Selection of appropriate clinical endpoints for examining the efficacy of investigational agents for non‐small cell lung cancer is of vital importance in clinical trial design. This review provides an overview of the study designs of clinical trials for approved agents in non‐small cell lung cancer and focuses on the validity of alternative endpoints for such trials.
Keywords: Carcinoma, Non‐small cell lung cancer, Antineoplastic agents, Surrogate endpoint, Food and Drug Administration drug approval
Abstract
Based on the positive results of various clinical trials, treatment options for non‐small cell lung cancer (NSCLC) have expanded greatly over the last 25 years. While regulatory approvals of chemotherapeutic agents for NSCLC have largely been based on improvements in overall survival, recent approvals of many targeted agents for NSCLC (afatinib, crizotinib, ceritinib, osimertinib) have been based on surrogate endpoints such as progression‐free survival and objective response. As such, selection of appropriate clinical endpoints for examining the efficacy of investigational agents for NSCLC is of vital importance in clinical trial design. This review provides an overview of clinical trial endpoints previously utilized for approved agents for NSCLC and highlights the key efficacy results for these trials. Trends for more recent approvals in NSCLC, including those for the immunotherapeutic agents nivolumab and pembrolizumab, are also discussed. The results of a correlative analysis of endpoints from 18 clinical trials that supported approvals of investigational agents in clinical trials for NSCLC are also presented.
Implications for Practice.
While improving survival remains the ultimate goal of oncology clinical trials, overall survival may not always be the most feasible or appropriate endpoint to assess patient response. Recently, several investigational agents, both targeted agents and immunotherapies, have gained U.S. Food and Drug Administration approval in non‐small cell lung cancer based on alternate endpoints such as progression‐free survival or response rate. An understanding of the assessment of response and trial endpoint choice is important for future oncology clinical trial design.
Introduction
Within the U.S., an estimated 224,390 new cases of lung cancer and 158,080 related deaths were expected in 2016, making lung cancer the leading cause of cancer‐related deaths [1]. The majority (83%) of lung cancers are classified as non‐small cell lung cancer (NSCLC) [1], and treatment of NSCLC in the absence of a targetable mutation typically entails surgery, radiation therapy, and platinum‐based chemotherapy, used either alone or in combination depending on disease status [2]. Several chemotherapeutic drugs have been approved by the U.S. Food and Drug Administration (FDA) for first‐line treatment of NSCLC, including cisplatin, vinorelbine, paclitaxel, gemcitabine, pemetrexed, and docetaxel (also approved for second‐line use) [3], [4].
With the advent of molecular testing, which is now standard for treatment of adenocarcinomas, targeted therapies have been implemented into the treatment regimen for NSCLC, with several agents approved by the FDA based on efficacy demonstrated in clinical trials in specific NSCLC patient populations [2]. To date, the majority of FDA‐approved drugs for NSCLC, including bevacizumab and erlotinib, have shown significant improvement in overall survival (OS) in clinical trials [5]. However, FDA guidelines have recently stated that substantial and robust differences in time to progression or progression‐free survival (PFS), which are endpoints commonly used in clinical trials as surrogates for OS, can also be used to support accelerated FDA approval of investigational agents [5]. In addition to the magnitude of the effect, the FDA has recommended examination of the risk‐benefit profile of a drug in order for PFS to be considered as the primary endpoint in support of its approval. Unlike earlier trials that evaluated targeted agents for NSCLC, more recent studies have established PFS or objective response as the primary endpoint [6], [7], [8], [9], [10], [11]. Because clinical trials have to be designed in order to ultimately ensure regulatory approval of the investigational agent, selection of the appropriate endpoint prior to the start of the study is essential. This review will provide an overview of the study designs of historical and recent clinical trials for approved agents in NSCLC and will focus on the validity of alternative endpoints for such trials.
Materials and Methods
Published clinical trials supporting FDA approval of investigational agents for NSCLC treatment have been included in this review (Table 1). For the correlation analysis of clinical trial endpoints (Table 2), published randomized controlled trials that demonstrated significant improvements in the indicated endpoints and led to FDA approval for the listed chemotherapeutic, targeted, and immunotherapeutic agents were included.
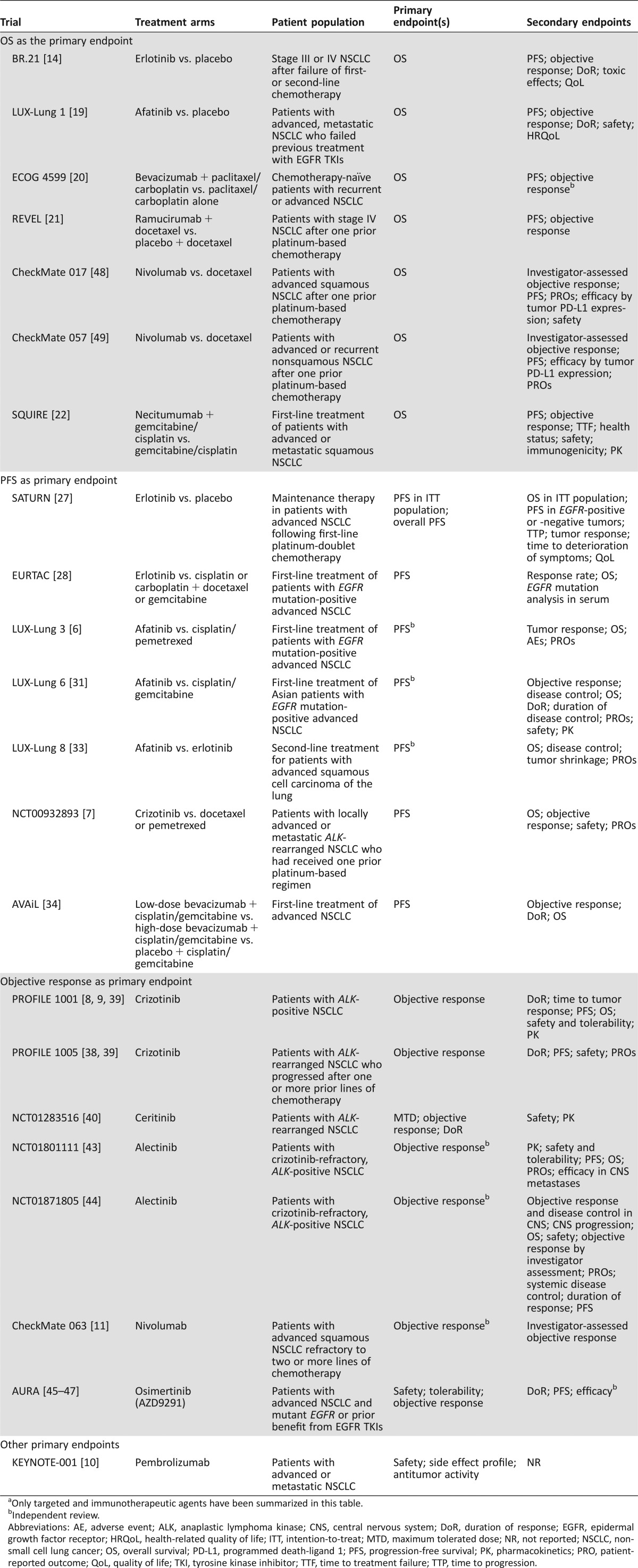
Table 1. Study designs of clinical trials for approved agents for NSCLCa.
