Abstract
Lessons Learned.
Weekly nanoparticle albumin‐bound‐paclitaxel (75 mg/m2) in combination with carboplatin (area under the curve 6 mg/mL/min) in elderly patients with previously untreated, advanced non‐small cell lung cancer showed favorable efficacy, was well tolerated, and showed less neuropathic toxicity.
This modified regimen offers potential for the treatment of elderly patients.
Background.
The CA031 trial suggested weekly nanoparticle albumin‐bound‐paclitaxel (nab‐PTX) was superior in efficacy to paclitaxel (PTX) once every 3 weeks when combined with carboplatin (CBDCA) for advanced non‐small cell lung cancer (NSCLC) patients; a subgroup analysis of elderly patients looked promising. In a multicenter phase II trial, we prospectively evaluated the efficacy and tolerability of modified CBDCA plus weekly nab‐PTX for elderly patients with untreated advanced NSCLC.
Methods.
Eligible patients received CBDCA (area under the curve [AUC] 6 mg/mL/min) on day 1 and nab‐PTX (75 mg/m2) on days 1, 8, and 15 every 4 weeks. The primary endpoint was an overall response rate (ORR), and secondary endpoints were progression‐free survival (PFS), overall survival (OS), and toxicity.
Results.
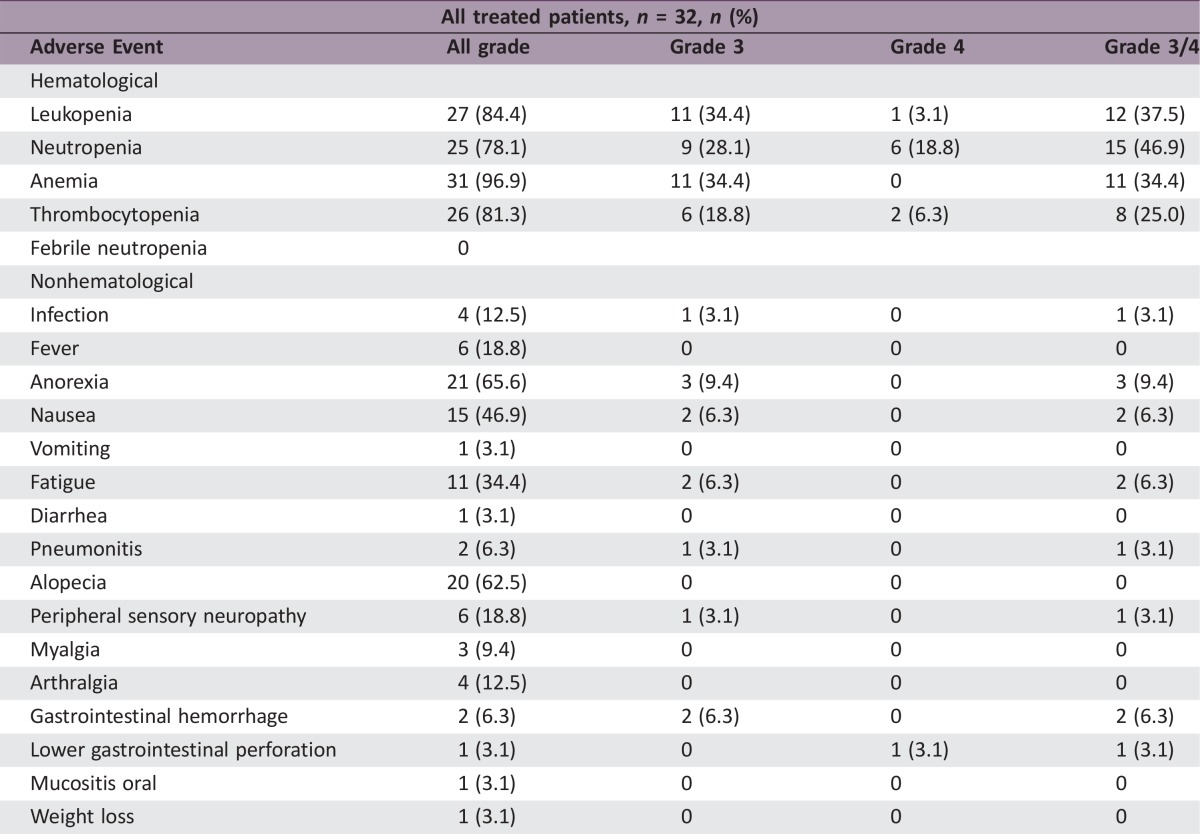
Of 32 patients (median age of 78 years), 84% were male, 56% had stage IV NSCLC, and 56% had squamous cell carcinoma. ORR and disease control rates were 50% (95% confidence interval (CI): 33–67) and 94% (95% CI: 85–100), respectively. Median PFS and OS were 6.4 months (95% CI: 4.8–8.0) and 17.5 months (95% CI: 11.9–23.1), respectively. Grade ≥3 toxicities were neutropenia (47%), leukopenia (38%), anemia (34%), thrombocytopenia (25%), and anorexia (9%). Febrile neutropenia and treatment‐related deaths were not observed.
