Abstract
Fatigue is a common and debilitating symptom, affecting 42–89% of end-stage kidney disease patients, persisting even in pre–dialysis care and stable kidney transplantation, with huge repercussions on functioning, quality of life and patient outcomes. This paper presents a critical review of current evidence for the role of psychological factors in renal fatigue. To date, research has concentrated primarily on the contribution of depression, anxiety and subjective sleep quality to the experience of fatigue. These factors display consistent and strong associations with fatigue, above and beyond the role of demographic and clinical factors. Considerably less research is available on other psychological factors, such as social support, stress, self-efficacy, illness and fatigue-specific beliefs and behaviours, and among transplant recipients and patients in pre-dialysis care. Promising evidence is available on the contribution of illness beliefs and behaviours to the experience of fatigue and there is some indication that these factors may vary according to treatment modality, reflecting the differential burdens and coping necessities associated with each treatment modality. However, the use of generic fatigue scales casts doubt on what specifically is being measured among dialysis patients, illness-related fatigue or post-dialysis-specific fatigue. Therefore, it is important to corroborate the available evidence and further explore, qualitatively and quantitatively, the differences in fatigues and fatigue-specific beliefs and behaviours according to renal replacement therapy, to ensure that any model and subsequent intervention is relevant and grounded in the experiences of patients.
Keywords: anxiety, depression, fatigue, kidney disease, sleep quality
Introduction
Fatigue is a complex array of symptoms that has been described as ‘extreme and persistent tiredness, weakness or exhaustion-mental, physical, or both’ [1–3]. It has consistently emerged as a common and debilitating symptom across chronic conditions [4–7]. Likewise, fatigue is a common complaint in end-stage kidney disease (ESKD), affecting from 42% to 89% of patients, and is present across the full spectrum of chronic kidney disease (CKD), affecting patients not yet requiring renal support, to patients on dialysis, and persisting in patients who have subsequently received a renal transplant [3, 8].
In ESKD, fatigue has huge repercussions on functioning and quality of life, further impairing patients’ daily functioning, motivation and social engagement [9–14], and contributing to poorer sleep quality and increased bodily pain [13–17]; however, these associations are likely to be bidirectional. Above all, there is also evidence to suggest that fatigue may contribute directly to clinical outcomes, increasing the risk of cardiac events [18] and mortality [19]. Given the significance of fatigue and its consequences on patient outcomes and quality of life, timely and effective management of fatigue represents a clinical priority. Currently, no consistent and theory-led treatment model of fatigue exists, with management relying primarily on pharmacological treatments targeting anaemia or involving patients in exercise [3]. A review of pharmacological treatments for fatigue among dialysis patients reached the conclusion that no medications can be recommended for the prevention of fatigue, and complete and prolonged relief from fatigue is rare [8]. Exercise-based interventions, on the other hand, have been criticized for being unsuitable for patients with multi-morbidities, disabilities and in poorer health [20].
To date, a number of reviews have attempted to synthesize the available research on the factors associated with fatigue in this patient population [3, 8, 21, 22]. These reviews provided interesting insights on the prevalence of renal fatigue [3, 8], the type of fatigue measurements relied upon [3, 8, 22] and the outcomes associated with fatigue [3, 8, 22]. Overall, these reviews unanimously indicate that the findings are often mixed and inconclusive, with little success in identifying demographic, social-situational and clinical factors that are consistently associated with the experience of fatigue.
These reviews concentrated mainly on dialysis patients and did not differentiate between studies reporting fatigue versus vitality outcomes [3, 8, 21, 22]. The concept of vitality is considered to be at the opposite end to fatigue on a fatigue–vitality continuum [3]. Some debate exists on the comprehensiveness of the concept of vitality, capturing a reduction in energy levels, but not necessarily the negative aspects of fatigue, such as weakness, lack of motivation and difficulty with concentration [22]. Given that the vitality subscale of the SF-36 is the most widely used instrument in the dialysis population as a marker of fatigue [23, 24], it is important to assess whether systematic differences exist in factors associated with fatigue versus vitality.
Overall, the evidence for the role of demographic and social-situational factors in the experience of fatigue is mostly weak and inconsistent (e.g. [25–34]). Similarly, in relation to clinical factors, there is generally a lack of evidence for the significant variation in fatigue according to dialysis adequacy, serum albumin, haemoglobin or other clinical factors [e.g. 27, 29, 33, 35]. This may be due to the relative homogeneity of clinical values, maintained within the recommended ranges across many studies [3, 8]. However, indisputably, poorly managed patients are likely to report worse outcomes and exacerbated symptoms, including fatigue [e.g. 36–40]. Findings are generally in agreement on the negative impact of physical inactivity [31–33, 41] and comorbidities on fatigue [28, 29, 33, 35, 42]. A greater number of concomitant conditions may lead to worse overall health and functioning, and increased stress and burden imposed by additional treatment requirements [3]. While, poor physical functioning may contribute to fatigue, fatigue may also lead to inability to engage in activities; therefore, the direction of this association is unclear given the prevalence of cross-sectional research [8].
To our knowledge, this is the first narrative review providing a more comprehensive and in-depth overview of the role of psychological and cognitive-behavioural factors in the experience of fatigue in the renal population. Also, differences in factors associated with fatigue as compared with vitality and variations by renal replacement therapy (RRT) were explored. In order to develop theory-based and effective interventions for fatigue in ESKD, it is important to identify potentially modifiable factors that contribute to fatigue above and beyond the influence of demographic, social-situational and clinical factors, in each treatment modality.
Review questions
(i) What is the available evidence for the role of psychological and cognitive behavioural factors in ESKD fatigue?
(ii) Are there any differences in factors depending on outcome used: fatigue versus vitality?
(iii) Are there any differences in factors associated with fatigue/vitality depending on the type of RRT?
Materials and methods
Search
In anticipation of a large volume of research examining the association between depression, anxiety, subjective sleep quality and fatigue, a narrative review approach was selected to synthesize the evidence on the aforementioned factors and capture what other psychological factors have been explored to date. This narrative review was guided by principles and methods of a systematic review. To identify relevant articles, the following databases were searched: (i) Embase (via Ovid), (ii) Medline (via Ovid), (iii) PsycInfo (via Ovid), (iv) Global Health (via Ovid) and (v) Web of Science. A combination of ESKD and fatigue terms was used (please see Box 1, in Supplementary Appendix A). The search strategy was adapted to each database. Alongside the electronic search, a manual search was also conducted to identify any additional articles. The search was limited to full-text articles to allow for adequate appraisal of the findings (Table 1). The studies were included if the sample consisted of adult (aged 18 years or older) ESKD patients, with their glomerular filtration rate (GFR) falling below 60 mL/min/1.73 m2, regardless of the RRT they were receiving or if they were in pre-dialysis care. Only articles that measured fatigue or vitality and reported explicit findings related to potential predictors of fatigue/vitality, measured by independent instruments, and not within-scale correlations, were included in this review. Psychological factors were defined as cognitive, emotional or behavioural factors typically considered modifiable in the context of psychosocial interventions. Intervention studies, studies assessing the consequences of fatigue on functioning, mortality or quality of life, or articles that included a fatigue subscale but failed to report discernible fatigue findings were excluded. Please see Supplementary Appendix B for further information.
