Abstract
Mitochondria are increasingly recognized as key players in genetic and acquired renal diseases. Most mitochondrial cytopathies that cause renal symptoms are characterized by tubular defects, but glomerular, tubulointerstitial and cystic diseases have also been described. For example, defects in coenzyme Q10 (CoQ10) biosynthesis and the mitochondrial DNA 3243 A>G mutation are important causes of focal segmental glomerulosclerosis in children and in adults, respectively. Although they sometimes present with isolated renal findings, mitochondrial diseases are frequently associated with symptoms related to central nervous system and neuromuscular involvement. They can result from mutations in nuclear genes that are inherited according to classic Mendelian rules or from mutations in mitochondrial DNA, which are transmitted according to more complex rules of mitochondrial genetics. Diagnosis of mitochondrial disorders involves clinical characterization of patients in combination with biochemical and genetic analyses. In particular, prompt diagnosis of CoQ10 biosynthesis defects is imperative because of their potentially reversible nature. In acute kidney injury (AKI), mitochondrial dysfunction contributes to the physiopathology of tissue injury, whereas mitochondrial biogenesis has an important role in the recovery of renal function. Potential therapies that target mitochondrial dysfunction or promote mitochondrial regeneration are being developed to limit renal damage during AKI and promote repair of injured tissue.
Mitochondria are key organelles that have an essential role in the life and death of cells. They derive from ancient Gram-negative bacteria similar to Rickettsia prowazekii, which began an endosymbiotic process with progenitors of eukaryotic cells more than 2 billion years ago1. Mitochondria have retained the structure of these bacteria; they have a highly permeable outer membrane and an inner membrane that is impermeable to most solutes and has several introflexions, named cristae2. Most importantly, mitochondria contain their own mitochondrial (mt)DNA, which is a relic of the ancestral endosymbiont genome and resembles prokaryotic DNA. The mitochondrial genome is composed of a single double-stranded circular loop that lacks introns, is not organized into chromatin and uses a different genetic code from that of eukaryotic cells3. mtDNA is present in multiple copies per mitochondrion, and transmission of mtDNA-linked traits does not follow classical Mendelian rules4 (TABLE 1). mtDNA encodes 37 genes, including 13 structural subunits of the mitochondrial respiratory chain. Proteins encoded by mtDNA represent only a small fraction of the total mitochondrial proteome; the majority of mitochondrial proteins are synthesized in the cytosol and imported into mitochondria via specialized systems5.
Table 1.
Characteristics of mitochondrial genetics
| Characteristic | Description |
|---|---|
| Maternal inheritance | Both genders might be affected by mtDNA mutations, but only females transmit mutations to their children |
| Heteroplasmy | Wild type and mutant mtDNA molecules can coexist in different proportions within cells of the same tissue or in different tissues of the same individual |
| Threshold effect | A mutation must affect a critical proportion (usually >70%) of the total mtDNA molecules within a cell or tissue to cause a biochemical effect resulting in a clinical phenotype |
| Random drift | Mutant and wild type mtDNA molecules segregate randomly in daughter cells during each cell division |
mtDNA, mitochondrial DNA.
The most important function of mitochondria is the generation of ATP through oxidative phosphorylation. They are also the site of essential metabolic pathways (including pyrimidine and haem biosynthesis as well as specific reactions of the urea cycle and the β-oxidation pathway) and they have key roles in thermogenesis, calcium homeostasis and control of the intrinsic apoptotic pathway6. Mitochondria are highly dynamic organelles; a complex mechanism of fusion and fission processes regulates the remodeling of cristae, which are essential for cytochrome c release and for the initiation of apoptosis7. They also have an essential role in tissue injury and repair processes, and have been implicated in various types of renal disorders, both inherited and acquired. In this Review we discuss the main types of mitochondrial cytopathies that can result in renal disease as well as the role of mitochondrial dysfunction in acute kidney injury (AKI).
The mitochondrial respiratory chain
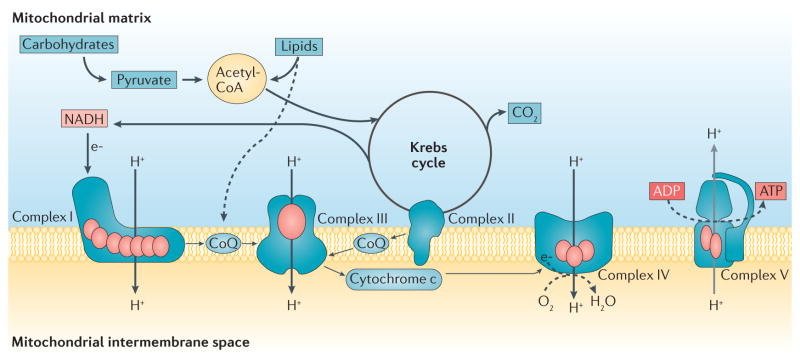
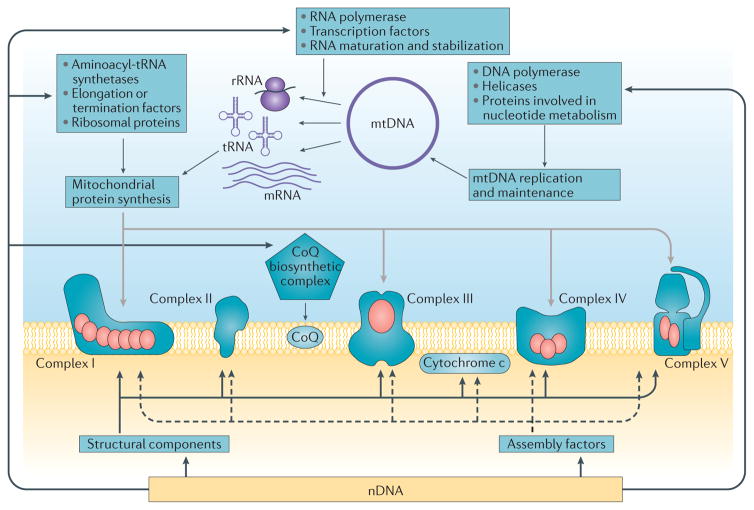
The mitochondrial respiratory chain is comprised of five enzymatic complexes and two electron carriers: coenzyme Q10 (CoQ10) and cytochrome c. Complexes I, II, III and IV transfer electrons from high-energy compounds generated by the reactions of the Krebs cycle (that is, NADH and FADH2), to molecular oxygen, and utilize the energy produced by these reactions to transfer protons from the mitochondrial matrix to the intermembrane space. These processes create an electrochemical gradient that is used by ATP synthase (also known as complex V) to synthesize ATP (FIG. 1). The respiratory chain complexes I, III and IV assemble to form super-complexes, which optimize electron flow and minimize the formation of reactive oxygen species (ROS)8. With the exception of complex II, which contains only nuclear (n) DNA-encoded subunits, the respiratory chain complexes are comprised of both mtDNA-encoded and nDNA-encoded subunits. Biogenesis of the respiratory chain also requires a large set of ancillary genes, which are encoded by nDNA. These assembly factors are necessary to import and direct structural subunits to the mitochondrial inner membrane, to stabilize assembly intermediates, and to synthesize and insert prosthetic groups into the holo-enzymes9. A large number of other nuclear gene products are required for the replication and maintenance of the mitochondrial genome, transcription and processing of mitochondrial RNA species, and synthesis of mitochondrial proteins10 (FIG. 2).
Figure 1. Mitochondrial energy metabolism and the respiratory chain.
Acetyl-coenzyme A (Acetyl-CoA) is the terminal product of carbohydrate and lipid metabolism, and is oxidized through the reactions of the Krebs cycle to produce CO2. The high energy electrons (e−) produced by these reactions enter the respiratory chain and eventually reduce molecular oxygen (02) to form water (H20). The energy released by this process is used to pump protons (H+) across the mitochondrial inner membrane and generate the electrochemical gradient that enables complex V to synthesize ATP. The red ovals represent mitochondrial DNA-encoded subunits of the respiratory chain complexes. CoQ, coenzyme Q.
Figure 2. Interplay of mitochondrial and nuclear genes in the biogenesis of the respiratory chain.
Mitochondrial (mt) DNA encodes 13 structural subunits of the respiratory chain complexes (red ovals) as well as two ribosomal (r)RNAs and 22 transfer (t)RNAs that are required for mitochondrial protein synthesis. Nuclear (n)DNA encodes all the other structural subunits of the respiratory chain complexes, cytochrome c, assembly factors, the enzymes required for coenzyme Q (CoQ) biosynthesis and proteins involved in mtDNA replication and maintenance and in mitochondrial protein synthesis.
Key points.
Healthy mitochondria are essential for normal kidney function; mitochondrial cytopathies can result in renal disease and mitochondrial damage has a role in the pathophysiology of acute kidney injury (AKI)
Although mitochondrial diseases are characterized by maternal inheritance, many mitochondrial disorders are caused by mutations in nuclear genes and are inherited according to classic Mendelian rules
Most mitochondrial diseases with kidney involvement cause tubular defects; however, mutations in the coenzyme Q10 (CoQ10) biosynthesis pathway and the mtDNA 3243 A>G mutation primarily cause glomerular disease
Diagnosis of genetic mitochondrial disorders increasingly relies on new sequencing techniques, but thorough biochemical and clinical characterization of patients is essential to guide these analyses
In AKI, mitochondrial dysfunction occurs primarily in the proximal tubule and participates in the physiopathology of tissue damage; mitochondrial biogenesis represents a crucial step in the recovery phase
Potential therapies that target mitochondrial dynamics, mitophagy and/or mitochondrial biogenesis might limit renal damage during AKI and promote recovery of kidney function
The electron carrier CoQ10 is a small lipophilic molecule comprising a quinone group and an isoprene tail. In the respiratory chain, coenzyme Q shuttles electrons from complexes I and II to complex III. CoQ10 is also a key antioxidant, a modulator of apoptosis, and a cofactor for several other dehydrogenases. Biosynthesis of CoQ10 requires at least 13 proteins (encoded by nuclear COQ genes)11, which are assembled into a multi-enzyme complex localized in the mitochondrial matrix12. Cytochrome c is synthesized in the cytosol, imported by a non-canonical mechanism into the mitochondrial intermembrane space and covalently bound to a haem group by holocytochrome c-type synthase.13 The main role of cytochrome c is the transfer of electrons from complex III to complex IV of the respiratory chain, but this small protein is also an essential component of the intrinsic apoptotic pathway.
Genetic mitochondrial defects
Mitochondrial dysfunction is a common finding in many pathological conditions and might be the direct consequence of a specific genetic defect or the result of a variety of environmental noxae. Although in principle, the term ‘mitochondrial disorder’ should be used to indicate any defect affecting mitochondrial enzymes or structural proteins, in clinical practice this term is generally used to indicate defects that directly or indirectly affect mitochondrial oxidative phosphorylation14. Genetic diseases involving oxidative phosphorylation can be caused by defects in mtDNA (with maternal inheritance or through de novo mutations) or in nDNA (associated with classic Mendelian genetics; TABLE 2). A third group of disorders includes mtDNA anomalies that are secondary to defects in nuclear genes controlling mtDNA maintenance10. Even if these disorders are associated with abnormalities in mtDNA, they are transmitted as autosomal dominant or recessive diseases10. Disease-causing mutations that result in defects in oxidative phosphorylation have been reported in >100 genes15. Although each individual defect is rare, the overall prevalence of mitochondrial disorders in the general population is probably greater than 1 in 5,000 (REF. 16).
Table 2.
