Abstract
Distal renal tubular acidosis caused by missense mutations in kidney isoform of anion exchanger 1 (kAE1/SLC4A1), the basolateral membrane Cl−/HCO3− exchanger of renal alpha-intercalated cells, has been extensively investigated in heterologous expression systems but rarely in human kidneys. The preferential apical localization of distal renal tubular acidosis (dRTA)-associated kAE1 mutants R901X, G609R and M909T in cultured epithelial monolayers has not been examined in human kidney. Here, we present kidney tissues from dRTA-affected siblings heterozygous for kAE1 G609R, characterized by predominant absence rather than mistargeting of kAE1 in intercalated cells. Thus, studies of heterologous recombinant expression of mutant proteins should be, whenever possible, interpreted in comparison to affected patient tissues.
Keywords: distal renal tubular acidosis, genetics, kAE1, kidney isoform of anion exchanger 1, slc4a1
Background
Familial distal renal tubular acidosis (dRTA) is caused by impaired renal acid–base homeostasis and can be accompanied by nephrocalcinosis, nephrolithiasis, hyperchloremia, hypokalemia and polyuria. If not diagnosed and treated early with bicarbonate replacement, patients display elevated risks of pyelonephritis and progression to end-stage renal disease (ESRD). The underlying defects in collecting duct acid secretion by alpha-intercalated cells (α-IC) reflect recessive loss-of-function mutations of ATP6B1 (vacuolar H+-ATPase β1 subunit) [1], ATP6VOA4 (vacuolar H+-ATPase α4 subunit) [2, 3] and CA2 (carbonic anhydrase II) [4, 5], as well as dominant and recessive loss-of-function mutations of SLC4A1 [kidney isoform of anion exchanger 1 (kAE1)] [6–12]. Although heterologous expression of these mutant gene products has allowed proposal of disease mechanisms, the pathogenic mechanisms of these mutant proteins in dRTA patient kidneys remain unclear.
kAE1 mediates electroneutral Cl−/HCO3− exchange across the basolateral membrane of α-IC. Localization of human kAE1 polypeptide expressed in polarized Madin-Darby canine kidney (MDCK) cell monolayers is also basolateral. However, dRTA-associated kAE1 mutants exhibit either retention in endoplasmic reticulum or Golgi (mutants R598H, S613F, C479W) or surface expression in nonpolarized or exclusively apical patterns (R901X, G609R, M909T) [12–17]. In contrast, the few reported histological studies of familial dRTA patient kidneys report partial or complete absence of kAE1 without evidence for apical membrane accumulation [18–22].
We report here the near-absence of detectable kAE1 in intercalated cells of ESRD nephrectomy specimens from siblings with dRTA secondary to the autosomal dominant mutation G609R in the kAE1 product of the SLC4A1 gene, in contrast to preferential apical localization of kAE1 G609R previously demonstrated in MDCK cell monolayers.
Case report
The 38-year-old male proband (III:1) was referred for recurrent, symptomatic nephrolithiasis diagnosed 10 years previously and was managed by lithotripsy and cysto-ureteroscopic extraction of calculi. Most stones contained 90% calcium phosphate/10% calcium oxalate. A few contained carbonate–apatite. Serum [HCO3−] was 20–23 mM, serum calcium 9.1 mg/dL, blood urea nitrogen 12 mg/dL, serum creatinine 0.9 mg/dL and hematological indices were normal. A 24-h urine (3.85 L containing 1949 mg creatinine) collected while on citrate therapy (25 mEq/day) was remarkable hypocitraturia, with for values of pH 7.06, calcium 430 mg (normal <250), oxalate 41 mg (normal <40), citrate 58 mg (normal >450) and urate 0.67 g (normal <0.8). A second, consecutive day collection showed similar results.
