the same treatment was significantly different at different treatment times. The MDA content of TL1, TL2 and TL3 increased significantly by 0.77 mg g-1 FW, 0.97 mg g-1 FW and 1.23 mg g-1 FW, respectively at 15 d. However, the soluble protein content decreased with time (Fig 4B). Compared to that of NL, the soluble protein content of TL1, TL2 and TL3 was 21.90%, 28.86% and 35.88% lower at 5 d, respectively; 14.93%, 29.87% and 39.98% lower at 10 d, respectively; and 14.59%, 29.18% and 46.69% lower at 15 d, respectively. There were significant differences between different treatments at 10 and 15 d, but the same treatment did not differ significantly at different treatment times.
Fig 4.
Effects of low light treatment on the MDA (A) content and the soluble protein (B) content in leaves of purple pak-choi seedlings. The data are the mean of three replicates, with SDs shown as vertical bars. The means marked with different letters indicate significant differences between treatments at p<0.05, as determined with Duncan’s multiple range test. Different lower-case letters at each time point indicate significant differences between treatments. FW is the abbreviation of fresh weight.
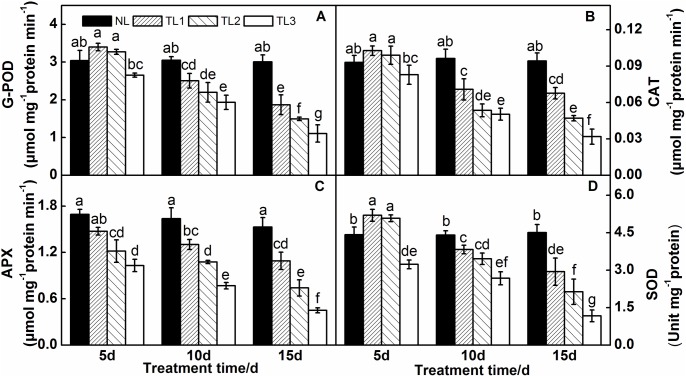
Antioxidant enzyme analysis
Low light stress resulted in significant changes of enzymatic activities of G-POD, CAT, APX, and SOD (Fig 5). For both the TL1 and TL2 leaves, the enzymatic activities of G-POD, CAT and SOD increased clearly after 5 d of the low light treatment (Fig 5A, 5B and 5D); however, the activities of these three enzymes decreased markedly after 10 d. G-POD activity decreased by 34.75% (TL1) and 50.31% (TL2), the CAT enzyme activity decreased by 28.26% (TL1) and 50.15% (TL2), and the SOD enzyme activity decreased by 34.75% (TL1) and 52.57% (TL2) relative to those of the NL leaves after 15 d. In contrast to TL1 and TL2, the G-POD, CAT and SOD enzyme activities decreased gradually from 5 d to 15 d in TL3.
Fig 5.
Effects of low light on guaiacol peroxidase (G-POD) (A), catalase (CAT) (B), ascorbate peroxidase (APX) (C) and superoxide dismutase (SOD) (D) activities in the leaves of purple pak-choi seedlings. The data are the mean of three replicates, with SDs shown as vertical bars. The means marked with different letters indicate significant differences between treatments at p<0.05, as determined with Duncan’s multiple range test. Different lower-case letters at each time point indicate significant differences between treatments.
For all the treatments, the changes in APX enzyme activities showed the same patterns as they were lower in TL1, TL2 and TL3 than those in NL leaves at 5, 10 and 15 d (Fig 5C). The APX activities of TL1, TL2 and TL3 leaves, decreased markedly by 14.82%, 28.09% and 39.11% at 5 d, respectively; by 20.36%, 34.29% and 53.03% at 10 d, respectively; and by 28.59%, 51.57% and 70.56% at 15 d, respectively.
