On May 18, 2016, the U.S. Food and Drug Administration approved atezolizumab for the treatment of patients with locally advanced or metastatic urothelial carcinoma whose disease progressed during or following platinum‐containing chemotherapy or within 12 months of neoadjuvant or adjuvant treatment with platinum‐containing chemotherapy. This article summarizes key review findings that supported this approval.
Keywords: Locally advanced or metastatic urothelial carcinoma, Bladder cancer, Platinum‐containing chemotherapy, Atezolizumab, Immunotherapy
Abstract
Until recently in the United States, no products were approved for second‐line treatment of advanced urothelial carcinoma. On May 18, 2016, the U.S. Food and Drug Administration approved atezolizumab for the treatment of patients with locally advanced or metastatic urothelial carcinoma whose disease progressed during or following platinum‐containing chemotherapy or within 12 months of neoadjuvant or adjuvant treatment with platinum‐containing chemotherapy. Atezolizumab is a programmed death‐ligand 1 (PD‐L1) blocking antibody and represents the first approved product directed against PD‐L1. This accelerated approval was based on results of a single‐arm trial in 310 patients with locally advanced or metastatic urothelial carcinoma who had disease progression after prior platinum‐containing chemotherapy. Patients received atezolizumab 1,200 mg intravenously every 3 weeks until disease progression or unacceptable toxicity. Key efficacy measures were objective response rate (ORR), as assessed by Independent Review per RECIST 1.1, and duration of response (DoR). With a median follow‐up of 14.4 months, confirmed ORR was 14.8% (95% CI: 11.1, 19.3) in all treated patients. Median DoR was not reached and response durations ranged from 2.1+ to 13.8+ months. Of the 46 responders, 37 patients had an ongoing response for ≥ 6 months. The most common adverse reactions (≥20%) were fatigue, decreased appetite, nausea, urinary tract infection, pyrexia, and constipation. Infection and immune‐related adverse events also occurred, including pneumonitis, hepatitis, colitis, endocrine disorders, and rashes. Overall, the benefit‐risk assessment was favorable to support accelerated approval. The observed clinical benefits need to be verified in confirmatory trial(s).
Implications for Practice.
This accelerated approval of atezolizumab for second‐line use in advanced urothelial carcinoma provides patients with an effective, novel treatment option for the management of their disease. This represents the first immunotherapy approved in this disease setting.
Introduction
Urothelial carcinoma is the most common malignancy in the urinary tract system and accounts for approximately 16,000 deaths annually in the U.S. [1], [2]. Although most urothelial carcinomas are nonmuscle invasive at diagnosis and can be managed effectively with surgical resection and/or intravesical therapies, approximately 10–15% of patients may develop invasive, locally advanced, and metastatic urothelial carcinoma [3]. Approximately 10% of patients have regionally advanced or metastatic disease at diagnosis [1]. Standard of care for patients with advanced disease is platinum‐containing chemotherapy in combination with gemcitabine [2]. However, most patients experience disease progression or intolerance to treatment during or after platinum‐containing chemotherapy, and their survival time is 5–10 months on average [4], [5], [6], [7], [8].
No FDA‐approved second‐line therapy for the disease was available prior to the approval of atezolizumab in May 2016. Outside the U.S., vinflunine is approved as a second‐line treatment, which is associated with a response rate of 9% and median response duration of 7.4 months [4]. Studies of other chemotherapeutics such as taxanes in the disease setting showed low response rates and considerable toxicities [6], [7], [8]. Median response durations with other cytotoxic chemotherapies were about 5 months [5], [8]. To date, no studies of chemotherapeutics in the second‐line disease setting have shown a statistically significant improvement in overall survival.
Atezolizumab (TECENTRIQ, Genentech, Inc.) is an Fc‐engineered, humanized, monoclonal antibody that binds to programmed death‐ligand 1 (PD‐L1) and inhibits its interactions with the PD‐1 and B7.1 receptors. This releases the PD‐L1/PD‐1 mediated inhibition of the immune response, including reactivation of the anti‐tumor immune response.
In a phase 1 study, no dose‐limiting toxicity was observed when atezolizumab was administered intravenously (IV) from 0.01 mg/kg to 20 mg/kg. A fixed dose of atezolizumab (1,200 mg) administered every 3 weeks was chosen for further clinical studies. This dose corresponds to approximately 17 mg/kg in patients of 70 kg. Based on a population pharmacokinetic analysis, the terminal half‐life of atezolizumab was 27 days and the steady state could be achieved after 6 to 9 weeks (2 to 3 cycles) of repeated dosing. Mild or moderate renal impairment or mild hepatic impairment had no clinically significant effect on the systemic exposure to atezolizumab.
