Abstract
Abnormalities in zinc homeostasis are indicated in many human diseases, including Alzheimer disease (AD). 63Zn-zinc citrate was developed as a positron emission tomography (PET) imaging probe of zinc transport and used in a first-in-human study in 6 healthy elderly individuals and 6 patients with clinically confirmed AD. Dynamic PET imaging of the brain was performed for 30 minutes following intravenous administration of 63Zn-zinc citrate (∼330 MBq). Subsequently, body PET images were acquired. Urine and venous blood were analyzed to give information on urinary excretion and pharmacokinetics. Regional cerebral 63Zn clearances were compared with 11C-Pittsburgh Compound B (11C-PiB) and 18F-fluorodeoxyglucose (18F-FDG) imaging data. 63Zn-zinc citrate was well tolerated in human participants with no adverse events monitored. Tissues of highest uptake were liver, pancreas, and kidney, with moderate uptake being seen in intestines, prostate (in males), thyroid, spleen, stomach, pituitary, and salivary glands. Moderate brain uptake was observed, and regional dependencies were observed in 63Zn clearance kinetics in relationship with regions of high amyloid-β plaque burden (11C-PiB) and 18F-FDG hypometabolism. In conclusion, zinc transport was successfully imaged in human participants using the PET probe 63Zn-zinc citrate. Primary sites of uptake in the digestive system accent the role of zinc in gastrointestinal function. Preliminary information on zinc kinetics in patients with AD evidenced regional differences in clearance rates in correspondence with regional amyloid-β pathology, warranting further imaging studies of zinc homeostasis in patients with AD.
Keywords: 63Zn, PET, zinc homeostasis, Alzheimer disease
Zinc is an essential metal in the body, which is a functional requirement for more than 300 metabolic enzymes and plays fundamental roles in protein structure and protein–protein interactions.1 The tertiary, quaternary, and quinary structures of proteins depend on zinc and other metal ions, which in turn affect protein aggregation and protein interactions with other proteins, DNA/RNA, and lipids. Zinc deficiency is a nutritional disorder affecting approximately 2 billion people in the developing world,2 while excess zinc consumption can cause detrimental effects of ataxia, lethargy, and copper deficiency. Abnormalities in zinc homeostasis have been implied in metabolic syndrome, diabetes, and diabetic complications.3 Zinc homeostasis may play an important role in certain cancer types, including pancreatic cancer,4 prostate cancer,5 and breast cancer.6 Also, zinc is associated with the aggregation of β-amyloid proteins that accumulate in brains of patients with Alzheimer disease (AD).7 Metal chelation therapy is under investigation for the treatment of AD, with the intent of altering zinc and copper binding within amyloid-β protein deposits in the brain.8 A recent study suggests that AD may have 3 distinct subtypes with regard to pathophysiology, with 1 being associated with zinc deficiency.9
The potential role of positron emission tomography (PET) imaging in assessing metal homeostasis in brains of patients with AD has recently motivated the development of 18F-CABS13 as a zinc ionophore molecule that is able to permeate the blood–brain barrier (BBB) and shows differences in brain uptake in APP/PS1 mice relative to age-matched wild-type mice.10 Clearly, information on the dynamics of the metal ions themselves in preclinical models of AD and in patients with AD would provide important information that could support (or call into question) the so-called “metals hypothesis” of AD.11 According to this hypothesis, zinc and copper may play a key role in the formation and stabilization of neurotoxic oligomers of amyloid β-proteins.11 To address this need, we have recently developed 63Zn-zinc citrate as a PET probe of zinc transport.12 The 38.5-minute radioisotopic half-life of 63Zn allows imaging studies of duration up to 2 to 3 hours to evaluate biodistribution and moderately rapid turnover processes in tissues. Biodistribution of 63Zn-zinc citrate in mice was found to be predominantly within the gastrointestinal (GI) tract, with low but significant uptake in brain regions.12
In the present study, we report data for the first time on 63Zn-zinc kinetics obtained in human participants. The biodistribution of 63Zn-zinc in the form of zinc citrate was assessed by PET imaging in healthy elderly individuals and in patients with AD after intravenous administration of radiotracer. Attention was paid to the measurement of cerebral uptake and clearance kinetics in order to evaluate any differences in zinc kinetics in patients with AD relative to healthy individuals. The studies show extensive distribution of 63Zn-zinc citrate in the liver, pancreas, and GI tract, with certain differences in regional brain kinetics in patients with AD corresponding to the known regional amyloid-β pathology.
Methods
Preparation of 63Zn-Zinc Citrate
63Zn was produced in a low-energy cyclotron (GE PETtrace, GE HealthCare, Waukesha, WI) via the 63Cu(p, n)63Zn reaction in an in-house-developed solution target, as previously described.12 The final product, 63Zn-zinc citrate was prepared using an isotonic 4% sodium citrate United States Pharmacopeia solution (Fenwal Inc, Lake Zurich, Illinois) and passed standard quality control tests for Current Good Manufacturing Practice for radiopharmaceutical production.12 Specific activity of 63Zn in the radiopharmaceutical product was 19.4 ± 14.6 GBq/μmol at the end of synthesis. Iron and copper ions were present at concentrations of 2.7 ± 2.5 mg/L and 0.18 ± 0.16 mg/L, respectively. Injection volumes ranged from 3 to 7 mL.
Human Participants
From April 2014 to September 2014, 6 healthy elderly individuals (2 males and 4 females; age 55-77) and 6 patients with AD (3 males and 3 females; age 63-88) were enrolled into the study. The participant characteristics are shown in Table 1. All patients were participants of an ongoing clinical trial on imaging evaluation of AD within the Mayo Clinic Alzheimer’s Research Center and had undergone clinical and imaging diagnostic (magnetic resonance [MR], 11C-Pittsburgh Compound B [11C-PiB]PET and 18F-fluorodeoxyglucose [18F-FDG]PET) testing within the last 9 months prior to the study. Healthy participants showed no evidence of AD-related abnormalities on 11C-PiB-PET or 18F-FDG-PET scans indicative of AD. All patients with AD met the National Institute of Neurological and Communicative Disorders and Stroke (NINCDS) criteria for probable AD and had a Mini-Mental State Examination score at screening between 10 and 24 inclusive. All participants were given the Short Test of Mental Status (STMS). The mean STMS score for healthy participants and patients with AD was 36.3/38 and 19.0/38, respectively. Patients with AD also had unequivocal evidence of amyloid-β plaque burden on 11C-PiB-PET. All participants were requested to abstain from intake of food, vitamins, and cough medications that contain zinc beginning from 8 pm in the evening prior to the study. Participants were instructed to maintain fasting but drink plenty of water in the morning prior to the study to be well hydrated. 63Zn-zinc citrate was administered at about 11 am on the day of the study. The study protocol was approved by the institutional review board of the Mayo Clinic, and all participants provided informed consent.
Table 1.
