By applying an exploratory cross‐sectional survey, this study evaluates current referral practices of a large sample of community‐based physicians caring for post‐treatment cancer survivors. The accessibility, availability, and benefits of psycho‐oncology services are explored, as well as who provides psychosocial support and predictors for impeded referral to services.
Keywords: Neoplasms, Clinical oncology, Health psychology, General practice, Health services research
Abstract
Background.
As persons of trust, community‐based physicians providing survivorship care (e.g., general practitioners [GPs]) often serve as the primary contacts for cancer survivors disclosing distress. From the perspective of physicians providing survivorship care for cancer patients, this study explores (a) the accessibility, availability, and potential benefits of psycho‐oncology services; (b) whether physicians themselves provide psychosocial support; and (c) predictors for impeded referrals of survivors to services.
Methods.
In a cross‐sectional survey, all GPs and community‐based specialists in a defined region were interviewed. In addition to descriptive analyses, categorical data were investigated by applying chi‐square tests. Predictors for impeded referrals were explored through logistic regression.
Results.
Of 683 responding physicians, the vast majority stated that survivors benefit from psycho‐oncology services (96.8%), but the physicians also articulated that insufficient coverage of psycho‐oncology services (90.9%) was often accompanied by impeded referrals (77.7%). A substantial proportion (14.9%) of physicians did not offer any psychosocial support. The odds of physicians in rural areas reporting impeded referrals were 1.91 times greater than the odds of physicians in large urban areas making a similar report (95% confidence interval [1.07, 3.40]).
Conclusion.
Most community‐based physicians providing survivorship care regard psycho‐oncology services as highly beneficial. However, a large number of physicians report tremendous difficulty referring patients. Focusing on those physicians not providing any psychosocial support, health policy approaches should specifically (a) raise awareness of the role of physicians as persons of trust for survivors, (b) highlight the effectiveness of psycho‐oncology services, and (c) encourage a proactive attitude toward the assessment of unmet needs and the initiation of comprehensive care.
Implications for Practice.
Community‐based physicians providing survivorship care for cancer patients regard psycho‐oncology services as a highly reasonable and beneficial addition to medical care. In light of insufficient local coverage with services, difficulties with seamless referrals constitute a major challenge for physicians. Apart from emphasizing the effectiveness of psycho‐oncology services and proactive attitudes toward the assessment of unmet needs, future policies should focus on the integration of medical and psychosocial follow‐up of cancer survivors, especially in rural areas.
Background and Rationale
Cancer survivors have a higher prevalence of mental disorders than the general population across various tumor types [1], [2], [3]. During disease trajectory, one in three cancer survivors suffers from clinically relevant mental health problems, most frequently depressive or anxiety disorders that require professional psychosocial support. Psychosocial interventions have shown to provide efficient amelioration [4], [5], [6]. Therefore, the current National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology recommend regular screening for distress during patients’ initial visits and then at appropriate intervals, particularly with changing disease or treatment status [7]. In cases of evident moderate or severe distress, the guidelines request oncologists refer patients to specialized psycho‐oncology services (i.e., psychiatrists/psychotherapists, counseling services, social workers, or peer support groups). Within the context of highly efficient psychosocial interventions being available, a wide consensus on best practice for distress in cancer survivors has been established on the level of formal recommendations [8].
However, it has recently been reported that distress screening and timely transition of severely distressed cancer survivors to mental health services are often not implemented in routine clinical practice [9], [10]. This trend especially holds true for primary care practices, where survivorship care for cancer survivors usually takes place. At this stage of the disease trajectory, the healthcare needs of post‐treatment cancer survivors mainly focus on psychosocial support [11], [12]. However, one in two community‐based physicians (CBPs) is not broadly involved in psychosocial care, according to self‐reported practices [13], [14]. Consequently, a substantial proportion of cancer survivors do not receive adequate treatment [3], [15]. Lack of information regarding existing specialized services and lack of referrals have consistently been reported as main barriers by survivors [15], [16], [17]. In contrast, the perspectives of physicians, who conduct survivorship care on current practice of referrals to mental health services, have been rarely assessed [15]. In a U.S. survey, fewer than half of the responding oncologists reported referring survivors to psychosocial services [12]. There is some evidence, mostly from qualitative findings or surveys relying on small and heterogeneous samples, that these physicians describe reluctance to psychosocial approaches, late referrals to appropriate services, and, most importantly, lack of referral systems [18], [19], [20]. Availability of services appears to be a major problem, specifically in rural areas, where services are fewer while the likelihood of being affected by distress is assumed to be higher [21], [22], [23]. Even if services do exist, according to survivors, they are often not accessible or not tailored to cancer survivors [24], [25]. Nevertheless, general practitioners (GPs) and CBPs following up on cancer survivors are often the most important contact persons for survivors regarding medical and psychological problems, as well as functioning as gatekeepers to secondary care [6], [11]. However, to the best of our knowledge, extensive data from CBPs involved in survivorship care on their attitudes toward psycho‐oncology services, perceived barriers, and their referral practice and own involvement are lacking.
Therefore, by applying an exploratory cross‐sectional survey, this study aims for an evaluation of current referral practice in a large sample of CBPs caring for post‐treatment cancer survivors. First, we will present results for three physician groups stratified by different board certifications (GPs, gynecologists, urologists) regarding their perspective on accessibility, availability, and potential benefits of psycho‐oncology services overall, as well as the physicians’ own involvement in the provision of psychosocial support for cancer survivors. According to the German National Guideline for Psychosocial Assessment, Counseling and Treatment of Adult Cancer Patients, psycho‐oncology care is defined as nonpharmacological (education, resource‐oriented interventions, psychotherapeutic techniques) [26] or psychopharmacological interventions [27] that are provided by specialized cancer counseling centers, psychotherapists/psychiatrists, or physicians. Within the German healthcare system in which our study took place, CBPs treat patients with a variety of health problems, including complex multimorbidity. They usually do not employ psychologists or social workers. However, a supplementary qualification in biobehavioral medicine and psychosocial support is available for practicing medical specialists (called “psychosomatic basic care”) [28]. The 80‐hour training program leading to this degree comprises basic theoretical and clinical instruction on both fundamental psychotherapeutic and psychopharmacological interventions, along with communication training with patients [28]. Psychosomatic basic care is also an integral part of specialist training for primary care physicians (PCPs), gynecologists and urologists. However, this training program is not tailored to the special needs of cancer patients; therefore, trained physicians may also need to refer to other services. Second, we will analyze the data with regard to the degree of urbanization. Third, we will further explore physician‐sided predictors for impeded referrals, which we defined as prompt secondary specialist care not being readily available for burdened cancer patients. Addressing both clinicians and policy‐makers, we will provide information on the current “real‐world” practice of interface management between CBPs providing cancer survivorship care and mental health specialists, and clarify potential gaps in psycho‐oncology care.