Early clinical studies showed that atezolizumab is active in numerous malignancies, including advanced urothelial carcinoma [9], [10]. Based on the preliminary clinical evidence showing a confirmed response rate of 50% (95% CI: 29.3%, 70.7%) in 20 patients with high PD‐L1 expression as determined by the prototype assay, atezolizumab received a Breakthrough Therapy designation in May 2014 for the treatment of patients with advanced urothelial carcinoma who have received prior platinum‐containing chemotherapy. The application also received priority review, and the proposed indication was approved in May 2016. Herein, we summarize key review findings that supported this approval.
Trial Design
A single‐arm, open‐label study (IMvigor 210, ClinicalTrials.gov Identifier NCT02108652) of atezolizumab was conducted in patients with treatment‐naïve, advanced urothelial carcinoma who were ineligible for cisplatin‐containing chemotherapy (Cohort 1) or who had disease progression during or following a prior platinum‐based chemotherapy regimen (Cohort 2). Major efficacy outcome measures included confirmed objective response rate (ORR) as assessed by Independent Review Facility (IRF) using RECIST 1.1 and duration of response (DoR). The U.S. Food and Drug Administration (FDA) review focused primarily on patients enrolled in Cohort 2 [12].
To be eligible for Cohort 2, patients were required to have disease progression during or following prior platinum‐containing chemotherapy, or within 12 months of neoadjuvant or adjuvant chemotherapy. Disease progression was assessed per RECIST 1.1. Patients were also required to have an Eastern Cooperative Oncology Group (ECOG) performance score of 0 or 1. Exclusion criteria included history of autoimmune disease, active or corticosteroid‐dependent brain metastases, administration of a live, attenuated vaccine within 4 weeks prior to enrollment, or administration of systemic immunostimulatory agents (e.g., interleukin‐2) within 6 weeks or systemic immunosuppressive medications (e.g., glucocorticoids, azathioprine) within 2 weeks prior to enrollment.
Patients received an IV infusion of 1,200 mg of atezolizumab on Day 1 of a 3‐week cycle until unacceptable toxicity, disease progression, or symptomatic progression. Tumor response assessments were performed every 9 weeks for the first 54 weeks and every 12 weeks thereafter. For patients who met RECIST 1.1 criteria for disease progression, continued treatment with atezolizumab was allowed at the discretion of the investigator if they were clinically stable and continued to benefit from treatment. Safety assessments began from the first dose of atezolizumab and continued at regular intervals (prior to each dose) until 30 days after the last dose of atezolizumab. All adverse events (AEs) were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, Version 4.0 (NCI CTCAE 4.0).
Objective response rate (ORR) is defined as the proportion of patients who had a confirmed complete or partial response per RECIST v 1.1 among patients with measurable disease at baseline who received at least one dose of study treatment. Duration of response was defined as the time from the initially documented partial response or complete response to evidence of disease progression as determined by the IRF or death due to any cause, whichever occurred first.
PD‐L1 expression status in tumor specimens was prospectively assessed using an analytically validated assay (VENTANA PD‐L1 [SP142]) at a central laboratory. Depending on the percentage of PD‐L1 stained tumor‐infiltrating immune cells (ICs) within the tumor area, patients were classified into two groups: PD‐L1 expression of ≥5% (covering ≥5% of the tumor area) and PD‐L1 expression of <5% (covering <5% of the tumor area). The results of PD‐L1 expression were blinded to patients, investigators, and trial sites and were retrospectively used for pre‐specified analyses of ORR and DoR in the two groups.
An additional phase 1 (PCD4989g) cohort that enrolled patients with advanced urothelial bladder cancer (UBC) was included in the submission. This UBC cohort started by enrolling patients with PD‐L1 expression positive tumors according to the prototype PD‐L1 assay and was later opened to patients regardless of PD‐L1 expression. This led to a population that was enriched for PD‐L1 expression [11]. For those with additional evaluable tumor specimens, PD‐L1 expression status was later reevaluated using the SP142 assay. For this cohort, both ORR and DoR were also assessed by IRF per RECIST 1.1.
Results
IMvigor 210 enrolled 310 patients in Cohort 2. Nineteen percent had disease progression following neoadjuvant or adjuvant chemotherapy. Key demographic and disease characteristics of these patients are summarized in Table 1.