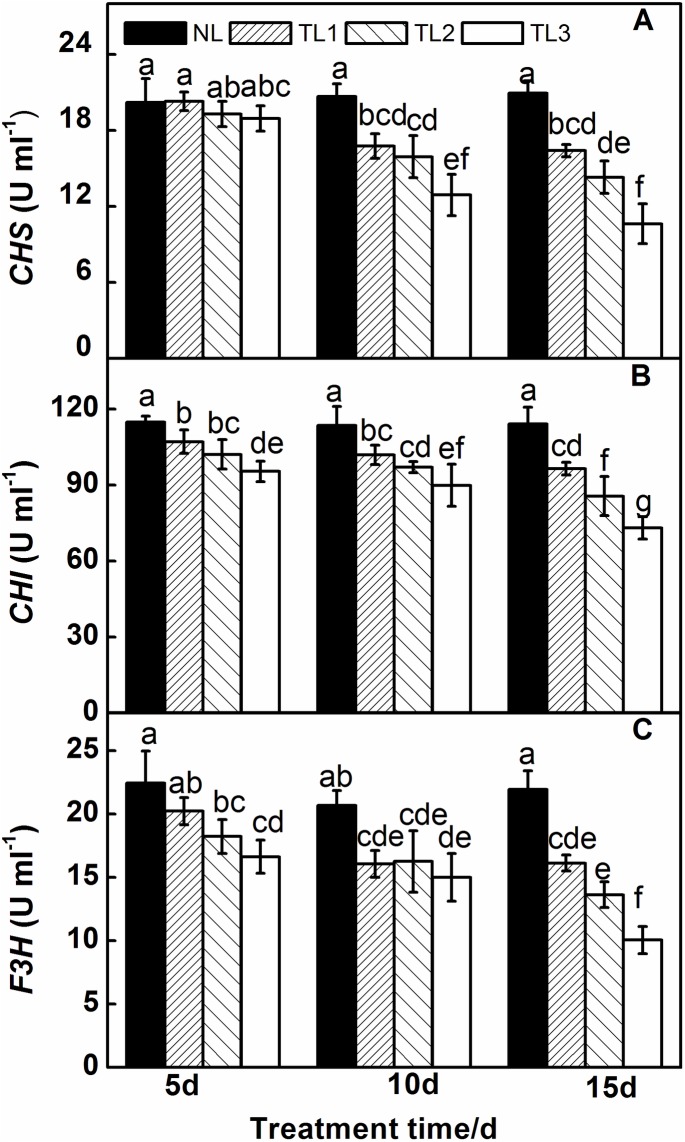
Anthocyanin biosynthetic enzyme analysis
Three key enzymes (CHS, CHI, F3H) in the anthocyanin biosynthetic pathway, affected significantly under low light stress, are shown in Fig 6. The CHS activity in NL, TL1, TL2 and TL3 leaves did not differ significantly at 5 d (Fig 6A), while it decreased slowly at 10 d. The activity was 21.70% (TL1), 31.72% (TL2) and 49.28% (TL3) lower than that in NL at 15 d. The CHI activity in TL1, TL2 and TL3 leaves was significantly lower than that in NL leaves at 5 d, 10 d and 15 d (Fig 6B). The TL2 and TL3 treatments were 11.75% and 18.69% lower at 15 d, respectively, than that at 10 d.
Fig 6. Effects of low light on chalcone synthase (CHS), chalcone-flavanone isomerase (CHI), flavanone-3- hydroxylase hydroxylation (F3H), activities in the leaves of purple pak-choi seedlings.
The data are the means of three replicates, with SDs shown as vertical bars. The means marked with different letters indicate significant difference between treatments at p<0.05, as determined with Duncan’s multiple range test. Different lower-case letters at each time point indicate significant differences between treatments.
The F3H activity in NL, TL1, TL2 and TL3 leaves was 22.44 U ml-1, 20.22 U ml-1, 18.22 U ml-1 and 16.62 U ml-1 at 5 d under low light stress, respectively (Fig 6C). At 10 d, the F3H activity of TL1, TL2 and TL3 leaves decreased by 22.28%, 21.32% and 37.37% relative to that of NL leaves, although F3H activity was not significantly different among TL1, TL2 and TL3. However, the F3H activity in NL, TL1, TL2 and TL3 leaves at 15 d was 21.93 U ml-1, 16.13 U ml-1, 13.26 U ml-1 and 10.04 U ml-1, respectively. The F3H activity in TL1, TL2 and TL3 was significantly lower than that in NL. In our experiment, the trend of DFR activity in all treatments was similar to that observed for F3H activity, and the ANS activity of treated leaves was significantly lower than that of NL leaves exposed to low light stress at 15 d. However, the ANR activity of NL, TL1, TL2 and TL3 leaves did not differ significantly at 5 d, 10 d or 15 d.
Discussion
Photosynthetic pigments play an important role in photosynthesis as they can assimilate and transfer light energy. Therefore, the pigments contents directly affect the photosynthetic efficiency. Chlorophylls are one of the most important pigments and represent a significant index of photosynthetic capacity [53]. In general, chlorophyll content will decrease after exposure to low light stress. In our study, chlorophyll a content was decreased when purple pak-choi was exposed to low light (TL1, TL2 and TL3). In addition, as the duration of exposure increased, the chlorophyll a content exhibited increasingly serious damage. However, the level of chlorophyll b, which absorbs diffuse light with a short wavelength, increased temporarily at 5 d and 10 d, but decreased at 15 d in response to low light stress. Chlorophyll b is responsible for transferring light energy in photosynthesis and could capture more energy to improve the utilization efficiency under low light stress.
