Neutrophil‐lymphocyte ratio is a measure of systemic inflammation that appears prognostic in localized and advanced non‐small cell lung cancer. Increased systemic inflammation portends a poorer prognosis in cancer patients. This study explores whether low neutrophil‐lymphocyte ratio at diagnosis is associated with improved overall survival in patients with locally advanced non‐small cell lung cancer.
Keywords: Neutrophil‐lymphocyte ratio, Non‐small cell lung cancer, Locally advanced, Prognosis
Abstract
Background.
Neutrophil‐lymphocyte ratio (NLR) is a measure of systemic inflammation that appears prognostic in localized and advanced non‐small cell lung cancer (NSCLC). Increased systemic inflammation portends a poorer prognosis in cancer patients. We hypothesized that low NLR at diagnosis is associated with improved overall survival (OS) in locally advanced NSCLC (LANSCLC) patients.
Patients and Methods.
Records from 276 patients with stage IIIA and IIIB NSCLC treated with definitive chemoradiation with or without surgery between 2000 and 2010 with adequate data were retrospectively reviewed. Baseline demographic data and pretreatment peripheral blood absolute neutrophil and lymphocyte counts were collected. Patients were grouped into quartiles based on NLR. OS was estimated using the Kaplan‐Meier method. The log‐rank test was used to compare mortality between groups. A linear test‐for‐trend was used for the NLR quartile groups. The Cox proportional hazards model was used for multivariable analysis.
Results.
The NLR was prognostic for OS (p < .0001). Median survival in months (95% confidence interval) for the first, second, third, and fourth quartile groups of the population distribution of NLR were 27 (19–36), 28 (22–34), 22 (12–31), and 10 (8–12), respectively. NLR remained prognostic for OS after adjusting for race, sex, stage, performance status, and chemoradiotherapy approach (p = .004).
Conclusion.
To our knowledge, our series is the largest to demonstrate that baseline NLR is a significant prognostic indicator in LANSCLC patients who received definitive chemoradiation with or without surgery. As an indicator of inflammatory response, it should be explored as a potential predictive marker in the context of immunotherapy and radiation therapy.
Implications for Practice.
Neutrophil‐lymphocyte ratio measured at the time of diagnosis was associated with improved overall survival in 276 patients with stage IIIA and IIIB non‐small cell lung cancer (NSCLC) treated with definitive chemoradiation with or without surgery. To our knowledge, our series is the largest to demonstrate that baseline neutrophil‐lymphocyte ratio is a significant prognostic indicator in locally advanced NSCLC patients who received definitive chemoradiation with or without surgery. Neutrophil‐lymphocyte ratio is an inexpensive biomarker that may be easily utilized by clinicians at the time of locally advanced NSCLC diagnosis to help predict life expectancy.
摘要
背景. 中性粒细胞‐淋巴细胞比值(NLR)是全身炎症的衡量指标, 而后者在局部晚期非小细胞肺癌(NSCLC)中具有预后作用。癌症患者全身炎症增加意味着预后较差。我们假设局部晚期NSCLC(LANSCLC)患者诊断时NLR较低与总生存期(OS)改善相关。
患者和方法. 我们回顾性分析了2000年至2010年期间接受确定性放化疗联合或不联合手术治疗且具有充分数据的276例IIIA期和IIIB期NSCLC患者的记录。采集基线人口统计学数据和治疗前外周血中性粒细胞和淋巴细胞绝对计数。根据NLR四分位数对患者进行分组。采用Kaplan‐Meier法估计OS。采用时序检验比较各组的死亡率。采用线性趋势检验分析各个NLR四分位数组。使用Cox比例风险模型进行多变量分析。
结果. NLR是OS的预后指标(p<0.0001)。NLR人群分布的第一、二、三和四四分位数组的中位生存期(95%置信区间)分别为27(19‐36)、28(22‐34)、22(12‐31)和10(8‐12)个月。校正人种、性别、分期、体力状态和放化疗方法后, NLR仍然是OS的预后指标(p=0.004)。
结论. 据我们所知, 本研究是规模最大的证明基线NLR是接受过确定性放化疗联合或不联合手术治疗的LANSCLC患者的显著预后指标的研究。NLR是炎症反应的指标, 应将其作为免疫治疗和放射治疗的潜在预测标志物进行探索。The Oncologist 2017;22:737–742
对临床实践的提示:在276例接受过确定性放化疗联合或不联合手术治疗的IIIA期和IIIB期NSCLC患者中, 诊断时测定的中性粒细胞‐淋巴细胞比值与总生存期改善相关。据我们所知, 本研究是规模最大的证明基线中性粒细胞‐淋巴细胞比值是接受过确定性放化疗联合或不联合手术治疗的局部晚期NSCLC患者的显著预后指标的研究。临床医生在诊断局部晚期NSCLC时可使用中性粒细胞‐淋巴细胞比值作为生物标志物, 该方法成本低廉且简单易行, 可帮助预测预期寿命。
Introduction
It is widely recognized that inflammation plays an integral role in both the development and propagation of various cancers, including lung carcinoma [1–3]. The human immune system functions to maintain tissue homeostasis by destroying pathogens and eliminating damaged cells. It is believed that chronic inflammation causes dysregulation of the innate and adaptive immune system, leading to tissue injury, loss of tissue architecture, and eventual malignant transformation [1], [2]. Chronic inflammation also leads to the release of proinflammatory and immunomodulatory mediators, such as cytokines and chemokines, which attract leukocytes and create a microenvironment favorable to the development and progression of tumors [1], [3]. Neutrophils and other myeloid cells take part in the inflammatory response. Activated neutrophils stimulated by tumor necrosis factor‐alpha release pro‐angiogenic factors, such as vascular endothelial growth factor and pro‐angiogenic chemokines CXCL8 and CXCL1, in vitro and, thus, may stimulate tumor angiogenesis in vivo [4]. In vitro studies have also suggested that direct interactions between neutrophils and tumor cells cause the release of inflammatory mediators, which may promote tumor growth specifically in non‐small cell lung cancer (NSCLC) [5].