Table 1.
Summary of instruments used to measure fatigue, depression, anxiety and subjective sleep quality
| Reference | RRT | Fatigue | Depression | Anxiety | Subjective sleep quality |
|---|---|---|---|---|---|
| (1) Bai et al. [31] | HD | Lin’s Fatigue scale [43] | CES-D | ||
| (2) Bossola et al. [44] | HD | SF-36 vitality | BDI | HARS | Athens Insomnia Scale |
| (3) Bossola et al. [45] | HD | SF-36 vitality | BDI | HARS | |
| (4) Bossola et al. [46] | HD | SF-36 vitality | BDI | ||
| (5)Bossola et al. [47] | HD | Six closed questions, pertaining to the domains of general tiredness, emotional tiredness, cognitive tiredness, sleepiness, weakness and lack of energy | GDS | ||
| (6) Bossola et al. [48] | HD | SF-36 vitality | BDI | HARS | |
| (7) Cardenas and Kutner [49] | HD & PD | VAS-F | SDS | ||
| (8) Chan et al. [29] | Tx | MFI-20 | HADS-D | HADS-A | PSQI |
| (9) Chen et al. [50] | HD | SF-36 vitality & CFQ | HADS-D | HADS-A | |
| (10) Chilcot et al. [33] | HD | CFQ | HADS-D | HADS-A | |
| (11) DePasquale et al. [51] | HD | SF-36 vitality | SCL-90 R-Depression | SCL-90 R-Anxiety | |
| (12) Dubin et al. [52] | HD | Two closed questions around fatigue after dialysis sessions. Severe PDF was defined as a ‘yes’ answer to the latter question. | Deemed depressed if: (i) diagnosis of depression, mood disorder in medical history, or (ii) prescribed antidepressants. | ||
| (13) Garcia et al. [53] | HD | SF-36 vitality | HDRS | ||
| (14) García-Llana et al. [14] | HD | SF-36 vitality | BDI | STAI | |
| (15) Goedendorp et al. [32] | Tx | CIS | BDI-PC | Sleeping problems were assessed using the subscale sleep/rest of the SIP | |
| (16) Jhamb et al. [15] | HD & PD | SF-36 vitality | Antidepressants | Benzodiazepines | Three items assess sleep initiation (‘Have trouble falling asleep?’), sleep maintenance (‘Awaken during sleep and have trouble falling asleep again?’) and sleep adequacy (‘Get enough sleep to feel rested upon waking in the morning?’). |
| (17) Jhamb et al. [16] | HD | SF-36 vitality | KDQoL sleep quality and sleeping pills | ||
| (18) Jhamb et al. [35] | Pre-dialysis, HD & PD | FACIT-F | PHQ-9 | PSQI | |
| (19) Joshwa et al. [54] | HD | FSS | BDI | PSQI | |
| (20) Kalender et al. [55] | CKD conservative management, HD & CAPD | SF-36 vitality | BDI | ||
| (21) Karakan et al. [56] | HD | PFS | BDI | ||
| (22) Kim and Son [40] | HD | VAS-F | SDS | ||
| (23) Koyama et al. [18] | HD | Fatigue Scale (developed by Fukuda et al. [57]) | KDQoL sleep quality | ||
| (24) Leinau et al. [58] | HD | BFI | PHQ-9 | ||
| (25) Letchmi et al. [30] | HD | MFI-20 | DASS 21 | DASS 21 | |
| (26) Lin et al. [59] | CAPD | SF-36 vitality and presence or absence of fatigue was studied according to the monthly clinical records of the primary nurses | BDI | ||
| (27) Liu [26] | HD | Mandarin Chinese Fatigue Assess Scale (Chung [60]) | Taiwanese Depression Questionnaire | ||
| (28) Lobbedez et al. [42] | HD & PD | MFI-20 | GDS | ||
| (29) Martínez-Sanchis et al. [61] | HD, CAPD, & Tx | SF-36 vitality | HADS-D | HADS-A | PSQI |
| (30) Martins et al. [62] | HD | SF-36 vitality | Cognitive-Somatic Anxiety Scale (Silva et al. [63]) | ||
| (31) McCann and Boore [41] | HD | MFI-20, VAS-F, SF-36 vitality | HADS-D | HADS-A | Overall Sleep Problems Index |
| (32) Oliveira et al. [64] | HD | SF-36 vitality | BDI | ||
| (33) Rodrigue et al. [34] | Pre- and post-Tx | FSI & MFI-20 | PSQI | ||
| (34) Santos [65] | HD | SF-36 vitality | CES-D | ||
| (35) Sayin et al. [28] | HD, PD, & Tx | SF-36 vitality | BDI | STAI | |
| (36) Senol et al. [12] | PD | FSS, SF-36 vitality | BDI | ||
| (37) Sklar et al. [66] | HD | Patients were regarded to suffer from PDF if they spontaneously offered this complaint when asked the open-ended question, ‘Do you feel better or worse after dialysis? If worse, please specify in which way(s)’. A complaint of fatigue would then be probed further with questions directed at its duration, frequency, and intensity, allowing for the creation of a fatigue index (one third of the sum of these three parameters, each rated from 1 to 5). | BDI | ||
| (38) Taskapan et al. [67] | HD | SF-36 vitality | HDRS | HARS | |
| (39) Unruh et al. [68] | HD & PD | SF-36 vitality | Three items assess sleep initiation (‘Have trouble falling asleep?’), sleep maintenance (‘Awaken during sleep and have trouble falling asleep again?’), and sleep adequacy (‘Get enough sleep to feel rested upon waking in the morning?’). | ||
| (40) Vazquez et al. [69] | HD | SF-36 vitality | BDI | STAI-S | |
| (41) Wang et al. [70] | HD | FACIT-F | HADS-D | ||
| (42) Wang et al. [71] | HD | FACIT-F | HADS-D | HADS-A | PSQI |
| (43) Wang et al. [72] | HD | FACIT-F | PSQI | ||
| (44) Williams et al. [73] | HD | VAS-F | HADS-D | HADS-A |
HD, haemodialysis; PD, peritoneal dialysis; Tx, transplant; CAPD, continuous ambulatory peritoneal dialysis; CES-D, Center for Epidemiological Studies Depression; BDI, Beck Depression Inventory; HARS, Hamilton Anxiety Rating Scale; GDS, Geriatric Depression Scale; VAS-F, fatigue visual analogue scale; SDS, Self-Rating Depression Scale; MFI-20: Multi-dimensional Fatigue Inventory; HADS, Hospital Anxiety and Depression Scale; PSQI, Pittsburgh Sleep Quality Index; CFQ, Chalder Fatigue Questionnaire; SCL-90 R, Symptom Checklist-90-Revised; PDF, post-dialysis fatigue; HDRS, Hamilton Depression Rating Scale; STAI, State-Trait Anxiety Inventory; CIS, Checklist Individual Strength; BDI-PC, Beck Depression Inventory–Primary Care; FACIT-F, Functional Assessment of Chronic Illness Therapy-Fatigue; PHQ-9, The Patient Health Questionnaire; FSS, Fatigue Severity Scale; PFS, Piper Fatigue Scale; DASS 21, Depression Anxiety Stress Scale; KDQoL, The Kidney Disease Quality of Life Questionnaire; FSI, Fatigue Symptom Inventory; BFI, Brief Fatigue Inventory; SIP, Sickness Impact Profile 8.
