Abstract
This commentary summarizes current knowledge on the clinical presentation, management, and outcomes of the inflammatory arthritis which may occur as an immune‐related adverse evet of immune checkpoint inhibitor therapy. Herein, we propose a new algorithm aimed at assisting oncologists in the diagnosis and management of this immune‐related adverse event.
Introduction
Immune checkpoint inhibitors (ICIs) targeting cytotoxic T‐lymphocyte associated protein‐4 (CTLA‐4), programmed cell death protein‐1 (PD‐1), and its ligand PD‐L1, are established cancer immunotherapies for solid tumor and hematologic malignancies [1], [2], [3], [4], [5], [6], [7], [8], [9], [10]. At present, ipilimumab, nivolumab, pembrolizumab, and the ipilimumab/nivolumab combination are U.S. Food and Drug Administration approved for metastatic melanoma [1], [2], [11]; nivolumab, pembrolizumab, and atezolizumab monotherapy for non‐small cell lung cancer (NSCLC) [3], [4], [5], [6]; nivolumab monotherapy for renal cell carcinoma (RCC), head and neck squamous carcinoma, and Hodgkin's lymphoma [7], [8], [12]; pembrolizumab for head and neck squamous carcinoma [13]; and atezolizumab for urothelial carcinoma [9]. In general, anti‐PD‐1/PD‐L1 monotherapy is associated with a relatively mild toxicity profile [14]. However, immune‐related adverse events (irAEs) may develop and lead to disabling symptoms that can be challenging to diagnose and manage [15]. Awareness of the clinical presentations and management of these toxicities is essential as the use of these agents expands.
The clinical features and outcomes of patients with irAEs, such as colitis [16], thyroid dysfunction [17], hypophysitis [18], [19], skin rash [20], [21], and pneumonitis [22], have been described, with accompanying algorithms for irAE diagnosis and management [14]. In general, irAE management includes drug‐holding, tapering doses of corticosteroids, and specific immunosuppression for clinically severe cases, such as infliximab for colitis and mycophenolate for hepatitis [14]. However, inflammatory arthritis (IA) secondary to ICIs is a less comprehensively reported irAE, without a described management approach. Herein, we summarize what is known about the clinical presentation, management, and outcomes of patients who develop IA with ICIs, and, based on these data, propose a new algorithm aimed at assisting treating oncologists to diagnose and manage this irAE.
Inflammatory Arthritis with Immune Checkpoint Blockade
Inflammatory arthritis that occurs with ICIs has been reported in a small number of clinical trials, with an estimated incidence of 1%–7% [11], [23], [24]. These reports, however, do not provide specific details about how arthritis was defined, types of arthritis seen, management, or outcomes. Arthralgia, which may or may not represent IA, is reported more frequently in clinical trials and ranges from 4% to 22%. Isolated case reports of IA with ICIs include: (a) two patients with polyarticular IA and tenosynovitis that occurred after 11 months and 14 months of pembrolizumab therapy for metastatic melanoma [25] and (b) a patient with advanced RCC who developed non‐erosive Jaccoud's arthropathy [26] and a second patient with advanced NSCLC who developed psoriasis with psoriatic arthritis, both after treatment with nivolumab [27]. Inflammatory arthritis management was reported for the latter patient, and included treatment with methotrexate and a corticosteroid taper for both skin and joint involvement.
