Therapy options for renal cell carcinoma are developing rapidly. This systematic review focuses on the current guidelines for treatment of renal cell carcinoma.
Keywords: Guidelines, Recommendations, Renal cell carcinoma, Surgery, Therapy
Abstract
The landscape of local and systemic therapy of renal cell carcinoma (RCC) is rapidly changing. The increase in the incidental finding of small renal tumors has increased the application of nephron‐sparing procedures, while ten novel agents targeting the vascular endothelial growth factor (VEGF) or the mammalian target of rapamycin pathways, or inhibiting the interaction of the programmed death 1 receptor with its ligand, have been approved since 2006 and have dramatically improved the prognosis of metastatic RCC (mRCC). These rapid developments have resulted in continuous changes in the respective Clinical Practice Guidelines/Expert Recommendations. We conducted a systematic review of the existing guidelines in MEDLINE according to the Preferred Reporting Items for Systematic Review and Meta‐Analyses statement, aiming to identify areas of agreement and discrepancy among them and to evaluate the underlying reasons for such discrepancies. Data synthesis identified selection criteria for nonsurgical approaches in renal masses; the role of modern laparoscopic techniques in the context of partial nephrectomy; selection criteria for cytoreductive nephrectomy and metastasectomy in mRCC; systemic therapy of metastatic non‐clear‐cell renal cancers; and optimal sequence of available agents in mRCC relapsed after anti‐VEGF therapy as the major areas of uncertainty. Agreement or uncertainty was not always correlated with the availability of data from phase III randomized controlled trials. Our review suggests that the combination of systematic review and critical evaluation can define practices of wide applicability and areas for future research by identifying areas of agreement and uncertainty among existing guidelines.
Implications for Practice.
Currently, there is uncertainity on the role of surgery in MRCC and on the choice of available guidelines in relapsed RCC. The best practice is individualization of targeted therapies. Systematic review of guidelines can help to identify unmet medical needs and areas of future research.
Introduction
Renal cell carcinoma (RCC) accounts for 2%–3% of all new adult malignancies [1]. The proportion of small and incidental renal tumors has significantly increased owing to the widespread use of abdominal imaging. Consequently, more than 50% of RCCs are currently detected incidentally [2]. Nevertheless, the incidence of all stages of RCC has increased over the past several years, contributing to a steadily increasing mortality rate per unit population.
The management of RCC has undergone substantial changes, with novel surgical and systemic strategies fundamentally altering the approach to this disease. In localized RCC, surgical practice has reduced morbidity and has advanced toward less invasive resection approaches, which achieve comparable oncological outcomes to the traditional nephrectomy [3]. Although surgery remains the most important and probably the only curative approach in RCC, the prognosis of metastatic RCC (mRCC) has also been improved in recent years. An enhanced understanding of the underlying biology of RCC has led to systemic therapy targeting the vascular endothelial growth factor (VEGF) and the mammalian target of rapamycin (mTOR) pathways or inhibiting the interaction of the programmed death 1 receptor with its ligand. Ten novel agents blocking these pathway elements have demonstrated efficacy and offer useful treatment options for patients with mRCC.
Several national and international urological and medical oncology societies and associations have published guidelines on RCC [4], [5], [6], [7], [8], [9], [10], [11]. Nevertheless, their utility in everyday practice may be associated with a variety of limitations. Practical issues and a difficulty for clinicians in the community to follow all the new available information have been suggested as possible causes [12]. In addition, issues associated with the development of guidelines may limit adherence in everyday practice. For example, variation in the definition of the levels of evidence (LoE) and grading of recommendations (GoR) result in differences in the strength of recommendations regarding the various treatment modalities. This variation underlines the considerable heterogeneity in the development and reporting of guidelines. For these reasons, the Institute of Medicine (IOM) established standards for developing trustworthy Clinical Practice Guidelines (CPGs) [13]. In a recent review of lung, breast, prostate, and colorectal cancers guidelines, a significant diversion from these standards has been detected [12]. Our group also reported similar findings for nonmetastatic bladder cancer [14]. Such evaluation for CPGs in RCC has not been carried out so far.
Apart from their wide and timely distribution, the identification of common statements as well as discrepancies among existing guidelines might be of further value. Areas of agreement represent recommendations that should be applied in order to improve the management of RCC patients, and this sets clear targets for our efforts at local, national, and international level. In contrast, issues associated with uncertainty represent the targets for future research and shift the balance from evidence‐based medicine to justified clinical practice based on personal experience and regional conditions and expertise. This need is highly relevant in a rapidly evolving field, such as the treatment of RCC. We hereby present the results of a systematic review of the current RCC guidelines and the critical evaluation of the evidence produced by this review.
Materials and Methods
A systematic review was performed according to the Preferred Reporting Items for Systematic Review and Meta‐Analyses guidelines [15]. The protocol of this study was submitted to the Institutional Review Board of Alexandra Hospital, Medical University of Athens, Greece, and is available upon request. Eligible articles were identified by a MEDLINE search of bibliographical databases for the period from January 1, 2008, up to October 31, 2015. The search strategy included the following keywords: (recommendation [ti] OR recommendations [ti] OR consensus [ti] OR guideline [ti] OR guidelines [ti] OR consultation [ti]) AND (society OR societies OR college OR association OR associations) AND ((renal OR kidney OR “clear cell”) AND (carcinoma OR carcinomas OR cancer OR cancers OR neoplasm OR neoplasms)).
All studies providing CPGs/Expert Recommendations regarding the treatment of renal carcinoma were considered eligible. CPGs referring exclusively to pathological or radiological guidelines or follow up or versions of guidelines for patients’ information were not eligible. Only articles in English were included. Two investigators (FZ and AD), working independently, searched the literature and extracted data from each eligible study. In addition, all references of the retrieved articles were checked in order to identify additional potentially eligible articles. In case of multiple guidelines, only the most recent publication was included. Finally, full‐length recommendations, if available, were also cross checked against the relevant papers in order to retrieve additional information. Respective guideline manuals were also reviewed if necessary to clarify methodological or ethical issues.
Following the completion of identifying eligible papers, two investigators (AB and KS) independently scored each one by using the eight standards and the 20 subcriteria outlined by the IOM (supplemental online Table 1) [13]. A criterion was fulfilled only when all subcriteria were also fulfilled. Furthermore, we systematically reviewed literature from June 15, 2012, to October 31, 2015, for recent publications on phase III randomized controlled trials (RCTs), systematic reviews on RCTs, and meta‐analyses of RCTs in order to take into consideration level 1 evidence not included in the published guidelines. The following algorithm was applied: ((renal[ti] OR kidney[ti] OR “clear cell”[ti]) AND (carcinoma[ti] OR carcinomas[ti] OR cancer[ti] OR cancers[ti] OR neoplasm[ti] OR neoplasms[ti])) AND (“phase 3”[ti] OR “phase III”[ti] OR meta‐analysis[ti]). The last revision of our manuscript (December 2016) also took into account the changes in European Society for Medical Oncology (ESMO) and National Comprehensive Cancer Network (NCCN) guidelines, which had been implemented after October 31, 2015. Finally, we synthesized available guidelines in order to highlight common statements as well as distinct differences among them, to search for the reasons behind these discrepancies and to associate recommendations with common practice.
Results
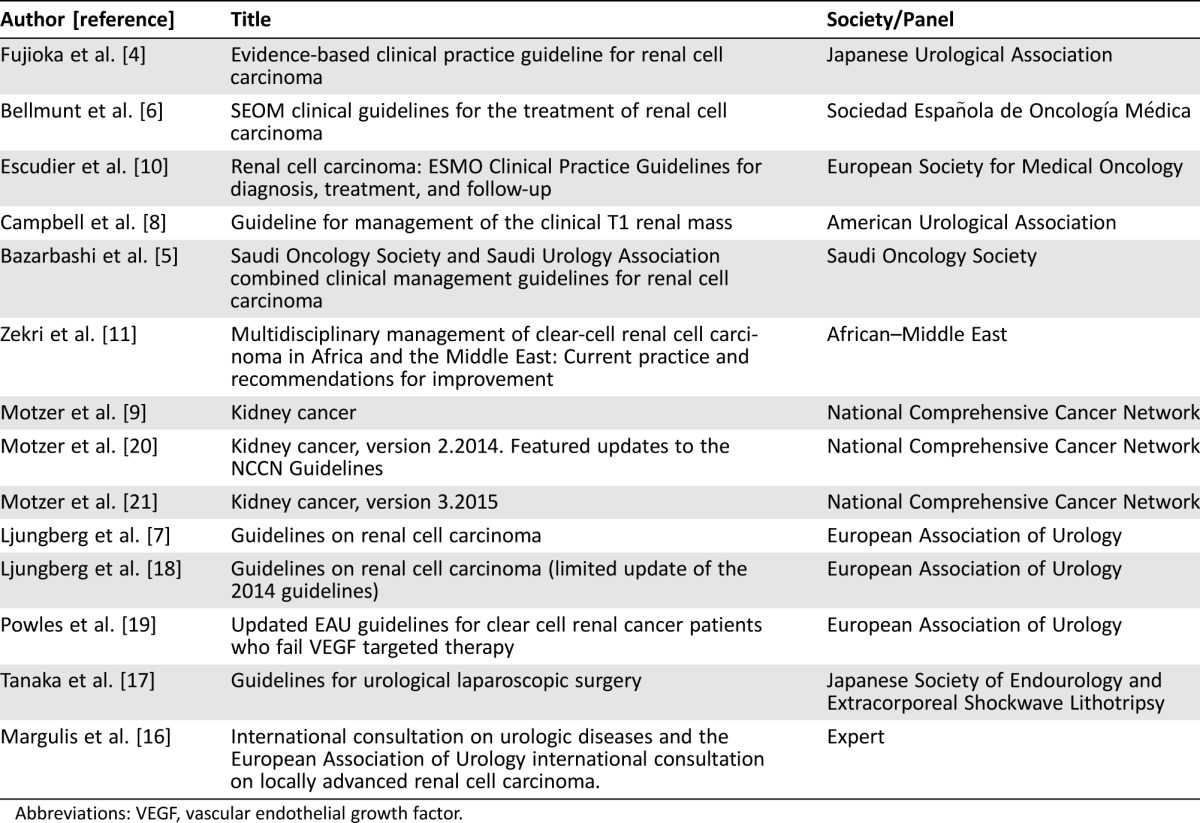
The search strategy retrieved 103 articles providing possible CPGs/Expert Recommendations regarding the treatment of renal carcinoma. Of these articles, 75 were irrelevant, 17 were excluded due to restrictions (i.e., language restrictions, existence of updated publications, etc.), and 11 were eligible [4], [5], [6], [7], [8], [10], [11], [16], [17], [18], [19]. After searching the references of all reviews and remaining articles, three additional articles were also included [9], [20], [21]. Overall, 14 papers published between 2008 and 2015 were eligible for the systematic review (Table 1). The aforementioned stages are illustrated in detail in Figure 1.
