Abstract
Nanomaterials have become essential components for the development of biosensors since such nanosized compounds were shown to clearly increase the analytical performance. The improvements are mainly related to an increased surface area, thus providing an enhanced accessibility for the analyte, the compound to be detected, to the receptor unit, the sensing element. Nanomaterials can also add value to biosensor devices due to their intrinsic physical or chemical properties and can even act as transducers for the signal capture. Among the vast amount of examples where nanomaterials demonstrate their superiority to bulk materials, the combination of different nano-objects with different characteristics can create phenomena which contribute to new or improved signal capture setups. These phenomena and their utility in biosensor devices are summarized in a non-exhaustive way where the principles behind these synergetic effects are emphasized.
Keywords: nanomaterials, biosensors, hybrids, carbon, metals, semiconductors, energy transfer
1. Introduction
The particularity of biosensors, compared to classic sensors, is that the sensing element, also called the receptor unit, is a biological entity or a bioinspired compound which confers an excellent selectivity towards the analyte to be detected. The unique specificity of such bioreceptors represents the main advantage within all sensor devices and the development of biosensors has become a huge research topic since highly complex solutions like blood can be analyzed for one specific target [1,2,3]. Biosensors are mainly used for the monitoring of diseases and are based on the recognition event of immune systems, viruses, bacteria, or cells, but also find utility for the detection of chemicals like blood sugar or pollutants [4,5]. One challenge is the signal capture during the biological recognition event [6,7].
Voltammetric biosensors rely on a redox process where the involved electron transfers are proportional to the analyte concentration [8]. For instance, the enzyme glucose oxidase (GOx) recognizes very specifically β-d-glucose, which is oxidized to gluconolactone. The reduced enzyme generally regenerates itself by reducing oxygen to hydrogen peroxide [9], a electroactive molecule which can be detected by the electrode. For immunosensing and the detection of DNA, more sophisticated setups are needed since an immune reaction or a hybridization of DNAs does not produce an electrochemical signal. For these cases, labeled secondary antibodies or DNA strands have to be involved after the recognition event where these labels will give the electrochemical signal. To avoid such supplemental time consuming preparation steps, electrochemical impedance spectroscopy (EIS) represents a very appropriate tool for immune and DNA sensors. EIS works with alternating currents (ACs) of small amplitude within a wide range of frequencies. The biorecognition event changes the sensing capacitance and interfacial electron transfer resistance of the electrode leading to a highly sensitive signal capture down to the femtomolar range [10,11].
Gravimetric biosensors are mostly piezoelectric devices where the detection of biological targets provokes a change of the resonance frequency related to the mass of the analyte [12,13]. One famous example is quartz crystal microbalance (QCM) but also micro- (or nano-) mechanical cantilever setups [14] are promising candidates for highly sensitive label free transduction techniques.
Most optical biosensors are based on a change in fluorescence or color during or after the recognition event [15]. As for electrochemical biosensors, some techniques need the use of supplemental labelling steps to introduce a photosensitive probe. Label-free optical detection can be achieved using surface plasmon resonance (SPR), which is a highly sensitive and quantitative transduction technique. The principle is based on the change of light-induced electron oscillations (surface plasmons) in the conduction band of metallic coatings (usually gold) when the dielectric constant of its environment changes [16]. This is the case, among others, for immune reactions or DNA hybridization where the recognition event changes the oscillation frequency which results in an angle change of the reflected light, its change of intensity, refractive index, or its phase [17,18].
The use of nanomaterials clearly already enhances the signal capture of all these transduction techniques used for biosensing thanks to their enhanced specific surface which allows the immobilization of an enhanced amount of bioreceptor units with an improved accessibility for the analytes. The advantages of different nanomaterials for biosensors are summarized in many review articles [19,20,21,22,23,24,25,26,27]. Here, we want to present some selected examples of synergetic effects achievable by combining different nanomaterials, thus enabling new or original transduction of biorecognition events.
2. Nanoparticles
Nanoparticles have become important components in biosensing devices since almost every material can be shaped into nanosized structures, thus conferring specific properties to the sensing element [28]. For instance, noble metal particles like silver and gold are famous for their localized resonant surface plasmons tremendously enhancing SPR or Raman signals [29,30,31]. These and other materials like platinum nanoparticles [32], or metal oxide nanoparticles [33] also provide improvements in catalysis and conductivity in electrochemical biosensors, while original setups were developed using magnetic nanoparticles [34]. Since many of these materials shows synergetic effects with other nano-objects, several examples will be described in more detail in the following sections. The most exploited synergetic effect between nanostructured materials is based on non-radiative energy transfer using upconverting nanoparticles (UCNPs) and quantum dots (QDs).
2.1. Upconverting Nanoparticles
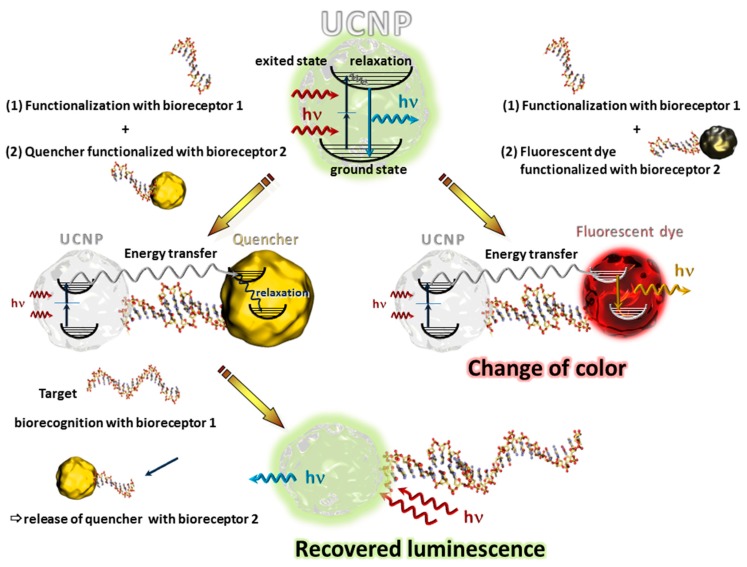
UCNPs have the capacity to absorb several photons in the infrared range and to convert this absorbed energy into an emission in the visible range via a nonlinear optical process [35]. Contrary to common multiphoton absorption materials, these nanoparticles do not need high excitation densities for efficient anti-Stokes type emission. The phenomenon of high wavelength absorption and low wavelength emission strongly depends on the ion-ion distance of a dopant (mostly lanthanides) in a host material (generally Na+ or Ca2+ fluorides). The confinement of the lanthanides in the matrix also determines the color of emitted light [36,37]. UCNPs became promising alternatives to other fluorescent labels for biosensing applications since they have very low background emissions and the high excitation wavelength does not provoke luminescence or absorption effects with other components of the biosensor [38,39]. UCNPs can be used as simple labels but more interesting are transduction principles based on non-radiative resonance energy transfer (RET) from the exited UCNPs to an acceptor where it should be emphasized that lanthanides are luminescent and not fluorescent and the RET is called luminescent resonant energy transfer (LRET) contrary to fluorescent (or Förster) resonant energy transfer (FRET) using fluorescent dyes [40]. The acceptor also plays a crucial role for this type of transduction because RET can only be achieved at corresponding quantum yields and cross sections, donor-acceptor distances, and their spectral emission and absorption overlap [41]. Furthermore, when the acceptor is a fluorescent probe, it can be excited by the UCNP leading to a change of the emission spectrum or simply to a change of the color of emitted light, or, when the acceptor is not a fluorophore, this results in the quenching of the emission of UCNPs [42] as illustrated in Figure 1.
Figure 1.
Schematic presentation of an UCNP and its anti-stokes type emission (top) and their functioning as bioanalytical transducer using a nanosized quencher (left) or a fluorescent dye (right).
Many approaches have been proposed relying on a quenching effect. For instance, Wang et al. demonstrated LRET between biotin-functionalized UCNPs and biotin-functionalized gold nanoparticles in the presence of avidin, which served here as the analyte which brings the two nano-objects in close contact leading to a linear reduction of the intensity of emitted light as a function of the avidin concentration [42]. A more sophisticated strategy was applied for the detection of thrombin in human plasma [43]. The specific thrombin aptamer was attached to the UCNPs while its luminescence is quenched in presence of carbon nanoparticles which form weak interactions with the aptamer. When the analyte is added, the carbon nanoparticles are released due to the stronger interaction between the aptamer and thrombin leading to a linear luminescence increase. A similar transduction principle was chosen by Zhang et al. who modified UCNPs with concanavalin A which interacts with saccharides. As quencher, chitosan-labeled graphene oxide was chosen and, due to the concanavalin A-chitosan interaction, the two components are assembled in close contact leading to the extinction of light. Then, glucose was used as analyte which forms stronger interaction with concanavalin A than chitosan leading to a glucose concentration dependent increase of emitted light [44].