Genetic defects that impair mitochondrial function
| Defect | Description and examples |
|---|---|
| mtDNA | |
| Mitochondrial protein synthesis | Point mutations or gross rearrangements in mtDNA usually impair mitochondrial protein synthesis as a whole, resulting in combined defects of several respiratory chain complexes10; complex II is spared, as only nuclear genes encode this complex Many point mutations affect genes that encode tRNAs or rRNAs The most frequent gross rearrangement is the recurrent 4,977 base pair ‘common’ deletion142 The renal phenotype is usually tubulopathy; glomerular defects are associated with the mtDNA 3243 A>G mutation |
| Structural subunits | Mutations in mtDNA-encoded structural subunits usually cause defects in individual respiratory chain complexes |
| nDNA | |
| Assembly factors | Assembly factors are not part of the respiratory chain, but are necessary for its biogenesis; mutations in genes that encode assembly factors are usually referred to as ‘indirect hits’ Some assembly factors are required for specific steps in the biogenesis of individual complexes (for example COX10 encodes a farnesyltransferase that is involved in the biosynthesis of the heme group of complex IV143), whereas others have shared functions and their mutations consequently affect multiple complexes, often including complex II (for example, mutations in LYRM4, which is required for the synthesis of iron–sulphur clusters, impair the activities of complexes I, II and III144) |
| Electron carriers | Mutations in genes that are required for CoQ10 biosynthesis cause primary CoQ10 deficiency Secondary CoQ10 deficiency is associated with mutations in nDNA genes that are not directly involved in CoQ10 biosynthesis, such as APTX and ETFDH, as well as with several mtDNA defects89; therefore, a reduction in CoQ10 levels does not necessarily indicate a mutation in a COQ gene Secondary forms of CoQ10 deficiency are probably much more frequent than primary forms Mutations in cytochrome c are transmitted as autosomal dominant traits and associated with familial thrombocytopenia; the disease pathogenesis results from deregulation of apoptosis rather than the bioenergetic defect |
| Mitochondrial dynamics | The most common defects in genes that control mitochondrial dynamics involve the profusion proteins OPA1 and mitofusin-2 In addition to promoting mitochondrial fusion, OPA1 is essential for the control of cristae remodelling and the formation of supercomplexes145, which increase the efficiency of oxidative phosphorylation Mitofusin-2 tethers mitochondria to the endoplasmic reticulum; this process is probably important for calcium metabolism146 |
| Mitochondrial protein synthesis | Genes that are involved in mitochondrial protein synthesis include those that encode aminoacyl–tRNA synthases, mitochondrial ribosomal proteins, elongation factors, proteins that are involved in the maturation of mRNAs and tRNAs, and other components of the translation machinery10 Mutations in these genes typically result in combined defects that spare complex II |
| mtDNA maintenance | Mutations in genes that encode proteins involved in mtDNA replication or nucleotide metabolism cause secondary mtDNA defects, including depletion (reduction in copy number), multiple deletions (resulting in the tissue-specific presence of mtDNA species with various types of gross deletions) and specific point mutations147 Defects in mtDNA maintenance are usually associated with combined deficiencies Some defects alter the lipid milieu of the mitochondrial inner membrane and indirectly impair oxidative phosphorylation15; for example, TAZ mutations result in in Barth syndrome, which is characterized by abnormal cardiolipin metabolism OPA1 and mitofusin-2 are also required for mtDNA maintenance and their deficiencies cause mtDNA depletion by unclear mechanisms145 |
| Structural subunits | Nuclear gene defects might directly involve structural subunits of individual complexes, most commonly complex I |
CoQ10, coenzyme Q10; mRNA, messenger RNA; mtDNA, mitochondrial DNA; nDNA, nuclear DNA; OPA1, optic atrophy 1 (also known as dynamin-like 120 kDa protein, mitochondrial); tRNA, transfer RNA.
In general, mtDNA mutations are heteroplasmic, whereas polymorphisms are homoplasmic (that is, they affect all mitochondrial genomes in an individual). A few examples of homoplasmic mutations also exist, such as the three common mutations that are associated with Leber hereditary optic neuropathy17 and the mtDNA 1555A>G mutation in the 12S rRNA gene, which causes deafness after exposure to aminoglycosides18. These mutations usually have a fairly mild phenotype with selective tissue involvement (either the optic nerve or the cochlea) and their expression is modulated by specific mtDNA haplogroups, nuclear background and epigenetic factors18.
As complete disruption of oxidative phosphorylation is not compatible with life, residual activity is always present, either because mutations are hypomorphic19 or redundancy in the system enables a minimal number of functional complexes to be assembled. In the case of mtDNA, heteroplasmy ensures that a small amount of wild-type mitochondrial genome is always expressed, enabling the synthesis of a minimal amount of functional respiratory chain complexes to sustain extra-uterine life. Notably, cells with high turnover (such as haematopoietic cell precursors) express lower levels of mutated mtDNA than those with low turnover (such as skeletal muscle and possibly renal cells) as a result of the natural selection of cells with higher percentages of wild-type mtDNA, which replicate more efficiently20. Renal disease has been reported in patients with genetic defects involving assembly factors, CoQ10 biosynthesis, mtDNA translation and mtDNA maintenance (TABLE 3).
Table 3.
Nuclear gene defects that affect respiratory chain biogenesis
| Category | Gene | Renal phenotype | Refs |
|---|---|---|---|
| Structural subunits | None yet described | NA | NA |
|
| |||
| Electron carriers (including CoQ biosynthesis) | PDSS1 | SRNS | 73 |
| PDSS2 | SRNS | 72 | |
| COQ2 | SRNS | 71 | |
| COQ6 | SRNS | 68 | |
| ADCK4 | SRNS | 69 | |
| COQ9 | Tubulopathy | 44 | |
|
| |||
| Assembly factors | COX10 | Tubulopathy | 148 |
| SURF1 | Tubulopathy* | 149 | |
| BCS1L | Tubulopathy | 150 | |
| UQCC2 | Tubulopathy | 151 | |
| TMEM70 | Tubulopathy* | 152 | |
|
| |||
| mtDNA translation | MRPS22 | Tubulopathy | 153 |
| YARS2 | Tubulopathy | 154 | |
| SARS2 | Tubulopathy | 155 | |
|
| |||
| mtDNA maintenance | RRM2B | Tubulopathy | 156 |
| TWINKLE | Tubulopathy | 157 | |
| MPV17 | Tubulopathy | 158 | |
| SUCLA2 | Tubulopathy* | 159 | |
| DGUOK | Tubulopathy* | 160 | |
|
| |||
| Lipid milieu | None yet described | NA | NA |
|
| |||
| Mitochondrial dynamics | None yet described | NA | NA |
CoQ, coenzyme Q; mtDNA, mitochondrial DNA; NA, not applicable; SRNS, steroid-resistant nephrotic syndrome.
Occasional manifestation.
Clinical features of mitochondrial diseases
In general, defects in oxidative phosphorylation produce two major effects: a reduction in ATP production and an increase in ROS production. A direct relationship between the magnitudes of these effects is not always present; for example, mild CoQ10 deficiency can result in a substantial increase in ROS production without significantly impairing ATP production, whereas severe CoQ10 deficiency causes an important bioenergetic defect without a substantial increase in ROS production21. Defects in electron carriers also affect apoptosis because cytochrome c and CoQ10 have important roles in this process. CoQ10 is a modulator of the mitochondrial permeability transition pore and acts as an antiapoptotic factor, whereas mutations in cytochrome c cause deregulation of apoptosis, which is more clinically relevant than the associated bioenergetic defect21–23.
Not surprisingly, the tissues that are most severely affected by defects in oxidative phosphorylation are those that are most reliant on aerobic metabolism for ATP production, such as the central nervous system and skeletal muscle. The majority of mitochondrial disorders, therefore, present with some degree of encephalomyopathy10. However, given the ubiquitous distribution of mitochondria, virtually all tissues and organs might be affected by mitochondrial diseases (TABLE 4).
Table 4.
Mitochondrial disorders: non renal effects
| Organ or system | Common manifestation |
|---|---|
| Endocrine system | Diabetes mellitus |
| Hypoparathyroidism | |
|
| |
| Gastrointestinal tract | Intestinal dysmotility |
| Pseudo-obstruction | |
| Malabsorption | |
|
| |
| Heart | Hypertrophic (rarely dilated) cardiomyopathy |
| Conduction defects | |
|
| |
| Hematologic system | Sideroblastic anaemia |
| Thrombocytopenia | |
| Neutropenia | |
|
| |
| Liver | Liver failure |
|
| |
| Nervous system | Psychomotor retardation and/or regression |
| Dementia | |
| Seizures | |
| Myoclonus | |
| Migrane | |
| Ataxia | |
| Spasticity | |
| Dystonia | |
| Stroke-like episodes | |
| Leukoencephalopathy | |
| Peripheral neuropathy | |
|
| |
| Sensory system | Deafness |
| Blindness | |
| Optic nerve atrophy | |
| Retinitis pigmentosa | |
| Cataracts | |
| Progressive external ophthalmoplegia | |
|
| |
| Skeletal muscles | Muscle weakness |
| Exercise intolerance | |
| Myoglobinuria | |
In the kidneys, mitochondrial disorders can result in various forms of tubulopathies, tubulointerstitial nephritis, cystic renal disease or glomerular disease, most commonly focal segmental glomerulosclerosis (FSGS)20. Renal symptoms are rarely isolated and commonly form part of a multisystemic disorder. Exceptions include some mtDNA mutations and some cases of CoQ10 deficiency in which renal dysfunction might be the only clinical manifestation at presentation24. In general, the coexistence of neuromuscular symptoms and renal defects should raise suspicion of a mitochondrial defect25. Some symptoms, such as sensorineural deafness or cardiomyopathy, might remain subclinical and require systematic testing. Specific skin and hair lesions have also been described26. The first symptoms of mitochondrial defects develop within the first weeks of life in approximately one-third of patients; more than 80% of patients are symptomatic by the age of 2 years27.
Renal tubular disorders
After the brain, the kidneys have the highest oxygen consumption per dry weight of tissue, owing to the intense reabsorption and excretion processes that occur in the renal tubules, particularly in cells of the proximal tubule, distal convoluted tubule and connecting segments, which are very rich in mitochondria. Most of the chemical gradients necessary to reabsorb and excrete solutes from the crude glomerular filtrate arise from the basolateral Na-K ATPase. In intact kidneys, approximately 1 mmol of oxygen is estimated to be required for the reabsorption of 20–30 mEq of sodium28,29. In addition to glucose, the kidney oxidizes fatty acids and amino acids to meet this constant metabolic demand.
Unsurprisingly, many mitochondrial disorders are characterized by various degrees of tubular dysfunction. The most severe form of tubulopathy is complete Fanconi syndrome with low-molecular-weight proteinuria, reflecting global dysfunction of the proximal epithelial cells, which can be associated with more distal tubular defects20,27,30–36. Fanconi syndrome has also been reported in children with specific mitochondrial syndromes, including Kearns-Sayre syndrome, Pearson syndrome, Leigh encephalopathy and CoQ10 deficiency20,27,30,31,37–46. More frequently, patients present with partial defects, including isolated renal tubular acidosis (RTA), aminoaciduria, glycosuria or a combination of the above20,27,45,47–51. In some children, a Bartter-like phenotype has been reported52,53. Some patients might also present with isolated hypermagnesuria20. Tubular defects are frequently not recognized because their clinical manifestations are often mild or overshadowed by more severe neurological symptoms. In a systematic study of 42 patients with mitochondrial disorders, half had renal tubular dysfunction, but only eight had overt disease, suggesting that the prevalence of renal involvement in mitochondrial cytopathies is underestimated51.
Mutations involving both nuclear and mitochondrial genes have been described to cause tubular defects. In general, consistent phenotypes that link mutations in a given gene to a specific tubular defect have not been identified20. Some mutations do, however, tend to be characterized more frequently by certain renal phenotypes; for example, mutations in BCS1L, UQCC2 or FBXL4, which are involved in oxidative phosphorylation, frequently cause proximal renal tubular acidosis47,48. A homozygous p.Ser78Gly mutation in BCS1L produces a specific clinical phenotype called GRACILE syndrome, which is characterized by intrauterine growth retardation, fulminant lactic acidosis, aminoaciduria and liver haemosiderosis, and is usually fatal in the neonatal period54. This syndrome is found almost exclusively in Finnish patients.