Participant Characteristics, Clinical Parameters, and Cognitive Test Results.
| Participant | Sex (F/M) | Age, years | Body Weight, kg | Healthy (H) Participants or Patients With AD | Diabetic (D) or Nondiabetic (N) | Plasma Glucose, mg/dL | Plasma Zinc, μg/mLa | Plasma Copper, μg/mL | Urine Zinc, μg/L | STMS | MMSE |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 64 | 66 | H | N | 76 | 0.82 | 1.31 | 126b | 38/38 | |
| 2 | M | 88 | 62 | AD | D | 129c | 0.81 | 0.93 | 1384b | 21/38d | NA |
| 3 | M | 63 | 89 | H | N | 89 | 0.84 | 1.06 | 169b | 35/35 | |
| 4 | M | 63 | 88 | AD | N | 81 | 0.86 | 0.94 | 108b | 27/38 | 24 |
| 5 | M | 69 | 73 | H | N | 74 | 0.88 | 1.14 | 377 | 32/38 | |
| 6 | M | 78 | 77 | AD | N | 92 | 0.70 | 0.89 | 106b | 8/38 | 10 |
| 7 | F | 62 | 56 | H | N | 87 | 0.78 | 1.06 | 33b | 36/38 | |
| 8 | F | 55 | 105 | H | N | 90 | 0.96 | 1.22 | 409 | 37/38 | |
| 9 | F | 70 | 70 | AD | D | 181c | 0.92 | 1.34 | 872b | 25/38 | 21 |
| 10 | F | 76 | 53 | AD | N | 85 | 0.70 | 1.05 | 42b | 24/38 | 19 |
| 11 | F | 77 | 64 | H | N | 84 | 0.66 | 1.05 | 225b | 37/38 | |
| 12 | F | 74 | 71 | AD | N | 97 | 0.75 | 1.46e | 346 | 22/38 | 16 |
Abbreviations: AD, Alzheimer disease; F, female; M, male; MMSE, Mini-Mental State Examination; NA, not available; STMS, Short Test of Mental Status; SUV, standardized uptake value.
aAll plasma zinc levels were within the normal range (0.66-1.1 μg/mL).
bUrine zinc levels outside the normal range (300-600 μg/L).
cFasting plasma glucose outside the normal range (70-100 mg/dL).
dLast STMS performed 3 years prior.
ePlasma copper level above the normal range (0.75-1.45 μg/mL).
Imaging Protocol
The imaging protocol is illustrated in Figure 1. After voiding of the bladder, participants were positioned in a GE 690XT PET/computed tomography (CT) scanner (GE HealthCare, Waukesha, WI) in a supine position. Intravenous catheters were placed in both arms for radiotracer injection and blood sampling. Computed tomography scans of the head were initially acquired in the preparation for a dynamic PET data acquisition over the brain for the first 30 minutes following commencement of intravenous administration. 63Zn-zinc citrate (300-370 MBq) was administered over 1 minute into one of the venous catheters. The frame sequence for the initial dynamic PET acquisition was 15 × 4, 8 × 15, 4 × 30, and 5 × 300 seconds. Following the dynamic brain scan, the participants were allowed a 15-minute break from the scanner, during which time voided their bladders. Two subsequent head to thigh (trunk) PET/CT scans were acquired at 45 to 70 minutes and 95 to 120 minutes postinjection (PI) with 15 to 20 minutes of break in between. The last PET/CT scan was completed at ∼2 hours PI. Venous blood samples (3-4 mL) were collected in heparinized tubes and placed on ice, beginning at 5 minutes PI and continuing over the imaging period. Urine was collected after each PET scan and measured for urine volume and 63Zn concentration as measured in a calibrated γ counter.
Figure 1.
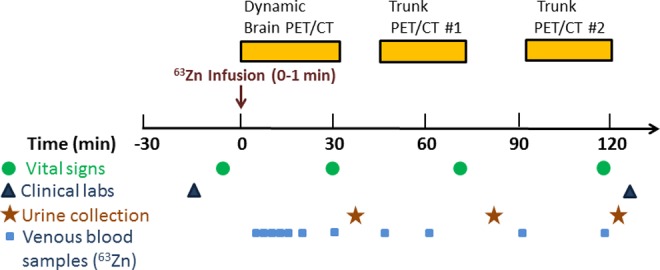
Experimental protocol.
Measurements in Venous Blood and Urine Samples
Ice-chilled blood samples were centrifuged at 3000g for 5 minutes to obtain plasma. 63Zn concentrations in whole blood and plasma were measured using a calibrated γ counter. 63Zn excretion in urine was calculated from the product of urine volume and 63Zn concentration.
Positron Emission Tomography Image Analysis
Magnetic resonance imaging (MRI; 3 T) and PET/CT scans using 11C-PiB were acquired and analyzed as described in detail elsewhere.13 The 63Zn-PET/CT and MR images were transferred to a workstation running PMOD (version 3.5, Fusion Toolbox; PMOD Technologies, Zurich, Switzerland) for analysis. The CT images for 63Zn-zinc citrate were registered to the patient’s MR images using a rigid transformation based on normalized mutual information. The same transformation was applied to the dynamic 63Zn-PET series. In the case of the whole-body series, only those 63Zn-PET and CT slices that contain the head were registered to the MRI. Next, the participant’s MRI was spatially normalized to an MRI template using a nonlinear warping procedure. The template was a T1-weighted MRI data set having 91 × 109 matrix with 91 slices and 2 mm3 voxels.14 The same transformation was then applied to all PET series. A predefined atlas of volumes of interest (VOIs) was defined for the template. The atlas of VOIs was subsequently superimposed on the PET images and time–activity curves (TACs) were generated. The uptake from the whole-body data was incorporated into the TACs from the dynamic data. An “AD-global” VOI was created by inclusion of VOIs that have importance in AD (cingulate, precuneus, anterior cingulate, parietal lobe, prefrontal, temporal, pre- and postcentral regions). 11C-PiB-PET cortical uptake was normalized to cerebellar gray matter to obtain the standardized uptake value ratio (SUVR). All patients with AD showed amyloid-β-positive SUVR >1.5 for the AD-global VOI. All healthy participants showed amyloid-β-negative SUVR <1.5 for the AD-global VOI.
Safety Measurements for 63Zn-Zinc Citrate Administration
Vital signs (heart rate, systolic and diastolic blood pressures, respiratory rate, temperature) were measured prior to 63Zn-zinc citrate administration and at 30, 70, and 115 minutes PI. Venous blood samples were taken prior to 63Zn-zinc citrate administration and at ∼130 minutes PI for a panel of clinical laboratory tests to evaluate the safety of the radiotracer administration.
Pharmacokinetics of 63Zn-Zinc Citrate in Venous Blood Samples
Peak time was defined as the time of maximum measurement (minimum of t >1 minute) with maximum observed concentration. Half-clearance times were defined as the time from peak concentration to an estimated 50% of peak concentration. Concentrations were modeled for each individual for whole-blood and plasma separately using the γ distribution, with time t = 0 defined as the time at peak for each participant. Plateau was defined as the first interval of time starting after t = 20 minutes with an observed change in concentration less than 0.05/minute. Time to plateau was defined as the end point of the interval in which plateau had occurred in both whole blood and plasma. Between-group comparisons of pharmacokinetic characteristics were performed using Wilcoxon rank sum tests.