The possibility to transfer upconverted energy to fluorophores thus changing the emission band after a biorecognition event has been extensively studied by Mattsson et al. [45]. For the proof of concept the biotin-streptavidin binding event was also used here as model recognition system. Streptavidin-modified UCNPs were attached to biotin-modified quantum dots leading to a change of the emission wavelength (the one for the quantum dots). In the presence of free biotin, mixed emissions or only the UCNPs’ luminescence could be observed. The possibility to calibrate the intensities of different wavelengths might represent a promising platform for multiplex biosensing.
However, one drawback of UCNPs for biosensing applications has to be noted. All mentioned examples need an excitation wavelength of 980 nm which is right in the absorption band of water and heats the sample. This inconvenience can be overcome by doping UCNPs with neodymium ions lowering the excitation wavelength to 808 nm. Sample heating can thus be avoided which is of particular importance for in vivo bioimaging [46] and this approach also shows advantages in the monitoring of enzymatic reactions [47].
2.2. Quantum Dots
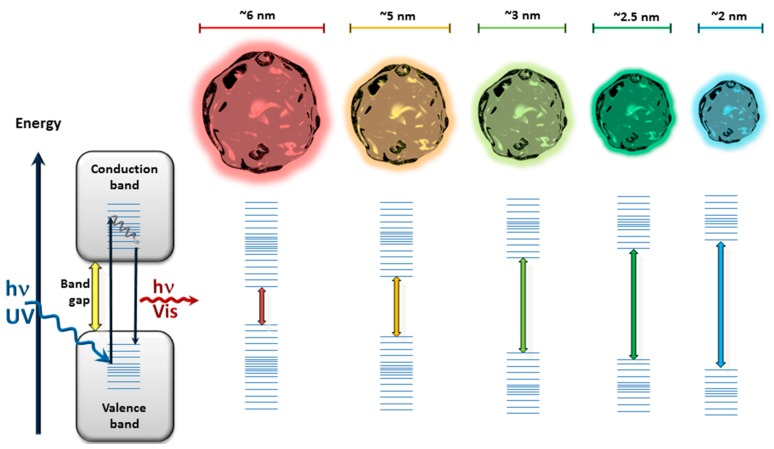
QDs have become almost the nanomaterial of choice for fluorescence-based transduction in bioanalytics. QDs are luminescent semiconducting nanocrystals principally based on cadmium chalcogenides [48,49,50]. Most of them are available as core-shell particles coated with ZnS or CdS for enhanced quantum yields and photostability [51,52]. They can absorb in a large wavelength range but have a narrow emission spectrum which is dependent on the particle size [53] (Figure 2).
Figure 2.
Illustration of QDs with different sizes and the related band gaps leading to different emission wavelengths after excitation with UV light.
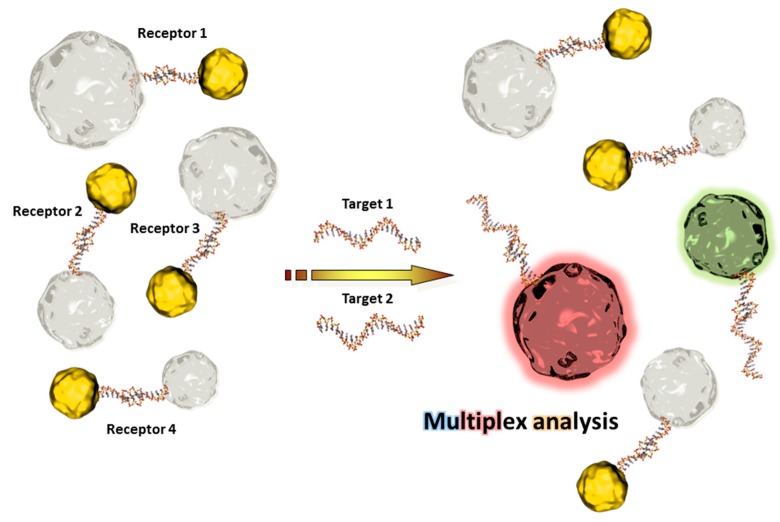
The availability of QDs with different emission wavelengths has made them promising candidates for multiplexed analysis [54,55,56], as depicted in Figure 3. Furthermore, a final coating of QDs allows efficient functionalization with bioreceptor units and can overcome possible toxicity issues [57].
Figure 3.
Scheme of a multiplex sensing principle using QDs and quenchers.
QDs are, as UCNPs, excellent optical transducers in combination with other nanomaterials. The principle is mostly based on the release of a quencher after the recognition event and the recovery of fluorescence. This strategy is particularly efficient for aptamer- and DNA sensors [58,59]. As a representative example, the assembly of a QD-labeled receptor DNA with a shorter corresponding DNA tagged with a gold nanoparticle is cut by the (longer) analyte DNA due to its higher hybridization kinetics. The gold nanoparticle is released and the QDs start to emit light again where the intensity is proportional to the analyte concentration [60,61] (Figure 3).
Gold nanoparticles are not only used as non-radiative quenchers, but can also act as antennas for increased fluorescence of QDs due to their high plasmonic behavior. When gold nanoparticles are localized at around 30 nm to the QD surface, the gold nanoparticle provokes an increase of the excitation rates of the QDs and hence the intensity of the fluorescence [62]. Further non-radiative energy transfer leading to QD fluorescence can be achieved using emitting protein labels which eliminate the need of external excitation light source [63]. There are also charge transfer quenching and chemiluminescence resonance energy transfer phenomena [64] to complete the most common applied principles of FRET-based biosensing using QDs [65,66,67].
QDs were also combined with magnetic nanoparticles for improved biodetection [68]. The magnetic nanoparticles are used for the separation of biological analytes in complex media like blood or any type of body fluids. In detail, receptor unit-modified magnetic nanoparticles are introduced in the analyte solution and interact specifically with the target molecule. The particles then migrate in a magnetic field until settling to form a deposit. The remaining solution can then be removed and the deposit can even be rinsed to eliminate any trapped species. QDs functionalized with a secondary receptor unit interact with the analyte on the magnetic particles and can quantify the detection via the intensity of the QD emission. A more sophisticated setup was proposed by Kurt et al. [69]. QDs and UCNPs, functionalized with different aptamers for different targets, served as recognition and transduction element in combination with magnetic nanoparticles modified with corresponding short DNA strands. The principle is based on the affinity interaction between aptamers and DNA linking weakly the magnetic nanoparticles with QDs and UCNPs which can then be separated from the solution in a magnetic field. In presence of the analytes (here the pathogens Salmonella typhimurium and Staphylococcus aureus), the DNA-aptamer link is broken by the competitive interaction with the target and the luminescent particles remain in solution after applying a magnetic field. The Salmonella typhimurium-UCNP and Staphylococcus aureus-QD assemblies can then be removed by washing thus leading to reduced intensities of emitted light. The authors observed a linear decrease of luminescence intensity with the analyte concentration. For the multiplex sensing setup, the remaining Staphylococcus aureus specific QDs and Salmonella typhimurium specific UCNPs are exited at 325 nm and 980 nm. UCNP excitation at 980 nm cannot excite the QDs since the energy of photons is below the band gap of the QDs. The authors observed negligible excitation of the QDs by the emission of UCNPs at 470 nm and could also exclude FRET. This setup might be a promising strategy for facilitated multiplex analysis but will need materials with narrower excitation and emission lines to prevent overlap or crosstalk effects.
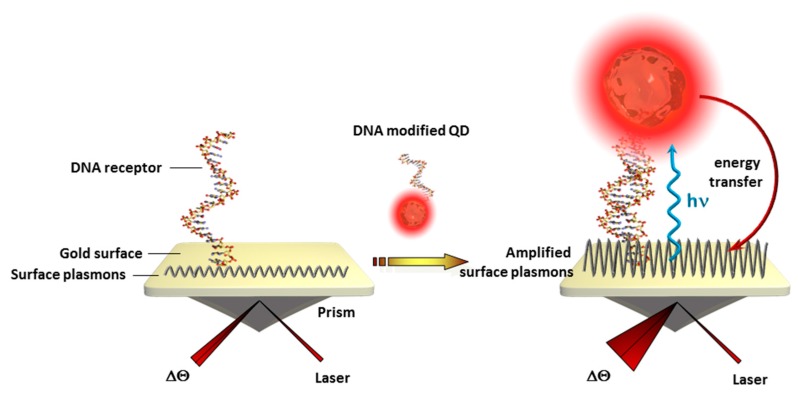
Besides FRET with other nanomaterials, QDs can also interact with propagating surface plasmons of gold surfaces leading to light emission of the QDs or the light induced excited state of QDs are transferred to the surface plasmons [70] as illustrated in Figure 4. The second effect led to clear signal enhancements in SPR setups where a 25-fold increase was observed for ss-DNA and a 50-fold increase could be obtained with prostate-specific antigens compared to bare gold surfaces. [71].
Figure 4.
Schematic illustration of SPR signal amplification after the biorecognition event using QD labeled biomarkers.
QDs also show remarkable properties in electrochemical biosensing devices in combination with CNTs [72]. The distribution of these semiconductors within a CNT composite matrix forms domains with altered conductivities behaving like a microelectrode array. This phenomenon results in a clear reduction of the double layer capacitance and thus to an improved noise signal ratio. This setup was validated for the detection of hydrogen peroxide and ascorbic acid.