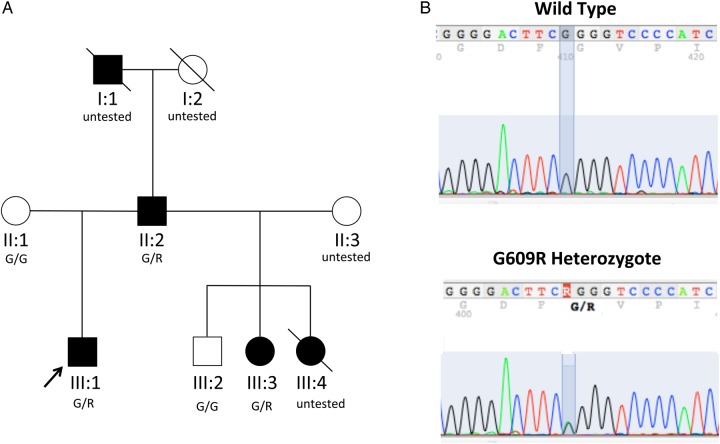
Teenage onset of nephrolithiasis in the proband's 74-year-old father (II:2) and in half-sisters III.3 (age 51 years) and III:4 (deceased at age 33 years from substance abuse) led to ESRD and transplant, without further stone disease. Autopsy of the 40-year-old paternal grandfather (I:1) revealed previously undiagnosed nephrocalcinosis. Half-brother III:2 was clinically unaffected. This personal and family history led to diagnosis of autosomal dominant dRTA in proband III:1, who has remained asymptomatic for 3 years on 15 mEq potassium citrate twice daily.
Genetic screening detected the heterozygous G609R variant of SLC4A1 (reported previously [12]) in the proband (III:1), his affected father (II:2) and surviving half-sister (III:3), whereas his unaffected half-brother (III:2) and mother expressed only wild-type alleles (Figure 1). Both affected half-sisters underwent nephrectomies for pain relief in the setting of recurrent nephrolithiasis and progressive pyelonephritis. Histopathological findings of chronic pyelonephritis, nephrocalcinosis and focal segmental glomerulosclerosis (FSGS) in the deceased sister III:4 were reported previously [25], without molecular diagnosis.
Fig. 1.
Pedigree of dRTA family. (A) The heterozygous kAE1/SLC4A1 mutation G609R was detected in affected family members (filled symbols), but not in unaffecteds (open symbols). SLC4A1 genotype status is listed under symbols. (B) The heterozygous SLC4A1 mutation c.1825G>A encoding the SLC4A1/AE1 missense substitution G609R was detected in the proband (III:1) by DNA sequencing of both strands of PCR-amplified cDNA prepared from total RNA isolated from whole blood, as previously described [23]. The cosegregating mutation was validated in genomic DNA of other family members by sequencing across SLC4A1 exon 15, as previously described [24]. Written consent was obtained under protocols approved by the Clinical Investigation Committees of Yale University School of Medicine and Beth Israel Deaconess Medical Center.
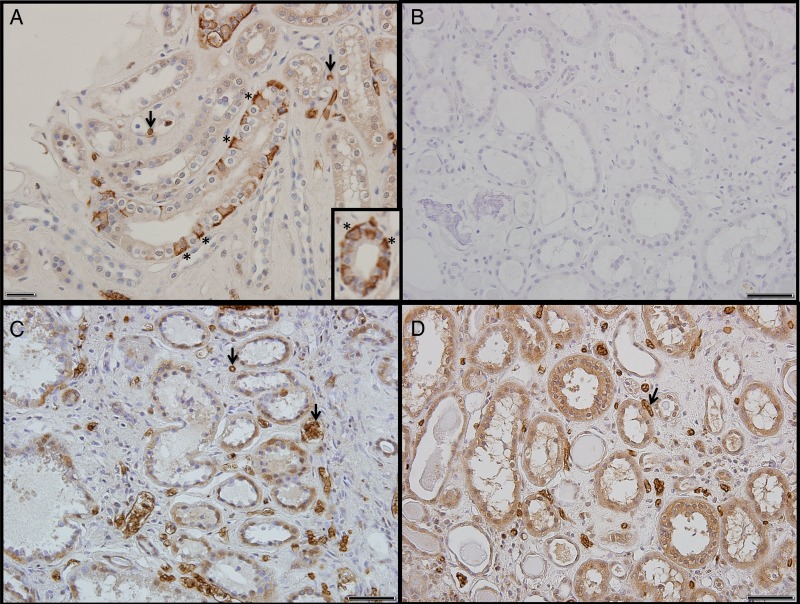
Sections of normal renal cortex from an unaffected portion of a kidney resected for carcinoma (Figure 2A and inset) served as positive controls for immunostaining (antibody validated in the Slc4a1−/− mouse) [31]. AE1 was localized in the basolateral membrane of collecting duct intercalated cells (asterisks), but not in adjacent AE1-negative principal cells. AE1 was also present in membranes of erythrocytes (black arrows), mostly within capillaries. Lack of tubular or erythroid staining in the absence of primary antibody (Figure 2B) confirmed the absence of tissue reactivity by peroxidase-coupled antibody in control and patient tissue.
