Abstract
Background: The impact of multiple socio-economic deprivation on patient outcomes in primary renal diseases is unknown. We aimed to assess whether risk of death or requiring renal replacement therapy (RRT) in patients with primary glomerulonephritis (GN) was higher in patients living in an area of multiple socio-economic deprivation.
Methods: Patients undergoing native renal biopsy between 2000 and 2014 were identified. Baseline demographics, postcode at time of biopsy, follow-up blood pressure, proteinuria and time to death or RRT were recorded. The Scottish Index of Multiple Deprivation (SIMD) is a multidimensional model used to measure deprivation based on postcode. Using SIMD, patients were separated into tertiles of deprivation.
Results: A total of 797 patients were included, 64.2% were male with mean age of 54.1 (standard deviation 17.0) years. Median follow-up was 6.3 (interquartile range 3.7–9.4) years during which 174 patients required RRT and 185 patients died. Patients in the most deprived tertile of deprivation were significantly more likely to die than those in the least deprived tertile [hazard ratio (HR) 2.2, P < 0.001], independent of age, baseline serum creatinine and blood pressure. They were not more likely to require RRT (P = 0.22). The increased mortality risk in the most deprived tertile was not uniform across primary renal diseases, with the association being most marked in focal segmental glomerulosclerosis (HR 7.4) and IgA nephropathy (HR 2.7) and absent in membranous nephropathy.
Conclusion: We have demonstrated a significant independent 2-fold increased risk of death in patients with primary GN who live in an area of multiple socio-economic deprivation at the time of diagnosis as compared with those living in less deprived areas.
Keywords: biopsy, FSGS, glomerulonephritis, IgA nephropathy, membranous nephropathy
Introduction
In the general population, living in an area of multiple deprivation has been shown to be associated with a significantly reduced life expectancy compared with living in relative affluence [1]. Furthermore, the incidence of chronic kidney disease (CKD) is higher in areas of socio-economic deprivation [2–4] and patients who live in deprived areas are more likely to develop end-stage renal disease (ESRD) [5].
In a healthcare system that is free at the point of access, we have previously demonstrated a significant association between multiple socio-economic deprivation and the incidence of IgA nephropathy (IgAN), an association not seen in other primary glomerulopathies [6]. What is not clear, however, is whether living in an area of socio-economic deprivation impacts upon patient outcomes in primary renal diseases.
The influence of socio-economic deprivation on health outcomes is complex, with deprivation used as a surrogate for factors including access to healthcare, health beliefs and behaviours, biological factors such as genetics, and environmental factors including housing, water supply and diseases [7]. It might be hypothesized that once a condition is diagnosed and patients are treated, population level inequalities would no longer apply, particularly in a healthcare setting that is free at the point of use.
We aimed to assess whether risk of death or requiring renal replacement therapy (RRT) was higher in patients with primary glomerulonephritis (GN) living in an area of multiple deprivation.
Materials and methods
Study population
The Glasgow Renal and Transplant Unit provides renal services to the West of Scotland, population 1.6 million. Ninewells Hospital Dundee (NWD) is located in the East of Scotland and covers a population of 400 000. In 2007 the population of Scotland was 5 144 200 [8], and therefore the two hospitals cover over a third of the Scottish population.
Data sources
In all, 2827 consecutive adult patients who underwent native renal biopsy between 2000 and 2012 (2000–14 at NWD) in the two centres were identified. Of these, 859 patients had a diagnosis of primary GN. Patients with minimal change nephropathy (n = 62) were excluded from both cohorts as this is not a progressive proteinuric disease, leaving a cohort of 797 patients.
Using the Electronic Renal Patient Record, renal function, proteinuria, blood pressure and postcode at the time of biopsy were recorded. The mean value for blood pressure, and median values for serum creatinine and urine protein to creatinine ratio during follow-up for individual patients were recorded. Clinic blood pressure every 3 months for the first 2 years after the diagnosis were recorded were available. Time to RRT for ESRD or death was noted.
Measure of multiple deprivation
The Scottish Index of Multiple Deprivation (SIMD) is a relative ranking of all the geographical areas of Scotland produced by the Scottish Government [9, 10]. The SIMD uses a multidimensional model to assess deprivation, using 38 indicators across 7 domains. Each domain is weighted according to its importance in the overall concept of multiple deprivation and robustness of the indicators comprising the domain. The domains are as follows: employment, income, crime, housing, health including standardized mortality ratio, alcohol and drug misuse, education and geographical and convenience access.