Table 1. Publications used for data synthesis.
Abbreviations: VEGF, vascular endothelial growth factor.
Figure 1.
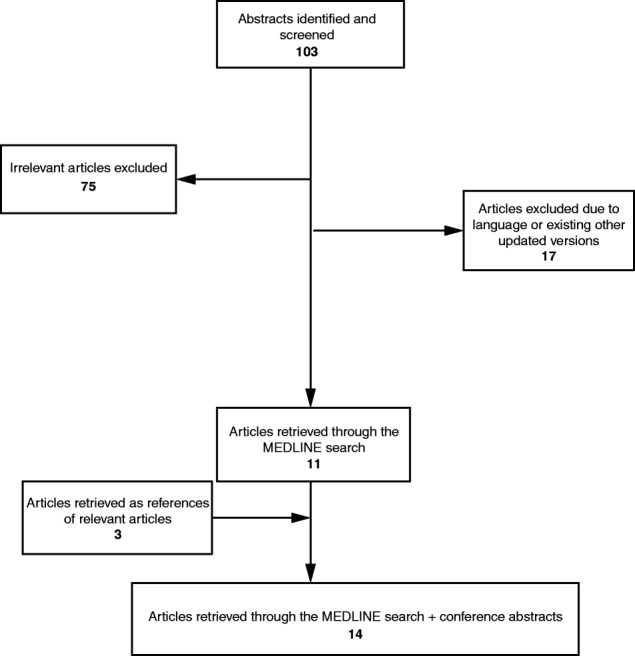
Outline of selection strategy.
Evaluation According to IOM Criteria
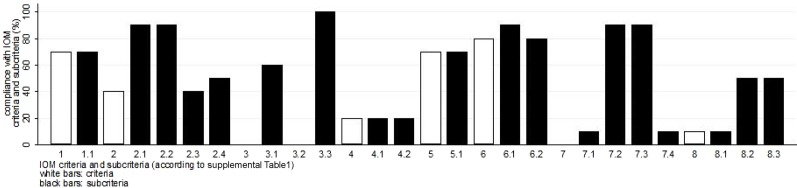
Compliance with IOM criteria for all included papers is shown in Figure 2. There was no representation of patients or the public in any CPGs or Recommendations panels. The methodology of the development of guidelines was described in all but one case (Saudi Oncology Society [SOS]), but the use of systematic reviews was specifically reported only by the American Urological Association (AUA) and the European Association of Urology (EAU). AUA was the only society that made the final draft of their guidelines available for public comment, while the NCCN was the only organization that included a full spectrum of stakeholders in the process of developing their guidelines.
Figure 2.
Adherence of the selected publications to the IOM criteria (definitions of criteria and sub‐criteria in supplemental online Table 1). Bars represent percentage of the selected publications, which adhere to each criterion or subcriterion. No bar indicates that no paper adhered to this subcriterion.
Abbreviations: IOM, Institute of Medicine.
Data Synthesis
Pathology, Staging, and Risk Assessment.
Review of guidelines regarding pathology, staging, and risk assessment is not within the scope of this review. The respective classifications used in the reviewed guidelines and in this review are described in detail in the supplemental online material.
Treatment of RCC.
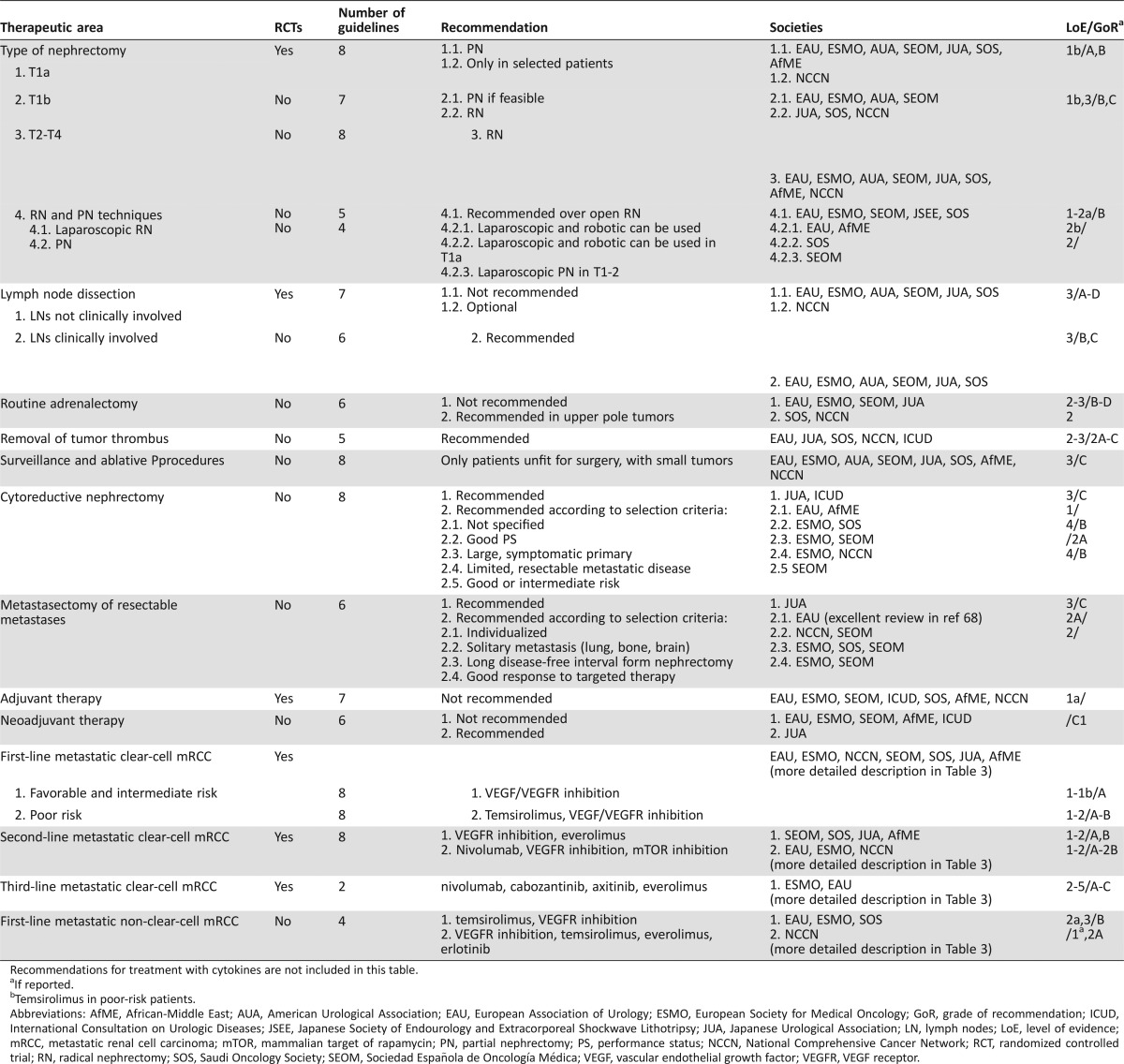
EAU, ESMO, Japanese Urological Association (JUA), African‐Middle East (AfME), Sociedad Española de Oncología Médica (SEOM), and SOS published guidelines for the whole spectrum of RCC management. The NCCN guidelines were focused on systemic therapy, while the AUA, the 2011 EAU International Consultation on Urologic Diseases (ICUD), and the Japanese Society of Endourology and Extracorporeal Shockwave Lithotripsy (JSEE) published guidelines on localized or locoregional disease. The strength of the recommendations was mainly based on the LoE of the available data. The definitions of LoE were similar across all papers, with the availability of phase III RCTs (and/or meta‐analyses of RCTs) universally accepted as representing the highest LoE. We thus speculated that unanimity across guidelines would be associated with the availability of such data and diversions with the lack of it. We therefore stratified our results according to the availability of such evidence (Table 2).