Fig. 2.
kAE1 immunoperoxidase localization in kidney sections from ‘normal’ patients and dRTA patients carrying the heterozygous kAE1 missense mutation G609R. (A) Basolateral kAE1 staining of intercalated cells (asterisks) shown in longitudinal and oblique section of collecting ducts from a non-cancerous, ‘normal’ kidney. Arrows indicate eAE1-stained erythrocytes in capillaries. (Inset) Basolateral kAE1 staining of intercalated cells (asterisks) in a transverse section of collecting duct from a kidney resected for renal cancer. (B) Kidney section from patient III:3, processed in the absence of primary anti-AE1 antibody, serving as control for diffuse background staining in other panels. (C and D) Kidney sections from patient III:3 (C) and from patient III:4 (D) stained with AE1 antibody, but not detecting immunostaining pattern characteristic of α-IC cells. Arrows indicate eAE1-positive erythrocytes in capillaries. Formalin-fixed, paraffin-embedded kidney tissue from prior nephrectomies were studied with written consent from proband's half-sister and her mother. Frozen sections were unavailable. Two-micrometer sections on polysine-coated slides (Fisher, Atlanta, GA, USA) were deparaffinized and rehydrated. Mounted sections were subjected to antigen retrieval (10 mM citric acid, pH 6.0, for 30 min at 90°C). Endogenous peroxidase was quenched with 3% H2O2 for 15 min. Sections were blocked with 2.5% normal horse serum at room temperature for 40 min, then incubated 40 min with 1:200 dilution of peptide affinity-purified antibody SA6 to mouse AE2 C-terminal aa 1224–1237 [26, 27] that cross-reacts with human kAE1 in conditions in which immunostaining of human AE2 is minimal [27–30]. Specific immunolabeling was detected with the ImmPRESS HRP Anti-Rabbit IgG (Peroxidase) Polymer Detection Kit (Vector Laboratories, Burlingame, CA, USA). Sections were developed with diaminobenzidine, counterstained with hematoxylin, dehydrated and mounted in Permount. Negative control sections were incubated without primary antibody. Scale bars, 50 µm.
Paraffin blocks from affected individuals III:3 and III:4 revealed grossly apparent regions of calcification. Sections from blocks with minimal calcification revealed chronic fibrotic and inflammatory changes consistent with the clinical diagnoses of chronic pyelonephritis and nephrocalcinosis. Tubules were separated by enlarged fibrotic interstitial spaces containing congested vessels. kAE1 immunostaining in tubular cells appeared absent, although eAE1 immunostaining was evident in red blood cells (arrows) of peritubular vessels and glomeruli. In some tubules, flattened epithelial cells appeared compressed by luminal debris and casts. In other tubules, granular, heterogeneous immunostaining throughout the cells suggested antigen aggregation or precipitation. The less intense, diffusely intracellular tubular immunostain, widely but not uniformly distributed among proximal tubular and other structures (Figure 2C and D), was consistent with low-level expression of the immunologically cross-reactive, more widely expressed AE2 anion exchanger, or with nonspecific immunostaining.