Increased systemic inflammation portends a poorer prognosis in cancer patients. Neutrophil‐lymphocyte ratio (NLR), calculated as the absolute neutrophil count (ANC) divided by the absolute lymphocyte count (ALC) within the peripheral blood, has been shown to correlate with prognosis in various malignancies, including pancreatic, breast, prostate, and lung cancers [6], [7], [8], [9], [10].
Neutrophil‐lymphocyte ratio has been evaluated in both localized and advanced NSCLC and appears prognostic in these patient populations [10], [11], [12]. Takahashi et al. [10] demonstrated that a preoperative NLR ≥2.5 in stage I NSCLC patients treated with complete resection was associated with statistically significantly decreased 5‐year overall survival (OS). Cedrés et al. [11] found that a pretreatment NLR ≥5 was associated with decreased OS in patients with stage IV NSCLC. NLR was utilized as a component of another inflammation‐based prognostic score, the advanced lung cancer inflammation index (ALI), which was also found to be an independent predictor of survival in patients with metastatic NSCLC [13].
Locally advanced NSCLC (LANSCLC, stage IIIA/B, American Joint Committee on Cancer (AJCC) Cancer Staging Manual, 7th edition, https://cancerstaging.org/references-tools/Pages/What-is-Cancer-Staging.aspx) is a common subset of NSCLC. Prior studies that evaluated the NLR in LANSCLC have been limited by small patient sample sizes and mostly pathologic stage III disease discovered after resection, precluding any real conclusions regarding the prognostic value of the NLR in this subset. In this study, we hypothesized that a low NLR measured at the time of diagnosis is associated with improved OS in patients with locally advanced (stage IIIA and IIIB) non‐small cell lung cancer.
Methods
Three hundred and eleven patients diagnosed with stage III NSCLC at the University of Maryland Greenebaum Cancer Center between January 2000 and December 2010 were retrospectively reviewed. All patients had biopsy‐proven NSCLC and presented with clinical stage IIIA or IIIB disease based on the AJCC 7th edition tumor‐node‐metastasis (TNM) classification and staging system. Baseline patient demographic data, including age, race, sex, Eastern Cooperative Oncology Group (ECOG) performance status, and tobacco use at the time of diagnosis were recorded. Patients with incomplete medical information were excluded, resulting in a total of 276 patients included in our analysis. Pretreatment ANC and pretreatment ALC from the peripheral blood were also collected. All ANC and ALC values were obtained between the time of cancer diagnosis and treatment initiation. The lab values closest to the time of treatment initiation were used. A pretreatment NLR was calculated for each patient, defined as the ANC divided by the ALC.
After diagnosis, each patient was assessed by a multidisciplinary team including a medical oncologist, radiation oncologist, and thoracic surgeon. All patients underwent standard workup, which included systemic imaging including positron emission tomography (PET), PET/CT (computerized tomography), CT, and/or bone scan, brain imaging consisting of magnetic resonance imaging (MRI) or CT with contrast, and routine blood work prior to treatment. Patients had documentation of mediastinal disease by cervical mediastinosopy, biopsy by endoscopic ultrasound, or PET. All patients were treated with definitive chemoradiation therapy with or without surgery. Radiation was most commonly administered with concurrent chemotherapy. Patients who were deemed potential trimodality treatment candidates (chemoradiotherapy and surgical resection) prior to treatment initiation either underwent surgery upfront followed by chemoradiotherapy or received concurrent chemoradiotherapy and were reassessed after chemoradiotherapy for surgical resection. Those potential trimodality treatment candidates who received upfront chemoradiation and experienced pathologic mediastinal clearance of disease underwent surgical resection if they were still considered surgical candidates; they subsequently received consolidative chemotherapy if clinically appropriate. Additional chemotherapy was also recommended to those with adequate performance status who did not have mediastinal lymph node clearance and did not have progressive disease. After completion of therapy, patients were followed with physical examination and CT of the chest every 3–4 months for the first 2–3 years, followed by every 4–6 months for another year, and annually thereafter.