Quality assessment
All identified studies underwent a quality assessment using a modified version of the Effective Public Health Practice Project (EPHPP) quality assessment tool that covers any quantitative study design to provide a descriptive overview of the quality of the current evidence base. Overall quality scores for each study were not computed. Relevant considerations appropriate to the studies were added to the assessment list, in particular: what study design was used, whether a power calculation was reported, what type of recruitment was used, what percentage of selected individuals agreed to participate, whether participants could be deemed representative of the population, whether reliable and valid instruments were used, whether analyses were appropriate, whether the P-value was adjusted for multiple analyses and whether confidence intervals were reported (where appropriate). A full description of the ratings for each study can be found in Supplementary Appendix C.
Analysis
Data from the included studies were extracted using a data extraction form that was adapted from the data collection checklist by the Cochrane Effective Practice and Organisation of Care Review Group to suit the purpose of this review. Key aspects of data extraction included: patient demographics, clinical and illness-related information, fatigue instrument used, investigated psychological factors, data analyses, and results for the associations between the investigated psychological factors and fatigue as an outcome. A narrative synthesis was conducted to provide a textual summary of the findings, pertaining to the reported associations between psychological factors and fatigue as an outcome, across all the included studies, and to identify any difference according to treatment modality and fatigue measurement used [74].
Results
Fifty-four studies were identified that reported on the association between psychological variables and fatigue, mainly within investigations of the predictors of quality of life in this setting, rather than fatigue-specific studies. The vast majority of these studies were cross-sectional (N = 46), relying on single-centre recruitment (N = 35). Sample sizes ranged from 33 [74] to 1798 [16]. On the whole, there was a lack of consideration of power across the studies. Similarly, in most studies (N = 52) alpha was not adjusted for multiple analyses. There was great variability in inclusion and exclusion criteria from study to study, especially evident with regards to dialysis vintage (see Supplementary Appendix C). Samples varied considerably, owing to the differences in research aims, participant selection criteria and RRT from study to study (see Supplementary Appendix C). Forty-three studies were exclusive to dialysis patients, with four studies consisting of mixed samples of both dialysis patients and transplant recipients. Only four studies included solely transplant recipients, and one study followed patients from pre-transplantation to post-transplantation. None of the studies consisted exclusively of patients in pre-dialysis care, but two studies included patients not undergoing dialysis alongside dialysis patients. Although there was variation in the instruments used, predominantly studies relied on established and validated instruments. Analyses were in general appropriate given the different research aims across the studies (see Supplementary Appendix C).
Psychological and cognitive behavioural factors
To date, attention has been predominantly paid to depression, anxiety and subjective sleep quality with regard to fatigue, mainly among dialysis patients (N = 44; see Table 1). From here onwards, studies from Table 1 are referred to using their assigned reference numbers. Limited research is available regarding other psychological and cognitive constructs that have been previously implicated in fatigue in other chronic illnesses [75–78].
Depression and anxiety
Depression and fatigue overlap significantly in how they are experienced, and depression often manifests itself in lethargy and a feeling of weakness and tiredness in the general population, as well as in patient populations [8, 79–83]. Therefore, drawing conclusive interpretations as to the causality of this association is not possible [3]. Thirty-eight studies were identified that looked at the relationship between depression and fatigue/vitality. The majority (N = 36) found a consistent significant association between the two, with depressed patients reporting greater levels of fatigue and lower vitality (study numbers 1–10, 12–16, 18–22, 24, 26–29, 31–32, 34–38, 40–42, 44 in Table 1).
There is also evidence to suggest a significant association between anxiety and fatigue among dialysis patients and transplant recipients, where out of 18 studies reporting on this association, 15 found a significant positive association between the two (study numbers 2, 3, 6, 8–10, 14, 16, 30, 31, 35, 38, 40, 42, 44 in Table 1). Overall, some studies found that depression and other mood disorders can explain up to 38–46% of the variance in fatigue (study numbers 27, 44 in Table 1).
Only a small minority of studies failed to corroborate the association between depression, anxiety and fatigue. This may be explained by sample differences, where Letchmi et al. (2011)’s study (study number 25 in Table 1) was conducted in Malaysia and the lack of association between these factors may be explained by cultural differences and stigma associated with psychological distress in Asian cultures [84, 85]. In fact, the prevalence of anxiety and depression was low in Letchmi et al.’s sample (study numbers 25 in Table 1). Any inconsistent findings may also be explained by the great variability in the measurement of fatigue, depression and anxiety, the likely reason for the disparity in DePasquale et al. ’s study (study number 11 in Table 1).
Subjective sleep quality
Even though the evidence for the role of objective sleep parameters, such as total sleep duration (e.g. study number 35 in Table 1) in the experience of fatigue seems to be weak, 13 studies were identified that examined the association between subjective sleep quality and fatigue. There was unanimous agreement with regards to the significant association between poorer subjective sleep quality and greater fatigue and lower vitality (study numbers 2, 8, 15–19, 23, 31, 33, 39, 42–43 in Table 1). Only one study failed to find a significant association between self-reported daytime sleepiness and vitality among peritoneal dialysis patients [86], whereas two studies were in support of this association [35, 54]. The prevalence of daytime sleepiness was low and the presence of restless leg syndrome (RLS), sleep apnoea and other sleep-disturbing factors were selected as exclusion criteria in the former study [86].
Social support
The relationship between social support and fatigue in renal patients has not been studied extensively [3, 21]. Out of 11 studies identified here, 7 found that severely fatigued renal patients perceive lower social support from friends and family [32, 72, 87–90]; this was further elaborated by Akin et al. (2014) [25] reporting that as levels of loneliness and fatigue increased, self-care abilities decreased, suggesting that greater fatigue severity and lower social support can have a detrimental influence on functioning and wellbeing. However, other studies did not find a significant association between social support and fatigue/vitality in the renal patient population [28, 40, 66, 73]. Great variability was evident in the measurement of social support, from studies using different self-report social support-specific scales [32, 40, 62, 66, 72, 73, 87] or social support scores extrapolated from other instruments, like the Health-Promoting Lifestyle Profile scale [88]. Given the mixed and limited evidence pool, drawing any conclusions is premature, as any differences can be also confounded by other factors, such as cultural differences and marital status.
