This study assesses the impact of salvage systemic chemotherapy on the surgical approach and outcome of high‐risk locally advanced rectal cancer patients who are inoperable or candidates for extensive surgical procedures after standard long‐course chemoradiotherapy.
Keywords: Locally advanced rectal cancer, Neoadjuvant chemoradiotherapy, Salvage systemic chemotherapy, Beyond total mesorectal excision surgery, Inoperable rectal cancer
Abstract
Background.
The potential of chemotherapy as salvage treatment after failure of neoadjuvant chemoradiotherapy for locally advanced rectal cancer (LARC) has never been explored. We conducted a single‐center, retrospective analysis to address this question.
Patients and Methods.
Patients with newly diagnosed LARC who were inoperable or candidates for extensive (i.e., beyond total mesorectal excision [TME]) surgery after long‐course chemoradiotherapy and who received salvage chemotherapy were included. The primary objective was to estimate the proportion of patients who became suitable for TME after chemotherapy.
Results.
Forty‐five patients were eligible (39 candidates for extensive surgery and 6 unresectable). Previous radiotherapy was given concurrently with chemotherapy in 43 cases (median dose: 54.0 Gy). Oxaliplatin‐ and irinotecan‐based salvage chemotherapy was administered in 40 (88.9%) and 5 (11.1%) cases, respectively. Eight patients (17.8%) became suitable for TME after chemotherapy, 10 (22.2%) ultimately underwent TME with clear margins, and 2 (4.4%) were managed with a watch and wait approach. Additionally, 13 patients had extensive surgery with curative intent. Three‐year progression‐free survival and 5‐year overall survival in the entire population were 30.0% (95% confidence interval [CI]: 15.0–46.0) and 44.0% (95% CI: 26.0–61.0), respectively. For the curatively resected and “watch and wait” patients, these figures were 52.0% (95% CI: 27.0–73.0) and 67.0% (95% CI: 40.0–84.0), respectively.
Conclusion.
Systemic chemotherapy may be an effective salvage strategy for LARC patients who fail to respond to chemoradiotherapy and are inoperable or candidates for beyond TME surgery. According to our study, one out of five patients may become resectable or be spared from an extensive surgery after systemic chemotherapy.
Implications for Practice.
High‐quality evidence to inform the optimal management of rectal cancer patients who are inoperable or candidates for beyond total mesorectal excision surgery following standard chemoradiotherapy is lacking. We show for the first time that systemic chemotherapy may be beneficial and result in one out of five poor prognosis patients becoming resectable or being spared from an extensive surgical approach. Although mores studies are needed to confirm these data, administering salvage systemic chemotherapy in this setting may have the potential to minimize morbidity associated with extensive surgical procedures and improve long‐term oncological outcome.
摘要
背景. 尚未探索过局部晚期直肠癌(LARC)患者在新辅助放化疗失败后使用化疗挽救治疗的可能性。我们通过一项单中心回顾性分析来阐明这一问题。
患者和方法. 本研究纳入了在长期放化疗后不能手术或符合扩大手术[即, 超出全直肠系膜切除术(TME)范围]标准且接受挽救化疗的新诊断LARC患者。主要目的是估计化疗后适合行TME的患者比例。
结果. 共有45例合格患者(39例符合扩大手术标准, 6例无法手术切除)。43例患者之前同步接受放疗和化疗(中位剂量:54.0 Gy)。分别有40例(88.9%)和5例(11.1%)患者接受基于奥沙利铂和伊立替康的挽救化疗。8例(17.8%)患者在化疗后符合TME标准;10例(22.2%)患者的肿瘤边界清晰, 最终行TME;2例(4.4%)患者采取观察等待的方法进行管理。此外, 13例患者出于根治性目的行扩大手术。总人群的3年无进展生存率和5年总生存率分别为30.0%[95%置信区间(CI):15.0‐46.0]和44.0%(95%CI:26.0‐61.0)。在行根治性切除术和”观察等待”的患者中, 3年无进展生存率和5年总生存率分别为52.0%(95%CI:27.0‐73.0]和67.0%(95%CI:40.0‐84.0)。
结论. 对于放化疗无效且不能手术或符合扩大手术(即, 超出TME范围)标准的LARC患者, 全身化疗可能是一种有效的挽救治疗策略。本研究表明, 20%的患者在全身化疗后可能进行手术切除或无需接受扩大手术。The Oncologist 2017;22:728–736
对临床实践的提示:对于标准放化疗后不能手术或符合扩大手术(即, 超出TME范围)标准的直肠癌患者而言, 尚无高质量的证据可供确定其最佳管理方法。本研究首次表明全身化疗具有潜在获益, 预后不良的患者在接受全身化疗后有20%可能进行手术切除或无需接受扩大手术。虽然需要开展更多研究来证实以上数据, 但在本研究条件下, 挽救性全身化疗有望将扩大手术的相关发病率降至最低并改善长期肿瘤学预后。
Introduction
Surgical resection according to the principles of total mesorectal excision (TME) is the mainstay of treatment for localized primary rectal cancer [1]. Routine adoption of TME and quality control of the resection specimens have led to a significant reduction of local recurrences and improvement of survival [1], [2]. Preoperative short‐course radiotherapy or long‐course chemoradiotherapy have further improved complete excision and local recurrence rates, but the impact of these treatments on long‐term outcome of patients with resectable tumors is controversial [3], [4].
Over the last decade, the term locally advanced rectal cancer (LARC) has been increasingly used, mostly as a result of the continuous efforts to implement risk‐adapted treatment strategies. While there has not been consensus on the exact definition of LARC, it is clear that this entity includes a spectrum of heterogeneous cancers at one end of which are tumors that require extensive surgical approaches (i.e., beyond the TME planes or exenterative‐type procedures) to achieve clear margins and unresectable tumors [5], [6]. Residual cancer within a distance of ≤1 mm from the circumferential resection margin (CRM) and involvement of adjacent organs (i.e., T4b) have been reported in approximately 1%–33% and 10% of rectal cancer patients, respectively [7], [8]. These high‐risk tumors can be reliably identified at baseline by high‐resolution magnetic resonance imaging (MRI) [9], and patients are routinely treated with long‐course chemoradiotherapy with the aim to not only reduce the risk of local recurrence but also downstage or downsize the tumor and allow a standard TME procedure with >1 mm clearance of tumor to the radial margins.
It has been reported, however, that among patients with tumor involvement of the mesorectal fascia at baseline, 20% and 8% still have a predicted (i.e., imaging‐based) CRM involvement and a positive pathological CRM after chemoradiotherapy, respectively [10]. Similarly, among patients who undergo chemoradiotherapy for tumors that are unresectable at diagnosis, 8% will remain inoperable and 28% will require an exenterative‐type resection [11]. Poor response to chemoradiotherapy in these patients may have important clinical implications. A positive CRM is unanimously considered as one of the most powerful prognostic factors in rectal cancer due to its association with increased risk of both local and distant recurrence, and poor survival especially after administration of preoperative radiotherapy [8]. Also, while exenterative‐type surgical procedures can still achieve clear margins and compensate for inadequate tumor downstaging or downsizing after neoadjuvant therapy, these are likely burdened with higher rates of postoperative morbidity and mortality, as well as deterioration of quality of life compared with standard TME [12], [13]. If, despite such extensive surgery, these patients succumb to distant metastatic disease (that now largely exceeds local recurrence as the main cause of death from rectal cancer), then the negative impact on quality of life may not be justified.