Conclusion.
Modified CBDCA plus weekly nab‐PTX demonstrated significant efficacy and acceptable toxicities in elderly patients with advanced NSCLC.
Abstract
经验总结
• 白蛋白结合型紫杉醇纳米颗粒(75 mg/m2)每周一次给药联合卡铂(曲线下面积6 mg/mL/min)治疗既往未经治疗的晚期非小细胞肺癌老年患者时疗效和耐受性良好, 且神经毒性较低。
• 这一改良方案在老年患者的治疗中具有潜在应用价值。
摘要
背景. CA031试验表明, 与卡铂(CBDCA)联合治疗晚期非小细胞肺癌(NSCLC)患者时, 白蛋白结合型紫杉醇纳米颗粒(nab‐PTX, 每周一次)的疗效优于紫杉醇(PTX, 每3周一次);老年患者的亚组分析显示该方案前景良好。在一项多中心II期试验中, 我们前瞻性评价了CBDCA联合每周一次nab‐PTX改良方案治疗未经治疗的晚期NSCLC老年患者的疗效和耐受性。
方法. 合格患者在每4周的第1天接受CBDCA[曲线下面积(AUC)6 mg/mL/min], 并在第1、8和15天接受nab‐PTX(75 mg/m2)。主要终点为总缓解率(ORR), 次要终点为无进展生存期(PFS)、总生存期(OS)和毒性。
结果. 32例患者(中位年龄78岁)中有84%为男性, 56%患有IV期NSCLC, 56%患有鳞状细胞癌。ORR和疾病控制率分别为50%[95%置信区间(CI):33‐67]和94%(95% CI:85‐100)。中位PFS和OS分别为6.4个月(95% CI:4.8‐8.0)和17.5个月(95% CI:11.9‐23.1)。≥3级毒性包括中性粒细胞减少症(47%)、白细胞减少症(38%)、贫血(34%)、血小板减少症(25%)和厌食(9%)。未观察到发热性中性粒细胞减少症和治疗相关死亡。
结论. CBDCA联合每周一次nab‐PTX改良方案在晚期NSCLC老年患者中表现出显著的疗效和可接受的毒性。The Oncologist 2017;22:640–e59
Discussion
This prospective study was designed to investigate the efficacy and safety of modified CBDCA combined with weekly nab‐PTX as first‐line chemotherapy in elderly patients with advanced NSCLC. Socinski et al. analyzed the efficacy and toxicity of CBDCA combined with nab‐PTX in elderly patients (≥70 years old) in the CA031 study and revealed the ORR was 32% and the median OS was 19.9 months [1]. An ORR of 50% and an OS of 17.5 months in the present study are consistent with the CA031 study, although our study was intended for patients ≥75 years of age, and the median weekly dose intensity was less than that used in the CA031 study (45 mg/m2 vs. 73.4 mg/m2).