The largest series of ICI‐related IA was published by Johns Hopkins investigators, and included nine patients (metastatic melanoma = 3, NSCLC = 4; small cell lung carcinoma = 1; RCC = 1) treated with ipilimumab/nivolumab (n = 7) and/or nivolumab (n = 2), respectively, who developed moderate to severe toxicity (grade 2: n = 5, grade 3: n = 4) [28]. After a comprehensive rheumatologic assessment, the clinical presentation of IA in these patients was found to resemble three distinct clinical phenotypes: (a) rheumatoid arthritis (RA; 6), (b) reactive arthritis (2), and (c) seronegative spondyloarthritis (1). Patients with the RA‐like IA presented with joint pain and swelling in the upper and lower extremities, followed by classic symmetrical swelling of the proximal interphalangeal joints, metacarpophalangeal joints, and/or wrists over time. Additionally, these patients tended to require higher corticosteroid doses (1–2 mg/kg/day prednisone/equivalent), when compared with patients with de novo RA (usually no more than 10–20 mg/day), and these patients’ symptoms persisted following ICI discontinuation. Patients with reactive‐arthritis‐like IA (n = 2) had arthritis, conjunctivitis, and urethritis, and were successfully treated using infliximab and 1–2 mg per kg of prednisone per day for two weeks followed by adalimumab, respectively. Lastly, one patient in this series had a large joint oligoarthritis, consistent with seronegative spondyloarthritis, and was managed with 40 mg of prednisone per day. Imaging in these cases (musculoskeletal ultrasound [US] = 3, musculoskeletal magnetic resonance imaging [MRI] = 1) demonstrated joint effusions, synovial proliferation, and increased vascularity, with bony erosions shortly after the onset of symptoms in two patients. Synovial fluid analysis completed in a subset of patients (4/9) demonstrated an inflammatory picture with high polymorphonuclear cells (polymorphonuclear leukocytes [PMNS]: 70%, white blood cell count [WBC] range: 9,854–28,400 cells/mm3). Autoantibody assessments were performed but none were positive for rheumatoid factor and anti‐cyclic citrullinated peptide antibodies, while three patients had slightly elevated anti‐nuclear antibody levels.
Proposed Management Algorithm for Immune‐Related Inflammatory Arthritis
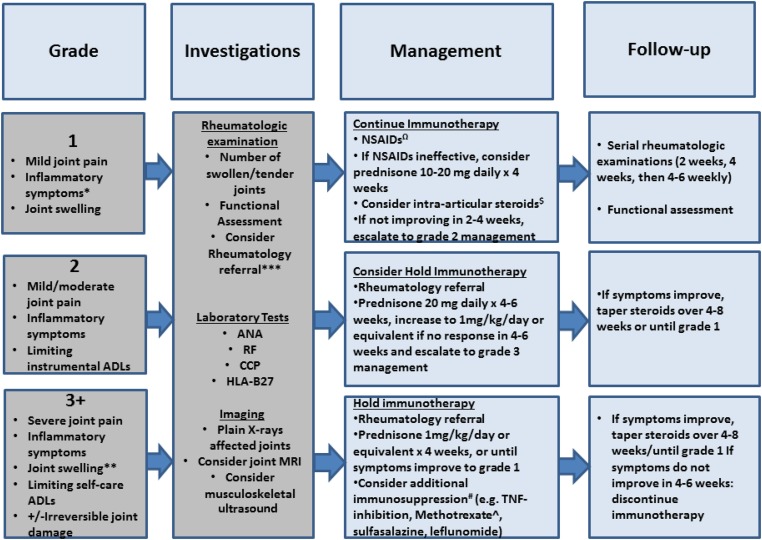
The diagnostic evaluation and treatment of irAEs thus far have been based on clinical experience from patients treated with these agents on clinical studies or as standard‐of‐care [14], and may be summarized in the form of algorithms [29], [30], stratified according to the National Institutes of Health Common Toxicity Criteria for Adverse Events [31]. While prior studies have indicated that this system may underestimate the severity and functional implications of rheumatologic AEs [32], it is a widely utilized oncology classification system. Based on the clinical data presented above, we propose an algorithm for ICI‐related IA (Fig. 1). We note that the recommendations are based on a limited number of cases in our early clinical experience.
Figure 1.
A proposed algorithm for the diagnostic workup and management of inflammatory arthritis that can occur with immune checkpoint blockade, stratified by Common Toxicity Criteria for Adverse Events grade. *, Inflammatory symptoms: Joint stiffness after sleep or inactivity, improvement of symptoms with movement or heat. **, Joint swelling refers to the clinical finding on examination, and may encompass soft tissue swelling, joint effusion, or synovitis. ***, Consider referral to rheumatology if persistent symptoms for >4 weeks, grade 2+toxicity (Common Toxicity Criteria for Adverse Events criteria [31]), or patients require >20 mg prednisone per day that does not taper to <10 mg per day within 4 weeks. Ω, For example, naproxen 500 mg twice a day or meloxicam 7.5–15 mg daily orally for 4–6 weeks. $, Intra‐articular steroid injection: If only 1–2 joints affected, and low dose prednisone (10 mg per day) or NSAIDs not effective. #, Avoid tocilizumab and tofacitinib due to potential bowel side effects. Avoid abatacept due to abrogation of checkpoint inhibition and potentially detrimental effects on tumor response. ^, Methotrexate (MTX) should be administered at a starting dose of 15 mg weekly dose with daily folic acid supplementation. Titrate up to a maximum of 25 mg weekly, or switch to injectable MTX if can't tolerate oral.