Current international guidelines suggest that radiotherapy dose escalation (i.e., additional 10–20 Gy beyond conventional dose), intraoperative radiotherapy, or brachytherapy could be considered for patients with close or positive margins, T4, or unresectable tumors after standard neoadjuvant therapy [14]. However, data to support these approaches are scarce and there is uncertainty regarding their efficacy [15], [16], [17], [18]. Notably, although mechanisms of radio‐ and chemotherapy‐resistance may differ and full dose systemic chemotherapy may provide a noncross resistant treatment to deliver after failure of chemoradiotherapy, the use of this strategy in this setting has never been investigated [19].
In this article, we report the results of a single institution, retrospective study that was designed to assess the impact of salvage systemic chemotherapy on the surgical approach and outcome of high‐risk LARC patients who are still inoperable or candidates for extensive surgical procedures despite the use of standard long‐course chemoradiotherapy.
Methods
All patients who were last seen in consultation at the Royal Marsden NHS Foundation Trust between April 2004 and July 2015 following a diagnosis of rectal cancer were reviewed and checked against the following study inclusion criteria: (a) histological confirmation of adenocarcinoma, (b) distal edge of the luminal tumor within 15 cm of the anal verge as assessed by baseline MRI, (c) newly diagnosed tumors (i.e., recurrent tumors excluded), (d) no evidence of distant metastases at diagnosis, (e) tumor deemed to be unresectable or requiring extensive surgery (i.e., beyond the TME planes) following completion of neoadjuvant long‐course (chemo) radiotherapy and restaging MRI as per treating surgeon or multidisciplinary team (MDT) assessment, (f) systemic chemotherapy administered as salvage treatment after long‐course chemoradiotherapy with the intent to enable an R0 resection within the TME planes, and (g) full medical records available for data extraction.
According to the common practice at our institution over the study period, eligible patients underwent an MRI of the pelvis and a computerized tomography (CT) scan of the thorax, abdomen, and pelvis at baseline for the purpose of tumor staging. The same scans were repeated after completion of neoadjuvant chemoradiotherapy and every 3 months during administration of salvage systemic chemotherapy. At each time point, these were prospectively reviewed at weekly institutional MDT meetings (involving gastrointestinal radiologists, medical oncologists, radiation oncologists, colorectal surgeons, and pathologists) where tumor resectability was assessed and a recommendation was made regarding the next management plan. In particular, MRI reassessment included evaluation of tumor regression grade (mrTRG), depth of extramural spread for tumor or fibrosis, and relationship in mm of tumor to the TME plane. For patients requiring exenterative surgery, the MRI assessment also included documentation of the compartments and/or organs involved by tumor [20].
Generally, radiotherapy was conformally computed tomography planned and delivered by a two‐phase technique (i.e., Phase I: 45 Gy in 25 fractions to the primary tumor and pelvic lymph nodes; Phase II = 5.4 in 3 fractions or 9 Gy in 5 fractions to the assessable tumor with a 2 cm margin in all directions). Following the MDT recommendation to consider salvage systemic treatment, the selection of the chemotherapy regimen was left to the discretion of the treating oncologist who decided based on a number of cinical parameters including age, performance status, and comorbidities. For patients who underwent curative surgical resection, follow‐up included outpatient visits every 3 months for the first year, every 6 months for years 2 and 3, and every year for years 4 and 5. A CT scan of the thorax, abdomen, and pelvis was done yearly for the first 3 years (MRI of the pelvis was performed as required). A carcinoembryonic antigen test was repeated at each visit. Follow‐up colonoscopies were performed within 12 months of surgery and, in the absence of significant findings, every 3 years thereafter.
Data on demographics; clinico‐pathological characteristics at baseline; neoadjuvant treatments; imaging at baseline, after chemoradiotherapy and after salvage systemic chemotherapy; pathology from resection specimens; adjuvant treatments; disease; and survival status at the time of the analysis were retrospectively collected for each patient using the institutional electronic patient record system and entered into a database. Also, predicted type of surgery required based on imaging performed after completion of chemoradiotherapy and after systemic chemotherapy and type of surgery actually performed were annotated by reviewing the MDT recommendations (or surgical consultations if final decision was made at a later stage by the treating surgeon) and the operation notes, respectively. Pelvic MRI scans were retrospectively reviewed by a specialized gastrointestinal radiologist for the purpose of assessing some imaging parameters whenever corresponding data could not be extrapolated from the original radiology report.
The primary objective of the study was to assess the proportion of patients who were deemed to be unresectable or candidates for extensive surgery after chemoradiotherapy and became suitable (based on preoperative imaging) for TME after salvage systemic chemotherapy. Secondary objectives included the proportion of patients who underwent TME surgery, rate of R0 resection, response to salvage systemic chemotherapy as assessed by imaging‐based parameters [including T downstaging, N downstaging, 30% reduction of intraluminal cranio‐caudal tumor length, change of extramural venous invasion (EMVI) status, change of CRM status, mrTRG], progression‐free survival (PFS) and overall survival (OS) in the overall study population and in the curatively resected (i.e., R0 or R1 resection) population, and pattern of treatment failure.
T and N downstaging were defined as reduction of at least one level in T and N staging, respectively, between baseline MRI and posttreatment MRI or histopathological staging. Tumor regression grade was defined as previously reported [21]. In brief, mrTRG 1 indicated radiological complete response (i.e., no evidence of residual tumor signal), mrTRG 2 good response (i.e., predominant fibrosis signal intensity with minimal residual tumor), mrTRG 3 moderate response (i.e., mixed areas of low signal fibrosis and intermediate signal intensity), mrTRG 4 minor response (i.e., persistent intermediate signal intensity with minimal low signal fibrosis), and mrTRG 5 no response (i.e., intermediate signal intensity, same appearances as original tumor). All survival outcomes were calculated from the start of salvage systemic chemotherapy. Progression‐free survival was defined as the time from the start of systemic chemotherapy to the date of progression (or unresectable disease based on either preoperative imaging or intraoperative findings for those patients who did not undergo curative surgery) or death from any cause. Overall survival was defined as time from start of systemic chemotherapy to date of death from any cause. Alive patients were censored at date of last follow‐up. Both PFS and OS were analysed using the Kaplan‐Meier method.
The study was approved by the Clinical Research and Development Department at the Royal Marsden NHS Foundation Trust. Due to the retrospective nature of the analysis, consent from patients included in the study was not required.
