A unique case of brainstem encephalitis, in a patient with malignant melanoma being treated with ipilimumab and pembrolizumab, is reported.
Abstract
Checkpoint inhibitors are increasingly being used in the treatment of malignant melanoma and other cancers. With the use of such therapies, autoimmune‐mediated adverse events in the central and peripheral nervous system are likely to occur more frequently. We report a unique case of brainstem encephalitis with a sudden lethal outcome following ipilimumab and pembrolizumab therapy in a patient with malignant melanoma. The autopsy showed a diffuse nodular activation of microglia in the whole encephalon with prominent intraparenchymal and perivascular lymphocytic infiltration of the brainstem. Non‐infectious brainstem encephalitis is a well‐recognized subset of paraneoplastic encephalitis. Brainstem involvement is usually accompanied by a wide spectrum of signs and symptoms, which were not observed in this case. The timing of the clinical symptoms as well as the histopathological findings suggest an autoimmune‐adverse event of ipilimumab and pembrolizumab administration rather than a paraneoplastic disorder. In the presence of neurological symptoms, immediate cessation of the immunotherapy and immunosuppressive therapy may lead to successful therapeutic intervention, as described in previous reports. Therefore, it is crucial that physicians are aware of the possible side effects of immunotherapies on the nervous system.
Implications for Practice.
Metastatic melanoma patients treated with the anti‐CTLA‐4 inhibitor ipilimumab have a high utilization of various types of health care services, such as inpatient hospital stays or doctor visits. There are differences across countries regarding patterns of health care utilization and economic burden of the disease. Health care services are used more frequently after patients experience progression of their disease. The study highlights that better therapies leading to durable response in patients with metastatic melanoma have the potential to decrease health care costs and patient burden in terms of hospitalizations and other health care services.
Introduction
Novel immunotherapies are widely used in the treatment of metastatic melanoma and other cancers. Ipilimumab and pembrolizumab, both human monoclonal antibodies, enhance antitumor activity by activating T cells [1], [2]. There are an increasing number of reports on autoimmune‐mediated adverse events of such therapies on the central and peripheral nervous system [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16]. Brainstem encephalitis associated with cancer has been described in cases with paraneoplastic disorders but not after treatment formetastatic melanoma.This is the first description of a fatal case of brainstemencephalitis after treatment with ipilimumab and pembrolizumab.
Case report
An otherwise healthy 60‐year‐old woman with a 1.5 mm thick nevoid melanoma (pT2b) on the left thigh was referred to our dermatooncology clinic in 2012. At the time of the primary diagnosis, there was no clinical or radiological evidence of metastases. The melanoma was re‐excised with a 1 cm safety margin and a sentinel lymph‐node biopsy was performed, showing no metastases. In 2015, radiological follow‐up with positron emission tomography–computed tomography (PET‐CT) scan showed three metabolically active in‐transit metastases on the left thigh, which were excised. Mutation status was positive for the BRAFV600E mutation. Three months later, radiological follow‐up of the brain with magnetic resonance imaging (MRI) and PET‐CT revealed a new lesion in the right gyrus frontalis medius as well as bilateral pulmonary nodules, both suspicious for metastases. Neurosurgical resection of the histologically confirmed brain metastases was followed by adjuvant radiotherapy with a total of 30 Gray. Subsequently, the patient was treated with the anti‐CTLA‐4 antibody ipilimumab (3 mg/kg IV) every 3 weeks for a total of 4 doses.