Table 1. Key baseline characteristics of patients in cohort 2 of IMvigor 210.
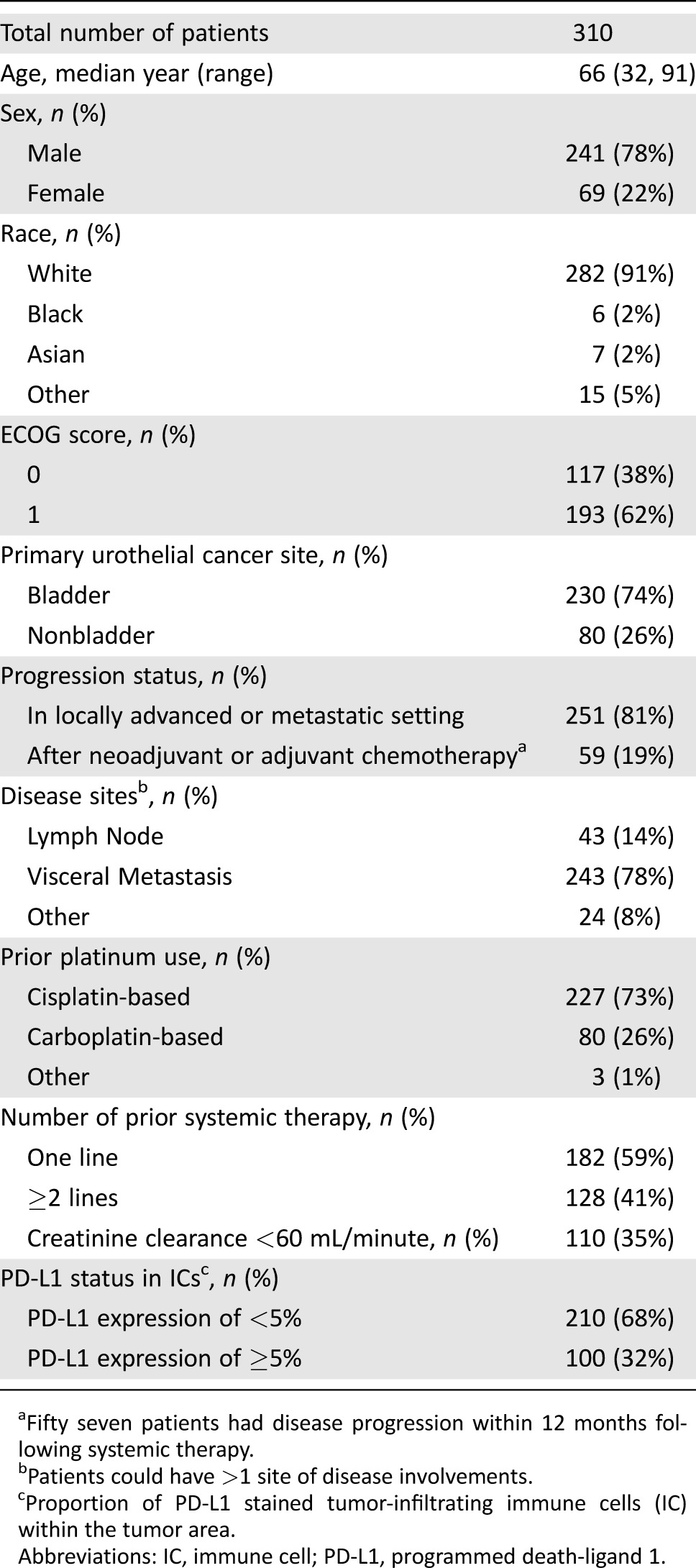
Fifty seven patients had disease progression within 12 months following systemic therapy.
Patients could have >1 site of disease involvements.
Proportion of PD‐L1 stained tumor‐infiltrating immune cells (IC) within the tumor area.
Abbreviations: IC, immune cell; PD‐L1, programmed death‐ligand 1.
As of the data cutoff, 19% of patients were on study treatment and 81% of patients discontinued study treatment. Of the discontinued patients, 86% were due to disease progression and 5% due to adverse events. The median treatment duration was 12.3 weeks (range: 0.1, 46 weeks).
Efficacy.
