Melanoma is a particularly immunogenic cancer, and immune checkpoint inhibitors have been extensively studied in this tumor type. This review focuses on the incidence of serious neurologic immune‐related adverse events, specifically encephalitis, in patients with advanced melanoma treated with nivolumab alone or in sequence or combination with ipilimumab. Practical guidance is provided for the diagnosis and management of treatment‐related encephalitis associated with nivolumab and ipilimumab.
Keywords: Encephalitis, Neurologic adverse events, Immune‐related adverse events, Melanoma, Immune checkpoint inhibitors, Case series
Abstract
Background.
Despite unprecedented efficacy across multiple tumor types, immune checkpoint inhibitor therapy is associated with a unique and wide spectrum of immune‐related adverse events (irAEs), including neurologic events ranging from mild headache to potentially life‐threatening encephalitis. Here, we summarize neurologic irAEs associated with nivolumab and ipilimumab melanoma treatment, present cases of treatment‐related encephalitis, and provide practical guidance on diagnosis and management.
Methods.
We searched a Global Pharmacovigilance and Epidemiology database for neurologic irAEs reported over an 8‐year period in patients with advanced melanoma receiving nivolumab with or without ipilimumab from 12 studies sponsored by Bristol‐Myers Squibb. Serious neurologic irAEs were reviewed, and relationship to nivolumab or ipilimumab was assigned.
Results.
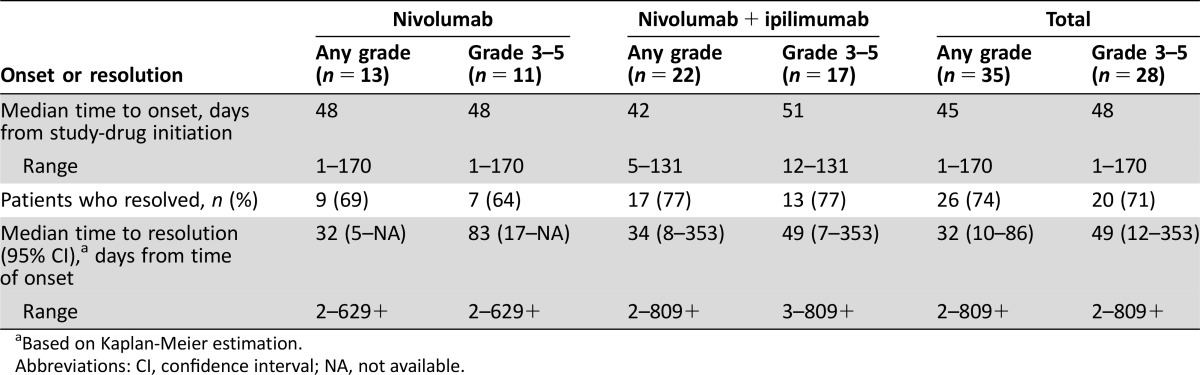
In our search of 3,763 patients, 35 patients (0.93%) presented with 43 serious neurologic irAEs, including neuropathy (n = 22), noninfective meningitis (n = 5), encephalitis (n = 6), neuromuscular disorders (n = 3), and nonspecific adverse events (n = 7). Study drug was discontinued (n = 20), interrupted (n = 8), or unchanged (n = 7). Most neurologic irAEs resolved (26/35 patients; 75%). Overall, median time to onset was 45 days (range 1–170) and to resolution was 32 days (2–809+). Median time to onset of encephalitis was 55.5 days (range 18–297); four cases resolved and one was fatal.
Conclusion.
Both oncologists and neurologists need to be aware of signs and symptoms of serious but uncommon neurologic irAEs associated with checkpoint inhibitors. Prompt diagnosis and management using an established algorithm are critical to minimize serious complications from these neurologic irAEs.
Implications for Practice.
With increasing use of checkpoint inhibitors in cancer, practicing oncologists need to be aware of the potential risk of neurologic immune‐related adverse events and be able to provide prompt treatment of this uncommon, but potentially serious, class of adverse events. We summarize neurologic adverse events related to nivolumab alone or in combination with ipilimumab in patients with advanced melanoma from 12 studies and examine in depth 6 cases of encephalitis. We also provide input and guidance on the existing neurologic adverse events management algorithm for nivolumab and ipilimumab.
Introduction
In the past 5 years, immune checkpoint inhibitors have produced unprecedented, durable clinical benefit in patients with advanced melanoma [1], [2], [3], [4], [5], as well as other tumor types. However, reactivation of antitumor immunity may be accompanied by a wide range of inflammatory and immune‐related adverse events (irAEs) that have been observed with ipilimumab, an inhibitor of cytotoxic T‐lymphocyte antigen 4 (CTLA‐4), as well as with nivolumab and pembrolizumab, inhibitors of programmed death 1 (PD‐1) [1], [2], [3], [4], [5], [6]. The pattern and incidence of irAEs vary with each inhibitor, but high‐grade events tend to be less frequent with PD‐1 than with CTLA‐4 inhibitors [7]. The most commonly reported irAEs in the melanoma population are skin‐related (30%–55% of patients), including pruritus and rash, and gastrointestinal events (12%–37% of patients), including diarrhea and colitis [1], [2], [3], [4], [5]. Hepatic and endocrine events have also been consistently observed.
Treatment with immune checkpoint inhibitors has also been associated with neurologic irAEs, a relatively uncommon class of events generally reminiscent of paraneoplastic syndromes [8]. These neurologic disorders typically develop in cancer patients when an antitumor immune response damages the nervous system due to cross‐reactivity against an antigen expressed by both tumor cells and healthy neurons [9], [10], [11]. At present, it is unclear whether neurologic irAEs associated with the use of immune checkpoint inhibitors are the result of such shared onco‐neural antigens or are related to the unmasking of a previously suppressed autoimmune condition [12].
Serious and sometimes fatal neurologic irAEs have been reported with ipilimumab, including sensory and motor neuropathy, Guillain‐Barré syndrome (GBS), and myasthenia gravis [13]. Other reported neurologic irAEs include inflammatory myopathy, aseptic meningitis with cerebrospinal fluid (CSF) lymphocytosis, and chronic inflammatory demyelinating polyneuropathy [12], [14], [15]. In a pooled analysis of nearly 1,500 patients with melanoma treated with ipilimumab, the incidence of neurologic irAEs was 0.1% [14], [16]; however, this incidence has been indicated to be an underestimation possibly related to lack of recognition or underreporting, as these irAEs are often transient peripheral sensorimotor neuropathies [15]. Peripheral neuropathies were predominant in this pooled analysis, with 4.5% of patients reporting peripheral sensory neuropathy, 0.9% reporting peripheral neuropathy, and 0.6% reporting peripheral motor neuropathy; however, these neuropathies were reported separately as treatment‐related AEs instead of irAEs [14].
In a pooled analysis of nearly 1,500 patients with melanoma treated with ipilimumab, the incidence of neurologic irAEs was 0.1%; however, this incidence has been indicated to be an underestimation possibly related to lack of recognition or underreporting, as these irAEs are often transient peripheral sensorimotor neuropathies.