Methods
Study Design and Setting
We conducted a prospective, cross‐sectional survey with GPs and office‐based consultants (gynecologists and urologists) to whom cancer patients are usually referred to after finishing active treatment. In Germany, where this study occurred, as in many other countries (except in the U.S., where formal guidelines on cancer survivorship care exist), no formal and concise delineation of responsibility for cancer follow‐up care exists [29]. Consequently, the above‐mentioned physician groups were assumed to carry out at least major parts of this follow‐up care. The current survey was embedded in a larger evaluation study within the German National Cancer Plan, entitled “Comparison of two psychosocial cancer care models for rural areas: the P‐O‐LAND study,” wherein two demographically comparable study regions in southern Germany with 1.2 million inhabitants were assessed. This study was approved by the Ethics Committee of Heidelberg Medical School (Registration‐No. S‐300/2013) and is reported according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) standards [30].
Participants
For representative sampling, all physicians with offices located in these areas were eligible. Physicians offering cancer survivorship care were identified from the mandatory registries of the regional Associations of Statutory Health Insurance Physicians. Individuals confirmed that they provided cancer survivorship care, although no minimum number of treated survivors was defined. For data collection, all eligible subjects were simultaneously asked to complete an anonymous paper‐and‐pencil self‐reported questionnaire and return it either via fax or postage‐paid envelopes. We explicitly encouraged participants to answer honestly, and we aimed for a high response rate by offering individual monetary compensation of $11 (U.S. dollar) and by reminding nonresponders with up to three subsequent postal mailings [31].
Measurements and Variables
The one‐page self‐reported questionnaire comprised 12 items and was developed based on a focus group of PCPs during a continuing education seminar. The physicians’ statements were converted to items with which participants could agree or disagree. In addition to items regarding physicians’ perceptions toward accessibility (items 1 and 2), availability (item 5), and benefit (items 3 and 4) of psychosocial care, we also asked the physicians to indicate their own practice patterns (items 6 and 12) and specialized qualifications (items 10 and 11). Three items collected knowledge regarding offers of and wait times for psychosocial services (items 7 to 9) and are not analyzed within this manuscript. The questionnaire was pretested for content validity and interpretation reliability with three physicians not participating in the survey. The survey is available as a supplementary document online (supplemental online Appendix 1). To objectively classify practice location into “rural” and “urban,” we followed the stratification of the degree of urbanization (DEGURBA) introduced by the European Commission, which distinguishes three types of areas: (a) densely populated areas (hereinafter named “large urban areas”), (b) intermediate density areas (“small urban areas”), and (c) thinly populated areas (“rural areas”) [32]. Based on the population grid, the DEGURBA classification has been implemented as the European standard for all surveys to harmonize previous spatial concepts.
Statistical Methods
Data analysis was conducted using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). Apart from descriptive analyses, categorical data were investigated by applying chi‐square tests. Where applicable, Pearson's correlation coefficient r was computed as a standard measure of effect size for findings. For coefficient r, small, medium, and large effect sizes were .10, .30, and .50, respectively [33]. To predict the dichotomous outcome variable “impeded referral (no = 0/yes = 1)”, we fitted a linear logistic regression model for discrete response data [34], [35]. We applied the method of maximum likelihood and effect coding of classification variables. Based on prior knowledge, sociodemographic and practice‐related predictors served as explanatory variables. To investigate representativeness of the sample, nonresponder analyses running Pearson's chi‐square tests for gender, specialization, and area type were conducted. To account for missing data, we computed imputation‐adjusted statistics for sample survey data by applying hot deck imputation (PROC SURVEYIMPUTE with approximate Bayesian bootstrap technique with 5 imputed datasets). However, comparison of the results with those from the complete case analysis showed no major differences; therefore, the results from the complete case analysis are presented. For all analyses, statistical significance was evaluated with a type 1 error of 5% (two‐tailed). Due to the explanatory approach of our analysis, we omitted the Bonferroni correction for controlling the family‐wise error rate.
Results
Sample Characteristics
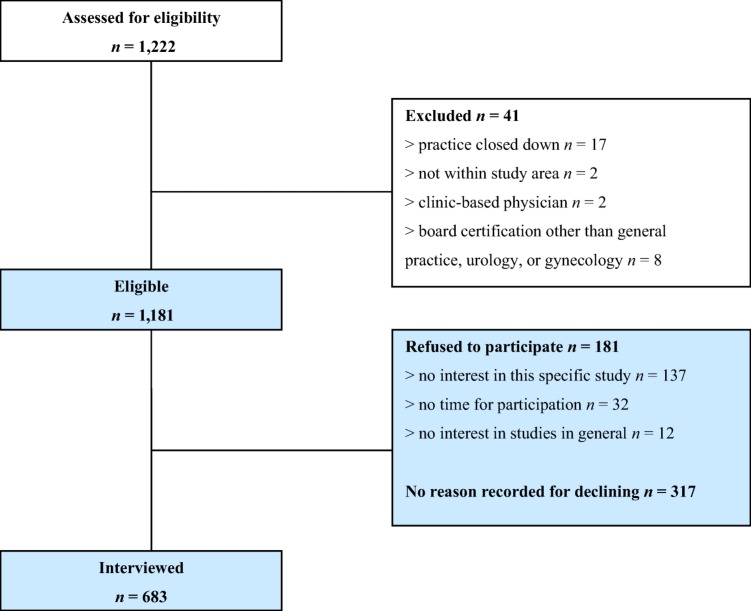
Of the 1,193 eligible physicians who all received an invitation to participate, 683 responded and were included in the analysis (Fig. 1). The total response rate amounted to 57%, with response rates of 59% (n = 221) from rural, 59% (n = 350) from small urban, and 50% (n = 112) from large urban areas. Subjects who passively or actively refused participation did not differ significantly from those included with respect to gender (p = .401) or board certification (GP, gynecology, urology) (p = .573). Subjects from rural and small urban areas responded more frequently than those from large urban areas: χ2(2) = 6.50 (p < .05). However, with r = .06, 95% confidence interval (CI) (.00, .11), the determined effect size was small. In other aspects, representativeness could not be assessed. Overall, the main reasons for nonparticipation assessed during follow‐up calls were lack of interest in the study and time constraints. The socio‐demographic characteristics of the participating physicians are depicted in Table 1.
Figure 1.
Flow diagram of recruitment.
Table 1. Socio‐demographic characteristics of participating physicians providing survivorship care.