Results
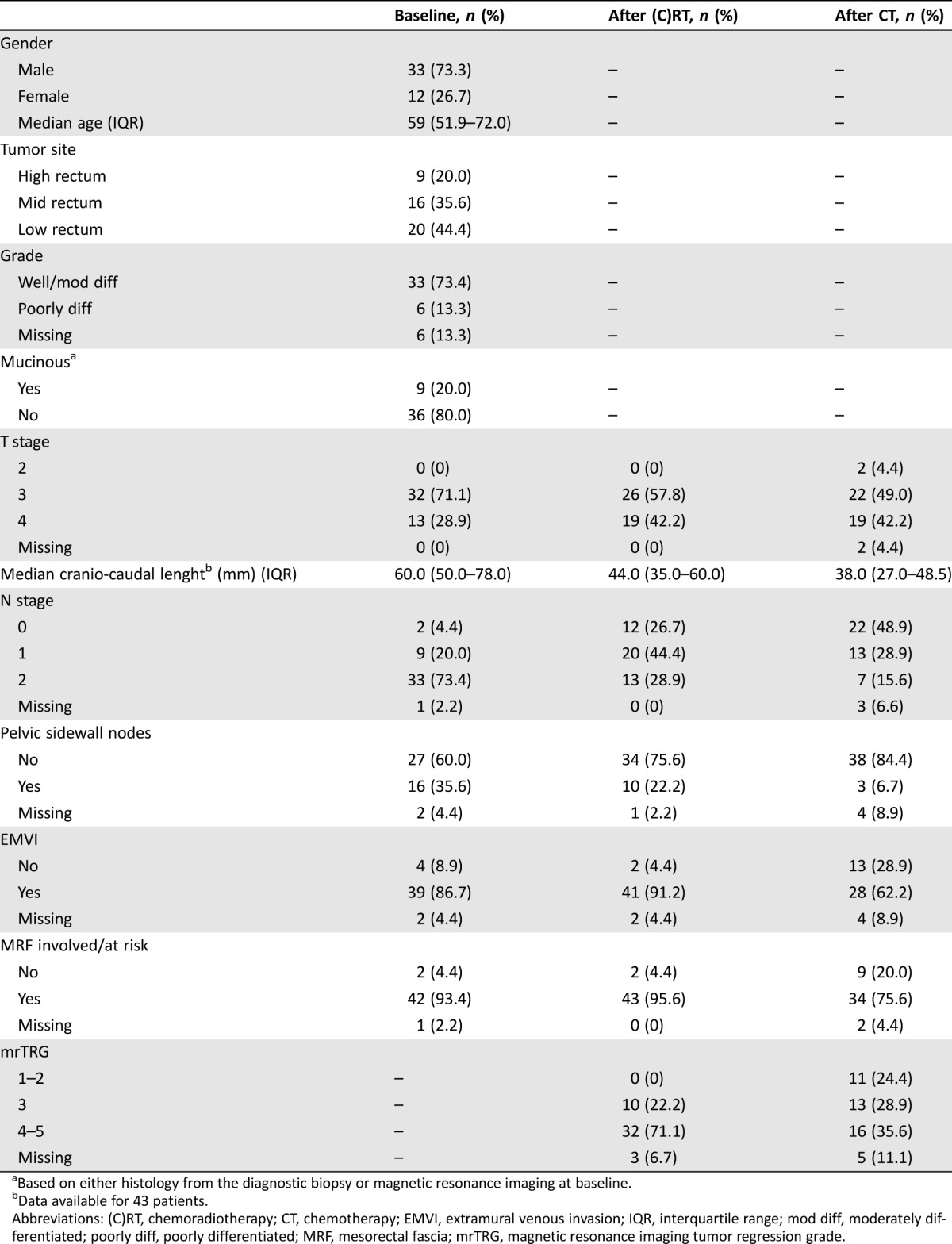
A total of 45 patients who were diagnosed between December 2001 and May 2015 met the study inclusion criteria. The majority of these (n = 38, 84.4%) were diagnosed after January 2010. Patient demographics and characteristics at baseline are shown in Table 1. There was a predominance of males (73.3%), and median age was 59 [interquartile range (IQR): 51.9–72.0]. All patients had ≥T3 tumors, and the vast majority of them had mid or low rectal cancers (80.0%), N2 disease (73.4%), EMVI (86.7%), and predicted CRM involvement (93.4%). Poorly differentiated and mucinous tumors (based on either histology from the diagnostic biopsy or staging MRI) were found in 13.3% and 20.0% of cases, respectively.
Table 1. Demographics and patient characteristics at baseline, after neoadjuvant chemoradiotherapy, and after salvage systemic chemotherapy.
Based on either histology from the diagnostic biopsy or magnetic resonance imaging at baseline.
Data available for 43 patients.
Abbreviations: (C)RT, chemoradiotherapy; CT, chemotherapy; EMVI, extramural venous invasion; IQR, interquartile range; mod diff, moderately differentiated; poorly diff, poorly differentiated; MRF, mesorectal fascia; mrTRG, magnetic resonance imaging tumor regression grade.
All patients received upfront fractionated pelvic radiotherapy. Median dose of radiotherapy was 54.0 Gy (IQR: 54.0–54.0; range: 34.0–55.8) and median duration of treatment was 42 days (IQR: 41.0–43.0; range: 13.0–57.0). In all cases, with the exception of two patients, radiotherapy was given concurrently with chemotherapy. This mostly consisted of single agent capecitabine (n = 41, 95.3%). One patient received a combination of fluorouracil and oxaliplatin, while in one other case capecitabine was replaced by raltitrexed due to preexisting patient cardiovascular comorbidities. Median time from the completion of radiotherapy to the restaging pelvic MRI scan was 31 days (IQR: 28.0–35.0; range: 21.0–80.0). Details of tumor characteristics after chemoradiotherapy and response to treatment are reported in Tables 1 and 2, respectively. After MDT discussion and/or surgical consultation, 39 patients (86.7%) were deemed to be candidates for beyond TME surgery, while 6 (13.3%) were considered inoperable (in three cases, tumor unresectability was confirmed during explorative surgery).
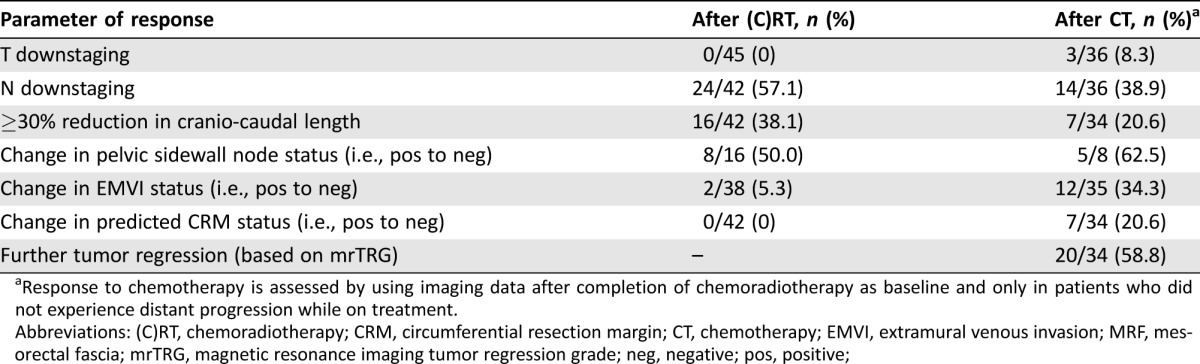
Table 2. Imaging‐based response to neoadjuvant chemoradiotherapy and salvage systemic chemotherapy.
Response to chemotherapy is assessed by using imaging data after completion of chemoradiotherapy as baseline and only in patients who did not experience distant progression while on treatment.