Neurologic irAEs have been less widely reported with nivolumab and pembrolizumab. However, dizziness and peripheral and sensory neuropathy, as well as cases of facial and abducens nerve paresis, demyelination, autoimmune neuropathy, GBS, and myasthenia gravis, have been observed in nivolumab clinical trials [12], [17], [18], [19], whereas sensory neuropathy, GBS, myasthenia gravis, and partial seizures have been seen with pembrolizumab [20], [21], [22]. Additionally, a recent retrospective study of 496 patients with melanoma treated with pembrolizumab or nivolumab in 15 centers in Germany and Switzerland found paresthesia, paresis/paralysis, and polyneuropathy in 3 (0.6%) patients; seizures in 2 (0.4%); and GBS, (meningo)‐radiculitis, aphasia, and parkinsonoid syndrome combined with bradykinesia in 1 patient (0.2%) [23].
Of particular note are reports of immune‐mediated encephalitis because of its potentially fatal nature [17], [24], [25], [26], [27], [28], as well as two cases of posterior reversible encephalopathy syndrome and acute encephalopathy toxicity, respectively [29], [30], [31], seen with nivolumab, pembrolizumab, and ipilimumab.
Encephalitis is a rare condition involving acute inflammation of the brain in association with clinical evidence of neurologic dysfunction [32]. Diagnosis can be challenging, and the etiology is unclear in many patients, with an infectious cause found in 40%–70% of cases [33]. One patient with non‐small cell lung cancer treated in a clinical trial with nivolumab for more than 7 months developed fatal limbic encephalitis, despite discontinuation of nivolumab and administration of corticosteroids [17]. In 2015, the U.S. Food and Drug Administration issued an ongoing postmarketing requirement for enhanced pharmacovigilance to evaluate the incidence, severity, outcomes, and associated clinical and laboratory findings of immune‐mediated encephalitis following exposure to nivolumab [34]. This study, expected to be completed in 2021, will collect, classify, and analyze data on all types of moderate to severe neurologic deterioration in patients exposed to nivolumab.
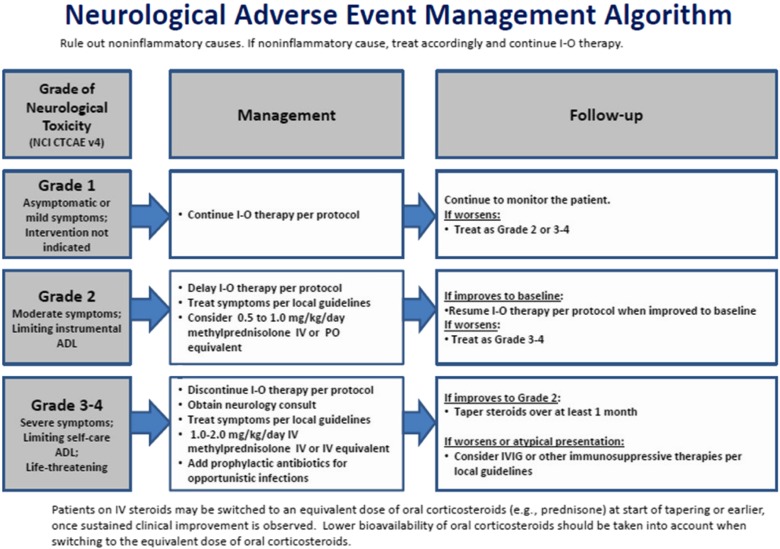
Although there is an established, published algorithm for managing neurologic irAEs associated with nivolumab and ipilimumab (Fig. 1) [35], the heterogeneous presentation of these events makes them difficult to diagnose and treat. With increasing use of immune checkpoint inhibitors across multiple tumor types, practicing oncologists need to be aware of these uncommon, treatment‐associated, potentially serious neurologic irAEs, as prompt recognition and intervention are critical to patient safety and improved outcomes.
Figure 1.
Neurologic adverse event management algorithm (reprinted from the supplementary appendix from [35], with permission from the Massachusetts Medical Society).
Abbreviations: ADL, activities of daily living; I‐O, immuno‐oncology; IV, intravenous; IVIG, intravenous immunoglobulins; NCI CTCAE, National Cancer Institute Common Toxicity Criteria for Adverse Events; PO, oral/by mouth.
Melanoma is a particularly immunogenic cancer, and immune checkpoint inhibitors have been extensively studied in this tumor type. Therefore, we searched the Bristol‐Myers Squibb Global Pharmacovigilance and Epidemiology database to determine the incidence of serious neurologic irAEs, specifically encephalitis, in patients with advanced melanoma treated with nivolumab alone or in sequence or combination with ipilimumab across 12 clinical trials and expanded access programs (EAPs). In this review, we summarize the neurologic irAEs identified and present each case of treatment‐related encephalitis observed in the study population. We also provide practical guidance on the diagnosis and management of treatment‐related encephalitis associated with nivolumab and ipilimumab.