3. Carbon Nanomaterials
Carbon is a privileged material for biosensing applications, especially for electrochemical transduction due to its excellent conductivity and biocompatibility [73]. Carbon appears in many different allotropes based on graphite (sp2), diamond (sp3) and intermittent sp2-sp3 hybridized macroscopic structures generally called amorphous carbon, from which vast amounts of substructures can be synthesized [74]. For electrochemical biosensing, glassy carbon, doped diamond, and graphite are standard materials for electrodes [75]. Their nanostructured part in the form of carbon nanotubes [76], fullerenes [77], or graphene [78] partly became the material of choice for improved performances of bioanalytical devices [79]. More recently, fluorescent carbon nanodots have attracted attention as non-toxic alternatives to quantum dots for optical biosensing and bioimaging [80]. Efficient functionalization techniques were established for carbon nanomaterials which allow the formation of bioassemblies and to combine the beneficial properties with those of other nanosized materials [81]. This also allows reproducible processing and shaping to obtain the desired properties. An elegant way to assemble different materials is the formation of composites. As an example for electrochemical transduction, carbon paste electrodes provide unlimited possibilities to combine any type of carbon material with (nanosized) fillers conferring improved performances to the biosensor device. Selected examples and procedures of customized carbon paste-based biosensors were summarized by Muñoz et al. [82]. The following section presents some further examples of successful combinations of nanostructured carbon allotropes with other materials with synergetic effects for enhanced biosensor performance.
3.1. Graphene
Graphene has become a fashionable material for biosensing because it is considered less toxic than CNTs [83,84]. Even though graphene is per definition not a nanomaterial [85], it is worth summarizing some examples of its synergetic effects with other nano-objects since it belongs to the rich carbon allotrope family.
For electrochemical transduction, graphite-based layered materials are used in bulk form but are also often called graphene or graphene-like 2D materials [86]. Obtained after mechanic exfoliation [87,88], chemical oxidation of graphite [89] and/or subsequent reduction [90], these carbon materials are represented in many biosensor application examples [91] such as electrochemical immunosensors [92] or enzymatic biosensors [93]. In terms of synergetic hybrid materials, and similar to CNT hybrids, many different metal nanoparticles like gold [94], platinum [95,96,97,98,99], or palladium [100], or metal oxide nanoparticles [101] clearly improved the sensing performance when combined with graphene and graphene-like 2D materials.
For optical transduction, graphene materials can also act as non-radiant energy acceptors in FRET-based biosensors using organic dye- [102] or quantum dot [103] -labeled bioreceptors like DNAs, aptamers, or proteins [104]. Graphene oxide itself shows photoluminescence and can act as both, energy donor and energy acceptor [105] with excellent quenching efficiency [106].
In particular, graphene can interact with DNA or oligonucleotide receptors in a non-covalent and reversible manner, contrary to CNTs, and these dye-labeled receptors desorb after the recognition, recovering the fluorescence of the labels. This principle could even be applied for a multiplexed colorimetric DNA sensor [107]. Weak interactions with graphene oxide can also be obtained with antibodies labeled with QDs, which were exploited for the detection of the model pathogen E. coli [108]. The fluorescence of a corresponding antibody-modified QD is quenched in the presence of graphene oxide and recovered after the recognition event with the bacteria. This setup was successfully applied on nanocellulose-based papers for fluorescence biosensing using QDs and UCNPs [109]. Furthermore, such papers also provide an excellent platform for colorimetric sensing of biorelevant chemicals when functionalized with silver or gold nanoparticles.
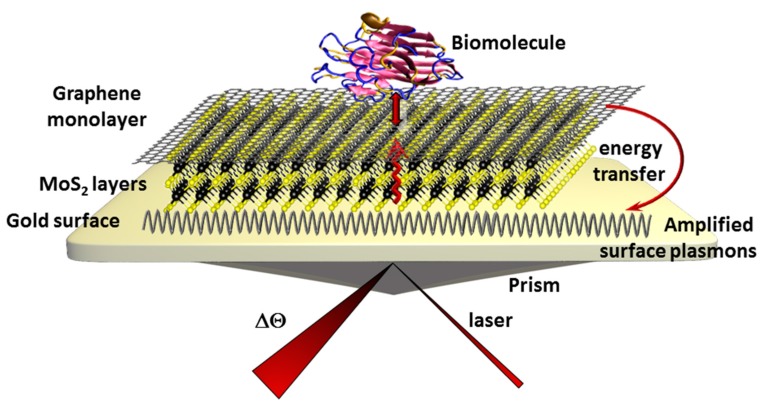
Furthermore, real monolayer graphene provides impressive beneficial properties in resonant plasmon transduction techniques [110]. Firstly predicted by theoretical models, these plasmonic properties of a single layer of graphene can interact with the surface plasmons of gold surfaces thus significantly amplifying the optical sensitivity of surface plasmon resonance (SPR) sensors [111]. By excitation in the visible light range [112] the propagation constant of surface plasmon polaritons (SPPs) is changed and the refractive index response in particular is amplified [113]. An almost two- fold increase of the SPR signal could be obtained just in presence of a graphene monolayer on gold which was validated in a highly sensitive anti-cholera toxin SPR sensor [114]. This phenomenon can in theory be further optimized using intermittent MoS2 layers [115]. Due to the improved optical absorption efficiency, the graphene-MoS2 layer can transfer this energy to the underlying gold layer thus further exciting and amplifying the resonant surface plasmons (Figure 5).
Figure 5.
Principle of improved SPR signals after adsorption of a biomolecules using MoS2 as intermittent layer between monolayer graphene and the gold surface.
The authors calculated an up to 500-fold increase of phase sensitivity of the SPR signal with theoretic models when a biomolecule is adsorbed on the graphene layer via π-π stacking interactions. The authors unfortunately did not precise which biomolecule these calculations were based on.
3.2. Carbon Nanotubes
Carbon nanotubes (CNTs) can be seen as seamlessly rolled up graphene with one to up to hundreds of concentric wall layers and provide excellent 1D conductivity and high aspect ratios which form entangled porous structures in bulk, drastically increasing the accessible surface area of electrodes [116,117,118]. Furthermore, efficient and reliable functionalization methods were developed for the immobilization of bioreceptor units on CNTs without altering the biological activity [119].
CNTs were confined with Pt nanoparticles in a Nafion matrix for improved DNA sensing using daunomycin, a redox active compound which intercalates hybridized DNAs [120]. Single stranded receptor DNA was immobilized on this composite and was exposed to different concentrations of the analyte, the corresponding ssDNA. Since daunomycin only intercalates after the recognition event, the differential pulse voltammetric signal increased for the electrocatalytic reduction of the electrochemical probe. The combination of the enhanced specific surface area of CNTs and the catalytic properties of Pt led to clearly improved performances compared to setups using the individual compounds.
CNT-gold nanoparticle (AuNPs) assemblies clearly improve electrochemical transduction due to enhanced electron transfer rates between an enzymatically generated substrate and the AuNPs-CNT composites. A highly sensitive choline biosensor was developed based on choline oxidase modified AuNPs-CNT electrodes [121]. After the enzyme-catalyzed oxidation of choline, hydrogen peroxide is released which is finally oxidized on the electrocatalytic nanocomposite electrode. Beside the beneficial effect of AuNP-CNT assemblies for electrochemical biosensors [122,123], other metal or metal oxide nanoparticles showed improved biosensing performances when combined with carbon nanotubes. Most examples describe the improved electrocatalytic oxidation of enzymatically generated H2O2 using cobalt hexacyanoferrate nanoparticles [124], Pt nanoparticles [125], or ZnO nanoparticles [126] while for this example CNT-graphene hybrids were used. There are many further examples of using different nanomaterials in combination with CNTs which are summarized in reference [127].
3.3. C60 Fullerenes and Carbon Dots
C60 is the first fully characterized carbon nano-object and is classified as a 0D material. Its molecular structure is composed of 12 five-membered rings surrounded by a total of 20 six-membered rings and it obeys perfectly Euler’s rule [128]. The particular electrochemical properties of C60 [129] evoked much attention for its possible application as a redox mediator in enzymatic biosensors [130]. C60 also showed remarkably enhancements of the specific surface of electrodes and was used as a building block for original nanoscaffolds [131]. An electrochemical aptasensor was reported where an electrode surface is modified with onion-like mesoporous graphene sheets, gold nanoparticles, and a first aptamer receptor. Prussian Blue-modified gold nanoparticles were adsorbed on amine-functionalized C60 together with a second aptamer receptor and alkaline phosphatase as label. After the recognition event of the model target platelet-derived growth factor B-chain, and the formation of the sandwich structure, the immobilized enzyme label hydrolyzes ascorbic acid phosphate to ascorbic acid which is then oxidized on the Prussian Blue/gold nanoparticles/C60 electrode. Further examples describe the combination of C60 with mostly gold or platinum nanoparticles for improved electrochemical [132] or gravimetric [133] immunosensors, or an electrochemiluminescent aptasensor [134] but all of them rely on the electrochemical behavior of C60 or the capability to enhance the surface area and not on synergetic phenomena between these nano-objects.