Scotland is geographically separated into 6505 areas (datazones) based on postcode with an average of 750 people living in each. This ranking provides a relative measure of deprivation, whereby one postcode area can be ranked relative to another, but cannot be used to determine ‘how much’ more deprived one area is compared with another.
Using the 2009 SIMD rankings, patients were separated into tertiles of deprivation: Tertile 1 (SIMD 2009) datazones 1–2168 (most deprived); Tertile 2 (SIMD 2009) datazones 2169–4336; Tertile 3 (SIMD 2009) datazones 4337–6505 (least deprived). The postcode of the patient undergoing biopsy was then used to determine his or her deprivation tertile.
Statistical analysis
Biopsy rate per million population per year was calculated. Survival analysis was conducted using Cox proportional hazards model. Statistics were calculated using IBM SPSS Statistics version 22.
Ethics
The Scottish Renal Biopsy Registry has multisite ethical approval for data collection and epidemiological analysis.
Results
Baseline demographics
A total of 797 patients were included in the study cohort. In all, 64.2% were male with mean age of 54.1 (standard deviation 17.0) years. Over 98% of patients were Caucasian. In all, 295 patients had a diagnosis of IgAN [12.6 biopsies per million population (PMP)/year], 189 focal segmental glomerulosclerosis (FSGS) (8.1 biopsies PMP/year), 185 membranous glomerulonephropathy (MGN) (7.9 biopsies PMP/year) and 128 had other causes of primary glomerulonephritis (membranoproliferative GN, undifferentiated GN, fibrillary and post-infectious GN). Table 1 demonstrates the baseline demographics of these groups. At baseline, patients with MGN were older (mean age 59.7 years versus 54.1 years overall) and had a lower mean serum creatinine at diagnosis than other primary renal diseases.
Table 1.
Baseline demographics and outcomes—all patients and by primary renal disease
| All cases (n = 797) | MGN, n = 185 (23.2%) | IgAN, n = 295 (37.0%) | FSGS, n = 189 (23.7%) | Other, n = 128 (16.1%) | |
|---|---|---|---|---|---|
| Male (n, %) | 512 (64.2%) | 111 (60.0%) | 211 (71.5%) | 109 (57.7%) | 81 (63.3%) |
| Age (years) | 54.1 (17.0) | 59.7 (16.0) | 50.3 (16.4) | 53.8 (16.8) | 55.6 (17.0) |
| Baseline serum creat (μmol/L/1.73 m2) | 128 (90–207) | 100 (79–150) | 144 (100–230) | 126 (90–181) | 164 (100–310) |
| Baseline uPCR (mg/mmol) | 346 (154–702) | 317 (128–624) | 343 (169–691) | 411 (178–748) | 302 (170–797) |
| SBP baseline (mmHg) | 144 (24) | 142 (25) | 144 (20) | 143 (28) | 145 (23) |
| DBP baseline (mmHg) | 80 (13) | 77 (12) | 81 (12) | 80 (12) | 78 (15) |
| SBP follow-up (mmHg) | 134 (31) | 133 (26) | 133 (32) | 135 (33) | 135 (35) |
| DBP follow-up (mmHg) | 74 (17) | 72 (14) | 76 (18) | 74 (18) | 73 (18) |
| uPCR follow-up (mg/mmol) | 122 (49–314) | 117 (40–323) | 130 (51–312) | 109 (45–348) | 125 (60–373) |
| Follow-up (years) | 6.3 (3.8–9.4) | 6.2 (4.0–9.4) | 6.2 (3.6–8.9) | 7.1 (4.8–10.5) | 5.3 (1.8–9.8) |
| RRT (n, %) | 174 (21.8%) | 27 (14.6%) | 80 (27.1%) | 33 (17.5%) | 34 (26.6%) |
| Dead (n, %) | 185 (23.2%) | 51 (27.6%) | 57 (19.3%) | 41 (21.7%) | 36 (28.1%) |
| Time to RRT (days) | 2022 (752–3218) | 2115 (1281–3386) | 1863 (706–2976) | 2395 (1332–3485) | 1430 (213–2924) |
| Time to death (days) | 2358 (1419–3535) | 2299 (1495–3415) | 2283 (1361–3304) | 2680 (1853–3822) | 1924 (701–3731) |
| Death or RRT (n, %) | 296 (37.1%) | 69 (37.3%) | 113 (38.3%) | 61 (32.3%) | 53 (41.4%) |
| Time to death or RRT (days) | 2027 (752–3230) | 2145 (1281–3386) | 1863 (706–3001) | 2380 (1258–3420) | 1430 (213–2924) |
Mean (standard deviation) or median (interquartile range). uPCR, urinary protein to creatinine ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; follow-up, mean or median during first 2 years of follow-up; T1–3, Tertiles 1–3.