Table 2. Summary of recommendations included in the reviewed guidelines for the treatment of mRCC according to the availability of RCTs.
Recommendations for treatment with cytokines are not included in this table.
If reported.
bTemsirolimus in poor‐risk patients.
Abbreviations: AfME, African‐Middle East; AUA, American Urological Association; EAU, European Association of Urology; ESMO, European Society for Medical Oncology; GoR, grade of recommendation; ICUD, International Consultation on Urologic Diseases; JSEE, Japanese Society of Endourology and Extracorporeal Shockwave Lithotripsy; JUA, Japanese Urological Association; LN, lymph nodes; LoE, level of evidence; mRCC, metastatic renal cell carcinoma; mTOR, mammalian target of rapamycin; PN, partial nephrectomy; PS, performance status; NCCN, National Comprehensive Cancer Network; RCT, randomized controlled trial; RN, radical nephrectomy; SOS, Saudi Oncology Society; SEOM, Sociedad Española de Oncología Médica; VEGF, vascular endothelial growth factor; VEGFR, VEGF receptor.
1. 1. Phase III RCTs Available.
1.1. Radical versus Partial Nephrectomy in Localized RCC (T1‐2N0M0). Surgery is recommended to achieve cure in localized RCC. The functional benefits of nephron‐sparing procedures have driven the indication of partial nephrectomy (PN). The equivalence of the oncological outcome with that of radical nephrectomy (RN) had been suggested by several nonrandomized studies [22], [23], [24], [25], and noninferiority was proven in a phase III RCTs [26] in solitary renal tumors <5 cm. Survival rates were 72.5% after RN versus 64.4% after PN after more than 9 years of follow‐up, while cancer‐specific survival (CSS) was 98.5% versus 97%, respectively. Based on these data, all guidelines recommend PN as the standard treatment in patients with T1a tumors [4], [8], [10], [11].
Such unanimity was not observed for T1b tumors. Whenever technically feasible, PN was recommended by EAU and ESMO [10], [18]. On the contrary, JUA [4] and SOS [5] retained RN as their standard, while SEOM [6] and AUA [8] adopted a more balanced approach accepting both as standards, underlying the increased surgical risk associated with PN and the increased probability of chronic kidney disease associated with RN. Notably, JUA and AUA guidelines were reported in 2012 and 2009, thus predating much of the recent data on PN, including the results of the randomized study [26].
2.
1.2. Lymph Node Dissection. In patients with localized disease and no clinical evidence of positive lymph nodes, lymph node (LN) dissection (LND) during nephrectomy is not recommended by EAU, ESMO, SEOM, and ICUD. The recommendation against LND was based on the results of an RCT performed to evaluate the role of routine lymphadenectomy for RCC (clinical T1‐3N0M0) [27] and failed to demonstrate any significant difference in CSS between the study groups. In contrast to the other guidelines, SOS considers regional LND (within Gerota's fascia) as an integral part of RN [5]. This diversion probably reflects skepticism regarding the above trial, because most patients were at low risk of developing LN metastases, and the majority received limited and unstandardized LND.
The management of grossly involved lymph nodes is still a matter of controversy. Nonrandomized data suggest improved outcomes in patients with complete removal of clinically involved LNs [28], [29], [30], [31]. Only three of the reviewed papers make relevant recommendations. EAU guidelines state conservatively that “clinically involved nodes could be excised for staging and symptom control” (GoR C) [18], while ICUD and SOS have issued more definitive statements [5], [16].
3.
1.3. Adjuvant Systemic Therapy. Adjuvant cytokine treatment was not recommended (LoE 1A) [5], [6], [7], [9], [10], [16]. A randomized study published in 2014 showed that the combination of 5‐fluorouracil, interferon‐a (IFNa), and interleukin‐2 (IL‐2) did not produce a survival benefit, while toxicity was considerable [32]. Based on the significant efficacy of targeted therapies in mRCC, five randomized studies have been initiated. Recently, the results of two of them were reported [33], [34]. One study (ASSURE) showed no benefit from adjuvant sunitinib or sorafenib [33], whereas the other (S‐TRAC) showed a prolongation of disease‐free survival from adjuvant sunitinib (6.8 years versus 5.6 years for placebo, p = .03) among patients with localized clear‐cell RCC at high risk for tumor recurrence after nephrectomy (pT ≥ 3 and/or pN+) [34]. Sunitinib therapy was associated with significantly higher incidence of adverse events. The reasons for the difference between these two studies are unclear, whereas mature overall survival (OS) data are not yet available. Sunitinib has not yet been approved as adjuvant therapy, while guidelines including the results of S‐TRAC have not yet been published.
4.
1.4. First‐Line Systemic Therapy in mRCC. Although treatment with IL‐2 and IFNa remain acceptable options, their use has been very limited, as anti‐VEGF agents and mTOR inhibitors (mTORIs) have shown superiority to IFNa [35], [36], [37], [38]. The availability of multiple options created the need for criteria to select the most appropriate treatment for the individual patient. Currently, the two most accepted criteria are risk group and histological subtype. Because all first‐line randomized trials required a clear‐cell component, high LoE exist only for this histological subtype. Risk stratification was mainly based on the Memorial Sloan Kettering Cancer Center (MSKCC) model, although the International mRCC Database Consortium (IMDC) criteria have been used in some of the more recent trials (detailed description of both criteria in supplemental online Table 2). Due to rapid drug development, some of the recommendations (i.e., JUA 2012, ICUD) were outdated, while others published several updates of their main publications (NCCN [20], [21]).
1.4.1. Favorable and Intermediate Risk. The importance of starting systemic therapy of mRCC with anti‐VEGF/VEGF receptor (VEGFR) agents is highlighted by a recent randomized, phase II study, which showed that starting treatment with the mTORI everolimus followed by sunitinib produced inferior results compared with the reverse sequence [39]. Three standards, sunitinib, pazopanib, and the combination bevacizumab + IFNa (B‐IFNa), are unanimously recommended by EAU, ESMO, NCCN, SEOM, AfME, and SOS. ICUD and JUA did not include pazopanib because these publications predate its approval.
In spite of the general consent, there are notable differences among the EAU, ESMO, and NCCN recommendations (Table 3). EAU recommends sunitinib and pazopanib for all risk groups and B‐IFNa for favorable and intermediate‐risk patients. ESMO, JUA, and SOS recommend all three standards only for favorable and intermediate‐risk patients. NCCN gives GoR 1 to all three standards for all risk groups, while also including axitinib in the acceptable options with GoR 2A. This recommendation was based on numerically promising progression‐free survival (PFS), reported in an RCT (which, however, did not fulfill the initial statistical hypothesis) and a randomized phase II study using dose titration of axitinib [40], [41].
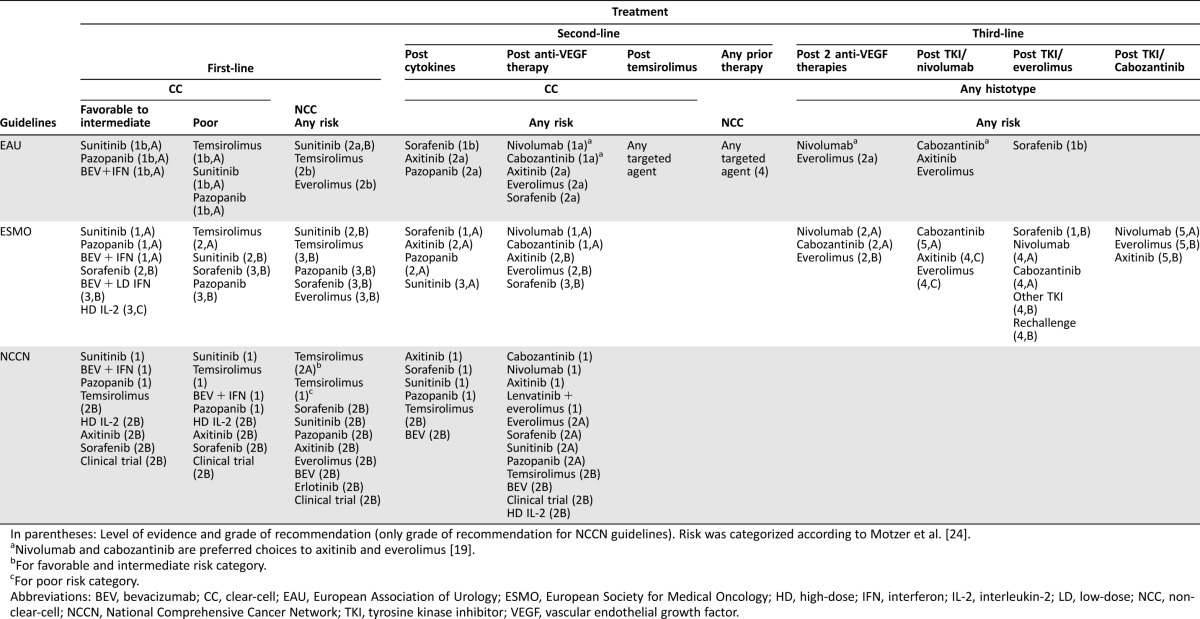
Table 3. EAU, ESMO, and NCCN recommendations for the treatment of metastatic renal cancer.