Carbon dots or carbon quantum dots, sometimes also called graphene quantum dots, can be considered further 0D carbon nanomaterials. Accidently discovered as a side product during arc discharge synthesis of single walled CNTs [135], these carbon QDs are promising candidates to replace heavy metal semiconductor QDs since they are still considered as a non-toxic carbon material with very satisfying quantum yields where even upconverting nanoparticles could be isolated and studied [136,137]. The fluorescence phenomenon is related to isolated domains of conjugated sp2 carbon surrounded by diamond like sp3 carbon [138] as depicted in Figure 6.
Figure 6.
Sketch of a carbon QD with its defined sp2 domains isolated and surrounded with diamond- like carbon which is highly oxidized on the surface of the particle.
The fluorescence is also influenced by the mostly carboxylated surface which confers carbon QDs excellent solubility, but also strong pH-dependent fluorescence emission [139]. Even when great progress was achieved in the synthesis and isolation of carbon QDs with specific properties, the controlled synthesis of defined domain distribution and surface functionalities leading to distinguished absorption and emission spectra, as it is the case for semiconductor QDs, remains a challenge [140,141]. In terms of biosensing applications, carbon QDs show similar performances as semiconductor QDs concerning FRET-based biosensing and as fluorescence labels [80]. Efficient FRET between gold nanoparticles and carbon QDs could be achieved when each nano-object is modified with a corresponding antibody-antigen system [142]. In the presence of the analyte, in this example an organic pollutant, these assemblies are broken, leading to the recovery of fluorescence. Based on the same principle, a DNA sensor was proposed using assemblies of carbon QDs and fluorescence dye quenching each other whereby fluorescence reappears after the recognition event [143]. It would be interesting to study the efficiency and performances of carbon QDs and semiconductor QDs under identical condition to gain insight into the real potential of carbon QDs. It might be assumed that such studies will be reported in the near future.
4. Conclusions
Synergetic effects of different nanoparticles became promising tools for highly sensitive biodetection applications where the FRET effect is at the moment the most promising example where QDs were shown to be particularly versatile when combined with nanosized acceptors. Carbon QDs or UCNPs are promising candidates with lower toxicity issues to one day replace semiconductor QDs. In regards of the steady growing availability of different nanomaterials with different properties revealing new phenomena when in contact, other original electronic, electrochemical, or magnetic transduction methods can be developed. There remains one famous example of a nano-object with synergetic properties to which a section was not dedicated in this review: gold nanoparticles. This is simply due to the fact that nanosized gold was mentioned with almost all discussed materials and to avoid repetition, a separate discussion about gold-hybrids was intentionally omitted. However, for more information, examples of the beneficial properties of gold nanoparticle hybrids for biosensing and diagnostics are summarized in reference [144].
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.De Corcuera J.I.R., Cavalieri R.P. Encyclopedia of Agricultural, Food, and Biological Engineering. Taylor & Francis; Abingdon, UK: 2007. Biosensors; pp. 119–123. [Google Scholar]
- 2.Tothill I.E., Turner A.P.F. Biosensors. In: Caballero B., editor. Encyclopedia of Food Sciences and Nutrition. 2nd ed. Academic Press; Oxford, UK: 2003. pp. 489–499. [Google Scholar]
- 3.Cosnier S. Electrochemical Biosensors. Pan Stanford Publishing; Singapore: 2015. [Google Scholar]
- 4.Mehrotra P. Biosensors and their applications: A review. J. Oral Biol. Craniofacial Res. 2016;6:153–159. doi: 10.1016/j.jobcr.2015.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X., Lu X., Chen J. Development of biosensor technologies for analysis of environmental contaminants. Trends Environ. Anal. Chem. 2014;2:25–32. doi: 10.1016/j.teac.2014.04.001. [DOI] [Google Scholar]
- 6.Mohanty S.P., Kougianos E. Biosensors: A tutorial review. IEEE Potentials. 2006;25:35–40. doi: 10.1109/MP.2006.1649009. [DOI] [Google Scholar]
- 7.Perumal V., Hashim U. Advances in biosensors: Principle, architecture and applications. J. Appl. Biomed. 2014;12:1–15. doi: 10.1016/j.jab.2013.02.001. [DOI] [Google Scholar]
- 8.Ronkainen N.J., Halsall H.B., Heineman W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010;39:1747–1763. doi: 10.1039/b714449k. [DOI] [PubMed] [Google Scholar]
- 9.Wang H.-C., Lee A.-R. Recent developments in blood glucose sensors. J. Food Drug Anal. 2015;23:191–200. doi: 10.1016/j.jfda.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baur J., Gondran C., Holzinger M., Defrancq E., Perrot H., Cosnier S. Label-free femtomolar detection of target DNA by impedimetric DNA sensor based on poly(pyrrole-nitrilotriacetic acid) film. Anal. Chem. 2010;82:1066–1072. doi: 10.1021/ac9024329. [DOI] [PubMed] [Google Scholar]
- 11.Giroud F., Gorgy K., Gondran C., Cosnier S., Pinacho D.G., Marco M.-P., Sánchez-Baeza F.J. Impedimetric Immunosensor Based on a Polypyrrole-Antibiotic Model Film for the Label-Free Picomolar Detection of Ciprofloxacin. Anal. Chem. 2009;81:8405–8409. doi: 10.1021/ac901290m. [DOI] [PubMed] [Google Scholar]
- 12.Skládal P. Piezoelectric biosensors. TrAC Trends Anal. Chem. 2016;79:127–133. doi: 10.1016/j.trac.2015.12.009. [DOI] [Google Scholar]
- 13.Johannsmann D. The Quartz Crystal Microbalance in Soft Matter Research: Fundamentals and Modeling. Springer; Cham, Switzerland: 2015. Gravimetric Sensing; pp. 191–204. [Google Scholar]
- 14.Meisam O., Malakoutian M.A., Mohammadmehdi C., Oroojalian F., Haghiralsadat F., Yazdian F. A Label-Free Detection of Biomolecules Using Micromechanical Biosensors. Chin. Phys. Lett. 2013;30:068701. [Google Scholar]
- 15.Damborský P., Švitel J., Katrlík J. Optical biosensors. Essays Biochem. 2016;60:91. doi: 10.1042/EBC20150010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelly K.L., Coronado E., Zhao L.L., Schatz G.C. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment. J. Phys. Chem. B. 2002;107:668–677. doi: 10.1021/jp026731y. [DOI] [Google Scholar]
- 17.Wijaya E., Lenaerts C., Maricot S., Hastanin J., Habraken S., Vilcot J.-P., Boukherroub R., Szunerits S. Surface plasmon resonance-based biosensors: From the development of different SPR structures to novel surface functionalization strategies. Curr. Opin. Solid State Mater. Sci. 2011;15:208–224. doi: 10.1016/j.cossms.2011.05.001. [DOI] [Google Scholar]
- 18.Guo X. Surface plasmon resonance based biosensor technique: A review. J. Biophotonics. 2012;5:483–501. doi: 10.1002/jbio.201200015. [DOI] [PubMed] [Google Scholar]
- 19.Hou S., Zhang A., Su M. Nanomaterials for Biosensing Applications. Nanomaterials. 2016;6:58. doi: 10.3390/nano6040058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holzinger M., Le Goff A., Cosnier S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014;2:63. doi: 10.3389/fchem.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aragay G., Pino F., Merkoçi A. Nanomaterials for Sensing and Destroying Pesticides. Chem. Rev. 2012;112:5317–5338. doi: 10.1021/cr300020c. [DOI] [PubMed] [Google Scholar]