The three tertiles of multiple socio-economic deprivation were very similar in terms of age and gender distribution (Table 2). During follow-up, patients in the least deprived tertile achieved better blood pressure control in the first 2 years after diagnosis.
Table 2.
Baseline demographics and outcomes by deprivation tertile
| All cases (n = 797) | T1 — most deprived (n = 378) | T2 (n = 220) | T3 — least deprived (n = 199) | |
|---|---|---|---|---|
| Male (n, %) | 512 (64.2) | 234 (61.9) | 148 (67.3) | 130 (65.3) |
| Age (years) | 54.1 (17.0) | 53.8 (16.4) | 54.7 (16.8) | 54.2 (18.2) |
| Baseline serum creat (μmol/L/1.73 m2) | 128 (90–207) | 130 (95–220) | 128 (90–181) | 120 (85–192) |
| Baseline uPCR (mg/mmol) | 346 (154–702) | 370 (143–689) | 315 (148–714) | 360 (179–736) |
| SBP baseline (mmHg) | 144 (24) | 146 (24) | 142 (26) | 141 (21) |
| DBP baseline (mmHg) | 80 (13) | 81 (13) | 79 (12) | 77 (11) |
| SBP follow-up (mmHg) | 134 (31) | 134 (30) | 138 (26) | 128 (36) |
| DBP follow-up (mmHg) | 74 (17) | 74 (17) | 76 (15) | 71 (20) |
| uPCR follow-up (mg/mmol) | 122 (49–314) | 125 (48–299) | 111 (42–314) | 124 (61–363) |
| RRT (n, %) | 174 (21.8%) | 90 (23.8%) | 49 (22.3%) | 35 (20.1%) |
| Dead (n, %) | 185 (23.2%) | 107 (28.3%) | 51 (23.2%) | 27 (13.6%) |
| Time to RRT (days) | 2022 (752–3218) | 1973 (634–3215) | 2025 (771–3134) | 2148 (1056–3403) |
| Time to death (days) | 2358 (1419–3535) | 2325 (1354–3436) | 2326 (1493–3149) | 2399 (1500–3684) |
| Follow-up (days) | 2299 (1367–3446) | 2308 (1334–3425) | 2280 (1302–3264) | 2372 (1438–3661) |
| Death or RRT (n, %) | 296 (37.1%) | 158 (41.8%) | 82 (37.3%) | 56 (28.1%) |
| Time to death or RRT (days) | 2027 (752–3230) | 1982 (635–3215) | 2024 (771–3134) | 2169 (1056–3403) |
Mean (standard deviation) or median (interquartile range). uPCR, urinary protein to creatinine ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; follow-up, mean or median during first 2 years of follow-up; T1–3, Tertiles 1–3.
Outcomes
Overall, 174 patients required RRT, 185 died and 296 required RRT or died. Eight patients were on RRT at the time of biopsy and were excluded from analyses of risk of requiring RRT. Median follow-up was 6.3 (interquartile range 3.7–9.4) years. More patients with IgAN or ‘Other’ primary renal diseases required RRT (Table 1). Time to RRT or death was shorter in patients with ‘Other’ primary renal diseases.
Patients in the most deprived tertile (Tertile 1) were significantly more likely to die than those in Tertiles 2 or 3 (P < 0.001). They were also significantly more likely to reach the combined endpoint of death or RRT (P = 0.005) but not the single endpoint of requiring RRT (P = 0.22) (Table 3).
Table 3.
