Abstract
Purpose
While the relationship of asthma and coronary heart disease (CHD) (a specific manifestation of cardiovascular disease) has not been described consistently, we tried to defined this relation and explore the influence of gender and asthma status (child- and adult-onset asthma) on this issue.
Methods
We searched published reports that described the relationship of asthma and CHD.
Results
Eleven trials were identified, covering 666,355 subjects. Asthma overall was significantly associated with CHD both for prospective trials (HR 1.34 [1.09,1.64], P = 0.005) and for retrospective trials(OR 1.29 [1.13,1.46], P = 0.001), when compared to individuals without asthma. Subgroup analysis split by gender indicated that females with asthma were significantly associated with CHD (HR 1.40 [1.20,1.62], P<0.001), but males with asthma were not significantly related with CHD (HR 1.19 [0.98,1.44], P = 0.07). For the four subgroups (Females with adult-onset asthma,males with adult-onset asthma,females with child-onset asthma,and males with child-onset asthma), pooled analysis of two trials indicated that only females with adult-onset asthma were significantly associated with CHD (HR 2.06 [1.32,3.19], P<0.001).
Conclusions
Our data indicated that asthma was associated with CHD, and the relationship between them seemed to derived mostly from females with adult-onset asthma. Considering the limits of our study, these findings should be taken with caution.
Introduction
Asthma characterized by bronchospasms and airflow obstruction is a chronic inflammatory disease of the airway and affects about 300 million people worldwide. Inflammation has been reported to play an important role in the pathogenesis of atherosclerosis [1–3]. Although there had been many trials examining the relationship of impaired lung function and cardiovascular disease (CVD)[4–9], only few trials relating asthma to CVD were reported. Recently, several trials supporting the relationship of asthma and CVD has been reported[10–11]. All the papers supported the relationship of asthma and CVD, but they all related distinct specific CVD outcomes (myocardial infarction, stroke, coronary heart disease (CHD), hypertension, heart failure or angina) to asthma, sometimes with distinct results for the same endpoint. The study by Lee suggested that CHD is the principal manifestation of CVD related with asthma[12], and supported by some trials [13–17]. Because of the chronic inflammatory nature of both asthma and CHD, inflammatory reaction might be a potential link between the CHD and asthma. Meanwhile, asthma was not significantly associated with CHD in some other trials[18–22]. So, we undertook this pooled analysis to clarify this relationship of asthma and CHD. There were some trials that presented the possible association split by gender, indicating this association may be stronger for females[13,15,17]. So, we also undertook subgroup analysis to explore the influence of gender on this association. Furthermore, asthma is not one unique disease, but a aggregation of different underlying subtypes with distinct causes [23–25]. Adult- and child-onset asthma vary regarding gender distribution, asthma triggers, and systemic inflammation[23–25]. In this pooled analysis, we also examined the possible influence of age of asthma onset on this association.
Methods
Literature search strategy
The Cochrane Controlled Trial Register, Embase, Medline, and the Science Citation Index were searched using the medical subject headings “asthma”, “cardiovascular disease” “coronary heart disease”, and “ischemic heart disease”. Reference lists of selected reports were also hand-searched. This pooled analysis was approved by the institutional review boards of Weifang People’s Hospital, in accordance with the Helsinki Declaration.
Selection of studies
Trials were included for this analysis if they met the following criteria: (1) They were published up to October, 2016. (2) They tried to clarify the association of asthma and coronary heart disease. (3) They had to provide the data of hazard ratio (HR) or odds ratio (OR) for CHD when compared asthma patients with individuals without asthma. Multiple reports about a single trial were considered as one. All potential trials were reviewed by two investigators separately(L.D.W and Z.X.S.).
Outcome measures
The primary outcome was to clarify the association of asthma and CHD, and evaluate the possible influence of gender and age of asthma onset on this association.
Quality assessment
The Newcastle-Ottawa Scale (S1 Table) was used to assess the quality of each enrolled trial. This measure assesses aspects of methodology in observational trials associated with study quality, including case selection, comparability of population and ascertainment of exposure to risks.