Efficacy results are shown in Table 2. With a median follow‐up time of 14.4 months, IRF‐confirmed ORR was 14.8% (95% CI: 11.1%, 19.3%) in all treated patients. Both CRs and PRs were observed. Median time to onset of response was 2.1 months (range: 1.6, 8.3). Median DoR in responders was not reached, and the observed response durations ranged from 2.1+ to 13.8+ months. Of the responders, 37 (80%) had ongoing responses of ≥6 months, of which 6 (13%) had ongoing responses of ≥12 months at the time of the analysis [11].
Table 2. Efficacy results of cohort 2 of IMvigor 210.
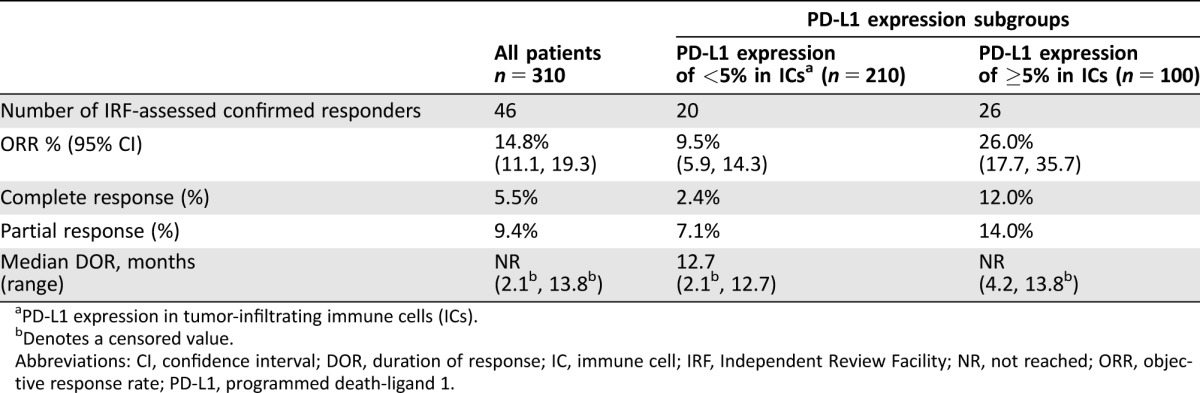
PD‐L1 expression in tumor‐infiltrating immune cells (ICs).
Denotes a censored value.
Abbreviations: CI, confidence interval; DOR, duration of response; IC, immune cell; IRF, Independent Review Facility; NR, not reached; ORR, objective response rate; PD‐L1, programmed death‐ligand 1.
Responses were also observed in the two PD‐1 expression subgroups. The confirmed ORR was 9.5% (95% CI: 5.9%, 14.3%) in patients with PD‐L1 expression of <5% and 26.0% (95% CI: 17.7%, 35.7%) in those with PD‐L1 expression of ≥5% in ICs. There were 5 CRs (2.4%) in patients with PD‐L1 expression of <5% and 12 CRs (12%) in those with PD‐L1 expression of ≥5%, suggesting that the PD‐L1 expression increased the possibility of a CR but did not preclude the development of a CR. There was no difference in time to onset of responses between the two subgroups. Median DoR was 12.7 months (2.1+, 12.7) in the subgroup with PD‐L1 expression of <5% and was not reached (4.2, 13.8+) in the subgroup with PD‐L1 expression of ≥5%. In either subgroup, most responders (70–80%) had ongoing responses of ≥6 months and some (10–15%) had sustained responses of ≥12 months [11]. These results suggest that atezolizumab‐induced responses were reasonably durable in either PD‐L1 expression subgroup despite a noticeable difference in ORR.
In 59 patients with disease progression following neoadjuvant or adjuvant therapy, the IRF‐confirmed ORR was 22.0% (95% CI: 12.3%, 34.7%). Median DoR was 12.7 months (range: 4.1, 12.7). Atezolizumab was active in patients with nonbladder urothelial carcinoma, visceral metastases, or prior BCG treatment. Patients with lymph node only metastases had a higher response rate compared with patients with visceral metastases (30% vs. 10%), suggesting that tumor burden and location may affect the treatment effect of atezolizumab. Durable responses were observed in all of these subgroups [11].
Findings from the phase 1 UBC cohort were consistent with the above results. This cohort enrolled 94 patients and had a median follow up of 20 months, approximately 6 months longer than that in Cohort 2 of IMvigor 210 [11]. Ninety‐eight percent of the patients received prior platinum‐containing chemotherapy [11]. Confirmed ORR, as assessed by IRF per RECIST 1.1, was 25.5% (95% CI: 17.1%, 35.6%), with 9 CRs and 15 PRs reported. Median DOR in all responders was not reached (range: 2.9, 26.3+ months), with ongoing responses of ≥6 months observed in 92% of the responders and of ≥12 months in 58% of the responders. The higher ORR might be related to the enrichment of patients with PD‐L1 expression in ICs of their tumor specimens.