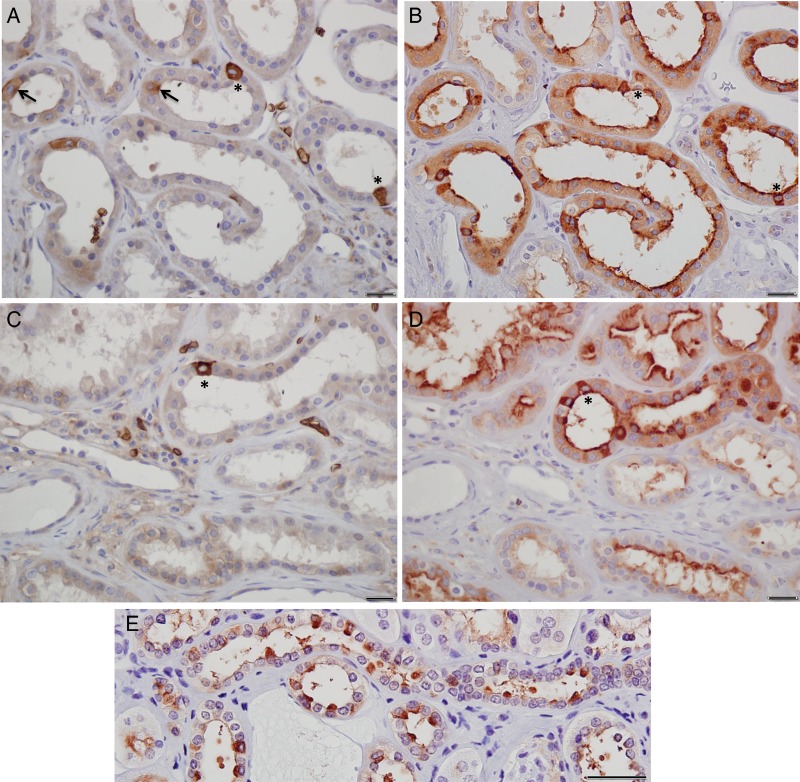
In fewer than 5% of visual fields, cells expressing abundant kAE1 were evident (Figure 3A and C, asterisks). These cells (Figure 3B and D, asterisks) were a minority among the many intercalated cells detectable in near-consecutive sections by immunostaining of vH+-ATPase (Figure 3B and D; compare with control in Figure 3E). Extremely rare tubular cells with modest apical or apicobasal enhancement of kAE1 staining (Figure 3A, arrows) were also noted.
Fig. 3.
Immunoperoxidase localization of kAE1 and vH-ATPase in kidney sections from dRTA patient III:3. (A and C) Non-representatative regions of tissue in which AE1 staining (diaminobenzidine substrate, Vector Laboratories) is evident in diffuse distribution in occasionally encountered intercalated cells (asterisks). Less frequently encountered are candidate intercalated cells with basal-apical or apical enhancement of kAE1 staining (arrows in A). Red blood cells are evident in interstitial spaces. (B and D) Near-consecutive sections showing intercalated cells with diffuse vH+-ATPase staining (NovaRed substrate, Vector Laboratories) with occasional apical enhancement. Asterisks indicate kAE1-positive cells in (A) and (B). Apical enhancement of vH+-ATPase staining is also evident in other collecting duct cells. (E) Unaffected ‘control’ kidney stained for vH+-ATPase. Scale bars: 20 µm (A–D) and 50 µm (E).
Discussion
The heterozygous kAE1 G609R mutation was previously reported in one large kindred with autosomal dominant dRTA characterized by nephrocalcinosis, nephrolithiasis and progression to ESRD [12]. The recombinant kAE1 G609R polypeptide carrying an N-terminal HA-tag was stably expressed in polarized MDCK epithelial cell monolayers, where it accumulated predominantly at the cellular apical membrane. Other dominant dRTA-associated kAE1 missense mutant polypeptides accumulating predominantly in apical membranes of MDCK cells include the N-terminally HA-tagged variant 901X [18] and the N-terminally EGFP-tagged M909T [17], studies that defined a class I PDZ-binding motif at the kAE1 C-terminus. However, subcellular localization of these mutants in affected human kidney remains unknown.