Our results indicated that the chlorophyll a content under low light stress was decreased in purple pak-choi resulting in photosynthetic damage, but the chlorophyll b content was increased to resist low light stress. Ma et al. [54] found that the chlorophyll a content did not change under low light, while the chlorophyll b content increased. Lakshmi and Singh [55,56] stated that chlorophyll a and chlorophyll b both increased under low light stress, whereas Bell [38] found that the chlorophyll content of Agrostis stolonifera decreased after long-term exposure to low light stress, and so that the plants exhibited etiolating, withering and dying when the light shade was to 95%.
Our results were consistent with a previous study that found the carotenoid and anthocyanin contents decreased under low light stress [57]. Anthocyanin is a specific characteristic of purple pak-choi. The reduction of anthocyanin content led to green plants or severe etiolation of plants. In our experiment, the carotenoid and anthocyanin levels were reduced to 0.048 mg g-1 FW and 0.045 mg g-1 FW, respectively, when the shade was more than 75% (TL3) at 15 d, and the color of the purple pak-choi became light green. Nielsen [58] reported that high light intensity promoted anthocyanin synthesis and accumulation so that the purple basil leaves were darker purple than those grown under low light. Shading pears and apples during cold conditions for 2 d reduced the accumulation of anthocyanin and increased their photosensitivity [59].
Photosynthesis is the fundamental physiological process that provides energy and carbon assimilation for plant growth [60], but it is often inhibited and damaged due to its sensitivity to low light stress [61]. In the present study, the leaf photosynthetic parameters Pn, Gs, Ci and Tr showed significant responses to low light levels. The Pn and Tr of TL1, TL2 and TL3 leaves were significantly decreased after exposure to low light, whereas the Ci and Gs of TL1 and TL2 first showed an increase after 5 d and then decreased at 10 d and 15 d. The proportion of shading determined the extent of the decrease. Our results showed that the purple pak-choi seedlings receiving shade treatment exhibited impaired photosynthetic capacity due to reduced Pn, Gs, Ci and Tr. In TL1 and TL2, Ci and Gs were increased to adapt to the low light environment in the short term and finally decreased to a level less than that in the NL plants. These results are consistent with the findings of Holmgren [62], who concluded that short-term shading caused elevated carbon dioxide levels in Schefflera seedling diachyma cells. Crookston et al [63] found that the Pn decreased by 38% under low light treatment in soybeans, which was the major cause of the decline in leaf photosynthetic rate. Fay and Knapp [64] also found that the Gs and Tr of soybean were decreased by half after exposure to shaded light for 9 min. However, Duli and Derrick [65] found that the decrease of Pn in cotton was not related to Gs and Tr under low light, instead the reduction of Pn resulted from the photosynthetic electron transfer capability. Therefore, the light responses in terms of photosynthesis were different among various species.
Proteins are required for biological activity, and thus the response to biotic or abiotic stress will undoubtedly be reflected in protein content and composition. We found that the soluble protein contents of TL1, TL2 and TL3 leaves were significantly lower than those in NL leaves at 5 d, 10 d and 15 d, and they decreased further as the shade increased, indicating that the accumulation of soluble protein was inhibited under low light stress leading to decreased production [56,66]. These findings were consistent with the results of Cockshull et al [67], who proposed that low light intensity was harmful to soluble proteins in tomato plants.
Low light stress causes different types and levels of damage to plant cells. One type involves the destruction of the membrane integrity for leaf blade cells, which leads to increased cell permeability and intracellular conductivity. MDA, which is produced during lipid peroxidation, is an important index of cell damage under stress. In the current study, the MDA content in purple pak-choi increased under low light stress, indicating that the degree of lipid peroxidation in the cell membrane is related to the duration of low light treatment and the degree of shaded light.