- 22.Ge X., Asiri A.M., Du D., Wen W., Wang S., Lin Y. Nanomaterial-enhanced paper-based biosensors. TrAC Trends Anal. Chem. 2014;58:31–39. doi: 10.1016/j.trac.2014.03.008. [DOI] [Google Scholar]
- 23.Song Y., Luo Y., Zhu C., Li H., Du D., Lin Y. Recent advances in electrochemical biosensors based on graphene two-dimensional nanomaterials. Biosens. Bioelectron. 2016;76:195–212. doi: 10.1016/j.bios.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 24.Oliveira S.F., Bisker G., Bakh N.A., Gibbs S.L., Landry M.P., Strano M.S. Protein functionalized carbon nanomaterials for biomedical applications. Carbon. 2015;95:767–779. doi: 10.1016/j.carbon.2015.08.076. [DOI] [Google Scholar]
- 25.Chimene D., Alge D.L., Gaharwar A.K. Two-Dimensional Nanomaterials for Biomedical Applications: Emerging Trends and Future Prospects. Adv. Mater. 2015;27:7261–7284. doi: 10.1002/adma.201502422. [DOI] [PubMed] [Google Scholar]
- 26.Ju H., Zhang X., Wang J. NanoBiosensing. Springer; New York, NY, USA: 2011. Nanomaterials for Immunosensors and Immunoassays; pp. 425–452. [Google Scholar]
- 27.Lei J., Ju H. Signal amplification using functional nanomaterials for biosensing. Chem. Soc. Rev. 2012;41:2122–2134. doi: 10.1039/c1cs15274b. [DOI] [PubMed] [Google Scholar]
- 28.El-Ansary A., Faddah L.M. Nanoparticles as biochemical sensors. Nanotechnol. Sci. Appl. 2010;3:65–76. doi: 10.2147/NSA.S8199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Doria G., Conde J., Veigas B., Giestas L., Almeida C., Assunção M., Rosa J., Baptista P.V. Noble Metal Nanoparticles for Biosensing Applications. Sensors. 2012;12:1657–1687. doi: 10.3390/s120201657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liao H., Nehl C.L., Hafner J.H. Biomedical applications of plasmon resonant metal nanoparticles. Nanomedicine. 2006;1:201–208. doi: 10.2217/17435889.1.2.201. [DOI] [PubMed] [Google Scholar]
- 31.Anker J.N., Hall W.P., Lyandres O., Shah N.C., Zhao J., Van Duyne R.P. Biosensing with plasmonic nanosensors. Nat. Mater. 2008;7:442–453. doi: 10.1038/nmat2162. [DOI] [PubMed] [Google Scholar]
- 32.Rioux R.M., Song H., Grass M., Habas S., Niesz K., Hoefelmeyer J.D., Yang P., Somorjai G.A. Monodisperse platinum nanoparticles of well-defined shape: Synthesis, characterization, catalytic properties and future prospects. Top. Catal. 2006;39:167–174. doi: 10.1007/s11244-006-0053-2. [DOI] [Google Scholar]
- 33.Taurino I., Sanzò G., Antiochia R., Tortolini C., Mazzei F., Favero G., De Micheli G., Carrara S. Recent advances in Third Generation Biosensors based on Au and Pt Nanostructured Electrodes. TrAC Trends Anal. Chem. 2016;79:151–159. doi: 10.1016/j.trac.2016.01.020. [DOI] [Google Scholar]
- 34.Li X., Wei J., Aifantis K.E., Fan Y., Feng Q., Cui F.-Z., Watari F. Current investigations into magnetic nanoparticles for biomedical applications. J. Biomed. Mater. Res. Part A. 2016;104:1285–1296. doi: 10.1002/jbm.a.35654. [DOI] [PubMed] [Google Scholar]
- 35.Haase M., Schäfer H. Upconverting Nanoparticles. Angew. Chem. Int. Ed. Engl. 2011;50:5808–5829. doi: 10.1002/anie.201005159. [DOI] [PubMed] [Google Scholar]
- 36.Heer S., Kömpe K., Güdel H.U., Haase M. Highly Efficient Multicolour Upconversion Emission in Transparent Colloids of Lanthanide-Doped NaYF4 Nanocrystals. Adv. Mater. 2004;16:2102–2105. doi: 10.1002/adma.200400772. [DOI] [Google Scholar]
- 37.Wang F., Deng R., Wang J., Wang Q., Han Y., Zhu H., Chen X., Liu X. Tuning upconversion through energy migration in core–shell nanoparticles. Nat. Mater. 2011;10:968–973. doi: 10.1038/nmat3149. [DOI] [PubMed] [Google Scholar]
- 38.Zhang F. Upconversion Nanoparticles for Biosensing. In: Zhang F., editor. Photon Upconversion Nanomaterials. Springer; Berlin/Heidelberg, Germany: 2015. pp. 255–284. [Google Scholar]
- 39.Achatz D.E., Ali R., Wolfbeis O.S. Luminescent Chemical Sensing, Biosensing, and Screening Using Upconverting Nanoparticles. In: Prodi L., Montalti M., Zaccheroni N., editors. Luminescence Applied in Sensor Science. Springer; Berlin/Heidelberg, Germany: 2011. pp. 29–50. [DOI] [PubMed] [Google Scholar]
- 40.Su Q., Feng W., Yang D., Li F. Resonance Energy Transfer in Upconversion Nanoplatforms for Selective Biodetection. Acc. Chem. Res. 2017;50:32–40. doi: 10.1021/acs.accounts.6b00382. [DOI] [PubMed] [Google Scholar]
- 41.Lakowicz J.R. Principles of Fluorescence Spectroscopy, Third Edition. J. Biomed. Opt. 2008;13:029901. doi: 10.1117/1.2904580. [DOI] [Google Scholar]
- 42.Wang L., Yan R., Huo Z., Wang L., Zeng J., Bao J., Wang X., Peng Q., Li Y. Fluorescence Resonant Energy Transfer Biosensor Based on Upconversion-Luminescent Nanoparticles. Angew. Chem. Int. Ed. Engl. 2005;44:6054–6057. doi: 10.1002/anie.200501907. [DOI] [PubMed] [Google Scholar]
- 43.Wang Y., Bao L., Liu Z., Pang D.-W. Aptamer Biosensor Based on Fluorescence Resonance Energy Transfer from Upconverting Phosphors to Carbon Nanoparticles for Thrombin Detection in Human Plasma. Anal. Chem. 2011;83:8130–8137. doi: 10.1021/ac201631b. [DOI] [PubMed] [Google Scholar]
- 44.Zhang C., Yuan Y., Zhang S., Wang Y., Liu Z. Biosensing Platform Based on Fluorescence Resonance Energy Transfer from Upconverting Nanocrystals to Graphene Oxide. Angew. Chem. Int. Ed. Engl. 2011;50:6851–6854. doi: 10.1002/anie.201100769. [DOI] [PubMed] [Google Scholar]
- 45.Mattsson L., Wegner K.D., Hildebrandt N., Soukka T. Upconverting nanoparticle to quantum dot FRET for homogeneous double-nano biosensors. RSC Adv. 2015;5:13270–13277. doi: 10.1039/C5RA00397K. [DOI] [Google Scholar]
- 46.Wang Y.-F., Liu G.-Y., Sun L.-D., Xiao J.-W., Zhou J.-C., Yan C.-H. Nd3+-Sensitized Upconversion Nanophosphors: Efficient In Vivo Bioimaging Probes with Minimized Heating Effect. ACS Nano. 2013;7:7200–7206. doi: 10.1021/nn402601d. [DOI] [PubMed] [Google Scholar]
- 47.Himmelstoß S.F., Wiesholler L.M., Buchner M., Muhr V., Märkl S., Baeumner A.J., Hirsch T. 980 nm and 808 nm Excitable Upconversion Nanoparticles for the Detection of Enzyme Related Reactions. Proc. SPIE. 2017;10077 doi: 10.1117/12.2252381. [DOI] [Google Scholar]
- 48.Murray C.B., Norris D.J., Bawendi M.G. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993;115:8706–8715. doi: 10.1021/ja00072a025. [DOI] [Google Scholar]
- 49.Park J., Joo J., Kwon S.G., Jang Y., Hyeon T. Synthesis of Monodisperse Spherical Nanocrystals. Angew. Chem. Int. Ed. Engl. 2007;46:4630–4660. doi: 10.1002/anie.200603148. [DOI] [PubMed] [Google Scholar]
- 50.Reiss P., Protière M., Li L. Core/Shell Semiconductor Nanocrystals. Small. 2009;5:154–168. doi: 10.1002/smll.200800841. [DOI] [PubMed] [Google Scholar]
- 51.Dabbousi B.O., Rodriguez-Viejo J., Mikulec F.V., Heine J.R., Mattoussi H., Ober R., Jensen K.F., Bawendi M.G. (CdSe)ZnS Core−Shell Quantum Dots: Synthesis and Characterization of a Size Series of Highly Luminescent Nanocrystallites. J. Phys. Chem. B. 1997;101:9463–9475. doi: 10.1021/jp971091y. [DOI] [Google Scholar]
- 52.Jaiswal J.K., Mattoussi H., Mauro J.M., Simon S.M. Long-term multiple color imaging of live cells using quantum dot bioconjugates. Nat. Biotech. 2003;21:47–51. doi: 10.1038/nbt767. [DOI] [PubMed] [Google Scholar]
- 53.Weller H. Colloidal Semiconductor Q-Particles: Chemistry in the Transition Region between Solid State and Molecules. Angew. Chem. Int. Ed. Engl. 1993;32:41–53. doi: 10.1002/anie.199300411. [DOI] [Google Scholar]