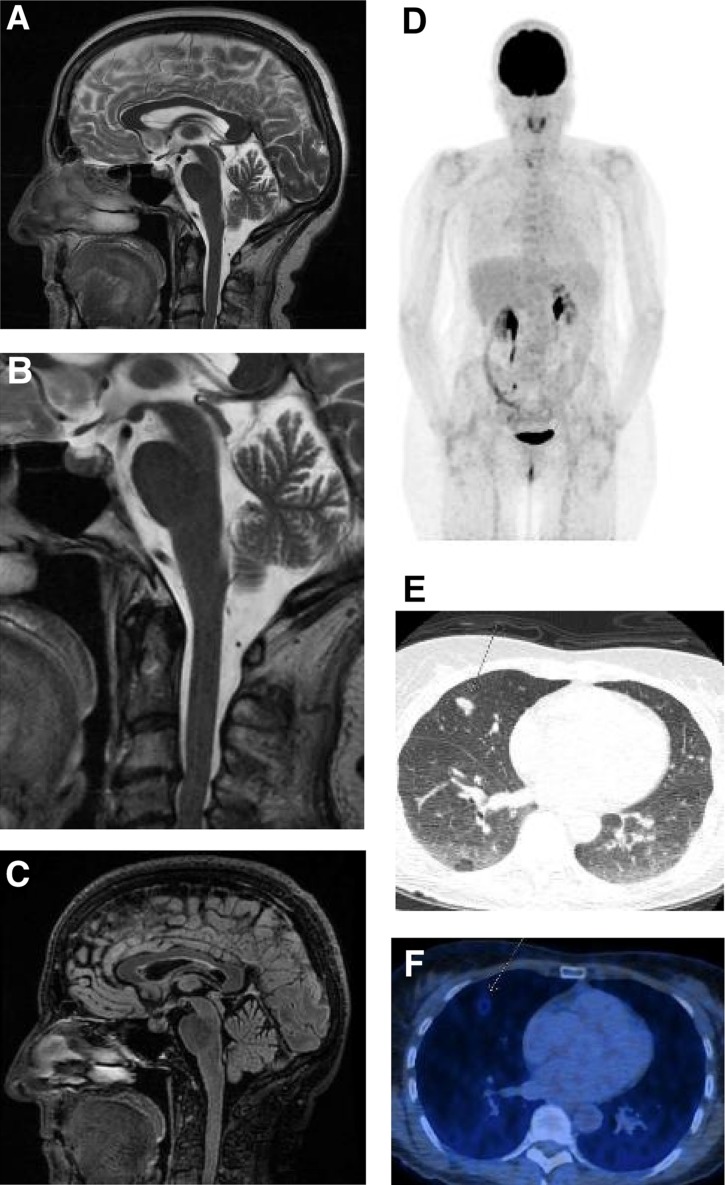
Three months after the last dose, the patient reported generalized tiredness, weakness, and loss of appetite. Serum endocrinological work‐up revealed a non‐significant cortisol shift without osmolality changes, which was not specific for hypopituitarism or adrenal insufficiency. No new metastases were found in the MRI of the brain (Fig. 1A–1C). The pituitary gland showed no focal metabolic enrichment, no enlargement, and therefore no signs of hypophysitis. A new PET‐CT scan demonstrated progression of the bilateral pulmonary nodules (Fig. 1D–1F).
Figure 1.
Staging 32 days before exitus (last staging before exitus) and 21 days before treatment start with pembrolizumab. (A): Magnetic resonance imaging brain: Overview of the brainstem without any signs of inflammation. No swelling or accentuation of the pituitary. The cerebral hemispheres show no focal lesions. (B): Detailed view of brainstem and pituitary gland. (C): Sagittal Fluid‐attenuated Inversion‐Recovery (FLAIR) sequence of the brain with pituitary and brainstem. (D): FDG‐PET/CT overview with several, barely detectable lesions in both lungs, that show minimal metabolic activity. (E): Computed tomography of the lungs with several small nodular infiltrates in the right middle lobe, compatible with metastases or granulomas. Prominent nodular infiltrates in the right middle lobe (arrow). (F): FDG‐PET/CT of the lungs with weak FDG uptake of the above nodular infiltrate.
Abbreviation: FDG‐PET/CT, 18F‐fluorodeoxyglucose (FDG)‐positron emission tomography (PET)/computed tomography (CT).
Subsequent therapy with pembrolizumab (2 mg/kg IV every 3 weeks), a PD‐1 antagonist, was initiated. Two weeks later, the patient unexpectedly passed away without any antecedent evidence of altered physical status.
An autopsy was performed. Surprisingly, the pulmonary nodules visible in the PET‐CT proved to be epithelioid granulomas. The Melanin‐A immunostain was negative. CD4 positive lymphocytic infiltrates were found in several organs, including the pituitary gland, thyroid, myocardium, lungs, liver, and adrenal glands without any clear signs of tissue damage.
