Abstract
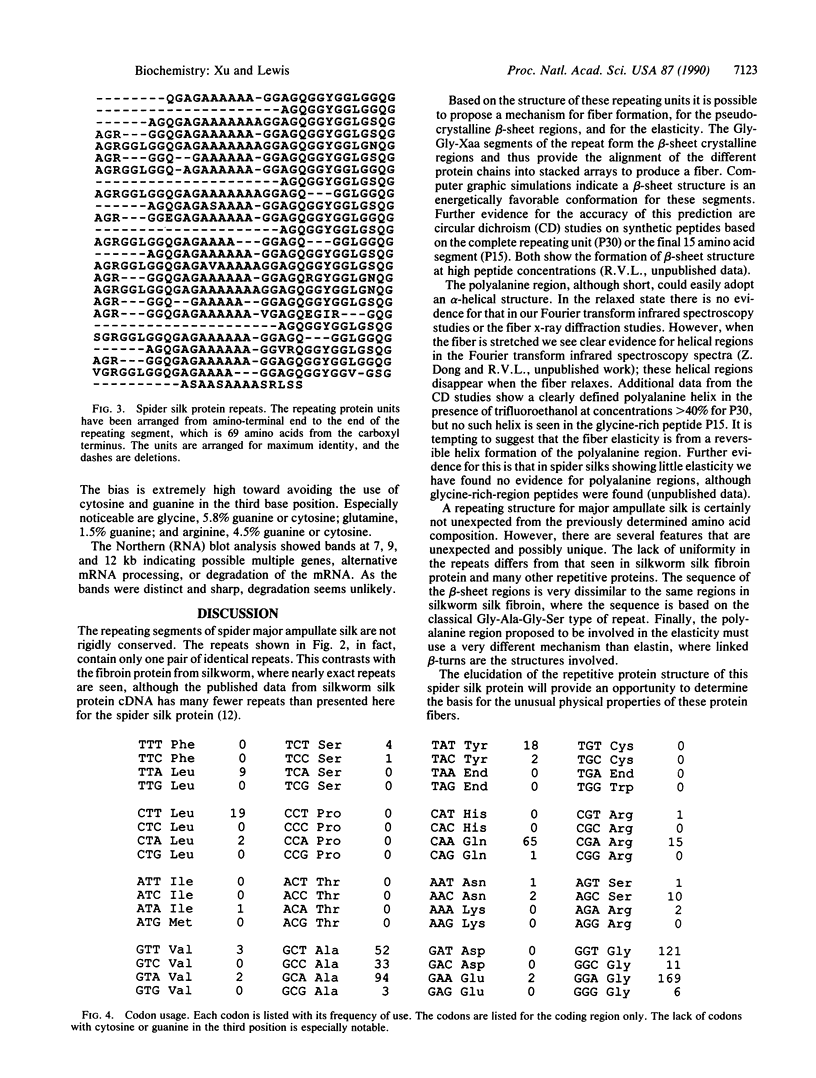
Spider major ampullate (dragline) silk is an extracellular fibrous protein with unique characteristics of strength and elasticity. The silk fiber has been proposed to consist of pseudocrystalline regions of antiparallel beta-sheet interspersed with elastic amorphous segments. The repetitive sequence of a fibroin protein from major ampullate silk of the spider Nephila clavipes was determined from a partial cDNA clone. The repeating unit is a maximum of 34 amino acids long and is not rigidly conserved. The repeat unit is composed of three different segments: (i) a 6 amino acid segment that is conserved in sequence but has deletions of 3 or 6 amino acids in many of the repeats; (ii) a 13 amino acid segment dominated by a polyalanine sequence of 5-7 residues; (iii) a 15 amino acid, highly conserved segment. The latter is predominantly a Gly-Gly-Xaa repeat with Xaa being alanine, tyrosine, leucine, or glutamine. The codon usage for this DNA is highly selective, avoiding the use of cytosine or guanine in the third position. A model for the physical properties of fiber formation, strength, and elasticity, based on this repetitive protein sequence, is presented.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita K., Ichimura S., Zama M., James T. C. Specific codon usage pattern and its implications on the secondary structure of silk fibroin mRNA. J Mol Biol. 1988 Oct 20;203(4):917–925. doi: 10.1016/0022-2836(88)90117-9. [DOI] [PubMed] [Google Scholar]
- Ogden R. C., Adams D. A. Electrophoresis in agarose and acrylamide gels. Methods Enzymol. 1987;152:61–87. doi: 10.1016/0076-6879(87)52011-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor D. W., Cordingley J. S., Butterworth A. E. Immunoprecipitation of surface antigen precursors from Schistosoma mansoni messenger RNA in vitro translation products. Mol Biochem Parasitol. 1984 Mar;10(3):305–318. doi: 10.1016/0166-6851(84)90029-x. [DOI] [PubMed] [Google Scholar]
- WARWICKER J. O. Comparative studies of fibroins. II. The crystal structures of various fibroins. J Mol Biol. 1960 Dec;2:350–362. doi: 10.1016/s0022-2836(60)80046-0. [DOI] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]