To date, two distinct familial mitochondrial tubular disorders have been identified. Mutations in the mitochondrial isoleucine tRNA gene (tRNAIle or MT-TI) that involve a critical nucleotide for codon–anticodon recognition have been associated with mitochondrial hypomagnesaemia in a large white kindred55. Symptoms segregated in the family following a maternal dominance modality and included at least one of the following: hypomagnesaemia, hypercholesterolaemia or hypertension. Serum Mg2+ levels were low in half of the family members in the maternal lineage and were associated with increased urinary Mg2+ excretion and decreased urinary Ca2+ excretion, suggesting a specific defect of the distal convoluted tubule55. The mechanisms that underlie Mg2+ losses in these patients are not fully understood; however, cells of the distal convoluted tubule have very high energy consumption56 and Mg2+ reabsorption in this segment requires ATP-dependent Na+ reabsorption55,57–59. In many cell models blocking oxidative phosphorylation impacts transcellular Na transport.
A second large family with autosomal dominant Fanconi syndrome characterized by prominent renal bicarbonate and phosphate losses was found to carry mutations in EHHADH60. The encoded protein, peroxisomal bifunctional enzyme, is involved in fatty acid oxidation and is primarily expressed in peroxisomes along the terminal segments of the proximal tubule. The mutation that segregates with disease in this family causes mistargeting of the protein to mitochondria, resulting in impaired mitochondrial oxidative phosphorylation with a dominant-negative effect. The latter finding is further substantiated by the absence of Fanconi syndrome in Ehhadh-knockout mice60.
Glomerular diseases
Podocytes are highly differentiated cells with limited replicative capacity. They are a major component of the glomerular filtration barrier, support the other capillary components in counteracting endocapillary pressure, synthesize major cytoskeletal proteins and extracellular matrix components, and have several immunological roles61. To maintain all of these functions, podocytes are particularly dependent on energy and are rich in mitochondria. Impairment of oxidative phosphorylation in podocytes results in excessive generation of ROS and in functional and structural alterations, resulting in disruption of the glomerular filtration barrier, proteinuria and ultimately the development of glomerular sclerotic lesions62,63. Podocyte mitochondrial dysfunctions can be acquired, such as in diabetic nephropathy64 and other chronic renal conditions65,66, or caused by genetic defects in mtDNA or nDNA. In addition to sporadic cases of glomerulopathies secondary to mutations in genes that encode mitochondrial proteins20, two major glomerular diseases have been identified: mitochondrial cytopathies secondary to genetic defects in the CoQ10 biosynthesis pathway and those that are caused by the mtDNA 3243 A>G mutation in the tRNALeu(UUR) gene.
CoQ10 biosynthesis defects
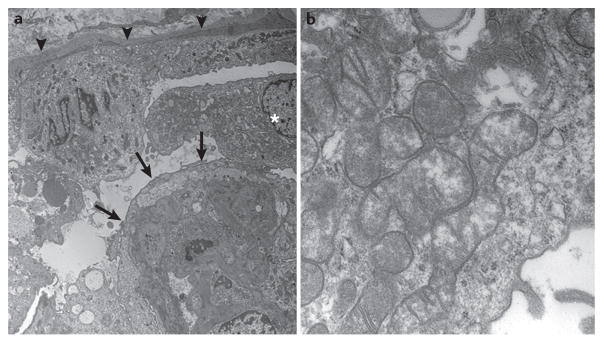
Glomerular involvement in these disorders can be isolated or occur as part of a multisystemic disease with a variable age of onset. In most cases, renal involvement is characterized by steroid-resistant proteinuria or nephrotic syndrome with or without haematuria, which usually progresses to chronic renal failure, and FSGS lesions in renal biopsy samples. High numbers of abnormal mitochondria in the cytoplasm of podocytes might sometimes be visible on electron microscopy (FIG. 3).
Figure 3. Electron microscopy images of a renal biopsy sample obtained from a patient with a COQ2 mutation.
a | The parietal epithelium of the Bowman capsule (arrowheads) appears healthy and contains a normal number of mitochondria. By contrast, the urinary space is occupied by swollen podocytes (asterisk) that show extensive foot-process fusion (arrows). b | Enlarged view of a podocyte showing the cytoplasm packed with mitochondria, several of which are dysmorphic.
Diseases resulting from defects in CoQ10 biosynthesis are receiving increasing attention as a growing number of potentially treatable defects are recognized. These diseases are characterized by broad molecular and clinical heterogeneity, which is related to the large number of enzymes involved in CoQ10 biosynthesis and the possibility of redundancy in different organs. The clinical relevance of this group of mitochondrial cytopathies is related to their response to oral supplementation with CoQ10 (REFS 67–69), a treatment that is unparalleled in other mitochondrial diseases. Early diagnosis of affected patients might prevent the development of irreversible neurological lesions and reverse the renal phenotype67.
Genetic defects that affect CoQ10 synthesis result in mitochondrial dysfunction and excessive production of ROS, which damage and ultimately cause apoptosis of podocytes. Interestingly, patients with idiopathic FSGS can have partial CoQ10 deficiencies that might affect their podocyte biology and participate in the development of FSGS lesions70. To date, mutations in nine genes involved in the synthesis of CoQ10 have been shown to cause primary CoQ10 deficiency (PDSS1, PDSS2, COQ2, COQ4, COQ6, COQ7, ADCK3, ADCK4 and COQ9). Mutations in these genes produce a heterogeneous clinical picture, ranging from fatal multisystem disease to isolated steroid resistant nephrotic syndrome (SRNS) or encephalopathy24. Mutations in COQ2 (REF. 71), PDSS2 (REF. 72), COQ6 (REF. 68), ADCK4 (REF. 69) and PDSS1 (REF. 73) have been associated with glomerular involvement.
COQ2
The first genetic defect that was identified in patients with primary CoQ10 deficiency was a mutation in COQ2, which encodes 4-hydroxybenzoate-polyprenyl transferase, the enzyme that catalyses the second step in the mitochondrial CoQ10 biosynthetic pathway71. To date, COQ2 mutations have been reported in 15 children from 10 unrelated families; 11 of these patients had glomerular involvement74–78. SRNS usually developed within the first year of life or in the neonatal period, and often represented the first symptom of the disease, with or without neurologic symptoms. However, not all patients with COQ2 mutations develop renal lesions79 and some show renal involvement later in the course of their disease78. Various histologic lesions have been reported; in most cases the renal histology showed FSGS, including one case of collapsing glomerulopathy, but crescentic glomerulonephritis or mild mesangial proliferation have also been reported75. On electron microscopy, podocytes appear swollen and packed with abnormal mitochondria75 (FIG. 3). The nephrotic syndrome is characterized by a rapid decline in renal function that does not recur after kidney transplantation. Prompt treatment with high doses of CoQ10 (30 mg/kg) has been shown to halt the progression of the disease, substantially improve proteinuria and reverse the clinical manifestations related to nephrotic syndrome67.
PDSS1 and PDSS2
PDSS2 encodes a subunit of the enzyme required for synthesis of the decaprenyl tail of CoQ10. In humans, the active form of this enzyme forms a heterotetramer comprising two PDSS1 and two PDSS2 units. To date, PDSS2 mutations have been identified in four patients with glomerular mitochondrial cytopathies associated with CoQ10 deficiency from two unrelated families72,80. The first family, with three affected siblings, was originally described in 2006 (REF. 81). All three children presented with progressive encephalopathy and SRNS; two children underwent successful renal transplantation at the ages of 8 years and 9 years, whereas the third child died at 8 years of age as a consequence of rapid neurological deterioration. Treatment with oral CoQ10 (5 mg/kg per day) improved the neurologic symptoms in the surviving children over 3 years of follow-up. In the second family, the patient presented at 3 months of age with seizures and hypotonia. He subsequently developed cortical blindness and nephrotic syndrome and died at 8 months of age because of severe refractory focal status epilepticus72. His brain MRI was compatible with Leigh syndrome. From 3 months of age, this child was treated with oral CoQ10 (50 mg per day) with no apparent clinical improvement. The reasons for this lack of response are unclear, but the treatment might have been started too late, when neurological and renal lesions could no longer regress. Two patients with PDSS1 mutations have also been described: the first showed no renal abnormalities82 whereas the second presented with nephrotic syndrome73.
A mouse model (kd/kd) harbouring a spontaneous homozygous missense mutation in Pdss2 recapitulates the human renal phenotype and does not show major extra-renal defects83. In this model, CoQ10 supplementation is effective in preventing the onset of renal disease84. Interestingly, treatment with the antioxidant and hypolipidemic compound probucol is also effective in preventing renal lesions in these mice85. Whether this beneficial effect is related to the antioxidant properties of probucol or whether the drug stimulates CoQ9 biosynthesis in these animals is unclear. No clinical data on probucol are available, but other antioxidants, such as idebenone (a soluble analogue of CoQ10) do not rescue defects that result in a reduction in the activity of complex II+III86 and seem to be ineffective at ameliorating symptoms in animal models80. The role of ROS in the pathogenesis of glomerulopathy in the kd/kd mouse model is supported by the observation that CoQ9 deficiency is ubiquitous in these animals, but a significant increase in ROS production is present only in the kidneys, where tissue damage occurs87.
COQ6
COQ6 encodes a mono-oxygenase, which catalyses the C5 hydroxylation step of the quinone ring. Mutations in this gene have been described in 11 patients from five families68. All of the affected children presented with SRNS and sensorineural deafness, generally at older ages than those reported for patients with COQ2 mutations. Proteinuria was diagnosed between 0.2 years and 6 years of age (median 1.2 years) and renal function deteriorated rapidly to reach end-stage renal disease (ESRD) between 0.4 years and 9 years of age (median 1.7 years). Five children died at a median age of 5 years. The most frequent renal histological picture (seen in seven patients) was FSGS; diffuse mesangial sclerosis was diagnosed in one biopsy sample. Facial dysmorphism and neurological impairment, including seizures, white matter abnormalities and ataxia, were also reported68. Notably the uniformity of the phenotype, and in particular the renal involvement, could reflect selection bias as all of the patients were identified from a SRNS cohort. A yeast complementation study that tested all of the mutated COQ6 alleles reported to date, showed that the defect could be rescued by vanillic acid or 2,4-dihydroxybensoic acid (DHB)19. These nontoxic analogues of the ring precursor of CoQ10 are able to bypass the enzymatic defect. DHB has also been shown to be effective in fibroblasts from patients with COQ7 mutations88.
ADCK4
ADCK4 is the human orthologue of the yeast COQ8 gene (L. Salviati, unpublished data), which encodes an atypical kinase involved in the regulation of CoQ10 biosynthesis. In yeast, overexpression of ADCK4 stabilizes the CoQ multienzyme biosynthetic complex, even in the absence of any of its components89. Mutations in ADCK4 account for the highest number of patients with renal disease secondary to CoQ10 biosynthesis defects reported to date: 38 patients from 18 families have been retrospectively described69,90. These patients typically presented with proteinuria and SRNS and most had a renal histological picture of FSGS, including a small number of patients with a collapsing variant. Extrarenal symptoms were present in a minority of patients, differed between affected patients and included mild neurologic disturbances and a single case of dilated cardiomyopathy. A patient who was treated with oral CoQ10 showed partial remission90. Compared with other CoQ10 biosynthesis defects, mutations in ADCK4 seem to result in a less severe clinical entity, with a more prominent renal phenotype, higher age at onset of SRNS (usually 10–20 years), slower progression to ESRD and good patient survival owing to the lack of extrarenal manifestations.
The relatively mild phenotype observed in patients with ADCK4 defects is probably related to the fact that the encoded enzyme has a modulatory function without catalytic activity, enabling residual CoQ10 synthesis even in the complete absence of this protein. In animal models ADCK4 knockout caused reduced podocyte motility in vitro, which could be reversed by adding CoQ10 to the culture medium69. In mice null mutations in other COQ genes prevent CoQ10 biosynthesis and are not compatible with life11. Mutations in ADCK3, a paralogue of ADCK4, also causes CoQ10 deficiency, but the resulting phenotype is completely different from that of ADCK4 mutations and includes cerebellar ataxia and encephalopathy without renal disease91,92. The functional relationship between these two genes requires further study.