Statistical analysis
All these analyses were undertaken using a random-effects model which could provided a more conservative result. The heterogeneity among these trials was evaluated using Cochrane χ2 test and quantified with the I2 statistic. We also undertook subgroup analyses to sought the source of heterogeneity. Publication bias was evaluated with Egger's test. All meta-analyses were undertaken with Review Manager (version 5.3; The Cochrane Collaboration, Oxford, England) and Stata ver. 12.0 software (College Station, TX). Statistical significance was defined as a P value of less than 0.05.
Results
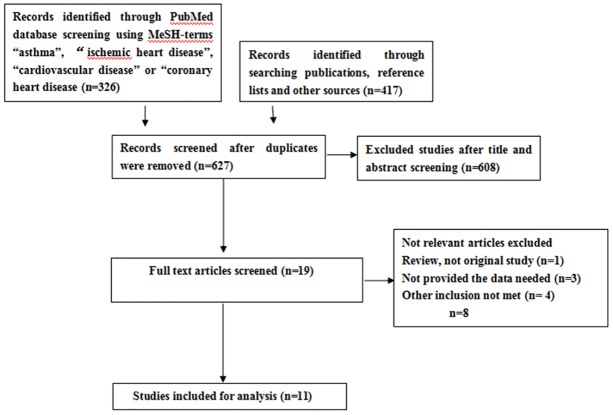
A comprehensive search of the Cochrane Controlled Trial Register, Embase, Medline, and the Science Citation Index attained 627 articles, of which 11 trials met the predefined inclusion criteria (Fig 1), covering 666,355 subjects totally[12–22]. Their characteristics were summarized in Table 1. These studies quality assessed by Newcastle-Ottawa Scale items was shown in S2 Table.
Fig 1. PRISMA flow diagram.
Table 1. Characteristics of included studies.
| year | Study type | Study Region | Study population | Study years | Mean Age | No. of Subjects | Adjusted factors | |
|---|---|---|---|---|---|---|---|---|
| Asthma | Nonasthma | |||||||
| Chung 2014[13] | Retrospective | Taiwan | Communities | 1996–2011 | 50.5 | 50.9 | 38 840 | Age, sex, and comorbidities of hypertension,Diabetes, hyperlipidemia, stroke, heart failure, COPD and smoking. |
| Colak 2015[14] | Prospective | Denmark | Communities | 2003–2013 | 53/60/56* | 56 | 40 649 | Age, sex, body mass index, leisure time physical activity, education,annual household income, alcohol consumption, cumulative tobacco consumption, systolic and diastolic blood pressure, total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, triglycerides, use of cholesterol-lowering medication, and presence of diabetes. |
| Iribarren 2004[18] | Retrospective | USA | Communities | 1964–1973 | 40 for men 41 for women |
41 for men 40 for women |
13047 | Age, race/ethnicity, education level, smoking status, alcohol consumption, body mass index, serum total cholesterol, white blood cell count, hypertension, diabetes, parental history of coronary heart disease, and occupational exposures |
| Iribarren 2012[15] | Prospective | USA | Communities | 1996–2008 | NA | NA | 407 190 | Diabetes, hypertension, hyperlipidemia, body mass index, and smoking status |
| Lee 2012[12] | Retrospective | USA | Communities | 1999–2006 | 53.1/37.7▲ | 49.9 | 16943 | Age, systolic blood pressure, HDL cholesterol, BMI, hs-CRP, smoking, and diabetes mellitus. |
| Liss 2000[19] | Retrospective | Canada | Hospitalization | 1980–1996 | NA | NA | 2400 | Period of birth, time period of accident, and sex. |
| Onufrak 2008[20] | Prospective | USA | Communities | 1987–2001 | 53.3/55.4★ 52.9/54.3● |
54.3☆ 53.7○ |
14 567 | Age,body mass index, black race,diabetes mellitus, hypertension, education level, low-and high-density lipoprotein cholesterol, and physical activity. |
| Prosser 2010[16] | Retrospective | British Columbia | Communities | 1996/97 | NA | NA | 111780 | Adult service user (ASU) population, the age distribution. |
| Schanen 2005[21] | Prospective | USA | Communities | 1987–1998 | 54 | 54 | 13501 | Age, sex, race/centre, HDL cholesterol, LDL cholesterol, systolic blood pressure, hypertension medication use, smoking status, pack years,W/H ratio, diabetes diagnosis, and sport score. |
| Toren 1996[22] | Prospective | Sweden | Outpatient | 1962–1986 | NA | NA | 262 | Smoking |