Abbreviations: ADL, activity of daily living; ANA, anti‐nuclear antibody; CCP, anti‐cyclic citrullinated peptide; HLA‐B27, human leukocyte antigen B27; NSAIDs, nonsteroidal anti‐inflammatory drugs; RF, rheumatoid factor; TNF, tumor necrosis factor.
In this algorithm, we assert that treating physicians should clinically assess patients with suspected ICI‐related IA for joint pain and swelling, as well as “inflammatory” symptoms, including: joint stiffness, morning accentuation of stiffness and pain, limited range of motion, and improvement of joint symptoms with movement or heat. Relevant investigations should include a panel of inflammatory and serologic markers, as well as radiologic imaging of affected joints (US/MRI) to identify erosive disease, for which corticosteroids would be inadequate to prevent further damage, and additional immunosuppression may be warranted. Rheumatology referral should be considered for recommendations regarding choice and interpretation of imaging, as well as management of patients with moderate to severe symptoms (grade 2+), symptoms lasting >4 weeks, or a requirement of >20 mg of prednisone per day that cannot be tapered to <10mg within 4 weeks. Rheumatologists can assist with classification of joints involved, help to differentiate arthralgia from true IA, and evaluate for related symptoms such as enthesitis, dactylitis, and axial IA. In patients with one joint disproportionately affected or resistant to treatment, metastatic disease should be considered.
To treat ICI‐related IA, nonsteroidal anti‐inflammatory drugs can be used for supportive management and intra‐articular steroids as local therapy for those with limited involvement of accessible joints. Intra‐articular steroids may be particuarly useful when larger joints are involved and when there are fewer than three joints affected. Grade 1 cases should be managed supportively, and ICIs may be continued with close monitoring for moderate and persistent symptoms that require intervention (grade 2). In the largest series of IA [28], all five patients with grade 2 IA had improvement and/or resolution with intra‐articular steroids with or without prolonged prednisone taper. However, the four patients with grade 3 IA initially treated with prednisone 40 mg for moderate symptoms went on to require higher doses (prednisone 1 mg/kg/day or equivalent) and additional immunosuppression. Therefore, patients with grade 2 IA may start with 20 mg of prednisone per day and have their management adjusted based on IA response. For grade 2 toxicity, we therefore recommend that clinicians consider holding immunotherapy since IA may worsen, while also considering whether an individual's cancer is likely to benefit from continued ICIs. In grade 3 cases, additional immunosuppression used successfully included: methotrexate (MTX), etanercept, infliximab, and adalimumab. Methotrexate, sulfasalazine, and leflunomide can be considered for use as steroid‐sparing agents for IA. Methotrexate monotherapy has been used successfully in several patients, after initial publication of the nine cases included in the above series. Notably, two patients from an extended series including these nine patients were successfully treated with MTX and subsequently able to discontinue corticosteroids and reduce to daily low‐dose corticosteroids, respectively [33]. The caveat to the use of these agents is that the time to onset can be 6–12 weeks or longer. A 6–12 week period may not be acceptable for patients who plan to re‐initiate immunotherapy or patients with a limited life expectancy. Additionally, the combination of MTX and tumor necrosis factor (TNF)‐inhibition may be more efficacious than MTX alone in selected cases [27], [34], [35], [36], [37]. The choice of TNF‐inhibition should also be considered depending on the presence of other irAEs, as monoclonal antibodies (e.g., infliximab, adalimumab) may be preferable to soluble receptors (etanercept) in those with immune‐related colitis, and tocilizumab and tofacitinib should likely be avoided due to possible potentiation of gastrointestinal perforation [38], [39]. In addition, abatacept, which augments T‐cell activation through CD28, may need to be avoided lest it abrogate the effect of anti‐CTLA‐4 ICIs [40]. Prior to administration of immunosuppression, patients should also be assessed for chronic infections (e.g., hepatitis B, C, tuberculosis) and classified with a specific form of IA [41], [42]. There is limited experience with regard to recurrence or clinical outcomes of IA when a patient is rechallenged with immunotherapy after an initial episode of IA. Two patients have been successfully treated for grade 2 IA with prednisone and rechallenged with immunotherapy. One was rechallenged with the same anti‐PD‐1 agent and the other with a different anti‐PD‐1/PD‐L1 agent on a clinical trial without recurrence of IA. It is important to note that both of these patients were maintained on low dose steroids (7.5–10 mg daily of prednisone).