Overall survival time was defined as the time from date of diagnosis to the date of death from any cause. Patients who were alive at the date of last contact were analyzed as censored on that date. OS was estimated using the Kaplan‐Meier method and the Mantel‐Cox log‐rank test was used to compare OS between groups. A log‐rank linear test‐for‐trend was used for comparing OS in the NLR quartile groups. The Cox proportional hazards model was used to adjust for other prognostic factors in a multivariable analysis. Distributions of NLR in subgroups were compared using the Mann‐Whitney U test for two subgroups and the Kruskal‐Wallis test for more than two subgroups. All p values are two‐tailed and statistical significance was called for p < .05.
Results
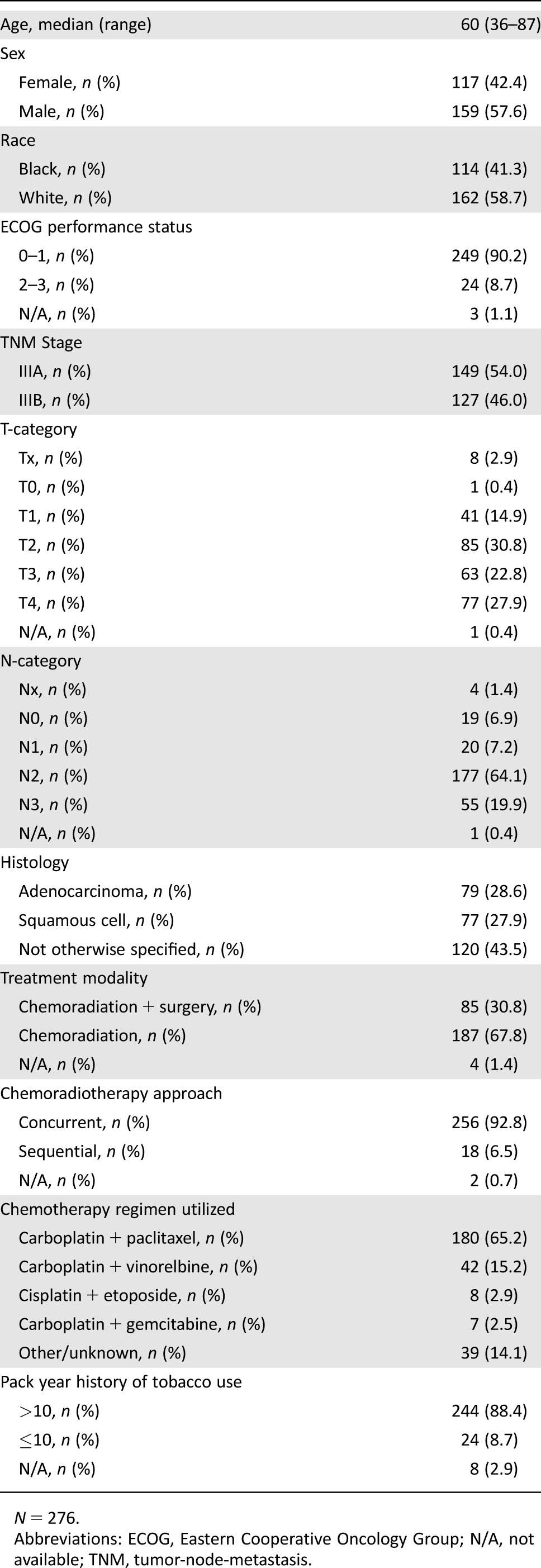
A total of 276 patients with adequate medical information were included in the final analysis. The baseline patient characteristics are shown in Table 1. Median age was 60 (range from 36–87 years). The majority of patients were male (57.6%), white (58.7%), had a performance status of 0–1 (90.2%), and had stage IIIA disease (54.0%). The most common histologic subtype was adenocarcinoma, followed by squamous cell carcinoma. Pretreatment laboratory evaluation revealed a median total white blood cell count of 8.1 (10th and 90th percentile, 5.2 and 13.8), median ANC of 5.5 (10th and 90th percentile, 3.2 and 10.2), and median ALC of 1.7 (10th and 90th percentile, 0.7 and 3.0). Median NLR was 3.3 (10th and 90th percentile, 1.6 and 9.3).
Table 1. Baseline patient characteristics.
N = 276.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; N/A, not available; TNM, tumor‐node‐metastasis.