Abbreviations: (C)RT, chemoradiotherapy; CRM, circumferential resection margin; CT, chemotherapy; EMVI, extramural venous invasion; MRF, mesorectal fascia; mrTRG, magnetic resonance imaging tumor regression grade; neg, negative; pos, positive;
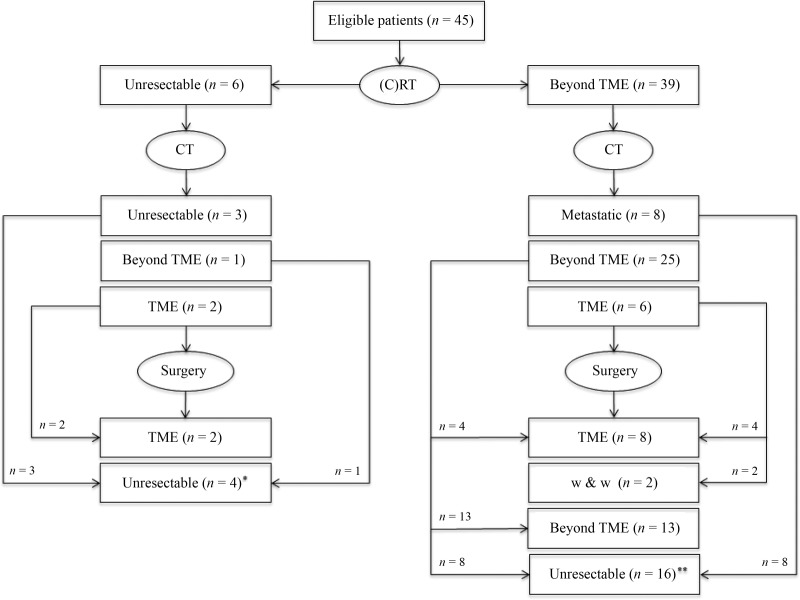
Systemic treatment after chemoradiotherapy is presented in Table 3. Doublet chemotherapy plus or minus bevacizumab was given to 44 patients (97.8%). In most cases, patients received an oxaliplatin‐based regimen (n = 40, 88.9%) while an irinotecan‐based regimen was used in five cases (11.1%, including one patients who started with single agent capecitabine and was subsequently switched to FOLFIRI). Treatment was administered for a median of 3.3 months (IQR: 2.4–5.3; range: 1.1–8.2) and in 16 patients (35.6%) this was continued beyond the first radiological assessment. Median time from treatment start to the first restaging pelvic MRI and preoperative pelvic MRI was 2.6 months (IQR: 2.4–3.1; range: 1.6–7.0) and 3.3 months (IQR: 2.5–5.4; range: 1.6–10.3), respectively. Details of tumor characteristics after salvage chemotherapy and incremental response to treatment (as compared to the postradiotherapy findings) are reported in Table 1 and 2. Eight patients (17.8%) were diagnosed with distant metastases during or after completion of chemotherapy. Among the remaining 37 patients, the MDT and/or treating surgeon considered 29 (64.4%) as still either inoperable (including one patient who became unresectable due to local progression while on chemotherapy) or candidates for beyond TME surgery, while 8 (17.8%) were deemed suitable for a TME approach. The latter group included two patients with unresectable tumors at baseline (as confirmed during explorative surgery) and two other patients who continued with the same chemotherapy despite the first MRI assessment after 3 months of treatment suggested that an extensive surgery would still be required (Fig. 1). Median time from the start of systemic chemotherapy to the first MRI showing that a TME was technically feasible was 5.3 months (IQR: 3.5–7.6; range: 3.0–8.5).
Table 3. Chemotherapy regimens used after chemoradiotherapy in the study population.
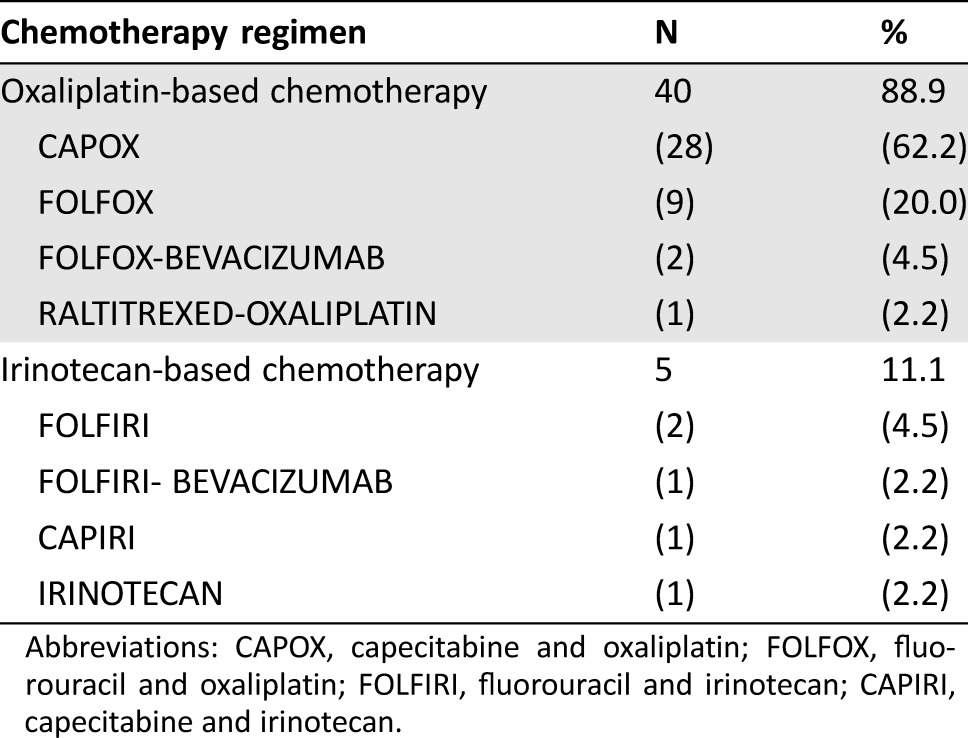
Abbreviations: CAPOX, capecitabine and oxaliplatin; FOLFOX, fluorouracil and oxaliplatin; FOLFIRI, fluorouracil and irinotecan; CAPIRI, capecitabine and irinotecan.
Figure 1.
Study flow diagram. Reasons why surgery was not performed included: *, Unresectable tumor (n = 3) and extensive surgery declined by patient (n = 1). **, Distant metastases (n = 8), risk/benefit ratio of an exenterative procedure felt unacceptable by patient/physician (n = 6), unresectable tumor (n = 1; tumor became unresectable due to local progression while awaiting patient decision regarding exenterative surgery), loss to follow‐up (n = 1).
Abbreviations: (C)RT, chemoradiotherapy; CT, chemotherapy; TME, beyond total mesorectal excision; w & w, watch and wait.
A total of 23 patients (51.1%) underwent surgery with a curative intent (one additional patient had an R2 palliative surgery that was required due to severe anal pain and MRI evidence of rectal perforation after chemotherapy). An extensive resection was undertaken in 13 cases, while 10 patients were ultimately amenable to TME surgery (i.e., 5 anterior resections and 5 abdominoperineal resections), including 4 who were deemed to be candidates for a beyond TME surgery according to the MDT and/or treating surgeon (Fig. 1). In these 4 cases, the interval between MRI after salvage chemotherapy and surgery was 30, 45, 87, and 91 days, respectively. An R0 resection (i.e., pathological CRM clear) was achieved in 21 cases, including all patients who underwent TME. A pathological complete response was observed in three patients (one in the extensive surgery group and two in the TME group) (Table 4). Reasons why surgery was not performed included the following: distant metastases (n = 8), unresectable primary tumor (n = 4; in one case, the tumor became unresectable due to local progression while awaiting patient decision regarding exenterative surgery), risk/benefit ratio of an exenterative procedure felt unacceptable by patient and/or physician (n = 6), and loss to follow‐up (n = 1). Two additional patients were proposed a “watch and wait” approach following radiological evidence of complete or almost complete tumor response. Postoperative adjuvant chemotherapy was administered in seven patients (including one patient who also had cyberknife treatment due to a positive margin).