Other mutations
Other genetic defects of CoQ10 biosynthesis, such as mutations in COQ4, COQ9 and COQ7, have not been linked to glomerular disease. Moreover, a patient with a COQ9 mutation had a tubulopathy without apparent glomerular involvement44. The reasons for this phenotypic discrepancy are unclear, but different degrees of destabilization of the CoQ10 biosynthetic complex by individual mutations might explain some of the variability93.
mtDNA 3243 A>G mutation
The mtDNA 3243 A>G mutation in the tRNALeu(UUR) gene is one of the most common mtDNA point mutations. This mutation was initially described in patients with mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes (MELAS) syndrome, a progressive neurodegenerative disorder that usually presents in children or young adults94. Approximately 80% of patients with MELAS syndrome harbour the mtDNA 3243 A>G mutation95, but other causative mtDNA mutations have also been reported43. The phenotypic expression of the mtDNA 3243 A>G mutation can be highly variable and causes a wide range of clinical manifestations, including muscle weakness, exercise intolerance, failure to thrive, developmental delay, progressive encephalopathy, migraine, stroke-like episodes, peripheral neuropathy and visual complaints due to ophthalmoplegia. Some patients present with myoclonic epilepsy with ragged red fibres (MERRF) syndrome or maternally inherited diabetes and deafness (MIDD).
Renal involvement is not very common in patients with MELAS syndrome. However, several patients with the mtDNA 3243 A>G mutation have developed proteinuria and renal failure, usually in association with other symptoms (such as diabetes and/or sensorineural hearing loss), but also as an isolated finding, at least at disease onset. The renal disease generally corresponds to a glomerulopathy with proteinuria, which is below the nephrotic range in two-thirds of patients. From the histological standpoint, most patients have FSGS lesions, but three cases of tubulointerstitial nephritis have also been described20. Approximately 80% of patients with renal involvement have some degree of deafness, so might be misdiagnosed with Alport syndrome. Overall, patients with the mtDNA 3243 A>G mutation seems to have less overt haematuria than those with Alport syndrome, and their renal biopsy samples do not show the typical ultrastructural findings of this disease. The absence of these features should always raise suspicion of the mitochondrial tRNALeu mutation. To date more than 30 patients with MELAS syndrome and renal involvement have been described in detail; approximately two-third of these patients were female and their age at diagnosis ranged from 14 years to 50 years20,96. The majority of patients were diagnosed with renal disease in their second or third decade of life and chronic kidney disease was present in half of these cases. Moderate neurologic symptoms were also present in the majority of patients.
A large-scale proteomic analysis of urine samples from adult patients with mitochondrial diseases showed that 75 of 117 participants carried the mtDNA 3243 A>G mutation97. Nearly half of the patients with this mutation had albuminuria and/or low-molecular-weight proteinuria, indicating that mtDNA 3243 A>G probably represents the most common mitochondrial disorder with renal involvement. Approximately half of the patients with this disorder presented with MIDD, whereas most of the remaining patients presented with MELAS or MERRF syndromes97.
Diagnosis of oxidative phosphorylation defects
Analysis of lactate levels
The diagnosis of defects in oxidative phosphorylation is a complex task that requires a combination of approaches10. As a functional respiratory chain is required for the oxidation of lactate (the final product of glycolysis) to carbon dioxide and water, the presence of increased lactate levels in serum or cerebrospinal fluid is an important finding. Such analyses can be integrated with magnetic resonance spectroscopy, which enables estimation of lactate levels in the brain98. These levels often fluctuate, however, and might be normal even in the presence of severe defects in oxidative phosphorylation99. The lactate-to-pyruvate ratio helps distinguish between oxidative phosphorylation disorders and other defects such as pyruvate dehydrogenase deficiency. Analysis of urinary organic acids might detect lactic aciduria and other abnormalities, such as dicarboxylic aciduria, which is a frequent, albeit nonspecific finding in patients with defects in oxidative phosphorylation24. Patients with mitochondrial renal disease often do not have constant hyperlactacidemia but might have elevated urinary lactate excretion. In addition, levels of fibroblast growth factor 21 are increased in patients who have mitochondrial disorders with significant muscle involvement100.
Neuroimaging
Neuroimaging often provides important clues to aid diagnosis. Focal lesions in deep grey matter structures, such as the putamen and basal ganglia, are among the most common findings, especially in paediatric patients101,102. Leigh syndrome, which is characterized by focal, bilateral, symmetric lesions involving basal ganglia and the periaqueductal grey matter, represents the effects of severe deficiencies in energy production in the central nervous system in infancy103. Older patients might present with stroke-like lesions in non-vascular territories, especially in the parieto-occipital region. These lesions are typical of MELAS syndrome101, but are also seen in other defects, including CoQ10 deficiencies74. Less-specific findings include cortical and cerebellar atrophy, as well as various white matter abnormalities.
Analysis of biopsy samples
Muscle biopsy is still considered the gold standard for diagnosis of oxidative phosphorylation defects10. Morphological analyses coupled with histochemical staining enables the detection of COX-deficient fibres and mitochondrial proliferation104. A uniform pattern points to a nDNA defect, whereas a mosaic distribution (owing to heteroplasmy) is suggestive of a mtDNA abnormality, which can occur as a result of a mutation in mtDNA or as a secondary effect of a mutation in a nuclear mtDNA maintenance gene. Oil-Red staining might detect lipid accumulation, which is often observed in CoQ10 deficiencies105.
Spectrophotometric measurements of enzymatic activities might distinguish between defects involving individual complexes and combined deficiencies. Analysis of the combined activity of complexes II and III, which require CoQ10 to shuttle electrons to complex III (FIG. 1), provides an indirect but reliable assessment of CoQ10 levels89. Finally, CoQ10 concentrations in muscle specimens can be measured using HPLC. Standardized analysis protocols for this technique have been developed and validated106,107. Similar analyses can be performed in cultured primary skin fibroblasts; however, some defects are not expressed in these cells. Cultured fibroblasts also enable functional studies.
In theory, CoQ10 analyses performed on muscle specimens can also be carried out on renal tissue75. Histochemical analyses of renal cortex samples might provide similar information to analyses of muscle specimens20; however, spectrophotometric analyses are more problematic because a surgical biopsy is necessary to obtain a large enough sample.
Next-generation sequencing
Next-generation sequencing approaches are revolutionizing the molecular diagnosis of mitochondrial disorders. The entire mtDNA can now be sequenced rapidly at low cost108. In patients with renal involvement, urinary sediment cells could be the optimal material for DNA extraction109. Likewise, in cases of nuclear defects, large gene panels or the entire exome can now be analysed19. In the past few years, numerous defects have been characterized at the molecular level using these techniques. Nonetheless, detailed phenotypic characterization of patients remains necessary to restrict the data analysis, which is time consuming and complicated.
Screening for CoQ10 deficiency
As timely diagnosis is crucial for the success of therapy, the possibility of CoQ10 deficiency should always be considered in patients with SRNS, particularly infants. No pathognomonic clinical features exist, but SRNS in association with neuromuscular symptoms or deafness should raise the suspicion of CoQ10 deficiency. Many patients, however, present with SRNS without extrarenal involvement at diagnosis. Moreover, although patients usually present in infancy or early childhood, onset of symptoms might occur later in life. The optimal diagnostic strategy for CoQ10 deficiency is still debated110. Traditional approaches require time and invasive procedures; such delay is not of critical importance in most disorders of oxidative phosphorylation, but might have dramatic consequences in the case of CoQ10 deficiencies. In principle, all individuals with isolated SRNS should be screened for CoQ10 deficiency, but performing a skin or muscle biopsy is not always possible. With new technological advances and cost reductions, screening using next-generation sequencing and specific gene panels is becoming a valuable diagnostic approach. Even if only 1% of patients with SRNS have CoQ10 deficiency111, the benefits of preventing ESRD and probably also neurological deterioration in these patients outweighs the cost of genetic screening, which is routinely performed in most cases. Systematic electron microscopy of renal biopsy samples could also enable rapid identification of many patients, as abnormal mitochondrial proliferation in podocytes is frequently observed75.
Mitochondrial dysfunction in acute kidney injury
As the renal tubules represent one of the most metabolically active epithelia in the human body, it is unsurprising that AKI — whether septic, ischaemic, or toxic in origin — involves early pathological changes in the mitochondria of the tubular epithelium112,113. These changes include decreased mitochondrial abundance, swelling of individual organelles, and disruption of the otherwise tightly stacked cristae. The proximal tubule is a primary site for mitochondrial disruption in AKI, but changes in the thick ascending limb and distal tubules have also been reported114. Evidence for mitochondrial involvement in human AKI was shown in early electron microscopy studies of specimens from patients who had died from septic shock115. Subsequent autopsy studies following sepsis and sequential biopsy studies during controlled renal ischaemia (for example, for nephrectomy) revealed similar lesions in mitochondria116,117.
Mitochondrial dysfunction in AKI typically accompanies ultrastructural pathology. For example, experimental cisplatin nephrotoxicity induces a decrease in the activity and expression of cytochrome c oxidase in the proximal tubule, but not in the distal nephron segments. This finding is consistent with the clinical observation that proximal tubular manifestations dominate the presentation of platinum-induced renal injury118. Comparison of a toxic form of AKI, glycerol-induced rhabdomyolysis, with post-ischaemic AKI showed that both conditions result in widespread loss of mitochondrial respiratory proteins from proximal tubules119, whereas experimental sepsis leads to a profound decrease in the expression and activity of multiple enzymatic components of the mitochondrial electron transport chain.112
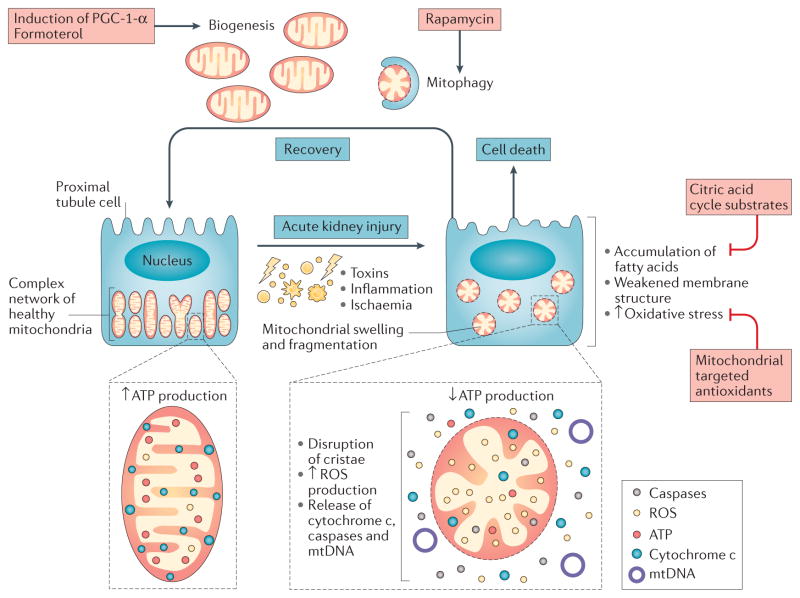
Injured mitochondria not only deprive the cell of ATP, but are an important source of molecules that amplify injury, precipitate cell death and induce inflammation (FIG. 4). ROS released from damaged mitochondria contribute to the oxidative stress widely reported in AKI. Structural disruption of mitochondria also releases cytochrome c, a trigger of apoptosis, as well as mtDNA, which can serve as a proinflammatory danger signal120. A highly orchestrated process of mitochondrial biogenesis, replication, and clearance via macroautophagy enables healthy cells to avoid the dangers of mitochondrial injury. Conversely, growing evidence indicates that mitochondria might be a compelling therapeutic target in multiple forms of AKI.
Figure 4. Mitochondrial injury and recovery during acute kidney injury (AKI).