All patients were treated with chemoradiation therapy with or without surgery. Of the included patients, 30.8% underwent surgical resection. Of the 85 patients who underwent surgery, 3 patients received surgery before receiving chemotherapy and radiation, and 82 patients received chemoradiotherapy followed by surgical resection. Definitive doses of radiation therapy were administered to each patient, with a median dose of 64.8 Gy (10th and 90th percentile, 57.1 Gy and 69.6 Gy). The majority of patients (92.8%) received chemotherapy administered concurrently with radiation therapy. A variety of chemotherapy regimens were employed, most commonly carboplatin (AUC = 2) and paclitaxel (45–50 mg/m2) (Table 1). A minority of patients received sequential chemotherapy followed by radiation therapy (6.5%). Among the 262 patients with complete data on the prescribed and delivered radiation doses, 94% of all patients received more than 95% of the prescribed dose of radiation therapy, and 93% of all patients received the full prescribed dose of radiation therapy (within ±0.01 Gy). At the time of our analysis, 223 patients were deceased and 53 patients were living. In living patients, the median follow‐up time was 4.7 years, ranging from 0.17 years to 14.6 years. Median OS was 21.5 months (95% confidence interval [CI] 17.6–25.4 months). Consolidation chemotherapy regimens differed depending on whether patients were enrolled in clinical trials and a variety of chemotherapy regimens were used. However, the most commonly used chemotherapy regimen for consolidation in either bimodality or trimodality therapy was carboplatin (AUC = 6) and paclitaxel (200 mg/m2).
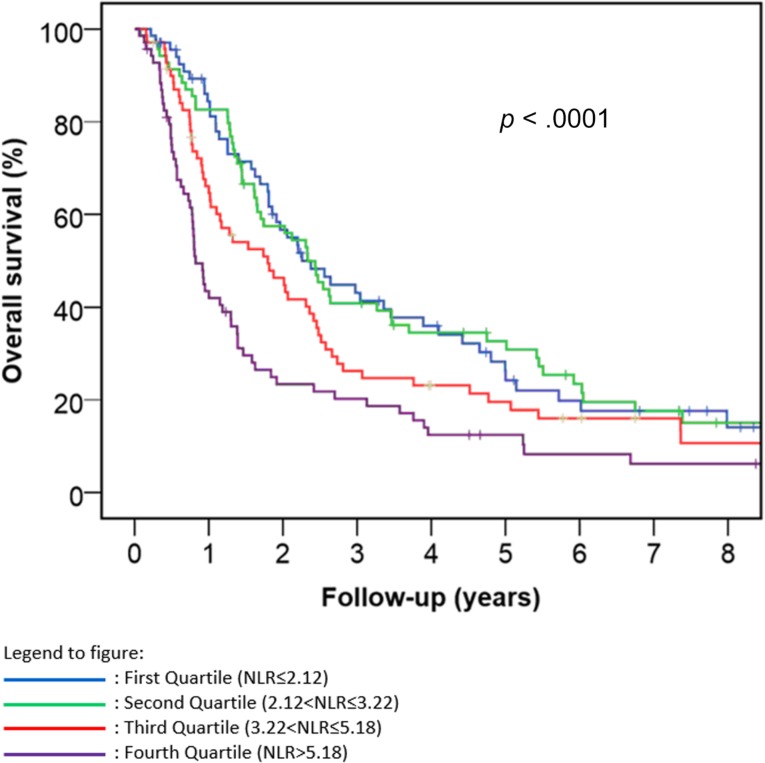
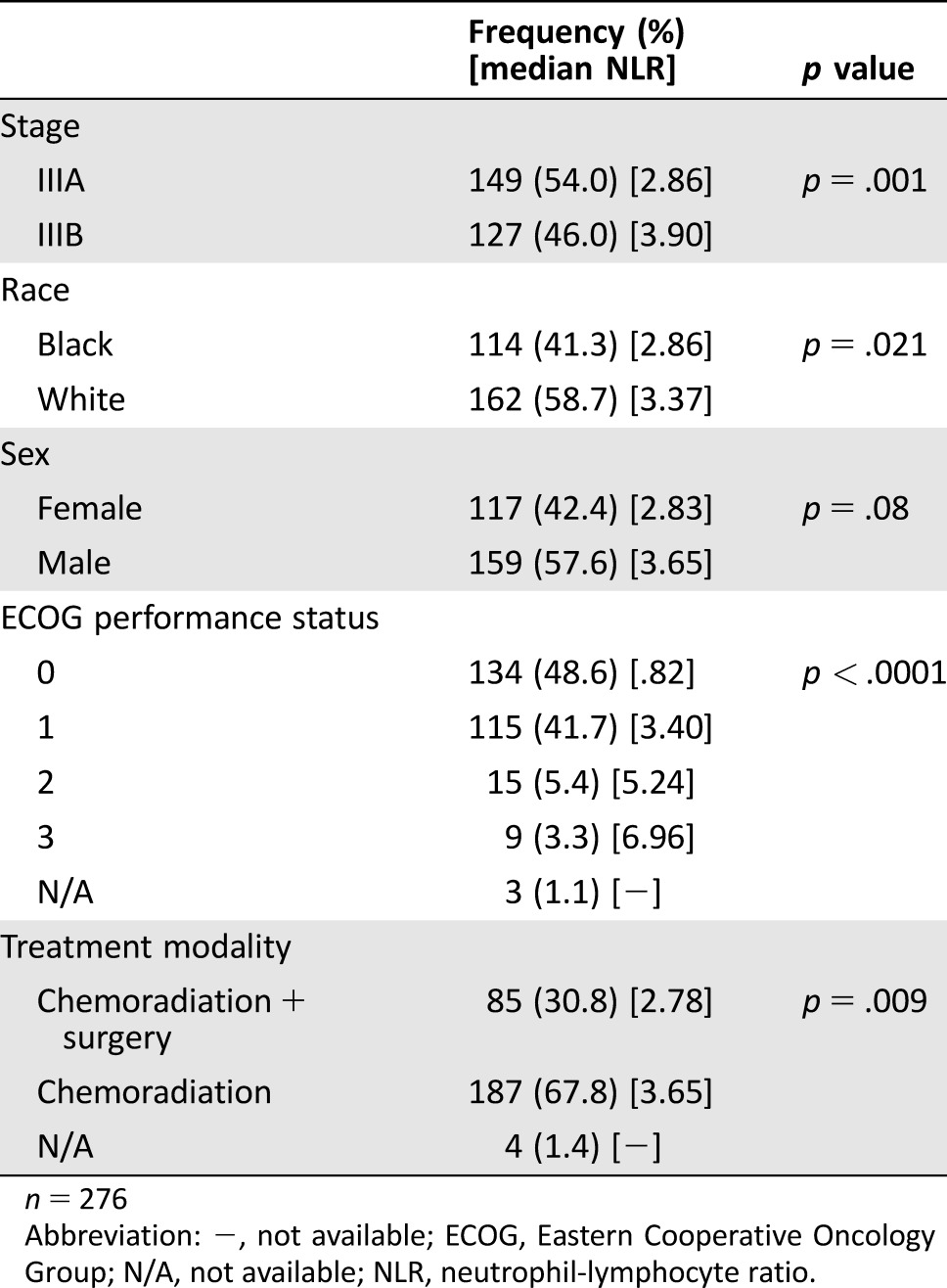
Neutrophil‐lymphocyte ratio was prognostic for OS (p < .0001) (Fig. 1). Median survival (95% CI) for the first, second, third, and fourth quartile groups of the population distribution of NLR were 27 (19–36) months, 28 (22–34) months, 22 (12–31) months and 10 (8–12) months, respectively. To facilitate comparison with reports in the literature, we also dichotomized the patient population using an NLR of 5 as the cut‐off point. Patients in our study with an NLR of <5 had a median OS of 26 months (95% CI 22–31 months), while patients with an NLR of ≥5 had a median OS of 11 months (95% CI 9–13 months; p < .00001). There was a statistically significant difference in NLR when comparing between stage IIIA and IIIB disease, black and white race, trimodality therapy and chemoradiation, and ECOG performance status. There was a