Only targeted and immunotherapeutic agents have been summarized in this table.
Independent review.
Abbreviations: AE, adverse event; ALK, anaplastic lymphoma kinase; CNS, central nervous system; DoR, duration of response; EGFR, epidermal growth factor receptor; HRQoL, health‐related quality of life; ITT, intention‐to‐treat; MTD, maximum tolerated dose; NR, not reported; NSCLC, non‐small cell lung cancer; OS, overall survival; PD‐L1, programmed death‐ligand 1; PFS, progression‐free survival; PK, pharmacokinetics; PRO, patient‐reported outcome; QoL, quality of life; TKI, tyrosine kinase inhibitor; TTF, time to treatment failure; TTP, time to progression.
Table 2. Correlative analysis of clinical trial endpoints supporting approval of investigational agents for NSCLC.
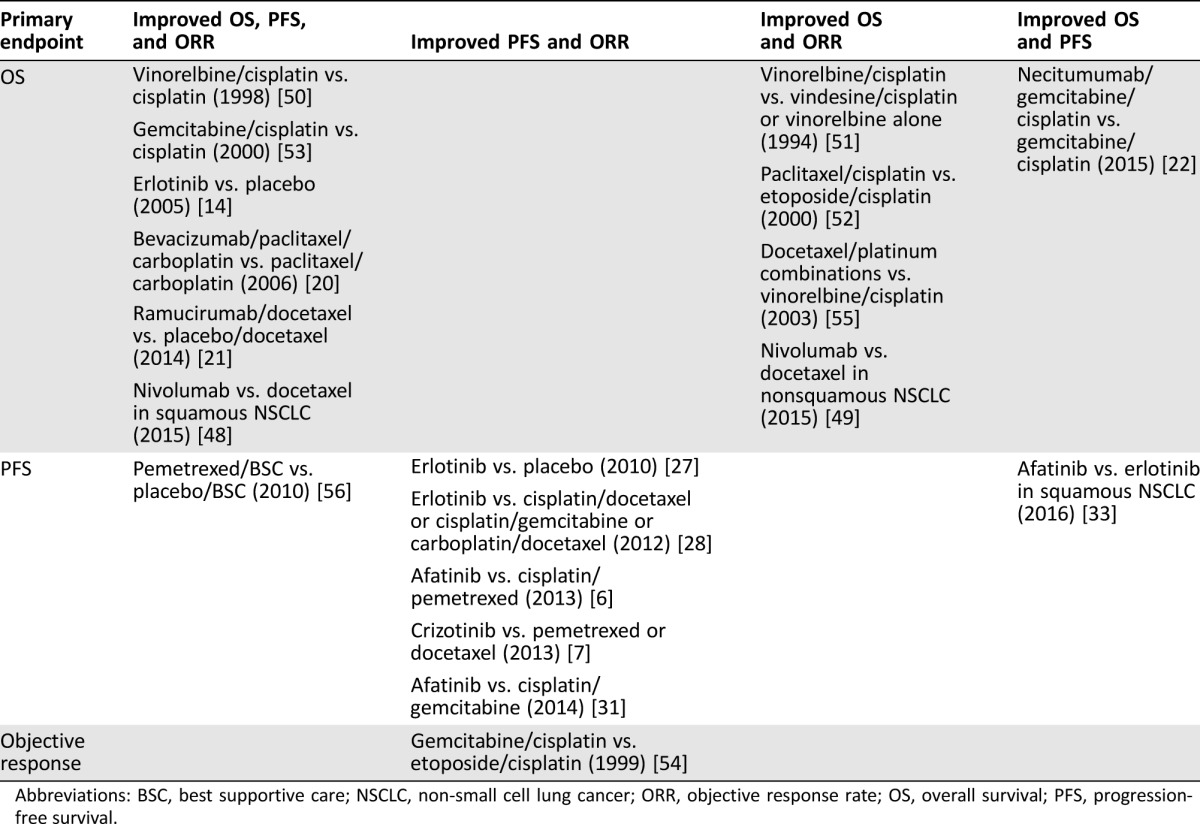
Abbreviations: BSC, best supportive care; NSCLC, non‐small cell lung cancer; ORR, objective response rate; OS, overall survival; PFS, progression‐free survival.
Clinical Trial Designs of Approved Targeted Agents for NSCLC
Primary and secondary endpoints of clinical trials of approved agents in NSCLC are summarized in Table 1.
Clinical Trials with OS as the Primary Endpoint
The gold standard primary endpoint for clinical trials in oncology has been OS, as it is not subject to investigator bias, it guides clinical practice decisions based on the potential risks and benefits of a particular therapy, and it is the clinical endpoint in which patients are most interested [12]. However, disadvantages of using OS as an endpoint have been noted, including the risk of confounding due to second‐line treatments or treatment crossovers and the length of time required for completion of trials, highlighting the need for alternative endpoints in certain contexts [12], [13].