Materials And Methods
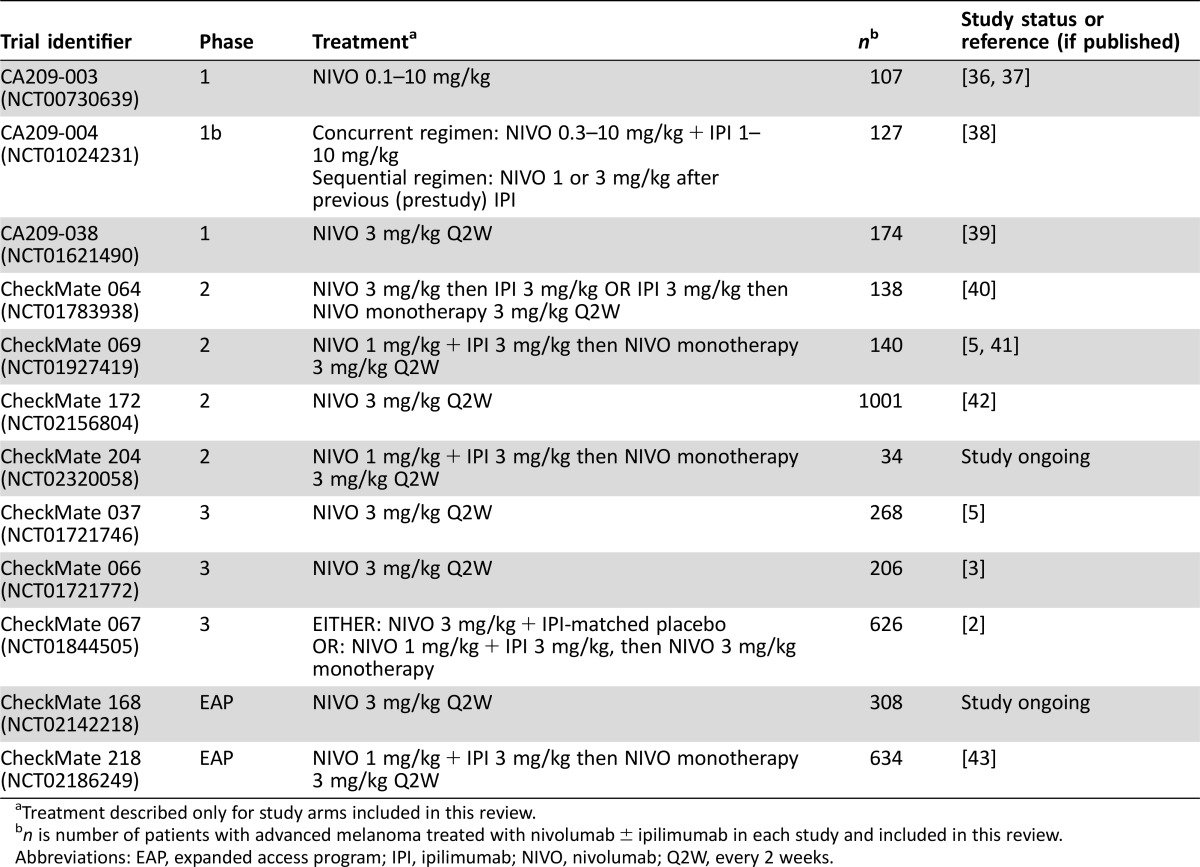
The Bristol‐Myers Squibb Global Pharmacovigilance and Epidemiology database was searched for serious neurologic irAEs reported between January 1, 2008, and February 3, 2016, in patients with advanced melanoma receiving nivolumab monotherapy or nivolumab in combination with ipilimumab, in one of 12 studies comprising three phase I trials, four phase II trials, three phase III trials, and two EAPs (Table 1) [2], [3], [5], [6], [36], [37], [38], [39], [40], [41], [42], [43]. Treatment‐emergent serious neurologic adverse events (AEs) were reviewed by three authors (H. Li, D. Reshef, and A. Avila), determined by consensus to be at least possibly related to nivolumab or ipilimumab, and included in this review. Serious neurologic events were those that required hospitalization or those that were considered life‐threatening or medically significant or that resulted in disability or death. Many of the patients in these studies were treated with both nivolumab and ipilimumab separately, in combination, or sequentially. Due to the variability of the studies in regard to data collection and analysis procedures, causality could not be definitively contributed to either nivolumab or ipilimumab separately.
Table 1. Summary of trials included in database search.
Treatment described only for study arms included in this review.
n is number of patients with advanced melanoma treated with nivolumab ± ipilimumab in each study and included in this review.
Abbreviations: EAP, expanded access program; IPI, ipilimumab; NIVO, nivolumab; Q2W, every 2 weeks.
The incidence of serious neurologic AEs in the pooled melanoma trial population from the database search was calculated, and AEs were summarized by type, time to onset and resolution, study‐drug action taken, and outcome based on resolution rate. Time to resolution was based on Kaplan‐Meier estimations. In addition, the six reported cases of encephalitis were investigated in detail and summarized herein.
Results
Neurologic irAEs
A total of 3,763 patients with advanced melanoma were treated with nivolumab monotherapy or combination therapy with nivolumab and ipilimumab in the 12 clinical studies or EAPs included in this review (Table 1). Thirty‐five of 3,763 patients (0.93%) presented with serious neurologic irAEs considered at least potentially related to study drug. These 35 patients had a median age of 63.5 years and were predominately male (74%). Most of these patients (n = 28) experienced only one neurologic irAE, but six patients had two and one patient had three neurologic irAEs. Of the seven patients experiencing more than one serious neurologic irAE, more patients received combination treatment (five patients) than nivolumab monotherapy (two patients).
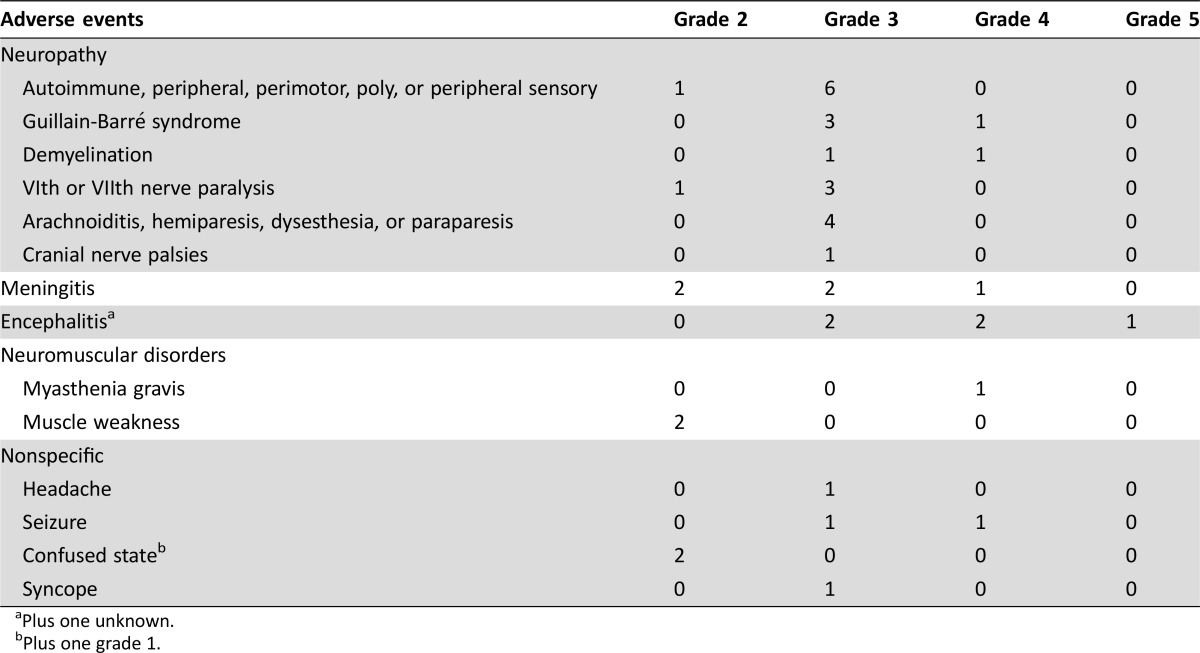
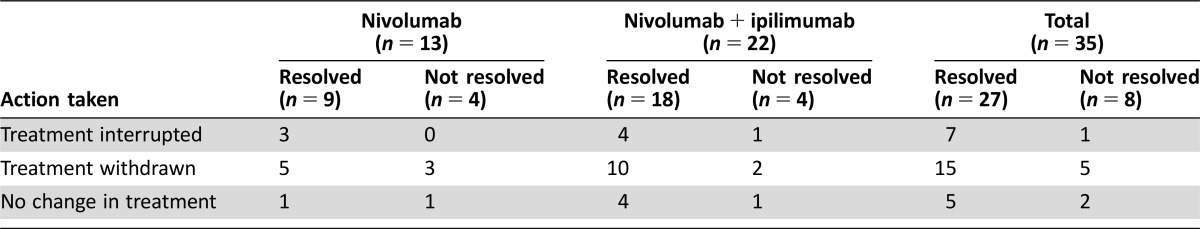
The types of neurologic irAEs observed included neuropathy (n = 22 patients), noninfective meningitis (n = 5 patients), encephalitis (n = 6 patients), neuromuscular disorders (n = 3 patients), and nonspecific events (n = 7 patients), which included headache, seizure, confused state, and syncope (see Table 2 for full list of events). Most of the neurologic events observed were grades 3–4 (32/43 events); one event was fatal (encephalitis, grade 5). Occurrence of a serious neurologic irAE commonly resulted in discontinuation of study drug (n = 20); dosing was interrupted in eight patients and unchanged in the remaining seven patients (Table 3). The majority of patients recovered with resolution of neurologic irAE (26/35, 74%), although some patients had events that resolved with sequelae (n = 7). In addition, there were four patients in whom the AEs had not resolved, three who were still recovering, and two for whom the outcome was unknown at the time of database search. Overall, median time to onset was 45 days for any‐grade serious neurologic AE (range 1–170) and 48 days for grade 3–5 AEs (1–170); median time to resolution was 32 (2–809+) and 49 (2–809+) days, respectively (Table 4).