Oxidative stress is activated under biotic and abiotic stresses and results in the abundant production of reactive oxygen species (ROS) [68]. POD, CAT, APX and SOD can scavenge H2O2 [69]. Almeselmani et al [70] and Dai. et al. [71] reported that the amelioration of oxidation resistance occurs due to antioxidant enzyme activity. In the present study, the activities of G-POD, CAT, and SOD increased in TL1 and TL2 leaves after 5 d of low light stress, but decreased in TL1, TL2 and TL3 at 10 d and 15 d. In contrast, in all low light treatments, the APX activity decreased. Our results indicate that low light stress could produce ROS and increase the activity of antioxidant enzymes. However, antioxidant enzyme activity is inhibited when stress exceeds a certain degree, and plants suffer oxidative damage when the ROS are not eliminated. This finding is consistent with the results reported by Zhang and Marcelo [72,73].
Light requirements and low temperature stimulated a series of enzymes in the anthocyanin biosynthetic pathway [74,75]. Takos et al [27] reported that light was the key environmental factor leading to anthocyanin synthesis in red apples. Many reports have described the function of enzymes in anthocyanin biosynthesis and anthocyanin accumulation. For example, DFR played an important role in anthocyanin biosynthesis in strawberry fruit [76]. CHS and DFR were critical in the process of anthocyanin biosynthesis in mature red peach and nectarine fruit [77]. In bayberry fruit, F3H, DFR and ANS levels were highly correlated with anthocyanin biosynthesis [78]. The results of the present study suggested that the enzyme activities of CHI, CHS, F3H, which are involved in the anthocyanin biosynthetic pathway, were decreased under low light stress for 10 d and 15 d, leading to a decline of anthocyanin. However, ANR activity did not show significant changes in any of the low light treatments. Therefore, ANR may not be regulated by low light stress. Our studies of low light in purple pak-choi are consistent with results in Arabidopsis thaliana [72], Perillafrutescens [75], Vitisvinifera [79] and Gerbera hybrida [80].
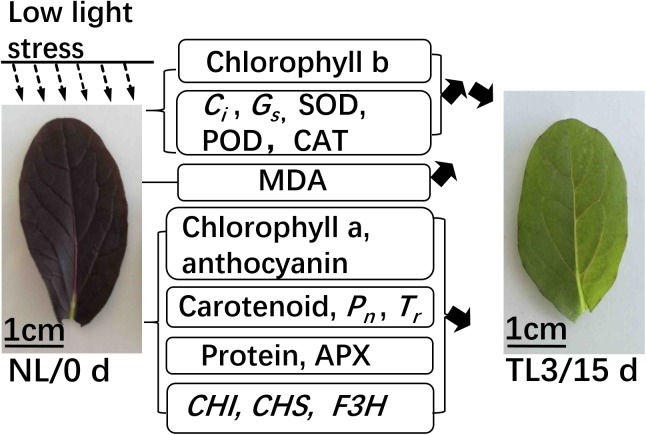
We summarized our conclusions to a model (Fig 7) to emphasize the physiological changes under light stress. At the early stage of low light treatment, the contents of chlorophyll b, Gs, Ci, SOD, POD, and CAT were increased to improve photosynthetic efficiency. When the low light stress was extended, it inhibited antioxidant enzyme activity and also suppressed the activity of anthocyanin biosynthesis enzymes, causing purple pak-choi seedlings to fade from purple to green. The physiological mechanism underlying the effects of low light stress on purple pak-choi were elucidated, demonstrating the hazards of low light and providing technical guidance for the cultivation of purple vegetables.
Fig 7. A model of purple pak-choi grown under low light stress.
The 45 degree upward sloping black arrow indicates a rise, and the 45 degree downward sloping black arrow indicates a descent.
Supporting information
(XLSX)
Acknowledgments
We thank Dr. Xuexia Wu and Xiaotao Ding for their technical assistance in the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information file.