In the trial that led to approval of the epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI) erlotinib (Tarceva, Genentech/Roche; South San Francisco, CA, https://www.gene.com/) for NSCLC in patients who previously received chemotherapy (BR.21), the primary endpoint was OS, with PFS, objective response, duration of response (DoR), toxic effects, and quality of life (QoL) designated as secondary endpoints [14]. Results from this trial revealed that erlotinib was associated with OS, PFS, and objective response rate (ORR) benefits in this specific patient population; subsequent studies demonstrated that erlotinib was associated with worse outcomes for patients with EGFR wild‐type tumors [15].
Afatinib (Gilotrif, Boehringer Ingelheim; Ingelheim, Germany, https://www.boehringer-ingelheim.com/) is an irreversible ErbB family blocker of EGFR, erb‐b2 receptor tyrosine kinase 2 (ErbB2), and ErbB4 [16, 17], with in vitro activity against ErbB3 phosphorylation [18]. Patients with advanced, metastatic NSCLC who failed previous treatment with EGFR TKIs were randomly assigned to receive afatinib or placebo in the LUX‐Lung 1 trial, which examined OS as the primary endpoint; secondary endpoints included PFS, objective response, response duration, safety, and health‐related QoL [19]. While no improvements in OS were noted for afatinib versus placebo, a robust PFS benefit was observed for afatinib compared with placebo in the overall pretreated population as well as in patients whose tumors harbored EGFR mutations.
The antiangiogenic vascular endothelial growth factor receptor (VEGFR) inhibitor bevacizumab (Avastin, Genentech/Roche) was evaluated in patients with advanced nonsquamous NSCLC following randomization into treatment groups consisting of either paclitaxel/carboplatin alone or with bevacizumab in the pivotal ECOG 4599 trial [20]. The primary endpoint of improved OS was achieved with the addition of bevacizumab to chemotherapy. Similarly, the secondary endpoints of PFS and ORR were also improved with the addition of bevacizumab to standard chemotherapy.
More recently, a phase III study evaluated ramucirumab (Cyramza, Eli Lilly and Company; Indianapolis, IN, https://www.lilly.com/), a monoclonal antibody that targets VEGFR‐2, in combination with docetaxel as second‐line treatment for stage IV NSCLC after platinum‐based therapy (REVEL) [21]. Significant OS (primary endpoint), PFS, and ORR improvements were observed with ramucirumab/docetaxel compared with placebo/docetaxel. Based on these positive findings, the FDA approved ramucirumab in combination with docetaxel for this indication in December 2014.
In November 2015, the FDA approved the anti‐EGFR monoclonal antibody necitumumab (Portrazza, Eli Lilly and Company) in combination with gemcitabine and cisplatin for the first‐line treatment of patients with advanced squamous NSCLC. This approval was based on the results of the open‐label, randomized SQUIRE trial comparing gemcitabine plus cisplatin with or without necitumumab. Results from the SQUIRE trial demonstrated significant improvements in OS (primary endpoint) and PFS but no improvement in ORR when necitumumab was added to gemcitabine/cisplatin chemotherapy [22].
Clinical Trials with PFS or Time to Progression as the Primary Endpoint
Recent trends in oncology clinical trials have shifted toward choosing surrogates such as PFS as the primary endpoint. While OS may be easier to examine in later‐line studies, when there may be less influence from subsequent therapies, there are several advantages to measuring PFS in clinical trials. These include shortened trial duration and faster time to drug approval [12]. Moreover, PFS can provide a clearer picture of the activity of an agent and allows for assessment of the duration of tumor control and measurement of tumor shrinkage and tumor stabilization, both of which are effects associated with most recently developed targeted agents [23]. On the other hand, disadvantages include the need for frequent and ongoing assessment of disease status, variable definitions regarding progression, and risk of bias [23], [24]. With respect to clinical trials for NSCLC, the Oncologic Drugs Advisory Committee recommended PFS as an endpoint for advanced NSCLC clinical trials in 2003 [12], but whether PFS can be used as a valid surrogate endpoint remains controversial [23], [25], [26].
PFS can provide a clearer picture of the activity of an agent and allows for assessment of the duration of tumor control and measurement of tumor shrinkage and tumor stabilization, both of which are effects associated with most recently developed targeted agents.
In contrast to the design of BR.21, PFS was selected as the primary endpoint in subsequent trials that led to the approval of erlotinib for maintenance treatment in advanced NSCLC (SATURN) [27] as well as first‐line treatment for patients with NSCLC harboring EGFR mutations (EURTAC) [28]. As a secondary endpoint in both of these trials, OS was prolonged with erlotinib versus placebo in the maintenance setting [27], but no OS benefit was observed with erlotinib compared with chemotherapy in patients with EGFR mutations, likely due to subsequent therapies [28], [29]. More specifically, subgroup analyses revealed no OS benefit with erlotinib for patients with del19 or L858R EGFR mutations [29]. Although improvements in PFS were noted in both trials, OS was designated as the regulatory endpoint for approval of erlotinib in maintenance treatment of advanced NSCLC.
In the LUX‐Lung 3 trial, patients with advanced nonsquamous NSCLC harboring EGFR mutations were stratified and assigned 2:1 to afatinib or cisplatin/pemetrexed [6]. The trial met its primary endpoint of PFS but failed to demonstrate an improvement in OS following treatment with afatinib. Nevertheless, the robust PFS benefit associated with afatinib in this trial led to its approval by the FDA for first‐line treatment of advanced NSCLC in patients harboring sensitizing EGFR mutations [30]. The LUX‐Lung 6 trial was a similarly designed trial comparing afatinib versus cisplatin/gemcitabine as first‐line treatment in Asian patients with advanced NSCLC harboring EGFR mutations [31]. Similar to the results from LUX‐Lung 3, a PFS but not OS benefit was observed in the overall population. Interestingly, a prespecified subgroup analysis of patients with del19 EGFR mutations indicated that afatinib prolonged OS compared with chemotherapy in both LUX‐Lung 3 and LUX‐Lung 6 [32]. The LUX‐Lung 8 trial compared afatinib versus erlotinib as second‐line treatment in patients with advanced squamous cell carcinoma of the lung [33]. At a median follow‐up of 18.4 months, PFS (primary endpoint) and OS (key secondary endpoint) were significantly greater in the afatinib group than in the erlotinib group. This PFS benefit (2.6 months with afatinib versus 1.9 months with erlotinib; hazard ratio, 0.81) led to the FDA approval of afatinib for the treatment of patients with metastatic, squamous NSCLC progressing after platinum‐based therapy [30].