Table 2. Individual neurologic adverse events observed in patients with advanced melanoma treated with melanoma with or without ipilimumab, by grade.
Plus one unknown.
Plus one grade 1.
Table 3. Action taken for patients with treatment‐related neurologic serious adverse events.
Table 4. Time to onset and resolution of treatment‐related neurologic serious adverse events.
Based on Kaplan‐Meier estimation.
Abbreviations: CI, confidence interval; NA, not available.
Encephalitis
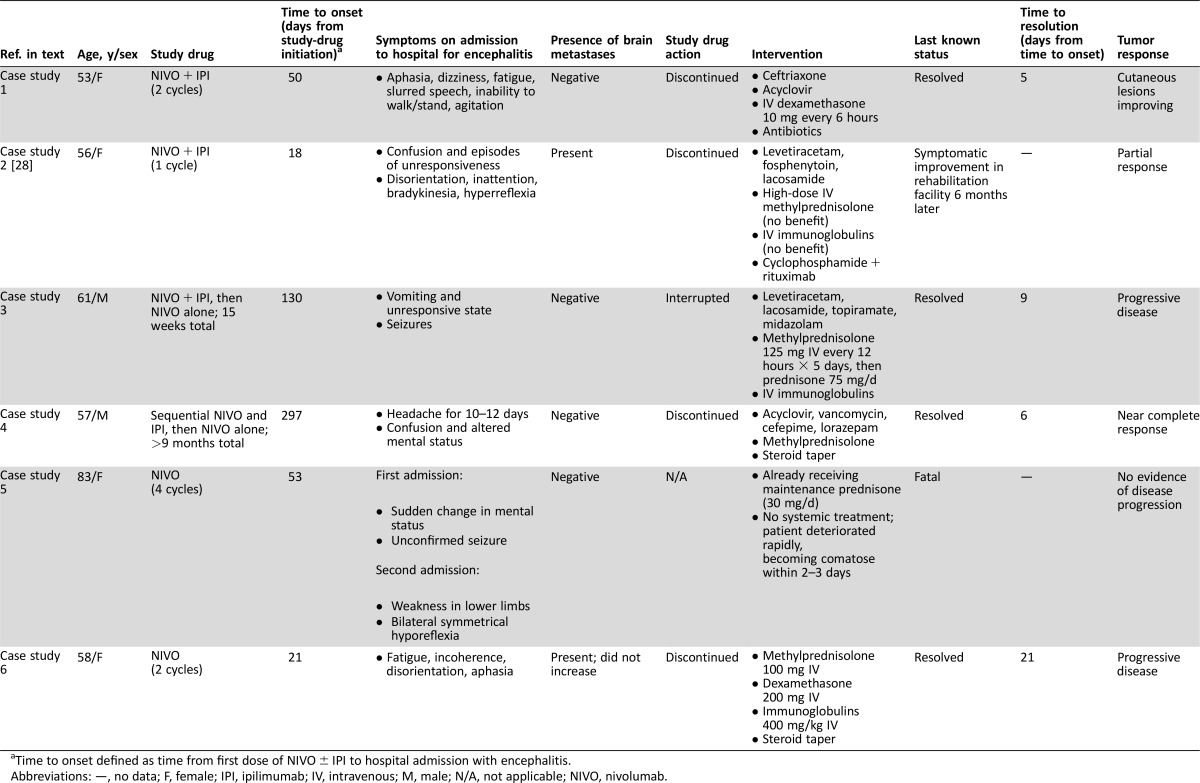
There were a total of six cases of encephalitis, five of which necessitated prolonged hospitalization (including two life‐threatening) and one that was fatal (Table 5) [28]. Median time to onset was 51.5 days (range of 18–297 days). Most patients presented with an altered mental state, characterized by signs and symptoms such as confusion, aphasia, and agitation; other signs and symptoms included difficulty walking or standing, seizure, and fatigue. Differential diagnoses included brain metastases, meningitis, and encephalitis of viral etiology. Several cases were treated empirically with antivirals and broad‐spectrum antibiotics before an infectious etiology was eliminated. In five of the six cases, encephalitis was ultimately managed by treatment with intravenous (IV) steroids, with IV immunoglobulins also required in three cases. Four of these five cases resolved in 5–21 days, but the fifth case was less responsive and the patient was discharged to a rehabilitation unit with slow improvement over a 6‐month period. Individual case histories are summarized below.
Table 5. Summary of patients with advanced melanoma who experienced encephalitis on treatment with NIVO with or without IPI.
Time to onset defined as time from first dose of NIVO ± IPI to hospital admission with encephalitis.
Abbreviations: —, no data; F, female; IPI, ipilimumab; IV, intravenous; M, male; N/A, not applicable; NIVO, nivolumab.
Case Study 1.