Toxicity.
All the 310 patients enrolled in Cohort 2 received at least one dose of atezolizumab and were included in the safety analysis. Immune‐mediated adverse events, defined as events requiring the use of systemic steroids with no alternate etiology, endocrine events, and other events thought to be immune‐related, are summarized in Table 3. Immune‐mediated adverse reactions were generally managed with administration of high‐dose (1–2 mg/kg prednisolone equivalent) corticosteroids followed by a taper and interruption of atezolizumab therapy. In total, 6.5% of patients experienced an immune‐mediated adverse event requiring systemic steroids and 2.3% of patients experienced hypothyroidism requiring hormone replacement. Other adverse events that were plausibly immune‐related and were not treated with systemic steroids included noninfectious diarrhea (Grade 1–4: 19%; Grade 3–4: 3%) and hyperglycemia/new‐onset diabetes mellitus (Grade 1–4: 4%; Grade 3–4: 0.6%). The pattern of immune‐related adverse events was generally consistent with other approved agents targeting the PD‐1/PD‐L1 pathway. Thyroid function tests were routinely collected only at baseline and end of study, such that the reported incidence of hypothyroidism may underestimate the true incidence.
Table 3. Atezolizumab immune‐related toxicities in cohort 2 of IMvigor 210.
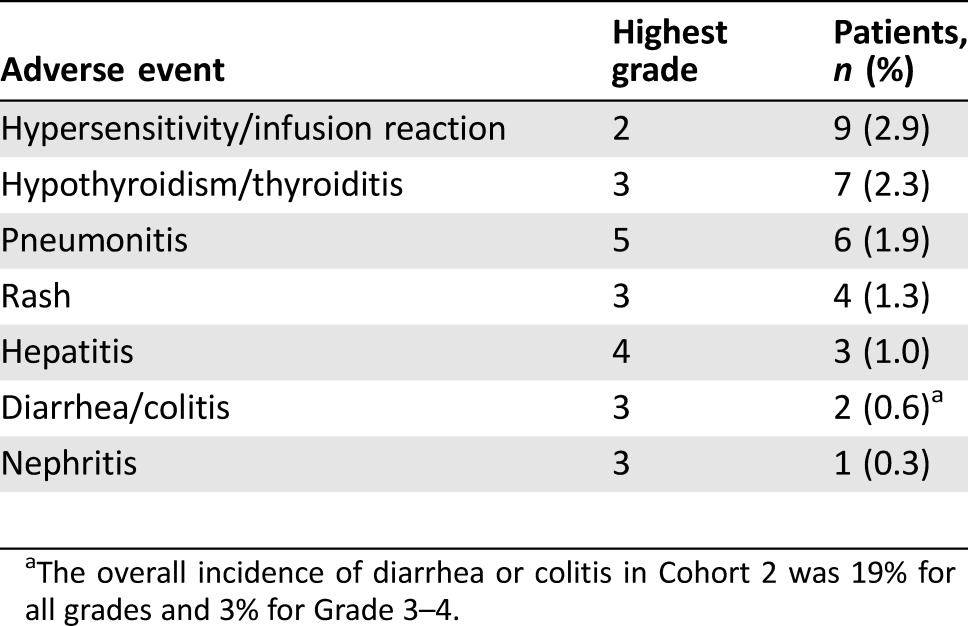
The overall incidence of diarrhea or colitis in Cohort 2 was 19% for all grades and 3% for Grade 3–4.
Three patients (0.9%) who were treated with atezolizumab experienced sepsis, pneumonitis, or intestinal obstruction that led to death. Ten patients discontinued atezolizumab due to an adverse event. The most common cause of discontinuation was infection, including sepsis, skin infection of the foot, and mycobacterial vertebral osteomyelitis leading to retroperitoneal hemorrhage.
The most common Grade 1‐4 adverse events occurring in at least 20% of patients treated with atezolizumab were fatigue (52%), decreased appetite (26%), nausea (25%), urinary tract infections (22%), pyrexia (21%), and constipation (21%). The most common Grade 3–4 adverse events occurring in at least 2% of patients treated with atezolizumab were urinary tract infection (9%), fatigue (6%), abdominal pain (4%), dyspnea (4%), hematuria (3%), and back/neck pain (2%). The laboratory abnormalities worsening from baseline to Grade 3–4 in at least 2% of patients treated with atezolizumab included lymphopenia (11%), hyponatremia (10%), anemia (6%), increased alkaline phosphatase (5%), hyperglycemia (5%), increased ALT (2%), increased AST (2%), and hypoalbuminemia (2%).