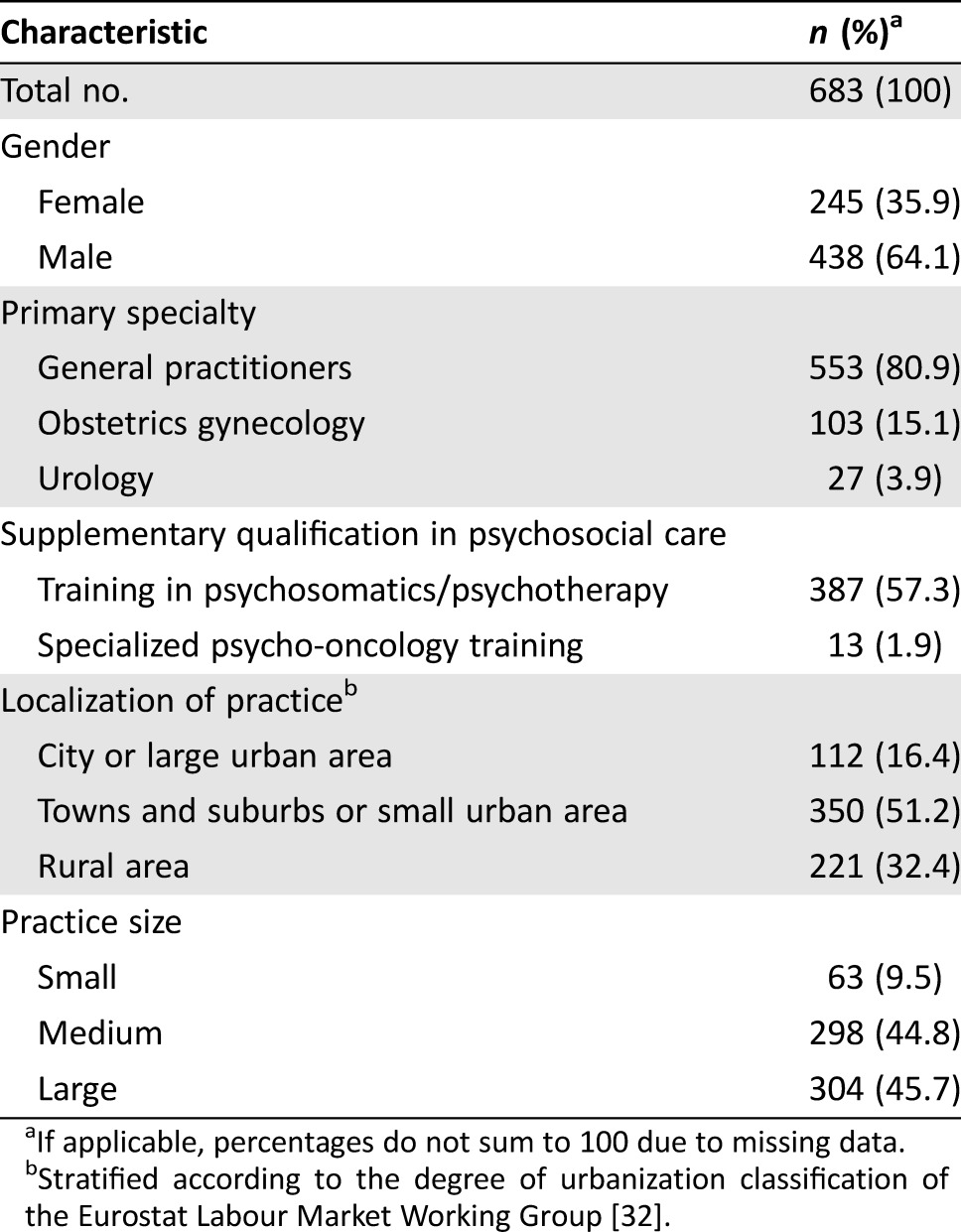
If applicable, percentages do not sum to 100 due to missing data.
Stratified according to the degree of urbanization classification of the Eurostat Labour Market Working Group [32].
Potential Benefits and Effectiveness of Psycho‐Oncology Care
Overall, almost all participating physicians viewed psychosocial care as a reasonable addition to medical care (97.3%) and stated that cancer survivors benefit from these services (96.8%) (Table 2). These ratings did not differ with regard to board certification, acquired supplementary psychosocial qualification, or localization of practice (Table 3).
Table 2. Overall reporting of accessibility, availability, and potential benefits of psycho‐oncology care.
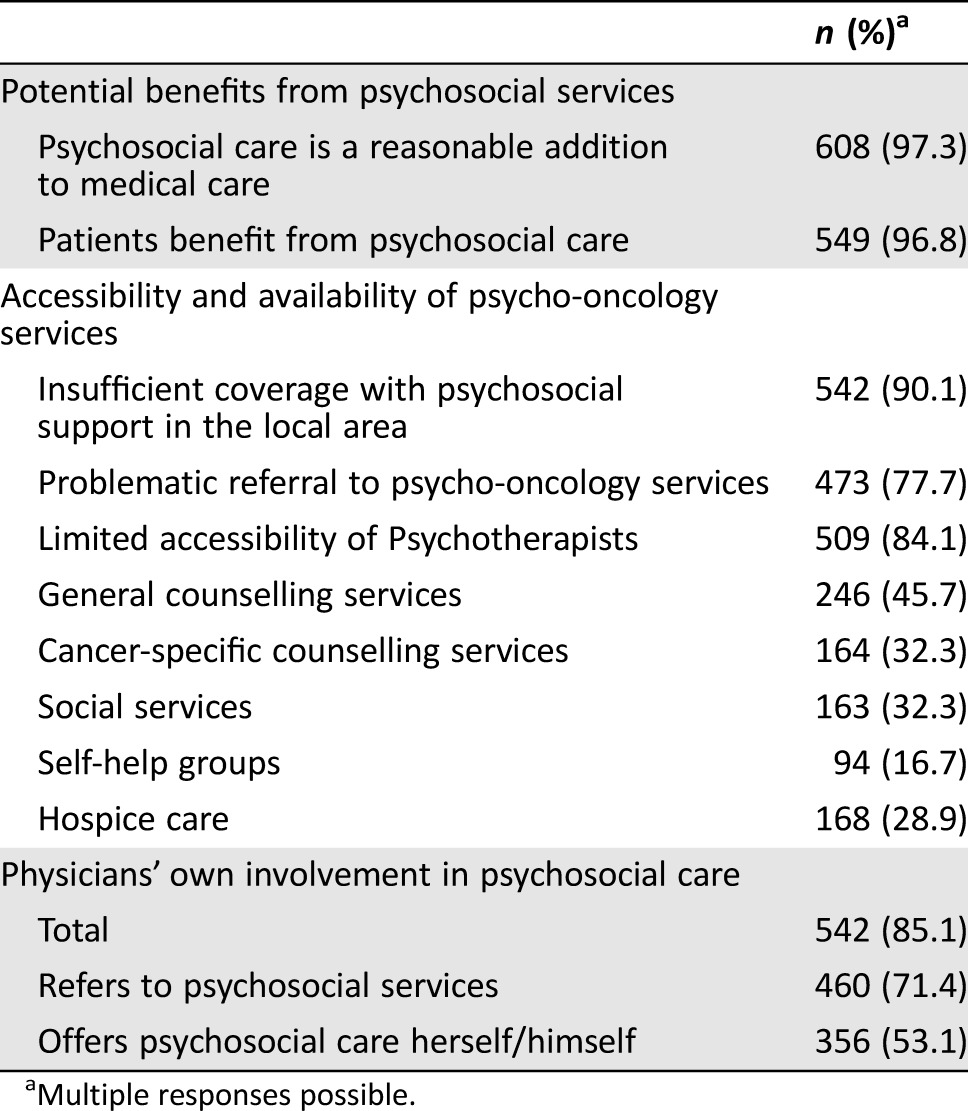
Multiple responses possible.