Funding Statement
This work was supported by the Shanghai green vegetable industry system, and SAAS Program for Excellent Research Team (SPERT). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Nagy F, Schafer E (2006) Photomorphogenesis in plants and bacteria. Dordrecht, The Netherlands: Springer. [Google Scholar]
- 2.Berenschot AS, Quecini V (2014) A reverse genetics approach identifies novel mutants in light responses and anthocyanin metabolism in petunia. Physiol Mol Biol Plants 20: 1–13. doi: 10.1007/s12298-013-0212-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang L, Deng F, Ren WJ, Yang WY (2012) Effects of shading on starch pasting characteristics of indica hybrid rice (Oryza sativa L.). PLoS One 8: e68220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demao J, Xia L (2001) Cultivar Differences in Photosynthetic Tolerance to Photooxidation and Shading in Rice (Oryza Sativa L.). Photosynthetica 39: 167–175. [Google Scholar]
- 5.Janardhan KV, Murty KS (1980) Effect of low light during vegetative stage on photosynthesis and growth attributes in rice. Indian J Plant Physi 23: 156–162. [Google Scholar]
- 6.Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55: 728–749. [DOI] [PubMed] [Google Scholar]
- 7.Ishida K (1989) Influence of respiration rate and metabolic substances on nodal position of first flower bud of eggplant seedlings. Engei Gakkai Zasshi 58: 657–664. [Google Scholar]
- 8.Vol N (1996) Stress and stress recovering by grapevines. Bot Helv 106: 73–84. [Google Scholar]
- 9.Callejónferre AJ, Manzanoagugliaro F, Díazpérez M, Carreñoortega A, Pérezalonso J (2009) Effect of shading with aluminised screens on fruit production and quality in tomato (Solanum lycopersicum L.) under greenhouse conditions. Span J Agric Res 7: 41–49. [Google Scholar]
- 10.Patten, Proebsting (1986) Effect of different artificial shading times and natural light intensities on the fruit quality of 'Bing' sweet cherry. J Am Soc Hortic Sci 111: 360–363. [Google Scholar]
- 11.Liu Q-h, Wu X, Chen B-c, Ma J-q, Gao J (2014) Effects of Low Light on Agronomic and Physiological Characteristics of Rice Including Grain Yield and Quality. Rice Science 21: 243–251. [Google Scholar]
- 12.Zhu P, Yang SM, Ma J, Li SX, Chen Y. (2008) Effect of Shading on the Photosynthetic Characteristics and Yield at Later Growth Stage of Hybrid Rice Combination. Acta Agronomica Sinica 34: 2003–2009. [Google Scholar]
- 13.Meng L, Chen WF, Li LX, Xu ZJ, Liu LX,Sun JW. (2002) Influence of Low Light on Stomatal Characters in Rice Leaves. Journal of Shenyang Agricultural University 33: 87–89. [Google Scholar]
- 14.Yang D, Duan LS, Xie HA, Li ZH, Huang TX (2011) Effect of pre-flowering light deficiency on biomass accumulation and physio-logical characteristics of rice. Chinese Journal of Eco-Agriculture 19: 347–352. [Google Scholar]
- 15.Sato K, Kim JM (1980) Relationships between environmental conditions and production- and consumption activities of individual leaves in the population of rice plant in a paddy field. 1. Changes in photosynthesis and dark respiration of individual leaves under field conditions. Jpn J Crop Sci. [Google Scholar]
- 16.Amirjani MR (2010) Effect of Salinity Stress on Growth, Mineral Composition, Proline Content, Antioxidant Enzymes of Soybean. American Journal of Plant Physiology 5: 350–360. [Google Scholar]
- 17.Singam K, Juntawong N, Cha-Um S, Kirdmanee C (2011) Salt stress induced ion accumulation, ion homeostasis, membrane injury and sugar contents in salt-sensitive rice (Oryza sativa L. spp. indica) roots under isoosmotic conditions. Afr J Biotechnol 10: 1340–1346. [Google Scholar]
- 18.Asada K (2006) Production and Scavenging of Reactive Oxygen Species in Chloroplasts and Their Functions 1. Plant Physiol 141: 391–396. doi: 10.1104/pp.106.082040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Volkov RA, Panchuk II, Mullineaux PM, Schöffl F (2006) Heat stress-induced H(2)O (2) is required for effective expression of heat shock genes in Arabidopsis. Plant Mol Biol 61: 733–746. doi: 10.1007/s11103-006-0045-4 [DOI] [PubMed] [Google Scholar]
- 20.Feild TS, Lee DW, Holbrook NM (2001) Why leaves turn red in autumn. The role of anthocyanins in senescing leaves of red-osier dogwood. Plant Physiol 127: 566–574. [PMC free article] [PubMed] [Google Scholar]