Table 4. Pathology findings from the resection specimens of patients who underwent surgery with a curative intent (n = 23).
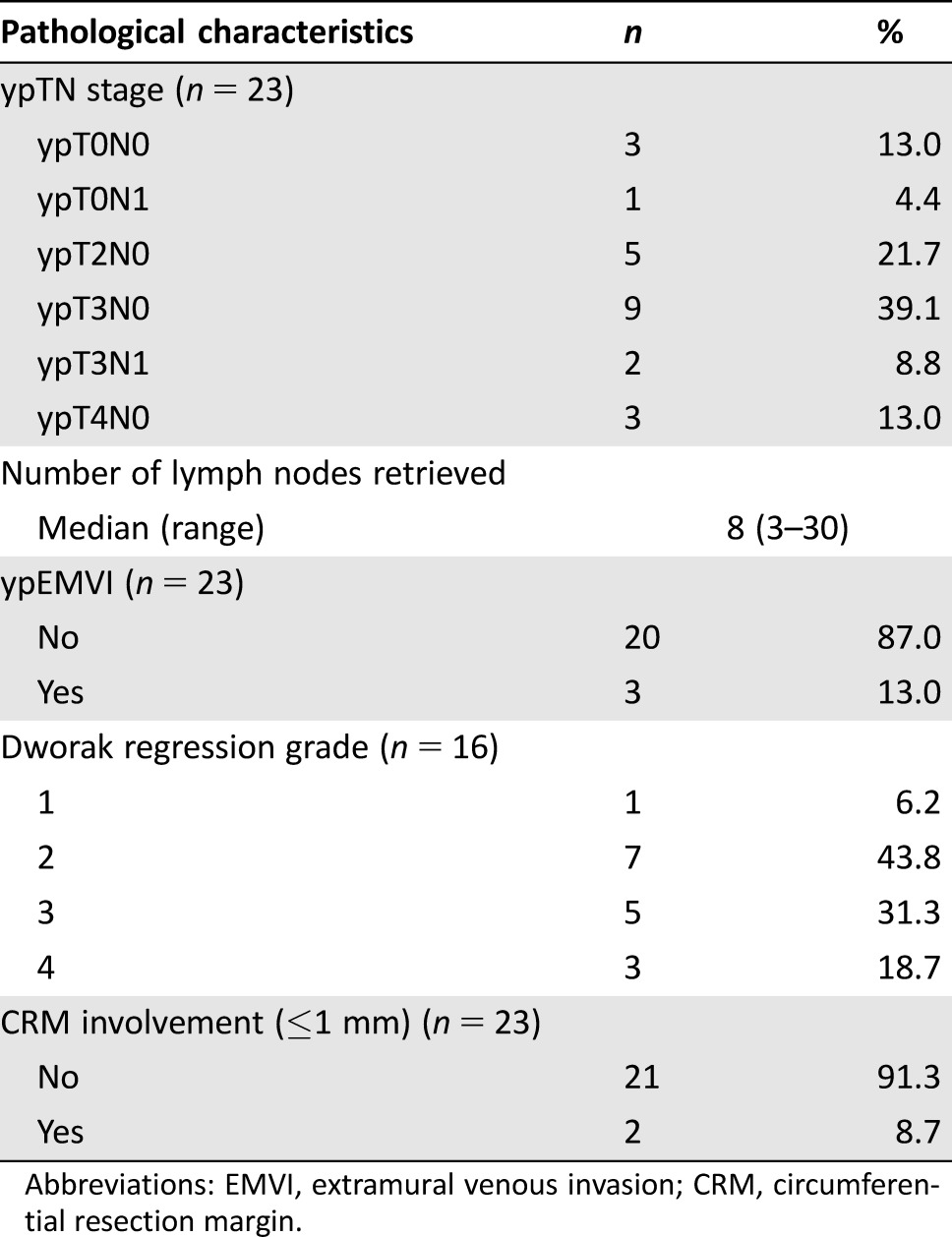
Abbreviations: EMVI, extramural venous invasion; CRM, circumferential resection margin.
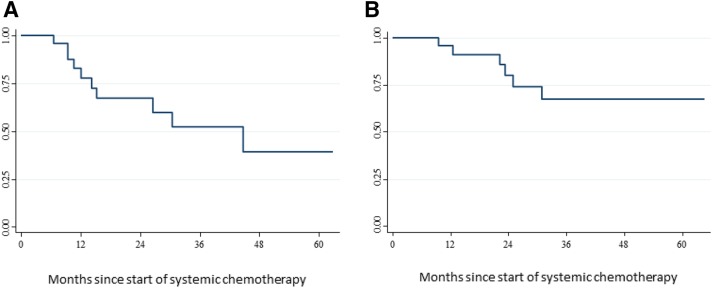
After a median follow‐up of 38.7 months (IQR: 24.7–43.1), in the overall study population, 3‐year PFS was 30.0% (95% CI: 15.0–46.0) and 5‐year OS was 44.0% (95% CI: 26.0–61.0). In the macroscopically radically resected and “watch and wait” population (n = 25), these figures were 52.0% (95% CI: 27.0–73.0) and 67.0% (95% CI: 40.0–84.0), respectively (median follow‐up for this patient population was 44.7 months (IQR: 14.2–not reached) (Fig. 2). At the time of this analysis, among patients who had curative resection, tumor recurrence was diagnosed in 10 cases (local recurrence alone in two, local and distant recurrence in one, and distant recurrence alone in seven). The two patients who were managed with “watch and wait” were alive and free of disease after 19.7 and 24.6 months from the start of salvage chemotherapy.
Figure 2.
Kaplan‐Meier curves for progression‐free survival (A) and overall survival (B) in patients who underwent radical resection (i.e., R0 or R1) or were managed with a “watch and wait” approach (n = 25).
Discussion
In this study, we have shown that administering systemic chemotherapy after poor response to neoadjuvant chemoradiotherapy may be an effective salvage strategy to allow a TME with clear surgical margins in some LARC patients who would otherwise be inoperable or candidates for an extensive surgery.
Over the last few years, an interest in the use of systemic chemotherapy in the preoperative setting has increasingly emerged. A number of strategies have been investigated, including induction chemotherapy before chemoradiotherapy [22], systemic chemotherapy (without radiotherapy) followed by surgery [23], and consolidation chemotherapy after chemoradiotherapy [24], all with encouraging results. Nevertheless, to our knowledge, the potential of chemotherapy as a salvage treatment for patients who achieve suboptimal response to chemoradiotherapy has never been explored.
Our series included a selected group of poor‐prognosis LARC patients as shown by the high proportion of tumors with prognostically unfavorable imaging characteristics at baseline (i.e., advanced TN stage, presence of EMVI, and predicted CRM involvement) and the lack of substantial downstaging or regression after long‐course chemoradiotherapy. More importantly, despite the use of standard neoadjuvant therapy, all patients were candidates for either an aggressive surgical approach (i.e., beyond the conventional TME planes) or a palliative treatment due to the local extent of their tumors. Further confirmation of the poor prognosis of our study population is provided by the modest long‐term survival outcomes, including a 3‐year PFS of 30.0% and a 5‐year OS of 42.8%, which lag far behind the corresponding historical figures for unselected LARC patients who are treated with neoadjuvant chemoradiotherapy [25].