A 53‐year‐old female with previously untreated advanced melanoma received 2 cycles of nivolumab 1 mg/kg IV plus ipilimumab 3 mg/kg IV every 3 weeks in a blinded phase III study. Forty‐seven days after the first dose of study drug, she developed a high fever of up to 106°F (41.1°C), for which she received naproxen, and was hospitalized 3 days later with aphasia, dizziness, fatigue, slurred speech, agitation, and inability to walk or stand; she later became noncommunicative. Magnetic resonance imaging (MRI) and computerized tomography (CT) of the brain were negative for metastasis and stroke, but electroencephalogram (EEG) showed diffuse, marked cerebral slowing. Lumbar puncture revealed leukocytosis (86% lymphocytes) in the CSF, protein 312 mg/dL, and glucose 43 mg/dL; culture and serology tests were negative. Other laboratory tests showed mild liver function test increases, with normal bilirubin. The patient was diagnosed with meningoencephalitis. The study‐drug combination was discontinued, and the patient was treated with ceftriaxone 2 g IV every 12 hours and acyclovir, as well as IV steroids (dexamethasone 10 mg every 6 hours) and antibiotics (vancomycin, meropenem, cefepime, and ampicillin). The patient's condition improved 3 days after hospitalization, and antibiotics were discontinued. The encephalitis resolved 5 days after the patient was admitted to the hospital, and the patient was discharged on levofloxacin; IV steroids were discontinued to minimize any negative effect on ongoing antitumor immune response, evidenced by improved subcutaneous lesions. The day after discharge from the hospital, the patient was ambulatory with improved symptoms. The etiology of the event has not been completely elucidated and could be due to the immune‐mediated effect of antitumor therapy or viral infection. Because extensive infectious workup performed on the patient was negative and no alternative etiology was indicated, the investigator deemed the event to be probably related to combination treatment with nivolumab and ipilimumab. Following initiation of nivolumab and ipilimumab combination therapy, the patient achieved a best response of progressive disease at 79 days and began treatment with dabrafenib and trametinib at 3 months. Additional subsequent therapy included pembrolizumab, resection, talimogene laherparepvec, and radiation. As of August, 2016, the patient had a survival time of 32 months.
Case Study 2.
Case study 2 has been reported previously [28] and is briefly summarized here. A 56‐year‐old female with advanced melanoma, including stable brain metastases, was enrolled in an EAP and received one dose of nivolumab 1 mg/kg IV plus ipilimumab 3 mg/kg IV. Two weeks following this dose, she experienced syncope and in another 4 days was hospitalized for worsening mental status and diagnosed with encephalitis. Paraneoplastic antibody testing was performed, and the patient was treated empirically with methylprednisolone 1,000 mg/d for 5 days followed by immunoglobulin 0.4 mg/kg/d for 5 days without significant improvement. The patient received two doses of rituximab, resulting in gradual improvement in mental status. The patient achieved a partial response 4 months following the nivolumab plus ipilimumab combination treatment. One month following the second dose of rituximab, the patient had evidence of disease progression in an external iliac lymph node, which was treated with radiation therapy. The patient remained in stable condition 12 months after combination treatment without further anticancer or immunosuppressive agent‐treatment.
Case Study 3.
A 61‐year‐old male with advanced melanoma was enrolled in an EAP. Over a period of approximately 15 weeks he received nivolumab 1 mg/kg IV plus ipilimumab 3 mg/kg IV (four doses), followed by nivolumab 3 mg/kg monotherapy (one dose). Following the first nivolumab single agent dose, he developed subtle but progressive altered mental status and eventually was found unresponsive at home. The patient was hospitalized requiring ventilator support and intensive care unit care. A CT scan without contrast of the head was normal, and a brain MRI showed abnormally high fluid‐attenuated inversion recovery (FLAIR) signal without evidence of brain metastasis. EEG showed seizure activity, prompting initiation of levetiracetam, lacosamide, topiramate, and midazolam. CSF analysis showed white blood cell (WBC) count of 14 (32 neutrophils and 25 lymphocytes), protein 85, and glucose 212, but was negative for infection. Two days after hospital admission, treatment with methylprednisolone (125 mg IV every 12 hours) was started for encephalitis. Due to refractory seizures related to encephalitis, methylprednisolone was increased to 1 gram IV daily, and IV immunoglobulin 1 g/kg was also given. Five days into hospitalization, the patient began to improve and no longer required ventilator support. Two days after extubation, his steroid medication was changed to oral prednisone 1 mg/kg. The encephalitis appeared to resolve 9 days after presentation to the hospital, and he was discharged on a slow steroid taper. In the absence of an alternative underlying cause, an immune‐mediated causal relationship between encephalitis and nivolumab or ipilimumab was considered probable, with seizures secondary to the encephalitis. Despite recovery from the AEs, study therapy was discontinued. He was subsequently treated with chemotherapy but died of progressive disease within 4 months.
Case Study 4.
A 57‐year‐old male patient with advanced melanoma received induction with sequential nivolumab 3 mg/kg IV every 2 weeks for 6 cycles (approximately 10 weeks) followed by ipilimumab 3 mg/kg IV every 3 weeks for 4 cycles (approximately 9 weeks), and then continued nivolumab 3 mg/kg every 2 weeks as part of a phase II study (approximately 16 weeks) for a total period of almost 10 months. After complaining of headache for 10–12 days, the patient was hospitalized with confusion and altered mental status; the study drug was discontinued. The patient could follow simple but not complex commands and could not complete sensory tests due to lack of attention. CT of the head without contrast showed no acute intracranial abnormality, and brain MRI 2 days later was also normal, with no evidence of brain metastasis. EEG was abnormal, with diffuse slowing and triphasic waves and phase reversal in the right frontal region; there was no sign of seizure activity. After a provisional diagnosis of encephalitis, the patient started empiric treatment with acyclovir, vancomycin, ampicillin, cefepime, lorazepam, and methylprednisolone. CSF analysis demonstrated leukocytosis of 52 and lymphocytic pleocytosis, but no infectious etiology was identified. Three days after admission to hospital, the patient's symptoms began to improve. On the sixth day after hospitalization, antibiotics were discontinued, the encephalitis was resolved (attributed to treatment with steroids), and the patient was discharged with prednisone taper over 48 days. The event was considered most likely immune‐mediated and related to nivolumab, with the possibility of a contributory role of ipilimumab not excluded. The patient has not received subsequent therapy, has achieved a best response of near complete response, and was without symptoms at the last follow‐up in August 2016.
Case Study 5.
An 83‐year‐old female patient with advanced melanoma received 4 doses of nivolumab 3 mg/kg IV, given every 2 weeks, in an EAP. The patient history included a primary malignancy in the nasal cavity that metastasized with predominant disease in bone and soft tissue. The patient had a cranial MRI 7 days after her fourth dose of nivolumab, due to the patient experiencing two episodes of sudden change in mental status. The MRI showed residual melanoma in the nasolacrimal duct and maxillary sinus, with some areas of hypersignal on the supratentorial white matter and FLAIR without a break in the hematocephalic barrier, probably caused by microangiopathy. The following day, the patient was hospitalized after an unconfirmed seizure; the patient made a full recovery from the event, was observed for 48 hours, and was discharged. At this time, the patient also developed a nonserious urinary infection with fever, which was controlled with broad‐spectrum cephalosporin antibiotics. Eleven days after her last dose of nivolumab, the patient was readmitted to the hospital with neurologic symptoms, including weakness in the lower limbs and bilateral symmetrical hyporeflexia, as well as rapidly advancing toxic encephalitis. The patient's neurologic symptoms deteriorated rapidly to a comatose state in a time span of 2–3 days, and she died 16 days after her final dose of nivolumab, with the cause of death reported as toxic encephalitis. The patient was on maintenance oral steroid treatment (prednisone 20 mg am and 10 mg pm) and did not receive high‐dose steroids or IV immunoglobulin for the event. Diagnostic tests did not reveal systemic bacterial infection. CSF showed elevated protein 103 (normal up to 60) but normal WBC count and normal glucose; CSF analysis was negative for bacterial and fungal staining. MRI brain imaging revealed no remarkable abnormality. Although other potential etiologies, such as viral infection, leptomeningeal disease, or metabolic encephalitis, were not ruled out, causality assessment indicated that the fatal encephalitis was related to nivolumab. At the time of the neurological events, the patient still had visible tumor in her nasal cavity, but no follow‐up tumor scans were performed. In addition, no postmortem examination was performed.