FDA approval for crizotinib (Xalkori, Pfizer; New London, CT, http://www.pfizer.com/), an inhibitor that targets the echinoderm microtubule‐associated protein‐like 4‐anaplastic lymphoma kinase (EML4‐ALK) fusion product, was based on the results of a phase III trial in which patients with locally advanced or metastatic ALK‐rearranged NSCLC who had received one prior platinum‐based regimen were treated with either crizotinib or chemotherapy (pemetrexed or docetaxel) [7]. A significant PFS benefit (the primary endpoint) along with increased ORR was associated with crizotinib versus chemotherapy, but no improvement in OS, a secondary endpoint, was observed.
Similar to the ECOG 4599 trial, the AVAiL placebo‐controlled phase III trial evaluated bevacizumab at 7.5‐mg/kg and 15‐mg/kg doses in combination with cisplatin/gemcitabine for first‐line treatment of advanced NSCLC [34]. The primary endpoint was amended from OS to PFS, and while a significant improvement in PFS was observed with bevacizumab, the secondary endpoint of improved OS was not met with this agent at either dose [35].
Clinical Trials with Objective Response as the Primary Endpoint
Objective response is often assessed by Response Evaluation Criteria in Solid Tumors (RECIST) and looks at both the complete response and partial response to therapy [12]. Advantages associated with using objective response in clinical trials include the ability to measure efficacy in single‐arm studies and the ability of investigators to attribute the effect to the drug and not to the natural history of the patient [12]. Objective response has also been proposed to be a more informative endpoint for discovering predictive biomarkers compared with OS [36]. On the other hand, objective response may not be able to capture the full long‐term benefits of treatment, and additional endpoints may be needed to validate the clinical benefit [12], [37].
Ongoing and completed phase I and II trials evaluating crizotinib in NSCLC have designated objective response as the primary endpoint [38], [39]. Significant improvements in ORR were observed in two single‐arm trials of crizotinib for locally advanced or metastatic ALK‐rearranged NSCLC, which contributed to the accelerated approval of crizotinib for this indication [9], [38], [39]. More recently, high response rates were noted in a completed phase I study for ceritinib (Zykadia, Novartis Pharmaceuticals; East Hanover, NJ, https://www.pharma.us.novartis.com/) in a similar patient population, including those who progressed after receiving crizotinib [40]. The high ORR observed with ceritinib led to FDA approval for this agent in patients with ALK‐rearranged NSCLC [41]. Another ALK inhibitor, alectinib (Alecensa, Genentech), was subsequently granted accelerated approval by the FDA in late 2015 for treatment of patients with ALK‐positive, metastatic NSCLC who have progressed on or are intolerant to crizotinib [42]. This approval was based on the high ORR (primary endpoint) of 50% and 48% observed in two pivotal single‐arm studies [43], [44].
Most recently, the FDA approved the mutant‐selective EGFR inhibitor osimertinib (Tagrisso, AstraZeneca Pharmaceuticals LP; Wilmington, DE, https://www.astrazeneca-us.com/) for the treatment of patients with advanced or metastatic NSCLC harboring T790M‐mutant EGFR who have progressed on EGFR‐TKI therapy. The approval was based on initial results from the AURA trial program evaluating the safety and efficacy of osimertinib (AZD9291) in NSCLC patients who progressed on EGFR‐TKI therapy. Primary endpoints of the phase I AURA study included safety, tolerability, and efficacy as measured by ORR, with secondary endpoints including DoR and PFS. Results from AURA demonstrated promising response rates in NSCLC patients harboring T790M EGFR mutations [45], [46], [47]. Due to the accelerated approval of osimertinib, additional studies are ongoing to validate its efficacy. These include phase III studies of first‐line osimertinib versus gefitinib or erlotinib in advanced, EGFR mutation‐positive NSCLC (FLAURA; ClinicalTrials.gov Identifier: NCT02296125) and osimertinib versus platinum‐based chemotherapy in advanced, T790M mutation‐positive NSCLC after progression on EGFR‐TKI therapy (AURA3; NCT02151981). PFS is the designated primary endpoint for both phase III trials, with OS and ORR among several secondary endpoints specified for both trials.
Recent Immunotherapy Approvals in NSCLC
Based on promising efficacy data, the FDA approved the immunotherapeutic agents nivolumab and pembrolizumab for NSCLC treatment in 2015. Nivolumab (Opdivo, Bristol‐Myers Squibb; Princeton, NJ, http://www.bms.com), a human anti‐programmed death‐1 antibody, gained approval in March 2015 for patients with advanced or metastatic squamous NSCLC following platinum‐based chemotherapy. Approval for this indication was based on the CheckMate 017 and CheckMate 063 trials demonstrating efficacy and safety of nivolumab. In the CheckMate 017 trial, nivolumab showed significant improvement in OS, the primary endpoint, compared with docetaxel [48]. Significant improvements in ORR and PFS were also observed with nivolumab compared with docetaxel for squamous NSCLC in this trial. The CheckMate 063 single‐arm trial specified objective response as the primary endpoint. Results from this trial demonstrated clinically meaningful antitumor activity for nivolumab along with a tolerable safety profile [11]. In October 2015, nivolumab was granted FDA approval for the treatment of advanced nonsquamous NSCLC with progression on or after platinum therapy based on data from the CheckMate 057 trial. The primary endpoint of this trial was OS, which, along with response rate, was significantly improved with nivolumab versus docetaxel, and levels of tumor programmed death‐ligand 1 (PD‐L1) expression were positively associated with clinical benefit [49]. Although nivolumab treatment did not improve PFS compared with docetaxel, the rate of PFS at 1 year was higher with nivolumab [49].
Pembrolizumab (Keytruda, Merck & Company; Whitehouse Station, NJ, http://www.merck.com) was approved in October 2015 for the treatment of advanced or metastatic NSCLC with expression of the immune checkpoint protein PD‐L1 as determined by an FDA‐approved test. The single‐arm phase I trial (KEYNOTE‐001) leading to FDA approval of pembrolizumab had primary objectives of evaluating safety, side effect profile, and response rate. Results from KEYNOTE‐001 demonstrated efficacy of pembrolizumab and an acceptable side effect profile in advanced NSCLC patients [10]. The efficacy of pembrolizumab was improved in patients whose tumors expressed PD‐L1 in at least 50% of the tumor cells, supporting the predictive value of PD‐L1 expression in response to pembrolizumab [10]. Ongoing phase III trials of pembrolizumab have designated a variety of primary endpoints to validate its efficacy in NSCLC, including disease‐free survival (DFS), PFS, and OS (NCT02504372, NCT02578680, NCT02220894, NCT02142738, and NCT01905657).