Discussion
The FDA review found that treatment with atezolizumab had a favorable benefit‐risk profile in patients with locally advanced or metastatic urothelial carcinoma who had disease progression during or after platinum‐containing chemotherapy. Treatment with atezolizumab elicited confirmed objective antitumor responses in approximately 15–25% of patients in single‐arm studies. Although the median response durations were not reached at the data cutoff for the response analyses, the majority of responding patients maintained their response for ≥6 months and some for ≥12 months. These response durations were longer than the reported response durations with single‐agent chemotherapy used in the same disease setting [4], [5], [8]. The safety profile shows that most patients tolerated atezolizumab and that the common adverse reactions (≥20%) were fatigue, decreased appetite, nausea, urinary tract infection, pyrexia, and constipation. The reported immune‐related adverse events were similar to those observed in approved PD‐1 targeted products such as pembrolizumab and nivolumab. Taken together, the evidence, as summarized in our Benefit‐Risk Assessment in Table 4 [11], is considered sufficient for accelerated approval of atezolizumab for the intended clinical use. This provides the first nonchemotherapeutic treatment in this disease setting, which has likely addressed an unmet medical need in the population.
Table 4. Benefit‐risk assessments of atezolizumab for second‐line use in advanced urothelial carcinoma.
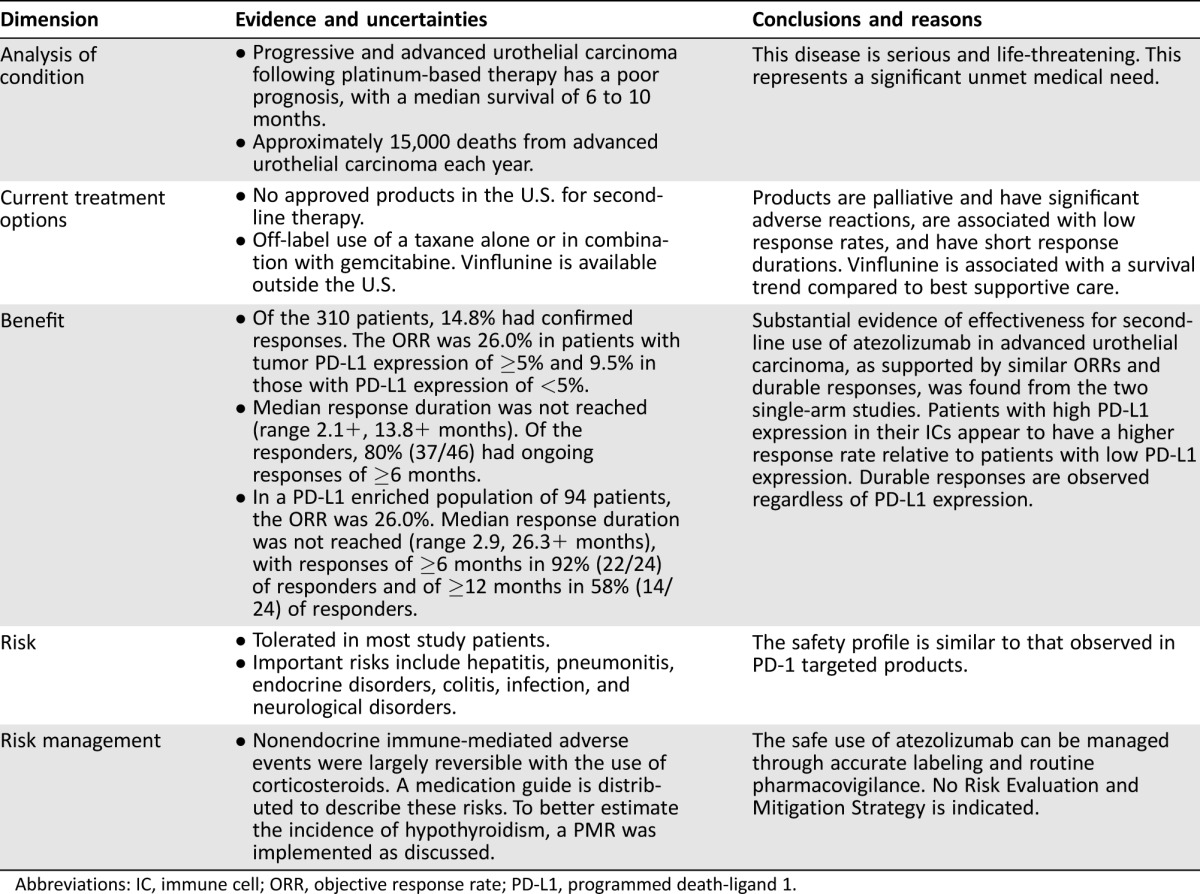
Abbreviations: IC, immune cell; ORR, objective response rate; PD‐L1, programmed death‐ligand 1.