Incidence of death or RRT by tertile of deprivation
| Category | T1 (n = 378) | T2 (n = 220) | T3 (n = 199) | P |
|---|---|---|---|---|
| RRT (n = 174) | 90 (24%) | 49 (22%) | 35 (18%) | 0.22 |
| Death (n = 185) | 107 (28%) | 51 (23%) | 27 (14%) | <0.001 |
| Death or RRT (n = 296) | 158 (42%) | 82 (37%) | 56 (28%) | 0.005 |
T1–3, Tertiles 1 (most deprived) to 3 (least deprived). Comparison by chi-square test.
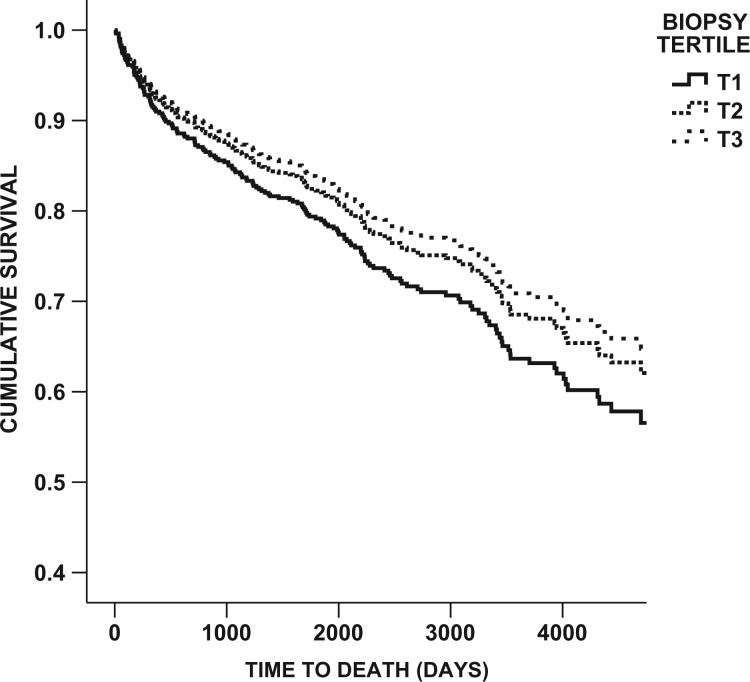
Using a multivariate Cox proportional hazards model, living in a deprivation Tertile 1 area was independently associated with a greater than 2-fold increase in the risk of death, independent of age, baseline serum creatinine and blood pressure (P = 0.001) (Table 4, Figure 1). Primary renal disease was not an independent predictor of risk of death, nor was gender. Similarly, living in deprivation Tertile 1 (most deprived) was independently associated with a 50% increase in the risk of death or requiring RRT compared with those living in Tertiles 2 or 3, less deprived areas (P = 0.011).
Table 4.
Multivariate analysis of time to death
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| Factor | HR | CI HR | P | HR | CI HR | P |
| Age | 1.073 | 1.061–1.085 | <0.001 | 1.082 | 1.066–1.097 | <0.001 |
| Creatinine baseline | 1.002 | 1.002–1.003 | <0.001 | 1.003 | 1.002–1.004 | <0.001 |
| uPCR baseline | 1.00 | 0.99–1.0 | 0.317 | |||
| SIMD Tertile 1 | 2.17 | 1.42–3.31 | <0.001 | 2.26 | 1.373–3.719 | 0.001 |
| SBP baseline | 1.01 | 1.003–1.018 | 0.007 | 1.001 | 0.994–1.008 | 0.761 |
| uPCR median over 1st 2y | 1.00 | 0.99–1.01 | 0.844 | |||
Time to death (185 deaths) by Cox proportional hazards model in the whole cohort. Non-significant univariate predictors: baseline diastolic blood pressure or follow-up blood pressure, protein:creatinine ratio (uPCR). HR, hazard ratio; CI, confidence interval; SBP, systolic blood pressure.
Fig. 1.
Cox regression survival curve of time to death (days)—all cases, separated by tertile of deprivation. P = 0.001. T1–3, Tertiles 1–3.
In a univariate Cox proportional hazards modelling, living in an area categorized as deprivation Tertile 1 was significantly associated with an increased risk of requiring RRT (P = 0.04) but this effect was not independent of serum creatinine at baseline or baseline systolic blood pressure (Table 5).
Table 5.