In parentheses: Level of evidence and grade of recommendation (only grade of recommendation for NCCN guidelines). Risk was categorized according to Motzer et al. [24].
Nivolumab and cabozantinib are preferred choices to axitinib and everolimus [19].
For favorable and intermediate risk category.
For poor risk category.
Abbreviations: BEV, bevacizumab; CC, clear‐cell; EAU, European Association of Urology; ESMO, European Society for Medical Oncology; HD, high‐dose; IFN, interferon; IL‐2, interleukin‐2; LD, low‐dose; NCC, non‐clear‐cell; NCCN, National Comprehensive Cancer Network; TKI, tyrosine kinase inhibitor; VEGF, vascular endothelial growth factor.
ESMO adds the option of a period of observation before starting treatment, especially in patients with limited tumor burden and few symptoms [10]. This recommendation is based on the outcome of patients who crossed over to an active agent after a brief period of treatment with placebo, which seemed similar to that of upfront active treatment (LoE 2, GoR C) [42]. The low GoR reflects the absence of validated criteria for selection of patients suitable for this approach.
1.4.2. Poor Risk. ESMO, JUA, EAU, SEOM, AfME, ICUD, and SOS consider temsirolimus as the standard for these patients. EAU and NCCN accredit sunitinib (which is also mentioned as an option by the other guidelines) and pazopanib with the same LoE and/or GoR as temsirolimus despite the lack of supporting phase III studies (LoE 1b, GoR A). The position of these two societies appears highly acceptable by everyday practice, as shown by real‐world data from US and International MRCC Database Consortium (IMDC) [43], [44]. Recently, the results of a phase II randomized trial (CABOSUN) were reported: cabozantinib, a tyrosine kinase inhibitor (TKI) of VEGFR2, mesenchymal‐epithelial transition factor (MET), and apoptotic cell recognition receptor (AXL), demonstrated a significant benefit in overall response rate (ORR) and progression free survival (PFS) over sunitinib in untreated intermediate and poor risk patients with mRCC [45]. The impact of these results on the future guidelines and on the treatment paradigm in mRCC cannot yet be determined.
5.
1.5. Second‐Line Systemic Therapy in mRCC. For the few patients who received primary treatment with cytokines, sorafenib, axitinib, and pazopanib are recommended by EAU, ESMO, and NCCN, albeit with different LoEs and strengths of recommendation (Table 3).
Currently, most patients with mRCC will receive anti‐VEGF/VEGFR therapy. Most patients will experience progression after a median time of 9–11 months. It is widely accepted that the optimal method to define progression is not yet determined. Furthermore, only around 50% of patients failing first‐line treatment will undergo second‐line treatment [44], which may imply an inherent selection bias. The widely applied practice of continuing the same agent in the (ill‐defined) “slow progressors” [46] as well as the offer of “treatment holidays” in the case of successful first‐line therapy in patients achieving complete response [47] further complicate the development of guidelines in this setting.
Following the RECORD‐1 and AXIS trials [48], [49], axitinib and everolimus emerge as the most accepted standards in the guidelines, which were published after the approval of these agents (EAU, ESMO, SEOM, SOS, NCCN, AfME). In most cases, sorafenib also received strong recommendation, as the AXIS trial confirmed its efficacy in post‐TKI setting [49]. Again, NCCN considers many agents as valid options, in spite of lack of randomized data for many of them. In contrast to first‐line recommendations, there is considerable diversion in the LoE and GoR given by the different societies (Table 3). The major reason for this discrepancy is the fact that none of these trials included a pure second‐line post anti‐VEGFR population; around 30% of the AXIS patients received first‐line cytokines, while only 21% of the RECORD‐1 patients had received only one previous line of therapy.
EAU and NCCN consider both axitinib and everolimus absolutely equal in terms of LoE, while ESMO considers that only the data of axitinib is of level I, while the reverse is quoted regarding the GoR (Table 3). Therefore, current guidelines do not aid the choice between axitinib and everolimus. A direct comparison between these two standards is lacking, while information from nonrandomized comparisons are inconclusive [50], [51]. For the above reasons, the value of discussing the available choices in the context of the patient's way of life and interests and taking into consideration the different toxicity profiles cannot be overestimated.
Recently, nivolumab, an immune checkpoint inhibitor, and cabozantinib have shown OS superiority over everolimus in second‐line therapy of mRCC [52], [53] and have been granted U.S. Food and Drug Administration (FDA) approval. EAU and ESMO have already endorsed these agents as new second‐line standards [19], [54], although limited availability may influence the choice in this setting. Finally, the concept of monotherapy in relapsed mRCC has been challenged by the randomized phase II trial, which showed that the combination of everolimus and a VEGFR TKI, lenvantinib, produced superior PFS compared with everolimus alone [55]. Lenvatinib (in combination with everolimus) has been granted FDA approval, but, based on the size of the supporting study, is currently included only in the updated version of NCCN guidelines [56].
6.
1.6. Third‐Line Systemic Therapy in mRCC. For patients who received everolimus after first‐line TKI, sorafenib is recommended, based on a phase III RCT that showed efficacy and safety of both dovitinib and sorafenib, which were compared [57]. OS was similar in both arms and approval of dovitinib was not pursued. According to the results of the previously mentioned randomized trials, EAU and ESMO have included nivolumab and cabozantinib in their recommendations (Table 3) [19], [54].
7. 2. No Phase III RCTs Available.
2.1. Surveillance and Ablative Procedures. These strategies are mainly applicable for small (T1a) renal tumors. Approximately 80% of all clinical T1a renal masses are malignant, and of these, about 20% to 30% demonstrate potentially aggressive histologic features. Criteria to accurately define the risk of tumor progression that could preclude nonsurgical approaches or lead to unsalvageable systemic metastases do not exist. Population‐based analyses show a significantly lower cancer‐specific mortality for patients with T1a RCC treated with surgery compared with nonsurgical management [58]. However, the same benefit was not confirmed in older (>75 years old), comorbid patients with small renal masses (<40 mm) and in patients with limited life expectancy. Similarly, the available data does not allow for any definitive conclusions regarding morbidity or surgical and oncological outcomes for cryoablation and radiofrequency ablation [59], [60], [61], [62], [63], [64], [65], and no recommendations can be safely made. Based on the available data, ESMO and SEOM recommend surveillance or ablative procedures in elderly patients with significant comorbidities and small solid renal tumors (<3–4 cm), high surgical risk, solitary kidney, compromised renal function, hereditary RCC, or multiple bilateral tumors (LoE III, GoR C) [6], [10]. EAU, SOS, and JUA make more general statements regarding elderly patients with small tumors and comorbidities [5], [7], [8].
8.
2.2. RN and PN Techniques. Both RN and PN can be theoretically performed by open or laparoscopic procedures. In addition to the traditional laparoscopy, robotic techniques have also been used. Few studies comparing one technique with another have included randomization in their design [66], [67], [68], [69]. Nevertheless, their design was flawed by including a very small number of patients, and their results cannot be considered of equally high LoE as properly designed RCTs.
Large comparative studies of open versus laparoscopic RN (LRN) in T1‐2N0M0 RCC reported similar results in disease‐free survival (DFS), OS, and CSS [17], [18], [20], [21], [70], [71], [72], while LRN is associated with lower morbidity [18]. Based on these data, LRN is recommended in patients with T1 or T2 disease not treatable by PN [5], [6], [10], [17], [18]. Not surprisingly, the strongest statement in favor of the laparoscopic approach comes from JSEE, which is dedicated to the promotion of research in urologic laparoscopy and considers laparoscopic RN as the standard in stage I disease [17].
Nonrandomized studies suggest similar oncological outcomes after open versus laparoscopic PN (LPN) [73], [74], but recommendations are less definitive compared with the statements for RN. EAU and SOS do not provide specific indications for LPN and only state that PN can be performed, either with an open, pure laparoscopic or robotic approach, based on the surgeon's expertise and skills (LoE 2b). On the other hand, SEOM definitively recommends LPN in stage I tumors, if technically feasible [6]. Recent reports have shown significantly lower estimated blood loss, shorter warm ischemia time, and comparable perioperative outcomes after robotic compared with pure laparoscopic PN [75], [76], [77]. Nevertheless, there are no reliable data regarding the oncological outcomes of robot‐assisted laparoscopic RN or PN [18], and specific recommendations for this technique were not made.
9.
2.3. Adrenalectomy. Ipsilateral routine adrenalectomy during PN or RN does not provide surgical advantage [78] and is not recommended when there is no radiological or intraoperative evidence of adrenal involvement [10]. In contrast to the other guidelines, SOS recommends adrenalectomy in upper pole tumors (LoE 2).
10.