Immunohistochemical study of kidney biopsies from 11 molecularly undiagnosed, primary dRTA patients pre-end-stage revealed absence of AE1 in seven specimens, reduction of AE1 in three and normal abundance in one [19]. In one patient with autoimmune polyendocrinopathy and dRTA of unknown etiology, kAE1 immunostaining was absent on kidney biopsy [32]. Additional reports have documented absence in kidney of immunoreactive vH+-ATPase in idiopathic dRTA [20] and absent renal immunoreactivity to vH+-ATPase and AE1 in Sjogren syndrome-associated dRTA [21, 22]. Human kidney kAE1 immunolocalization has been previously reported for two dRTA patients with known causative mutations. The resected end-stage kidney of a dRTA patient heterozygous for kAE1 R589H showed lack of immunospecific staining for AE1 and apparent reduction in intercalated cell number [28]. A kidney biopsy from a dRTA patient heterozygous for kAE1 S613F revealed reduced intercalated cell numbers with either intracellular or absent kAE1 immunostaining [22]. The preserved erythrocyte immunostaining for eAE1 in sections of kidneys expressing kAE1 mutants R589H, S613F and G609R reflects the erythrocyte's increased tolerance for nominal AE1 haploinsufficiency compared with that of α-IC cells [7, 22, 28], perhaps explained in part by erythroid-specific expression of the chaperone protein, glycophorin A [33].
We have presented kAE1 immunostaining in resected kidney specimens of two siblings from a family with dRTA cosegregating with the heterozygous dRTA mutation SLC4A1 G609R. Intercalated cells were present in these end-stage kidney sections characterized by interstitital fibrosis and cellular atrophy, but kAE1-expressing intercalated cells were severely reduced in number and with reduced levels of expression. Only very rare cells exhibited potentially apical or apicobasal enhancement of kAE1-immunoreactive staining in the dRTA kidney tissue blocks available to us.
The finding of FSGS in the resected kidneys of both half-sisters III:3 and III:4 is of interest, in view of the identification of SLC4A1 as a protein-binding partner of nephrin [34]. Indeed, immunoreactive AE1 has been detected in mouse podocytes in culture and in mouse kidney glomeruli, and Ae1−/− mice exhibited glomerulomegaly, glomerular basement membrane thickening, mesangial expansion and incompletely penetrant albuminuria [34]. The clinical presentation of dRTA can be complex, as recently demonstrated by a novel autosomal recessive dRTA associated with the homozygous SLC4A1 mutation F524del initially diagnosed as a case of nephronophthisis [35]. Moreover, SLC4A1 mRNA abundance in isolated murine glomeruli as determined by SAGE may be as high as 8% of that in isolated aldosterone-sensitive distal nephron [36], and mouse glomerular SLC4A1 expression has been confirmed in subsequent microarray studies [37, 38]. In contrast, RNAseq of microdissected rat kidney revealed that SLC4A1 mRNA in isolated glomeruli is only <0.1% of the level in isolated collecting ducts [39]. Comparable human data remain unavailable. Immunoreactive kAE1 was not evident in non-erythroid glomerular cells in our end-stage tissue samples.
The plasticity of polarized expression of heterologous transgene products was recently highlighted by demonstration of cell-line-specific differences in polarized membrane protein sorting, attributable to differential expression of mu-adaptins [40]. Such cell-line-specific differences in protein trafficking proteomes may explain classical differences in polarized expression among independent clones of the same cell type [41]. Similar differences in SLC4A1 dRTA mutant polypeptide targeting were recently observed in polarized cell lines and in mice. The dominant dRTA mutant SLC4A1 R589H retained in the endoplasmic reticulum of polarized MDCK monolayers is localized normally at the basolateral membrane in two mouse kidney epithelial cell lines. Moreover, the corresponding mouse AE1 mutation R607H also localizes to basolateral membranes of α-IC cells in intact kidney, but with reduced expression levels in reduced numbers of immunologically identifiable α-IC cells (Hennings JC, Eladari, D, Huebner C, et al., manuscript in revision). Thus, mutant membrane transporter targeting phenotypes can reflect genetic backgrounds of host cells or organisms.