Tubular epithelial cells in the proximal tubule and outer medulla are heavily invested with mitochondria in order to generate the ATP necessary for solute transport. Diverse aetiologies of AKI injure the mitochondria, leading to organellar swelling and fragmentation. Injured mitochondria, in turn, release an array of proinflammatory and injurious molecules, such as reactive oxygen species (ROS), which, if unchecked, promote cell death. Experimental findings suggest that recovery from AKI might require the clearance of injured mitochondria through mitophagy and the replenishment of mitochondrial mass through mitochondrial biogenesis, a process mediated by the transcriptional co-activator peroxisome proliferator-activated receptor-γ co-activator 1-α (PGC-1-α). Examples of potential preventive and therapeutic strategies are highlighted in pink boxes. mtDNA, mitochondrial DNA.
Fatty acids
Although comprehensive discussion of mitochondrial energy metabolism in AKI is beyond the scope of this Review, the roles of fatty acids and ROS need to be highlighted. Fatty acids are the most efficient source of mitochondrial ATP generation, but their intracellular accumulation can result in lipotoxicity. During ischaemia, a mismatch develops between ongoing hydrolysis of membrane phospholipids and reduced clearance of these fatty acids via re-esterification and mitochondrial fatty acid oxidation. This imbalance leads to the accumulation of non-esterified fatty acids (NEFAs), which can act as detergents that weaken the membrane structure, culminating in apoptosis. Biochemical interventions to reduce NEFAs (for example, by applying citric acid cycle substrates) protect freshly isolated proximal tubules from hypoxia–reoxygenation injury and restore normal ATP production121–123. In transgenic mice that overexpress the transcription factor peroxisome proliferator-activated receptor-α, protection against ischaemic AKI is associated with restoration of normal fatty acid metabolism124. Finally, the sequestration of noxious fatty acids into the storage form of triglycerides might be an endogenous adaptive response to injury. Triglyceride accumulation in cortical and medullary segments of nephrons seems to be a hallmark of diverse renal injuries, ranging from acute obstruction to experimental sepsis and ischaemia–reperfusion injury (IRI)125,126.
Reactive oxygen species
During normal mitochondrial metabolism, a large concentration gradient of hydrogen ions across the inner mitochondrial membrane provides the energy for the phosphorylation of ADP to ATP. When components of the electron transport chain are downregulated, disassembled, spatially displaced or altered, the movement of electrons can become dysregulated, resulting in the generation of excess ROS. Mitochondria seem to be a major source of excess ROS during acute cellular injury as a result of inflammation or ischaemic stress. Although ROS have vital signalling roles in healthy cells, excess levels can lead to catalytic free-radical damage to all classes of macromolecules. Generic antioxidants might have limited therapeutic potential in AKI, but two different classes of mitochondria-targeted antioxidants seem promising in preclinical models. One class of such molecules, an example of which is MitoQ, covalently links the antioxidant ubiquinone to a lipophilic cation that ‘locks’ the compound into mitochondria127,128. Another therapeutic strategy involves linking the antioxidant chemical to a peptide that provides mitochondrial targeting, for example a Szeto-Schiller peptide129. Experiments using mitochondria-targeted antioxidants have confirmed that mitochondria are an important source of ROS during various types of renal injury and shown that reduction of mitochondria-derived ROS can ameliorate AKI130,131. These molecules are currently being examined in clinical settings of excess mitochondrial ROS generation, such as IRI.
Mitochondrial dynamics
Ischaemic and toxic forms of AKI are characterized by marked mitochondrial fragmentation. The fragmented mitochondria are potential sources of ROS, cytochrome c, mitochondrial DNA and other potentially injurious molecules. Inhibition of mitochondrial fission by genetically or pharmacologically blocking dynam-in-related protein 1 (Drp1) has been shown to protect cultured renal tubular cells from stress-induced apoptosis and attenuate AKI following ischaemia–reperfusion or cisplatin exposure113. Experimental pigment nephropathy can also be ameliorated by Drp1 inhibition132. Although complementary experiments with gain-of-function mutations remain to be performed, these findings suggest that altered mitochondrial dynamics are a key feature of AKI and a potential therapeutic target. Consistent with this hypothesis, experimental evidence suggests that the NAD-dependent protein deacetylase sirtuin 3 might attenuate cisplatin-induced mitochondrial fragmentation and protect against experimental AKI133.
Mitophagy
Safe disposal of fragmented mitochondria via mitophagy might protect stressed cells from death and ameliorate AKI. Renal IRI has been shown to induce mitophagy in renal tubules134, and mice that lack the autophagy regulator Atg7 show increased sensitivity to cisplatin nephrotoxicity135. Drugs that induce mitophagy, such as rapamycin, merit further exploration as therapeutic strategies to enhance the clearance of injury-propagating fragmented mitochondria and accelerate recovery after AKI.
Mitochondrial biogenesis
To maintain a steady pool of mitochondria, losses to mitophagy must be replenished by the expansion of mitochondrial mass. An array of nuclear transcription factors and co-activators are involved in mitochondrial biogenesis. The best studied co-activator is peroxisome proliferator-activated receptor-γ co-activator 1-α (PGC-1-α), which is highly expressed in the most metabolically active organs, including the heart, kidney, brains, skeletal muscle and liver136. In the kidney PGC-1-α expression reflects the relative distribution of mitochondria; the highest expression is in the cortex, followed by the tubules with much lower levels in the glomerulus112. In ischaemic and septic AKI, an initial decrease in PGC-1-α expression is followed by a return to normal levels as organ function recovers, suggesting a role of this co-activator in AKI recovery112,119. Consistent with this hypothesis, specific knockout of Ppargc1a, which encodes PGC-1-α, from the proximal tubule blunted renal recovery following experimental sepsis112. Signals from innate inflammatory pathways might result in downregulation of PGC-1-α during infection137,138.
Data from gain-of-function experiments also suggest that targeting mitochondrial biogenesis might attenuate renal injury and/or accelerate recovery from AKI. In cultured proximal tubular cells, induction of PGC-1-α after (but not before) oxidant exposure accelerated recovery of mitochondrial function139. To identify pharmacological stimulators of mitochondrial biogenesis, Jesinkey et al. screened a large library of small molecules in model cellular systems. One such compound, the β-adrenergic agonist formoterol, stimulated mitochondrial biogenesis, reduced necrosis and improved kidney function in mice that had been subjected to renal IRI140. Further studies are required to determine whether PGC-1-α is required for formoterol-dependent renoprotection, and to delineate the underlying mechanisms. However, these findings are promising because they suggest the translational utility of unbiased cell-based drug screens targeting mitochondrial processes to identify agents that might aid recovery from established AKI.
Conclusions
Healthy mitochondria are essential for normal renal health and mutations that directly or indirectly impair mitochondrial function or assembly can cause renal disease. The genetics of mitochondrial disorders is complex and can follow various patterns of inheritance. Although most patients with renal disease resulting from a mitochondrial disorder have a tubulopathy, two well-defined glomerular diseases in patients with mitochondrial cytopathies have been described: FSGS resulting from defects in the CoQ10 biosynthesis pathway and FSGS secondary to the mtDNA 3243 A>G mutation. These latter diseases are particularly important because defects in CoQ10 biosynthesis might be rescued by oral CoQ10 supplementation and renal diseases caused by the mtDNA 3243 A>G mutation are transmitted following a maternal pattern of inheritance and associated with extrarenal symptoms that need to be monitored.
In most cases, however, mitochondrial damage is acquired. Injury to tubular mitochondria represents an early event during AKI. As injured mitochondria release multiple noxious factors, the cellular processes that occur upstream and downstream of this event are of substantial interest. Research into the effects of targeting mitochondrial dynamics, mitophagy and biogenesis has yielded consistent and exciting results that suggest the potential of manipulating these processes to ameliorate AKI. New approaches developed to treat acquired mitochondrial damage might also be potentially beneficial in some genetic mitochondrial disorders. Other alternative strategies, such as pronuclear transfer — a technique for mitochondrial replacement — might also represent potentially valuable approaches in some diseases, but ethical considerations need to be addressed before such techniques can be adopted in clinical practice141.
Acknowledgments
Mitochondrial studies in S.M.P.’s laboratory are supported by R01-DK0950972. Studies in L.S.’s laboratory are supported by Telethon grants 14187 and 13222.
Biographies
Francesco Emma is Chief of the Department of Paediatric Specialties, Chief of Paediatric Nephrology and Director of the Nephrology Laboratory at the Bambino Gesù Children’s Hospital in Rome, Italy. He trained in medicine and paediatrics at the Catholic University of Louvain in Belgium and then undertook a fellowship in Paediatric Nephrology at Children’s Hospital, Harvard Medical School, USA. His research interests are focused on the physiopathology of inherited renal disorders, in particular cystinosis and mitochondrial renal diseases, and of steroid-sensitive nephrotic syndrome.
Giovanni Montini is Professor of Paediatrics at the University of Milan and Chief of the Paediatric Nephrology and Dialysis Department of the Fondazione IRCCS Ca’ Granda in Milan, Italy. He began his career in Padua, Italy and has also worked at various other institutions in Italy and abroad, including Guy’s Hospital and Great Ormond Street Hospital for Children in London, UK. He is an accomplished researcher, who has published over 130 peer-reviewed manuscripts, and is also a member of the editorial boards of several paediatric journals. His areas of expertise include renal transplantation, urinary tract infections, urinary tract malformations, chronic kidney damage and nephropathy in mitochondrial diseases.
Samir Parikh is Associate Professor of Medicine at Harvard Medical School, USA, and staff physician in the Division of Nephrology at Beth Israel Deaconess Medical Center, USA. His laboratory studies molecular mechanisms of critical illnesses and his group provided the initial description of peroxisome proliferator-activated receptor-γ co-activator 1-α as an important factor for functional recovery from sepsis-associated acute kidney injury. His laboratory has been funded by the National Institutes of Health, the Amgen Medical Institute, the Klarman foundation, the American Society of Nephrology, the American Heart Association, and the American Diabetes Association. Dr Parikh received his undergraduate degree from Harvard, his medical degree from Vanderbilt, and his postgraduate training at Beth Israel Deaconess Medical Center, USA.
Leonardo Salviati is Associate Professor of Medical Genetics at the Department of Woman and Child Health, University of Padova, Italy. He received his medical training at the University of Padova. He was a Postdoctoral Fellow in the Department of Neurology, Columbia University, New York, USA from 1999 to 2002, and was appointed Assistant Professor of Medical Genetics at the University of Padova in 2005. His research has focused on the molecular bases and pathogenesis of mitochondrial disorders with particular attention to coenzyme Q10 deficiency.
Footnotes
Author contributions
All authors researched the data, made a substantial contribution to discussion of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Competing interests statement
The authors declare no competing interests.