2.4. Inferior Vena Caval Thrombus. Despite the absence of high LoE, there was unanimity regarding the management of RCC with venous thrombi. Open RN with the goal of removing the kidney and the tumor thrombus and obtaining negative margins was considered the standard of care [4], [5], [7], [9], [10], [16]. Selection of patients has been suggested by certain guidelines. Absence of metastatic disease and an acceptable performance status (PS) are considered selection criteria by NCCN (GoR 2A) [9] and EAU (LoE 3, GoR C) [7]. Surgical fitness was the only criterion recommended by ICUD (LoE 1) [16].
11.
2.5. Neoadjuvant Systemic Therapy. This modality was discussed only in the context of locally advanced disease and especially when tumor thrombi are present [4], [10], [16]. There is anecdotal evidence that tumor thrombus downstaging can be achieved with neoadjuvant targeted agents (LoE 4) [79], [80], [81]. However, a larger case review found only a marginal reduction of tumor thrombi [16]. Only JUA includes neoadjuvant therapy in its therapeutic algorithm (GoR C1).
12.
2.6. Surgery in Metastatic Disease. Cancer patients who are diagnosed with synchronous metastases are normally treated with systemic therapy. Nevertheless, local treatment of the primary as well as metastatic sites should be considered in mRCC, as this management can lead to long DFS even without the use of systemic therapy [82], [83].
2.6.1. Cytoreductive Nephrectomy. In most patients with RCC, nephrectomy usually precedes the occurrence of metastases, since most patients are diagnosed without metastatic disease. Nevertheless, 20%–30% of patients are diagnosed with synchronous metastases. Cytoreductive nephrectomy (CN) was associated with a significant survival benefit in patients undergoing treatment with IL‐2 or IFN‐a (LoE 1A). Nevertheless, for the majority of patients who receive first‐line therapy with the current standards, that is, targeted agents, recommendations [4], [5], [6], [7], [9], [10], [11] are based on retrospective series [84], [85], [86]. Although it is uniformly accepted that CN should be considered in patients with synchronous metastases, there is disagreement on the appropriate population for CN. A detailed description of the selection criteria suggested by each paper is included in Table 2. Regarding the type of nephrectomy, patient numbers in LRN studies are too small, and further research is needed.
2.6.2. Local Therapy of Metastatic Sites. There is a uniform recommendation in favor of metastasectomy of resectable metastases [4], [5], [7], [9], [10], although NCCN limits this recommendation to single metastatic sites. This recommendation is based on data from retrospective comparative studies, which point towards an OS and CSS benefit and delay of systemic therapy from complete metastasectomy [87]. The decision for metastasectomy should be individualized (LoE 3, GoR C), but criteria for selection are either not mentioned or differ from each other. PS, risk profiles, patient preference, and alternative techniques to achieve local control are used by EAU [7]; ESMO includes solitary or easily accessible pulmonary metastases, solitary resectable intra‐abdominal metastasis, a long DFS after nephrectomy, or a partial response in metastases to systemic therapy [10]; and SEOM considers resection of up to four lung metastases and solitary metastases in other locations, in long DFS, or as consolidation after stabilization of disease under targeted therapy [6]. Only EAU offers site‐specific recommendations for metastasectomy, based on a recently conducted systematic review of mainly retrospective series [84]. With the exception of brain and possibly bone metastases, metastasectomy is the recommended local treatment for most sites (LoE 3). For bone and brain metastases, stereotactic radiotherapy and radiosurgery can be offered in selected cases (GoR C). No systemic therapy after complete metastasectomy is recommended.
13.
2.7. Systemic Treatment of Non‐Clear Cell mRCC. There are no published phase III RCTs including specifically non‐clear‐cell (NCC) renal carcinomas. Therefore, all available information comes from phase II studies, real‐world evidence, and subgroup analyses of randomized trials that included NCC carcinomas [38]. Most studies included papillary and chromophobe carcinomas.
Not surprisingly, LoE and GoR for this group are low, ranging from 2a to 3 and 2 to 2B, respectively, while recommendations are limited to first‐line treatment and show considerable variability. EAU considers sunitinib, everolimus, and temsirolimus as the recommended options; ESMO and SOS consider temsirolimus, sunitinib, and sorafenib; and NCCN quotes a wide range of agents, including all approved agents for RCC with the addition of the epidermal growth factor receptor inhibitor erlotinib. Interestingly, there is a GoR 1 for temsirolimus in NCC, poor‐risk patients by NCCN. A recent literature review and a meta‐analysis confirmed the above options, which, however, are less effective compared with clear‐cell tumors, leading to the conclusion that the optimal treatment for these tumors remains unclear [88], [89]. Recently, two randomized phase II studies comparing sunitinib and everolimus in NCC tumors (ASPEN) [90] or NCC with clear cell (cc) with at least 20% sarcomatoid features (ESPN) [91] did not demonstrate superiority of everolimus over sunitinib, while there was a trend in favor of sunitinib. Based on these findings, ESMO, EAU, and SEOM recommend sunitinib as the standard in their more recent guidelines, although LoE is expectedly low [6], [18], [54].
Discussion
To our knowledge, this is the first review of existing CPGs on RCC. We believe that our methodology offers a useful tool for the utilization of guidelines in clinical practice and can be applied to all tumor types. The combination of systematic review and critical evaluation led to the identification of areas of agreement and uncertainty among different guidelines, which was a major goal of this study. The major areas of uncertainty were as follows: (a) selection criteria for nonsurgical approaches in renal masses; (b) the role of modern laparoscopic techniques, especially in the context of PN; (c) selection criteria for CN and metastasectomy in mRCC; (d) systemic therapy of metastatic NCC renal cancers; € optimal sequence of available agents in mRCC relapsed after anti‐VEGF/VEGFR therapy. We speculated that the degree of consensus among different guidelines would be associated with the LoE and, in particular, the existence (or not) of phase III RCTs to support recommendations. Thus, areas of uncertainty would be characterized by low LoE due to the absence of phase III RCTs. This hypothesis was only partially true. For example, it applies for the first four areas of uncertainty mentioned above, but not for the last one; although several options for second‐ or third‐line treatment were proposed, based on phase III RCTs, no reliable criteria for treatment selection exist in this setting. In contrast, several recommendations, such as the increasing acceptance of laparoscopic techniques, the recommendation against adrenalectomy, and the established role of surgery in metastatic disease were universally accepted although not supported by RCTs. These findings indicate that issues such as surgical morbidity, personal experience, and justified surgical practice are strongly taken into consideration in the development of guidelines. RCTs are of utmost importance for the development of guidelines and the evolution of clinical practice, but their usefulness could be enhanced by the involvement of academia in their design, as the mere proof of the superiority of one agent versus another does not always answer relevant clinical problems.
Regarding our limitations in choosing the most effective sequence of available agents, it is evident that a “one sequence fits all” situation does not exist. To complicate things further, the RCTs leading to the approval and their inclusion in guidelines of agents in this setting varied in their design regarding the primary endpoint. PFS was the primary endpoint in RECORD‐1, AXIS, and METEOR, while OS was the primary endpoint in CheckMate 025. Agents supported by OS benefit are considered as more valid options in current guidelines, although lack of such benefit could be due to the limited power of a study designed to show a PFS benefit. This in combination with the unmet need for effective therapies in NCC carcinomas highlights the fact that the significant progress in the molecular characterization of renal tumors [92], [93], the extensive research on the mechanisms of resistance, and the effort to identify useful predictive molecular markers [94], [95] have not yet been captured in the current treatment paradigm.
Several differences among the reviewed guidelines were detected. Most papers concentrated either on surgical or medical therapies depending on the background of the respective society. Nevertheless, in all but three cases recommendations on all aspects of the management of RCC were included, and adequate representation of urologists and medical oncologists was ensured. This underlines the common belief that a multimodality approach will ensure the optimal management of RCC. The papers reviewed cover a broad spectrum of societies with noticeable cultural and economic differences. This was frequently reflected in their recommendations; a wide spectrum of approaches between “minimal recommendations” and “detailed description of all possible options” was adopted by the various panels, depending on the aim of the development of guidelines, the targeted audience, and reimbursement issues. It could also be argued that the recommendations of existing guidelines should be rated according to the size of the audience they influence or the quality of the methodology that was used for their development. The IOM standards have been proposed for the latter purpose [12], [13]. We found that certain standards, that is, representation of patients or public and availability for public comment prior to publication, have not been introduced in almost any of the reviewed guidelines, similar to previous reports for breast, lung, prostate, colorectal, and bladder cancer [12], [14]. A systematic review of the literature was included in the methodology of 4 of the 14 eligible publications. Not overlooking the fact that a single society (EAU) contributed three of these four publications, this represents an improvement over our previous review on bladder cancer, which revealed that none of the 15 eligible publications reported this methodology [14]. The RCC publications were more recent, due to the more rapid evolvement of the treatment paradigm, and this may have allowed familiarization of the authors with more advanced methodology and application of a higher level of standards in the development of guidelines. It has to be underlined that the IOM standards have not been widely accepted yet, and some of them have been considered “too vague and subjective to be analyzed” [96]. For this reason, the evaluation presented here is purely descriptive, and we did not use it to exclude any of the publications considered eligible or to compare the quality of the reviewed guidelines. On the contrary, we equally considered all recommendations. We believe that the development of guidelines represents an essential effort toward the optimization of cancer care and should be sought at local and national levels as much as at the international level. On the other hand, the effort to establish widely acceptable criteria for uniform development of high‐quality guidelines should continue.