Our data are consistent with reduction or loss of kAE1 polypeptide expression as a dRTA mechanism in patients heterozygous for kAE1 missense mutation G609R. With the rare possible exceptions noted (Figure 3A), our data do not support apical mistargeting of mutant polypeptide as a pathogenic mechanism in human kidney. However, establishment of a definitive human kidney targeting phenotype of dRTA mutant kAE1 G609R must await availability of morphologically intact kidney tissue from dRTA patients of defined genotype.
Authors’ contribution
A.A.V. wrote the manuscript, recruited patients and collected tissues. B.E.S. carried out genotyping and reviewed the manuscript. Z.K.Z. performed immunohistochemistry and reviewed the manuscript. N.D.A. provided paraffin-embedded fixed tissues and reviewed the manuscript. N.K.D. wrote the manuscript and recruited patients. S.L.A. wrote the manuscript, carried out genotyping and performed immunohistochemistry.
Conflict of interest statement
The results presented in this paper have not been published previously.
Acknowledgements
We acknowledge support from NIH postdoctoral training grant T32-DK007276 (to A.A.V.) and from the Harvard Digestive Diseases Center NIH-DK34854 (to S.L.A.).
References
- 1. Karet F, Finberg K, Nelson R et al. . Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet 1999; 21: 84–90 [DOI] [PubMed] [Google Scholar]
- 2. Smith A, Skaug J, Choate K et al. . Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat Genet 2000; 26: 71–75 [DOI] [PubMed] [Google Scholar]
- 3. Smith A, Finberg K, Wagner C et al. . Molecular cloning and characterization of Atp6n1b: a novel fourth murine vacuolar H+-ATPase a-subunit gene. J Biol Chem 2001; 276: 42382–42388 [DOI] [PubMed] [Google Scholar]
- 4. Sly W, Hewett-Emmett D, Whyte M et al. . Carbonic anhydrase II deficiency identified as the primary defect in the autosomal recessive syndrome of osteopetrosis with renal tubular acidosis and cerebral calcification. Proc Natl Acad Sci USA 1983; 80: 2752–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shah G, Bonapace G, Hu P et al. . Carbonic anhydrase II deficiency syndrome (osteopetrosis with renal tubular acidosis and brain calcification): novel mutations in CA2 identified by direct sequencing expand the opportunity for genotype-phenotype correlation. Hum Mutat 2004; 24: 272. [DOI] [PubMed] [Google Scholar]
- 6. Bruce L, Cope D, Jones G et al. . Familial distal renal tubular acidosis is associated with mutations in the red cell anion exhanger (band 3, AE1) gene. J Clin Invest 1997; 100: 1693–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jarolim P, Shayakul C, Prabakaran D et al. . Autosomal dominant distal renal tubular acidosis is associated in three families with heterozygosity for the R589H mutation in the AE1 (band 3) Cl−/HCO3− exchanger. J Biol Chem 1998; 273: 6380–6388 [DOI] [PubMed] [Google Scholar]
- 8. Karet F, Gainza F, Györy A et al. . Mutations in the chloride-bicarbonate exchanger gene AE1 cause autosomal dominant but not autosomal recessive distal renal tubular acidosis. Proc Natl Acad Sci USA 1998; 95: 6337–6342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weber S, Soergel M, Jeck N et al. . Atypical distal renal tubular acidosis confirmed by mutation analysis. Pediatr Nephrol 2000; 15: 201–204 [DOI] [PubMed] [Google Scholar]
- 10. Cheidde L, Vieira T, Lima P et al. . A novel mutation in the anion exchanger 1 gene is associated with familial distal renal tubular acidosis and nephrocalcinosis. Pediatrics 2003; 112(Pt 1): 1361–1367 [DOI] [PubMed] [Google Scholar]