- 54.Geißler D., Charbonnière L.J., Ziessel R.F., Butlin N.G., Löhmannsröben H.-G., Hildebrandt N. Quantum Dot Biosensors for Ultrasensitive Multiplexed Diagnostics. Angew. Chem. Int. Ed. Engl. 2010;49:1396–1401. doi: 10.1002/anie.200906399. [DOI] [PubMed] [Google Scholar]
- 55.Petryayeva E., Algar W.R. Multiplexed Homogeneous Assays of Proteolytic Activity Using a Smartphone and Quantum Dots. Anal. Chem. 2014;86:3195–3202. doi: 10.1021/ac500131r. [DOI] [PubMed] [Google Scholar]
- 56.Algar W.R., Khachatrian A., Melinger J.S., Huston A.L., Stewart M.H., Susumu K., Blanco-Canosa J.B., Oh E., Dawson P.E., Medintz I.L. Concurrent Modulation of Quantum Dot Photoluminescence Using a Combination of Charge Transfer and Förster Resonance Energy Transfer: Competitive Quenching and Multiplexed Biosensing Modality. J. Am. Chem. Soc. 2017;139:363–372. doi: 10.1021/jacs.6b11042. [DOI] [PubMed] [Google Scholar]
- 57.Biju V., Itoh T., Ishikawa M. Delivering quantum dots to cells: Bioconjugated quantum dots for targeted and nonspecific extracellular and intracellular imaging. Chem. Soc. Rev. 2010;39:3031–3056. doi: 10.1039/b926512k. [DOI] [PubMed] [Google Scholar]
- 58.Zhang C.-Y., Yeh H.-C., Kuroki M.T., Wang T.-H. Single-quantum-dot-based DNA nanosensor. Nat. Mater. 2005;4:826–831. doi: 10.1038/nmat1508. [DOI] [PubMed] [Google Scholar]
- 59.Freeman R., Girsh J., Willner I. Nucleic Acid/Quantum Dots (QDs) Hybrid Systems for Optical and Photoelectrochemical Sensing. ACS Appl. Mater. Interfaces. 2013;5:2815–2834. doi: 10.1021/am303189h. [DOI] [PubMed] [Google Scholar]
- 60.Dyadyusha L., Yin H., Jaiswal S., Brown T., Baumberg J.J., Booy F.P., Melvin T. Quenching of CdSe quantum dot emission, a new approach for biosensing. Chem. Commun. 2005:3201–3203. doi: 10.1039/b500664c. [DOI] [PubMed] [Google Scholar]
- 61.Dai Z., Zhang J., Dong Q., Guo N., Xu S., Sun B., Bu Y. Adaption of Au Nanoparticles and CdTe Quantum Dots in DNA Detection. Chin. J. Chem. Eng. 2007;15:791–794. doi: 10.1016/S1004-9541(08)60004-X. [DOI] [Google Scholar]
- 62.Maye M.M., Gang O., Cotlet M. Photoluminescence enhancement in CdSe/ZnS-DNA linked-Au nanoparticle heterodimers probed by single molecule spectroscopy. Chem. Commun. 2010;46:6111–6113. doi: 10.1039/c0cc00660b. [DOI] [PubMed] [Google Scholar]
- 63.So M.-K., Xu C., Loening A.M., Gambhir S.S., Rao J. Self-illuminating quantum dot conjugates for in vivo imaging. Nat. Biotech. 2006;24:339–343. doi: 10.1038/nbt1188. [DOI] [PubMed] [Google Scholar]
- 64.Huang X., Li L., Qian H., Dong C., Ren J. A Resonance Energy Transfer between Chemiluminescent Donors and Luminescent Quantum-Dots as Acceptors (CRET) Angew. Chem. Int. Ed. Engl. 2006;118:5264–5267. doi: 10.1002/ange.200601196. [DOI] [PubMed] [Google Scholar]
- 65.Algar W.R., Tavares A.J., Krull U.J. Beyond labels: A review of the application of quantum dots as integrated components of assays, bioprobes, and biosensors utilizing optical transduction. Anal. Chim. Acta. 2010;673:1–25. doi: 10.1016/j.aca.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 66.Frasco M., Chaniotakis N. Semiconductor Quantum Dots in Chemical Sensors and Biosensors. Sensors. 2009;9:7266–7286. doi: 10.3390/s90907266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Petryayeva E., Algar W.R., Medintz I.L. Quantum Dots in Bioanalysis: A Review of Applications Across Various Platforms for Fluorescence Spectroscopy and Imaging. Appl. Spectrosc. 2013;67:215–252. doi: 10.1366/12-06948. [DOI] [PubMed] [Google Scholar]
- 68.Moro L., Turemis M., Marini B., Ippodrino R., Giardi M.T. Better together: Strategies based on magnetic particles and quantum dots for improved biosensing. Biotechnol. Adv. 2017;35:51–63. doi: 10.1016/j.biotechadv.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 69.Kurt H., Yüce M., Hussain B., Budak H. Dual-excitation upconverting nanoparticle and quantum dot aptasensor for multiplexed food pathogen detection. Biosens. Bioelectron. 2016;81:280–286. doi: 10.1016/j.bios.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 70.Wei H., Ratchford D., Li X., Xu H., Shih C.-K. Propagating Surface Plasmon Induced Photon Emission from Quantum Dots. Nano Lett. 2009;9:4168–4171. doi: 10.1021/nl9023897. [DOI] [PubMed] [Google Scholar]
- 71.Malic L., Sandros M.G., Tabrizian M. Designed Biointerface Using Near-Infrared Quantum Dots for Ultrasensitive Surface Plasmon Resonance Imaging Biosensors. Anal. Chem. 2011;83:5222–5229. doi: 10.1021/ac200465m. [DOI] [PubMed] [Google Scholar]
- 72.Muñoz J., Bastos-Arrieta J., Muñoz M., Muraviev D., Céspedes F., Baeza M. CdS quantum dots as a scattering nanomaterial of carbon nanotubes in polymeric nanocomposite sensors for microelectrode array behavior. J. Mater. Sci. 2016;51:1610–1619. doi: 10.1007/s10853-015-9484-0. [DOI] [Google Scholar]
- 73.Săndulescu R., Tertiş M., Cristea C., Bodoki E. New Materials for the Construction of Electrochemical Biosensors. In: Rinken T., editor. Biosensors—Micro and Nanoscale Applications. InTech; Rijeka, Croatia: 2015. pp. 1–36. [Google Scholar]
- 74.Zahra K., Majid M., Mircea V.D. Main Allotropes of Carbon: A Brief Review. In: Mihai V.P., Marius Constantin M., editors. Sustainable Nanosystems Development, Properties, and Applications. IGI Global; Hershey, PA, USA: 2017. pp. 185–213. [Google Scholar]
- 75.Uslu B., Ozkan S.A. Electroanalytical Application of Carbon Based Electrodes to the Pharmaceuticals. Anal. Lett. 2007;40:817–853. doi: 10.1080/00032710701242121. [DOI] [Google Scholar]
- 76.Tîlmaciu C.-M., Morris M.C. Carbon nanotube biosensors. Front. Chem. 2015;3:59. doi: 10.3389/fchem.2015.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pilehvar S., De Wael K. Recent Advances in Electrochemical Biosensors Based on Fullerene-C60 Nano-Structured Platforms. Biosensors. 2015;5:712–735. doi: 10.3390/bios5040712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Celik N., Balachandran W., Manivannan N. IET Circuits, Devices and Systems. Volume 9. Institution of Engineering and Technology; Stevenage, UK: 2015. Graphene-based biosensors: Methods, analysis and future perspectives; pp. 434–445. [Google Scholar]
- 79.Yáñez-Sedeño P., Campuzano S., Pingarrón J. Carbon Nanostructures for Tagging in Electrochemical Biosensing: A Review. J. Carbon Res. 2017;3:3. doi: 10.3390/c3010003. [DOI] [Google Scholar]
- 80.Shi H., Wei J., Qiang L., Chen X., Meng X. Fluorescent Carbon Dots for Bioimaging and Biosensing Applications. J. Biomed. Nanotechnol. 2014;10:2677–2699. doi: 10.1166/jbn.2014.1881. [DOI] [PubMed] [Google Scholar]
- 81.Bardhan N.M. 30 years of advances in functionalization of carbon nanomaterials for biomedical applications: A practical review. J. Mater. Res. 2016;32:107–127. doi: 10.1557/jmr.2016.449. [DOI] [Google Scholar]
- 82.Muñoz J., Baeza M. Customized Bio-Functionalization of Nanocomposite Carbon Paste Electrodes for Electrochemical Sensing: A Mini Review. Electroanalysis. 2017 doi: 10.1002/elan.201700087. [DOI] [Google Scholar]
- 83.Yang W., Ratinac K.R., Ringer S.P., Thordarson P., Gooding J.J., Braet F. Carbon Nanomaterials in Biosensors: Should You Use Nanotubes or Graphene? Angew. Chem. Int. Ed. Engl. 2010;49:2114–2138. doi: 10.1002/anie.200903463. [DOI] [PubMed] [Google Scholar]