Cognitive behavioural factors
Illness and symptom perceptions and associated behaviours have often emerged as dominant constructs in adjustment, quality of life and outcomes across chronic conditions [90, 91]. Illness perceptions refer to organized beliefs about a particular condition, which can then determine coping behaviours [92, 93]. Illness perceptions consist of five dominant dimensions: (i) identity (how symptoms are experienced and attributed to the illness); (ii) cause (beliefs about causes of the illness); (iii) timeline (beliefs about the duration of the illness, cyclical, acute or chronic); (iv) consequences (beliefs about the impact of the illness); and (v) control/cure (beliefs regarding the controllability/curability of the illness) [92, 93]. The contribution of illness perceptions and coping behaviours to the experience of fatigue has been extensively documented in the literature across a range of chronic conditions (e.g. study numbers [75, 94] in Table 1). However, these factors have been so far overlooked in ESKD. Two studies examined the contribution of beliefs about the illness to fatigue; holding a severe illness identity and greater consequence beliefs were associated with lower levels of vitality in a hierarchical regression model, but none of the other illness beliefs—timeline, personal control, illness coherence, and emotional representations—emerged as significant with regards to vitality [27]. The second study examined appraisals of the illness among haemodialysis (HD) and continuous ambulatory peritoneal dialysis (CAPD) patients. Only the appraisal of the illness as a threat was significantly correlated with greater fatigue in CAPD, whereas appraisal of the illness neither as a loss nor as a challenge were associated with fatigue [95].
No research is currently available regarding fatigue-specific beliefs and behaviours in this patient population. A prospective study, led by the authors, is currently underway, evaluating fatigue severity and fatigue-related functional impairment over time and assessing what fatigue beliefs and behaviours, alongside demographic and clinical variables, contribute to fatigue over time. The baseline cross-sectional data from this study revealed that an overall negative perception of fatigue, all-or-nothing and avoidance/resting behaviours in response to fatigue were significant predictors of greater fatigue severity, above and beyond the role of demographic and clinical factors, as well as distress [33].
Other psychological factors
Six studies have explored additional psychological factors in relation to fatigue/vitality. There is evidence in support of the negative influence of worrying and stress on energy levels [14, 88, 96]. One study also reported a significant association between self-efficacy and decreased fatigue [96] and illness intrusiveness and greater fatigue [97]. Additionally, there is some indication that greater perception of health responsibility and health–promoting behaviours can contribute to higher levels of energy in transplant recipients [88].
An interesting insight emerged from Sayin et al. ’s study [28], demonstrating a link between illness knowledge and energy levels, where poorer illness knowledge was associated with lower vitality. Support for this link was evident in a trial, where following an educational intervention, illness knowledge became significantly associated with lower fatigue; however, no association between the two was evident at baseline [98].
A recent study indicated that subjective perception of exertion following exercise was the only independent predictor of physical fatigue in transplant (Tx) recipients, irrespective of objective parameters of physical fitness [99].
The role of fatigue measurement
Across the literature, the evidence for the role of psychological factors in fatigue seems to be generally consistent regardless of instruments used. In particular, studies that used the vitality sub-scale of the SF-36 reported comparable findings. In fact, high correlations exist between fatigue and vitality [12, 35, 41], supporting the use of vitality as a marker of fatigue. An interesting finding emerged from one study, where only subscales of the Multi-dimensional Fatigue Inventory, specifically general fatigue and mental fatigue, were significantly associated with anxiety, but there was no association between anxiety and fatigue measured by the vitality subscale of the SF-36 and visual analogue scale [41]. Fatigue-specific instruments may capture a wider spectrum of manifestations of tiredness as compared with the vitality subscale of the SF-36 or any single item scales. In fact, since the vitality subscale consists of only four items, whether it can adequately represent all the facets of fatigue is questionable [100]. There is a lack of studies comparing directly the vitality subscale of the SF-36 with fatigue-specific instruments to discern whether subtle systematic differences exist in the instruments’ abilities to capture relationships between the experience of fatigue and psychological predictors. Additionally, none of the currently available fatigue instruments have been specifically developed for renal fatigue. The qualitative experience of fatigue may differ between different patient populations. Therefore, the content of generic fatigue instruments may fail to capture the relevant to renal patients’ fatigue-related experiences [101], in particular with regards to illness-related fatigue and post-dialysis-specific fatigue experiences.
Discussion
The aim of this narrative review was to build on previous reviews, by concentrating exclusively on the currently available evidence on the role of psychological factors in renal fatigue, across the full spectrum of ESKD. There was agreement between studies on the important role of depression, anxiety and subjective sleep quality in the experience of fatigue. Considerably less research is available on other psychological factors, such as social support, stress, self-efficacy, beliefs and behaviours. Promising evidence is available on the contribution of illness beliefs and behaviours to the experience of fatigue and there is some indication that these factors may vary according to treatment modality, reflecting the differential burdens and coping necessities associated with each treatment modality. Prolonged perception of the illness as a threat may lead patients to feeling burnt out, but this held only among CAPD, but not HD patients [95]. Differential burdens and challenges imposed by each treatment modality may explain why illness beliefs and fatigue levels vary. In fact, a recent study found that self-care HD patients have significantly greater personal control and illness coherence beliefs compared with in-centre patients [102]. There was also evidence for the role of stress and worrying in fatigue, suggesting that any additional burden, emotional or physical, may have detrimental effects on energy levels. Poorer illness knowledge may indirectly contribute to vitality via treatment non-adherence and greater illness-related distress.
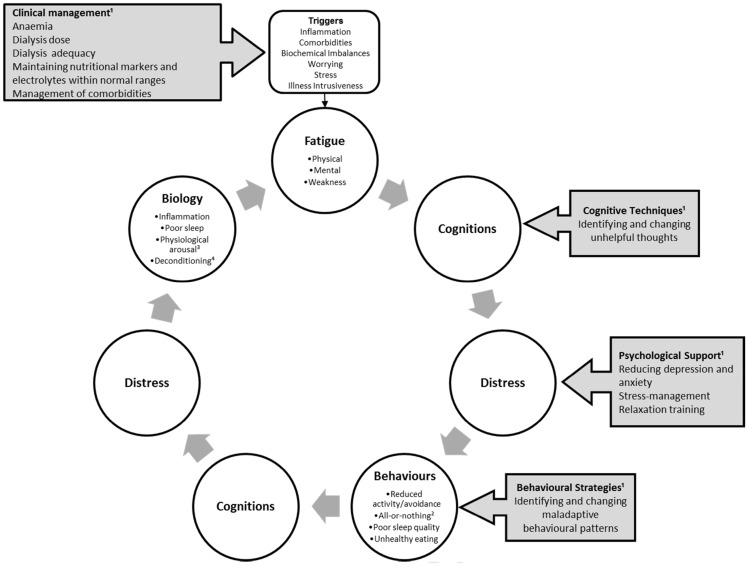
Across studies, when cognitive-behavioural and psychological factors were introduced into the models, many demographic and clinical factors ceased to be significant, with cognitive-behavioural and psychological factors explaining 14.6–46% of unique fatigue/vitality variance [26, 27, 29, 31, 33, 47, 73]. Furthermore, the association between some demographic, social-situational and clinical factors may be explained by means of psychological factors. For example, the positive relationship between age and fatigue has been conventionally explained by age-related factors like differences in dialysis vintage, physical functioning and increased comorbidities [3]. However, this age–fatigue relationship has not been support unanimously across studies in ESKD [30, 103]. This inconsistency may be explained by psychological factors, with younger patients possibly perceiving a greater impact of the illness on their lives and functioning, therefore holding more severe and maladaptive illness beliefs, which in turn leading to greater fatigue. Similarly, any link between marital status and fatigue may be the product of differences in social support, acting on fatigue indirectly. Employment status can act as a marker of age and physical activity, but it simultaneously may be an indication of the perceived role of patients and can also act as a distraction. In relation to education, patients with low educational attainment may find it more difficult to understand their illness and the importance of adhering to every aspect of treatment, while patients with greater educational attainment may be more aware of the severity and consequences of the illness. Similarly, some studies have reported a link between worsening renal function and fatigue in Tx recipients [29, 104]. A possible explanation may be that for Tx recipients worsening renal function represents a risk for graft loss, possibly leading to increased distress, worry and fear [104]. Overall, these findings suggest that the degree of renal function may not directly impact on energy levels, but rather through increased distress. Therefore, as Figure 1 postulates, clinical factors may trigger fatigue, but it is likely to be maintained by psychological processes, leading to a vicious cycle of negative illness and fatigue beliefs, increased distress and maladaptive behaviours. This is based on integrated fatigue models formulated in other chronic conditions, in particular Chronic Fatigue Syndrome and multiple sclerosis (MS) [105, 106].