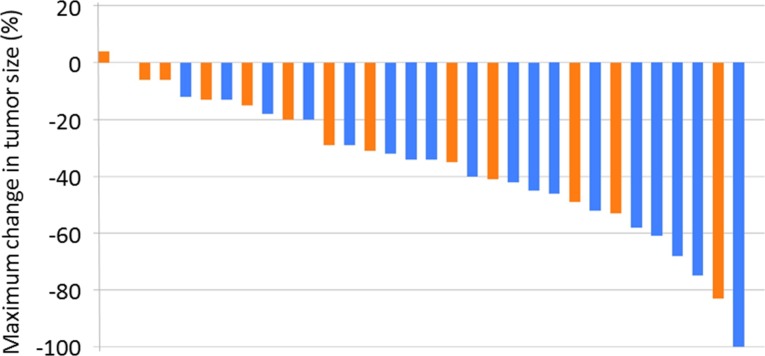
Figure 1.
Tumor shrinkage by histological type. Blue bars: squamous cell carcinoma, n = 18. Orange bars: adenocarcinoma, n = 14.
Quoix et al. recently reported on IFCT‐0501, a phase III study comparing CBDCA (AUC 6 mg/mL/min on day 1 every 4 weeks) plus PTX (90 mg/m2 on days 1, 8, and 15 every 4 weeks) with monotherapy with vinorelbine or gemcitabine in elderly patients with advanced NSCLC [2]. The median OS was significantly longer in the doublet group than in the monotherapy group. Similar to the IFCT‐0501 trial, we previously demonstrated the high efficacy of CBDCA (AUC 6 mg/mL/min on day 1 every 4 weeks) plus weekly PTX (70 mg/m2 on days 1, 8, and 15 every 4 weeks) compared with single‐agent docetaxel for elderly patients with NSCLC [3]. We have also reported on a randomized phase II trial of weekly PTX combined with CBDCA (same as above) versus standard PTX combined with CBDCA (210 mg/m2 and AUC 6 mg/mL/min on day 1 every 3 weeks) for elderly patients with advanced NSCLC [4]. Regarding the efficacy of CBDCA plus nab‐PTX, the ORR and PFS (50%, 6.4 months) attained are consistent with the results achieved with a CBDCA plus weekly PTX regimen in our previous two studies (55%, 6.0 months and 54%, 6.6 months, respectively) [3], [4].
Regarding the toxicity of modified carboplatin plus weekly nab‐paclitaxel, its adverse event (AE) profile was tolerable. Nonhematological toxicities were acceptable, with the most frequent AEs being anorexia, alopecia, and nausea. Also, the relatively lower incidences of sensory neuropathy resulting in the discontinuation of treatment were similar to those seen in patients treated with CBDCA plus nab‐PTX in the CA031 study [1], [5]. Our data support the feasibility of a modified regimen of CBDCA plus a lower dose of nab‐PTX for elderly patients. In addition to fewer AEs, because nab‐PTX does not contain solvents, unlike PTX, it is not usually necessary to take premedications, such as H1 and H2 blockers and steroids, to prevent allergic reactions prior to the administration of nab‐PTX [6], [7], [8], [9]. Therefore, nab‐PTX may be a more acceptable drug option than PTX for elderly lung cancer patients.