trend toward lower NLR in female patients versus male patients; however, this did not reach statistical significance (p = .08; Table 2). In a multivariable Cox proportional hazards analysis stratified by whether the patients had surgery, NLR remained prognostic for OS (p = .004) after adjusting for race, sex, stage, ECOG performance status, and chemoradiotherapy approach (concurrent vs. sequential). Going from one quartile of the NLR distribution to the next quartile, the hazard ratio was 1.219 with a 95% CI of 1.065–1.395. Separate analyses of the prognostic effect of NLR in patients treated with or without surgery also showed a significant effect in both groups (p = .013 and p = .025, respectively).
Figure 1.
Kaplan‐Meier curve of overall survival stratified by neutrophil‐lymphocyte ratio quartile. Abbreviation: NLR, neutrophil‐lymphocyte ratio.
Table 2. Baseline patient characteristics and neutrophil‐lymphocyte ratio.
n = 276
Abbreviation: −, not available; ECOG, Eastern Cooperative Oncology Group; N/A, not available; NLR, neutrophil‐lymphocyte ratio.
Discussion
Use of prognostic biomarkers, such as the NLR, at the time of NSCLC diagnosis may provide clinicians with valuable information regarding a patient's predicted life expectancy. An elevated NLR correlates with a high degree of inflammation and reflects a state of neutrophilia and/or lymphopenia [14]. Immune cells within the tumor microenvironment itself also play a role in the inflammatory response, which can promote cancer growth. Neutrophils recruited into the tumor stroma exert pro‐tumorigenic effects by inhibiting apoptosis, promoting angiogenesis, and stimulating the formation of metastases [15], creating an inflammatory environment favorable for malignant growth. Nonregulatory CD4+ helper T cells and CD8+ cytotoxic T cells are known to play an important role in immunosurveillance. High densities of stromal CD4+ cells and stromal CD8+ T cells, as well as simultaneous localization of CD4+ and CD8+ T cells within the tumor stroma, have all been found to be favorable independent prognostic factors in patients with NSCLC [16], [17]; therefore, a relative lymphopenia may be reflective of decreased host defense mechanisms that allow for cancer development. A recent retrospective study found that an NLR >2.7 was significantly associated with decreased density of CD4+ immune cells in the tumor microenvironment of gastric cancer [18], although additional studies are needed to evaluate the correlation between NLR and immune cells within the tumor microenvironment in NSCLC patients.