- 84.Pumera M. Graphene in biosensing. Mater. Today. 2011;14:308–315. doi: 10.1016/S1369-7021(11)70160-2. [DOI] [Google Scholar]
- 85.Potočnik J. Commission Recommendation of 18 October 2011 on the definition of nanomaterial. Off. J. Eur. Union. 2011;54:38–40. [Google Scholar]
- 86.Bonaccorso F., Lombardo A., Hasan T., Sun Z., Colombo L., Ferrari A.C. Production and processing of graphene and 2D crystals. Mater. Today. 2012;15:564–589. doi: 10.1016/S1369-7021(13)70014-2. [DOI] [Google Scholar]
- 87.Paton K.R., Varrla E., Backes C., Smith R.J., Khan U., O’Neill A., Boland C., Lotya M., Istrate O.M., King P., et al. Scalable production of large quantities of defect-free few-layer graphene by shear exfoliation in liquids. Nat. Mater. 2014;13:624–630. doi: 10.1038/nmat3944. [DOI] [PubMed] [Google Scholar]
- 88.Coleman J.N. Liquid Exfoliation of Defect-Free Graphene. Acc. Chem. Res. 2012;46:14–22. doi: 10.1021/ar300009f. [DOI] [PubMed] [Google Scholar]
- 89.Hummers W.S., Offeman R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958;80:1339. doi: 10.1021/ja01539a017. [DOI] [Google Scholar]
- 90.Kuila T., Mishra A.K., Khanra P., Kim N.H., Lee J.H. Recent advances in the efficient reduction of graphene oxide and its application as energy storage electrode materials. Nanoscale. 2013;5:52–71. doi: 10.1039/C2NR32703A. [DOI] [PubMed] [Google Scholar]
- 91.Morales-Narváez E., Baptista-Pires L., Zamora-Gálvez A., Merkoçi A. Graphene-Based Biosensors: Going Simple. Adv. Mater. 2017;29 doi: 10.1002/adma.201604905. [DOI] [PubMed] [Google Scholar]
- 92.Campuzano S., Pedrero M., Nikoleli G.P., Pingarrón J.M., Nikolelis D.P. Hybrid 2D-nanomaterials-based electrochemical immunosensing strategies for clinical biomarkers determination. Biosens. Bioelectron. 2017;89:269–279. doi: 10.1016/j.bios.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 93.Pavlidis I.V., Patila M., Bornscheuer U.T., Gournis D., Stamatis H. Graphene-based nanobiocatalytic systems: Recent advances and future prospects. Trends Biotechnol. 2014;32:312–320. doi: 10.1016/j.tibtech.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 94.Hong W., Bai H., Xu Y., Yao Z., Gu Z., Shi G. Preparation of Gold Nanoparticle/Graphene Composites with Controlled Weight Contents and Their Application in Biosensors. J. Phys. Chem. C. 2010;114:1822–1826. doi: 10.1021/jp9101724. [DOI] [Google Scholar]
- 95.Dey R.S., Raj C.R. Development of an Amperometric Cholesterol Biosensor Based on Graphene−Pt Nanoparticle Hybrid Material. J. Phys. Chem. C. 2010;114:21427–21433. doi: 10.1021/jp105895a. [DOI] [Google Scholar]
- 96.Claussen J.C., Kumar A., Jaroch D.B., Khawaja M.H., Hibbard A.B., Porterfield D.M., Fisher T.S. Nanostructuring Platinum Nanoparticles on Multilayered Graphene Petal Nanosheets for Electrochemical Biosensing. Adv. Funct. Mater. 2012;22:3399–3405. doi: 10.1002/adfm.201200551. [DOI] [Google Scholar]
- 97.Borisova B., Sánchez A., Jiménez-Falcao S., Martín M., Salazar P., Parrado C., Pingarrón J.M., Villalonga R. Reduced graphene oxide-carboxymethylcellulose layered with platinum nanoparticles/PAMAM dendrimer/magnetic nanoparticles hybrids. Application to the preparation of enzyme electrochemical biosensors. Sens. Actuators B Chem. 2016;232:84–90. doi: 10.1016/j.snb.2016.02.106. [DOI] [Google Scholar]
- 98.Loaiza O.A., Lamas-Ardisana P.J., Añorga L., Jubete E., Ruiz V., Borghei M., Cabañero G., Grande H.J. Graphitized carbon nanofiber–Pt nanoparticle hybrids as sensitive tool for preparation of screen printing biosensors. Detection of lactate in wines and ciders. Bioelectrochemistry. 2015;101:58–65. doi: 10.1016/j.bioelechem.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 99.Vanegas D.C., Taguchi M., Chaturvedi P., Burrs S., Tan M., Yamaguchi H., McLamore E.S. A comparative study of carbon-platinum hybrid nanostructure architecture for amperometric biosensing. Analyst. 2014;139:660–667. doi: 10.1039/C3AN01718D. [DOI] [PubMed] [Google Scholar]
- 100.Zeng Q., Cheng J.-S., Liu X.-F., Bai H.-T., Jiang J.-H. Palladium nanoparticle/chitosan-grafted graphene nanocomposites for construction of a glucose biosensor. Biosens. Bioelectron. 2011;26:3456–3463. doi: 10.1016/j.bios.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 101.Halder A., Zhang M., Chi Q. Electrocatalytic Applications of Graphene–Metal Oxide Nanohybrid Materials. In: Norena L.E., Wang J.-A., editors. Advanced Catalytic Materials—Photocatalysis and Other Current Trends. InTech; Rijeka, Croatia: 2016. [DOI] [Google Scholar]
- 102.Li F., Huang Y., Yang Q., Zhong Z., Li D., Wang L., Song S., Fan C. A graphene-enhanced molecular beacon for homogeneous DNA detection. Nanoscale. 2010;2:1021–1026. doi: 10.1039/b9nr00401g. [DOI] [PubMed] [Google Scholar]
- 103.Dong H., Gao W., Yan F., Ji H., Ju H. Fluorescence Resonance Energy Transfer between Quantum Dots and Graphene Oxide for Sensing Biomolecules. Anal. Chem. 2010;82:5511–5517. doi: 10.1021/ac100852z. [DOI] [PubMed] [Google Scholar]
- 104.Ma H., Wu D., Cui Z., Li Y., Zhang Y., Du B., Wei Q. Graphene-Based Optical and Electrochemical Biosensors: A Review. Anal. Lett. 2012;46:1–17. doi: 10.1080/00032719.2012.706850. [DOI] [Google Scholar]
- 105.Morales-Narváez E., Merkoçi A. Graphene Oxide as an Optical Biosensing Platform. Adv. Mater. 2012;24:3298–3308. doi: 10.1002/adma.201200373. [DOI] [PubMed] [Google Scholar]
- 106.Morales-Narvaez E., Perez-Lopez B., Pires L.B., Merkoci A. Simple Forster resonance energy transfer evidence for the ultrahigh quantum dot quenching efficiency by graphene oxide compared to other carbon structures. Carbon. 2012;50:2987–2993. doi: 10.1016/j.carbon.2012.02.081. [DOI] [Google Scholar]
- 107.He S., Song B., Li D., Zhu C., Qi W., Wen Y., Wang L., Song S., Fang H., Fan C. A Graphene Nanoprobe for Rapid, Sensitive, and Multicolor Fluorescent DNA Analysis. Adv. Funct. Mater. 2010;20:453–459. doi: 10.1002/adfm.200901639. [DOI] [Google Scholar]
- 108.Morales-Narváez E., Hassan A.-R., Merkoçi A. Graphene Oxide as a Pathogen-Revealing Agent: Sensing with a Digital-Like Response. Angew. Chem. Int. Ed. Engl. 2013;52:13779–13783. doi: 10.1002/anie.201307740. [DOI] [PubMed] [Google Scholar]
- 109.Morales-Narváez E., Golmohammadi H., Naghdi T., Yousefi H., Kostiv U., Horák D., Pourreza N., Merkoçi A. Nanopaper as an Optical Sensing Platform. ACS Nano. 2015;9:7296–7305. doi: 10.1021/acsnano.5b03097. [DOI] [PubMed] [Google Scholar]
- 110.Islam M.S., Kouzani A.Z. Variable Incidence Angle Localized Surface Plasmon Resonance Graphene Biosensor; Proceedings of the 2011 IEEE/ICME International Conference on Complex Medical Engineering; Harbin, China. 22–25 May 2011; pp. 58–63. [Google Scholar]
- 111.Wu L., Chu H.S., Koh W.S., Li E.P. Highly sensitive graphene biosensors based on surface plasmon resonance. Opt. Express. 2010;18:14395–14400. doi: 10.1364/OE.18.014395. [DOI] [PubMed] [Google Scholar]
- 112.Bruna M., Borini S. Optical constants of graphene layers in the visible range. Appl. Phys. Lett. 2009;94:031901. doi: 10.1063/1.3073717. [DOI] [Google Scholar]
- 113.Jacek G., Dawn T.H.T. Graphene-based waveguide integrated dielectric-loaded plasmonic electro-absorption modulators. Nanotechnology. 2013;24:185202. doi: 10.1088/0957-4484/24/18/185202. [DOI] [PubMed] [Google Scholar]