Table 3. Comparisons of observed frequencies between physicians stratified by office localizationa.
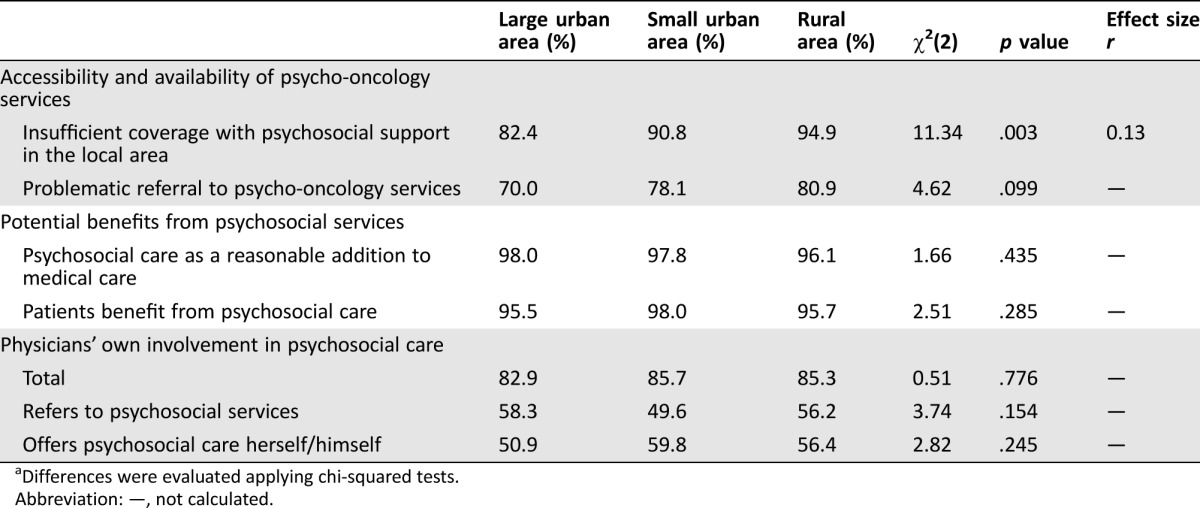
Differences were evaluated applying chi‐squared tests.
Abbreviation: —, not calculated.
Availability and Accessibility of Psycho‐Oncology Services
In contrast to the benefit of psycho‐oncology services for cancer survivors observed by the physicians, the vast majority of physicians articulated insufficient local coverage with psycho‐oncology services (90.9%) often accompanied by impeded referrals (77.7%) (Table 2). According to 84.1% of the physicians, accessibility of psychotherapists was particularly difficult. More seamless referrals were observed with counseling services and self‐help groups. With regard to urban‐rural disparities, insufficient coverage from the physicians’ perspectives was significantly higher in rural areas, χ2(2) = 11.34 (p < .01), although this effect was small, with r = .13 (95% CI [.05, .21]) (Table 3). Concerning impeded referrals, this difference did not reach the significance level, but a clear trend showing more difficult transitions in rural areas was observed (p = .099). We did not observe area‐specific differences in relation to the above‐mentioned types of psycho‐oncology services. A subgroup analysis revealed that the few physicians who disagreed with the benefits of psychosocial services were more likely to report insufficient coverage and difficult referrals: χ2(1) = 5.69 (p < .05 with r = .10, 95% CI [.02, .18]).
Physicians’ Own Involvement in the Provision of Psychosocial Support
Although approximately 9 out of 10 physicians offering cancer survivorship care either referred cancer survivors to psycho‐oncology services (71.4%) or offered basic psychosocial care themselves (53.1%), a substantial percentage (14.9%) (n = 95) neither offered any form of psychosocial support themselves nor referred cancer survivors to existing services (Table 2). This observation did not differ significantly when we accounted for degree of urbanization (Table 3). Less surprisingly, physicians with supplementary psychosocial qualifications were more likely to deliver psychosocial support themselves: χ2(1) = 21.15 (p < .0001, relative risk [RR] = 1.14, 95% CI [1.03, 1.27]). However, physicians who provided psychosocial support were generally as likely to refer cancer survivors to specialized services as those who did not offer support: χ2(1) = 2.44 (p = .12, RR = 0.88, 95% CI [0.74, 1.04]).
Physician‐Sided Predictors for Impeded Referral of Cancer Survivors to Psycho‐Oncology Services
To predict impeded referrals from physician‐sided factors, we conducted a logistic regression with socio‐demographic characteristics as predictor variables (gender, board certification: GP/gynecologist/urologist, acquired supplementary psychosocial qualification, localization of practice with regard to degree of urbanization, and office size) and an observation of impeded referral (yes/no) as a dichotomous outcome variable. The findings are presented in Table 4. Regarding individual predictors, we observed a trend for localization of practice: Wald's χ2(2) = 5.04 (p = .081). When specifying contrasts, we detected that the odds of physicians in rural areas stating difficulties with referring cancer survivors to local psychosocial services were 1.91 times greater than the odds of physicians in large urban areas stating similar difficulties (odds ratio [OR] = 1.91, 95% CI [1.07, 3.40]). With respect to board certification, we observed that the odds of GPs exhibiting problems with referrals were 1.72 times greater than the odds of gynecologists (OR = 1.72, 95% CI [1.03, 2.88]). Regarding measures of association, the c‐statistic (c = .604) indicated that the presented model correctly predicted a higher probability of observations with the event outcome (impeded referral) compared with the probability of nonevent observations (referral unproblematic) for 60.4% of all possible pairs of physicians. Therefore, it seems likely that physicians with offices located in rural areas perceive more problems with referrals to specialized services than do physicians in urban areas.
Table 4. Physician‐sided predictors for impeded referral of cancer survivors to psycho‐oncology services (probability modeled for impeded referral = “yes”).