| Yun 2012[17] | Retrospective | USA | Hospitalization | 1964–1983 | 15.1 | 15.1 | 7176 | Diabetes mellitus; coronary heart disease; rheumatoid arthritis; inflammatory bowel disease. |
Abbreviations: NA not available. 53/60/56* 53 for Never-Smokers asthma; 60 for Former Smokers asthma; 56 for Current Smokers asthma. 53.1/37.7▲ 53.1 for Adult onset asthma; 37.7 for Child onset asthma. 53.3/55.4★ 53.3 for men with Child-Onset Asthma; 55.4 for men with Adult-Onset Asthma. 54.3☆ for men with No Asthma. 52.9/54.3● 52.9 for women with Child-Onset Asthma; 54.3 for women with Adult-Onset Asthma. 53.7○ for women with No Asthma.
As shown in Fig 2, pooled analysis indicated that asthma overall was significantly associated with CHD both for prospective trials (HR 1.34 [1.09,1.64], P = 0.005) and for retrospective trials(OR 1.29 [1.13,1.46], P = 0.001), when compared to individuals without asthma. The values for heterogeneity test were high for these prospective trials (I2 = 77%, p = 0.001), low for these retrospective trials (I2 = 43%, p = 0.12). Egger's test indicated no publication bias(P = 0.759 for prospective subgroup, P = 0.457 for retrospective subgroup). A funnel plot of publication bias was shown in S1 Fig.
Fig 2. Meta-analysis of the association between asthma and coronary heart disease(CHD).

HR, Hazard Ratio; OR Odds Ratio; CI, 95% confidence interval; Random, random-effects model.
When males with asthma were isolated,the increased risk for CHD were insignificant (HR 1.19 [0.98,1.44], P = 0.07)(Fig 3). Subgroup analysis split by gender indicated that only females with asthma attained a significant increased risk for CHD(HR 1.40 [1.20,1.62], P<0.001)(Fig 3). The significant relationship between females with asthma and CHD remained stable in sensitivity analysis when excluding any one of these trials. But only when excluding the Iribarren 2004 trial [17], the association of males with asthma and CHD became significantly(RR 1.29 [1.19, 1.40], P<0.001). The values for heterogeneity test were (I2 = 80%, p<0.001) for females with asthma subgroup,and (I2 = 89%, p<0.001) for males with asthma subgroup, respectively. No publication bias was shown for each subgroup (P = 0.90, 0.99, respectively). Meanwhile, the test for heterogeneity among genders indicated no statistical difference (P = 0.20) in Fig 3.
Fig 3. Subgroup meta-analysis of the association between asthma and coronary heart disease(CHD) by sex (females and males).
HR, Hazard Ratios; CI, 95% confidence interval; Random, random-effects model.
In gender and age of asthma onset stratified analyses, pooled analysis of two trials [12,20] indicated that a significant association with CHD was only shown in females with adult-onset asthma(HR 2.06 [1.32,3.19], P<0.001), but not in males with adult-onset asthma (HR 0.81 [0.48, 1.39], P = 0.45), females with child-onset asthma(HR 0.91 [0.50, 1.65],P = 0.75), and males with child-onset asthma(HR 0.75 [0.24, 2.33],P = 0.62)(Fig 4). All results remained stable in sensitivity analysis. The values for heterogeneity test were (I2 = 20%, p = 0.26) for females with adult-onset asthma, males with adult-onset asthma(I2 = 0%, p = 0.41),females with child-onset asthma (I2 = 0%, p = 0.76) and males with child-onset asthma(I2 = 81%, p = 0.02). In addition, there were no overall significant asthma-CHD association when pooling these two studies(HR 1.17 [0.81, 1.69], P = 0.41), and no significant association in gender-specific analysis (Males: HR 0.85 [0.51, 1.40], P = 0.10; Females: HR 1.54 [0.92, 2.57],P = 0.10).