Statistical Analysis
Summary statistics are shown as mean ± standard deviation (SD) for continuous variables and N (%) for categorical variables. Participants were divided into 2 groups: normal, healthy adults and patients with AD based on the characteristics described in Human Participants section. Between-group comparisons for baseline demographics and clinical characteristics used Wilcoxon rank sum test or Fisher exact test for continuous and categorical variables, respectively. Between-group comparisons for 63Zn-zinc citrate, %dose/organ, 11C-PiB, and 18F-FDG were performed using Wilcoxon rank sum tests. Due to the limited power to include multiple main effects in 1 model, for comparisons either between preinjection and PI characteristics (with the exception of vital signs) or between PET scans, results are presented in 2 ways: first, a between-time comparison was performed using Wilcoxon signed rank tests; second, differences in the amount of change between groups was assessed using Wilcoxon rank sum tests. For 11C-PiB, regional cerebral uptake was normalized to cerebellar uptake. For 18F-FDG, regional cerebral uptake was normalized to pons uptake. Vital signs (heart rate, temperature, respiratory rate, systolic and diastolic blood pressures) were taken once preinjection and 3 times PI and were compared using Kruskal-Wallis rank sum tests. Reported P values have not been corrected for multiple testing. All Wilcoxon tests were exact, 2-sided tests. Statistical analysis was performed using R (version 3.1.1; Vienna, Austria).
Results
Participant Characteristics
The participant characteristics are shown in Table 1. All participants showed plasma zinc concentrations in the normal range of 0.66 to 1.1 μg/mL. Two of 6 patients with AD were diabetic (participants 2 and 9). Both of the diabetic patients with AD showed abnormally high plasma glucose and urinary zinc (>600 μg/L) concentrations, the latter being consistent with the previous reports of hyperzincuria in type 2 diabetics in the absence of neurodegeneration.15,16 Nevertheless, the plasma zinc levels in these 2 participants were in the normal range. The majority of nondiabetic participants showed abnormally low concentrations of zinc in urine (<300 μg/L), possibly due to their fasting state and heavy consumption of water in the morning of the study as instructed. One healthy elderly participant (data not shown) was excluded post hoc from the study because of an abnormally low fasting plasma zinc concentration indicative of a state of zinc depletion.
Safety Measurements for 63Zn-Zinc Citrate Administration
Measurements of vital signs (heart rate, systolic and diastolic blood pressures, respiratory rate, temperature) and a comprehensive panel of clinical laboratory tests for the assessment of radiopharmaceutical safety are reported in Supplemental Information. These tests showed the 63Zn-zinc citrate administration to be well tolerated with very few results outside the normal range and no pattern indicative of an effect of radiotracer administration across participants. There was not sufficient evidence to conclude that a difference exists between the experimental groups nor that the vitals at any time point were different than another; all P values were >0.2, with the exception of between-group comparison at baseline respiratory rate (P = 0.138).
Pharmacokinetics of 63Zn-Zinc Citrate in Venous Blood
Figure 2 shows representative curves for venous whole–blood and plasma concentrations of 63Zn radioactivity following intravenous administration of 63Zn-zinc citrate. The pharmacokinetic properties are summarized in Table 2. Peak concentration of 63Zn in venous plasma and in whole blood occurred within 10 minutes. The ratio of concentrations of 63Zn in whole blood to plasma increased over time to a plateau value of 0.878 ± 0.117 in the healthy group and 0.869 ± 0.138 in the AD group at 1 hour, evidencing the transport of 63Zn from plasma to erythrocytes. Some transport into erythrocytes may have occurred after samples were withdrawn from the participants, although the samples were placed immediately on ice to slow down this process. Plasma half-clearance times were approximately 8 minutes (healthy: 7.06 ± 2.19, AD: 8.39 ± 1.61). There were no statistically significant differences in the pharmacokinetic parameters for patients with AD versus healthy participants.
Figure 2.
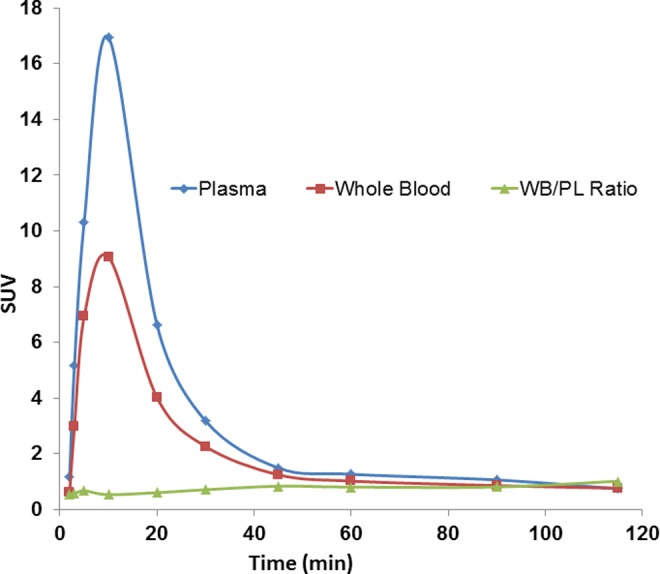
Representative kinetics of 63Zn radioactivity in venous whole blood and plasma of healthy participant. The ratio of concentrations of 63Zn in whole blood to plasma increased over time, evidencing transport of 63Zn from plasma to erythrocytes.
Table 2.
Pharmacokinetic Properties of 63Zn-Zinc Citrate.a
| Property | Healthy Participants | Patients With AD |
|---|---|---|
| Time of peak, plasma, minutes | 8.25 ± 2.87 | 7.60 ± 2.51 |
| Peak concentration, plasma (SUV) | 11.00 ± 5.44 | 10.93 ± 2.50 |
| Half-clearance time, plasma, minutes | 7.06 ± 2.19 | 8.39 ± 1.61 |
| Time of peak, whole blood, minutes | 9.50 ± 0.58 | 6.90 ± 2.61 |
| Peak concentration, whole blood (SUV) | 6.52 ± 2.27 | 7.04 ± 1.20 |
| Half-clearance time, whole blood, minutes | 9.078 ± 1.93 | 10.35 ± 2.51 |
| WB:plasma ratio at plateau | 0.878 ± 0.117 | 0.869 ± 0.138 |
| Time of ratio plateau, minutes | 56.25 ± 7.5 | 57.00 ± 6.71 |
Abbreviations: AD, Alzheimer disease; SUV, standardized uptake value; WB, whole blood.
aNo statistically significant differences were found on comparing AD and healthy groups.