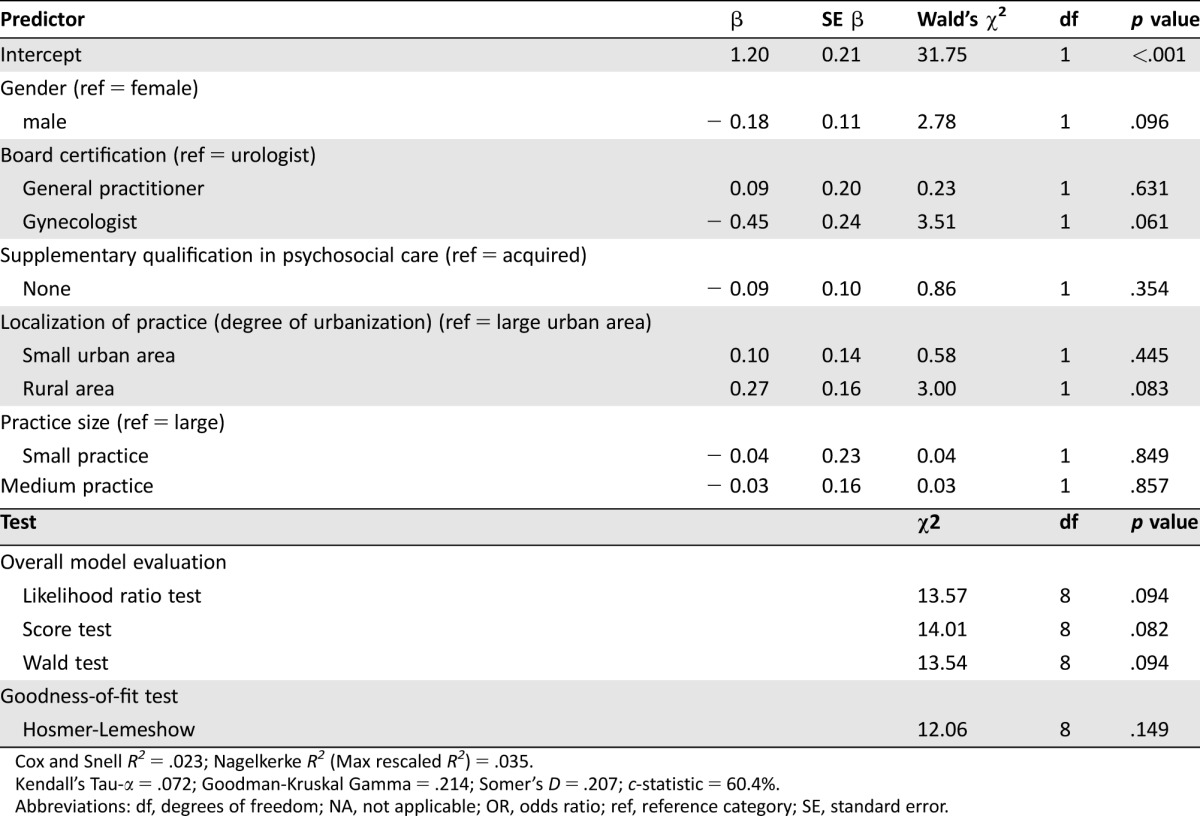
Cox and Snell R2 = .023; Nagelkerke R2 (Max rescaled R2) = .035.
Kendall's Tau‐α = .072; Goodman‐Kruskal Gamma = .214; Somer's D = .207; c‐statistic = 60.4%.
Abbreviations: df, degrees of freedom; NA, not applicable; OR, odds ratio; ref, reference category; SE, standard error.
Discussion
To the best of our knowledge, this is one of the first studies that examined the perspective of CBPs on psychosocial cancer survivorship care. This study provides a thorough examination of the potential benefits and accessibility of psycho‐oncology services in urban and rural areas. Furthermore, we presented findings on physicians’ own involvement in the provision of psychosocial support and physician‐sided predictors for impeded referrals to specialized services. First, the vast majority of responding physicians described substantial benefits of psycho‐oncology services and significant benefits for cancer survivors. Second, although a majority of physicians provided some nature of psychosocial support themselves, one in six physicians entirely refrain from doing so. Third, in contrast to the aforementioned results, an overwhelming proportion of practitioners indicated insufficient local coverage of psycho‐oncology services, along with impeded referral processes. Fourth, when compared with large urban areas, practices located in a rural area emerged as the main predictor for impeded referrals. These key results indicate an extra shortage of psycho‐oncology services, especially in rural areas, which is highly problematic when combined with the situation in which a treating physician does not provide such services, either.
With respect to the overall benefits of psycho‐oncology services, our findings corroborate previous investigations emphasizing the great benefit for cancer survivors to receive psycho‐oncology services assigned by their CBPs. In a sample interviewed by Giudice et al., 98.2% of physicians stated the beneficial effects of psycho‐oncology services, although only 56.0% of physicians were actually able to offer psychosocial support themselves [19]. Our comparable results on physician‐reported benefits of psycho‐oncology services are also in accordance with another practice‐based report stating positive attitude and subjective norm of health professionals as predictors for referrals to these services [23]. Nevertheless, we would like to emphasize that quality of psychosocial care is not only a function of physician acknowledgement of patient benefit; it also includes patient‐provider communication, integration of biomedical and psychosocial care, training and professional development, and ongoing evaluation throughout a continuum of care [36].
Concerning the physicians’ own involvement, we replicated the report of 70.0% of providers referring patients to psycho‐oncology services that was found in a U.S. sample by Eakin and Strycker [16]. Remarkably, but reinforced by subsequent work [12], the authors emphasized the discrepancy between providers’ reported referral rates and estimates of actual patient use. Nevertheless, there also seems to be a constant number of physicians who do not become involved in the provision of psychosocial support. Specifically, Kam et al. found that 11.3% of oncology professionals never referred their patients even to a basic form of psychosocial support, such as a cancer helpline [23]. In our sample, a similar percentage (15%) of the physicians solely focused on medical treatment in their survivorship care without considering psychosocial support or referral. This observation is in remarkably high contrast to the recently observed 4‐week prevalence of mental disorders in 31.8% of cancer patients, who consequently need psychosocial support [2]. From the physicians’ perspective, recent analyses have revealed that lack of ownership, poor education regarding distress in cancer, insufficient consultation time, and lack of access to appropriately qualified mental health specialists constitute the main barriers for implementing clinical pathways in psychosocial follow‐up care [12], [15], [37], [38]. Other authors have raised the hypothesis that difficulties in addressing distress may be the result of a collusion of avoidance between physicians and patients in light of a life‐threatening illness [19]. For nonreferrals, lack of awareness of available psychosocial services has been discussed as a main barrier [16], [17]. Nevertheless, previous data also indicate that awareness is not a key impediment to referral, but attitudes to these services seem to be a main predictor [23]. The latter linkage is in accordance with the results of our subgroup analysis on physicians who negated the benefits of psycho‐oncology services, although the direction of this association could not be assessed within our design. However, reflection of physicians’ own values is a key factor for oncologists throughout the trajectory of a cancer disease [39]. In our study, the vast majority of participating physicians rated both benefits and efficacy as high, and they were willing to engage themselves in psychosocial support (85%). Accordingly, in a U.S. survey, Muriel and colleagues reported that 95% of the responding oncologists delivered some form of psychosocial support [12].