Multivariate analysis of time to RRT
| Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| Factor | HR | CI HR | P | HR | CI HR | P |
| Creatinine baseline | 1.006 | 1.005–1.007 | <0.001 | 1.006 | 1.006–1.007 | <0.001 |
| SIMD Tertile 1 | 1.517 | 1.016–2.265 | 0.042 | |||
| SBP baseline | 1.014 | 1.007–1.022 | <0.001 | 1.011 | 1.001–1.02 | 0.03 |
| SBP follow-up | 1.013 | 1.003–1.023 | 0.01 | |||
| DBP baseline | 1.022 | 1.008–1.036 | 0.002 | |||
Time to RRT (165 events—9 occurred prior to biopsy and were excluded) by Cox proportional hazards model in the whole cohort. Non-significant univariate predictors were follow-up diastolic blood pressure, baseline or follow-up urinary protein excretion and age. HR, hazard ratio; CI, confidence interval; SBP, systolic blood pressure; DBP, diastolic blood pressure.
To establish whether this effect is the same regardless of primary renal disease, the sensitivity analyses were repeated examining membranous nephropathy, IgAN and FSGS alone. Table 6 details the incidence of the primary endpoints by primary renal disease. In patients with membranous nephropathy, living in an area of multiple socio-economic deprivation is not associated with an increased risk of death (P = 0.87) or requirement for RRT (P = 0.64). Living in an area of multiple socio-economic deprivation at the time of a diagnosis of IgAN is independently associated with a 2.7-fold increased risk of death (P = 0.03), independent of age, serum creatinine at diagnosis and blood pressure. There was not an increased risk of requiring RRT in these patients. The same pattern was seen in FSGS, where deprivation Tertile 1 conferred a 7.4-fold increased risk of death (P = 0.002), independent of age, serum creatinine at baseline and blood pressure. However, living in a more deprived area was not a significant predictor of risk of requiring RRT in patients with FSGS.
Table 6.
Primary endpoints by primary renal disease
| Membranous nephropathy | ||||
|---|---|---|---|---|
| Category | T1 (n = 93) | T2 (n = 47) | T3 (n = 45) | P |
| RRT (n = 27) | 13 (14%) | 10 (21%) | 4 (9%) | 0.236 |
| Death (n = 51) | 25 (27%) | 14 (30%) | 12 (27%) | 0.925 |
| Death or RRT (n = 69) | 32 (34%) | 21 (45%) | 16 (36%) | 0.476 |
| IgAN | ||||
| Category | T1 (n = 143) | T2 (n = 85) | T3 (n = 67) | P |
| RRT | 44 (31%) | 19 (22%) | 17 (25%) | 0.36 |
| Death | 37 (26%) | 14 (17%) | 6 (9%) | 0.011 |
| Death or RRT | 66 (46%) | 27 (32%) | 20 (30%) | 0.026 |
| FSGS | ||||
| Category | T1 (n = 78) | T2 (n = 57) | T3 (n = 57) | P |
| RRT | 16 (20.5%) | 10 (18.5%) | 7 (12.3%) | 0.448 |
| Death | 25 (32.1%) | 12 (22.2%) | 4 (7.0%) | 0.002 |
| Death or RRT | 31 (39.7%) | 19 (35.2%) | 11 (19.3%) | 0.037 |
T1–3, Tertiles 1–3; T1, most socio-economically deprived area. Estimate of significance using chi-square test. P < 0.05 = significant.
Cause of death
The cause of death was not available for all patients but 14 patients died of ischaemic heart disease, 10 patients died of other vascular disease, 19 died of sepsis, 10 deaths were due to malignancy and 10 patients died of other causes.
Discussion
In this study, we have demonstrated a significant 2-fold increased risk of death in patients with primary GN, who live in an area of multiple socio-economic deprivation at the time of diagnosis, compared with those living in relative affluence. This association is independent of age, baseline renal function, blood pressure control and proteinuria. Patients living in deprived areas were not more likely to require RRT for ESRD.
This is the first study to look at the impact of deprivation on patients with a diagnosis of primary GN and not simply CKD, which encompasses a multitude of causes and associations [11]. The overall death rate of 23% in the study, during a median follow-up of 6.3 years, is remarkable and highlights that a diagnosis of primary GN carries significant burden for patients, strengthening the argument for aggressive risk factor modification. Although the cause of death was only available for a minority of patients, the causes were diverse and not purely cardiovascular or malignancy-related.