- 114.Singh M., Holzinger M., Tabrizian M., Winters S., Berner N.C., Cosnier S., Duesberg G.S. Non-covalently functionalized monolayer graphene for sensitivity enhancement of surface plasmon resonance immunosensors. J. Am. Chem. Soc. 2015;137:2800–2803. doi: 10.1021/ja511512m. [DOI] [PubMed] [Google Scholar]
- 115.Zeng S., Hu S., Xia J., Anderson T., Dinh X.-Q., Meng X.-M., Coquet P., Yong K.-T. Graphene–MoS2 hybrid nanostructures enhanced surface plasmon resonance biosensors. Sens. Actuators B Chem. 2015;207:801–810. doi: 10.1016/j.snb.2014.10.124. [DOI] [Google Scholar]
- 116.Battigelli A., Ménard-Moyon C., Da Ros T., Prato M., Bianco A. Endowing carbon nanotubes with biological and biomedical properties by chemical modifications. Adv. Drug Deliv. Rev. 2013;65:1899–1920. doi: 10.1016/j.addr.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 117.Le Goff A., Holzinger M., Cosnier S. Enzymatic biosensors based on SWCNT-conducting polymer electrodes. Analyst. 2011;136:1279–1287. doi: 10.1039/c0an00904k. [DOI] [PubMed] [Google Scholar]
- 118.Wang J. Carbon-Nanotube Based Electrochemical Biosensors: A Review. Electroanalysis. 2005;17:7–14. doi: 10.1002/elan.200403113. [DOI] [Google Scholar]
- 119.Ménard-Moyon C., Kostarelos K., Prato M., Bianco A. Functionalized Carbon Nanotubes for Probing and Modulating Molecular Functions. Chem. Biol. 2010;17:107–115. doi: 10.1016/j.chembiol.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 120.Zhu N., Chang Z., He P., Fang Y. Electrochemical DNA biosensors based on platinum nanoparticles combined carbon nanotubes. Anal. Chim. Acta. 2005;545:21–26. doi: 10.1016/j.aca.2005.04.015. [DOI] [Google Scholar]
- 121.Wu B., Ou Z., Ju X., Hou S. Carbon Nanotubes/Gold Nanoparticles Composite Film for the Construction of a Novel Amperometric Choline Biosensor. J. Nanomater. 2011;2011:464919. doi: 10.1155/2011/464919. [DOI] [Google Scholar]
- 122.Zhang H., Meng Z., Wang Q., Zheng J. A novel glucose biosensor based on direct electrochemistry of glucose oxidase incorporated in biomediated gold nanoparticles–carbon nanotubes composite film. Sens. Actuators B Chem. 2011;158:23–27. doi: 10.1016/j.snb.2011.04.057. [DOI] [Google Scholar]
- 123.Wu B.-Y., Hou S.-H., Yin F., Zhao Z.-X., Wang Y.-Y., Wang X.-S., Chen Q. Amperometric glucose biosensor based on multilayer films via layer-by-layer self-assembly of multi-wall carbon nanotubes, gold nanoparticles and glucose oxidase on the Pt electrode. Biosens. Bioelectron. 2007;22:2854–2860. doi: 10.1016/j.bios.2006.11.028. [DOI] [PubMed] [Google Scholar]
- 124.Yang M., Jiang J., Yang Y., Chen X., Shen G., Yu R. Carbon nanotube/cobalt hexacyanoferrate nanoparticle-biopolymer system for the fabrication of biosensors. Biosens. Bioelectron. 2006;21:1791–1797. doi: 10.1016/j.bios.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 125.Yang M., Yang Y., Liu Y., Shen G., Yu R. Platinum nanoparticles-doped sol–gel/carbon nanotubes composite electrochemical sensors and biosensors. Biosens. Bioelectron. 2006;21:1125–1131. doi: 10.1016/j.bios.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 126.Hwa K.-Y., Subramani B. Synthesis of zinc oxide nanoparticles on graphene–carbon nanotube hybrid for glucose biosensor applications. Biosens. Bioelectron. 2014;62:127–133. doi: 10.1016/j.bios.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 127.Kumar S., Ahlawat W., Kumar R., Dilbaghi N. Graphene, carbon nanotubes, zinc oxide and gold as elite nanomaterials for fabrication of biosensors for healthcare. Biosens. Bioelectron. 2015;70:498–503. doi: 10.1016/j.bios.2015.03.062. [DOI] [PubMed] [Google Scholar]
- 128.Kroto H.W., Heath J.R., O’Brien S.C., Curl R.F., Smalley R.E. C60: Buckminsterfullerene. Nature. 1985;318:162–163. doi: 10.1038/318162a0. [DOI] [Google Scholar]
- 129.Chlistunoff J., Cliffel D., Bard A.J. Electrochemistry of fullerene films. Thin Solid Films. 1995;257:166–184. doi: 10.1016/0040-6090(94)05703-6. [DOI] [Google Scholar]
- 130.Afreen S., Muthoosamy K., Manickam S., Hashim U. Functionalized fullerene (C60) as a potential nanomediator in the fabrication of highly sensitive biosensors. Biosens. Bioelectron. 2015;63:354–364. doi: 10.1016/j.bios.2014.07.044. [DOI] [PubMed] [Google Scholar]
- 131.Han J., Zhuo Y., Chai Y., Yuan R., Xiang Y., Zhu Q., Liao N. Multi-labeled functionalized C60 nanohybrid as tracing tag for ultrasensitive electrochemical aptasensing. Biosens. Bioelectron. 2013;46:74–79. doi: 10.1016/j.bios.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 132.Li Y., Fang L., Cheng P., Deng J., Jiang L., Huang H., Zheng J. An electrochemical immunosensor for sensitive detection of Escherichia coli O157:H7 using C60 based biocompatible platform and enzyme functionalized Pt nanochains tracing tag. Biosens. Bioelectron. 2013;49:485–491. doi: 10.1016/j.bios.2013.06.008. [DOI] [PubMed] [Google Scholar]
- 133.Pan N.-Y., Shih J.-S. Piezoelectric crystal immunosensors based on immobilized fullerene C60-antibodies. Sens. Actuators B Chem. 2004;98:180–187. doi: 10.1016/j.snb.2003.09.034. [DOI] [Google Scholar]
- 134.Zhuo Y., Ma M.-N., Chai Y.-Q., Zhao M., Yuan R. Amplified electrochemiluminescent aptasensor using mimicking bi-enzyme nanocomplexes as signal enhancement. Anal. Chim. Acta. 2014;809:47–53. doi: 10.1016/j.aca.2013.09.060. [DOI] [PubMed] [Google Scholar]
- 135.Xu X., Ray R., Gu Y., Ploehn H.J., Gearheart L., Raker K., Scrivens W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004;126:12736–12737. doi: 10.1021/ja040082h. [DOI] [PubMed] [Google Scholar]
- 136.Xiao A., Wang C., Chen J., Guo R., Yan Z., Chen J. Carbon and Metal Quantum Dots toxicity on the microalgae Chlorella pyrenoidosa. Ecotoxicol. Environ. Saf. 2016;133:211–217. doi: 10.1016/j.ecoenv.2016.07.026. [DOI] [PubMed] [Google Scholar]
- 137.Zheng X.T., Ananthanarayanan A., Luo K.Q., Chen P. Glowing Graphene Quantum Dots and Carbon Dots: Properties, Syntheses, and Biological Applications. Small. 2015;11:1620–1636. doi: 10.1002/smll.201402648. [DOI] [PubMed] [Google Scholar]
- 138.Baker S.N., Baker G.A. Luminescent Carbon Nanodots: Emergent Nanolights. Angew. Chem. Int. Ed. Engl. 2010;49:6726–6744. doi: 10.1002/anie.200906623. [DOI] [PubMed] [Google Scholar]
- 139.Liu H., Ye T., Mao C. Fluorescent Carbon Nanoparticles Derived from Candle Soot. Angew. Chem. Int. Ed. Engl. 2007;46:6473–6475. doi: 10.1002/anie.200701271. [DOI] [PubMed] [Google Scholar]
- 140.Wang Y., Hu A. Carbon quantum dots: Synthesis, properties and applications. J. Mater. Chem. C. 2014;2:6921–6939. doi: 10.1039/C4TC00988F. [DOI] [Google Scholar]
- 141.Lim S.Y., Shen W., Gao Z. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015;44:362–381. doi: 10.1039/C4CS00269E. [DOI] [PubMed] [Google Scholar]
- 142.Bu D., Zhuang H., Yang G., Ping X. An immunosensor designed for polybrominated biphenyl detection based on fluorescence resonance energy transfer (FRET) between carbon dots and gold nanoparticles. Sens. Actuators B Chem. 2014;195:540–548. doi: 10.1016/j.snb.2014.01.079. [DOI] [Google Scholar]
- 143.Li H., Zhang Y., Wang L., Tian J., Sun X. Nucleic acid detection using carbon nanoparticles as a fluorescent sensing platform. Chem. Commun. 2011;47:961–963. doi: 10.1039/C0CC04326E. [DOI] [PubMed] [Google Scholar]
- 144.Kim J.E., Choi J.H., Colas M., Kim D.H., Lee H. Gold-based hybrid nanomaterials for biosensing and molecular diagnostic applications. Biosens. Bioelectron. 2016;80:543–559. doi: 10.1016/j.bios.2016.02.015. [DOI] [PubMed] [Google Scholar]