In this study, we confirmed our hypothesis that low NLR measured at the time of diagnosis is associated with improved OS in patients with LANSCLC receiving multimodality therapy. NLR remained significantly prognostic even after adjusting for other variables including race, sex, stage, performance status, and chemoradiotherapy approach (concurrent vs. sequential). To our knowledge, our series is the largest to demonstrate that NLR is a prognostic indicator in NSCLC patients with locally advanced diseases treated with definitive chemoradiotherapy, with or without surgery. The majority of published studies evaluating NLR in NSCLC included no or only a small proportion of stage IIIA or IIIB patients in their analyses and did not evaluate patients who were receiving definitive chemoradiotherapy. The few studies that have included larger groups of patients with stage III disease were surgical series. Choi et al. [19] retrospectively evaluated the relationships between preoperative NLR and postoperative nonsteroidal anti‐inflammatory drug use on recurrence‐free survival and OS within a cohort of stage I–III NSCLC patients who underwent surgical resection. Among the 280 patients with World Health Organization tumor stage III disease included in their analysis, no survival impact was seen with high preoperative NLR (≥5). In this analysis, only 25% of the patients received adjuvant chemotherapy even though 47% of the surgically resected patients had stage II or III disease, the stages most likely to benefit from adjuvant chemotherapy [20], and this may have impacted these results. Furthermore, this analysis did not evaluate patients who received definitive chemoradiotherapy.
Zhang et al. [21] retrospectively evaluated preoperative NLR in patients with primary operable NSCLC and included 239 patients with pathological stage IIIA disease. Stage IIIA patients with low NLR (≤2.3) had significantly better OS (p < .001). Patients were excluded from this study if they had received preoperative chemotherapy or radiotherapy. The authors note that the main treatment among the study participants post‐surgically was chemotherapy or radiation, either alone or in combination, and routine NSCLC chemotherapy regimens were utilized; no additional details about the nonsurgical management were reported. Patients with stage IIIB disease were not included in the analysis; therefore, the study's results are not applicable to a large proportion of patients determined to have stage III disease at the time of lung cancer diagnosis.
A recently published meta‐analysis included 14 published studies with 3,656 patients and evaluated the association between NLR and OS and progression‐free survival (PFS) in NSCLC [15]. Patients with a high pretreatment NLR had inferior OS (HR 1.70; 95% CI 1.39–2.09) and PFS (HR 1.63; 95% CI 1.27–2.09). This meta‐analysis included patients with all stages of NSCLC and did not perform subgroup analyses specifically evaluating patients with locally advanced disease. Many previous studies have evaluated the NLR as a continuous variable to determine an appropriate cut‐off value that can be used to easily determine prognosis. In the meta‐analysis published by Gu et al. described above, the investigators performed a subgroup analysis that demonstrated that a cut‐off NLR of 5 was most consistently prognostic [15]. For comparison, we also analyzed our data utilizing a cut‐off point of 5. Patients in our study with an NLR of <5 had a median OS of 26 months, while patients with an NLR of ≥5 had a median OS of 11 months. This additional analysis provides further support to our hypothesis that low NLR at the time of diagnosis is associated with improved survival outcomes.
To date, the NLR has not been employed routinely in any disease setting. We believe that the current paper adds to the literature indicating that this is a routinely available test that may help with future studies.
Our study has some possible limitations. The data utilized in our study were collected from a single institution, which may affect the generalizability of our conclusions to other patient populations. Given its retrospective nature, certain aspects of our data collection could not be standardized; it was not possible for pretreatment blood tests to be collected from a defined baseline time point. However, all labs were taken from a pretreatment time point in close proximity to treatment initiation, and we would not expect large fluctuations in laboratory values prior to therapy initiation. In the absence of available banked blood samples, our NLR calculations were based on complete blood count measurements obtained from patients’ medical records, which may also reduce the standardization of this measurement. However, we believe that this actually increases the validity of our findings, as this indicates that such an evaluation can be done under standard clinical conditions.
We were also unable to control for tumor genotype in our study, given the retrospective nature of our analysis. The study's tumor samples were collected prior to the period when there was knowledge and/or availability of such testing (2000–2010). Our NLR calculations may have been confounded by other causes of increased neutrophil counts or inflammation, such as active infection or concomitant nonsteroidal anti‐inflammatory drug or steroid use, factors that are difficult to determine on retrospective review and warrant future prospective evaluation. Nevertheless, the association between NLR and OS in our study was highly significant.