Trial Information
- Disease
Lung cancer – NSCLC
- Stage of disease/treatment
Metastatic/Advanced
- Prior therapy
None
- Type of study – 1
Phase II
- Type of study – 2
Single arm
- Primary endpoint
Overall response rate
- Secondary endpoint
Progression free survival
- Secondary endpoint
Overall survival
- Secondary endpoint
Toxicity
- Additional details of endpoints or study design
- Patients. Eligible patients were aged 75 years or older, with histologically or cytologically confirmed NSCLC that was classified as either clinical stage IIIb, IV, or a postoperative recurrence. Other eligibility criteria for patients included being previously untreated and having had at least one unidimensionally measurable lesion according to the Response Evaluation Criteria in Solid Tumors (RECIST), an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, and an estimated life expectancy >12 weeks. Laboratory criteria included a hemoglobin concentration ≥9 g/dL, a neutrophil count ≥ 1,500/mm3, a platelet count ≥100,000/mm3, aspartate transaminase and alanine transaminase ≤100 IU/L, serum creatinine 1.5 mg/dL, and PaO2 ≥60 mmHg. Exclusion criteria included peripheral neuropathy with Common Terminology Criteria for Adverse Events (CTCAE) grade ≥2, prior treatment with PTX as adjuvant chemotherapy, progressive brain metastases, or uncontrolled third‐space fluid retention prior to study entry. Patients were also excluded if they had an active infection, interstitial pneumonia, active lung fibrosis, an active synchronous cancer, or a history of allergy or hypersensitivity to drugs. An institutional review board at each hospital approved this study, and written, informed consent was obtained from all enrolled patients.
- Study Design and Treatment Plan. This multicenter, single arm, phase II study evaluated the efficacy and safety of a 75 mg/m2 infusion of nab‐PTX on days 1, 8, and 15, followed by a CBDCA AUC of 6 mg/mL/min (per Calvert formula) on day 1 every 4 weeks, as first‐line chemotherapy in patients with advanced NSCLC. Treatment of at least four cycles was encouraged unless disease progression or unacceptable toxicities were observed. Second‐line chemotherapy or other treatments after this study were not prohibited by the protocol. Patients who had an epidermal growth factor receptor (EGFR) mutation received EGFR tyrosine kinase inhibitor after progression to this study treatment according to the protocol.
- Assessment of Efficacy and Safety Endpoints. The primary endpoint of this study was the ORR and the rate of complete response (CR) and/or partial response (PR) as confirmed by a blinded, centralized, independent review according to RECIST. Computed tomography scans were performed every 4 weeks from screening until disease progression. Secondary endpoints were PFS, OS, and toxicity profile. The follow‐up period was 18 months after the last patient enrollment.
- Statistical Methods. The minimum number of patients enrolled in the present study was 32 assuming an expected response rate (RR) of 40% and a threshold RR of 20% with an α error of 0.05 (one‐sided) and a β error of 0.2. Assuming that about 10% of patients would eventually not qualify, at least 35 patients needed to be recruited. The primary analysis of the ORR was planned in qualified patients, defined as receiving at least one course of nab‐PTX and CBDCA. PFS and OS were analyzed using Kaplan‐Meier methods. Safety analyses summarized AEs by maximum CTCAE grade, and whether or not they were serious, during the entire treatment period.
- Investigator's Analysis
Active and should be pursued further
Drug Information
- Drug 1
- Generic/Working name
Nanoparticle albumin‐bound‐paclitaxel
- Trade name
Abraxane
- Company name
Taiho Pharma
- Drug type
Chemotherapy
- Drug class
Microtubule‐targeting agent
- Dose
75 milligrams (mg) per square meter (m2)
- Route
IV
- Schedule of administration
Day 1, 8, and 15 every 4 weeks
- Drug 2
- Generic/Working name
Carboplatin
- Trade name
Paraplatin
- Company name
Bristol‐Myers Squibb
- Drug type
Chemotherapy
- Drug class
Platinum compound
- Dose
AUC 6 mg/mL/min
- Route
IV
- Schedule of administration
Day 1 every 4 weeks
Patient Characteristics for Phase II Control Arm
- Number of patients, male
27
- Number of patients, female
5
- Stage
-
IIIb: 7
IV: 18
Postoperative recurrence: 7
- Age
Median (range): 78(75–86)
- Number of prior systemic therapies
Median (range): 0
- Performance status: ECOG
-
0 — 16
1 — 16
2 —
3 —
unknown —
- Other: EGFR mutation status
-
Mutant: 2