Biodistribution of 63Zn-Zinc Citrate
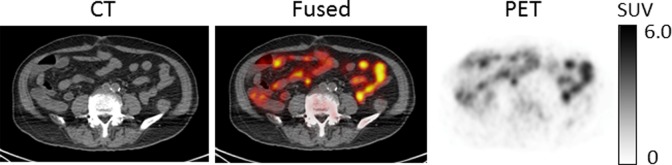
Figure 3 shows PET/CT images of 63Zn-zinc citrate in representative participants. Prominent uptake was observed in the liver, with pancreas, kidneys, spleen, and intestines showing moderate levels of uptake. Intestinal uptake was consistent across duodenum, jejunum, and ileum (Figure 4). This suggests that intestinal uptake of 63Zn resulted from the transport of radiotracer from blood, not only the possible secretion into the upper small bowel by liver, pancreas, or gallbladder. Brain uptake was low but apparent on images. No qualitative differences were observed in the PET images of whole-body distribution of 63Zn-zinc citrate in patients with AD relative to healthy participants. Also, no qualitative differences could be seen in the images from the last trunk PET/CT study at 95 to 120 minutes in comparison with the images acquired at 45 to 70 minutes.
Figure 3.
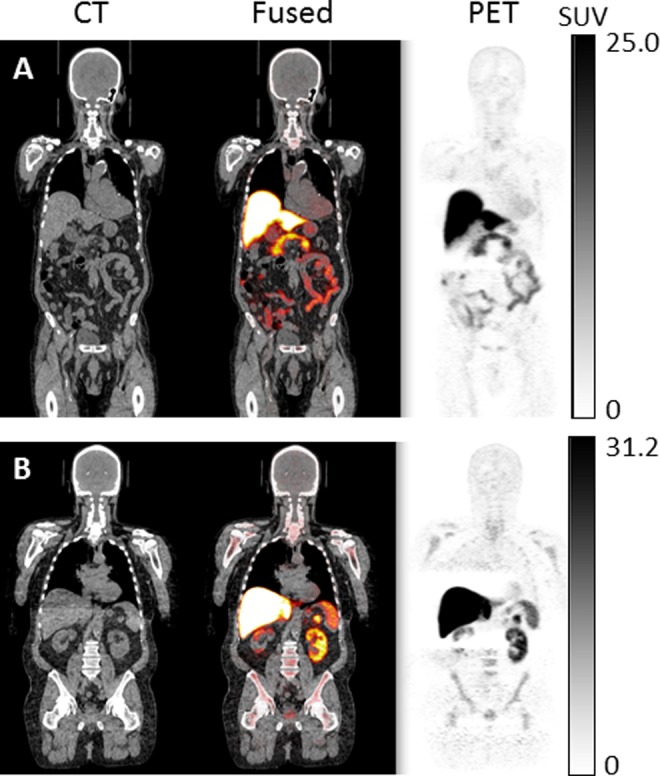
Positron emission tomography (PET)/computed tomography (CT) images in representative patient with Alzheimer disease (AD; A) and healthy elderly participant (B) at 45 to 70 minutes postadministration of 63Zn-zinc citrate. Hepatic uptake is largely prominent, while lower uptake by pancreas, spleen, kidneys and intestines, bone marrow, and brain was observed. No qualitative differences were observed in the PET images of whole-body distribution of 63Zn-zinc citrate in patients with AD relative to healthy participants.
Figure 4.
Abdominal positron emission tomography (PET)/computed tomography (CT) images of 63Zn-zinc citrate distribution acquired 45 to 70 minute postinjection (PI) show intraluminal distribution in intestines.
Table 3 shows the biodistribution (standardized uptake values [SUVs]) for 63Zn-zinc citrate obtained from head to thigh PET/CT images acquired at 45 to 70 minutes and 95 to 120 minutes PI. Earlier uptake data are reported in Table S1. Tissues with the highest uptake were liver, pancreas, and kidney. The whole-organ uptakes in liver and pancreas were 51.2% ± 5.5% and 1.07% ± 0.60%, respectively, across all participants, and these values were not statistically different for patients with AD relative to healthy participants. Thus, hepatic uptake of intravenously administered 63Zn-zinc citrate was hugely predominant and clearance of 63Zn by the liver was negligible between the first and second trunk PET scans. Moderate uptake was seen in pituitary, salivary glands, thyroid, spleen, stomach, intestines, prostate (in males), and bone marrow. Moderately low uptake was found across brain regions with SUVs ranging 0.3 to 0.5. This level of uptake in brain regions could not be explained by 63Zn radioactivity in blood vessels of the brain as it was much higher (SUV ∼ 0.4, Table 3) than the product of blood volume (∼5%) and whole-blood concentration (SUV ∼ 1, Figure 3). Urinary excretion of 63Zn was very minimal at 2 hours and similar for the 2 participant groups (healthy 0.041% ± 0.020%; AD 0.047% ± 0.052%). The 2 diabetic patients with AD showed the highest urinary excretions (0.133% and 0.086%), consistent with hyperzincuria in these participants.
Table 3.
Biodistribution (SUV) of 63Zn-Zinc Citrate in Healthy Elderly Participants and Patients With AD.a
| Tissue | PET Scan at 45-70 Minutes | PET Scan at 95-120 Minutes | ||
|---|---|---|---|---|
| Healthy | Patients With AD | Healthy | Patients With AD | |
| AD-global | 0.39 ± 0.05 | 0.37 ± 0.07 | 0.35 ± 0.05 | 0.35 ± 0.06 |
| Parietal | 0.36 ± 0.05 | 0.34 ± 0.06 | 0.33 ± 0.04 | 0.33 ± 0.06 |
| Cingulate, precuneus | 0.41 ± 0.05 | 0.38 ± 0.06 | 0.37 ± 0.05 | 0.37 ± 0.05 |
| Prefrontal cortex | 0.37 ± 0.05 | 0.36 ± 0.07 | 0.33 ± 0.05 | 0.33 ± 0.06 |
| Orbitofrontal | 0.39 ± 0.06 | 0.36 ± 0.06 | 0.32 ± 0.06 | 0.33 ± 0.08 |
| Lateral temporal | 0.39 ± 0.05 | 0.37 ± 0.08 | 0.35 ± 0.05 | 0.34 ± 0.07 |
| Primary visual cortex | 0.50 ± 0.06 | 0.46 ± 0.09 | 0.44 ± 0.06 | 0.44 ± 0.08 |
| Cerebellum | 0.46 ± 0.05 | 0.46 ± 0.09 | 0.41 ± 0.06 | 0.41 ± 0.08 |
| Anterior cingulate | 0.34 ± 0.05 | 0.31 ± 0.06 | 0.30 ± 0.05 | 0.28 ± 0.05 |
| Occipital cortex | 0.44 ± 0.05 | 0.41 ± 0.08 | 0.40 ± 0.05 | 0.40 ± 0.07 |