Correlative Analysis of Clinical Trial Endpoints Supporting FDA Approval
Since 1990, 19 randomized controlled clinical trials have been identified that supported FDA approval of nontargeted and targeted agents for NSCLC [6], [7], [14], [20], [21], [22], [27], [28], [31], [33], [48], [49], [50], [51], [52], [53], [54], [55], [56]. Among the 18 trials that reported both OS and PFS endpoints (one trial reported only OS and ORR [51]), only nine trials reported improvements in both OS and PFS with the investigational agent (Table 2); among the remaining trials that did not achieve both endpoints, six trials reported improvements in only PFS, and three trials reported an improvement in only OS. Likewise, among 19 trials that reported both OS and objective response endpoints, 11 trials reported improvements in both OS and objective response with the investigational agent, and six trials reported improvements in only objective response with the investigational agent. It is worth noting that several of the trials that demonstrated PFS but not OS benefits reported crossover of patients between treatment arms [6], [7], [28]. Additionally, while the individual LUX‐Lung 3 and LUX‐Lung 6 trials demonstrated improvements only in PFS with afatinib in the overall population, a prespecified analysis of patients with del19 EGFR mutations demonstrated an OS benefit with afatinib versus chemotherapy in both trials [32], illustrating the utility of well‐defined molecular biomarkers in determining outcomes in clinical trials. Similarly, while median PFS did not favor nivolumab over docetaxel in nonsquamous NSCLC, the rates of PFS and OS at 1 year were higher with nivolumab in this trial [49]. Patients with higher expression of PD‐L1 at predefined levels demonstrated improved PFS with nivolumab compared with docetaxel, further highlighting the importance of biomarkers of response in NSCLC.
Discussion and Clinical Perspectives
Historically, clinical trials for NSCLC have relied on improved OS as the primary endpoint for regulatory submission and approval of investigational agents. However, FDA approvals for afatinib, crizotinib, ceritinib, osimertinib, nivolumab, and pembrolizumab for the treatment of specific patient populations with advanced NSCLC in 2013 to 2015 have highlighted increases in the use of appropriate surrogate endpoints in establishing the clinical benefit of investigational agents. These shifts to include surrogate endpoints may be influenced by the increasing number of targeted agents in clinical development.
In particular, PFS has become a widely used surrogate endpoint in many oncology clinical trials, including trials for NSCLC, due to the shorter follow‐up required as well as the lack of crossover effects or confounding in patients receiving multiple lines of therapy [12]. Discussion around this trend toward designation of PFS as the primary endpoint for NSCLC trials is increasing, and clinicians and investigators are considering its utilization in NSCLC trial design [23], [25], [57]. In support of this viewpoint, a meta‐analysis of 60 randomized NSCLC trials demonstrated that PFS is a valid surrogate endpoint for OS in studies evaluating chemotherapy and radiotherapy in patients with locally advanced lung cancers [58]. Another meta‐analysis of phase II trials for advanced NSCLC revealed that failure‐free survival or PFS at 12 weeks is a strong predictor of subsequent patient survival [59]. Furthermore, an analysis of 14 randomized controlled trials in NSCLC showed a strong correlation between ORR and PFS, but an association between ORR and OS or between PFS and OS could not be established, possibly because of crossover or longer OS with targeted therapies [60]. Analyses of these trials at the patient level demonstrated that responders had more favorable PFS and OS compared with nonresponders, thereby further strengthening the argument for using ORR and PFS as primary endpoints for approval [60].
The investigational drug and its mechanism of action should be considered when deciding on clinical trial endpoints. Immunotherapeutic agents such as nivolumab and pembrolizumab are often associated with delayed response kinetics, potentially due to the time needed for immune cell expansion and invasion into the tumor [61]. These kinetics may complicate the assessment of response, when tumor growth, normally characterized as disease progression, may occur before the onset of response [61], [62]. In an effort to capture the differences in response kinetics and sequence often seen with immunotherapies, new response criteria were proposed to allow for immunotherapy‐specific assessment of patient response [62]. The role of these immune‐related response criteria in clinical trial design and interpretation is unclear, as the trials leading to approval of nivolumab and pembrolizumab in NSCLC primarily used RECIST criteria for assessment of response [10], [11], [48], [49]. The trials comparing nivolumab with docetaxel in squamous and nonsquamous NSCLC also designated OS as the primary endpoint [48], [49], suggesting that the effects of immune‐mediated responses on choosing trial endpoints are not well understood. As we learn more about these agents, new trial designs and endpoints are likely to be adopted to truly assess the clinical benefit of these drugs.
Immunotherapeutic agents such as nivolumab and pembrolizumab are often associated with delayed response kinetics, potentially due to the time needed for immune cell expansion and invasion into the tumor. These kinetics may complicate the assessment of response, when tumor growth, normally characterized as disease progression, may occur before the onset of response.
Endpoint choice can also vary based on the clinical development strategy. Traditional approvals of targeted agents in NSCLC, such as erlotinib and afatinib, were based on time‐to‐event endpoints (e.g., OS, DFS, or PFS), which can lengthen the approval process. Recent accelerated drug approvals in NSCLC (e.g., osimertinib) have instead been based on ORR and/or DoR in single‐arm studies. This allows agents with promising efficacy to be utilized more rapidly, with the expectation that confirmatory randomized controlled trials measuring a time‐to‐event endpoint will follow. This strategy is supported by recent results showing correlation between response rates and PFS [60]. Thus, surrogate endpoints can predict clinical benefit and may allow more rapid drug approval to improve the treatment of NSCLC.