Case Study 6.
A 58‐year‐old female with advanced melanoma metastatic to the brain received nivolumab 3 mg/kg every 2 weeks as part of a phase II study. The patient had received stereotactic radiotherapy followed by 4 cycles of ipilimumab in the 5 months before starting nivolumab treatment, but progression of her malignancy continued. Seven days after the patient's second dose of nivolumab, she was admitted to the hospital with increasing fatigue, incoherence, disorientation, and aphasia, initially considered as grade 4 encephalitis and later diagnosed as nivolumab‐related grade 3 encephalitis. Study therapy was discontinued. Physical and neurologic examination did not reveal any abnormalities, and laboratory tests were substantially unchanged from baseline. Brain MRI showed no stroke, and brain metastases were unchanged or smaller, with no new lesions. CSF showed WBC count 18 with elevated lymphocytes 92% (0–70) and was negative for autoimmune antibodies. No infectious etiology was identified. The patient's condition gradually deteriorated, with increased confusion and aphasia. She began treatment with methylprednisolone 100 mg (1–2 mg/kg) 13 days after her last dose of nivolumab. After 6 days of treatment without any neurological improvement, steroids were increased to dexamethasone 200 mg daily combined with a 5‐day course of IV immunoglobulins 400 mg/kg/d. The patient's condition gradually improved over 11 days on steroids, at which point she began a steroid taper. Following resolution of her encephalitis 4 days later, she was discharged. The patient's best response on nivolumab was progressive disease. The patient started subsequent therapy 2 months following the encephalitis hospitalization and died of progressive disease 3 months later.
Practical Guidance on the Diagnosis and Treatment of Immune‐Mediated Encephalitis
Presentation and Diagnosis.
Diagnosis of encephalitis of any etiology can be a challenge, as patients present with a broad range of clinical symptoms that may include alteration in consciousness, confusion and memory problems, fever, and headache, with or without focal neurologic signs such as seizure [32], [33]. In our experience, we have found in particular that headache, fever, tiredness or weakness (focal/diffuse), confusion or delirium, memory problems, sleepiness, hallucinations, altered mental status, seizures, speech difficulty, and stiff neck encompass the spectrum of signs and symptoms associated with immune‐mediated encephalitis. It is critical that patients are educated about the possibility that these symptoms could be due to their immune checkpoint inhibitor therapy and understand the importance of reporting symptoms quickly, because early recognition and appropriate intervention potentially lead to higher likelihood of resolution.
Differential diagnoses to be considered include metastatic neoplasm or paraneoplastic syndromes, autoimmune disease, stroke, vasculitis, collagen vascular disorders, and drug reactions, which may all be confused with infectious encephalitis [32], [33]. Primary infectious encephalitis should be distinguished from post‐infectious encephalitis or peri‐encephalitis or encephalomyelitis, which is caused by immune‐mediated demyelination and often occurs after previous infection or immunization. Immune‐mediated encephalitis associated with immune checkpoint inhibitors in patients receiving nivolumab with or without ipilimumab can be diagnosed in cases in which no infectious agent is identified, in which post‐infectious encephalitis is unlikely based on clinical history of the patient, and in which other (differential) diagnoses have also been ruled out.
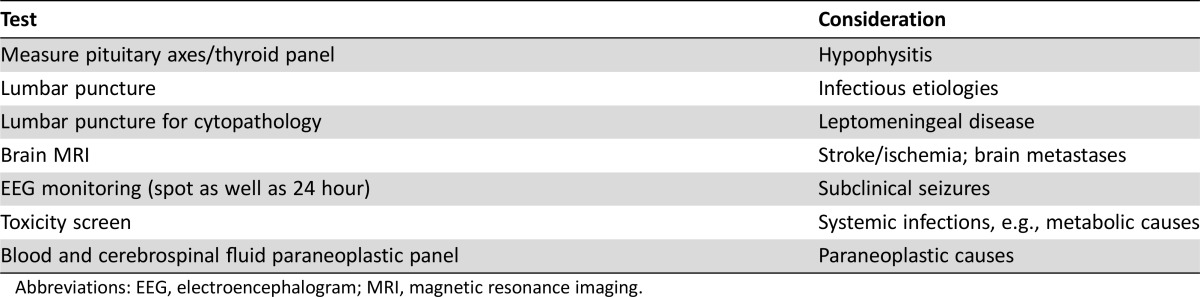
A typical diagnostic work‐up should include measuring the pituitary axes to rule out hypophysitis; a lumbar puncture to rule out infectious etiologies and leptomeningeal disease; brain MRI to rule out stroke/ischemia and brain metastases; EEG monitoring (possibly both spot EEG as well as 24‐hour monitoring) to rule out subclinical seizures; a toxicity screen; both blood and CSF paraneoplastic panel; thyroid panel; and standard complete blood count with differential panel.
In the cases presented here, timing of presentation relative to start of checkpoint blockade was variable (ranging from a few weeks to nearly 10 months) with no difference noted between single‐agent nivolumab and nivolumab plus ipilimumab combination. For health care providers, patients, and caregivers, it is important to remember that presentation can occur early or later. A typical diagnostic work‐up should include measuring the pituitary axes to rule out hypophysitis; a lumbar puncture to rule out infectious etiologies and leptomeningeal disease; brain MRI to rule out stroke/ischemia and brain metastases; EEG monitoring (possibly both spot EEG as well as 24‐hour monitoring) to rule out subclinical seizures; a toxicity screen; both blood and CSF paraneoplastic panel; thyroid panel; and standard complete blood count with differential panel (Table 6). An interesting observation in the cases presented here is the lymphocytosis (>80%) in the CSF in at least two of the cases, as has been reported in previous cases [44], [45]. Additionally, it is important to consider that neurologic AEs may occur regardless of whether CNS metastases are present.
Table 6. Typical workup for patients with new‐onset neurologic findings.
Abbreviations: EEG, electroencephalogram; MRI, magnetic resonance imaging.
Management.