| Medial temporal | 0.39 ± 0.06 | 0.36 ± 0.06 | 0.35 ± 0.07 | 0.33 ± 0.04 |
| Pons | 0.45 ± 0.08 | 0.42 ± 0.09 | 0.36 ± 0.05 | 0.38 ± 0.09 |
| Pituitary | 3.00 ± 0.48 | 2.78 ± 0.65 | 2.61 ± 0.42 | 2.65 ± 0.261 |
| Parotid gland | 2.56 ± 1.10 | 2.43 ± 0.42 | 2.47 ± 0.92 | 2.64 ± 0.55 |
| Submandibular gland | 3.17 ± 0.67 | 3.14 ± 0.51 | 3.32 ± 0.62 | 3.12 ± 0.68 |
| Thyroid | 3.94 ± 1.01 | 4.15 ± 1.93 | 4.17 ± 1.05 | 4.26 ± 1.70 |
| Lung | 0.27 ± 0.12 | 0.30 ± 0.15 | 0.30 ± 0.09 | 0.31 ± 0.11 |
| Heart | 0.81 ± 0.22 | 1.10 ± 0.17 | 0.76 ± 0.21 | 1.17 ± 0.14 |
| Breast (females) | 0.04 ± 0.04 | 0.09 ± 0.12 | 0.05 ± 0.06 | 0.10 ± 0.11 |
| Liver | 33.2 ± 3.1 | 30.6 ± 7.5 | 34.3 ± 5.0 | 31.3 ± 7.9 |
| Spleen | 4.46 ± 0.60 | 5.00 ± 0.70 | 4.12 ± 0.71 | 4.85 ± 0.48 |
| Pancreas | 12.1 ± 4.0 | 13.0 ± 3.8 | 13.5 ± 3.4 | 12.4 ± 2.9 |
| Stomach | 4.34 ± 1.08 | 3.96 ± 0.87 | 4.68 ± 0.88 | 5.07 ± 1.07 |
| Ileum | 5.66 ± 1.13 | 4.68 ± 0.41 | 5.47 ± 1.56 | 4.77 ± 1.89 |
| Kidney | 9.94 ± 1.02 | 10.72 ± 1.69 | 10.09 ± 1.30 | 10.80 ± 1.79 |
| Prostate (males) | 3.15 ± 0.04 | 3.14 ± 2.07 | 3.90 ± 0.52 | 3.67 ± 3.02 |
| Testicles (males) | 0.57 ± 0.36 | 0.70 ± 0.27 | 0.42 ± 0.08 | 0.62 ± 0.02 |
| Bone | 0.24 ± 0.10 | 0.21 ± 0.11 | 0.24 ± 0.24 | 0.25 ± 0.12 |
| Bone marrow | 1.41 ± 0.20 | 1.40 ± 0.31 | 1.42 ± 0.47 | 1.50 ± 0.54 |
| Skeletal muscle | 0.17 ± 0.03 | 0.16 ± 0.06 | 0.16 ± 0.02 | 0.18 ± 0.04 |
Abbreviations: AD, Alzheimer disease; PET, positron emission tomography; SUV, standardized uptake value.
aNo statistically significant differences were found on comparing AD and healthy groups at these time points.
Brain Kinetics of 63Zn-Zinc Citrate
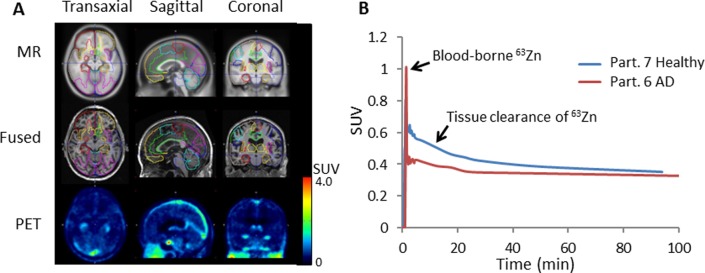
A 30-minute dynamic PET acquisition was commenced over the brain at the beginning of the 63Zn-zinc citrate administration. In addition to these early data, the cerebral regions in the 2 later trunk PET/CT scans were available for completing the regional 63Zn TACs out to 2 hours. Atlas-based cerebral regions were defined on the MRI images obtained for each participant and then transferred for quantitative region-of-interest evaluation on the PET images, as illustrated in Figure 5A. As mentioned previously, the absolute uptake data did not shown any differences between AD and healthy experimental groups (Table 3). However, clearance kinetics over the period 27.5 to 97.5 minutes in several regions were significantly slower in patients with AD relative to healthy participants (Figures 5B and 6). To provide quantitative indices of clearance rate, we evaluated the percentage changes in SUV exhibited within the brain regions between various time points. Standardized uptake value changes were significantly smaller in patients with AD for the following regions: AD global region, left parietal lobe, left prefrontal cortex, left lateral temporal lobe, orbitofrontal cortex, and occipital cortex (P < 0.05). All of these regions were also characterized by 11C-PiB accumulation indicative of amyloid-β plaque. Regional cerebral 63Zn clearance values from 47.5 to 92.5 minutes are shown in Figure 7 in relationship with SUVRs for 11C-PiB and 18F-FDG in the same regions. Again, 63Zn clearance was lower in patients with AD in several regions correlating with abnormalities on the 11C-PiB and 18F-FDG scans, although variability in 63Zn clearance values were relatively larger. On the other hand, several regions not typically associated with 11C-PiB positive uptake (medial temporal, cerebellum, and pons) did not show statistically significant 63Zn SUV changes between the AD and healthy groups.
Figure 5.
A, Regional image analysis of brain kinetics of 63Zn-zinc citrate. T1-weighted magnetic resonance (MR) images are shown at top and coregistered 63Zn-PET images at 45 to 50 minutes postinjection (PI) are shown at the bottom. Outlines of volumes of interest (VOIs) are shown in various colors on the MR and fused images. Low levels of zinc uptake (SUV < 0.5) were observed across brain. B, Representative left prefrontal cortex time–activity curves in patients with AD and healthy participants.
Figure 6.
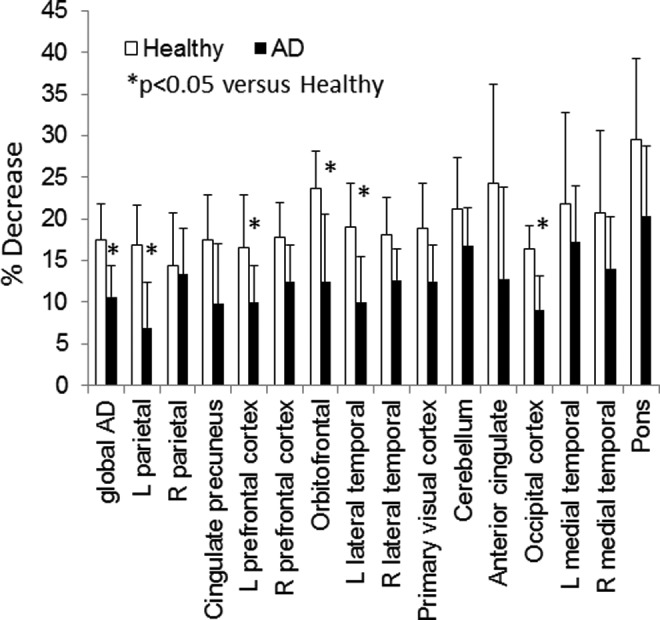
Regional cerebral clearances of 63Zn between 27.5 and 92.5 minutes postinjection (PI).