While the correlative analysis of clinical trial endpoints for FDA‐approved agents revealed some discordance between OS and PFS benefits in approximately half of the clinical trials that were reviewed, effects of crossover and subsequent lines of therapy on OS must be taken into consideration. Consequently, evaluation of individual OS and PFS data for each patient with respect to their treatment history would likely provide valuable information regarding the validity of PFS as a surrogate endpoint for NSCLC clinical trials. Additionally, the role of alternate endpoints such as PFS in demonstrating clinical benefit will likely be impacted by patient selection. The triple angiokinase inhibitor nintedanib (Vargatef, Boehringer Ingelheim) was approved by the European Medicines Agency in combination with docetaxel for the treatment of locally advanced, metastatic, or locally recurrent NSCLC of adenocarcinoma histology after first‐line chemotherapy. This approval was based on results of the LUME‐Lung 1 trial, which demonstrated a significant improvement in PFS (primary endpoint) with nintedanib in patients with advanced NSCLC of all histologies [63]. An overall survival benefit was seen only in NSCLC patients with adenocarcinoma, illustrating the potential impact of patient selection on the correlation between trial endpoints. Importantly, the approval of nintedanib in the European Union also indicates that regulatory agencies may differ in their acceptance of surrogate trial endpoints.
While many of the recent clinical trials for NSCLC have designated PFS as the primary endpoint, the topic remains controversial, and several investigators have questioned the validity of PFS as a surrogate endpoint for OS in NSCLC, especially in settings in which the magnitude of PFS benefit is small [26]. To address some of the concerns surrounding the use of PFS as an endpoint, the FDA has recommended the inclusion of sensitivity analyses in the design of clinical trials to evaluate the robustness of PFS as an endpoint in phase III trials and to determine the likelihood of the results being oversimplified [23], [64]. Additionally, clinical studies could be designed with coprimary endpoints such as OS and PFS, thus allowing faster assessments of response while having adequate power to show OS benefit.
Conclusion
The use of surrogate endpoints in clinical trials for NSCLC allows investigators to obtain information regarding the efficacy of an investigational agent that OS by itself cannot provide, and these endpoints should be valid for approval where appropriate. However, clear guidance on the use of these endpoints should be implemented, as currently no specific guidelines have been established regarding the use of PFS or objective response as the primary endpoint. Clinical trials for both afatinib and crizotinib in patients with NSCLC demonstrate examples in which robust PFS benefits have led to recent FDA approvals and highlight increasing regulatory acceptance of PFS as a surrogate endpoint in clinical trials for NSCLC. Careful planning and inclusion of appropriate endpoints and analyses with respect to the type of investigational agent and the patient population being evaluated will help ensure that clinical benefit of future investigational agents is properly assessed.
Acknowledgments
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors. The authors received no direct compensation related to the development of the manuscript. Writing, editorial support, and formatting assistance was provided by Lauren Fink, PhD, of MedErgy, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Incorporated (BIPI). BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Author Contributions
Conception and Design: Charu Aggarwal, Hossein Borghaei
Collection and/or Assembly of Data: Charu Aggarwal, Hossein Borghaei
Data Analysis and Interpretation: Charu Aggarwal, Hossein Borghaei
Manuscript Writing: Charu Aggarwal, Hossein Borghaei
Final Approval of Manuscript: Charu Aggarwal, Hossein Borghaei
Disclosures
Charu Aggarwal: Genentech, Bristol‐Myers Squibb, Eli Lilly (C/A). Hossein Borghaei: Bristol‐Myers Squibb, Lilly, Celgene, Merck, Pfizer, Trovagene, EMD‐Serono, Boehringer Ingelheim, Genentech (C/A), Bristol‐Myers Squibb, Celgene (H), Merck/Celgene, Millennium (RF).
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.American Cancer Society. Cancer facts & figures, 2016. Available at https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2016.html. Accessed March 10, 2017.
- 2.National Comprehensive Cancer Network . NCCN clinical practice guideline in oncology. Non‐small cell lung cancer. Version 6.2015. Available at https://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed March 10, 2017.
- 3.Alimta® (pemetrexed disodium) [package insert]. Indianapolis, IN: Eli Lilly and Company, 2009.
- 4. Bunn P, Pazdur R, Burke L et al. Workshop summary on endpoints for approval of cancer drugs for lung cancer. 2003. Available at https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/CancerDrugs/ucm094744.pdf. Accessed March 10, 2017.
- 5.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research et al. Guidance for industry: Clinical trial endpoints for the approval of non‐small cell lung cancer drugs and biologics. 2015. Available at https://www.fda.gov/downloads/drugs/guidances/ucm259421.pdf. Accessed March 10, 2017.