Based on the regulatory requirements for accelerated approval [13], the clinical benefit of atezolizumab should be verified in confirmatory trial(s) in urothelial carcinoma. Currently, a randomized, active‐controlled trial in the same disease setting is ongoing. This trial, registered as NCT02302807 at ClinicalTrials.gov, compares atezolizumab with Investigator‐choice of chemotherapy. The primary endpoint is overall survival.
Regarding the possibility that the incidence of hypothyroidism may be underestimated, the FDA mandated a postmarketing requirement (PMR) to evaluate thyroid function studies on a more frequent basis in atezolizumab trials to better characterize the true incidence of thyroid toxicity.
For this approved indication, use of the PD‐L1 assay may be helpful but is not mandated for selection of patients to receive atezolizumab, because durable responses were observed regardless of PD‐L1 expression levels in tumor‐infiltrating ICs. Although the response rate in patients with PD‐L1 expression of ≥5% in ICs was modestly higher than that in those with PD‐L1 expression of <5% in ICs, there were CRs in those with PD‐L1 expression of <5%. Overall, there was no adequate clinical evidence to limit the use of atezolizumab to the former group. Given the few treatment options available in this disease setting, it is important to make atezolizumab clinically available to patients who may benefit from atezolizumab. The role of PD‐L1 expression in tumor‐infiltrating ICs and the related assay in selection of patients may be better understood if the ongoing randomized trial has positive results. The data in this application were primarily from a single‐arm study without a control, making it very difficult to assess whether PD‐L1 expression in ICs is prognostic and/or predictive in advanced urothelial carcinoma [14].
During the development and regulatory assessments of atezolizumab, similar antitumor activity was reported for other PD‐1 or PD‐L1 targeted products (e.g., avelumab, durvalumab, pembrolizumab, and nivolumab) in advanced urothelial carcinoma [15], [16], [17], [18]. In small populations of approximately 30 to 80 study patients, reported response rates were approximately 18‐30% and durable responses were observed. This suggests a class effect of PD‐1/PD‐L1‐targeted products in the disease. The recently released results of a randomized phase 3 trial of pembrolizumab in the same second‐line disease setting showed an improvement in overall survival when compared with Investigator‐selected chemotherapy [19]. Upon verification, this may provide additional evidence to support use of PD‐1/PD‐L1‐targeted products in the treatment of advanced urothelial carcinoma. Successful developments of these products and timely evaluations of them will help broaden patient access to novel effective treatments.
Conclusion
Our review found adequate evidence to support the current accelerated approval of atezolizumab for second‐line use in patients with advanced urothelial carcinoma who have had prior platinum‐containing chemotherapy. This approval is based on the observed improvement in surrogates, including objective response rate and duration of response. For continued approval, the clinical benefit of atezolizumab should be verified in patients with advanced urothelial carcinoma.