Conclusion
With a growing pipleine of ICIs, the use of these agents for cancer therapy is expanding rapidly. There is, thus, a critical need to gain familarity with immune‐related toxicities and their management. Immune checkpoint inhibitor‐induced IA is an underappreciated irAE that may be clinically severe, rapidly destructive, disabling, and impactful on quality of life. Published data from clinical trials likely underestimates the true incidence of IA, due to rheumatologic symptoms that may be recorded separately, but together represent one clinical syndrome [32]. Immune checkpoint inhibitor‐related IA in reported studies and published cases [25], [26], [27], [28] exhibit a range of appearances and require special management considerations. Thus, we, as a multi‐disciplinary team composed of rheumatologists, oncologists, clinical trialists, and both rheumatology and oncology laboratory researchers, put forward our provisional diagnostic and management recommendations of this phenomenon. This algorithm will require validation in larger datasets in the future. The oncology community should be made aware of the diagnostic evaluation of these patients, which includes comprehensive musculoskeletal examinations, laboratory assessments that may not be routinely completed in oncology clinics, and radiologic assessments for structural changes affecting management choices. In addition, there are important management considerations that are distinct from other irAEs, including the use of intra‐articular steroids as local therapy, starting lower doses of corticosteroids initially for moderately symptomatic cases, as well as the use of immunosuppressants, such as MTX and a TNF‐inhibitor, alone or in combination.
Acknowledgements
We received financial support from P30‐AR053503 Core D from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (C.O.B.), NIH grant DE‐12354‐15A1 (A.N.B.), the Jerome L. Greene Foundation Scholar Award (L.C.C.), and the Jerome L. Greene Foundation Discovery Award (C.O.B. and J.N.).
Contributed equally
Contributed equally
Disclosures
Jarushka Naidoo: Bristol‐Myers Squibb, AstraZeneca (C/A, H), Merck (RF); Patrick M. Forde: Bristol‐Myers Squibb, AstraZeneca, Celgene, Boehringer (C/A), Bristol‐Myers Squibb, AstraZeneca, Kyowa, Novartis (RF); Evan J. Lipson: Bristol‐Myers Squibb, EMD Serono, Merck, Novartis (C/A), Genentech, Merck (RF); Hans J. Hammers: Bristol‐Myers Squibb, Pfizer (C/A); Bristol‐Myers Squibb (RF); William Sharfman: Merck, Bristol‐Myers Squibb, Novartis, Castle Biosciences (C/A), Bristol‐Myers Squibb, Merck (RF); Dung T. Le: Merck (C/A), Merck, Bristol‐Myers Squibb, Aduro Biotech (RF); Alan N. Baer: Bristol‐Myers Squibb (C/A); Clifton O. Bingham: Bristol‐Myers Squibb (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Weber JS, D'Angelo SP, Minor D et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti‐CTLA‐4 treatment (CheckMate 037): A randomised, controlled, open‐label, phase 3 trial. Lancet Oncol 2015;16:375–384. [DOI] [PubMed] [Google Scholar]
- 2. Larkin J, Chiarion‐Sileni V, Gonzalez R et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med 2015;373:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brahmer J, Reckamp KL, Baas P et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med 2015;373:123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Borghaei H, Paz‐Ares L, Horn L et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med 2015;373:1627–1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Herbst RS, Baas P, Kim DW et al. Pembrolizumab versus docetaxel for previously treated, PD‐L1‐positive, advanced non‐small‐cell lung cancer (KEYNOTE‐010): A randomised controlled trial. Lancet 2016;387:1540–1550. [DOI] [PubMed] [Google Scholar]
- 6. Fehrenbacher L, Spira A, Ballinger M et al. Atezolizumab versus docetaxel for patients with previously treated non‐small‐cell lung cancer (POPLAR): A multicentre, open‐label, phase 2 randomised controlled trial. Lancet 2016;9:1540–1550. [DOI] [PubMed] [Google Scholar]