Figure 7.
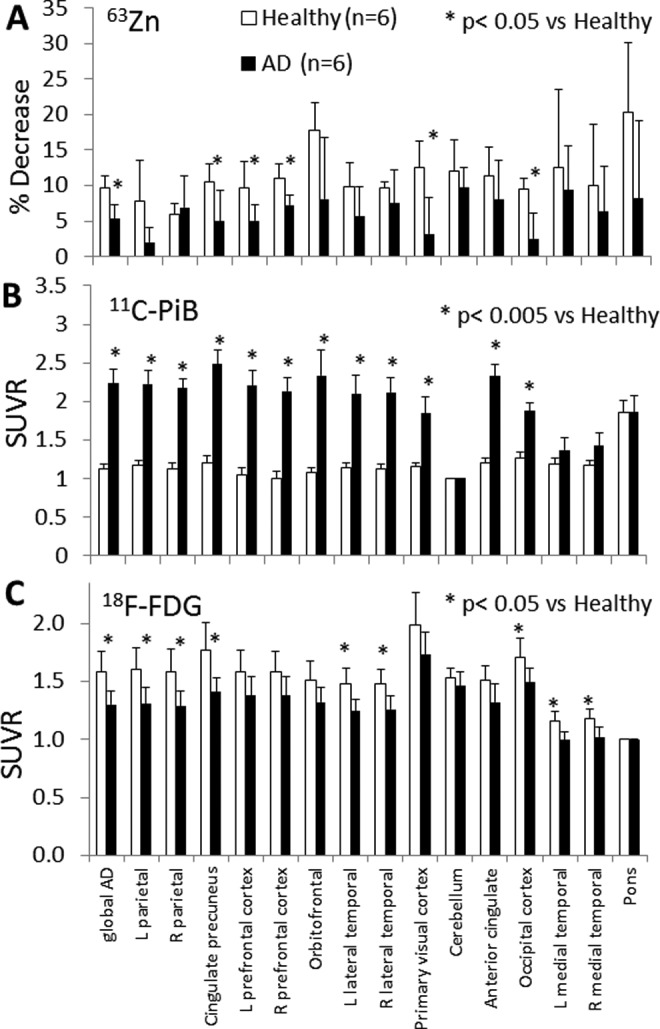
A, Regional cerebral clearances of 63Zn between 47.5 and 92.5 minutes postinjection (PI). B, Standardized uptake value ratios (SUVRs) for 11C-PiB normalized to the cerebellum. C, The SUVRs for 18F-FDG normalized to the pons.
Discussion
This first-in-human PET imaging study of zinc distribution after intravenous administration of 63Zn-zinc citrate was aimed at answering 2 primary questions. First, what are the major tissues that take up zinc and what potential clinical role might 63Zn-PET play in these tissues? Second, can 63Zn-PET be useful to evaluate the uptake and turnover of zinc in brains of patients with AD? The answer to the first question was predominantly liver, with moderately high uptake of zinc in the pancreas, kidney, and GI tract. Since zinc homeostasis critically depends on the balance of intake from diet and excretion (primarily through the gut), it was not surprising to find the locus of initial distribution of 63Zn in organs of the digestive system, including liver and pancreas. The findings that more than half of the injected dose was found to be taken up by liver and hepatic 63Zn concentration was stable up to at least 2 hours PI underscore the importance of liver for storage and redistribution of zinc. Zinc deficiency is well documented in a variety of liver diseases.17 Disturbances of zinc homeostasis are associated with hepatitis B infection,18 cirrhosis,19 alcoholic liver disease,20 nonalcoholic fatty liver disease,21 hepatocellular carcinoma,22 and malabsorption.23 Subnormal hepatic zinc content is a common finding in these disease states. Thus, PET imaging of hepatic zinc kinetics using 63Zn as probe may play a role in evaluating a variety of diseases that affect the liver. The pancreas also showed high and persistent uptake of 63Zn-zinc citrate in this study. Zinc plays an important role in a variety of pancreatic and β-cell functions, including insulin storage and secretion24 and secretion of zinc-associated pancreatic enzymes into the duodenum.25 Indeed, zinc homeostasis suffers in diseases of the pancreas, including chronic pancreatitis26 and diabetes.24 The pancreas was readily imaged by 63Zn-PET, although differentiation of β-cell uptake from acinar pancreatic uptake would be precluded by the very small β-cell volume fraction. Renal uptake of 63Zn-zinc citrate was also high and undiminished from the first and second trunk PET scans. The urinary excretion of 63Zn radioactivity was found to be minimal (<0.1%), consistent with the known fate of zinc in the kidney to be uptaken by epithelial cells of the renal tubular system.27 The moderate uptake of 63Zn-zinc citrate across the small intestine supports the important role of zinc in digestion. The finding of 63Zn radioactivity in the lumen of the ileum (Figure 4) as early as an hour (the earliest image of the abdomen) after intravenous administration argues that intestinal absorption of zinc is significant and may provide zinc as an essential nutrient to intestinal microbiota. Evidence has been found for high-affinity zinc transporters in several bacterial species that populate within the intestines,28 which could explain the high luminal accumulation of 63Zn. This finding underscores the importance of the rapid exchange of zinc between blood and small intestine relevant to overall zinc homeostasis and the ability of 63Zn-PET to provide noninvasive assessment of this exchange. It is possible that 63Zn-PET could provide a new tool to evaluate intestinal zinc dynamics in patients presenting with zinc deficiency, for example, patients with Crohn’s disease29 and inflammatory bowel disease.30
The second (and primary) aim of this study was to obtain data on zinc uptake and turnover in the brains of patients with AD. Since zinc is known to bind with high affinity to amyloid-β protein31 and be an essential component of amyloid β-protein aggregates in AD brains,32 we postulated that 63Zn kinetics after intravenous administration of 63Zn-zinc citrate might be different in amyloid-β plaque-rich regions in AD brains in comparison with the same regions in healthy elderly participants. This study recruited elderly patients already enrolled in an ongoing multiyear longitudinal imaging study within the Mayo Clinic Alzheimer’s Research Center utilizing MR, FDG-PET, and 11C-PiB-PET. 11C-PiB-PET has been extensively validated as a PET probe of regional cerebral amyloid-β plaque burden in aging brain and AD.33 Transport of 63Zn-zinc citrate across the BBB into the brain was evidenced by SUVs of around 0.5 following the vascular washout phase in the first 4 to 7 minutes postadministration (Figure 5B). Measurements of the initial uptake into brain regions at 12.5 minutes PI did not show regional variation and were not statistically different in patients with AD compared to healthy participants. However, 63Zn clearance rates from brain regions did show statistically significant differences with respect to brain region and the experimental group. Slower 63Zn clearance rates were found in patients with AD in the following regions (Figure 6, P < .05): AD global (comprised of cingulate, precuneus, anterior cingulate, left and right parietal lobes, left and right prefrontal cortices, left and right temporal lobes, and pre- and postcentral regions), cingulate, precuneus, left and right prefrontal cortices, left parietal, left lateral temporal, orbitofrontal cortex, primary visual cortex, and occipital cortex. The brain regions comprising the “AD global” region are all regions that typically show elevated 11C-PiB-PET accumulation in patients with AD. Slower 63Zn clearance may result from enhanced binding of 63Zn-zinc in cerebral tissue, possibly to AD-related protein aggregates. In addition to the well-established binding properties of zinc to amyloid-β plaque, there is recent evidence that zinc also binds to tau protein, another protein that accumulates in AD brains in aggregate form and is strongly implicated in the pathophysiology of AD.34 Furthermore, there are elevated levels of soluble oligomers of amyloid-β in AD brain that could also represent binding sites for intracerebral zinc.35