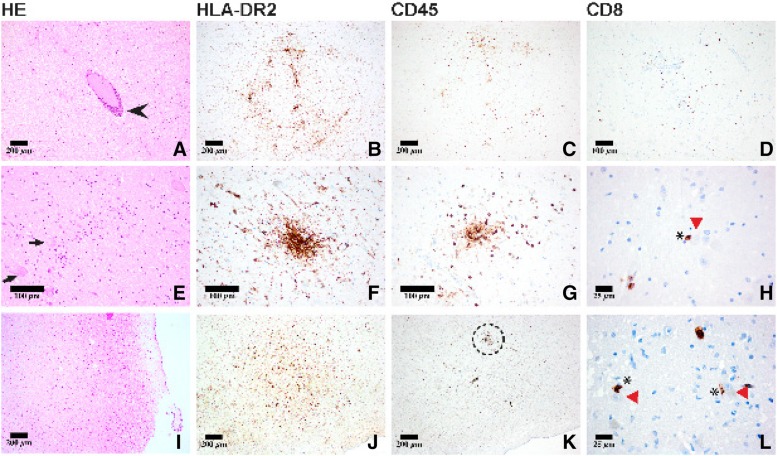
Histological examination of the brain, however, showed diffuse and nodular microglial activation within the cerebral hemispheres, with accentuation in the brainstem. In the pons and medulla in particular there were diffuse and perivascular lymphocytic infiltrates as well as diffuse and nodular activated microglial infiltrates, highlighted with immunohistochemical stains (CD45 and HLA‐DR2, respectively; Fig. 2). The lymphocytic infiltrates consisted mainly of cytotoxic CD8+ T‐lymphocytes, often with direct proximity to neurons in brainstem regions that modulate cardiovascular and respiratory function. The lymphocytes in the brain did not immunoreact with PD‐1 and PD‐L1. In contrast, a pulmonary lymph node showed scattered PD‐1 positive lymphocytes, whereas PD‐L1 was negative. The Melanin‐A immunostain was negative. No pathogens could be detected in the Brown‐Brenn, Grocott, and Ziehl‐Neelsen stains for microorganisms. Immunohistochemical staining for neurotropic viruses (Cytomegalovirus, Measles Virus, Herpes Simplex Virus, SV40, Toxoplasmosis, Varicella Zoster Virus, Human Herpes Virus 6) were all negative. In the absence of an infectious or neoplastic etiology, an autoimmune‐mediated brainstem encephalitis involving cardiovascular and respiratory centers was considered to represent the cause of death. The autoimmune reaction was interpreted to be due to prior immunotherapy with ipilimumab and pembrolizumab.
Figure 2.
Neuropathological work‐up of the brain (pons: A–H; I–L: medulla oblongata) showed both perivascular (A, arrowhead) and diffuse (E, arrows: neurons) lymphocytic infiltrates and microglial activation concentrated in the brainstem as highlighted by CD45 and HLA‐DRA2 immunostains, respectively (D, H, I). On occasion, direct proximity to neurons was observed (H, L: asterisk, CD8+ T‐lymphocyte; red arrowhead, neurons in the pons and medulla) suggesting the formation of an “immunological synapse” to important cardiovascular and respiratory neuronal centers in the brainstem (L: close‐up of dashed area seen in K).
Discussion
To our knowledge, we describe the first fatal case of encephalitis, predominantly localized to the brainstem, following a novel immunotherapeutic intervention for metastatic melanoma. A wide spectrum of signs and symptoms usually accompany brainstem encephalitis including diplopia, ptosis, dizziness, nausea, vomiting, dysarthria, dysphonia, sensorineural deafness, and central hypoventilation [17]. Unfortunately, no such findings were apparent in the current case, which hampered antemortem diagnosis. Although inflammation was not restricted to the brainstem, inflammatory infiltrates were found in the pons and medulla, suggesting the formation of an “immunological synapse” to important cardiovascular and respiratory neuronal centers. Importantly, this finding is likely responsible for the lethal outcome (Fig. 2). Inflammation concentrated in the brainstem is a recognized subset of paraneoplastic encephalitis [18]. For example, Barnett et al. [19] described a case of paraneoplastic brainstem encephalitis in a patient with lung adenocarcinoma that was associated with the expression of Ma proteins. Paraneoplastic encephalitis, which can affect any part of the nervous system, has been reported to accompany a variety of systemic neoplasms [18]. Importantly, paraneoplastic syndromes induced by a tumour may be present before the cancer has been identified [18].
The timing of the onset of the symptoms after the administration of ipilimumab and pembrolizumab as well as histopathological findings in the brain suggests an autoimmune‐mediated adverse event rather than a classic paraneoplastic disorder. The complete absence of PD‐1 positive lymphocytes in the brain could possibly be explained by the antecedent immunomodulating therapy.