Fig 4. Subgroup meta-analysis of the association between asthma and coronary heart disease(CHD) by sex and age of asthma onset (child- and adult-onset).
HR, Hazard Ratios; CI, 95% confidence interval; Random, random-effects model.
Discussion
Our pooled analysis indicated that patients with asthma had an increased risk of 32% for CHD occurence when compared to individuals without asthma. Since patients with asthma are usually treated with corticosteroids to control their airway inflammation, it would be proper to analyze the possible influence of steroids on this association of asthma and CHD. Since both males and females with asthma use these medicines, steroids would not totally explain why males with asthma was not found to be significantly associated with CHD. Why relationships of asthma and CHD in females are stronger than that observed in males still remains unclear. It has been suggested that estrogen enhances proinflammatory cytokines released from macrophages, monocytes, and vascular cells, all of which would intrigue asthma[26–27]. The occurrence of asthma in females was associated with shifts in estrogen levels, which incidence increased after puberty and peaked during the onset of menopause. Females with asthma may be particularly susceptible to estrogen-modulated alterations in inflammatory cytokine regulation. Because of the chronic inflammatory nature of CHD, the inflammatory cytokine alterations might be a potential link between the CHD and asthma[24]. Asthma also has been found to be associated with coronary artery spasm. Both coronary artery disease and coronary artery spasm are inflammatory diseases. Although gender is not a risk factor, more men than women have coronary artery spasm. However, racial heterogeneity shows that in Caucasians, females are more likely to develop coronary artery spasm.
Asthma has been recognized not as a uniform disease, but an aggregation of distinct conditions[23–25]. Child-onset asthma differs from adult-onset asthma in several aspects, including its distribution in males and females and its immunologic characteristics. To further elucidate possible reasons forming the relationship of asthma and CHD, we decided to split the population by two criterias: gender (males and females), age of asthma onset (adult and child-onset). Pooling analysis of two trials[12,20] had indicated that the significant association was only observed in females with adult-onset asthma, but not in females with child-onset asthma or males with either adult- or child-onset asthma. Additionally, the findings of no overall asthma-CHD association and no association in gender-specific analysis when pooling the two studies might strengthen the relationship between females with adult-onset asthma with CHD. One possible explanation for the lack of the relationship between CHD and child-onset asthma is that child-onset asthma derives from a distinct allergic basis from adult-onset asthma which has been associated with gender and environmental irritants. Because adult-onset asthma stems from more intrinsic causes inside the body, such as hormones and stiffening of chest walls. These intrinsic factors could increase CHD risk. Another possible reason was that cigarette smoking had been shown to affect adult-onset asthma, and was also a risk factor for CHD [28–30].
However, these findings should be interpreted with caution: Firstly, we used abstracted data, whereas an individual patient data-based pooled analysis would have provided a more precise estimate of the relationship of asthma and CHD. Current asthma symptoms, smoking and obesity had been shown to be important factors in phenotypes of adult-onset asthma [29–30]. They could be analyzed well with individual patient data. Secondly, medication information including dosages for asthma is lacking, because steroids could be an important confounding factor. Thirdly, the studies were relatively heterogeneous with respect to patient population, disease status, and study design. Given this clinical difference (Table 1) and statistical heterogeneity (Fig 2) among them, our decision to this association could be questioned.
The data supports the conclusion that asthma was associated with CHD, and the relationship between them seemed to derived mostly from females with adult-onset asthma. Considering the limits of our study, these findings should be taken with caution.