Conclusion
In conclusion, this systematic review and critical evaluation of existing guidelines in RCC identified similarities and discrepancies in certain areas. An effort for wide application of the former should be made, while the latter represent areas of unmet medical needs and, therefore, targets for future research. Close collaboration of urologists and medical oncologists in the whole spectrum of this disease ensures optimal management and outcome for these patients.
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
The existing guidelines on genitourinary cancer were the subject of a multidisciplinary national congress organized by the Hellenic Genitourinary Cancer Group (HGUCG; Athens, Greece; November 2014). Site‐specific consensus sessions were led by international experts who also serve as co‐authors of this review (BE, CS, BD). The conclusions of these sessions were published on the HGUCG website (www.eeoogek.gr) and served as the theoretical background for the initiation of the systematic review and the critical evaluation of RCC guidelines, which are the subject of this paper.
Footnotes
For Further Reading: Daniel Keizman, David Sarid, Jae L. Lee et al. Outcome of Patients With Metastatic Chromophobe Renal Cell Carcinoma Treated With Sunitinib. The Oncologist 2016;21:1212–1217.
Implications for Practice: Data on the activity of sunitinib in metastatic chromophobe renal cell carcinoma (mchRCC) are limited. This study analyzed the activity of sunitinib in a cohort of mchRCC patients. Of 36 patients with mchRCC who were treated with first‐line sunitinib, 78% achieved a clinical benefit. Median PFS and OS were 10 and 26 months, respectively. Treatment outcome was not significantly different between mchRCC patients and individually matched metastatic clear cell RCC patients.
Author Contributions
Concepts and Design: Charalampos Deliveliotis, Flora Zagouri, Aristotle Bamias, Meletios Dimopoulos
Provision of Study Material or Patients: Flora Zagouri, Aristotle Bamias, Bernard Escudier, Cora Sternberg, Athanassios Dellis, Bob Djavan, Kimon Tzannis, Loukas Kontovinis, Konstantinos Stravodimos, Athanassios Papatsoris, Dionysios Mitropoulos, Constantine A. Constantinides
Collection and/or Assembly Data: Charalampos Deliveliotis, Flora Zagouri, Aristotle Bamias, Cora Sternberg, Athanassios Dellis, Bob Djavan, Kimon Tzannis, Loukas Kontovinis, Konstantinos Stravodimos, Athanassios Papatsoris, Dionysios Mitropoulos, Constantine A. Constantinides
Data Analysis and Interpretation: Charalampos Deliveliotis, Flora Zagouri, Aristotle Bamias, Bernard Escudier, Cora Sternberg, Athanassios Dellis, Bob Djavan, Kimon Tzannis, Loukas Kontovinis, Konstantinos Stravodimos, Athanassios Papatsoris, Dionysios Mitropoulos, Constantine A. Constantinides, Meletios Dimopoulos
Manuscript Writing: Charalampos Deliveliotis, Flora Zagouri, Aristotle Bamias, Bernard Escudier, Cora Sternberg, Athanassios Dellis, Bob Djavan, Kimon Tzannis, Loukas Kontovinis, Konstantinos Stravodimos, Athanassios Papatsoris, Dionysios Mitropoulos, Constantine A. Constantinides, Meletios Dimopoulos
Final Approval of Manuscript: Charalampos Deliveliotis, Flora Zagouri, Aristotle Bamias, Bernard Escudier, Cora Sternberg, Athanassios Dellis, Bob Djavan, Kimon Tzannis, Loukas Kontovinis, Konstantinos Stravodimos, Athanassios Papatsoris, Dionysios Mitropoulos, Constantine A. Constantinides, Meletios Dimopoulos
Disclosures
Aristotle Bamias: Pfizer (C/A), Novartis (H, RF); Bernard Escudier: Pfizer, Novartis, BMS, Exelixis, Roche (C/A); Cora Sternberg: Novartis, Pfizer, Bristol‐Myer Squibb, Ipsen, Nektar, AstraZeneca (H); Flora Zagouri: Novartis (H); Loukas Kontovinis: Novartis, Roche, Amgen (H), Novartis, Amgen, Roche, Boehringer (RF); Meletios Dimopoulos: Celgene, Novartis, Amgen, Janssen (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supplementary Information
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics 2013. CA Cancer J Clin 2013;63:11–30. [DOI] [PubMed] [Google Scholar]
- 2. Novara G, Ficarra V, Antonelli A et al. Validation of the 2009 TNM version in a large multi‐institutional cohort of patients treated for renal cell carcinoma: Are further improvements needed? Eur Urol 2010;58:588–595. [DOI] [PubMed] [Google Scholar]
- 3. Leibovich BC, Blute ML, Cheville JC et al. Nephron sparing surgery for appropriately selected renal cell carcinoma between 4 and 7 cm results in outcome similar to radical nephrectomy. J Urol 2004;17:1066–1070. [DOI] [PubMed] [Google Scholar]
- 4. Fujioka T, Obara W; Committee for Establishment of the Clinical Practice Guideline for the Management of Renal Cell Carcinoma and the Japanese Urological Association. Evidence‐based clinical practice guideline for renal cell carcinoma: The Japanese Urological Association 2011 update. Int J Urol 2012;19:496–503. [DOI] [PubMed] [Google Scholar]
- 5. Bazarbashi S, Alkhateeb S, Abusamra A et al. Saudi Oncology Society and Saudi Urology Association combined clinical management guidelines for renal cell carcinoma. Urol Ann 2014;6:286–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bellmunt J, Puente J, Garcia de Muro J et al. SEOM clinical guidelines for the treatment of renal cell carcinoma. Clin Transl Oncol 2014;16:1043–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ljungberg B, Bensalah K, Bex A et al. Guidelines on renal cell carcinoma. European Association of Urology 2014. Available at https://uroweb.org/wp-content/uploads/10-Renal-Cell-Carcinoma_LR.pdf. Accessed March 23, 2017.
- 8. Campbell SC, Novick AC, Belldegrun A et al; Practice Guidelines Committee of the American Urological Association. Guideline for management of the clinical T1 renal mass. J Urol 2009;182:1271–1279. [DOI] [PubMed] [Google Scholar]
- 9. Motzer RJ, Agarwal N, Beard C et al. NCCN clinical practice guidelines in oncology: Kidney cancer. J Natl Compr Canc Netw 2009;7:618–630. [DOI] [PubMed] [Google Scholar]
- 10. Escudier B, Porta C, Schmidinger M et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2014;25(suppl 3):iii49–iii56. [DOI] [PubMed] [Google Scholar]
- 11. Zekri J, Dreosti LM, Ghosn M et al. Multidisciplinary management of clear‐cell renal cell carcinoma in Africa and the Middle East: Current practice and recommendations for improvement. J Multidiscip Healthc 2015:8;335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reames BN, Krell RW, Ponto SN et al. Critical evaluation of oncology clinical practice guidelines. J Clin Oncol 2013;31:2563–2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Institute of Medicine. Clinical practice guidelines we can trust. Washington, DC: National Academies Press 2011:1–130. [PubMed]
- 14. Zagouri F, Peroukidis S, Tzannis K et al. Current clinical practice guidelines on chemotherapy and radiotherapy for the treatment of non‐metastatic muscle‐invasive urothelial cancer: A systematic review and critical evaluation by the Hellenic Genito‐Urinary Cancer Group (HGUCG). Crit Rev Oncol Hematol 2015;93:36–49. [DOI] [PubMed] [Google Scholar]
- 15. Liberati A, Altman DG, Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 2009;62:e1–e34. [DOI] [PubMed] [Google Scholar]
- 16. Margulis V, Master VA, Cost NG et al. International consultation on urologic diseases and the European Association of Urologyinternational consultation on locally advanced renal cell carcinoma. Eur Urol 2011;60:673–683. [DOI] [PubMed] [Google Scholar]
- 17. Tanaka M, Ono Y, Matsuda T et al. Guidelines for urological laparoscopic surgery. Int J Urol 2009;16:115–125. [DOI] [PubMed] [Google Scholar]
- 18. Ljungberg B, Bensalah K, Bex A et al. Guidelines on renal cell carcinoma. European Association of Urology 2015. Available at http://uroweb.org/wp-content/uploads/10-Renal-Cell-Carcinoma_LR1.pdf. Accessed March 23, 2017.