- 7. Motzer RJ, Escudier B, McDermott DF et al. Nivolumab versus everolimus in advanced renal‐cell carcinoma. N Engl J Med 2015;373:1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ansell SM, Lesokhin AM, Borrello I et al. PD‐1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenberg JE, Hoffman‐Censits J, Powles T et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum‐based chemotherapy: A single‐arm, multicentre, phase 2 trial. Lancet 2016;387:1909–1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robert C, Schachter J, Long GV et al. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med 2015;372:2521–2532. [DOI] [PubMed] [Google Scholar]
- 12. Ferris RL, Blumenschein G, Jr., Fayette J et al. Nivolumab for recurrent squamous‐cell carcinoma of the head and neck. N Engl J Med 2016;375:1856–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seiwert TY, Burtness B, Mehra R et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE‐012): An open‐label, multicentre, phase 1b trial. Lancet Oncol 2016;17:956–965. [DOI] [PubMed] [Google Scholar]
- 14. Naidoo J, Page DB, Li BT et al. Toxicities of the anti‐PD‐1 and anti‐PD‐L1 immune checkpoint antibodies. Ann Oncol 2015;26:2375–2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weber JS, Kahler KC, Hauschild A. Management of immune‐related adverse events and kinetics of response with ipilimumab. J Clin Oncol 2012;30:2691–2697. [DOI] [PubMed] [Google Scholar]
- 16. Beck KE, Blansfield JA, Tran KQ et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T‐lymphocyte‐associated antigen 4. J Clin Oncol 2006;24:2283–2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Abdel‐Rahman O, ElHalawani H, Fouad M. Risk of endocrine complications in cancer patients treated with immune check point inhibitors: a meta‐analysis. Future Oncol 2016;12:413–425. [DOI] [PubMed] [Google Scholar]
- 18. Min L, Hodi FS, Giobbie‐Hurder A et al. Systemic high‐dose corticosteroid treatment does not improve the outcome of ipilimumab‐related hypophysitis: A retrospective cohort study. Clin Cancer Res 2015;21:749–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iwama S, De Remigis A, Callahan MK et al. Pituitary expression of CTLA‐4 mediates hypophysitis secondary to administration of CTLA‐4 blocking antibody. Sci Transl Med 2014;6:230ra245. [DOI] [PubMed] [Google Scholar]
- 20. Naidoo J, Schindler K, Querfeld C et al. Autoimmune bullous skin disorders with immune checkpoint inhibitors targeting PD‐1 and PD‐L1. Cancer Immunol Res 2016;4:383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Minkis K, Garden BC, Wu S et al. The risk of rash associated with ipilimumab in patients with cancer: A systematic review of the literature and meta‐analysis. J Am Acad Dermatol 2013;69:e121–128. [DOI] [PubMed] [Google Scholar]
- 22. Naidoo J, Wang X, Woo KM et al. Pneumonitis in patients treated with anti–programmed death‐1/programmed death ligand 1 therapy. J Clin Oncol 2017;35:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bashey A, Medina B, Corringham S et al. CTLA4 blockade with ipilimumab to treat relapse of malignancy after allogeneic hematopoietic cell transplantation. Blood 2009;113:1581–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hodi FS, Lee S, McDermott DF et al. Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: A randomized clinical trial. JAMA 2014;312:1744–1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chan MM, Kefford RF, Carlino M et al. Arthritis and tenosynovitis associated with the anti‐PD1 antibody pembrolizumab in metastatic melanoma. J Immunother 2015;38:37–39. [DOI] [PubMed] [Google Scholar]
- 26. de Velasco G BB, Choueiri TK. Autoimmune arthropathy and uveitis as complications of programmed death 1 inhibitor treatment. Arthritis Rheumatol 2016;68:556–557. [DOI] [PubMed] [Google Scholar]
- 27. Law‐Ping‐Man S, Martin A, Briens E et al. Psoriasis and psoriatic arthritis induced by nivolumab in a patient with advanced lung cancer. Rheumatology (Oxford) 2016;55:2087–2089. [DOI] [PubMed] [Google Scholar]
- 28. Cappelli LC, Gutierrez AK, Baer AN et al. Inflammatory arthritis and sicca syndrome induced by nivolumab and ipilimumab. Ann Rheum Dis 2016;76:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Opdivo (nivolumab): Immune‐Mediated Adverse Reactions Management Guide. Available at http://www.opdivohcp.bmscustomerconnect.com/servlet/servlet.FileDownload?file=00Pi000000 Hj19REAR. Accessed March 31, 2017.