- 11. Siritippayawan S, Kirdpon S, Vasuvattakul S et al. . A de novo R589C mutation of anion exchanger 1 causing distal renal tubular acidosis. Pediatr Nephrol 2003; 18: 644–648 [DOI] [PubMed] [Google Scholar]
- 12. Rungroj N, Devonald M, Cuthbert A et al. . A novel missense mutation in AE1 causing autosomal dominant distal renal tubular acidosis retains normal transport function but is mistargeted in polarized epithelial cells. J Biol Chem 2004; 279: 13833–13838 [DOI] [PubMed] [Google Scholar]
- 13. Quilty J, Li J, Reithmeier R. Impaired trafficking of distal renal tubular acidosis mutants of the human kidney anion exchanger kAE1. Am J Physiol Renal Physiol 2002; 282: F810–F820 [DOI] [PubMed] [Google Scholar]
- 14. Toye A, Banting G, Tanner M. Regions of human kidney anion exchanger 1 (kAE1) required for basolateral targeting of kAE1 in polarised kidney cells: mis-targeting explains dominant renal tubular acidosis (dRTA). J Cell Sci 2004; 117(Pt 8): 1399–1410 [DOI] [PubMed] [Google Scholar]
- 15. Cordat E, Kittanakom S, Yenchitsomanus P et al. . Dominant and recessive distal renal tubular acidosis mutations of kidney anion exchanger 1 induce distinct trafficking defects in MDCK cells. Traffic 2006; 7: 117–128 [DOI] [PubMed] [Google Scholar]
- 16. Chu C, Woods N, Sawasdee N et al. . Band 3 Edmonton I, a novel mutant of the anion exchanger 1 causing spherocytosis and distal renal tubular acidosis. Biochem J 2010; 426: 379–388 [DOI] [PubMed] [Google Scholar]
- 17. Fry A, Su Y, Yiu V et al. . Mutation conferring apical-targeting motif on AE1 exchanger causes autosomal dominant distal RTA. J Am Soc Nephrol 2012; 23: 1238–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Devonald M, Smith A, Poon J et al. . Non-polarized targeting of AE1 causes autosomal dominant distal renal tubular acidosis. Nat Genet 2003; 33: 125–127 [DOI] [PubMed] [Google Scholar]
- 19. Han J, Kim G, Kim J et al. . Secretory-defect distal renal tubular acidosis is associated with transporter defect in H(+)-ATPase and anion exchanger-1. J Am Soc Nephrol 2002; 13: 1425–1432 [DOI] [PubMed] [Google Scholar]
- 20. Kang S, Kim J, Park J. Biopsy-proven type 1 renal tubular acidosis in a patient with metabolic acidosis. Korean J Intern Med 2012; 27: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Joo K, Jeon U, Han J et al. . Absence of H(+)-ATPase in the intercalated cells of renal tissues in classic distal renal tubular acidosis. Clin Nephrol 1998; 49: 226–231 [PubMed] [Google Scholar]
- 22. Walsh S, Turner C, Toye A et al. . Immunohistochemical comparison of a case of inherited distal renal tubular acidosis (with a unique AE1 mutation) with an acquired case secondary to autoimmune disease. Nephrol Dial Transplant 2007; 22: 807–812 [DOI] [PubMed] [Google Scholar]
- 23. Stewart A, Kedar P, Shmukler B et al. . Functional characterization and modified rescue of novel AE1 mutation R730C associated with overhydrated cation leak stomatocytosis. Am J Physiol Cell Physiol 2011; 300: C1034–C1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shmukler B, Kedar P, Warang P et al. . Hemolytic anemia and distal renal tubular acidosis in two Indian patients homozygous for SLC4A1/AE1 mutation A858D. Am J Hematol 2010; 85: 824–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Balogun R, Adams N, Palmisano J et al. . Focal segmental glomerulosclerosis, proteinuria, and nephrocalcinosis associated with renal tubular acidosis. Nephrol Dial Transplant 2002; 17: 308–310 [DOI] [PubMed] [Google Scholar]