Supporting information
(DOC)
(DOCX)
(TIF)
Acknowledgments
We are indebted to Wenjun Xu for assistance with data analysis and critiquing the manuscript.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Ridker PM, Hennekens CH, Burning JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 2000;342:836–43. doi: 10.1056/NEJM200003233421202 [DOI] [PubMed] [Google Scholar]
- 2.Lindahl B, Toss H, Siegbahn A, Venge P, Wallentin L. Markers of myocardial damage and inflammation in relation to long-term mortality in unstable coronary artery disease. FRISC study Group. N Engl J Med 2000;343:1139–47. doi: 10.1056/NEJM200010193431602 [DOI] [PubMed] [Google Scholar]
- 3.Danesh J, Whincup P, Walker M, Lennon L, Thomson A, Appleby P, et al. Lowgrade inflammation and coronary heart disease: prospective study and updated meta-analysis. Br Med J 2000;321:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enstrom G, Lind P, Hedblad B, Wollmer P, Stavenow L, Janzon L, et al. Lung function and cardiovascular risk: relationship with inflammation-sensitive plasma proteins. Circulation 2002;106:2555–60. [DOI] [PubMed] [Google Scholar]
- 5.Schroeder EB, Welch VL, Couper D, Nieto FJ, Liao D, Rosamond W, et al. Lung function and incident coronary heart disease. Am J Epidemiol 2003;158:1171–81. [DOI] [PubMed] [Google Scholar]
- 6.Sin DD, Wu L, Man FP. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest 2005; 127:1952–9. doi: 10.1378/chest.127.6.1952 [DOI] [PubMed] [Google Scholar]
- 7.Hole DJ, Watt G, Davey-Smith G, Hart C, Gillis C, Hawthorne V. Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective study. Br Med J 1996;313:711–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ochs-Balcom H, Grant B, Muti P, Sempos C, Freuenheim J, Trevisan M, et al. Pulmonary function and abdominal adiposity in the general population. Chest 2000;118:656–64. [DOI] [PubMed] [Google Scholar]
- 9.Lange P, Nyboe J, Appleyard M, Jensen G, Schnohr P. Spirometric findings and mortality in never-smokers. J Clin Epidemiol 1990;43:867–73. [DOI] [PubMed] [Google Scholar]
- 10.Dogra S, Ardern C, Baker J. The relationship between age of asthma onset and cardiovascular disease in Canadians. J Asthma 2007;44:848–54. [DOI] [PubMed] [Google Scholar]
- 11.Knoflach M, Kiechl S, Mayr A, Willeit J, Poewe W, Wick G. Allergenic rhinitis, asthma, and atherosclerosis in the Bruneck and ARMY Studies. Arch Intern Med 2005;165: 2521–6. doi: 10.1001/archinte.165.21.2521 [DOI] [PubMed] [Google Scholar]
- 12.Lee HM, Truong ST, Wong ND.Association of adult-onset asthma with specific cardiovascular conditions.Respir Med. 2012. July;106(7):948–53. doi: 10.1016/j.rmed.2012.02.017 [DOI] [PubMed] [Google Scholar]
- 13.Chung WS, Shen TC, Lin CL, Chu YH, Hsu WH, Kao CH.Adult asthmatics increase the risk of acute coronary syndrome: A nationwide population-based cohort study.Eur J Intern Med. 2014. December;25(10):941–5. doi: 10.1016/j.ejim.2014.10.023 [DOI] [PubMed] [Google Scholar]
- 14.Çolak Y, Afzal S, Nordestgaard BG, Lange P. Characteristics and Prognosis of Never-Smokers and Smokers with Asthma in the Copenhagen General Population Study. A Prospective Cohort Study. Am J Respir Crit Care Med. 2015. July 15;192(2):172–81. doi: 10.1164/rccm.201502-0302OC [DOI] [PubMed] [Google Scholar]
- 15.Iribarren C, Tolstykh IV, Miller MK, Sobel E, Eisner MD. Adult asthma and risk of coronary heart disease, cerebrovascular disease, and heart failure: a prospective study of 2 matched cohorts.Am J Epidemiol. 2012. December 1;176(11):1014–24. doi: 10.1093/aje/kws181 [DOI] [PubMed] [Google Scholar]