Our study retrospectively evaluated patients diagnosed and treated with stage III NSCLC over a 10‐year period, which introduces the possibility that there were changes in assessment and care. The AJCC TNM classification and staging system was also updated over this time period. In order to standardize the patients included in our study, the AJCC seventh edition was used to determine IIIA or IIIB disease status. The actual technology available for staging (e.g., PET, MRI, CT, etc.) did not change markedly during this period and neither did the nature of chemotherapy or radiation technique. Our study also employed a cross‐sectional design to evaluate the NLR prior to cancer treatment initiation; a longitudinal study design with serial NLR measurements throughout the course of treatment may provide additional insight into how the inflammatory response changes over time and influences prognosis. But, in a retrospective design, laboratory tests are not necessarily performed at pre‐defined time points throughout therapy. Additionally, the prognostic value of the NLR has not yet been validated in a prospective independent cohort of stage III NSCLC patients treated with definitive chemoradiation. This would be a consideration in future study designs.
As an indicator of inflammatory response, NLR also has potential application in the context of immunomodulatory therapy and therapies that are known to impact the immune system, such as radiation therapy [22]. Exciting advancements in lung cancer research and pharmaceutical development have occurred with the development and approval of agents such as nivolumab and pembrolizumab for the treatment of advanced NSCLC [23], [24], [25]. Anti‐PD‐1/PD‐L1 therapies are currently under evaluation as consolidation therapy after first‐line chemoradiation therapy in NSCLC patients with stage IIIA and IIIB disease (e.g., clinical trials NCT02125461 and NCT02434081).
Currently, it is unknown whether NLR at the time of diagnosis is a predictive biomarker for response to immune checkpoint inhibitors in NSCLC. However, in a retrospective analysis of 187 patients with metastatic melanoma who received ipilimumab, pretreatment NLR <5 was associated with a significantly superior PFS (HR = .38; 95% CI 0.22–.66; p < .0006) and OS (HR = .24; 95% CI .13–.36; p < .0001 [26]). Further research is warranted to evaluate whether NLR can be used to predict responsiveness to immunotherapies or whether NLR is simply a prognostic biomarker irrespective of the nature of the treatment (chemotherapy, immunotherapy, surgery, or radiation).
Conclusion
In conclusion, our study demonstrates that baseline NLR is prognostic for OS in stage III NSCLC patients treated with definitive chemoradiation, with or without surgery. NLR is an inexpensive biomarker that may provide clinicians with valuable information regarding a patient's predicted life expectancy at the time of LANSCLC diagnosis. Despite our study's retrospective nature that limited some aspects of the data collection and analysis, our results provide support for future prospective studies examining the relationship between NLR and survival in stage III NSCLC patients receiving definitive chemoradiation.
Acknowledgements
Josephine Feliciano has funded this project using the Paul Calabresi K12 Scholar award. Preliminary results of this study were presented as a poster at the 16th World Conference on Lung Cancer (September 2015).
Author Contributions
Concept and design: Katherine A. Scilla, Soren M. Bentzen, Martin J. Edelman, Josephine L. Feliciano
Provision of study materials or patients: Pranshu Mohindra, Steven J. Feigenberg, Martin J. Edelman, Josephine L. Feliciano
Collection and/or assembly of data: Katherine A. Scilla, Vincent K. Lam, Elizabeth M. Nichols, Melissa A. Vyfhuis, Neha Bhooshan
Data analysis and interpretation: Katherine A. Scilla, Soren M. Bentzen, Pranshu Mohindra, Steven J. Feigenberg, Martin J. Edelman, Josephine L. Feliciano
Manuscript writing: Katherine A. Scilla, Soren M. Bentzen, Pranshu Mohindra, Melissa A. Vyfhuis, Steven J. Feigenberg, Martin J. Edelman, Josephine L. Feliciano
Final approval of manuscript: Katherine A. Scilla, Soren M. Bentzen, Vincent K. Lam, Pranshu Mohindra, Elizabeth M. Nichols, Melissa A. Vyfhuis, Neha Bhooshan, Steven J. Feigenberg, Martin J. Edelman, Josephine L. Feliciano
Disclosures
Steven J. Feigenberg: Maryland Industrial Partnerships Grant (RF, ET); Martin J. Edelman: Bristol‐Myers Squibb, Lilly Oncology, Merck, Ariad, Genentech (C/A, H), Bristol‐Myers Squibb, Lilly Oncology, Merck, Adaptimmune (RF), Biomarker Strategies (OI); Josephine L. Feliciano: Merck, Genentech (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Fernandes JV, Cobucci RN, Jatobá CA et al. The role of the mediators of inflammation in cancer development. Pathol Oncol Res 2015;21:527–534. [DOI] [PubMed] [Google Scholar]
- 2. Bremnes RM, Al‐Shibli K, Donnem T et al. The role of tumor‐infiltrating immune cells and chronic inflammation at the tumor site on cancer development, progression, and prognosis: Emphasis on non‐small cell lung cancer. J Thorac Oncol 2011;6:824–833. [DOI] [PubMed] [Google Scholar]