Given the evidence that there is a slowing of 63Zn-zinc clearance from certain regions of the brain in patients with AD, there is potential that this measurement could serve as a noninvasive indication of zinc dyshomeostasis in the AD brain. Larger and more diverse study populations will be needed to establish the clinical potential of this technique for the evaluation of neuronal pathology. Also, the mechanistic basis for regional differences in 63Zn clearance rate has yet to be determined. As PET imaging agents of tau protein aggregates become more available, it will be of interest to correlate 63Zn kinetics with both amyloid-β and tau pathology. It will also be important to look at factors other than aberrant protein accumulations in brain that could affect intracerebral zinc turnover, such as zinc deficiency. Indeed, 1 mentally healthy participant (82 years) was excluded from analysis in this study on the basis of a subnormal plasma zinc concentration indicative of zinc deficiency. The 63Zn clearance rates across all brain regions for this participant were markedly lower than others in the healthy group (data not shown) consistent with the notion that brain kinetics of zinc may be abnormal in elderly participants who are in a zinc-deficient state.36
Several study limitations should be noted. Arterial input functions were not obtained that may allow investigation of more sophisticated models to regress the cerebral 63Zn TACs. Kinetic rate constants for blood–tissue and tissue–blood transport of 63Zn could not be assessed in this study. Clearance rates of 63Zn from tissue at later times were inferred from changes in SUV. Future work should employ arterial input functions to provide more meaningful quantitative indices of 63Zn kinetics in the brain. Another limitation of the study was the small size of the experimental groups (n = 6 each) and the limited selection of clinically diagnosed patients with AD that showed positive 11C-PiB scans and hypometabolism on FDG-PET scans. Future work is warranted in larger participant groups and inclusion of participants who have a range of cognitive impairment. Effects of potential perfusion changes in AD brain were not assessed in this study. Decreased perfusion in AD-relevant brain regions could be an alternative explanation for the slower clearance rate of 63Zn, although the lack of abnormality seen in the early uptake of 63Zn in the same regions would argue against a perfusion effect. The average age of the AD group (75 years) was greater than that of the healthy group (65 years), although not statistically significant (P = 0.06). The AD cohort included 2 participants who were diabetic. Since zinc dyshomeostasis has been noted in diabetics,15,16 this may represent a potential confound. However, the plasma zinc levels in the 2 diabetic patients were in the normal range, and separate analysis of 63Zn clearance rates with the diabetic patients being removed showed similar discrepancies to the healthy group. Finally, the level of signal in the brain is low, reflecting the transport limitation on zinc into the brain imposed by the BBB. Although the inclusion of a brain-penetrant zinc ionophore in the 63Zn formulation could potentially bring more 63Zn radioactivity into the brain, the use of such a carrier could alter the natural binding processes within the brain and increase the risk of adverse events. Future investigations (in preclinical models) with zinc-binding ionophores as carriers for 63Zn are anticipated. Should the 63Zn-PET scans show sensitivity to changes in cerebral zinc transport resulting from ionophore administration, this may be developed into a useful tool for monitoring ionophore therapies.
Conclusion
A first-in-human PET imaging study of 63Zn-zinc citrate was performed in patients with AD and healthy elderly participants. Prominent uptake of 63Zn radioactivity was seen in the liver (∼51% dose/organ), pancreas, kidney, and GI tract. Brain uptake was relatively low (SUVs ∼ 0.4) but sufficient to allow assessment of 63Zn uptake and clearance patterns on a regional basis. Although brain uptake was consistent across brain regions and experimental groups, slower clearance rates of 63Zn radioactivity were seen in several brain regions in patients with AD relative to healthy participants. The regions with slower 63Zn clearance corresponded to regions of known amyloid-β pathology on 11C-PIB-PET scans and lower uptake on FDG-PET scans. Positron emission tomography imaging with 63Zn-zinc citrate represents a new tool for noninvasive assessment of zinc dynamics in the living human body.
Supplementary Material
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Dr Lowe serves as a consultant for Piramal Imaging.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The work was supported by National Institute on Aging (AG16574), Department of Energy (DE-SC0008947), Mayo Clinic Alzheimer’s Research Center, and the Elsie and Marvin Dekelboum Family Foundation. Dr Lowe receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals
Supplemental Material: The online supplemental data are available at http://journals.sagepub.com/doi/suppl/10.1177/1536012116673793.