Despite a high value of psychosocial services, community‐based practitioners stated insufficient availability of services as a main problem. Within the German regular care setting, which is based on free healthcare provided by a statutory contribution system [40], capacities for psychosocial care are substantial, but long wait lists and the special needs of cancer survivors decrease accessibility. Urban‐rural disparities in coverage with psycho‐oncology services have been scarcely assessed thus far. Our finding that physicians in rural areas perceive more problems with referrals is in accordance with previous work. From the provider perspective, lack of local services for remote cancer survivors has previously been identified as a predictor of referrals to psycho‐oncology services [23]. However, in southwestern Germany, at least where the investigation took place, small differences in effect size existed. Accounting for the survivor perspective, Weaver et al. recently demonstrated yet again that even many years after their cancer diagnosis, rural cancer survivors are at a greater risk for a variety of poor health outcomes [25]. Recently, a systematic investigation demonstrated that patients in the Greater Munich area in Germany have fewer psychosocial services at a manageable distance from their home [21]. However, in this study, no group differences between urban and rural patients with reference to the frequency of both significant clinical distress and psychosocial services utilization were identified; however, overall acceptance of psychosocial support was rather low. Alternatively, the doctor‐patient relationship as the only significant predictor of mental health outcome was emphasized. Attributing minor importance to the availability of services, the authors hypothesize barriers, such as fear of stigmatization and prejudices toward psychotherapy, which are more common in rural areas [41]. Our study adds the physicians’ perspective to this body of knowledge and demonstrates that survivorship care providers located in truly rural areas indeed report more difficulties with referrals to psychosocial services. In conjunction with the above‐mentioned existing literature, multifactorial explanations not limited to the mere availability of local services must be taken into account.
Given the naturalistic context of the survey, some methodological limitations as sources of potential bias have to be discussed. First, we relied on self‐reports, a potential fallible source of data that may underestimate the proportion of unproblematic referrals as well as the local coverage with psycho‐oncology services due to recall bias. We suggest that future studies assess whether and how many referred patients receive adequate psychosocial services (according to GPs and to patients). Second, pretesting of our questionnaire was fairly limited, which may have resulted in reduced content validity for its items, especially for those who asked physicians to report their own behavior. Third, self‐reported behavior may not accurately reflect clinical practice. For feasibility reasons, in light of very limited staff time, we did not observe real clinical practices on site. Therefore, we dispensed with increasing the validity for measuring physicians’ actual behaviors [42]. Additionally, we agree with Forsythe et al. in emphasizing the need for studies that directly measure actual physician behavior by applying direct observation or record review [14]. Nevertheless, we tried to reduce the likelihood of social desirability and leniency bias by collecting data through self‐administered questionnaires in an anonymous mailed survey [43]. Fourth, concerning the DEGURBA classification, it should be noted that categorical definitions of urbanization have been demonstrated to mask hidden heterogeneity in very rural areas with regard to healthcare access outcomes [44]. Therefore, it cannot be ruled out that in some rural areas, coverage with and accessibility of psycho‐oncology services is actually better than overall results suggest. Fifth, the rather moderate response rate was in accordance with previous surveys of similar content in primary care but notes the possibility that physicians more sensitive to psychosocial issues were more likely to participate [12], [15], [45], [46]. Remarkably, it has been demonstrated that survey accuracy does not necessarily depend on high response rate [43], and the relatively large proportion of physicians indicating no provision of psychosocial support argues against a major recruitment bias. Sixth, regarding representativeness of the sample, subjects from rural and small urban areas were more likely to participate. However, this effect was very small, indicating at most a marginal impact on the sample composition. With reference to generalizability, our results may not directly be transferred to (a) patients recently diagnosed with cancer or undergoing treatment or (b) other countries with differently structured healthcare systems. Seventh, we were not able to fully explore the specificity of our results with regard to psycho‐oncology care, as we did not interview the participants on their referral experiences with other medical disciplines (e.g., cardiology or even psychiatry itself). Finally, the cross‐sectional nature of the study precludes causal inferences regarding associations between the degree of urbanization and insufficient coverage and impeded referral.
Conclusion
A fair number of cancer survivors experience severe psychological distress and articulate substantial unmet needs for specialized psychosocial support [3], [47]. The primary care physician, and, in many cases, the main provider of survivorship care, is usually the contact person for the survivor to address these needs and to initiate appropriate psychosocial treatment. Most physicians report tremendous difficulty with seamless referrals and insufficient local coverage with services. This gap within the clinical pathway can only partly be compensated by physicians’ own involvement in the delivery of psychosocial support, as indicated by the observation that, with respect to the referral of specialized services, the likelihood of physicians providing support did not differ from that of their counterparts who denied offering support. As a consequence, it can be assumed that this gap will lead more often to increased resource utilization of acute services (e.g., emergency departments) with higher healthcare costs as a result, as this phenomenon has been reported many times in earlier work [48], [49], [50]. The situation is more dramatic in rural areas, but insufficient referral is not only a matter of external factors. First and foremost, regarding those CBPs who do not provide any psychosocial support, future health policy approaches should specifically (a) inform CBPs of their role as persons of trust for cancer survivors, (b) highlight the effectiveness of existing psycho‐oncology services, and (c) encourage a proactive attitude toward assessment of unmet needs and initiation of comprehensive care. Most primary care physicians involved in survivorship care are willing to provide psychosocial support for patients. However, being confronted with many healthcare demands, PCPs are not always able to take over the responsibility of all aspects of psychosocial follow‐ups. Our findings reinforce the need for a coordinated approach to facilitate a formal transition from primary care to specialized services in currently fragmented healthcare systems. Specifically, policies advocating a proactive attitude toward assessment of unmet needs (e.g., survivorship care planning) and interventions to improve primary care‐based referrals (e.g., involvement of local secondary care providers in dissemination activities of structured referral sheets) should be strengthened [51], [52], [53].
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
We thank all participating physicians. The study was funded as part of the National Cancer Plan by the German Federal Ministry of Health (Bundesministerium fuer Gesundheit, BMG). The funder was not actively involved in conduct of the study. The authors had full access to all of the data in this study and take complete responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
For Further Reading: Joseph A. Greer, Lara Traeger, Heather Bemis et al. Pilot Randomized Controlled Trial of Brief Cognitive‐Behavioral Therapy for Anxiety in Patients with Terminal Cancer. The Oncologist 2012;17:1337–1345.