Fig. 1.
Preliminary biopsychosocial model of fatigue in end-stage kidney disease. This figure illustrates how fatigue can be triggered by illness, biochemical imbalances, or stress and worrying, and it can then be perpetuated and maintained by a vicious cycle of negative beliefs, depression and/or anxiety and maladaptive behavioural patterns. 1Grey boxes with arrows exemplify potential areas for intervention. Clinical management revolves around illness control and maintaining biochemical values within optimal ranges, as well as management of comorbidities. Cognitive techniques can be used to identify and change negative beliefs (e.g. catastrophizing) and to reduce depression and anxiety. Behavioural strategies, as part of psychotherapy, or physiotherapy can support patients in developing a stable activity pattern, and healthy sleep and dietary habits. 2All-or-nothing = behaviour characterized by bursts of activity followed by rest. ³Physiological arousal = in response to stress, chronic activation of the central and autonomic nervous systems as well as the endocrine system. 4Deconditioning = changes in body systems due to physical inactivity and disuses, such as loss in muscle strength and tone.
Limitations and future directions
Although there is promising evidence with regards to the role of psychological factors in the experience of fatigue in this setting, it is important to emphasize that most of the evidence originates from cross-sectional studies and therefore, no conclusive interpretations in relation to causality can be drawn. Considerably less research is available on psychological factors in fatigue among transplant recipients and patients in pre-dialysis care. Corroboration of the evidence for the role of social support, self-efficacy and illness intrusiveness in fatigue is necessary. Also, longitudinal studies are warranted to provide causal evidence, particularly for the associations between depression, anxiety, subjective sleep quality and fatigue. Given the evidence for differences in illness beliefs depending on RRT, it would be valuable for future research to compare fatigue-specific beliefs according to what RRT patients are receiving.
Conclusion
Fatigue is a prevalent and debilitating symptom across the whole spectrum of renal disease. Previous narrative reviews have mainly concentrated on fatigue in dialysis patients, indicating at present a poor understanding of the pathogenesis and risk factors of fatigue in this patient population. Given the important role psychological and cognitive-behavioural factors have played in cancer and MS fatigue, this review concentrated exclusively on the current evidence for the role of these factors in renal fatigue. The most consistent evidence emerged with regards to the role of depression; anxiety and subjective sleep quality in the experience of fatigue, in many studies the role of these factors was above and beyond that of demographic, social-situational and clinical factors. Considerably less research is available on other psychological constructs, but there are some promising findings on the role of illness perceptions and behavioural patterns in the experience of fatigue. Differences in illness beliefs and behaviours depending on RRT may also manifest themselves in the experience of fatigue, reflecting the differential burdens and restrictions of each treatment modality. However, the evidence base consists of single studies, and corroboration of these findings is warranted, particularly by longitudinal studies. Additionally, the currently available generic fatigue instruments may be unable to capture the differences between illness-related fatigue and post-dialysisity-specific fatigue; therefore, it is unclear what exactly the currently used fatigue instruments are measuring, possibly contributing to the incongruities in the literature. Given the modifiable nature of psychological and cognitive-behavioural factors, these can be targeted in interventions, as has been previously successfully done in other chronic conditions, in order to alleviate the levels of fatigue in patients with renal failure.
Funding
This work was funded by a Biomedical Research Studentship to Miss Federica Picariello from the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Conflict of interest statement
None declared.
Supplementary data
Supplementary data are available online at http://ckj.oxfordjournals.org.
Supplementary Material
References
- 1. Pawlikowska T, Chalder T, Hirsch S. et al. Population based study of fatigue and psychological distress. BMJ 1994; 308: 763–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. David A, Pelosi A, McDonald E. et al. Tired, weak, or in need of rest: fatigue among general practice attenders. BMJ 1990; 301: 1199–1202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Artom M, Moss-Morris R, Caskey F. et al. Fatigue in Advanced Kidney Disease. Kidney International, 2014; 86: 497–505 [DOI] [PubMed] [Google Scholar]
- 4. Wagner L, Cella D. Fatigue and cancer: causes, prevalence and treatment approaches. Br J Cancer 2004; 91: 822–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Putzki N, Katsarava Z, Vago S. et al. Prevalence and severity of multiple-sclerosis-associated fatigue in treated and untreated patients. Eur Neurol 2008; 59: 136–142 [DOI] [PubMed] [Google Scholar]
- 6. Wolfe F, Hawley DJ, Wilson K. The prevalence and meaning of fatigue in rheumatic disease. J Rheumatol 1996; 23: 1407–1417 [PubMed] [Google Scholar]
- 7. Henderson M, Safa F, Easterbrook P. et al. Fatigue among HIV‐infected patients in the era of highly active antiretroviral therapy. HIV Med 2005; 6: 347–352 [DOI] [PubMed] [Google Scholar]
- 8. Bossola M, Vulpio C, Tazza L. Fatigue in chronic dialysis patients. In: Seminars in Dialysis. Wiley Online Library, 2011; 24: 550–555 [DOI] [PubMed] [Google Scholar]
- 9. Heiwe S, Clyne N, Dahlgren MA. Living with chronic renal failure: patients' experiences of their physical and functional capacity. Physiother Res Int 2003; 8: 167–177 [DOI] [PubMed] [Google Scholar]
- 10. Bonner A, Wellard S, Caltabiano M. The impact of fatigue on daily activity in people with chronic kidney disease. J Clin Nurs 2010; 19: 3006–3015 [DOI] [PubMed] [Google Scholar]
- 11. Bossola M, Pellu V, Di Stasio E. et al. Self-reported physical activity in patients on chronic hemodialysis: correlates and barriers. Blood Purif 2014; 38: 24–29 [DOI] [PubMed] [Google Scholar]