References
- 1. Frassinetti S, Bronzetti G, Caltavuturo L, Cini M, Croce CD. The role of zinc in life: a review. J Environ Pathol Toxicol Oncol. 2006;25(3):597-610. doi:10.1615/JEnvironPatholToxicolOncol.v25.i3.40. [DOI] [PubMed] [Google Scholar]
- 2. Penny ME. Zinc supplementation in public health. Ann Nutr Metab. 2013;62(suppl 1):31-42. doi:10.1159/000348263. [DOI] [PubMed] [Google Scholar]
- 3. Miao X, Sun W, Fu Y, Miao L, Cai L. Zinc homeostasis in the metabolic syndrome and diabetes. Front Med. 2013;7(1):31-52. doi:10.1007/s11684-013-0251-9. [DOI] [PubMed] [Google Scholar]
- 4. Costello LC, Levy BA, Desouki MM, et al. Decreased zinc and downregulation of ZIP3 zinc uptake transporter in the development of pancreatic adenocarcinoma. Cancer Biol Ther. 2011;12(4):297-303. doi:10.4161/cbt.12.4.16356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kolenko V, Teper E, Kutikov A, Uzzo R. Zinc and zinc transporters in prostate carcinogenesis. Nat Rev Urol. 2013;10(4):219-226. doi:10.1038/nrurol.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Alam S, Kelleher SL. Cellular mechanisms of zinc dysregulation: a perspective on zinc homeostasis as an etiological factor in the development and progression of breast cancer. Nutrients. 2012;4(8):875-903. doi:10.3390/nu4080875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Craddock TJ, Tuszynski JA, Chopra D, et al. The zinc dyshomeostasis hypothesis of Alzheimer’s disease. PLoS One. 2012;7(3):e33552 doi:10.1371/journal.pone.0033552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lannfelt L, Blennow K, Zetterberg H, et al. ; PBT2-201-EURO study group. Safety, efficacy, and biomarker findings of PBT2 in targeting Abeta as a modifying therapy for Alzheimer’s disease: a phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008;7(9):779-786. doi:10.1016/S1474-4422(08)70167-4. [DOI] [PubMed] [Google Scholar]
- 9. Bredesen DE. Metabolic profiling distinguishes three subtypes of Alzheimer’s disease. Aging (Albany NY). 2015;7(8):595-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Liang SH, Holland JP, Stephenson NA, et al. PET neuroimaging studies of [18F]CABS13 in a double transgenic mouse model of Alzheimer’s disease and nonhuman primates. ACS Chem Neurosci. 2015;6(4):535-541. doi:10.1021/acschemneuro.5b00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bush AI, Tanzi RE. Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics. 2008;5(3):421-432. doi:10.1016/j.nurt.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DeGrado TR, Pandey MK, Byrne JF, et al. Preparation and preliminary evaluation of63Zn-zinc citrate as a novel PET imaging biomarker for zinc. J Nucl Med. 2014;55(8):1348-1354. doi:10.2967/jnumed.114.141218. [DOI] [PubMed] [Google Scholar]
- 13. Lowe VJ, Kemp BJ, Jack CR, Jr, et al. Comparison of 18F-FDG and PiB PET in cognitive impairment. J Nucl Med. 2009;50(6):878-886. doi:10.2967/jnumed.108.058529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vemuri P, Gunter JL, Senjem ML, et al. Alzheimer’s disease diagnosis in individual subjects using structural MR images: validation studies. Neuroimage. 2008;39(3):1186-1197. doi:10.1016/j.neuroimage.2007.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kinlaw WB, Levine AS, Morley JE, Silvis SE, McClain CJ. Abnormal zinc metabolism in type II diabetes mellitus. Am J Med. 1983;75(2):273-277. doi:10.1016/0002-9343(83)91205-6. [DOI] [PubMed] [Google Scholar]
- 16. Xu J, Zhou Q, Liu G, Tan Y, Cai L. Analysis of serum and urinal copper and zinc in Chinese Northeast population with the prediabetes or diabetes with and without complications. Oxid Med Cell Longev. 2013;2013:635214 doi:10.1155/2013/635214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kahn AM, Helwig HL, Redeker AG, Reynolds TB. Urine and serum zinc abnormalities in disease of the liver. Am J Clin Pathol. 1965;44(4):426-435. [DOI] [PubMed] [Google Scholar]
- 18. Gür G, Bayraktar Y, Özer D, Özdogan M, Kayhan B. Determination of hepatic zinc content in chronic liver disease due to hepatitis B virus. Hepatoqastroenterology. 1998;45(20):472-476. [PubMed] [Google Scholar]
- 19. Poo JL, Romero R, Rodriguez F, et al. Serum zinc concentrations in two cohorts of 153 healthy subjects and 100 cirrhotic patients from Mexico City. Dig Dis. 1995;13(2):136-142. [DOI] [PubMed] [Google Scholar]
- 20. Bode CJ, Hanisch P, Henning H, Koenig W, Richter FW, Bode C. Hepatic zinc content in patients with various stages of alcoholic liver disease and in patients with chronic active and chronic persistent hepatitis. Hepatology. 1988;8(6):1605-1609. [DOI] [PubMed] [Google Scholar]
- 21. Guo CH, Chen PC, Ko WS. Status of essential trace minerals and oxidative stress in viral hepatitis C patients with nonalcoholic fatty liver disease. Int J Med Sci. 2013;10(6):730-737. doi:10.7150/ijms.6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Franklin RB, Levy BA, Zou J, et al. ZIP14 zinc transporter downregulation and zinc depletion in the development and progression of hepatocellular cancer. J Gastrointest Cancer. 2012;43(2):249-257. doi:10.1007/s12029-011-9269-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walker BE, Dawson JB, Kelleher J, Lasowsky MS. Plasma and urinary zinc in patients with malabsorption syndromes or hepatic cirrhosis. Gut. 1973;14(12):943-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li YV. Zinc and insulin in pancreatic beta-cells. Endocrine. 2014;45(2):178-189. doi:10.1007/s12020-013-0032-x. [DOI] [PubMed] [Google Scholar]
- 25. Gjørup I, Petronijevic L, Rubinstein E, Andersen B, Worning H, Burcharth F. Pancreatic secretion of zinc and copper in normal subjects and in patients with chronic pancreatitis. Digestion. 1991;49(3):161-166. [DOI] [PubMed] [Google Scholar]
- 26. Rajesh G, Girish BN, Vaidyanathan K, Balakrishnan V. Diet, nutrient deficiency and chronic pancreatitis. Trop Gastroenterol. 2013;34(2):68-73. [DOI] [PubMed] [Google Scholar]
- 27. Kumar R, Prasad R. Functional characterization of purified zinc transporter from renal brush border membrane of rat. Biochim Biophys Acta. 2000;1509(1-2):429-439. doi:10.1016/S0005-2736(00)00325-4. [DOI] [PubMed] [Google Scholar]
- 28. Gielda LM, DiRita VJ. Zinc competition among the intestinal microbiota. MBio. 2012;3(4):e00171-12. doi:10.1128/mBio.00171-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brignola C, Belloli C, De Simone G, et al. Zinc supplementation restores plasma concentrations of zinc and thymulin in patients with Crohn’s disease. Aliment Pharmacol Ther. 1993;7(3):275-280. [DOI] [PubMed] [Google Scholar]
- 30. Alkhouri RH, Hashmi H, Baker RD, Gelfond D, Baker SS. Vitamin and mineral status in patients with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2013;56(1):89-92. doi:10.1097/MPG.0b013e31826a105d. [DOI] [PubMed] [Google Scholar]
- 31. Bush AI, Pettingell WH, Multhaup G, et al. Rapid induction of Alzheimer A beta amyloid formation by zinc. Science. 1994;265(5177):1464-1467. [DOI] [PubMed] [Google Scholar]
- 32. Religa D, Strozyk D, Cherny RA, et al. Elevated cortical zinc in Alzheimer disease. Neurology. 2006;67(1):69-75. [DOI] [PubMed] [Google Scholar]
- 33. Driscoll I, Troncoso JC, Rudow G, et al. Correspondence between in vivo 11C-PiB-PET amyloid imaging and postmortem, region-matched assessment of plaques. Acta Neuropathol. 2012;124(6):823-831. doi:10.1007/s00401-012-1025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang Y, Wu Z, Cao Y, Lang M, Lu B, Zhou B. Zinc binding directly regulates tau toxicity independent of tau hyperphosphorylation. Cell Rep. 2014;8(3):831-842. doi:10.1016/j.celrep.2014.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Selkow DJ, Masters CL. Biochemistry of amyloid β-protein and amyloid deposits in Alzheimer’s disease. Cold Spring Harb Perspect Med. 2012;2(6):a006262 doi:10.1101/cshperspect.a006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nuttall JR, Oteiza PI. Zinc and the aging brain. Genes Nutr. 2014;9(1):379 doi:10.1007/s12263-013-0379-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.