The findings of our study suggest that one out of five such high‐risk LARC patients can have their tumor resected or be spared from the consequences of an extensive surgery following the use of sequential systemic chemotherapy. Notably, all patients who underwent TME had negative surgical margin (i.e., R0 resection), and in two cases a pathological complete response was also observed. Moreover, two additional patients had the opportunity to avoid surgery and undergo a “watch and wait” approach due to the radiologic evidence of a clinical complete response at the end of chemotherapy. Although the absence of an appropriate control group and the unavailability of patient reported outcome data do not allow us to draw any definitive conclusion, it is likely that the change of surgical approach resulting from the administration of salvage systemic chemotherapy might have minimized the risk of tumor‐ and treatment‐related morbidities and translated into better quality of life and improved survival.
Delay of surgery resulting in an increased risk of tumor progression is one of the main concerns around the administration of chemotherapy in patients who are still amenable to an extensive resection after completion of standard chemoradiotherapy. In our study, however, local tumor progression precluding curative resection after salvage chemotherapy occurred only in 1 out of 39 potentially resectable patients (2.6%). Although a further 18% were diagnosed with distant metastases while on or soon after completion of chemotherapy, it is unlikely that these patients might have missed the chance of a potentially curative surgical resection. These patients are known to be at high risk of distant failure, and lack of response to chemoradiotherapy is a further high‐risk feature. Stretching the interval from chemoradiotherapy to surgery by administering sequential chemotherapy could actually provide an opportunity window to identify poor prognosis patients with rapidly progressing tumors and restrict exenterative‐type surgical resections to those who are most likely to benefit.
While the overall impact of systemic chemotherapy in this setting may appear promising (i.e., change of treatment approach in 26.7% of cases), it is possible that there is scope for yet further improvement. Virtually all study patients were treated with oxaliplatin‐ or irinotecan‐based doublet chemotherapy, whereas only a minority (6.7%) also received bevacizumab. It is legitimate to hypothesize that more aggressive regimens including doublet chemotherapy plus either anti‐angiogenic agents or anti‐epidermal‐growth‐factor‐receptor (EGFR) monoclonal antibodies and triplet chemotherapy (plus or minus biologics) could lead to higher tumor regression rates and increase the proportion of patients who become candidates for a TME surgery despite poor response to chemoradiotherapy [26], [27], [28], [29], [30]. We have previously demonstrated that adding cetuximab to neoadjuvant doublet chemotherapy (i.e., before chemoradiotherapy) significantly improves the objective response rate in locally advanced rectal patients with RAS wild‐type tumors [31], [32]. Furthermore, overexpression of EGFR has been reported to be a predictive factor of resistance to radiotherapy in rectal cancer, and using anti‐EGFR agents after failure of chemoradiotherapy may represent a rational strategy to target biologically aggressive tumor clones [33], [34], [35], [36], [37].
While some patients had a significant benefit in terms of surgical approach from the use of salvage chemotherapy, the majority of them (73.3%) were still deemed as inoperable or candidates for an extensive surgical procedure. One could argue that these patients may have received an unnecessary treatment, which, in addition to the abovementioned risk of tumor progression, could also be associated with increased toxicities and possibly detriment of quality of life. The design of our study does not allow us to estimate the benefit (if any) of salvage chemotherapy in this group of patients. However, we have shown that administering systemic chemotherapy after chemoradiotherapy led to some incremental tumor response (as indicated by a number of imaging‐based parameters), which, regardless of the type of surgical resection performed (i.e., TME or beyond TME), may have ultimately translated into improved outcome. Also, it should be noted that, in view of the high‐risk features of their tumors, these patients would be very likely to be proposed the same treatment after surgery, a setting where the efficacy of chemotherapy is yet to be demonstrated and the risk of toxicity and low compliance appears significantly higher compared with preoperative chemotherapy [38], [39].
Our analysis has a number of limitations in addition to the small sample size and the retrospective design. The definition of tumor resectability in this study was based on MRI imaging that has been previously reported to be as effective as pathology at predicting the likelihood of local recurrence, disease‐free survival, and overall survival [40]. Furthermore, mrTRG has also been validated as a method of predicting response to treatment [21], [41]. However, especially when nonhigh‐resolution techniques are employed and Magnetic Resonance Imaging and Rectal Cancer European Equivalence (MERCURY)‐defined criteria are not used, MRI is less specific in the assessment of parameters such as involvement of the mesorectal fascia after administration of neoadjuvant chemoradiotherapy [42], [43]. Nevertheless, it is unlikely that this may have led to an excess of patients who were considered to be inoperable or candidates for extensive surgical approaches after standard chemoradiotherapy and became suitable for TME after chemotherapy. In our study, 4 out of 26 patients (15%) who were deemed to be candidate for a beyond‐TME surgical approach after salvage chemotherapy ultimately underwent TME. While imaging cannot confidently rule out residual microscopic foci of cancer within the dense fibrotic tissue threatening or involving the mesorectal fascia, the discrepancy between the type of surgery that was recommended after completion of salvage chemotherapy and that which was actually carried out is more likely to reflect the willingness of some surgeons to cut through fibrotic tissue to allow a sphincter‐preserving surgery rather than an overall poor accuracy of MRI as such. This also reflects the learning curve of our MDT in relation to the management of patients with locally advanced tumors who are likely candidates for a beyond‐TME surgical approach. Moreover, the relatively long interval between MRI assessment after salvage chemotherapy and surgery may have accounted for at least some of the observed discrepancies. The contention that the results of our study are not significantly biased by the decision to rely on MRI for the assessment of tumor resectability and definition of surgical plan is supported by the fact that 2 out of 10 patients who underwent TME following chemotherapy were truly inoperable as reported by the treating surgeon during exploratory surgery after completion of chemoradiotherapy.
Another potential limitation is the median time interval from completion of chemoradiotherapy to tumor assessment, which in our study was lower (4.4 weeks) compared with what is now considered as the optimal standard by international guidelines and consensus statements on LARC beyond TME planes (6–8 weeks) [6], [14], [44]. Therefore, it cannot be excluded that some of the downstaging or downsizing effects that have been attributed to salvage systemic chemotherapy may actually be secondary to delayed radiotherapy‐induced tumor regression [45], [46]. However, it is worth noting that in two cases, tumor resectability according to the TME principles was achieved only after approximately 6 months of systemic chemotherapy, this being continued beyond the first MRI after 3 months of treatment showed that an extensive surgery was still required. This mitigates against a significant impact of possible confounding factors on the study results and further support a true “rescue effect” of salvage chemotherapy in this setting. Especially in this group of locally advanced tumors with no or minimal signs of tumor regression soon after completion of chemoradiotherapy, it is very unlikely that a substantial, chemoradiotherapy‐induced, delayed tumor regression may have occurred and led to a change in surgical approach. Indeed, studies suggest that the highest benefit from neoadjuvant chemoradiotherapy (in terms of either tumor downstaging, pathological complete response, or radical resection) is observed from 8 to 11 weeks after completion of treatment [46], [47]. Delaying surgery beyond this timeframe may actually increase the risk of positive resection margins, possibly as a result of tumor regrowth [47].