Wild: 16
Unknown: 14
- Cancer types or histologic subtypes
-
Adenocarcinoma: 14
Squamous cell carcinoma: 18
Primary Assessment Method for Phase II Control Arm
- Number of patients screened
35
- Number of patients enrolled
35
- Number of patients evaluable for toxicity
32
- Number of patients evaluated for efficacy
32
- Response assessment CR
n = 1 (3.1%)
- Response assessment PR
n = 15 (46.9%)
- Response assessment SD
n = 14 (43.8%)
- Response assessment PD
n = 2 (6.3%)
- Response assessment OTHER
n = 0 (0%)
- (Median) duration assessments PFS
6.4 months, CI: 4.8–8.0
- (Median) duration assessments OS
17.5 months, CI: 11.9–23.1
Assessment, Analysis, and Discussion
- Completion
Study completed
- Pharmacokinetics/Pharmacodynamics
Not Collected
- Investigator's assessment
Active and should be pursued further
Lung cancer is the most common cause of death from cancer in the elderly population. With many countries facing an aging population, the incidence of lung cancer in this age group is expected to increase. Consequently, it has become increasingly important to establish more effective treatments for elderly patients with advanced non‐small cell lung cancer (NSCLC). A recent IFCT‐0501 trial demonstrated that carboplatin (CBDCA) combined with weekly paclitaxel (PTX) would be advantageous, compared with vinorelbine or gemcitabine monotherapy, for elderly patients with previously untreated, advanced NSCLC [2]. Subsequently, the CA031 trial suggested that weekly nanoparticle albumin‐bound‐PTX (nab‐PTX) was superior in efficacy and safety compared with PTX once every 3 weeks when combined with CBDCA [1], [5]. In addition, a subgroup analysis of elderly patients in the CA031 trial yielded very promising data (a 34% overall response rate [ORR] and 8 months of progression‐free survival [PFS]), whereas in a Japanese subgroup analysis, many cases required the omission of treatments as well as a reduction in doses [10]. We therefore conducted this multicenter, nonrandomized, open‐label, phase II trial to prospectively evaluate the efficacy and tolerability of a modified CBDCA plus weekly nab‐PTX regimen for elderly patients with previously untreated, advanced NSCLC. The ORR met our primary endpoint and supported the favorable efficacy of this combination among an elderly population, as found in the CA031 trial [5].
We also evaluated the ORR by histologic type. Satouchi et al. reported that a Japanese subset analysis of the CA031 study showed more effectiveness in the squamous cell carcinoma subset than in nonsquamous cell carcinoma [10]. Although a similar tendency was shown in this study, because of the small sample size, a significant difference was not observed between adenocarcinoma and squamous cell carcinoma in terms of ORR and PFS. Recently, several studies have confirmed the effectiveness of nab‐PTX with CBDCA in patients with squamous cell carcinoma [11]. Further study is needed to determine the difference in effects for both histological types.
The present study had several limitations. First, this study used a small sample size and, for this reason, future phase III studies will be required to evaluate the effectiveness of this modified regimen, although promising results were obvious in this study. Concerning this limitation, the CAPITAL study, a randomized phase III trial of CBDCA plus nab‐PTX versus docetaxel for squamous NSCLC in the elderly, is ongoing in Japan. Second, the present study lacked a quality‐of‐life (QoL) assessment. Most elderly patients with advanced NSCLC do not have curative treatment options, and, therefore, the goal of therapy for such patients is a prolongation of survival without negatively impacting QoL. From this perspective, we plan to evaluate QoL in a future, randomized phase III trial of modified CBDCA plus nab‐PTX for elderly patients with advanced NSCLC.
In conclusion, weekly nab‐PTX (75 mg/m2) in combination with CBDCA (AUC 6 mg/mL/min) in elderly patients with previously untreated, advanced NSCLC showed favorable efficacy, was well tolerated, and showed less neuropathic toxicity. Therefore, nab‐PTX offers several distinct advantages over conventional PTX when combined with CBDCA. Based on our results, even if the high cost of nab‐PTX is considered, we believe that a modified CBDCA plus weekly nab‐PTX regimen should be used for elderly patients regarding efficacy and tolerability. Future phase III studies will be needed to further evaluate the role of weekly nab‐PTX in combination with CBDCA for such patients.