Our patient had been treated for metastatic melanoma for an extended period without prior evidence of a paraneoplastic syndrome. The infiltration of cytotoxic CD8+ T cell infiltrates in close proximity to neurons as well as the diffuse microglial infiltration suggesting an autoimmune‐adverse reaction targeting brainstem tissue. The direct interaction between cytotoxic CD8+ T cells and neurons is illustrated in Figure 2H, 2L. The direct contact between an antigen presenting cell, in this case the MHC1 complex of neuron, and the T‐cell receptor has been referred to as an “immunological synapse” [20]. This interaction results in T‐cell activation and may be responsible for the autoinflammation in the brainstem. Accordingly, immunotherapy is considered the most likely trigger for the brainstem encephalitis in this case.
Ipilimumab and pembrolizumab, both human monoclonal antibodies that enhance antitumor activity by T‐cell activation, are recognized to initiate autoimmune‐mediated events in the central and peripheral nervous system [2], [3]. For ipilimumab, several conditions of the peripheral nervous system have been described, including cranial peripheral neuropathy, multifocal radiculoneuropathy, chronic inflammatory demyelinating polyneuropathy (CIDP), Guillain‐Barré syndrome, meningo‐radiculo‐neuritis, myasthenia gravis, and Tolosa‐Hunt syndrome. Syndromes affecting the central nervous system (CNS), including transverse myelitis, aseptic meningitis, meningoencephalitis, necrotic myelopathy, and granulomatous CNS inflammation, have been reported following ipilimumab therapy [3], [4], [5], [6], [7], [8], [9], [10] (Table 1). Autoimmune‐mediated neurological side effects of pembrolizumab affecting the peripheral nervous system include CIDP, Guillain‐Barré syndrome, and myasthenia [11], [12]. Furthermore, limbic encephalitis following pembrolizumab, CNS demyelination after treatment with nivolumab, and lambrolizumab‐induced CNS toxicity with epilepsia partialis continua have been described [11], [12], [13], [14], [15] (Table 1).
Table 1. Neurologic manifestations of checkpoint inhibitors.

Abbreviations: Anti‐NMDA, anti‐N‐methyl‐D‐aspartate receptor antibodies; CIDP, chronic inflammatory demyelinating polyneuropathy; CNS, central nervous system.
Although the temporal relationship between treatment and the onset of symptoms points to ipilimumab, pembrolizumab represents another possible trigger of the brainstem encephalitis. The median time to onset of known adverse side effects reported is >1 month [21]. Notably, William et al. [16] described a case of autoimmune encephalitis associated with immune checkpoint inhibitors, namely ipilimumab and nivolumab. They identified anti‐N‐methyl‐D‐aspartate receptor antibodies in the cerebrospinal fluid, which confirmed autoimmunity as the cause of the disease.
Conceivably, the combination of ipilimumab and pembrolizumab may have a synergistic effect that increased the risk for developing neuroinflammation in the current case. The majority of the patients previously described were successfully treated by withdrawing immunotherapy, followed by immediate immunosuppressive therapy with high dose glucocorticoids, intravenous immunoglobulins, rituximab, and/or plasmapheresis [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13], [14], [15], [16].
Conclusion
Although brainstem encephalitis is often fatal, timely recognition of early signs/symptoms may lead to successful therapeutic intervention [22]. Therefore, it is of great importance to increase awareness among physicians of the possible side effects of immunotherapies on the nervous system.