Main strengths of our analysis are the adoption of a well‐defined, largely homogeneous management pathway for LARC patients for the duration of the study, and the collection of prospectively annotated recommendations from institutional MDT meetings that were regularly attended by a highly experienced team of clinicians, including specialized gastrointestinal radiologists and colorectal surgeons. Also, although the overall study period spanned over approximately 15 years, the vast majority of patients were treated within the last 5 years, thus reinforcing the contention that our findings are generalizable to the current clinical practice. This strategy is being tested in a prospective multicenter trial (TRIGGER) in which patients are randomized to an experimental arm of sequential systemic chemotherapy or deferral of surgery based on mrTRG after chemoradiotherapy (NCT02704520) [48].
Conclusion
Our retrospective analysis suggests that systemic chemotherapy could be a useful salvage strategy for high‐risk LARC patients who are still inoperable or require extensive surgical procedures after standard chemoradiotherapy. While administering chemotherapy in this setting may already be a relatively common practice in some centers, this has been largely empirical and not supported by any evidence. Herein we provide for the first time a valuable source of information on the potential of salvage chemotherapy that can be used in the decision‐making process whenever the prospect of an exenterative‐type resection or palliative treatment is envisaged after failure of standard neoadjuvant therapy. Prospective studies are certainly required to confirm our data and possibly assess the role of systemic chemotherapy in this setting against alternative therapeutic options.
Acknowledgments
We acknowledge the NIHR Biomedical Research Centre at The Royal Marsden NHS Foundation Trust and The Institute of Cancer Research for the support to this study.
Author Contributions
Conception/design: Francesco Sclafani, Gina Brown, David Cunningham, Ian Chau
Provision of study material or patients: Francesco Sclafani, Gina Brown, David Cunningham, Sheela Rao, Paris Tekkis, Diana Tait, Shahnawaz Rasheed, David Watkins, Naureen Starling, Andrew Wotherspoon, Ian Chau
Collection and/or assembly of data: Francesco Sclafani, Federica Morano, Chiara Baratelli
Data analysis and interpretation: Francesco Sclafani, Gina Brown, David Cunningham, Ian Chau
Manuscript writing: Francesco Sclafani, Gina Brown, David Cunningham, Shahnawaz Rasheed, Ian Chau
Final approval of manuscript: Francesco Sclafani, Gina Brown, David Cunningham, Sheela Rao, Paris Tekkis, Diana Tait, Federica Morano, Chiara Baratelli, Shahnawaz Rasheed, David Watkins, Naureen Starling, Andrew Wotherspoon, Ian Chau
Disclosures
David Cunningham: Amgen, AstraZeneca, Bayer, Celgene, Merrimack, Medimmune, Merck Serono, Sanofi (RF); Gina Brown: EISAI (C/A), NIHR BRC (RF); Sheela Rao: Roche, Merck, Amgen (C/A); Ian Chau: Eli‐Lilly, Bristol‐Meyers Squibb, Merck Sharp & Dohme Limited, Bayer, Roche, Five Prime Therapeutics (C/A), Eli Lilly, Pfizer (H); Janssen‐Cilag, Sanofi Oncology, Merck‐Serono (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1:1479–1482. [DOI] [PubMed] [Google Scholar]
- 2. Quirke P, Steele R, Monson J et al. Effect of the plane of surgery achieved on local recurrence in patients operated with operable rectal cancer: A prospective study using data from the MRC CR07 and NCIC‐CTGCO16 randomised clinical trial. Lancet 2009;373:821–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Van Gijn W, Marijnen CA, Nagtegaal ID et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12‐year follow‐up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011;12:575–582. [DOI] [PubMed] [Google Scholar]
- 4. Sauer R, Liersch T, Merkel S et al. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: Results of the German CAO/ARO/AIO‐94 randomized phase III trial after a median follow‐up of 11 years. J Clin Oncol 2012;30:1926–1933. [DOI] [PubMed] [Google Scholar]
- 5. Blomqvist L, Glimelius B. The ‘good', the ‘bad', and the ‘ugly' rectal cancers. Acta Oncol 2008;47:5–8. [DOI] [PubMed] [Google Scholar]
- 6. Beyond TME Collaborative. Consensus statement on the multidisciplinary management of patients with recurrent and primary rectal cancer beyond total mesorectal excision planes. Br J Surg 2013;100:1009–1014. [DOI] [PubMed] [Google Scholar]
- 7. Gunderson LL, Jessup JM, Sargent DJ et al. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol 2010;28:264–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nagtegaal ID, Quirke P. What is the role for the circumferential margin in the modern treatment of rectal cancer? J Clin Oncol 2008;26:303–312. [DOI] [PubMed] [Google Scholar]
- 9. Al‐Sukhni E, Milot L, Fruitman M et al. Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: A systematic review and meta‐analysis. Ann Surg Oncol 2012;19:2212–2223. [DOI] [PubMed] [Google Scholar]
- 10. Simpson GS, Eardley N, McNicol F. Circumferential resection margin (CRM) positivity after MRI assessment and adjuvant treatment in 189 patients undergoing rectal cancer resection. Int J Colorectal Dis 2014;29:585–590. [DOI] [PubMed] [Google Scholar]
- 11. Braendengen M, Tveit KM, Berglund A et al. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol 2008;26:3687–3694. [DOI] [PubMed] [Google Scholar]
- 12. Yang TX, Morris DL, Chua TC. Pelvic exenteration for rectal cancer: a systematic review. Dis Colon Rectum 2013;56:519–531. [DOI] [PubMed] [Google Scholar]
- 13. Palmer G, Martling A, Lagergren P. Quality of life after potentially curative treatment for locally advanced rectal cancer. Ann Surg Oncol 2008;15:3109–3117. [DOI] [PubMed] [Google Scholar]
- 14.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) ‐ Rectal Cancer, Version 2.2016. Available at https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf. Accessed October 10, 2016.