- 19. Powles T, Staehler M, Ljungberg B et al. Updated EAU guidelines for clear cell renal cancer patients who fail VEGF targeted therapy. Eur Urol 2016;69:4–6. [DOI] [PubMed] [Google Scholar]
- 20. Motzer RJ, Jonasch E, Agarwal N et al. Kidney cancer, version 2.2014. J Natl Compr Canc Netw 2014;12:175–182. [DOI] [PubMed] [Google Scholar]
- 21. Motzer RJ, Jonasch E, Agarwal N et al. Kidney cancer,vVersion 3.2015. J Natl Compr Canc Netw 2015;13:151–159. [DOI] [PubMed] [Google Scholar]
- 22. Crepel M, Jeldres C, Perrotte P et al. Nephron‐sparing surgery is equally effective to radical nephrectomy for T1BN0M0 renal cell carcinoma: A population‐based assessment. Urology 2010;75:271–275. [DOI] [PubMed] [Google Scholar]
- 23. Patard JJ, Shvarts O, Lam JS et al. Safety and efficacy of partial nephrectomy for all T1 tumors based on an international multicenter experience. J Urol 2004;171:2181–2185. [DOI] [PubMed] [Google Scholar]
- 24. Antonelli A, Ficarra V, Bertini R et al; members of the SATURN Project ‐ LUNA Foundation . Elective partial nephrectomy is equivalent to radical nephrectomy in patients with clinical T1 renal cell carcinoma: Results of a retrospective, comparative, multi‐institutional study. BJU Int 2012;109:1013–1018. [DOI] [PubMed] [Google Scholar]
- 25. Badalato GM, Kates M, Wisnivesky JP et al. Survival after partial and radical nephrectomy for the treatment of stage T1bN0M0 renal cell carcinoma (RCC) in the USA: A propensity scoring approach. BJU Int 2012;109:1457–1462. [DOI] [PubMed] [Google Scholar]
- 26. Van Poppel H, Da Pozzo L, Albrecht W et al. A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron‐sparing surgery and radical nephrectomy for low‐stage renal cell carcinoma. Eur Urol 2011;59:543–552. [DOI] [PubMed] [Google Scholar]
- 27. Blom JH, van Poppel H, Maréchal JM et al; EORTC Genitourinary Tract Cancer Group . Radical nephrectomy with and without lymph‐node dissection: Final results of European Organization for Research and Treatment of Cancer (EORTC) randomized phase 3 trial 30881. Eur Urol 2009;55:28–34. [DOI] [PubMed] [Google Scholar]
- 28. Pantuck AJ, Zisman A, Dorey F et al. Renal cell carcinoma with retroperitoneal lymph nodes: Role of lymph node dissection. J Urol 2003;169:2076–2083. [DOI] [PubMed] [Google Scholar]
- 29. Canfield SE, Kamat AM, Sanchez‐Ortiz RF et al. Renal cell carcinoma with nodal metastases in the absence of distance disease (clinical stage TxN1‐2M0): The impact of aggressive surgical resection on patient outcome. J Urol 2006;175: 864–869. [DOI] [PubMed] [Google Scholar]
- 30. Karakiewicz PI, Trinh Q‐D, Bhojani N et al. Renal cell carcinoma with nodal metastases in the absence of distant disease: Prognostic indicators of disease‐specific survival. Eur Urol 2007;51:1616–1624. [DOI] [PubMed] [Google Scholar]
- 31. Whitson JM, Harris CR, Reese AC et al. Lymphadenectomy improves survival of patients with renal cell carcinoma and nodal metastases. J Urol 2011;181:1615–1620. [DOI] [PubMed] [Google Scholar]
- 32. Aitchison M, Bray CA, Van Poppel H et al. Adjuvant 5‐flurouracil, alpha‐interferon and interleukin‐2 versus observation in patients at high risk of recurrence after nephrectomy for renal cell carcinoma: Results of a phase III randomised European Organisation for Research and Treatment of Cancer (Genito‐Urinary Cancers Group)/National Cancer Research Institute trial. Eur J Cancer 2014;50:70–77. [DOI] [PubMed] [Google Scholar]
- 33. Haas NB, Manola J, Uzzo RG et al. Adjuvant sunitinib or sorafenib for high‐risk, non‐metastatic renal‐cell carcinoma (ECOG‐ACRIN E2805): A double‐blind, placebo‐controlled, randomised, phase 3 trial. Lancet 2016;387:2008–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ravaud A, Motzer RJ, Pandha HS et al. Adjuvant sunitinib in high‐risk renal‐cell carcinoma after nephrectomy. N Engl J Med 2016;375:2246–2254. [DOI] [PubMed] [Google Scholar]
- 35. Motzer RJ, Hutson TE, Tomczak P et al. Sunitinib versus interferon alfa in metastatic renal‐cell carcinoma. N Engl J Med 2007;356:115–24. [DOI] [PubMed] [Google Scholar]
- 36. Escudier B, Pluzanska A, Koralewski P et al. Bevacizumab plus interferon alfa‐2a for treatment of metastatic renal cell carcinoma: A randomised, double‐blind phase III trial. Lancet 2007;370:2103–2111. [DOI] [PubMed] [Google Scholar]
- 37. Rini BI, Halabi S, Rosenberg JE et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol 2008;26:5422–5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hudes G, Carducci M, Tomczak P et al. Temsirolimus, interferon alfa, or both for advanced renal‐cell carcinoma. N Engl J Med 2007;356:2271–2281. [DOI] [PubMed] [Google Scholar]
- 39. Motzer RJ, Barrios CH, Kim TM et al. phase II randomized trial comparing sequential first‐line everolimus and second‐line sunitinib versus first‐line sunitinib and second‐line everolimus in patients with metastatic renal cell carcinoma. J Clin Oncol 2014;32:2765–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hutson TE, Lesovoy V, Al‐Shukri S et al. Axitinib versus sorafenib as first‐line therapy in patients with metastatic renal‐cell carcinoma: A randomized open‐label phase 3 trial. Lancet Oncol 2013;14:1287–1294. [DOI] [PubMed] [Google Scholar]
- 41. Rini BI, Melichar B, Ueda T et al. Axitinib with or without dose titration for first‐line metastatic renal‐cell carcinoma: A randomised double‐blind phase 2 trial. Lancet Oncol 2013;14:1233–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sternberg CN, Hawkins RE, Wagstaff J et al. A randomised, double‐blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: Final overall survival results and safety update. Eur J Cancer 2013;49:1287–1296. [DOI] [PubMed] [Google Scholar]
- 43. Hess G, Borker R, Fonseca E. Treatment patterns: Targeted therapies indicated for first‐line management of metastatic renal cell carcinoma in a real‐world setting. Clin Genitourin Cancer 2013;11:161–167. [DOI] [PubMed] [Google Scholar]
- 44. Ko JJ, Choueiri TK, Rini BI et al. First‐, second‐, third‐line therapy for mRCC: Benchmarks for trial design from the IMDC. Br J Cancer 2014;110:1917–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Choueiri TK, S. Halabi S, Sanford B et al. Cabozantinib versus sunitinib (CABOSUN) as initial targeted therapy for patients with metastatic renal cell carcinoma (mRCC) of poor and intermediate risk groups: The Alliance A031203 CABOSUN trial. 2017;35:591–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Escudier B, Albiges L, Blesius A et al. How to select targeted therapy in renal cell cancer. Ann Oncol 2010;21(suppl 7):vii59–vii62. [DOI] [PubMed] [Google Scholar]
- 47. Albiges L, Oudard S, Negrier S et al. Complete remission with tyrosine kinase inhibitors in renal cell carcinoma. J Clin Oncol 2012;30:482–487. [DOI] [PubMed] [Google Scholar]
- 48. Motzer RJ, Escudier B, Oudard S et al. Efficacy of everolimus in advanced renal cell carcinoma: A double‐blind, randomised, placebo‐controlled phase III trial. Lancet 2008;372:449–456. [DOI] [PubMed] [Google Scholar]
- 49. Rini BI, Escudier B, Tomczak P et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial. Lancet. 2011;378:1931–1939. [DOI] [PubMed] [Google Scholar]
- 50. Heng DY, Signorovitch J, Swallow E et al. Comparative effectiveness of second‐line targeted therapies for metastatic renal cell carcinoma: A systematic review and meta‐analysis of real‐world observational studies. PLoS One 2014;9:e114264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Elaidi R, Harbaoui A, Beuselinck B et al. Outcomes from second‐line therapy in long‐term responders to first‐line tyrosine kinase inhibitor in clear‐cell metastatic renal cell carcinoma. Ann Oncol 2015;26:378–385. [DOI] [PubMed] [Google Scholar]
- 52. Motzer RJ, Escudier B, McDermott DF et al. Nivolumab versus everolimus in advanced renal‐cell carcinoma. N Engl J Med. 2015;373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Choueiri TK, Escudier B, Powles T et al; METEOR Investigators. Cabozantinib versus everolimus in advanced renal cell carcinoma (METEOR): Final results from a randomised, open‐label, phase 3 trial. Lancet Oncol 2016;17:917–927. [DOI] [PubMed] [Google Scholar]
- 54. Escudier B, Porta C, Schmidinger M et al. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol 2016;27(suppl 5):v58–v68. [DOI] [PubMed] [Google Scholar]
- 55. Motzer RJ, Hutson TE, Glen H et al. Lenvatinib, everolimus, and the combination in patients with metastatic renal cell carcinoma: A randomised, phase 2, open‐label, multicentre trial. Lancet Oncol 2015;16:1473–1482. [DOI] [PubMed] [Google Scholar]
- 56.NCCN guidelines for kidney cancer. Version 2.2017, October 31, 2016. Available at www.NCCN.org. Accessed March 27, 2017.