In patients presenting with new‐onset moderate to severe neurologic signs or symptoms, nivolumab (and ipilimumab, if applicable) treatment should be withheld while other possible causes are eliminated. Once immune‐mediated encephalitis seems the most likely diagnosis, treatment should be permanently discontinued and corticosteroids (1–2 mg/kg/d IV methylprednisolone or IV equivalent per established guidelines) should be administered (Fig. 1). High‐dose corticosteroids of at least 2 mg/kg/d are often necessary for grade 3 or higher AEs. In our experience, we have found that discontinuing study drug and early intervention with high‐dose steroids were critical to resolution and better patient outcomes. Empiric antibiotics and antivirals are reasonable, as are empiric anti‐epileptics if there is a concern for seizures. If the symptoms worsen, IV immunoglobulins or other immunosuppressive therapies may be given in accordance with local guidelines. In patients with improvement of symptoms to grade 2, steroids can be tapered over a period of at least 1 month. Immunosuppression and the need for a long steroid taper can increase the risk of opportunistic infection, so antibiotic prophylaxis is recommended. We would not encourage re‐challenge in cases of grade 2 or higher neurologic irAEs.
Conclusion
Our database search identified an approximately 1% incidence of treatment‐related serious neurologic irAEs in a population of more than 3,700 patients with advanced melanoma treated with nivolumab, with or without ipilimumab, across 12 clinical trials and EAPs. There were six cases of immune‐mediated encephalitis, constituting an overall incidence of less than 0.2%. Four of the six cases treated with high‐dose IV steroids resolved within 3 weeks. Other cases of encephalitis associated with nivolumab treatment may have been seen outside the clinical trial setting, although we are currently unaware of any such reports. Additional encephalitis cases have been reported in association with ipilimumab [24], [25], [26], [29] and pembrolizumab [27], [30], [31].
The presenting symptoms of encephalitis are quite heterogeneous, causing difficulty in diagnosis. As with other irAEs, time to onset of encephalitis varies, emphasizing the need for patient and caregiver education and the health care provider's continuous monitoring and awareness. Early recognition and intervention with high‐dose steroids are important in the successful management and resolution of irAEs associated with immune checkpoint inhibitors [46] and are likely also to result in better outcomes for patients who develop encephalitis.
Acknowledgments
This study was supported by Bristol‐Myers Squibb, Princeton, New Jersey, USA. Professional medical writing and editorial assistance were provided by Melissa Kirk, Ph.D., C.M.P.P., and Cara Hunsberger at StemScientific, an Ashfield Company, funded by Bristol‐Myers Squibb.
Author Contributions
Conception/Design: James Larkin, Bartosz Chmielowski, Christopher D. Lao, F. Stephen Hodi, William Sharfman, Hewei Li, Daniel Reshef, Alexandre Avila, David A. Reardon
Provision of study material or patients: James Larkin, Bartosz Chmielowski, Christopher D. Lao, F. Stephen Hodi, William Sharfman, Jeffrey Weber, Karijn P. M. Suijkerbuijk, Sergio Azevedo
Collection and/or assembly of data: James Larkin, Bartosz Chmielowski, Christopher D. Lao, F. Stephen Hodi, William Sharfman, Jeffrey Weber, Karijn P. M. Suijkerbuijk, Sergio Azevedo, Hewei Li, Daniel Reshef, Alexandre Avila, David A. Reardon
Manuscript writing: James Larkin, Bartosz Chmielowski, Christopher D. Lao, F. Stephen Hodi, William Sharfman, Jeffrey Weber, Karijn P. M. Suijkerbuijk, Sergio Azevedo, Hewei Li, Daniel Reshef, Alexandre Avila, David A. Reardon
Final approval of manuscript: James Larkin, Bartosz Chmielowski, Christopher D. Lao, F. Stephen Hodi, William Sharfman, Jeffrey Weber, Karijn P. M. Suijkerbuijk, Sergio Azevedo, Hewei Li, Daniel Reshef, Alexandre Avila, David A. Reardon
Disclosures
James Larkin: Pfizer, Bristol‐Myers Squibb, Novartis, Merck Sharp & Dohme (RF); Bartosz Chmielowski: Bristol‐Myers Squibb, Merck, Eisai, Immunocore, Amgen (CA), Genentech, Janssen (H); F. Stephen Hodi: EMD Serono, Merck, Novartis (CA), Bristol‐Myers Squibb (RF); William Sharfman: Bristol‐Myers Squibb, Merck, Novartis, Castlebiosciences (CA), Bristol‐Myers Squibb, Merck (RF); Jeffrey Weber: named on a patent related to a predictive biomarker for ipilimumab (IP), Bristol‐Myers Squibb, Merck, GlaxoSmithKline, AstraZeneca, Genentech, EMD Serono (CA), Bristol‐Myers Squibb, Merck (RF), Altor, Celldex, CytoMx (OI); Karijn P. M. Suijkerbuijk: Bristol‐Myers Squibb (CA), Roche (Other, travel expenses); Hewei Li: Bristol‐Myers Squibb (E, OI); Daniel Reshef: Bristol‐Myers Squibb (E, OI); Alexandre Avila: Bristol‐Myers Squibb (E, OI); David A. Reardon: Abbvie, Amgen, Bristol‐Myers Squibb, Cavion, Celldex, EMD Serono, Genetech/Roche, Inovio, Juno Pharmaceuticals, Pfizer (CA), Abbvie, Amgen, Bristol‐Myers Squibb, Cavion, Celldex, EMD Serono, Genetech/Roche, Inovio, Juno Pharmaceuticals, Merck (H), Celldex Therapeutics, Incyte, Midatech (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Hodi FS, O'Day SJ, McDermott DF et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Larkin J, Chiarion‐Sileni V, Gonzalez R et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Robert C, Long GV, Brady B et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 2015;372:320–330. [DOI] [PubMed] [Google Scholar]
- 4. Robert C, Schachter J, Long GV et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–2532. [DOI] [PubMed] [Google Scholar]
- 5. Weber JS, D'Angelo SP, Minor D et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti‐CTLA‐4 treatment (CheckMate 037): A randomised, controlled, open‐label, phase 3 trial. Lancet Oncol 2015;16:375–384. [DOI] [PubMed] [Google Scholar]
- 6. Postow MA, Callahan MK, Wolchok JD. Immune checkpoint blockade in cancer therapy. J Clin Oncol 2015;33:1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boutros C, Tarhini A, Routier E et al. Safety profiles of anti‐CTLA‐4 and anti‐PD‐1 antibodies alone and in combination. Nat Rev Clin Oncol 2016;13:473–486. [DOI] [PubMed] [Google Scholar]
- 8. Lancaster E. Paraneoplastic disorders. Continuum (Minneap Minn) 2015;21:452–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pignolet BS, Gebauer CM, Liblau RS. Immunopathogenesis of paraneoplastic neurological syndromes associated with anti‐Hu antibodies: A beneficial antitumor immune response going awry. Oncoimmunology 2013;2:e27384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Steinman L. Conflicting consequences of immunity to cancer versus autoimmunity to neurons: Insights from paraneoplastic disease. Eur J Immunol 2014;44:3201–3205. [DOI] [PubMed] [Google Scholar]
- 11. Topalian SL, Sharpe AH. Balance and imbalance in the immune system: Life on the edge. Immunity 2014;41:682–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hottinger AF. Neurologic complications of immune checkpoint inhibitors. Curr Opin Neurol 2016;29:806–812. [DOI] [PubMed] [Google Scholar]
- 13.YERVOY® (ipilimumab) [package insert]. Princeton, NJ: Bristol‐Myers Squibb Company USA; 2015.