Living in an area of multiple socio-economic deprivation is known to confer an increased mortality risk compared with living in relative affluence. In Scotland, life expectancy is 13 years shorter among males born in the most deprived decile compared with the least deprived decile [1]. Scotland has the lowest life expectancy in Western Europe and Glasgow has the lowest life expectancy within Scotland, suggesting that the differences seen here may be augmented compared with other geographical areas.
In the general population in Scotland, at the age of 55 years (the average seen in this cohort), men can expect to live a further 25 years and women a further 28 years [12]. However, this does vary significantly by geography. Among the general population in SIMD Tertile 1, men have an average life expectancy of 23.0 years and women 26.2 years at the age of 55 years, while in Tertile 3 this rises to 27.7 years for men and 29.9 years for women. Therefore, it appears that the mortality risk seen in patients in this study is above the national average and that the difference seen with deprivation (2- to 7-fold increase in IgA and FSGS) is greater than would be expected. It also suggests that the absence of such an association in MGN is unusual.
The influence of multiple socio-economic deprivation on health has been described as a reflection of inequality in the distribution of power, money and resources in society. Inequalities lead to differences in income, employment, education and daily living conditions, and are linked to negative health behaviours including smoking, poor diet, alcohol misuse and other factors, all of which impact upon an individuals’ risk of morbidity and mortality [13]. Influencing these complex and long-term inequalities requires a population level social and economic intervention and is the focus of much research in Scotland. When reviewing patients in the clinic, it is important to bear in mind the environmental and contextual factors for each patient and to target interventions appropriately.
Whether the increased risk of death seen in this study among patients from deprived areas is an effect of health behaviours such as diet, smoking and alcohol, and independent of renal specific factors, was beyond the scope of this study. It would be impossible to separate out the influence of structural and environmental factors and of health behaviour with any degree of certainty. Perhaps renal disease and the impact upon cardiovascular risk [14] has a synergistic effect with these lifestyle choices. Scotland was one of the first country to ban smoking in public places and is attempting to introduce a minimum pricing strategy for alcohol; however, any impact of these measures on outcomes for patients with GN and other conditions will not be seen for many years.
The fact that the effect differs by primary renal disease, however, counts against a generic effect of CKD and reduced estimated glomerular filtration rate. The effect was most marked in patients with FSGS but was completely absent in patients with membranous nephropathy. Whether this is because patients with MGN are older at diagnosis and their burden of risk is already established or whether IgAN and FSGS have environmental associations is not clear. Also, the recent discovery of the anti-PLA2 receptor antibody [15] suggests that idiopathic MGN is a primary autoimmune condition and the natural history and systemic effects may differ from those of IgAN and FSGS, which have more systemic associations.
A previously reported correlation between low birth weight and renal disease is interesting [16], with lower nephron number resulting in nephromegaly and perhaps an increased susceptibility to glomerular disease. Low birth weight is more common in areas of socio-economic deprivation and might explain some of the increased risk seen.
The lack of association between deprivation status and risk of requiring RRT is curious in light of the strong association with mortality. The reported association between incidence of ESRD and requirement for RRT in patients from areas of multiple deprivation may have more to do with prevalence of disease than greater rates of progression. Predictors of requiring RRT in this study were renal function at baseline and blood pressure, both of which were associated with the risk of death. It is perhaps reassuring that in a health service free at the point of access, deprivation appears to have no impact upon renal outcomes, although access to healthcare is affected by more than cost. There may also be an issue of competing risk, with patients from more deprived areas dying before reaching ESRD and therefore any excess risk is lost.
Scotland, as a whole, is not racially diverse and over 90% of patients included in this study are White British, suggesting that differences in outcome are not related to race. Also, although there are wide differences in relative affluence in Scotland, it is a first world country where there is universal access to clean water, sanitation and health care. Communicable diseases are infrequent.
Further study is required to establish whether aggressive cardiovascular risk modification improves mortality rate in primary GN, where research tends to focus on the progression of disease to end-stage renal failure. It would also be valuable to investigate whether certain patient groups, such as those living in relative deprivation, benefit more than others from these interventions. Also, many primary renal diagnoses represent a spectrum of disease, collected together by histological appearance, which may reflect differing pathogenesis, and further study of environmental effects on renal disease is merited.