- 6. Sequist LV, Yang JC, Yamamoto N et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–3334. [DOI] [PubMed] [Google Scholar]
- 7. Shaw AT, Kim DW, Nakagawa K et al. Crizotinib versus chemotherapy in advanced ALK‐positive lung cancer. N Engl J Med 2013;368:2385–2394. [DOI] [PubMed] [Google Scholar]
- 8. Kwak EL, Bang YJ, Camidge DR et al. Anaplastic lymphoma kinase inhibition in non‐small‐cell lung cancer. N Engl J Med 2010;363:1693–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Camidge DR, Bang YJ, Kwak EL et al. Activity and safety of crizotinib in patients with ALK‐positive non‐small‐cell lung cancer: Updated results from a phase 1 study. Lancet Oncol 2012;13:1011–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Garon EB, Rizvi NA, Hui R et al. Pembrolizumab for the treatment of non‐small‐cell lung cancer. N Engl J Med 2015;372:2018–2028. [DOI] [PubMed] [Google Scholar]
- 11. Rizvi NA, Mazieres J, Planchard D et al. Activity and safety of nivolumab, an anti‐PD‐1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non‐small‐cell lung cancer (CheckMate 063): A phase 2, single‐arm trial. Lancet Oncol 2015;16:257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCain JA Jr. The ongoing evolution of endpoints in oncology. Manag Care (suppl) 2010;19:1–11. [Google Scholar]
- 13. Korn EL, Freidlin B, Abrams JS. Overall survival as the outcome for randomized clinical trials with effective subsequent therapies. J Clin Oncol 2011;29:2439–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shepherd FA, Rodrigues Pereira J, Ciuleanu T et al. Erlotinib in previously treated non‐small‐cell lung cancer. N Engl J Med 2005;353:123–132. [DOI] [PubMed] [Google Scholar]
- 15. Garassino MC, Martelli O, Broggini M et al. Erlotinib versus docetaxel as second‐line treatment of patients with advanced non‐small‐cell lung cancer and wild‐type EGFR tumors (TAILOR): A randomised controlled trial. Lancet Oncol 2013;14:981–988. [DOI] [PubMed] [Google Scholar]
- 16. Li D, Ambrogio L, Shimamura T et al. BIBW2992, an irreversible EGFR/HER2 inhibitor highly effective in preclinical lung cancer models. Oncogene 2008;27:4702–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Solca F, Dahl G, Zoephel A et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther 2012;343:342–350. [DOI] [PubMed] [Google Scholar]
- 18. Ioannou N, Seddon AM, Dalgleish A et al. Treatment with a combination of the ErbB (HER) family blocker afatinib and the IGF‐IR inhibitor, NVP‐AEW541 induces synergistic growth inhibition of human pancreatic cancer cells. BMC Cancer 2013;13:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Miller VA, Hirsh V, Cadranel J et al. Afatinib versus placebo for patients with advanced, metastatic non‐small‐cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX‐Lung 1): A phase 2b/3 randomised trial. Lancet Oncol 2012;13:528–538. [DOI] [PubMed] [Google Scholar]
- 20. Sandler A, Gray R, Perry MC et al. Paclitaxel‐carboplatin alone or with bevacizumab for non‐small‐cell lung cancer. N Engl J Med 2006;355:2542–2550. [DOI] [PubMed] [Google Scholar]
- 21. Garon EB, Ciuleanu TE, Arrieta O et al. Ramucirumab plus docetaxel versus placebo plus docetaxel for second‐line treatment of stage IV non‐small‐cell lung cancer after disease progression on platinum‐based therapy (REVEL): A multicentre, double‐blind, randomised phase 3 trial. Lancet 2014;384:665–673. [DOI] [PubMed] [Google Scholar]
- 22. Thatcher N, Hirsch FR, Luft AV et al. Necitumumab plus gemcitabine and cisplatin versus gemcitabine and cisplatin alone as first‐line therapy in patients with stage IV squamous non‐small‐cell lung cancer (SQUIRE): An open‐label, randomised, controlled phase 3 trial. Lancet Oncol 2015;16:763–774. [DOI] [PubMed] [Google Scholar]
- 23. Soria JC, Massard C, Le Chevalier T. Should progression‐free survival be the primary measure of efficacy for advanced NSCLC therapy? Ann Oncol 2010;21:2324–2332. [DOI] [PubMed] [Google Scholar]
- 24. Lebwohl D, Kay A, Berg W et al. Progression‐free survival: Gaining on overall survival as a gold standard and accelerating drug development. Cancer J 2009;15:386–394. [DOI] [PubMed] [Google Scholar]
- 25. Garon EB. Issues surrounding clinical trial endpoints in solid malignancies with a focus on metastatic non‐small cell lung cancer. Lung Cancer 2012;77:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sacher AG, Le LW, Leighl NB. Shifting patterns in the interpretation of phase III clinical trial outcomes in advanced non‐small‐cell lung cancer: The bar is dropping. J Clin Oncol 2014;32:1407–1411. [DOI] [PubMed] [Google Scholar]
- 27. Cappuzzo F, Ciuleanu T, Stelmakh L et al. Erlotinib as maintenance treatment in advanced non‐small‐cell lung cancer: A multicentre, randomised, placebo‐controlled phase 3 study. Lancet Oncol 2010;11:521–529. [DOI] [PubMed] [Google Scholar]
- 28. Rosell R, Carcereny E, Gervais R et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012;13:239–246. [DOI] [PubMed] [Google Scholar]
- 29.Tarceva® (erlotinib) tablets, for oral use [package insert]. Northbrook, IL: OSI Pharmaceuticals, LLC, 2015. [Google Scholar]
- 30.Gilotrif® (afatinib) tablets, for oral use [package insert]. Ridgefield, CT; Boehringer Ingelheim Pharmaceuticals, 2016.
- 31. Wu YL, Zhou C, Hu CP et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐Lung 6): An open‐label, randomised phase 3 trial. Lancet Oncol 2014;15:213–222. [DOI] [PubMed] [Google Scholar]
- 32. Yang JCH, Wu YL, Schuler M et al. Afatinib versus cisplatin‐based chemotherapy for EGFR mutation‐positive lung adenocarcinoma (LUX‐Lung 3 and LUX‐Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol 2015;16:141–151. [DOI] [PubMed] [Google Scholar]
- 33. Soria JC, Felip E, Cobo M et al. Afatinib versus erlotinib as second‐line treatment of patients with advanced squamous cell carcinoma of the lung (LUX‐Lung 8): An open‐label randomised controlled phase 3 trial. Lancet Oncol 2015;16:897–907. [DOI] [PubMed] [Google Scholar]
- 34. Reck M, von Pawel J, Zatloukal P et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first‐line therapy for nonsquamous non‐small‐cell lung cancer: AVAiL. J Clin Oncol 2009;27:1227–1234. [DOI] [PubMed] [Google Scholar]