References
- 1.Andersson SG, et al. The genome sequence of Rickettsia prowazekii and the origin of mitochondria. Nature. 1998;396:133–140. doi: 10.1038/24094. [DOI] [PubMed] [Google Scholar]
- 2.Frey TG, Mannella CA. The internal structure of mitochondria. Trends Biochem Sci. 2000;25:319–324. doi: 10.1016/s0968-0004(00)01609-1. [DOI] [PubMed] [Google Scholar]
- 3.Anderson S, et al. Sequence and organization of the human mitochondrial genome. Nature. 1981;290:457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- 4.Schon EA, DiMauro S, Hirano M. Human mitochondrial DNA: roles of inherited and somatic mutations. Nat Rev Genet. 2012;13:878–890. doi: 10.1038/nrg3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Opalinska M, Meisinger C. Metabolic control via the mitochondrial protein import machinery. Curr Opin Cell Biol. 2015;33:42–48. doi: 10.1016/j.ceb.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Dimmer KS, Scorrano L. (De)constructing mitochondria: what for? Physiology (Bethesda) 2006;21:233–241. doi: 10.1152/physiol.00010.2006. [DOI] [PubMed] [Google Scholar]
- 7.Scorrano L, et al. A distinct pathway remodels mitochondrial cristae and mobilizes cytochrome c during apoptosis. Dev Cell. 2002;2:55–67. doi: 10.1016/s1534-5807(01)00116-2. [DOI] [PubMed] [Google Scholar]
- 8.Acin-Perez R, Fernandez-Silva P, Peleato ML, Perez-Martos A, Enriquez JA. Respiratory active mitochondrial supercomplexes. Mol Cell. 2008;32:529–539. doi: 10.1016/j.molcel.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 9.Ghezzi D, Zeviani M. Assembly factors of human mitochondrial respiratory chain complexes: physiology and pathophysiology. Adv Exp Med Biol. 2012;748:65–106. doi: 10.1007/978-1-4614-3573-0_4. [DOI] [PubMed] [Google Scholar]
- 10.DiMauro S, Schon EA, Carelli V, Hirano M. The clinical maze of mitochondrial neurology. Nat Rev Neurol. 2013;9:429–444. doi: 10.1038/nrneurol.2013.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doimo M, et al. Genetics of coenzyme q10 deficiency. Mol Syndromol. 2014;5:156–162. doi: 10.1159/000362826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nguyen TP, et al. Molecular characterization of the human COQ5 C-methyltransferase in coenzyme Q10 biosynthesis. Biochim Biophys Acta. 2014;1841:1628–1638. doi: 10.1016/j.bbalip.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen JW. Cytochrome c biogenesis in mitochondria — systems III and V. FEBS J. 2011;278:4198–4216. doi: 10.1111/j.1742-4658.2011.08231.x. [DOI] [PubMed] [Google Scholar]
- 14.DiMauro S, Schon EA. Mitochondrial disorders in the nervous system. Annu Rev Neurosci. 2008;31:91–123. doi: 10.1146/annurev.neuro.30.051606.094302. [DOI] [PubMed] [Google Scholar]
- 15.Mayr JA, et al. Spectrum of combined respiratory chain defects. J Inherit Metab Dis. 2015;38:4198–4216. doi: 10.1007/s10545-015-9831-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorman GS, et al. Prevalence of nuclear and mitochondrial DNA mutations related to adult mitochondrial disease. Ann Neurol. 2015;77:753–759. doi: 10.1002/ana.24362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Petruzzella V, et al. Deep sequencing unearths nuclear mitochondrial sequences under Leber’s hereditary optic neuropathy-associated false heteroplasmic mitochondrial DNA variants. Hum Mol Genet. 2012;21:3753–3764. doi: 10.1093/hmg/dds182. [DOI] [PubMed] [Google Scholar]
- 18.Giordano C, et al. Pathogenesis of the deafness-associated A1555G mitochondrial DNA mutation. Biochem Biophys Res Commun. 2002;293:521–529. doi: 10.1016/S0006-291X(02)00256-5. [DOI] [PubMed] [Google Scholar]
- 19.Doimo M, et al. Effect of vanillic acid on COQ6 mutants identified in patients with coenzyme Q10 deficiency. Biochim Biophys Acta. 2014;1842:1–6. doi: 10.1016/j.bbadis.2013.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emma F, Bertini E, Salviati L, Montini G. Renal involvement in mitochondrial cytopathies. Pediatr Nephrol. 2012;27:539–550. doi: 10.1007/s00467-011-1926-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Quinzii CM, et al. Reactive oxygen species, oxidative stress, and cell death correlate with level of CoQ10 deficiency. FASEB J. 2010;24:3733–3743. doi: 10.1096/fj.09-152728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morison IM, et al. A mutation of human cytochrome c enhances the intrinsic apoptotic pathway but causes only thrombocytopenia. Nat Genet. 2008;40:387–389. doi: 10.1038/ng.103. [DOI] [PubMed] [Google Scholar]
- 23.De Rocco D, et al. Mutations of cytochrome c identified in patients with thrombocytopenia THC4 affect both apoptosis and cellular bioenergetics. Biochim Biophys Acta. 2014;1842:269–274. doi: 10.1016/j.bbadis.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 24.Desbats MA, et al. Primary coenzyme Q10 deficiency presenting as fatal neonatal multiorgan failure. Eur J Hum Genet. 2015;23:1254–1258. doi: 10.1038/ejhg.2014.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emma F, Montini G, Salviati L, Dionisi-Vici C. Renal mitochondrial cytopathies. Int J Nephrol. 2011;2011:609213. doi: 10.4061/2011/609213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bodemer C, et al. Hair and skin disorders as signs of mitochondrial disease. Pediatrics. 1999;103:428–433. doi: 10.1542/peds.103.2.428. [DOI] [PubMed] [Google Scholar]
- 27.Niaudet P, Rotig A. The kidney in mitochondrial cytopathies. Kidney Int. 1997;51:1000–1007. doi: 10.1038/ki.1997.140. [DOI] [PubMed] [Google Scholar]
- 28.Deetjen P. Measurement of metabolism during renal work. Int J Biochem. 1980;12:243–244. [PubMed] [Google Scholar]
- 29.Thurau K. Renal Na-reabsorption and O2-uptake in dogs during hypoxia and hydrochlorothiazide infusion. Proc Soc Exp Biol Med. 1961;106:714–717. doi: 10.3181/00379727-106-26451. [DOI] [PubMed] [Google Scholar]
- 30.Ogier H, et al. de Toni-Fanconi-Debré syndrome with Leigh syndrome revealing severe muscle cytochrome c oxidase deficiency. J Pediatr. 1988;112:734–739. doi: 10.1016/s0022-3476(88)80690-5. [DOI] [PubMed] [Google Scholar]
- 31.Niaudet P, et al. Deletion of the mitochondrial DNA in a case of de Toni-Debré-Fanconi syndrome and Pearson syndrome. Pediatr Nephrol. 1994;8:164–168. doi: 10.1007/BF00865468. [DOI] [PubMed] [Google Scholar]
- 32.Rotig A. Renal disease and mitochondrial genetics. J Nephrol. 2003;16:286–292. [PubMed] [Google Scholar]
- 33.Morris AA, et al. Neonatal Fanconi syndrome due to deficiency of complex III of the respiratory chain. Pediatr Nephrol. 1995;9:407–411. doi: 10.1007/BF00866711. [DOI] [PubMed] [Google Scholar]
- 34.Au KM, et al. Mitochondrial DNA deletion in a girl with Fanconi’s syndrome. Pediatr Nephrol. 2007;22:136–140. doi: 10.1007/s00467-006-0288-y. [DOI] [PubMed] [Google Scholar]
- 35.Kuwertz-Broking E, et al. Renal Fanconi syndrome: first sign of partial respiratory chain complex IV deficiency. Pediatr Nephrol. 2000;14:495–498. doi: 10.1007/s004670050802. [DOI] [PubMed] [Google Scholar]
- 36.Mochizuki H, et al. Mitochondrial encephalomyopathies preceded by de-Toni-Debré-Fanconi syndrome or focal segmental glomerulosclerosis. Clin Nephrol. 1996;46:347–352. [PubMed] [Google Scholar]
- 37.De Meirleir L, et al. Clinical and diagnostic characteristics of complex III deficiency due to mutations in the BCS1L gene. Am J Med Genet A. 2003;121A:126–131. doi: 10.1002/ajmg.a.20171. [DOI] [PubMed] [Google Scholar]
- 38.Liu HM, et al. A novel 3670-base pair mitochondrial DNA deletion resulting in multi-systemic manifestations in a child. Pediatr Neonatol. 2012;53:264–268. doi: 10.1016/j.pedneo.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 39.Tzoufi M, et al. A rare case report of simultaneous presentation of myopathy, Addison’s disease, primary hypoparathyroidism, and Fanconi syndrome in a child diagnosed with Kearns–Sayre syndrome. Eur J Pediatr. 2013;172:557–561. doi: 10.1007/s00431-012-1798-1. [DOI] [PubMed] [Google Scholar]
- 40.Pitchon EM, et al. Patient with Fanconi syndrome (FS) and retinitis pigmentosa (RP) caused by a deletion and duplication of mitochondrial DNA (mtDNA) Klin Monbl Augenheilkd. 2007;224:340–343. doi: 10.1055/s-2007-962854. [DOI] [PubMed] [Google Scholar]
- 41.Mori K, Narahara K, Ninomiya S, Goto Y, Nonaka I. Renal and skin involvement in a patient with complete Kearns–Sayre syndrome. Am J Med Genet. 1991;38:583–587. doi: 10.1002/ajmg.1320380417. [DOI] [PubMed] [Google Scholar]
- 42.Topaloglu R, et al. Two new cases with Pearson syndrome and review of Hacettepe experience. Turk J Pediatr. 2008;50:572–576. [PubMed] [Google Scholar]
- 43.O’Toole JF. Renal manifestations of genetic mitochondrial disease. Int J Nephrol Renovasc Dis. 2014;7:57–67. doi: 10.2147/IJNRD.S37887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duncan AJ, et al. A nonsense mutation in COQ9 causes autosomal-recessive neonatal-onset primary coenzyme Q10 deficiency: a potentially treatable form of mitochondrial disease. Am J Hum Genet. 2009;84:558–566. doi: 10.1016/j.ajhg.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee YS, et al. Mitochondrial tubulopathy: the many faces of mitochondrial disorders. Pediatr Nephrol. 2001;16:710–712. doi: 10.1007/s004670100637. [DOI] [PubMed] [Google Scholar]
- 46.Gilbert RD, Emms M. Pearson’s syndrome presenting with Fanconi syndrome. Ultrastruct Pathol. 1996;20:473–475. doi: 10.3109/01913129609016351. [DOI] [PubMed] [Google Scholar]
- 47.Ezgu F, et al. Severe renal tubulopathy in a newborn due to BCS1L gene mutation: effects of different treatment modalities on the clinical course. Gene. 2013;528:364–366. doi: 10.1016/j.gene.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 48.Gai X, et al. Mutations in FBXL4, encoding a mitochondrial protein, cause early-onset mitochondrial encephalomyopathy. Am J Hum Genet. 2013;93:482–495. doi: 10.1016/j.ajhg.2013.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tucker EJ, et al. Next-generation sequencing in molecular diagnosis: NUBPL mutations highlight the challenges of variant detection and interpretation. Hum Mutat. 2012;33:411–418. doi: 10.1002/humu.21654. [DOI] [PubMed] [Google Scholar]
- 50.Matsutani H, et al. Partial deficiency of cytochrome c oxidase with isolated proximal renal tubular acidosis and hypercalciuria. Child Nephrol Urol. 1992;12:221–224. [PubMed] [Google Scholar]
- 51.Martin-Hernandez E, et al. Renal pathology in children with mitochondrial diseases. Pediatr Nephrol. 2005;20:1299–1305. doi: 10.1007/s00467-005-1948-z. [DOI] [PubMed] [Google Scholar]
- 52.Emma F, et al. ‘Bartter-like’ phenotype in Kearns–Sayre syndrome. Pediatr Nephrol. 2006;21:355–360. doi: 10.1007/s00467-005-2092-5. [DOI] [PubMed] [Google Scholar]
- 53.Goto Y, et al. Renal tubular involvement mimicking Bartter syndrome in a patient with Kearns–Sayre syndrome. J Pediatr. 1990;116:904–910. doi: 10.1016/s0022-3476(05)80648-1. [DOI] [PubMed] [Google Scholar]
- 54.Visapaa I, et al. GRACILE syndrome, a lethal metabolic disorder with iron overload, is caused by a point mutation in BCS1L. Am J Hum Genet. 2002;71:863–876. doi: 10.1086/342773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wilson FH, et al. A cluster of metabolic defects caused by mutation in a mitochondrial tRNA. Science. 2004;306:1190–1194. doi: 10.1126/science.1102521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Reilly RF, Ellison DH. Mammalian distal tubule: physiology, pathophysiology, and molecular anatomy. Physiol Rev. 2000;80:277–313. doi: 10.1152/physrev.2000.80.1.277. [DOI] [PubMed] [Google Scholar]