- 21.Gould KS (2003) Nature's Swiss army knife: the diverse protective roles of anthocyanins in leaves. Biomed Res Int 2004: 314–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chalker-Scott L (1999) Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol 70: 1–9. [Google Scholar]
- 23.Lange H, Shropshire W, Mohr H (1971) An Analysis of Phytochrome-mediated Anthocyanin Synthesis. Plant Physiol 47: 649–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Batschauer A, Ehmann B, Schäfer E (1991) Cloning and characterization of a chalcone synthase gene from mustard and its light-dependent expression. Plant Mol Biol 16: 175–185. [DOI] [PubMed] [Google Scholar]
- 25.Neuhaus G, Bowler C, Kern R, Chua NH (1993) Calcium/calmodulin-dependent and -independent phytochrome signal transduction pathways. Cell 73: 937–952. [DOI] [PubMed] [Google Scholar]
- 26.Guo J, Wang MH (2010) Ultraviolet A-specific induction of anthocyanin biosynthesis and PAL expression in tomato (Solanum lycopersicum L.). Plant Growth Regul 62: 1–8. [Google Scholar]
- 27.Takos AM, Jaffe FW, Jacob SR, Bogs J, Robinson SP, Walker AR. (2006) Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples. Plant Physiol 142: 1216–1232. doi: 10.1104/pp.106.088104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo N, Cheng F, Wu J, Liu B, Zheng SN, Liang JL, et al. (2014) Anthocyanin biosynthetic genes in Brassica rapa. BMC Genomics 487: 135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mushtaq MA, Pan Q, Chen D, Zhang Q, Ge X, Li ZY. (2016) Comparative Leaves Transcriptome Analysis Emphasizing on Accumulation of Anthocyanins in Brassica: Molecular Regulation and Potential Interaction with Photosynthesis. Frontiers in Plant Science 7 doi: 10.3389/fpls.2016.00311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimada N, Aoki T, Sato S, Nakamura Y, Tabata S, Ayabe S. (2003) A cluster of genes encodes the two types of chalcone isomerase involved in the biosynthesis of general flavonoids and legume-specific 5-deoxy(iso)flavonoids in Lotus japonicus. Plant Physiol 131: 941–951. doi: 10.1104/pp.004820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koes RE, Quattrocchio F, Mol JNM (1994) The flavonoid biosynthetic pathway in plants: Function and evolution. Bioessays 16: 123–132. [Google Scholar]
- 32.Cominelli E, Gusmaroli G, Allegra D, Galbiati M, Wade HK, Jenkins GL,et al. (2008) Expression analysis of anthocyanin regulatory genes in response to different light qualities in Arabidopsis thaliana. J Plant Physiol 165: 886–894. doi: 10.1016/j.jplph.2007.06.010 [DOI] [PubMed] [Google Scholar]
- 33.Albert NW, Lewis DH, Zhang H, Irving LJ, Jameson PE, Davies KM. (2009) Light-induced vegetative anthocyanin pigmentation in Petunia. J Exp Bot 60: 2191–2202. doi: 10.1093/jxb/erp097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petroni K, Tonelli C (2011) Recent advances on the regulation of anthocyanin synthesis in reproductive organs. Plant Science 181: 219–229. doi: 10.1016/j.plantsci.2011.05.009 [DOI] [PubMed] [Google Scholar]
- 35.Huang ZA, Zhao T, Fan HJ, Wang N, Zheng SS, Ling HQ. (2012) The upregulation of NtAN2 expression at low temperature is required for anthocyanin accumulation in juvenile leaves of Lc-transgenic tobacco (Nicotiana tabacum L.). J Genet Genomics 39: 149–156. doi: 10.1016/j.jgg.2012.01.007 [DOI] [PubMed] [Google Scholar]
- 36.Lopez L, Carbone F, Bianco L, Giuliano G, Facella P, Perrotta G. (2012) Tomato plants overexpressing cryptochrome 2 reveal altered expression of energy and stress-related gene products in response to diurnal cues. Plant Cell Environ 35: 994–1012. doi: 10.1111/j.1365-3040.2011.02467.x [DOI] [PubMed] [Google Scholar]
- 37.Ambawat S, Sharma P, Yadav NR, Yadav RC (2013) MYB transcription factor genes as regulators for plant responses: an overview. Physiology Mol Biol Plants 19: 307–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li YC, Lin TC, Martin CE (2015) Leaf anthocyanin, photosynthetic light-use efficiency, and ecophysiology of the South African succulent Anacampseros rufescens (Anacampserotaceae). South African Journal of Botany 99: 122–128. [Google Scholar]