- 12. Senol V, Sipahioglu MH, Ozturk A. et al. Important determinants of quality of life in a peritoneal dialysis population in Turkey. Ren Fail 2010; 32: 1196–1201 [DOI] [PubMed] [Google Scholar]
- 13. Sabanciogullari S, Yilmaz FT, Gungor FI. et al. Sexual function in patients with chronic renal failure on hemodialysis and its effects on patients' perception of health and life satisfaction. Sex Disabil 2015; 33: 175–186 [Google Scholar]
- 14. García-Llana H, Remor E, Selgas R. Adherence to treatment, emotional state and quality of life in patients with end-stage renal disease undergoing dialysis. Psicothema 2013; 25: 79–86 [DOI] [PubMed] [Google Scholar]
- 15. Jhamb M, Argyropoulos C, Steel JL. et al. Correlates and outcomes of fatigue among incident dialysis patients. Clin J Am Soc Nephrol 2009; 4: 1779–1786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jhamb M, Pike F, Ramer S. et al. Impact of fatigue on outcomes in the hemodialysis (HEMO) study. Am J Nephrol 2011; 33: 515–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Neto JFR, Ferraz MB, Cendoroglo M. et al. Quality of life at the initiation of maintenance dialysis treatment - A comparison between the SF-36 and the KDQ questionnaires. Qual Life Res 2000; 9: 101–107 [DOI] [PubMed] [Google Scholar]
- 18. Koyama H, Fukuda S, Shoji T. et al. Fatigue is a predictor for cardiovascular outcomes in patients undergoing hemodialysis. Clin J Am Soc Nephrol 2010; 5: 659–666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bossola M, Di Stasio E, Antocicco M. et al. Fatigue is associated with increased risk of mortality in patients on chronic hemodialysis. Nephron 2015; 130: 113–118 [DOI] [PubMed] [Google Scholar]
- 20. Kosmadakis G, Bevington A, Smith A. et al. Physical exercise in patients with severe kidney disease. Nephron Clin Pract 2010; 115: c7–c16 [DOI] [PubMed] [Google Scholar]
- 21. Horigan AE. Fatigue in hemodialysis patients: a review of current knowledge. J Pain Symptom Manag 2012; 44: 715–724 [DOI] [PubMed] [Google Scholar]
- 22. Jhamb M, Weisbord SD, Steel JL. et al. Fatigue in patients receiving maintenance dialysis: a review of definitions, measures, and contributing factors. Am J Kidney Dis 2008; 52: 353–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kalantar-Zadeh K, Unruh M. Health related quality of life in patients with chronic kidney disease. Int Urol Nephrol 2005; 37: 367–378 [DOI] [PubMed] [Google Scholar]
- 24. Unruh ML, Weisbord SD, Kimmel PL. Psychosocial factors in patients with chronic kidney disease: health‐related quality of life in nephrology research and clinical practice Semin Dial 2005; 18: 82–90 [DOI] [PubMed] [Google Scholar]
- 25. Akin S, Mendi B, Ozturk B. et al. Assessment of relationship between self-care and fatigue and loneliness in haemodialysis patients. J Clin Nurs 2014; 23: 856–864 [DOI] [PubMed] [Google Scholar]
- 26. Liu H. Fatigue and associated factors in hemodialysis patients in Taiwan. Res Nurs Health 2006; 29: 40–50 [DOI] [PubMed] [Google Scholar]
- 27. Timmers L, Thong M, Dekker FW. et al. Illness perceptions in dialysis patients and their association with quality of life. Psychol Health 2008; 23: 679–690 [DOI] [PubMed] [Google Scholar]
- 28. Sayin A, Mutluay R, Sindel S. Quality of life in hemodialysis, peritoneal dialysis, and transplantation patients. Transplantation Proceedings 2007; 39: 3047–3053 [DOI] [PubMed] [Google Scholar]
- 29. Chan W, Bosch JA, Jones D. Predictors and consequences of fatigue in prevalent kidney transplant recipients. Transplantation 2013; 96: 987–994 [DOI] [PubMed] [Google Scholar]
- 30. Letchmi S, Das S, Halim H. et al. Fatigue experienced by patients receiving maintenance dialysis in hemodialysis units. Nurs Health Sci 2011; 13: 60–64 [DOI] [PubMed] [Google Scholar]
- 31. Bai Y-L, Lai L-Y, Lee B-O. et al. The impact of depression on fatigue in patients with haemodialysis: a correlational study. J Clin Nurs 2015; 24: 2014–2022 [DOI] [PubMed] [Google Scholar]
- 32. Goedendorp MM, Hoitsma AJ, Bloot L. et al. Severe fatigue after kidney transplantation: a highly prevalent, disabling and multifactorial symptom. Transplant Int 2013; 26: 1007–1015 [DOI] [PubMed] [Google Scholar]
- 33. Chilcot J, Moss-Morris R, Artom M. et al. Psychosocial and clinical correlates of fatigue in haemodialysis patients: the importance of patients’ illness cognitions and behaviours. Int J Behav Med 2015: 1–11 [DOI] [PubMed] [Google Scholar]
- 34. Rodrigue JR, Mandelbrot DA, Hanto DW. et al. A cross-sectional study of fatigue and sleep quality before and after kidney transplantation. Clin Transplant 2011; 25: E13–E21 [DOI] [PubMed] [Google Scholar]
- 35. Jhamb M, Liang K, Yabes J. et al. Prevalence and correlates of fatigue in chronic kidney disease and end-stage renal disease: are sleep disorders a key to understanding fatigue? Am J Nephrol 2013; 38: 489–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Izbirak G, Akan H, Mistik S. et al. Comparison of health-related quality of life of patients on hemodialysis and continuous ambulatory peritoneal dialysis. Turkiye Klinikleri Tip Bilimleri Dergisi 2010; 30: 1595–1602 [Google Scholar]
- 37. Merkus MP, Jager KJ, Dekker FW. et al. Quality of life in patients on chronic dialysis: self-assessment 3 months after the start of treatment. Am J Kidney Dis 1997; 29: 584–592 [DOI] [PubMed] [Google Scholar]
- 38. Santos PR, Franco Sansigolo Kerr LR. Clinical and laboratory variables associated with quality of life in Brazilian haemodialysis patients: a single-centre study. Revista Medica de Chile 2008; 136: 1264–1271 [PubMed] [Google Scholar]
- 39. Barrett BJ, Vavasour HM, Major A. et al. Clinical and psychological correlates of somatic symptoms in patients on dialysis. Nephron 1990; 55: 10–15 [DOI] [PubMed] [Google Scholar]
- 40. Kim H, Son G. Fatigue and its related factors in Korean patients on hemodialysis. Taehan Kanho Hakhoe Chi 2005; 35: 701–708 [DOI] [PubMed] [Google Scholar]
- 41. McCann K, Boore JR. Fatigue in persons with renal failure who require maintenance haemodialysis. J Adv Nurs 2000; 32: 1132–1142 [DOI] [PubMed] [Google Scholar]
- 42. Lobbedez T, Desbordes TE, Joly F. et al. Fatigue in elderly patients on dialysis. Nephrol Therapeutique 2008; 4: 584–589 [DOI] [PubMed] [Google Scholar]
- 43. Lin C, Lee Y, Hung C. et al. Development of a novel fatigue scale for hemodialysis patients. Formosan J Medicine 2006; 10: 422–428 [Google Scholar]