Acknowledgements
The authors thank all patients, families, and investigators participating in the NJLCG1301 study.
Footnotes
UMIN‐CTR Identifier: UMIN000010324
Sponsor: North Japan Lung Cancer Study Group
Principal Investigator: Eisaku Miyauchi
IRB Approved: Yes
Disclosures
Akira Inoue: Taiho (H); Shunichi Sugawara: AstraZeneca, Chugai, Nippon Boehringer Ingelheim, Pfizer, Taiho, Eli Lilly and Company, Novartis, Kyowa Hakko Kirin, Bristol‐Myers Squibb, Ono (H); Makoto Maemondo: Bristol‐Myers Squibb (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1. Socinski MA, Langer CJ, Okamoto I et al. Safety and efficacy of weekly nab®‐paclitaxel in combination with carboplatin as first‐line therapy in elderly patients with advanced non‐small‐cell lung cancer. Ann Oncol 2013;24:314–321. [DOI] [PubMed] [Google Scholar]
- 2. Quoix E, Zalcman G, Oster JP et al. Carboplatin and weekly paclitaxel doublet chemotherapy compared with monotherapy in elderly patients with advanced non‐small‐cell lung cancer: IFCT‐0501 randomised, phase 3 trial. Lancet 2011;378:1079–1088. [DOI] [PubMed] [Google Scholar]
- 3. Maemondo M, Inoue A, Sugawara S et al. Randomized phase II trial comparing carboplatin plus weekly paclitaxel and docetaxel alone in elderly patients with advanced non‐small cell lung cancer: North Japan Lung Cancer Group Trial 0801. The Oncologist 2014;19:352–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sakakibara T, Inoue A, Sugawara S et al. Randomized phase II trial of weekly paclitaxel combined with carboplatin versus standard paclitaxel combined with carboplatin for elderly patients with advanced non‐small‐cell lung cancer. Ann Oncol 2010;21:795–799. [DOI] [PubMed] [Google Scholar]
- 5. Socinski MA, Bondarenko I, Karaseva NA et al. Weekly nab‐paclitaxel in combination with carboplatin versus solvent‐based paclitaxel plus carboplatin as first‐line therapy in patients with advanced non‐small‐cell lung cancer: Final results of a phase III trial. J Clin Oncol 2012;30:2055–2062. [DOI] [PubMed] [Google Scholar]
- 6. Ten Tije AJ, Verweij J, Loos WJ et al. Pharmacological effects of formulation vehicles: Implications for cancer chemotherapy. Clin Pharmacokinet 2003;42:665–685. [DOI] [PubMed] [Google Scholar]
- 7. Desai N, Trieu V, Yao Z et al. Increased antitumor activity, intratumor paclitaxel concentrations, and endothelial cell transport of cremophor‐free, albumin‐bound paclitaxel, ABI‐007, compared with cremophor‐based paclitaxel. Clin Cancer Res 2006;12:1317–1324. [DOI] [PubMed] [Google Scholar]
- 8. Gupta N, Hatoum H, Dy GK. First line treatment of advanced non‐small‐cell lung cancer‐specific focus on albumin bound paclitaxel. Int J Nanomedicine 2014;9:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hirsh V. Nab‐paclitaxel for the management of patients with advanced non‐small‐cell lung cancer. Expert Rev Anticancer Ther 2014;14:129–141. [DOI] [PubMed] [Google Scholar]
- 10. Satouchi M, Okamoto I, Sakai H et al. Efficacy and safety of weekly nab‐paclitaxel plus carboplatin in patients with advanced non‐small cell lung cancer. Lung Cancer 2013;81:97–101. [DOI] [PubMed] [Google Scholar]
- 11. Simon GR. Nab‐Paclitaxel for the treatment of advanced squamous non‐small‐cell lung cancer: A comprehensive update. Clin Lung Cancer 2014;15:391–397. [DOI] [PubMed] [Google Scholar]