- 57.McCormick JA, Ellison DH. Distal convoluted tubule. Compr Physiol. 2015;5:45–98. doi: 10.1002/cphy.c140002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simon DB, et al. Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na–Cl cotransporter. Nat Genet. 1996;12:24–30. doi: 10.1038/ng0196-24. [DOI] [PubMed] [Google Scholar]
- 59.Soltoff SP. ATP and the regulation of renal cell function. Annu Rev Physiol. 1986;48:9–31. doi: 10.1146/annurev.ph.48.030186.000301. [DOI] [PubMed] [Google Scholar]
- 60.Klootwijk ED, et al. Mistargeting of peroxisomal EHHADH and inherited renal Fanconi’s syndrome. N Engl J Med. 2014;370:129–138. doi: 10.1056/NEJMoa1307581. [DOI] [PubMed] [Google Scholar]
- 61.Jefferson JA, Alpers CE, Shankland SJ. Podocyte biology for the bedside. Am J Kidney Dis. 2011;58:835–845. doi: 10.1053/j.ajkd.2011.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muller-Deile J, Schiffer M. The podocyte power-plant disaster and its contribution to glomerulopathy. Front Endocrinol (Lausanne) 2014;5:209. doi: 10.3389/fendo.2014.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saleem MA. 100 ways to kill a podocyte. Nephrol Dial Transplant. 2015 doi: 10.1093/ndt/gfu363. http://dx.doi.org/10.1093/ndt/gfu363. [DOI] [PubMed]
- 64.Higgins GC, Coughlan MT. Mitochondrial dysfunction and mitophagy: the beginning and end to diabetic nephropathy? Br J Pharmacol. 2014;171:1917–1942. doi: 10.1111/bph.12503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Che R, Yuan Y, Huang S, Zhang A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am J Physiol Renal Physiol. 2014;306:F367–F378. doi: 10.1152/ajprenal.00571.2013. [DOI] [PubMed] [Google Scholar]
- 66.Kawakami T, et al. Deficient autophagy results in mitochondrial dysfunction and FSGS. J Am Soc Nephrol. 2015;26:1040–1052. doi: 10.1681/ASN.2013111202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Montini G, Malaventura C, Salviati L. Early coenzyme Q10 supplementation in primary coenzyme Q10 deficiency. N Engl J Med. 2008;358:2849–2850. doi: 10.1056/NEJMc0800582. [DOI] [PubMed] [Google Scholar]
- 68.Heeringa SF, et al. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J Clin Invest. 2011;121:2013–2024. doi: 10.1172/JCI45693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ashraf S, et al. ADCK4 mutations promote steroid-resistant nephrotic syndrome through CoQ10 biosynthesis disruption. J Clin Invest. 2013;123:5179–5189. doi: 10.1172/JCI69000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gasser DL, et al. Focal segmental glomerulosclerosis is associated with a PDSS2 haplotype and, independently, with a decreased content of coenzyme Q10. Am J Physiol Renal Physiol. 2013;305:F1228–1238. doi: 10.1152/ajprenal.00143.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Quinzii C, et al. A mutation in para-hydroxybenzoate-polyprenyl transferase (COQ2) causes primary coenzyme Q10 deficiency. Am J Hum Genet. 2006;78:345–349. doi: 10.1086/500092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lopez LC, et al. Leigh syndrome with nephropathy and CoQ10 deficiency due to decaprenyl diphosphate synthase subunit 2 (PDSS2) mutations. Am J Hum Genet. 2006;79:1125–1129. doi: 10.1086/510023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vasta V, Merritt JL, 2nd, Saneto RP, Hahn SH. Next-generation sequencing for mitochondrial diseases: a wide diagnostic spectrum. Pediatr Int. 2012;54:585–601. doi: 10.1111/j.1442-200X.2012.03644.x. [DOI] [PubMed] [Google Scholar]
- 74.Salviati L, et al. Infantile encephalomyopathy and nephropathy with CoQ10 deficiency: a CoQ10-responsive condition. Neurology. 2005;65:606–608. doi: 10.1212/01.wnl.0000172859.55579.a7. [DOI] [PubMed] [Google Scholar]
- 75.Diomedi-Camassei F, et al. COQ2 nephropathy: a newly described inherited mitochondriopathy with primary renal involvement. J Am Soc Nephrol. 2007;18:2773–2780. doi: 10.1681/ASN.2006080833. [DOI] [PubMed] [Google Scholar]
- 76.McCarthy HJ, et al. Simultaneous sequencing of 24 genes associated with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol. 2013;8:637–648. doi: 10.2215/CJN.07200712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dinwiddie DL, et al. Diagnosis of mitochondrial disorders by concomitant next-generation sequencing of the exome and mitochondrial genome. Genomics. 2013;102:148–156. doi: 10.1016/j.ygeno.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scalais E, et al. Early myoclonic epilepsy, hypertrophic cardiomyopathy and subsequently a nephrotic syndrome in a patient with CoQ10 deficiency caused by mutations in para-hydroxybenzoate-polyprenyl transferase (COQ2) Eur J Paediatr Neurol. 2013;17:625–630. doi: 10.1016/j.ejpn.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 79.The Multiple-System Atrophy Research Collaboration. Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med. 2013;369:233–244. doi: 10.1056/NEJMoa1212115. [DOI] [PubMed] [Google Scholar]
- 80.Rotig A, et al. Quinone-responsive multiple respiratory-chain dysfunction due to widespread coenzyme Q10 deficiency. Lancet. 2000;356:391–395. doi: 10.1016/S0140-6736(00)02531-9. [DOI] [PubMed] [Google Scholar]
- 81.Rahman S, Clarke CF, Hirano M. 176th ENMC International Workshop: diagnosis and treatment of coenzyme Q10 deficiency. Neuromuscul Disord. 2012;22:76–86. doi: 10.1016/j.nmd.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mollet J, et al. Prenyldiphosphate synthase, subunit 1 (PDSS1) and OH-benzoate polyprenyltransferase (COQ2) mutations in ubiquinone deficiency and oxidative phosphorylation disorders. J Clin Invest. 2007;117:765–772. doi: 10.1172/JCI29089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peng M, et al. Primary coenzyme Q deficiency in Pdss2 mutant mice causes isolated renal disease. PLoS Genet. 2008;4:e1000061. doi: 10.1371/journal.pgen.1000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Saiki R, et al. Coenzyme Q10 supplementation rescues renal disease in Pdss2kd/kd mice with mutations in prenyl diphosphate synthase subunit 2. Am J Physiol Renal Physiol. 2008;295:F1535–F1544. doi: 10.1152/ajprenal.90445.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Falk MJ, et al. Probucol ameliorates renal and metabolic sequelae of primary CoQ deficiency in Pdss2 mutant mice. EMBO Mol Med. 2011;3:410–427. doi: 10.1002/emmm.201100149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lopez LC, et al. Treatment of CoQ10 deficient fibroblasts with ubiquinone, CoQ analogs, and vitamin C: time- and compound-dependent effects. PLoS ONE. 2010;5:e11897. doi: 10.1371/journal.pone.0011897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Quinzii CM, et al. Tissue-specific oxidative stress and loss of mitochondria in CoQ-deficient Pdss2 mutant mice. FASEB J. 2013;27:612–621. doi: 10.1096/fj.12-209361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Freyer C, et al. Rescue of primary ubiquinone deficiency due to a novel COQ7 defect using 2,4-dihydroxybensoic acid. J Med Genet. 2015;52:779–783. doi: 10.1136/jmedgenet-2015-102986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Desbats MA, Lunardi G, Doimo M, Trevisson E, Salviati L. Genetic bases and clinical manifestations of coenzyme Q10 (CoQ10) deficiency. J Inherit Metab Dis. 2015;38:145–156. doi: 10.1007/s10545-014-9749-9. [DOI] [PubMed] [Google Scholar]
- 90.Korkmaz E, et al. ADCK4-associated glomerulopathy causes adolescence-onset FSGS. J Am Soc Nephrol. 2015 doi: 10.1681/ASN.2014121240. http://dx.doi.org/10.1681/ASN.2014121240. [DOI] [PMC free article] [PubMed]
- 91.Mollet J, et al. CABC1 gene mutations cause ubiquinone deficiency with cerebellar ataxia and seizures. Am J Hum Genet. 2008;82:623–630. doi: 10.1016/j.ajhg.2007.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lagier-Tourenne C, et al. ADCK3, an ancestral kinase, is mutated in a form of recessive ataxia associated with coenzyme Q10 deficiency. Am J Hum Genet. 2008;82:661–672. doi: 10.1016/j.ajhg.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luna-Sanchez M, et al. The clinical heterogeneity of coenzyme Q10 deficiency results from genotypic differences in the Coq9 gene. EMBO Mol Med. 2015;7:670–687. doi: 10.15252/emmm.201404632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pavlakis SG, Phillips PC, DiMauro S, De Vivo DC, Rowland LP. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann Neurol. 1984;16:481–488. doi: 10.1002/ana.410160409. [DOI] [PubMed] [Google Scholar]
- 95.Mehrazin M, et al. Longitudinal changes of mtDNA A3243G mutation load and level of functioning in MELAS. Am J Med Genet A. 2009;149A:584–587. doi: 10.1002/ajmg.a.32703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seidowsky A, et al. Renal involvement in MELAS syndrome — a series of 5 cases and review of the literature. Clin Nephrol. 2013;80:456–463. doi: 10.5414/CN107063. [DOI] [PubMed] [Google Scholar]
- 97.Hall AM, et al. The urinary proteome and metabonome differ from normal in adults with mitochondrial disease. Kidney Int. 2015;87:610–622. doi: 10.1038/ki.2014.297. [DOI] [PubMed] [Google Scholar]
- 98.Wilichowski E, Pouwels PJ, Frahm J, Hanefeld F. Quantitative proton magnetic resonance spectroscopy of cerebral metabolic disturbances in patients with MELAS. Neuropediatrics. 1999;30:256–263. doi: 10.1055/s-2007-973500. [DOI] [PubMed] [Google Scholar]
- 99.Salviati L, et al. Novel SURF1 mutation in a child with subacute encephalopathy and without the radiological features of Leigh syndrome. Am J Med Genet A. 2004;128A:195–198. doi: 10.1002/ajmg.a.30073. [DOI] [PubMed] [Google Scholar]
- 100.Suomalainen A, et al. FGF-21 as a biomarker for muscle-manifesting mitochondrial respiratory chain deficiencies: a diagnostic study. Lancet Neurol. 2011;10:806–818. doi: 10.1016/S1474-4422(11)70155-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gropman AL. Neuroimaging in mitochondrial disorders. Neurotherapeutics. 2013;10:273–285. doi: 10.1007/s13311-012-0161-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bricout M, et al. Brain imaging in mitochondrial respiratory chain deficiency: combination of brain MRI features as a useful tool for genotype/phenotype correlations. J Med Genet. 2014;51:429–435. doi: 10.1136/jmedgenet-2013-102256. [DOI] [PubMed] [Google Scholar]
- 103.Leigh PN, Al-Sarraj S, DiMauro S. Subacute necrotising encephalomyelopathy (Leigh’s disease; Leigh syndrome) J Neurol Neurosurg Psychiatry. 2015;86:363–365. doi: 10.1136/jnnp-2012-304601. [DOI] [PubMed] [Google Scholar]
- 104.Sundaram C, et al. Contribution of muscle biopsy and genetics to the diagnosis of chronic progressive external opthalmoplegia of mitochondrial origin. J Clin Neurosci. 2011;18:535–538. doi: 10.1016/j.jocn.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 105.Trevisson E, DiMauro S, Navas P, Salviati L. Coenzyme Q deficiency in muscle. Curr Opin Neurol. 2011;24:449–456. doi: 10.1097/WCO.0b013e32834ab528. [DOI] [PubMed] [Google Scholar]
- 106.Spinazzi M, Casarin A, Pertegato V, Salviati L, Angelini C. Assessment of mitochondrial respiratory chain enzymatic activities on tissues and cultured cells. Nat Protoc. 2012;7:1235–1246. doi: 10.1038/nprot.2012.058. [DOI] [PubMed] [Google Scholar]