- 44. Bossola M., Luciani G, Tazza L. Fatigue and its correlates in chronic hemodialysis patients. Blood Purif 2009; 28: 245–252 [DOI] [PubMed] [Google Scholar]
- 45. Bossola M, Ciciarelli C, Conte G. et al. Correlates of symptoms of depression and anxiety in chronic hemodialysis patients. Gen Hosp Psychiatry 2010a; 32: 125–131 [DOI] [PubMed] [Google Scholar]
- 46. Bossola M, Luciani G, Giungi S. Anorexia, fatigue, and plasma interleukin-6 levels in chronic hemodialysis patients. Ren Fail 2010b; 32: 1049–1054 [DOI] [PubMed] [Google Scholar]
- 47. Bossola M, Di Stasio E, Antocicco M. et al. Qualities of fatigue in patients on chronic hemodial. Hemodial Int 2013; 17: 32–40 [DOI] [PubMed] [Google Scholar]
- 48. Bossola M, Di Stasio E, Giungi S. Fatigue is associated with serum interleukin-6 levels and symptoms of depression in 50 patients on chronic hemodialysis. J Pain Symptom Manag 2015; 49: 578–585 [DOI] [PubMed] [Google Scholar]
- 49. Cardenas DD, Kutner NG, The problem of fatigue in dialysis patients. Nephron 1982; 30: 336–340 [DOI] [PubMed] [Google Scholar]
- 50. Chen C-K, Tsai Y-C, Hsu H-J. et al. Depression and suicide risk in hemodialysis patients with chronic renal failure. Psychosomatics 2010; 51: 528–528 [DOI] [PubMed] [Google Scholar]
- 51. DePasquale C, Pistorio ML, Corona D. et al. Correlational study between psychic symptoms and quality of life among hemodialysis patients older than 55 years of age. Transplant Proc 2012; 44: 1876–1878 [DOI] [PubMed] [Google Scholar]
- 52. Dubin RF, Teerlink JR, Schiller NB. et al. Association of segmental wall motion abnormalities occurring during hemodialysis with post-dialysis fatigue. Nephrol Dial Transplant 2013; 28: 2580–2585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Garcia TW, Veiga JPR, da Motta LDC. et al. Depressed mood and poor quality of life in male patients with chronic renal failure undergoing hemodialysis. Revista Brasileira de Psiquiatria 2010; 32: 1–11 [DOI] [PubMed] [Google Scholar]
- 54. Joshwa B, Khakha DC, Mahajan S. Fatigue and depression and sleep problems among hemodialysis patients in a tertiary care center. Saudi J Kidney Dis Transplant 2012; 23: 729–735 [DOI] [PubMed] [Google Scholar]
- 55. Kalender B, Ozdemir A, Dervisoglu E. et al. Quality of life in chronic kidney disease: effects of treatment modality, depression, malnutrition and inflammation. Int J Clin Pract 2007; 61: 569–576 [DOI] [PubMed] [Google Scholar]
- 56. Karakan S, Sezer S, Ozdemir F. Factors related to fatigue and subgroups of fatigue in patients with end-stage renal disease. Clin Nephrol 2011; 76: 358–364 [DOI] [PubMed] [Google Scholar]
- 57. Fukuda S, Takashima S, Iwase M. et al. Development and validation of a new fatigue scale for fatigued subjects with and without chronic fatigue syndrome. In: Watanabe Y, Evengard B, Natelson BHet al. (eds). Fatigue Science for Human Health 2008: 89–102 [Google Scholar]
- 58. Leinau L, Murphy TE, Bradley E. et al. Relationship between conditions addressed by hemodialysis guidelines and non-ESRD-specific conditions affecting quality of life. Clin J Am Soc Nephrol. New York, NY: Springer, 2009; 4: 572–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lin J, Guo Q, Ye X. et al. The effect of social support and coping style on depression in patients with continuous ambulatory peritoneal dialysis in southern China. Int Urol Nephrol 2013; 45: 527–535 [DOI] [PubMed] [Google Scholar]
- 60. Chung LC. The clinical validation of defining characteristics and related factors in hemodialysis patients. Unpublished master's thesis. Tao Yuan, Taiwan, ROC: Chang Gung University, 1995 [Google Scholar]
- 61. Martínez-Sanchis S, Bernal MC, Montagud JV. et al. Quality of life and stressors in patients with chronic kidney disease depending on treatment. Spanish J Psychol 2015; 18: E25. [DOI] [PubMed] [Google Scholar]
- 62. Martins C, Duarte J, Chaves C. Contributions to the Quality of Life of Chronic Renal insufficient patients Procedia Social and Behavioral Sciences. 2015; 144–151 [Google Scholar]
- 63. Silva CF, Azevedo MH, Dias MR. Estudo Padronizado do Trabalho por Turnos: Versão Experimental. Bateria de escalas. Coimbra: Serviço de Psicologia Médica da Faculdade de Medicina da Universidade de Coimbra, 1994 [Google Scholar]
- 64. Oliveira CM, Costa SP, Costa LC. et al. Depression in dialysis patients and its association with nutritional markers and quality of life. J Nephrol, 2012; 25: 954. [DOI] [PubMed] [Google Scholar]
- 65. Santos PR. Depression and quality of life of hemodialysis patients living in a poor region of Brazil. Revista Brasileira de Psiquiatria 2011; 33: 332–337 [DOI] [PubMed] [Google Scholar]
- 66. Sklar AH, Riesenberg LA, Silber AK. et al. Postdialysis fatigue. Am J Kidney Dis 1996; 28: 732–736 [DOI] [PubMed] [Google Scholar]
- 67. Taskapan H, Ates F, Kaya B. et al. Psychiatric disorders and large interdialytic weight gain in patients on chronic haemodialysis. Nephrology 2005; 10: 15–20 [DOI] [PubMed] [Google Scholar]
- 68. Unruh ML, Buysse DJ, Dew MA. et al. Sleep quality and its correlates in the first year of dialysis. Clin J Am Soc Nephrol 2006; 1: 802–810 [DOI] [PubMed] [Google Scholar]
- 69. Vazquez I, Valderrábano F, Jofre R. et al. Psychosocial factors and quality of life in young hemodialysis patients with low comorbidity. J Nephrol 2003; 16: 886–894 [PubMed] [Google Scholar]
- 70. Wang SY, Zang XY, Liu JD. et al. Indicators and correlates of psychological disturbance in Chinese patients receiving maintenance hemodialysis: a cross-sectional study. Int Urol Nephrol 2015; 47: 679–689 [DOI] [PubMed] [Google Scholar]
- 71. Wang SY, Zang XY, Liu JD. et al. Psychometric properties of the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) in Chinese patients receiving maintenance dialysis. J Pain Symptom Manag 2015b; 49: 135–143 [DOI] [PubMed] [Google Scholar]
- 72. Wang S-Y, Zang X-Y, Fu S-H. et al. Factors related to fatigue in Chinese patients with end-stage renal disease receiving maintenance hemodialysis: a multi-center cross-sectional study. Ren Fail 2016; 38: 442–450 [DOI] [PubMed] [Google Scholar]
- 73. Williams AG, Crane PB, Kring D. Fatigue in African American women on hemodialysis. Nephrol Nurs J 2007; 34: 610–617, 644. [PubMed] [Google Scholar]
- 74. Popay J, Roberts H, Sowden A. et al. Guidance on the conduct of narrative synthesis in systematic reviews. ESRC Methods Program 2006; 15: 47–71 [Google Scholar]