- 30.Merck. Keytruda (pembrolizumab) prescribing information. 2015. Available at https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf. Accessed April 13, 2017.
- 31.Common Terminology Criteria for Adverse Events (CTCAE): Version 4.0. Available at http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf. Accessed March 31, 2017.
- 32. Woodworth T, Furst DE, Alten R et al. Standardizing assessment and reporting of adverse effects in rheumatology clinical trials II: The Rheumatology Common Toxicity Criteria v.2.0. J Rheumatol 2007;34:1401–1414. [PubMed] [Google Scholar]
- 33. Cappelli LC, Gutierrez AK, Baer AN et al. Rheumatologic Consequences of Immunotherapy to Treat Malignancies: The Tip of an Iceberg. In American College of Rheumatology AVR/ARHP Annual Meeting 2016. Washington, DC:, 2016.
- 34. Detert J, Bastian H, Listing J et al. Induction therapy with adalimumab plus methotrexate for 24 weeks followed by methotrexate monotherapy up to week 48 versus methotrexate therapy alone for DMARD‐naive patients with early rheumatoid arthritis: HIT HARD, an investigator‐initiated study. Ann Rheum Dis 2013;72:844–850. [DOI] [PubMed] [Google Scholar]
- 35. Breedveld FC, Weisman MH, Kavanaugh AF et al. The PREMIER study: A multicenter, randomized, double‐blind clinical trial of combination therapy with adalimumab plus methotrexate versus methotrexate alone or adalimumab alone in patients with early, aggressive rheumatoid arthritis who had not had previous methotrexate treatment. Arthritis Rheum 2006;54:26–37. [DOI] [PubMed] [Google Scholar]
- 36. St Clair EW, van der Heijde DM, Smolen JS et al. Combination of infliximab and methotrexate therapy for early rheumatoid arthritis: A randomized, controlled trial. Arthritis Rheum 2004;50:3432–3443. [DOI] [PubMed] [Google Scholar]
- 37. Maini R, St Clair EW, Breedveld F et al. Infliximab (chimeric anti‐tumour necrosis factor alpha monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: A randomised phase III trial. ATTRACT Study Group. Lancet 1999;354:1932–1939. [DOI] [PubMed] [Google Scholar]
- 38. Tanaka T, Ogata A, Narazaki M. Tocilizumab for the treatment of rheumatoid arthritis. Expert Rev Clin Immunol 2010;6:843–854. [DOI] [PubMed] [Google Scholar]
- 39. Xie F, Yun H, Bernatsky S et al. Risk for gastrointestinal perforation among rheumatoid arthritis patients receiving tofacitinib, tocilizumab, or other biologics. Arthritis Rheumatol 2016;68:2612–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ruderman EM, Pope RM. Drug insight: Abatacept for the treatment of rheumatoid arthritis. Nat Clin Pract Rheumatol 2006;2:654–660. [DOI] [PubMed] [Google Scholar]
- 41. Aletaha D, Neogi T, Silman AJ et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010;62:2569–2581. [DOI] [PubMed] [Google Scholar]
- 42. Rudwaleit M, Landewe R, van der Heijde D et al. The development of Assessment of SpondyloArthritis International Society classification criteria for axial spondyloarthritis (part I): Classification of paper patients by expert opinion including uncertainty appraisal. Ann Rheum Dis 2009;68:770–776. [DOI] [PubMed] [Google Scholar]