- 16.Prosser R, Carleton B, Smith A.The comorbidity burden of the treated asthma patient population in British Columbia.Chronic Dis Can. 2010. March;30(2):46–55. [PubMed] [Google Scholar]
- 17.Yun HD, Knoebel E, Fenta Y, Gabriel SE, Leibson CL, Loftus EV Jr, et al. Asthma and proinflammatory conditions: a population-based retrospective matched cohort study.Mayo Clin Proc. 2012. October;87(10):953–60. doi: 10.1016/j.mayocp.2012.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iribarren C, Tolstykh Irina V, Eisner Mark D. Are patients with asthma at increased risk of coronary heart disease? Int J Epidemiol 2004;33:743–8. doi: 10.1093/ije/dyh081 [DOI] [PubMed] [Google Scholar]
- 19.Liss GM, Tarlo SM, Macfarlane Y, Yeung KS. Hospitalization among workers compensated for occupational asthma.Am J Respir Crit Care Med. 2000. July;162(1):112–8. doi: 10.1164/ajrccm.162.1.9906108 [DOI] [PubMed] [Google Scholar]
- 20.Onufrak SJ, Abramson JL, Austin HD, Holguin F, McClellan WM, Vaccarino LV. Relation of adult-onset asthma to coronary heart disease and stroke. Am J Cardiol 2008;101:1247–52. doi: 10.1016/j.amjcard.2007.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schenen JG, Iribarren C, Shahar E, Punjabi NM, Rich SS, Sorlie PD, et al. Asthma and incident cardiovascular disease: the Atherosclerosis Risk in Communities Study. Thorax 2005;60:633–8. doi: 10.1136/thx.2004.026484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toren K, Lindholm NB. Do patients with severe asthma run an increased risk from ischaemic heart disease? Int J Epidemiol 1996;25:617–20. [DOI] [PubMed] [Google Scholar]
- 23.Hekking PP, Bel EH. Developing and emerging clinical asthma phenotypes.J Allergy Clin Immunol Pract. 2014. Nov-Dec;2(6):671–80. doi: 10.1016/j.jaip.2014.09.007 [DOI] [PubMed] [Google Scholar]
- 24.Kankaanranta H, Kauppi P, Tuomisto LE, Ilmarinen P. Emerging Comorbidities in Adult Asthma: Risks, Clinical Associations, and Mechanisms.Mediators Inflamm. 2016;2016:3690628 doi: 10.1155/2016/3690628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ilmarinen P, Tuomisto LE, Kankaanranta H. Phenotypes, Risk Factors, and Mechanisms of Adult-Onset Asthma.Mediators Inflamm. 2015;2015:514868 doi: 10.1155/2015/514868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xing D, Feng W, Miller AP, Weathington NM, Chen YF, Novak L, et al. Estrogen modulates TNF—induced inflammatory responses in rat aortic smooth muscle cells through estrogen receptor-beta activation. Am J Physiol Heart Circ Physiol 2007;292: H2607–H2612. [DOI] [PubMed] [Google Scholar]
- 27.Zaitsu M, Narita S, Lambert KC, Grady JJ, Estes DM, Curran EM, et al. Estradiol activates mast cells via a non-genomic estrogen receptor-alpha and calcium influx. Mol Immunol 2007;44:1977–1985. doi: 10.1016/j.molimm.2006.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Westerhof GA, Vollema EM, Weersink EJ, Reinartz SM, de Nijs SB, Bel EH. Predictors for the development of progressive severity in new-onset adult asthma.J Allergy Clin Immunol. 2014. November;134(5):1051–6.e2. doi: 10.1016/j.jaci.2014.05.005 [DOI] [PubMed] [Google Scholar]
- 29.Tuomisto LE, Ilmarinen P, Niemelä O, Haanpää J, Kankaanranta T, Kankaanranta H.A 12-year prognosis of adult-onset asthma: Seinäjoki Adult Asthma Study.Respir Med. 2016. August;117:223–9. doi: 10.1016/j.rmed.2016.06.017 [DOI] [PubMed] [Google Scholar]
- 30.Tommola M, Ilmarinen P, Tuomisto LE, Haanpää J, Kankaanranta T, Niemelä O, et al. The effect of smoking on lung function: a clinical study of adult-onset asthma.Eur Respir J. 2016. November;48(5):1298–1306. doi: 10.1183/13993003.00850-2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.