- 3. Coussens LM, Werb Z. Inflammation and cancer. Nature 2002;420:860–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Murdoch C, Muthana M, Coffelt SB et al. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer 2008;8:618–631. [DOI] [PubMed] [Google Scholar]
- 5. Hattar K, Franz K, Ludwig M et al. Interactions between neutrophils and non‐small cell lung cancer cells: Enhancement of tumor proliferation and inflammatory mediator synthesis. Cancer Immunol Immunother 2014;63:1297–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Templeton AJ, McNamara MG, Šeruga B et al. Prognostic role of neutrophil‐to‐lymphocyte ratio in solid tumors: A systematic review and meta‐analysis. J Natl Cancer Inst 2014;106:dju124. [DOI] [PubMed] [Google Scholar]
- 7. Luo G, Guo M, Liu Z et al. Blood neutrophil‐lymphocyte ratio predicts survival in patients with advanced pancreatic cancer treated with chemotherapy. Ann Surg Oncol 2015;22:670–676. [DOI] [PubMed] [Google Scholar]
- 8. Noh H, Eomm M, Han A. Usefulness of pretreatment neutrophil to lymphocyte ratio in predicting disease‐specific survival in breast cancer patients. J Breast Cancer 2013;16:55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nuhn P, Vaghasia AM, Goyal J et al. Association of pretreatment neutrophil‐to‐lymphocyte ratio (NLR) and overall survival (OS) in patients with metastatic castration‐resistant prostate cancer (mCRPC) treated with first‐line docetaxel. BJU Int 2014;114:E11–E17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Takahashi Y, Horio H, Hato T et al. Prognostic significance of preoperative neutrophil‐lymphocyte ratios in patients with stage I non‐small cell lung cancer after complete resection. Ann Surg Oncol 2015;22(Suppl 3):1324–1331. [DOI] [PubMed] [Google Scholar]
- 11. Cedrés S, Torrejon D, Martínez A et al. Neutrophil to lymphocyte ratio (NLR) as an indicator of poor prognosis in stage IV non‐small cell lung cancer. Clin Transl Oncol 2012;14:864–869. [DOI] [PubMed] [Google Scholar]
- 12. Forget P, Machiels JP, Coulie PG et al. Neutrophil:Lymphocyte ratio and intraoperative use of ketorolac or diclofenac are prognostic factors in different cohorts of patients undergoing breast, lung, and kidney cancer surgery. Ann Surg Oncol 2013;20(Suppl 3):650–660. [DOI] [PubMed] [Google Scholar]
- 13. Jafri SH, Shi R, Mills G. Advance lung cancer inflammation index (ALI) at diagnosis is a prognostic marker in patients with metastatic non‐small cell lung cancer (NSCLC): A retrospective review. BMC Cancer 2013;13:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Salciccioli JD, Marshall DC, Pimentel MA et al. The association between the neutrophil‐to‐lymphocyte ratio and mortality in critical illness: An observational cohort study. Crit Care 2015;19:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gu XB, Tian T, Tian XJ et al. Prognostic significance of neutrophil‐to‐lymphocyte ratio in non‐small cell lung cancer: A meta‐analysis. Sci Rep 2015;5:12493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Al‐Shibli KI, Donnem T, Al‐Saad S et al. Prognostic effect of epithelial and stromal lymphocyte infiltration in non‐small cell lung cancer. Clin Cancer Res 2008;14:5220–5227. [DOI] [PubMed] [Google Scholar]
- 17. Hiraoka K, Miyamoto M, Cho Y et al. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non‐small‐cell lung carcinoma. Br J Cancer 2006;94:275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Choi Y, Kim JW, Nam KH et al. Systemic inflammation is associated with the density of immune cells in the tumor microenvironment of gastric cancer. Ann Oncol 2016;27(Suppl 6):207–242, 10.1093/annonc/mdw371.29. [DOI] [PubMed] [Google Scholar]
- 19. Choi JE, Villarreal J, Lasala J et al. Perioperative neutrophil:Lymphocyte ratio and postoperative NSAID use as predictors of survival after lung cancer surgery: A retrospective study. Cancer Med 2015;4:825–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Pignon JP, Tribodet H, Scagliotti GV et al. Lung Adjuvant Cisplatin Evaluation: A pooled analysis by the LACE Collaborative Group. J Clin Oncol 2008;26:3552–3559. [DOI] [PubMed] [Google Scholar]
- 21. Zhang H, Xia H, Zhang L et al. Clinical significance of preoperative neutrophil‐lymphocyte vs platelet–lymphocyte ratio in primary operable patients with non‐small cell lung cancer. Am J Surg 2015;210:526–535. [DOI] [PubMed] [Google Scholar]
- 22. Park B, Yee C, Lee KM. The effect of radiation on the immune response to cancers. Int J Mol Sci 2014;15:927–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell Lung Cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Herbst RS, Baas P, Kim DW et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomized controlled trial. Lancet 2016;387(10027):1540–1550. [DOI] [PubMed] [Google Scholar]
- 26. Ferrucci PF, Gandini S, Battaglia A et al. Baseline neutrophil‐to‐lymphocyte ratio is associated with outcome of ipilimumab‐treated metastatic melanoma patients. Br J Cancer 2015;112:1904–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]