- 26. Alper S, Stuart-Tilley A, Simmons C et al. . The fodrin-ankyrin cytoskeleton of choroid plexus preferentially colocalizes with apical Na + K(+)-ATPase rather than with basolateral anion exchanger AE2. J Clin Invest 1994; 93: 1430–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stuart-Tilley A, Sardet C, Pouyssegur J et al. . Immunolocalization of anion exchanger AE2 and cation exchanger NHE-1 in distinct adjacent cells of gastric mucosa. Am J Physiol 1994; 266(Pt 1): C559–C568 [DOI] [PubMed] [Google Scholar]
- 28. Shayakul C, Jarolim P, Zachlederova M et al. . Characterization of a highly polymorphic marker adjacent to the SLC4A1 gene and of kidney immunostaining in a family with distal renal tubular acidosis. Nephrol Dial Transplant 2004; 19: 371–379 [DOI] [PubMed] [Google Scholar]
- 29. Sabolic I, Brown D, Gluck S et al. . Regulation of AE1 anion exchanger and H(+)-ATPase in rat cortex by acute metabolic acidosis and alkalosis. Kidney Int 1997; 51: 125–137 [DOI] [PubMed] [Google Scholar]
- 30. Breton S, Alper S, Gluck S et al. . Depletion of intercalated cells from collecting ducts of carbonic anhydrase II-deficient (CAR2 null) mice. Am J Physiol 1995; 269(Pt 2): F761–F774 [DOI] [PubMed] [Google Scholar]
- 31. Stehberger P, Shmukler B, Stuart-Tilley A et al. . Distal renal tubular acidosis in mice lacking the AE1 (band 3) Cl−/HCO3− exchanger (slc4a1). J Am Soc Nephrol 2007; 18: 1408–1418 [DOI] [PubMed] [Google Scholar]
- 32. van den Wildenburg M, Hoorn E, Mohebbi N et al. . Distal renal tubular acidosis with multiorgan autoimmunity: a case report. Am J Kidney Dis 2015; 65: 607–610 [DOI] [PubMed] [Google Scholar]
- 33. Tanphaichitr V, Sumboonnanonda A, Ideguchi H et al. . Novel AE1 mutations in recessive distal renal tubular acidosis. Loss-of-function is rescued by glycophorin A. J Clin Invest 1998; 102: 2173–2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu F, Saleem M, Kampik N et al. . Anion exchanger 1 interacts with nephrin in podocytes. J Am Soc Nephrol 2010; 21: 1456–1467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gee H, Otto E, Hurd T et al. . Whole-exome resequencing distinguishes cystic kidney diseases from phenocopies in renal ciliopathies. Kidney Int 2014; 85: 880–887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cheval L, Pierrat F, Dossat C et al. . Atlas of gene expression in the mouse kidney: new features of glomerular parietal cells. Physiol Genomics 2011; 2011: 3. [DOI] [PubMed] [Google Scholar]
- 37. Boerries M, Grahammer F, Eiselein S et al. . Molecular fingerprinting of the podocyte reveals novel gene and protein regulatory networks. Kidney Int 2013; 83: 1052–1064 [DOI] [PubMed] [Google Scholar]
- 38. Gharib S, Pippin J, Ohse T et al. . Transcriptional landscape of glomerular parietal epithelial cells. PLoS One 2014; 9: e105289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee J, Chou C, Knepper M. Deep sequencing in microdissected renal tubules identifies nephron segment-specific transcriptomes. J Am Soc Nephrol 2015; 26: 2669–2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schreiner R, Frindt G, Diaz F et al. . The absence of a clathrin adapter confers unique polarity essential to proximal tubule function. Kidney Int 2010; 78: 382–388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Stoops E, Caplan M. Trafficking to the apical and basolateral membranes in polarized epithelial cells. J Am Soc Nephrol 2014; 25: 1375–1386 [DOI] [PMC free article] [PubMed] [Google Scholar]