Author Contributions
Conception/design: Simon Bossart, Reinhard Dummer
Provision of study material or patients: Simon Bossart, Selina Thurneysen, Karl Frontzek, Henning Leske, Daniela Mihic, Joanna Mangana, Simone M. Goldinger, Reinhard Drummer, Hannes Nagel
Collection and/or assembly of data: Simon Bossart, Selina Thurneysen, Elisabeth Rushing, Karl Frontzek, Daniela Mihic, Hannes Nagel
Data analysis and interpretation: Simon Bossart, Elisabeth Rushing, Simone M. Goldinger, Reinhard Dummer
Manuscript writing: Simon Bossart, Elisabeth Rushing, Simone M. Goldinger, Reinhard Dummer
Disclosures
Simone M. Goldinger: Merck Sharp & Dohme, Bristol‐Myers Squibb, Roche, Novartis (CA, Other [travel support]); Reinhard Dummer: Novartis, Merck Sharp & Dohme, Bristol‐Myers Squibb, Roche, GlaxoSmithKline, Amgen, Takeda (CA, E); Novartis, Merck Sharp & Dohme, Bristol‐Myers Squibb, Roche, GlaxoSmithKline (RF); Novartis, Merck Sharp & Dohme, Bristol‐Myers Squibb, Roche, GlaxoSmithKline, Amgen (ET). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Robert C, Schachter J, Long GV et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–2532. [DOI] [PubMed] [Google Scholar]
- 2. Mahoney KM, Freeman GJ, McDermott DF. The Next Immune‐Checkpoint Inhibitors: PD‐1/PD‐L1 Blockade in Melanoma. Clin Ther 2015;37:764–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Voskens CJ, Goldinger SM, Loquai C et al. The price of tumor control: an analysis of rare side effects of anti‐CTLA‐4 therapy in metastatic melanoma from the ipilimumab network. PLoS One 2013;8:e53745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thaipisuttikul I, Chapman P, Avila EK. Peripheral neuropathy associated with ipilimumab: A report of 2 cases. J Immunother 2015;38:77–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Manousakis G, Koch J, Sommerville RB et al. Multifocal radiculoneuropathy during ipilimumab treatment of melanoma. Muscle Nerve 2013;48:440–444. [DOI] [PubMed] [Google Scholar]
- 6. Liao B, Shroff S, Kamiya‐Matsuoka C et al. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro Oncol 2014;16:589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilgenhof S, Neyns B. Anti‐CTLA‐4 antibody‐induced Guillain‐Barré syndrome in a melanoma patient. Ann Oncol 2011;22:991–3. [DOI] [PubMed] [Google Scholar]
- 8. Bompaire F, Mateus C, Taillia H et al. Severe meningo‐radiculo‐neuritis associated with ipilimumab. Invest New Drugs 2012;30:2407–2410. [DOI] [PubMed] [Google Scholar]
- 9. Stein MK, Summers BB, Wong CA et al. Meningoencephalitis Following Ipilimumab Administration in Metastatic Melanoma. Am J Med Sci 2015;350:512–513. [DOI] [PubMed] [Google Scholar]
- 10. Abdallah A‐O, Herlopian A, Ravilla R et al. Ipilimumab‐induced necrotic myelopathy in a patient with metastatic melanoma: A case report and review of literature. J Oncol Pharm Pract 2016;22:537–542. [DOI] [PubMed] [Google Scholar]
- 11. de Maleissye MF, Nicolas G, Saiag P. Pembrolizumab‐Induced Demyelinating Polyradiculoneuropathy N Engl J Med 2016;375:296–297. [DOI] [PubMed] [Google Scholar]
- 12. Zimmer L, Goldinger SM, Hofmann L et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side‐effects of anti‐PD‐1 therapy. Eur J Cancer 2016;60:210–225. [DOI] [PubMed] [Google Scholar]
- 13. Salam S, Lavin T, Turan A. Limbic encephalitis following immunotherapy against metastatic malignant melanoma. BMJ Case Rep 2016;pii:bcr 2016215012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Maurice C, Schneider R, Kiehl T‐R et al. Subacute CNS Demyelination after Treatment with Nivolumab for Melanoma. Cancer Immunol Res 2015;3:1299–1302. [DOI] [PubMed] [Google Scholar]
- 15. Mandel JJ, Olar A, Aldape KD et al. Lambrolizumab induced central nervous system (CNS) toxicity. J Neurol Sci 2014;344:229–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Williams TJ, Benavides DR, Patrice KA et al. Association of Autoimmune Encephalitis With Combined Immune Checkpoint Inhibitor Treatment for Metastatic Cancer. JAMA Neurol 2016;73:928–933. [DOI] [PubMed] [Google Scholar]
- 17. Saiz A, Bruna J, Stourac P et al. Anti‐Hu‐associated brainstem encephalitis. J Neurol Neurosurg Psychiatry 2009;80:404–407. [DOI] [PubMed] [Google Scholar]
- 18. Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med 2003;349:1543–1554. [DOI] [PubMed] [Google Scholar]
- 19. Barnett M, Prosser J, Sutton I et al. Paraneoplastic brain stem encephalitis in a woman with anti‐Ma2 antibody. J Neurol Neurosurg Psychiatry 2001;70:222–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Alarcón B, Mestre D, Martínez‐Martín N. The immunological synapse: A cause or consequence of T‐cell receptor triggering? Immunology 2011;133:420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merck. Prescribing Information for Keytruda 2014–2016. Available at https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf. Accessed March 29, 2017.
- 22. Tan IL, Mowry EM, Steele SU et al. Brainstem encephalitis: Etiologies, treatment, and predictors of outcome. J Neurol 2013;260:2312–2319. [DOI] [PMC free article] [PubMed] [Google Scholar]