- 39.Miki S, Wada KC, Takeno K (2015) A possible role of an anthocyanin filter in low-intensity light stress-induced flowering in Perilla frutescens var. crispa. Journal of Plant Physiology 175: 157 doi: 10.1016/j.jplph.2014.12.002 [DOI] [PubMed] [Google Scholar]
- 40.Zhou WX, Li CH, Liu TX, Wang XP, Yan ZG (2013) Effects of low-light stress on maize ear development and endogenous hormones content of two maize hybrids (Zea mays L.) with different shade-tolerance. Acta Ecological Inca 33: 4315–4323. [Google Scholar]
- 41.Meir S, Kochanek B, Glick A, Salim S, Lers A, Burd S, et al. (2010) Reduced petal pigmentation in lisianthus (Eustoma grandiflorum) flowers under low light conditions is associated with decreased expression of anthocyanin biosynthesis genes. Acta Horticulturae 877: 1735–1744. [Google Scholar]
- 42.Vale AP, Santos J, Brito NV, Peixoto V, Carvalho R, Rose E, et al. (2015) Light influence in the nutritional composition of Brassica oleracea sprouts. Food Chemistry 178: 292–300. doi: 10.1016/j.foodchem.2015.01.064 [DOI] [PubMed] [Google Scholar]
- 43.Johnson WA, Nechols JR, Cloyd RA, Rotenberg D, Kennelly MM (2016) Effect of Light Intensity on Brassica rapa Chemistry and Plutella xylostella (Lepidoptera: Plutellidae) Life History Traits". Journal of Entomological Science 47: 327–349. [Google Scholar]
- 44.Lichtenthaler HK, Wellburn AR (1983) Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem Soc Trans 603: 591–592. [Google Scholar]
- 45.Sukwattanasinit T, Burana-Osot J, Sotanaphun U (2007) Spectrophotometric method for quantitative determination of total anthocyanins and quality characteristics of roselle (Hibiscus sabdariffa). Planta Medica 73: 1517–1522. doi: 10.1055/s-2007-990262 [DOI] [PubMed] [Google Scholar]
- 46.Lee J, Rennaker C, Wrolstad RE (2008) Correlation of two anthocyanin quantification methods: HPLC and spectrophotometric methods. Food Chem 110: 782–786. [Google Scholar]
- 47.Jiang M, Zhang J (2001) Effect of abscisic acid on active oxygen species, antioxidative defence system and oxidative damage in leaves of maize [Zea mays] seedlings. Plant Cell Physiol 42: 1265–1273. [DOI] [PubMed] [Google Scholar]
- 48.Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 49.Cakmak I, Marschner H (1992) Magnesium Deficiency and High Light Intensity Enhance Activities of Superoxide Dismutase, Ascorbate Peroxidase, and Glutathione Reductase in Bean Leaves. Plant Physiol 98: 1222–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Durner J, Klessig DF (1996) Salicylic acid is a modulator of tobacco and mammalian catalases. J Biol Chem 271: 28492–28501. [DOI] [PubMed] [Google Scholar]
- 51.Nakano Y, Asada K (1980) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22: 867–880. [Google Scholar]
- 52.Giannopolitis CN, Ries SK (1977) Superoxide Dismutase: I. Occurrence in Higher Plants. Plant Physiol 59: 309–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang FJ (2007) Effect of Light Stress on the Content of Chloroplast Pigment and Taxol in the Leaves of Taxus chinensis var.mairei. Bulletin of Botanical Research 27: 556–558. [Google Scholar]
- 54.Ma ZQ, Li SS, Zhang MJ, Jiang SH, Xiao YL (2010) Light intensity affects growth, photosynthetic capability, and total flavonoid accumulation of Anoectochilus plants. Hortscience 45: 863–867. [Google Scholar]
- 55.Singh VP, Dey SK, Murty KS (1988) Effect of low light stress on growth and yield of rice. Indian J Plant Physi 31: 84–91. [Google Scholar]
- 56.Praba ML, Vanangamudi M, Thandapani V (2011) Effects of low light on yield and physiological attributes of rice. International Rice Research Notes 29. [Google Scholar]
- 57.Bell GE, Danneberger TK (1999) Temporal shade on creeping bentgrass turf. Crop Sci 39: 1142–1146. [Google Scholar]
- 58.Nielsen SL, Simonsen AM (2011) Photosynthesis and photoinhibition in two differently coloured varieties of Oxalis triangularis—the effect of anthocyanin content. Photosynthetica 49: 346–352. [Google Scholar]
- 59.Steyn WJ, Wand SJE, Jacobs G, Rosecrance RC, Roberts SC (2009) Evidence for a photoprotective function of low-temperature-induced anthocyanin accumulation in apple and pear peel. Physiologia Plantarum 136: 461–472. doi: 10.1111/j.1399-3054.2009.01246.x [DOI] [PubMed] [Google Scholar]