- 107.Montero R, et al. Analysis of coenzyme Q10 in muscle and fibroblasts for the diagnosis of CoQ10 deficiency syndromes. Clin Biochem. 2008;41:697–700. doi: 10.1016/j.clinbiochem.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 108.Payne BA, Gardner K, Coxhead J, Chinnery PF. Deep resequencing of mitochondrial DNA. Methods Mol Biol. 2015;1264:59–66. doi: 10.1007/978-1-4939-2257-4_6. [DOI] [PubMed] [Google Scholar]
- 109.Shanske S, et al. Varying loads of the mitochondrial DNA A3243G mutation in different tissues: implications for diagnosis. Am J Med Genet A. 2004;130A:134–137. doi: 10.1002/ajmg.a.30220. [DOI] [PubMed] [Google Scholar]
- 110.Yubero D, et al. Molecular diagnosis of coenzyme Q10 deficiency. Expert Rev Mol Diagn. 2015;15:1049–1059. doi: 10.1586/14737159.2015.1062727. [DOI] [PubMed] [Google Scholar]
- 111.Sadowski CE, et al. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J Am Soc Nephrol. 2015;26:1279–1289. doi: 10.1681/ASN.2014050489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tran M, et al. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest. 2011;121:4003–4014. doi: 10.1172/JCI58662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119:1275–1285. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Manny J, et al. Structural changes in the perfused canine kidney exposed to the direct action of endotoxin. Isr J Med Sci. 1980;16:153–161. [PubMed] [Google Scholar]
- 115.Trump BF, et al. The application of electron microscopy and cellular biochemistry to the autopsy. Hum Pathol. 1975;6:499–516. doi: 10.1016/s0046-8177(75)80068-2. [DOI] [PubMed] [Google Scholar]
- 116.Takasu O, et al. Mechanisms of cardiac and renal dysfunction in patients dying of sepsis. Am J Respir Crit Care Med. 2013;187:509–517. doi: 10.1164/rccm.201211-1983OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Parekh DJ, et al. Tolerance of the human kidney to isolated controlled ischemia. J Am Soc Nephrol. 2013;24:506–517. doi: 10.1681/ASN.2012080786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zsengeller ZK, et al. Cisplatin nephrotoxicity involves mitochondrial injury with impaired tubular mitochondrial enzyme activity. J Histochem Cytochem. 2012;60:521–529. doi: 10.1369/0022155412446227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Funk JA, Schnellmann RG. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol Renal Physiol. 2012;302:F853–F864. doi: 10.1152/ajprenal.00035.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang Q, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Feldkamp T, Kribben A, Roeser NF, Senter RA, Weinberg JM. Accumulation of nonesterified fatty acids causes the sustained energetic deficit in kidney proximal tubules after hypoxia-reoxygenation. Am J Physiol Renal Physiol. 2006;290:F465–F477. doi: 10.1152/ajprenal.00305.2005. [DOI] [PubMed] [Google Scholar]
- 122.Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci USA. 2000;97:2826–2831. doi: 10.1073/pnas.97.6.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bienholz A, et al. Substrate modulation of fatty acid effects on energization and respiration of kidney proximal tubules during hypoxia/reoxygenation. PLoS ONE. 2014;9:e94584. doi: 10.1371/journal.pone.0094584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Li S, et al. Transgenic expression of proximal tubule peroxisome proliferator-activated receptor-α in mice confers protection during acute kidney injury. Kidney Int. 2009;76:1049–1062. doi: 10.1038/ki.2009.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tannenbaum J, Purkerson ML, Klahr S. Effect of unilateral ureteral obstruction on metabolism of renal lipids in the rat. Am J Physiol. 1983;245:F254–F262. doi: 10.1152/ajprenal.1983.245.2.F254. [DOI] [PubMed] [Google Scholar]
- 126.Zager RA, Johnson AC, Hanson SY. Renal tubular triglyercide accumulation following endotoxic, toxic, and ischemic injury. Kidney Int. 2005;67:111–121. doi: 10.1111/j.1523-1755.2005.00061.x. [DOI] [PubMed] [Google Scholar]
- 127.Cocheme HM, et al. Mitochondrial targeting of quinones: therapeutic implications. Mitochondrion. 2007;7:S94–S102. doi: 10.1016/j.mito.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 128.Kelso GF, et al. Selective targeting of a redox-active ubiquinone to mitochondria within cells: antioxidant and antiapoptotic properties. J Biol Chem. 2001;276:4588–4596. doi: 10.1074/jbc.M009093200. [DOI] [PubMed] [Google Scholar]
- 129.Zhao K, et al. Cell-permeable peptide antioxidants targeted to inner mitochondrial membrane inhibit mitochondrial swelling, oxidative cell death, and reperfusion injury. J Biol Chem. 2004;279:34682–34690. doi: 10.1074/jbc.M402999200. [DOI] [PubMed] [Google Scholar]
- 130.Szeto HH, et al. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J Am Soc Nephrol. 2011;22:1041–1052. doi: 10.1681/ASN.2010080808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Mukhopadhyay P, et al. Mitochondrial-targeted antioxidants represent a promising approach for prevention of cisplatin-induced nephropathy. Free Radic Biol Med. 2012;52:497–506. doi: 10.1016/j.freeradbiomed.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tang WX, Wu WH, Qiu HY, Bo H, Huang SM. Amelioration of rhabdomyolysis-induced renal mitochondrial injury and apoptosis through suppression of Drp-1 translocation. J Nephrol. 2013;26:1073–1082. doi: 10.5301/jn.5000268. [DOI] [PubMed] [Google Scholar]
- 133.Morigi M, et al. Sirtuin 3-dependent mitochondrial dynamic improvements protect against acute kidney injury. J Clin Invest. 2015;125:715–726. doi: 10.1172/JCI77632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ishihara M, et al. Sestrin-2 and BNIP3 regulate autophagy and mitophagy in renal tubular cells in acute kidney injury. Am J Physiol Renal Physiol. 2013;305:F495–F509. doi: 10.1152/ajprenal.00642.2012. [DOI] [PubMed] [Google Scholar]
- 135.Jiang M, et al. Autophagy in proximal tubules protects against acute kidney injury. Kidney Int. 2012;82:1271–1283. doi: 10.1038/ki.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Puigserver P, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 137.Sweeney TE, Suliman HB, Hollingsworth JW, Piantadosi CA. Differential regulation of the PGC family of genes in a mouse model of Staphylococcus aureus sepsis. PLoS ONE. 2010;5:e11606. doi: 10.1371/journal.pone.0011606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sweeney TE, Suliman HB, Hollingsworth JW, Welty-Wolf KE, Piantadosi CA. A toll-like receptor 2 pathway regulates the Ppargc1a/b metabolic co-activators in mice with Staphylococcal aureus sepsis. PLoS ONE. 2011;6:e25249. doi: 10.1371/journal.pone.0025249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rasbach KA, Schnellmann RG. PGC-1α over-expression promotes recovery from mitochondrial dysfunction and cell injury. Biochem Biophys Res Commun. 2007;355:734–739. doi: 10.1016/j.bbrc.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 140.Jesinkey SR, et al. Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J Am Soc Nephrol. 2014;25:1157–1162. doi: 10.1681/ASN.2013090952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wrigley A, Wilkinson S, Appleby JB. Mitochondrial replacement: ethics and identity. Bioethics. 2015;29:631–638. doi: 10.1111/bioe.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schon EA, et al. A direct repeat is a hotspot for large-scale deletion of human mitochondrial DNA. Science. 1989;244:346–349. doi: 10.1126/science.2711184. [DOI] [PubMed] [Google Scholar]
- 143.Glerum DM, Tzagoloff A. Isolation of a human cDNA for heme A:farnesyltransferase by functional complementation of a yeast cox10 mutant. Proc Natl Acad Sci USA. 1994;91:8452–8456. doi: 10.1073/pnas.91.18.8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Lim SC, et al. Mutations in LYRM4, encoding iron–sulfur cluster biogenesis factor ISD11, cause deficiency of multiple respiratory chain complexes. Hum Mol Genet. 2013;22:4460–4473. doi: 10.1093/hmg/ddt295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Cogliati S, et al. Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell. 2013;155:160–171. doi: 10.1016/j.cell.2013.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 147.Hirano M, Lagier-Tourenne C, Valentino ML, Marti R, Nishigaki Y. Thymidine phosphorylase mutations cause instability of mitochondrial DNA. Gene. 2005;354:152–156. doi: 10.1016/j.gene.2005.04.041. [DOI] [PubMed] [Google Scholar]
- 148.Valnot I, et al. A mutation in the human heme A:farnesyltransferase gene (COX10) causes cytochrome c oxidase deficiency. Hum Mol Genet. 2000;9:1245–1249. doi: 10.1093/hmg/9.8.1245. [DOI] [PubMed] [Google Scholar]
- 149.Tay SK, et al. Unusual clinical presentations in four cases of Leigh disease, cytochrome C oxidase deficiency, and SURF1 gene mutations. J Child Neurol. 2005;20:670–674. doi: 10.1177/08830738050200080701. [DOI] [PubMed] [Google Scholar]
- 150.de Lonlay P, et al. A mutant mitochondrial respiratory chain assembly protein causes complex III deficiency in patients with tubulopathy, encephalopathy and liver failure. Nat Genet. 2001;29:57–60. doi: 10.1038/ng706. [DOI] [PubMed] [Google Scholar]
- 151.Tucker EJ, et al. Mutations in the UQCC1-interacting protein, UQCC2, cause human complex III deficiency associated with perturbed cytochrome b protein expression. PLoS Genet. 2013;9:e1004034. doi: 10.1371/journal.pgen.1004034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Magner M, et al. TMEM70 deficiency: long-term outcome of 48 patients. J Inherit Metab Dis. 2015;38:417–426. doi: 10.1007/s10545-014-9774-8. [DOI] [PubMed] [Google Scholar]
- 153.Saada A, et al. Antenatal mitochondrial disease caused by mitochondrial ribosomal protein (MRPS22) mutation. J Med Genet. 2007;44:784–786. doi: 10.1136/jmg.2007.053116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Nakajima J, et al. A novel homozygous YARS2 mutation causes severe myopathy, lactic acidosis, and sideroblastic anemia 2. J Hum Genet. 2014;59:229–232. doi: 10.1038/jhg.2013.143. [DOI] [PubMed] [Google Scholar]
- 155.Belostotsky R, et al. Mutations in the mitochondrial seryl-tRNA synthetase cause hyperuricemia, pulmonary hypertension, renal failure in infancy and alkalosis, HUPRA syndrome. Am J Hum Genet. 2011;88:193–200. doi: 10.1016/j.ajhg.2010.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bourdon A, et al. Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nat Genet. 2007;39:776–780. doi: 10.1038/ng2040. [DOI] [PubMed] [Google Scholar]
- 157.Prasad C, et al. Exome sequencing reveals a homozygous mutation in TWINKLE as the cause of multisystemic failure including renal tubulopathy in three siblings. Mol Genet Metab. 2013;108:190–194. doi: 10.1016/j.ymgme.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 158.El-Hattab AW, Scaglia F. Mitochondrial DNA depletion syndromes: review and updates of genetic basis, manifestations, and therapeutic options. Neurotherapeutics. 2013;10:186–198. doi: 10.1007/s13311-013-0177-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Carrozzo R, et al. SUCLA2 mutations are associated with mild methylmalonic aciduria, Leigh-like encephalomyopathy, dystonia and deafness. Brain. 2007;130:862–874. doi: 10.1093/brain/awl389. [DOI] [PubMed] [Google Scholar]
- 160.Dimmock DP, et al. Clinical and molecular features of mitochondrial DNA depletion due to mutations in deoxyguanosine kinase. Hum Mutat. 2008;29:330–331. doi: 10.1002/humu.9519. [DOI] [PubMed] [Google Scholar]