- 57. Motzer RJ, Porta C, Vogelzang, NJ et al. Dovitinib versus sorafenib for third‐line targeted treatment of patients with metastatic renal cell carcinoma: An open‐label, randomised phase 3 trial. Lancet Oncol 2014;15:286–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zini L, Perrotte P, Jeldres C et al. A population‐based comparison of survival after nephrectomy vs nonsurgical management for small renal masses. BJU Int 2009;103:899–904. [DOI] [PubMed] [Google Scholar]
- 59. Young EE, Castle SM, Gorbatiy V et al. Comparison of safety, renal function outcomes and efficacy of laparoscopic and percutaneous radio frequency ablation of renal masses. J Urol 2012;187:1177–1182. [DOI] [PubMed] [Google Scholar]
- 60. Olweny EO, Park SK, Tan YK et al. Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: Comparable oncologic outcomes at a minimum of 5 years of follow‐up. Eur Urol 2012;61:1156–1161. [DOI] [PubMed] [Google Scholar]
- 61. Stern JM, Gupta A, Raman JD et al. Radiofrequency ablation of small renal cortical tumours in healthy adults: Renal function preservation and intermediate oncological outcome. BJU Int 2009;104:786–789. [DOI] [PubMed] [Google Scholar]
- 62. Davenport MS, Caoili EM, Cohan RH et al. MRI and CT characteristics of successfully ablated renal masses: Imaging surveillance after radiofrequency ablation. AJR Am J Roentgenol 2009;192:1571–1578. [DOI] [PubMed] [Google Scholar]
- 63. Nisbet AA, Rieder JM, Tran VQ et al. Decision tree for laparoscopic partial nephrectomy versus laparoscopic renal cryoablation for small renal masses. J Endourol 2009;23:431–437. [DOI] [PubMed] [Google Scholar]
- 64. Berger A, Kamoi K, Gill IS et al. Cryoablation for renal tumors: Current status. Curr Opin Urol 2009;19:138–142. [DOI] [PubMed] [Google Scholar]
- 65. Kutikov A, Kunkle DA, Uzzo RG. Focal therapy for kidney cancer: A systematic review. Curr Opin Urol 2009;19:148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Desai MM, Strzempkowski B, Matin SF et al. Prospective randomized comparison of transperitoneal versus retroperitoneal laparoscopic radical nephrectomy. J Urol 2005;173:38–41. [DOI] [PubMed] [Google Scholar]
- 67. Nambirajan T, Jeschke S, Al‐Zahrani H et al. Prospective, randomizedcontrolled study: transperitoneal laparoscopic versus retroperitoneoscopic radical nephrectomy. Urology 2004;64:919–924. [DOI] [PubMed] [Google Scholar]
- 68. Nadler RB, Loeb S, Clemens JQ et al. A prospective study of laparoscopic radical nephrectomy for T1 tumors—Is transperitoneal, retroperitoneal or hand assisted the best approach? J Urol 2006;175:1230–1233. [DOI] [PubMed] [Google Scholar]
- 69. Dunn MD, Portis AJ, Shalhav AL et al. Laparoscopic versus open radical nephrectomy: A 9‐year experience. J Urol 2000;164:1153–1159. [PubMed] [Google Scholar]
- 70. Portis AJ, Yan Y, Landman J et al. Long‐term followup after laparoscopic radical nephrectomy. J Urol 2002;167:1257–1262. [PubMed] [Google Scholar]
- 71. Fenn NJ, Gill IS. The expanding indications for laparoscopic radical nephrectomy. BJU Int 2004;94:761–765. [DOI] [PubMed] [Google Scholar]
- 72. Lee SE, Ku JH, Kwak C et al. Hand assisted laparoscopic radical nephrectomy: comparison with open radical nephrectomy. J Urol 2003;170:756–759. [DOI] [PubMed] [Google Scholar]
- 73. Marszalek M, Meixl H, Polajnar M et al. Laparoscopic and open partial nephrectomy: A matched‐pair comparison of 200 Patients. Eur Urol 2009;55:1171–1178. [DOI] [PubMed] [Google Scholar]
- 74. Lane BR, Gill IS. 7‐year oncological outcomes after laparoscopic and open partial nephrectomy. J Urol 2010;183:473–479. [DOI] [PubMed] [Google Scholar]
- 75. Masson‐Lecomte A, Bensalah K, Seringe E et al. A prospective comparison of surgical and pathological outcomes obtained after robot‐assisted or pure laparoscopic partial nephrectomy in moderate to complex renal tumours: Results from a French multicentre collaborative study. BJU Int 2013;111:256–263. [DOI] [PubMed] [Google Scholar]
- 76. Aboumarzouk OM, Stein RJ, Eyraud R et al. Robotic versus laparoscopic partial nephrectomy: A systematic review and meta‐analysis. Eur Urol 2012;62:1023–1033. [DOI] [PubMed] [Google Scholar]
- 77. Bi L, Zhang C, Li K et al. Robotic partial nephrectomy for renal tumors larger than 4 cm: A systematic review and meta‐analysis. PLoS One 2013;8:e75050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lane BR, Tiong HY, Campbell SC et al. Management of the adrenal gland during partial nephrectomy. J Urol 2009;181:2430–2436. [DOI] [PubMed] [Google Scholar]
- 79. Karakiewicz PI, Suardi N, Jeldres C et al. Neoadjuvant sutent induction therapy may effectively down‐stage renal cell carcinoma atrial thrombi. Eur Urol 2008;53:845–848. [DOI] [PubMed] [Google Scholar]
- 80. Di Silverio F, Sciarra A, Parente U et al. Neoadjuvant therapy with sorafenib in advanced renal cell carcinoma with vena cava extension submitted to radical nephrectomy. Urol Int 2008;80:451–453. [DOI] [PubMed] [Google Scholar]
- 81. Shuch B, Riggs SB, LaRochelle JC et al. Neoadjuvant targeted therapy and advanced kidney cancer: Observations and implications for a new treatment paradigm. BJU Int 2008;102:692–696. [DOI] [PubMed] [Google Scholar]
- 82. Alt AL, Boorjian SA, Lohse CM et al. Survival after complete surgical resection of multiple metastases from renal cell carcinoma. Cancer 2011;117:2873–2882. [DOI] [PubMed] [Google Scholar]
- 83. Kwak C, Park YH, Jeong CW et al. Metastasectomy without systemic therapy in metastatic renal cell carcinoma: Comparison with conservative treatment. Urol Int 2007;79:145–151. [DOI] [PubMed] [Google Scholar]
- 84. Heng DY, Wells JC, Rini BI et al. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol 2014;66:704–710. [DOI] [PubMed] [Google Scholar]
- 85. Choueiri TK, Xie W, Kollmannsberger C et al. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol 2011;185:60–66. [DOI] [PubMed] [Google Scholar]
- 86. Bamias A, Tzannis K, Papatsoris A et al. Prognostic significance of cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma treated with first‐line sunitinib: A European multiinstitutional study. Clin Genitourin Cancer 2014;12:373–378 [DOI] [PubMed] [Google Scholar]
- 87. Dabestani S, Marconi L, Hofmann F et al. Local treatments for metastases of renal cell carcinoma: A systematic review. Lancet Oncol 2014;15:e549–e561. [DOI] [PubMed] [Google Scholar]
- 88. Bellmunt J, Dutcher J. Targeted therapies and the treatment of non‐clear cell renal cell carcinoma. Ann Oncol 2013;24:1730–1740. [DOI] [PubMed] [Google Scholar]
- 89. Vera‐Badillo FE, Templeton AJ, Duran I et al. systemic therapy for non‐clear cell renal cell carcinomas: A systematic review and meta‐analysis. Eur Urol 2015;67:740–988. [DOI] [PubMed] [Google Scholar]
- 90. Armstrong AJ, Halabi S, Eisen T et al. Everolimus versus sunitinib for patients with metastatic non‐clear cell renal cell carcinoma (ASPEN): A multicentre, open‐label, randomised phase 2 trial. Lancet Oncol 2016;17:378–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Tannir NM, Jonasch E, Albiges L et al. Everolimus versus sunitinib prospective evaluation in metastatic non‐clear cell renal cell carcinoma (ESPN): A randomized multicenter phase 2 trial. Eur Urol 2016;69:866–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Randall MJ, Millard F, Kurzrock R. Molecular aberrations, targeted therapy, and renal cell carcinoma: Current state‐of‐the‐art. Cancer Metastasis Rev 2014;33:1109–1124. [DOI] [PubMed] [Google Scholar]
- 93.Cancer Genome Atlas Research Network . Comprehensive molecular characterization of papillary renal‐cell carcinoma. N Engl J Med 2016;374:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Templeton AJ, Knox JJ, Lin X et al. Change in neutrophil‐to‐lymphocyte ratio in response to targeted therapy for metastatic renal cell carcinoma as a prognosticator and biomarker of efficacy. Eur Urol 2016;70:358–364. [DOI] [PubMed] [Google Scholar]
- 95. Kwiatkowski DJ, Choueiri TK, Fay AP et al. Mutations in TSC1, TSC2, and MTOR are associated with response to rapalogs in patients with metastatic renal cell carcinoma. Clin Cancer Res 2016;22:2445–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kung J, Miller RR, Mackowiak PA. Failure of clinical practice guidelines to meet institute of medicine standards: Two more decades of little, if any, progress. Arch Intern Med 2012;172:1628–1633. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.