Author Contributions
Conception/Design: Yang‐Min Ning, Daniel Suzman, V. Ellen Maher, Geoffrey Kim, Richard Pazdur
Collection and/or assembly of data: Yang‐Min Ning, Daniel Suzman, V. Ellen Maher, Lijun Zhang, Shenghui Tang
Data analysis and interpretation: Yang‐Min Ning, Daniel Suzman, V. Ellen Maher, Lijun Zhang, Shenghui Tang, Tiffany Ricks, Todd Palmby, Wentao Fu, Qi Liu, Kirsten B. Goldberg, Geoffrey Kim, Richard Pazdur
Manuscript writing: Yang‐Min Ning, Daniel Suzman, V. Ellen Maher, Kirsten B. Goldberg
Final approval of manuscript: Yang‐Min Ning, Daniel Suzman, V. Ellen Maher, Lijun Zhang, Shenghui Tang, Tiffany Ricks, Todd Palmby, Wentao Fu, Qi Liu, Kirsten B. Goldberg, Geoffrey Kim, Richard Pazdur
Disclosures
The authors indicated no financial relationships.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- 2.National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology: Bladder cancer, version I. 2016; NCCN.org
- 3. Park JC, Citrin DE, Agarwal PK et al. Mutimodal management of muscle invasive bladder cancer. Curr Probl Cancer 2014;38:80–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bellmunt J, Theodore C, Demkov T et al. Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum‐containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 2009;27:4454–4461. [DOI] [PubMed] [Google Scholar]
- 5. Sweeney CJ, Roth BJ, Kabbinavar FF et al. Phase II study of pemetrexed for second‐line treatment of transitional cell cancer of the urothelium. J Clin Oncol 2006;24:3451–7. [DOI] [PubMed] [Google Scholar]
- 6. Choueiri TK, Ross RW, Jacobus S et al. Double‐blind, randomized trial of docetaxel plus vandetanib versus docetaxel plus placebo in platinum‐pretreated metastatic urothelial cancer. J Clin Oncol 2011;30:507–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ko YJ, Canil CM, Mukherjee SD et al. Nanoparticle albumin‐bound paclitaxel for second‐line treatment of metastatic urothelial carcinoma: A single group, multicentre, phase 2 study. Lancet Oncol 2013;14: 769–76. [DOI] [PubMed] [Google Scholar]
- 8. Petrylak DP, Tagawa ST, Kohli M, et al. Docetaxel as monotherapy or combined with ramucirumab or icrucumab in second‐line treatment for locally advanced or metastatic urothelial carcinoma: An open‐label, three‐arm, randomized controlled Phase II trial. J Clin Oncol 2016;34:1500–1509. [DOI] [PubMed] [Google Scholar]
- 9. Cha E, Wallin J, and Kowanetz M. PD‐L1 inhibition with MPDL3280A for solid tumors. Semin Oncol 2015;42:484–487. [DOI] [PubMed] [Google Scholar]
- 10. Powles T, Eder JP, Fine GD et al. MPDL3280A (anti‐PD‐L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 2014;515:558–562. [DOI] [PubMed] [Google Scholar]
- 11.FDA Clinical and Statistical Reviews of Atezolizumab for Second‐line Use in Advanced Urothelial Carcinoma Available at: http://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/761034Orig1s000MedR.pdf. Accessed December 1, 2016.
- 12. Rosenberg JE, Hoffman‐Censits J, Powles T et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum‐based chemotherapy: a single‐arm, multicenter, phase 2 trial. Lancet 2016;387:1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson JR, Ning YM, Farrell A et al. Accelerated approval of oncology products: The food and drug administration experience. J Natl Cancer Inst 2011;103:636–644. [DOI] [PubMed] [Google Scholar]
- 14. Bellmunt J, Mullane SA, Werner L et al. Association of PD‐L1 expression on tumor‐infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol 2015;26: 812–817. [DOI] [PubMed] [Google Scholar]
- 15. Apolo AB, Infante JR, Hamid O, et al. Avelumab (MSB0010718C; anti‐PD‐L1) in patients with metastatic urothelial carcinoma from the JAVELIN solid tumor phase 1b trial: Analysis of safety, clinical activity, and PD‐L1 expression. J Clin Oncol 2016;34 (suppl; abstr 4514). [Google Scholar]
- 16. Massard C, Gordon MS, Sharma S et al. Safety and efficacy of durvalumab (MEDI4736), an anti–programmed cell death ligand‐1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol 2016;34:3119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elizabeth R. Plimack, Joaquim Bellmunt, Shilpa Gupta, et al. Pembrolizumab (MK‐3475) for advanced urothelial cancer: Updated results and biomarker analysis from KEYNOTE‐012. J Clin Oncol 2015;33 (suppl; abstr 4502).
- 18. Sharma P, Bono P, Kim JW et al: Efficacy and safety of nivolumab monotherapy in metastatic urothelial cancer (mUC): Results from the phase I/II CheckMate 032 study. J Clin Oncol 2016;34 (suppl; abstr 4501). [Google Scholar]
- 19. Bellmunt J, de Wit R, Vaughn DJ et al: KEYNOTE‐045: Open‐label, phase III study of pembrolizumab versus investigator's choice of paclitaxel, docetaxel, or vinflunine for previously treated advanced urothelial carcinoma. http://jitc.biomedcentral.com/articles/10.1186/s40425-016-0191‐4. Accessed December 2016.