- 75. Alsén P, Brink E, Persson L-O. et al. Illness perceptions after myocardial infarction: relations to fatigue, emotional distress, and health-related quality of life. J Cardiovascular Nurs 2010; 25: E1–E10 [DOI] [PubMed] [Google Scholar]
- 76. Broeckel JA, Jacobsen PB, Horton J. et al. Characteristics and correlates of fatigue after adjuvant chemotherapy for breast cancer. J Clin Oncol 1998; 16: 1689–1696 [DOI] [PubMed] [Google Scholar]
- 77. Irving K, Matcham F, Ali S. et al. Fatigue and functional disability in rheumatoid arthritis: evidence for a cognitive behavioural model. Rheumatology 2015; 54: i83–i84 [Google Scholar]
- 78. Skerrett TN, Moss-Morris R. Fatigue and social impairment in multiple sclerosis: the role of patients' cognitive and behavioral responses to their symptoms. J Psychosomatic Res 2006; 61: 587–593 [DOI] [PubMed] [Google Scholar]
- 79. Baldwin DS, Papakostas GI. Symptoms of fatigue and sleepiness in major depressive disorder. J Clin Psychiatry 2006; 67: 9–15 [PubMed] [Google Scholar]
- 80. Leone SS. A disabling combination: fatigue and depression. Br J Psychiatry 2010; 197: 86–87 [DOI] [PubMed] [Google Scholar]
- 81. Pae C-U, Lim H-K, Han C. Fatigue as a core symptom in major depressive disorder: overview and the role of bupropion. Expert Rev Neurother 2007; 7: 1251–1263 [DOI] [PubMed] [Google Scholar]
- 82. Skapinakis P, Lewis G, Meltzer H. Clarifying the relationship between unexplained chronic fatigue and psychiatric morbidity: results from a community survey in Great Britain. Int Rev Psychiatry 2003; 15: 57–64 [DOI] [PubMed] [Google Scholar]
- 83. Skapinakis P, Lewis G, Meltzer H. Clarifying the relationship between unexplained chronic fatigue and psychiatric morbidity: results from a community survey in Great Britain. Am J Psychiatry 2003; 15: 57–64 [DOI] [PubMed] [Google Scholar]
- 84. Wong DFK, Xuesong H, Poon A. et al. Depression literacy among Chinese in Shanghai, China: a comparison with Chinese-speaking Australians in Melbourne and Chinese in Hong Kong. Social Psychiatry Psychiatr Epidemiol 2012; 47: 1235–1242 [DOI] [PubMed] [Google Scholar]
- 85. Fung KM, Tsang HW, Corrigan PW. et al. Measuring self-stigma of mental illness in China and its implications for recovery. Int J Soc Psychiatry 2007; 53: 408–418 [DOI] [PubMed] [Google Scholar]
- 86. Bilgic A, Akman B, Sezer S. et al. Daytime sleepiness and quality of life in peritoneal dialysis patients. Ther Apher Dial 2011; 15: 565–571 [DOI] [PubMed] [Google Scholar]
- 87. Karadag E, Kilic SP, Metin O. Relationship between fatigue and social support in hemodialysis patients. Nurs Health Sci 2013; 15: 164–171 [DOI] [PubMed] [Google Scholar]
- 88. Houle N, Bohannon RW, Frigon L. et al. Health promoting behaviors, quality of life, and hospital resource utilization of patients receiving kidney transplants. Nephrol Nurs J 2002; 29: 35–40, 56. [PubMed] [Google Scholar]
- 89. Wight JP, Edwards L, Brazier J. et al. The SF36 as an outcome measure of services for end stage renal failure. Quality Health Care 1998; 7: 209–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hagger MS, Orbell S. A meta-analytic review of the common-sense model of illness representations. Psychol Health 2003; 18: 141–184 [Google Scholar]
- 91. Petrie KJ, Jago LA, Devcich DA. The role of illness perceptions in patients with medical conditions. Curr Opin Psychiatry 2007; 20: 163–167 [DOI] [PubMed] [Google Scholar]
- 92. Leventhal H, Benyamini Y, Brownlee S. et al. Illness representations: theoretical foundations. Percept Health Illness 1997; 2: 19–46 [Google Scholar]
- 93. Leventhal H, Nerenz DR, Purse J. Illness representations and coping with health threats. 1984, Lawrence Erlbaum Associates, Hillsdale: NJ. 219–252.
- 94. Jopson R, Moss-Morris R. The role of illness severity and illness representations in adjusting to multiple sclerosis. J Psychosomatic Res 2003; 54: 503–511 [DOI] [PubMed] [Google Scholar]
- 95. Nowak Z, Laudanski K. The perception of the illness with subsequent outcome measure in more favorable in continuos peritoneal dialysis vs hemodialysis in the framework of appraisal model of stress. Int J Med Sci 2014; 11: 291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Baak A. The association between worrying, self-efficacy and quality of life in renal patients on dialysis, in Faculty of Social & Behavioral Sciences2015, Leiden University. 1–32.
- 97. Devins GM, Mandin H, Hons RB. et al. Illness intrusiveness and quality of life in end-stage renal disease: comparison and stability across treatment modalities. Health Psychol 1990; 9: 117–142 [DOI] [PubMed] [Google Scholar]
- 98. Mohamed SA. The effectiveness of an educational intervention on fatigue in hemodialysis patients: a randomized controlled trial. J Nurs Health Sci 2014; 3: 40–50 [Google Scholar]
- 99. Chan W, Jones D, Bosch JA. et al. Cardiovascular, muscular and perceptual contributions to physical fatigue in prevalent kidney transplant recipients. Transplant Int 2016. [DOI] [PubMed] [Google Scholar]
- 100. O'Connor PJ. Evaluation of four highly cited energy and fatigue mood measures. J Psychosom Res 2004; 57: 435–441 [DOI] [PubMed] [Google Scholar]
- 101. Whitehead L. The measurement of fatigue in chronic illness: a systematic review of unidimensional and multidimensional fatigue measures. J Pain Symptom Manag 2009; 37: 107–128 [DOI] [PubMed] [Google Scholar]
- 102. Jayanti A, Foden P, Wearden A. et al. Illness beliefs in end stage renal disease and associations with self-care modality choice. PloS One 2016; 11: e0154299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Neipp M, Karavul B, Jackobs S. et al. Quality of life in adult transplant recipients more than 15 years after kidney transplantation. Transplantation 2006; 81: 1640–1644 [DOI] [PubMed] [Google Scholar]
- 104. Saracino A, Gollo I, Di Noia I. et al. Loss of renal function is associated with deterioration of health-related quality of life in kidney transplant patients. Transplant Proc 2008; 40: 3460–3465 [DOI] [PubMed] [Google Scholar]
- 105. Surawy C, Hackmann A, Hawton K. et al. Chronic fatigue syndrome: a cognitive approach. Behav Res Ther 1995; 33: 535–544 [DOI] [PubMed] [Google Scholar]
- 106. Van Kessel K, Moss-Morris R. Understanding multiple sclerosis fatigue: a synthesis of biological and psychological factors. J Psychosom Res 2006; 61: 583–585 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.