Abstract: Introduction. Patients with terminal cancer often experience marked anxiety that is associated with poor quality of life. Although cognitive‐behavioral therapy (CBT) is an evidence‐based treatment for anxiety disorders, the approach needs to be adapted to address realistic concerns related to having cancer, such as worries about disease progression, disability, and death. In this pilot randomized controlled trial (clinicaltrials.gov identifier NCT00706290), we examined the feasibility and potential efficacy of brief CBT to reduce anxiety in patients with terminal cancer. Methods. We adapted CBT by developing treatment modules targeting skills for relaxation, coping with cancer worries, and activity pacing. Adults with incurable malignancies and elevated anxiety based on the Hamilton Anxiety Rating Scale (HAM‐A) were randomly assigned to individual CBT or a waitlist control group. Primary outcomes included the number of completed CBT visits and the change in HAM‐A scores from baseline to 8‐week follow‐up per a treatment‐blind evaluator. The feasibility criterion was 75% adherence to the intervention. Results. We randomized 40 patients with terminal cancers to CBT (n = −0) or waitlist control (n = −0) groups; 70% completed posttreatment assessments. Most patients who received CBT (80%) participated in at least five of the required six therapy sessions. Analysis of covariance models, adjusted for baseline scores, showed that those assigned to CBT had greater improvements in HAMA scores compared to the control group, with an adjusted mean difference of −5.41 (95% confidence interval: −10.78 to −0.04) and a large effect size for the intervention (Cohen's d = 0.80). Conclusion. Providing brief CBT tailored to the concerns of patients with terminal cancer was not only feasible but also led to significant improvements in anxiety.
Author Contributions
Conception/design: Markus W Haun, Verena Zimmermann‐Schlegel, Mechthild Hartmann, Wolfgang Herzog
Collection and/or assembly of data: Markus W Haun, Verena Zimmermann‐Schlegel, Halina Sklenarova
Data analysis and interpretation: Markus W Haun, Verena Zimmermann‐Schlegel, Mechthild Hartmann, Halina Sklenarova
Manuscript Writing: Markus W Haun, Verena Zimmermann‐Schlegel, Mechthild Hartmann
Final approval of manuscript: Markus W Haun, Verena Zimmermann‐Schlegel, Mechthild Hartmann, Halina Sklenarova, Wolfgang Herzog
Disclosures
The authors indicated no financial relationships.
Supplementary Information
References
- 1. Haun MW, Sklenarova H, Villalobos M et al. Depression, anxiety and disease‐related distress in couples affected by advanced lung cancer. Lung Cancer 2014;86:274–280. [DOI] [PubMed] [Google Scholar]
- 2. Mehnert A, Brahler E, Faller H et al. Four‐week prevalence of mental disorders in patients with cancer across major tumor entities. J Clin Oncol 2014;32:3540–3546. [DOI] [PubMed] [Google Scholar]
- 3. Whitney RL, Bell JF, Bold RJ et al. Mental health needs and service use in a national sample of adult cancer survivors in the USA: Has psychosocial care improved? Psychooncology 2015;24:80–88. [DOI] [PubMed] [Google Scholar]
- 4. Faller H, Schuler M, Richard M et al. Effects of psycho‐oncologic interventions on emotional distress and quality of life in adult patients with cancer: Systematic review and meta‐analysis. J Clin Oncol 2013;31:782–793. [DOI] [PubMed] [Google Scholar]
- 5. Greer JA, Traeger L, Bemis H et al. A pilot randomized controlled trial of brief cognitive‐behavioral therapy for anxiety in patients with terminal cancer. The Oncologist 2012;17:1337–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Heins MJ, Korevaar JC, Rijken PM, et al. For which health problems do cancer survivors visit their General Practitioner? Eur J Cancer 2013;49:211–218. [DOI] [PubMed] [Google Scholar]
- 7. Holland JC, Andersen B, Breitbart WS et al. Distress management. J Natl Compr Canc Netw 2013;11:190–209. [DOI] [PubMed] [Google Scholar]
- 8. Jacobsen PB, Wagner LI. A new quality standard: the integration of psychosocial care into routine cancer care. J Clin Oncol 2012;30:1154–1159. [DOI] [PubMed] [Google Scholar]
- 9. Mitchell AJ, Vahabzadeh A, Magruder K. Screening for distress and depression in cancer settings: 10 lessons from 40 years of primary‐care research. Psychooncology 2011;20:572–584. [DOI] [PubMed] [Google Scholar]
- 10. Zebrack B, Kayser K, Sundstrom L et al. Psychosocial distress screening implementation in cancer care: An analysis of adherence, responsiveness, and acceptability. J Clin Oncol 2015;33:1165–1170. [DOI] [PubMed] [Google Scholar]
- 11. Hoekstra RA, Heins MJ, Korevaar JC. Health care needs of cancer survivors in general practice: A systematic review. BMC Fam Pract 2014;15:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Muriel AC, Hwang VS, Kornblith A et al. Management of psychosocial distress by oncologists. Psychiatr Serv 2009;60:1132–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bober SL, Recklitis CJ, Campbell EG et al. Caring for cancer survivors: A survey of primary care physicians. Cancer 2009;115(suppl 18):4409–4418. [DOI] [PubMed] [Google Scholar]
- 14. Forsythe LP, Alfano CM, Leach CR, et al. Who provides psychosocial follow‐up care for post‐treatment cancer survivors? A survey of medical oncologists and primary care physicians. J Clin Oncol 2012;30:2897–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dilworth S, Higgins I, Parker V et al. Patient and health professional's perceived barriers to the delivery of psychosocial care to adults with cancer: A systematic review. Psychooncology 2014;23:601–612. [DOI] [PubMed] [Google Scholar]
- 16. Eakin EG, Strycker LA. Awareness and barriers to use of cancer support and information resources by HMO patients with breast, prostate, or colon cancer: Patient and provider perspectives. Psychooncology 2001;10:103–113. [DOI] [PubMed] [Google Scholar]
- 17. Neumann M, Galushko M, Karbach U et al. Barriers to using psycho‐oncology services: A qualitative research into the perspectives of users, their relatives, non‐users, physicians, and nurses. Support Care in Cancer 2010;18:1147–1156. [DOI] [PubMed] [Google Scholar]
- 18. Absolom K, Holch P, Pini S et al. The detection and management of emotional distress in cancer patients: The views of health‐care professionals. Psychooncology 2011;20:601–608. [DOI] [PubMed] [Google Scholar]
- 19. Del Giudice ME, Leszcz M, Pritchard KI et al. Attitudes of Canadian oncology practitioners toward psychosocial interventions in clinical and research settings in women with breast cancer. Psychooncology 1997;6:178–189. [DOI] [PubMed] [Google Scholar]
- 20. Luxford K, Hill D, Bell R. Promoting the implementation of best‐practice guidelines using a matrix tool. Disease Management & Health Outcomes 2006;14:85–90. [Google Scholar]