- 14. Tarhini A. Immune‐mediated adverse events associated with ipilimumab CTLA‐4 blockade therapy: The underlying mechanisms and clinical management. Scientifica (Cairo) 2013;2013:857519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liao B, Shroff S, Kamiya‐Matsuoka C et al. Atypical neurological complications of ipilimumab therapy in patients with metastatic melanoma. Neuro Oncol 2014;16:589–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ibrahim RA, Berman DM, DePril V et al. Ipilimumab safety profile: Summary of findings from completed trials in advanced melanoma. J Clin Oncol 2011;29(suppl):8583a. [Google Scholar]
- 17.OPDIVO® (nivolumab) [package insert]. Princeton, NJ: Bristol‐Myers Squibb Company USA;2016.
- 18. Polat P, Donofrio PD. Myasthenia gravis induced by nivolumab therapy in a patient with non‐small‐cell lung cancer. Muscle Nerve 2016;54:507. [DOI] [PubMed] [Google Scholar]
- 19. Shirai T, Sano T, Kamijo F et al. Acetylcholine receptor binding antibody‐associated myasthenia gravis and rhabdomyolysis induced by nivolumab in a patient with melanoma. Jpn J Clin Oncol 2016;46:86–88. [DOI] [PubMed] [Google Scholar]
- 20. Mandel JJ, Olar A, Aldape KD et al. Lambrolizumab induced central nervous system (CNS) toxicity. J Neurol Sci 2014;344:229–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.KEYTRUDA® (pembrolizumab) [package insert]. Whitehouse Station, NJ: Merck & Co. USA, Inc.;2015.
- 22. Muro K, Chung HC, Shankaran V et al. Pembrolizumab for patients with PD‐L1‐positive advanced gastric cancer (KEYNOTE‐012): A multicentre, open‐label, phase 1b trial. Lancet Oncol 2016;17:717–726. [DOI] [PubMed] [Google Scholar]
- 23. Zimmer L, Goldinger SM, Hofmann L et al. Neurological, respiratory, musculoskeletal, cardiac and ocular side‐effects of anti‐PD‐1 therapy. Eur J Cancer 2016;60:210–225. [DOI] [PubMed] [Google Scholar]
- 24. Boyd K, Kalladka D, Overell J et al. Ipilimumab induced encephalitis: A case report. Immunome Res 2015;11:092. [Google Scholar]
- 25. Carl D, Grüllich C, Hering S et al. Steroid responsive encephalopathy associated with autoimmune thyroiditis following ipilimumab therapy: A case report. BMC Res Notes 2015;8:316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Conry RM, Sullivan JC, Nabors LB 3rd Ipilimumab‐induced encephalopathy with a reversible splenial lesion. Cancer Immunol Res 2015;3:598–601. [DOI] [PubMed] [Google Scholar]
- 27. Salam S, Lavin T, Turan A. Limbic encephalitis following immunotherapy against metastatic malignant melanoma. BMJ Case Rep 2016;2016:pii:bcr2016215012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Williams TJ, Benavides DR, Patrice KA et al. Association of autoimmune encephalitis with combined immune checkpoint inhibitor treatment for metastatic cancer. JAMA Neurol 2016;73:928–933. [DOI] [PubMed] [Google Scholar]
- 29. Maur M, Tomasello C, Frassoldati A et al. Posterior reversible encephalopathy syndrome during ipilimumab therapy for malignant melanoma. J Clin Oncol 2012;30:e76–e78. [DOI] [PubMed] [Google Scholar]
- 30. LaPorte J, Solh M, Ouanounou S. Posterior reversible encephalopathy syndrome following pembrolizumab therapy for relapsed Hodgkin's lymphoma. J Oncol Pharm Pract 2017;23:71–74. [DOI] [PubMed] [Google Scholar]
- 31. Khoja L, Maurice C, Chappell M et al. Eosinophilic fasciitis and acute encephalopathy toxicity from pembrolizumab treatment of a patient with metastatic melanoma. Cancer Immunol Res 2016;4:175–178. [DOI] [PubMed] [Google Scholar]
- 32. Tunkel AR, Glaser CA, Bloch KC et al. The management of encephalitis: Clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis 2008;47:303–327. [DOI] [PubMed] [Google Scholar]
- 33. Bloch KC, Glaser C. Diagnostic approaches for patients with suspected encephalitis. Curr Infect Dis Rep 2007;9:315–322. [DOI] [PubMed] [Google Scholar]
- 34. Kazandjian D, Suzman DL, Blumenthal G et al. FDA approval summary: Nivolumab for the treatment of metastatic non‐small cell lung cancer with progression on or after platinum‐based chemotherapy. The Oncologist 2016;21:634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Postow MA, Chesney J, Pavlick AC et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Topalian SL, Hodi FS, Brahmer JR et al. Safety, activity, and immune correlates of anti‐PD‐1 antibody in cancer. N Engl J Med 2012;366:2443–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Topalian SL, Sznol M, McDermott DF et al. Survival, durable tumor remission, and long‐term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 2014;32:1020–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wolchok JD, Kluger H, Callahan MK et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 2013;369:122–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Urba WJ, Martin‐Algarra S, Callahan M et al. Immunomodulatory activity of nivolumab monotherapy in patients with advanced melanoma. Paper presented at the AACR Annual Meeting; April 18–22, 2015; Philadelphia, PA.
- 40. Weber JS, Gibney G, Sullivan RJ et al. Sequential administration of nivolumab and ipilimumab with a planned switch in patients with advanced melanoma (CheckMate 064): An open‐label, randomised, phase 2 trial. Lancet Oncol 2016;17:943–955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hodi FS, Chesney J, Pavlick AC et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2‐year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol 2016;17:1558–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ascierto PA, Demidov LV, Garbe C et al. Nivolumab (NIVO) safety in patients with advanced melanoma (MEL) who have progressed on or after ipilimumab (IPI): a single‐arm, open‐label, multicenter, phase II study (CheckMate 172). J Clin Oncol 2016;34(suppl):9526a. [Google Scholar]
- 43. Chapman PB, Sznol M, Lao C et al. Safety data from an expanded access program (EAP) of nivolumab (NIVO) in combination with ipilimumab (IPI) in patients with advanced melanoma (MEL). J Clin Oncol 2016;34(suppl):9525a. [Google Scholar]
- 44. Yang JC, Hughes M, Kammula U et al. Ipilimumab (anti‐CTLA4 antibody) causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother 2007;30:825–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bompaire F, Mateus C, Taillia H et al. Severe meningo‐radiculo‐neuritis associated with ipilimumab. Invest New Drugs 2012;30:2407–2410. [DOI] [PubMed] [Google Scholar]
- 46. Champiat S, Lambotte O, Barreau E et al. Management of immune checkpoint blockade dysimmune toxicities: A collaborative position paper. Ann Oncol 2016;27:559–574. [DOI] [PubMed] [Google Scholar]