- 60.Lawlor DW (2009) Musings about the effects of environment on photosynthesis. Ann Bot 103: 543–549. doi: 10.1093/aob/mcn256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mittler R (2006) Abiotic stress, the field environment and stress combination. Trends Plant Sci 11: 15–19. doi: 10.1016/j.tplants.2005.11.002 [DOI] [PubMed] [Google Scholar]
- 62.Holmgren M (2000) Combined effects of shade and drought on tulip poplar seedlings: trade-off in tolerance or facilitation? Oikos 90: 67–78. [Google Scholar]
- 63.Crookston RK, Treharne KJ, Ludford P, Ozbun JL (1975) Response of beans to shading. Crop Sci 15: 412–416. [Google Scholar]
- 64.Fay PA, Knapp AK (1998) Responses to short-term reductions in light in soybean leaves: Effects of leaf position and drought stress. Int J Plant Sci 159: 805–811. [Google Scholar]
- 65.Duli Z, Derrick MO (1998) Influence of shade on mineral nutrient status of field-grown cotton. J Plant Nutr 21: 1681–1695. [Google Scholar]
- 66.Verheul MJ (2012) Effects of plant density, leaf removal and light intensity on tomato quality and yield. Acta Hortic 956: 365–372. [Google Scholar]
- 67.Cockshull KE, Graves CJ, Cave CRJ (1992) The influence of shading on yield of glasshouse tomatoes. J Hortic Sci 67: 11–24. [Google Scholar]
- 68.Qu AL, Ding YF, Jiang Q, Zhu C (2013) Molecular mechanisms of the plant heat stress response. Biochem Bioph Res Co 432: 203–207. [DOI] [PubMed] [Google Scholar]
- 69.Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7: 405–410. [DOI] [PubMed] [Google Scholar]
- 70.Almeselmani M, Deshmukh PS, Sairam RK, Kushwaha SR, Singh TP (2006) Protective role of antioxidant enzymes under high temperature stress. Plant Science 171: 382–388. doi: 10.1016/j.plantsci.2006.04.009 [DOI] [PubMed] [Google Scholar]
- 71.Dai AH, Nie YX, Yu B, Li Q, Lu LY, Bai JG. (2012) Cinnamic acid pretreatment enhances heat tolerance of cucumber leaves through modulating antioxidant enzyme activity. Environ Exp Bot 79: 1–10. [Google Scholar]
- 72.Pompelli MF, Martins SCV, Antunes WC, Chaves ARM, Damatta FM (2010) Photosynthesis and photoprotection in coffee leaves is affected by nitrogen and light availabilities in winter conditions. J Plant Physiol 167: 1052–1060. doi: 10.1016/j.jplph.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 73.Zhang XL, Jia XF, Yu B, Gao Y, Bai JG (2011) Exogenous hydrogen peroxide influences antioxidant enzyme activity and lipid peroxidation in cucumber leaves at low light. Scientia Horticulturae 129: 656–662. [Google Scholar]
- 74.Lipphardt S, Brettschneider R, Kreuzaler F, Schell J, Dangl JL (1988) UV-inducible transient expression in parsley protoplasts identifies regulatory cis-elements of a chimeric Antirrhinum majus chalcone synthase gene. EMBO J 7: 4027–4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gong Z, Yamazaki M, Sugiyama M, Tanaka Y, Saito K (1997) Cloning and molecular analysis of structural genes involved in anthocyanin biosynthesis and expressed in a forma-specific manner in Perilla frutescens. Plant Mol Biol 35: 915–927. [DOI] [PubMed] [Google Scholar]
- 76.Moyano E, Caballero JL (1998) A fruit-specific putative dihydroflavonol 4-reductase gene is differentially expressed in strawberry during the ripening process. Plant Physiol 117: 711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsuda T, Yamaguchi M, Honda C, Moriguchi T (2004) Expression of anthocyanin biosynthesis genes in the skin of peach and nectarine fruit. J Am Soc Hortic Sci 129: 857–862. [Google Scholar]
- 78.Niu SS, Xu CJ, Zhang WS, Bo Z, Xian L, Wang KL, et al. (2010) Coordinated regulation of anthocyanin biosynthesis in Chinese bayberry (Myrica rubra) fruit by a R2R3 MYB transcription factor. Planta 231: 887–899. doi: 10.1007/s00425-009-1095-z [DOI] [PubMed] [Google Scholar]
- 79.Matus JT, Loyola R, Vega A, Peña-Neira A, Bordeu E, Arce-Johnson P, et al. (2009) Post-veraison sunlight exposure induces MYB-mediated transcriptional regulation of anthocyanin and flavonol synthesis in berry skins of Vitis vinifera. J Exp Bot 60: 853–867. doi: 10.1093/jxb/ern336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meng X, Xing T, Wang X (2004) The role of light in the regulation of anthocyanin accumulation in Gerbera hybrida. Plant Growth Regul 44: 243–250. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLSX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information file.