- 15. Dubois JB, Bussieres E, Richaud P et al. Intra‐operative radiotherapy of rectal cancer: Results of the French multi‐institutional randomized study. Radiother Oncol 2011;98:298–303. [DOI] [PubMed] [Google Scholar]
- 16. Wiig JN, Giercksky KE, Tveit KM. Intraoperative radiotherapy for locally advanced or locally recurrent rectal cancer: Does it work at all? Acta Oncol 2014;53:865–876. [DOI] [PubMed] [Google Scholar]
- 17. Westermark LW, Jacobsen A, Qvortrup C et al. Long‐term results of a phase II trial of high‐dose radiotherapy (60 Gy) and UFT/l‐leucovorin in patients with non‐resectable locally advanced rectal cancer (LARC). Acta Oncol 2008;47:428–433. [DOI] [PubMed] [Google Scholar]
- 18. Sun Myint A, Lee CD, Snee AJ. High dose rate brachytherapy as a boost after preoperative chemoradiotherapy for more advanced rectal tumors: The Clatterbridge experience. Clin Oncol (R Coll Radiol) 2007;19:711–719. [DOI] [PubMed] [Google Scholar]
- 19. Ajani JA, Izzo JG, Lee JS. Chemotherapy and radiotherapy resistance: Complexity, reality, and promise. J Clin Oncol 2009;27:162–163. [DOI] [PubMed] [Google Scholar]
- 20. Georgiou PA, Tekkis PP, Constantinides VA et al. Diagnostic accuracy and value of magnetic resonance imaging (MRI) in planning exenterative pelvic surgery for advanced colorectal cancer. Eur J Cancer 2013;49:72–81. [DOI] [PubMed] [Google Scholar]
- 21. Patel UB, Taylor F, Blomqvist L et al. Magnetic resonance imaging‐detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol 2011;29:3753–3760. [DOI] [PubMed] [Google Scholar]
- 22. Chua YJ, Barbachano Y, Cunningham D et al. Neoadjuvant capecitabine and oxaliplatin before chemoradiotherapy and total mesorectal excision in MRI‐defined poor‐risk rectal cancer: A phase 2 trial. Lancet Oncol 2010;11:241–248. [DOI] [PubMed] [Google Scholar]
- 23. Schrag D, Weiser MR, Goodman KA et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: A pilot trial. J Clin Oncol 2014;32:513–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Garcia‐Aguilar J, Chow OS, Smith DD et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: A multicentre, phase 2 trial. Lancet Oncol 2015;16:957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hofheinz RD, Wenz F, Post S et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: A randomised, multicentre, non‐inferiority, phase 3 trial. Lancet Oncol 2012;13:579–588. [DOI] [PubMed] [Google Scholar]
- 26. Douillard JY, Oliner KS, Siena S et al. Panitumumab–FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med 2013;369:1023–1034. [DOI] [PubMed] [Google Scholar]
- 27. Van Cutsem E, Lenz HJ, Köhne CH et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol 2015;33:692–700. [DOI] [PubMed] [Google Scholar]
- 28. Yamazaki K, Nagase M, Tamagawa H et al. Randomized phase III study of bevacizumab plus FOLFIRI and bevacizumab plus mFOLFOX6 as first‐line treatment for patients with metastatic colorectal cancer (WJOG4407G). Ann Oncol 2016;27:1539–1546. [DOI] [PubMed] [Google Scholar]
- 29. Cremolini C, Loupakis F, Antoniotti C et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first‐line treatment of patients with metastatic colorectal cancer: Updated overall survival and molecular subgroup analyses of the open‐label, phase 3 TRIBE study. Lancet Oncol 2015;16:1306–1315. [DOI] [PubMed] [Google Scholar]
- 30. Fornaro L, Lonardi S, Masi G et al. FOLFOXIRI in combination with panitumumab as first‐line treatment in quadruple wild‐type (KRAS, NRAS, HRAS, BRAF) metastatic colorectal cancer patients: A phase II trial by the Gruppo Oncologico Nord Ovest (GONO). Ann Oncol 2013;24:2062–2067. [DOI] [PubMed] [Google Scholar]
- 31. Dewdney A, Cunningham D, Tabernero J et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high‐risk rectal cancer (EXPERT‐C). J Clin Oncol 2012;30:1620–1627. [DOI] [PubMed] [Google Scholar]
- 32. Sclafani F, Gonzalez D, Cunningham D et al. RAS mutations and cetuximab in locally advanced rectal cancer: Results of the EXPERT‐C trial. Eur J Cancer 2014;50:1430–1436. [DOI] [PubMed] [Google Scholar]
- 33. Azria D, Bibeau F, Barbier N et al. Prognostic impact of epidermal growth factor receptor (EGFR) expression on loco‐regional recurrence after preoperative radiotherapy in rectal cancer. BMC Cancer 2005;5:62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Giralt J, Eraso A, Armengol M et al. Epidermal growth factor receptor is a predictor of tumor response in locally advanced rectal cancer patients treated with preoperative radiotherapy. Int J Radiat Oncol Biol Phys 2002;54:1460–1465. [DOI] [PubMed] [Google Scholar]
- 35. Giralt J, de las Heras M, Cerezo L et al. The expression of epidermal growth factor receptor results in a worse prognosis for patients with rectal cancer treated with preoperative radiotherapy: A multicenter, retrospective analysis. Radiother Oncol 2005;74:101–108. [DOI] [PubMed] [Google Scholar]
- 36. Li S, Kim J‐S, Kim J‐M et al. Epidermal growth factor receptor as a prognostic factor in locally advanced rectal‐cancer patients treated with preoperative chemoradiation. Int J Radiat Oncol Biol Phys 2006;65:705–712. [DOI] [PubMed] [Google Scholar]
- 37. Milas L, Fang FM, Mason KA et al. Importance of maintenance therapy in C225‐induced enhancement of tumor control by fractionated radiation. Int J Radiat Oncol Biol Phys 2007;67:568–572. [DOI] [PubMed] [Google Scholar]
- 38. Breugom AJ, Swets M, Bosset JF et al. Adjuvant chemotherapy after preoperative chemoradiotherapy and surgery for patients with rectal cancer: A systematic review and meta analysis of individual patient data. Lancet Oncol 2015;16:200–207. [DOI] [PubMed] [Google Scholar]
- 39. Fernández‐Martos C, Pericay C, Aparicio J et al. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging‐defined, locally advanced rectal cancer: Grupo Cáncer de Recto 3 Study. J Clin Oncol 2010;28:859–865. [DOI] [PubMed] [Google Scholar]
- 40. Taylor FG, Quirke P, Heald RJ et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease‐free survival and local recurrence: 5‐year follow‐up results of the MERCURY study. J Clin Oncol 2014;32:34–43. [DOI] [PubMed] [Google Scholar]
- 41. Sclafani F, Brown G, Cunningham D et al. PAN‐EX: A pooled analysis of two trials of neoadjuvant chemotherapy followed by chemoradiotherapy in MRI‐defined, locally advanced rectal cancer. Ann Oncol 2016;27:1557–1565. [DOI] [PubMed] [Google Scholar]
- 42. Villegen RF, Beets GL, Lammering G et al. Mesorectal fascia invasion after neoadjuvant chemotherapy and radiation therapy for locally advanced rectal cancer: Accuracy of MR imaging for prediction. Radiology 2008;246:454–462. [DOI] [PubMed] [Google Scholar]
- 43. Oberholzer K, Junginger T, Heintz A et al. Rectal cancer: MR imaging of the mesorectal fascia and effect of chemoradiation on assessment of tumor involvement. J Magn Reson Imaging 2012;36:658–663. [DOI] [PubMed] [Google Scholar]
- 44. Glimelius B, Tiret E, Cervantes A et al. ESMO Guidelines Working Group . Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2013;24:(Suppl 6: vi81–vi88). [DOI] [PubMed] [Google Scholar]
- 45. Dhadda AS, Zaitoun AM, Bessell EM. Regression of rectal cancer with radiotherapy with or without concurrent capecitabine ‐ Optimising the timing of surgical resection. Clin Oncol (R Coll Radiol) 2009;21:23–31. [DOI] [PubMed] [Google Scholar]
- 46. Sloothaak DA, Geijsen DE, van Leersum NJ et al. Optimal time interval between neoadjuvant chemoradiotherapy and surgery for rectal cancer. Br J Surg 2013;100:933–939. [DOI] [PubMed] [Google Scholar]
- 47. Sun Z, Adam MA, Kim J et al. Optimal timing to surgery after neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Am J Coll Surg 2016;222:367–374. [DOI] [PubMed] [Google Scholar]
- 48.Magnetic Resonance Tumour Regression Grade as Biomarker for Stratified Management of Rectal Cancer Patients (TRIGGER). Available at https://clinicaltrials.gov/ct2/show/NCT02704520. Accessed April 2, 2017.