Our data relating to patients with primary renal disease are complete and no patients were lost to follow-up.
Limitations
This study is an observational study. Therefore, we only report associations and cannot determine causality. SIMD is a population measure of multiple deprivation, rather than an individual level measure, meaning that patients can live in a deprived area but may not in fact be deprived. More complete data on cause of death may have shed more light on the mechanism underlying the observed increased risk of death. A measure of comorbidity including diabetes would have been valuable. Information about medications taken during follow-up, such as anti-platelets, inhibitors of the renin angiotensin system and statin therapy might have been revealing. We were unable to determine whether the diagnosis of FSGS was primary or secondary, which would have been useful to ascertain.
Conclusions
In this novel study looking at the association between living in an area of multiple deprivation and being diagnosed with a primary glomerular disease, we have shown that patients who live in a deprived area have at least a 2-fold increased risk of death compared with those who live in relative affluence. This increased risk is greater than would be expected from the background population, suggesting an interplay between renal disease and the environment. Interestingly, no association with the risk of requiring RRT was seen, which may reflect the competing risk of death. The effect on mortality is not equal across all primary renal diseases and is absent in MGN but significantly increased in IgAN and FSGS, suggesting that further study should be directed towards the mechanisms of disease in these conditions.
Acknowledgements
The results presented in this article have not been published previously in whole or part, except in abstract format.
Conflict of interest statement
None declared.
References
- 1. Beeston CMG, Ford J, Wimbush E. et al. Health Inequalities Policy Review for the Scottish Ministerial Task Force on Health Inequalities. Edinburgh: NHS Health Scotland, 2014 [Google Scholar]
- 2. Hossain MP, Palmer D, Goyder E. et al. Social deprivation and prevalence of chronic kidney disease in the UK: workload implications for primary care. QJM 2012; 105: 167–175 [DOI] [PubMed] [Google Scholar]
- 3. Coresh J, Wei GL, McQuillan G. et al. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 2001; 161: 1207–1216 [DOI] [PubMed] [Google Scholar]
- 4. Al Qaoud TM, Nitsch D, Wells J. et al. Socioeconomic status and reduced kidney function in the Whitehall II Study: role of obesity and metabolic syndrome. Am J Kidney Dis 2011; 58: 389–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caskey FJ. Renal replacement therapy: can we separate the effects of social deprivation and ethnicity? Kidney Int Suppl (2011) 2013; 3: 246–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. McQuarrie EP, Mackinnon B, McNeice V. et al. The incidence of biopsy-proven IgA nephropathy is associated with multiple socioeconomic deprivation. Kidney Int 2014; 85: 198–203 [DOI] [PubMed] [Google Scholar]
- 7. Garcia-Garcia G, Jha V, Tao Li PK. et al. Chronic kidney disease (CKD) in disadvantaged populations. Clin Kidney J 2015; 8: 3–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. MacKenzie G. Scotland's Population 2011: The Registrar General’s Annual Review of Demographic Trends 157th Edition 2012
- 9. Scottish Government. Scottish Index of Multiple Deprivation 2009: General Report.Edinburgh, UK: Scottish Government, 2009
- 10. Scottish Government. Evaluation of Statistical Techniques in the Scottish Index of Multiple Deprivation Document by the University of Glasgow 2012
- 11. Hossain MP, Goyder EC, Rigby JE. et al. CKD and poverty: a growing global challenge. Am J Kidney Dis 2009; 53: 166–174 [DOI] [PubMed] [Google Scholar]
- 12. National Records of Scotland. Life Expectancy for Areas within Scotland. Edinburgh, UK: National Records of Scotland, 2014 [Google Scholar]
- 13. Scottish Government. Equally Well: Report of the Ministerial Task Force on Health Inequalities, Vol. 2 2008 [Google Scholar]
- 14.Scottish Health Survey 2010 - Volume 1: Main Report.2011
- 15. Beck LH, Jr, Bonegio RG, Lambeau G. et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 2009; 361: 11–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lackland DT, Bendall HE, Osmond C. et al. Low birth weights contribute to high rates of early-onset chronic renal failure in the Southeastern United States. Arch Intern Med 2000; 160: 1472–1476 [DOI] [PubMed] [Google Scholar]