- 21. Beraldi A, Kukk E, Nest A et al. Use of cancer‐specific mental health resources‐is there an urban‐rural divide? Support Care Cancer 2015;23:1285–1294. [DOI] [PubMed] [Google Scholar]
- 22. Butow PN, Phillips F, Schweder J et al. Psychosocial well‐being and supportive care needs of cancer patients living in urban and rural/regional areas: a systematic review. Support Care Cancer 2012;20:1–22. [DOI] [PubMed] [Google Scholar]
- 23. Kam LY, Knott VE, Wilson C, et al. Using the theory of planned behavior to understand health professionals' attitudes and intentions to refer cancer patients for psychosocial support. Psychooncology 2012;21:316–323. [DOI] [PubMed] [Google Scholar]
- 24. Fuchsia Howard A, Smillie K, Turnbull K et al. Access to medical and supportive care for rural and remote cancer survivors in Northern British Columbia. J Rural Health 2014;30:311–321. [DOI] [PubMed] [Google Scholar]
- 25. Weaver KE, Geiger AM, Lu L et al. Rural‐urban disparities in health status among US cancer survivors. Cancer 2013;119:1050–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hodges LJ, Walker J, Kleiboer AM et al. What is a psychological intervention? A meta‐review and practical proposal. Psychooncology 2011;20:470–478. [DOI] [PubMed] [Google Scholar]
- 27.Association of Scientific Medical Societies in Germany, German Cancer Society, German Cancer Aid. German Guideline Program in Oncology (GGPO). Retrieved from website: http://leitlinienprogramm-onkologie.de/English-Language.16.0.html
- 28. Zipfel S, Herzog W, Kruse J, et al. Psychosomatic Medicine in Germany: More Timely than Ever. Psychotherapy and Psychosomatics 2016;85:262–269. [DOI] [PubMed] [Google Scholar]
- 29. McCabe MS, Bhatia S, Oeffinger KC et al. American Society of Clinical Oncology statement: Achieving high‐quality cancer survivorship care. J Clin Oncol 2013;31:631–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. von Elm E, Altman DG, Egger M et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J Clin Epidemiol 2008;61:344–349. [DOI] [PubMed] [Google Scholar]
- 31. VanGeest JB, Johnson TP, Welch VL. Methodologies for improving response rates in surveys of physicians: A systematic review. Eval Health Prof 2007;30:303–321. [DOI] [PubMed] [Google Scholar]
- 32. Dijkstra L, Poelman H. Regional Working Paper 2014 ‐ A harmonised definition of cities and rural areas: The new degree of urbanisation: European Commission; 2014. http://ec.europa.eu/regional_policy/sources/docgener/work/2014_01_new_urban.pdf
- 33. Cohen J. A power primer. Psychol Bull 1992;112:155–159. [DOI] [PubMed] [Google Scholar]
- 34. Peng C‐YJ, Lee KL, Ingersoll GM. An introduction to logistic regression analysis and reporting. The Journal of Educational Research 2002;96:3–14. [Google Scholar]
- 35. Peng C‐YJ, So T‐SH. Logistic regression analysis and reporting: A primer. Understanding Statistics 2002;1:31–70. [Google Scholar]
- 36.Adler NE, Page AE, eds. Cancer care for the whole patient: Meeting psychosocial health needs. Washington, DC: National Academies Press, 2008. [PubMed]
- 37. Rankin NM, Butow PN, Thein T et al. Everybody wants it done but nobody wants to do it: An exploration of the barrier and enablers of critical components towards creating a clinical pathway for anxiety and depression in cancer. BMC Health Serv Res 2015;15:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Turner J. The changing landscape of cancer care ‐ the impact of psychosocial clinical practice guidelines. Psychooncology 2015;24:365–370. [DOI] [PubMed] [Google Scholar]
- 39. Mori M, Shimizu C, Ogawa A et al. A national survey to systematically identify factors associated with oncologists' attitudes toward end‐of‐life discussions: What determines timing of end‐of‐life discussions? The Oncologist 2015;20:1304–1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Diefenbacher A. Psychiatry and psychosomatic medicine in Germany: Lessons to be learned? Aust N Z J Psychiatry 2005;39:782–794. [DOI] [PubMed] [Google Scholar]
- 41. Andrykowski MA, Burris JL. Use of formal and informal mental health resources by cancer survivors: differences between rural and nonrural survivors and a preliminary test of the theory of planned behavior. Psychooncology 2010;19:1148–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peabody JW, Luck J, Glassman P et al. Measuring the quality of physician practice by using clinical vignettes: a prospective validation study. Ann Intern Med 2004;141:771–780. [DOI] [PubMed] [Google Scholar]
- 43. Krosnick JA. Survey research. Annual Review of Psychology 1999;50:537–567. [DOI] [PubMed] [Google Scholar]
- 44. Hall S, Kaufman J, Ricketts T. Defining urban and rural areas in U.S. epidemiologic studies. J Urban Health 2006;83:162–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fagerlind H, Kettis A, Glimelius B et al. Barriers against psychosocial communication: Oncologists' perceptions. J Clin Oncol 2013;31:3815–3822. [DOI] [PubMed] [Google Scholar]
- 46. Potosky AL, Han PK, Rowland J et al. Differences between primary care physicians' and oncologists' knowledge, attitudes and practices regarding the care of cancer survivors. J Gen Intern Med 2011;26:1403–1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Buchanan ND, King JB, Rodriguez JL et al. Changes among US cancer survivors: Comparing demographic, diagnostic, and health care findings from the 1992 and 2010 National Health Interview Surveys. ISRN Oncol 2013; 2013:238017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hewitt M, Rowland JH. Mental health service use among adult cancer survivors: Analyses of the National Health Interview Survey. J Clin Oncol 2002;20:4581–4590. [DOI] [PubMed] [Google Scholar]
- 49. Himelhoch S, Weller WE, Wu AW et al. Chronic medical illness, depression, and use of acute medical services among Medicare beneficiaries. Medical Care 2004;42:512–521. [DOI] [PubMed] [Google Scholar]
- 50. Kadan‐Lottick NS, Vanderwerker LC, Block SD et al. Psychiatric disorders and mental health service use in patients with advanced cancer. Cancer 2005;104:2872–2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Akbari A, Mayhew A, Al‐Alawi MA et al. Interventions to improve outpatient referrals from primary care to secondary care. Cochrane Database Syst Rev 2008:CD005471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chrischilles EA, McDowell BD, Rubenstein L et al. Survivorship care planning and its influence on long‐term patient‐reported outcomes among colorectal and lung cancer survivors: The CanCORS disease‐free survivor follow‐up study. J Cancer Surviv 2015;9:269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Li M, Kennedy EB, Byrne N et al. Systematic review and meta‐analysis of collaborative care interventions for depression on patients with cancer. Psychooncology 2016. [Epub ahead of print]. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.