- 35. Reck M, von Pawel J, Zatloukal P et al. Overall survival with cisplatin‐gemcitabine and bevacizumab or placebo as first‐line therapy for nonsquamous non‐small‐cell lung cancer: Results from a randomised phase III trial (AVAiL). Ann Oncol 2010;21:1804–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stewart DJ, Kurzrock R. Fool's gold, lost treasures, and the randomized clinical trial. BMC Cancer 2013;13:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johnson JR, Williams G, Pazdur R. End points and United States Food and Drug Administration approval of oncology drugs. J Clin Oncol 2003;21:1404–1411. [DOI] [PubMed] [Google Scholar]
- 38. Kim DW, Ahn MJ, Shi Y et al. Results of a global phase II study with crizotinib in advanced ALK‐positive non‐small cell lung cancer (NSCLC). J Clin Oncol 2012;(suppl 30):7533a. [Google Scholar]
- 39. Malik SM, Maher VE, Bijwaard KE et al. U.S. Food and Drug Administration approval: Crizotinib for treatment of advanced or metastatic non‐small cell lung cancer that is anaplastic lymphoma kinase positive. Clin Cancer Res 2014;20:2029–2034. [DOI] [PubMed] [Google Scholar]
- 40. Shaw AT, Kim DW, Mehra R et al. Ceritinib in ALK‐rearranged non‐small‐cell lung cancer. N Engl J Med 2014;370:1189–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Awad MM, Shaw AT. ALK inhibitors in non‐small cell lung cancer: Crizotinib and beyond. Clin Adv Hematol Oncol 2014;12:429–439. [PMC free article] [PubMed] [Google Scholar]
- 42. ® Alecensa (alectinib) capsules, for oral use [package insert]. South San Francisco, CA: Genentech USA, Inc, 2015. [Google Scholar]
- 43. Ou SI, Ahn JS, De Petris L et al. Alectinib in crizotinib‐refractory ALK‐rearranged non‐small‐cell lung cancer: A phase II global study. J Clin Oncol 2016;34:661–668. [DOI] [PubMed] [Google Scholar]
- 44. Shaw AT, Gandhi L, Gadgeel S et al. Alectinib in ALK‐positive, crizotinib‐resistant, non‐small‐cell lung cancer: A single‐group, multicentre, phase 2 trial. Lancet Oncol 2016;17:234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Janne PA, Yang JC, Kim DW et al. AZD9291 in EGFR inhibitor‐resistant non‐small‐cell lung cancer. N Engl J Med 2015;372:1689–1699. [DOI] [PubMed] [Google Scholar]
- 46. Janne PA, Ahn M, Kim D et al. A phase I study of AZD9291 in patients with EGFR‐TKI‐resistant advanced NSCLC – Updated progression‐free survival and duration of response data. Ann Oncol 2015;26(suppl 1):LBA3a. [Google Scholar]
- 47. Ramalingam SS, Yang JC‐H, Lee CK et al. AZD9291, a mutant‐selective EGFR inhibitor, as first‐line treatment for EGFR mutation‐positive advanced non‐small cell lung cancer (NSCLC): Results from a phase 1 expansion cohort. J Clin Oncol 2015;(suppl 33):8000a. [Google Scholar]
- 48. Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wozniak AJ, Crowley JJ, Balcerzak SP et al. Randomized trial comparing cisplatin with cisplatin plus vinorelbine in the treatment of advanced non‐small‐cell lung cancer: A Southwest Oncology Group study. J Clin Oncol 1998;16:2459–2465. [DOI] [PubMed] [Google Scholar]
- 51. Le Chevalier T, Brisgand D, Douillard JY et al. Randomized study of vinorelbine and cisplatin versus vindesine and cisplatin versus vinorelbine alone in advanced non‐small‐cell lung cancer: Results of a European multicenter trial including 612 patients. J Clin Oncol 1994;12:360–367. [DOI] [PubMed] [Google Scholar]
- 52. Bonomi P, Kim K, Fairclough D et al. Comparison of survival and quality of life in advanced non‐small‐cell lung cancer patients treated with two dose levels of paclitaxel combined with cisplatin versus etoposide with cisplatin: Results of an Eastern Cooperative Oncology Group trial. J Clin Oncol 2000;18:623–631. [DOI] [PubMed] [Google Scholar]
- 53. Sandler AB, Nemunaitis J, Denham C et al. Phase III trial of gemcitabine plus cisplatin versus cisplatin alone in patients with locally advanced or metastatic non‐small‐cell lung cancer. J Clin Oncol 2000;18:122–130. [DOI] [PubMed] [Google Scholar]
- 54. Cardenal F, Lopez‐Cabrerizo MP, Anton A et al. Randomized phase III study of gemcitabine‐cisplatin versus etoposide‐cisplatin in the treatment of locally advanced or metastatic non‐small‐cell lung cancer. J Clin Oncol 1999;17:12–18. [DOI] [PubMed] [Google Scholar]
- 55. Fossella F, Pereira JR, von Pawel J et al. Randomized, multinational, phase III study of docetaxel plus platinum combinations versus vinorelbine plus cisplatin for advanced non‐small‐cell lung cancer: The TAX 326 study group. J Clin Oncol 2003;21:3016–3024. [DOI] [PubMed] [Google Scholar]
- 56. Cohen MH, Cortazar P, Justice R et al. Approval summary: Pemetrexed maintenance therapy of advanced/metastatic nonsquamous, non‐small cell lung cancer (NSCLC). The Oncologist 2010;15:1352–1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hotta K, Suzuki E, Di Maio M et al. Progression‐free survival and overall survival in phase III trials of molecular‐targeted agents in advanced non‐small‐cell lung cancer. Lung Cancer 2013;79:20–26. [DOI] [PubMed] [Google Scholar]
- 58. Mauguen A, Pignon JP, Burdett S et al. Surrogate endpoints for overall survival in chemotherapy and radiotherapy trials in operable and locally advanced lung cancer: A re‐analysis of meta‐analyses of individual patients' data. Lancet Oncol 2013;14:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Mandrekar SJ, Qi Y, Hillman SL et al. Endpoints in phase II trials for advanced non‐small cell lung cancer. J Thorac Oncol 2010;5:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Blumenthal GM, Karuri SW, Zhang H et al. Overall response rate, progression‐free survival, and overall survival with targeted and standard therapies in advanced non‐small‐cell lung cancer: US Food and Drug Administration trial‐level and patient‐level analyses. J Clin Oncol 2015;33:1008–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Hoos A, Eggermont AMM, Janetzki S et al. Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst 2010;102:1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wolchok JD, Hoos A, O'Day S et al. Guidelines for the evaluation of immune therapy activity in solid tumors: Immune‐related response criteria. Clin Cancer Res 2009;15:7412–7420. [DOI] [PubMed] [Google Scholar]
- 63. Reck M, Kaiser R, Mellemgaard A et al. Docetaxel plus nintedanib versus docetaxel plus placebo in patients with previously treated non‐small‐cell lung cancer (LUME‐Lung 1): A phase 3, double‐blind, randomised controlled trial. Lancet Oncol 2014;15:143–155. [DOI] [PubMed] [Google Scholar]
- 64.US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research et al. Guidance for industry. Clinical trial endpoints for the approval of cancer drugs and biologics, 2007. Available at https://www.fda.gov/downloads/Drugs/…/Guidances/ucm071590.pdf. Accessed March 10, 2017.