The cost and value of cancer treatment is under scrutiny worldwide. The objective of this study was to evaluate the cost‐effectiveness of bevacizumab in the first‐line treatment of metastatic colorectal cancer (mCRC) from the perspective of payers across five selected countries, namely the U.S., U.K., Canada, Australia, and Israel.
Keywords: Cost, Cost‐effectiveness, Colorectal cancer, Economics, Value, Chemotherapy
Abstract
Background.
In the U.S., the addition of bevacizumab to first‐line chemotherapy in metastatic colorectal cancer (mCRC) has been demonstrated to provide 0.10 quality‐adjusted life years (QALYs) at an incremental cost‐effectiveness ratio (ICER) of $571,000/QALY. Due to variability in pricing, value for money may be different in other countries. Our objective was to establish the cost‐effectiveness of bevacizumab in mCRC in the U.S., U.K., Canada, Australia, and Israel.
Methods.
We performed the analysis using a previously established Markov model for mCRC. Input data for efficacy, adverse events, and quality of life were considered to be generalizable and therefore identical for all countries. We used country‐specific prices for medications, administration, and other health service costs. All costs were converted from local currency to U.S. dollars at the exchange rates in March 2016. We conducted one‐way and probabilistic sensitivity analyses (PSA) to assess the model robustness across parameter uncertainties.
Results.
Base case results demonstrated that the highest ICER was in the U.S. ($571,000/QALY) and the lowest was in Australia ($277,000/QALY). In Canada, the U.K., and Israel, ICERs ranged between $351,000 and $358,000 per QALY. PSA demonstrated 0% likelihood of bevacizumab being cost‐effective in any country at a willingness to pay threshold of $150,000 per QALY.
Conclusion.
The addition of bevacizumab to first‐line chemotherapy for mCRC consistently fails to be cost‐effective in all five countries. There are large differences in cost‐effectiveness between countries. This study provides a framework for analyzing the value of a cancer drug from the perspectives of multiple international payers.
Implications for Practice.
The cost‐effectiveness of bevacizumab varies significantly between multiple countries. By conventional thresholds, bevacizumab is not cost‐effective in metastatic colon cancer in the U.S., the U.K., Australia, Canada, and Israel.
Introduction
The cost and value of cancer treatment is now under close scrutiny worldwide. Several professional societies within the oncology community such as the American Society of Clinical Oncology and the European Society for Medical Oncology have recently developed tools that attempt to analyze the value of different treatment strategies [1], [2]. The value of cancer drugs can also be assessed with the use of cost‐effectiveness analyses. A challenge with these approaches is that drug prices differ significantly between countries [3], and one cannot simply extrapolate cost‐effectiveness data from one country to another [4].
A standard first‐line therapy for metastatic colorectal cancer is 5‐fluorouracil (5‐FU), leucovorin, and oxaliplatin (FOLFOX) [5]. Bevacizumab, a monoclonal antibody against the vascular endothelial growth factor A is widely used as an addition to the chemotherapeutic regimen in a number of countries based on data from randomized clinical trials [6], [7], [8]. 5‐FU, leucovorin, and irinotecan (FOLFIRI) is typically administered as a second‐line regimen for patients whose disease progresses on first‐line therapy [9]. While this sequence of therapy is common, FOLFOX and FOLFIRI can be used interchangeably in the first‐ and second‐line settings.
In the U.S. setting, first‐line bevacizumab was previously shown to provide an additional 0.10 quality‐adjusted life years (QALY) at a cost of $571,000 per QALY gained [10]. This result established evidence that first‐line bevacizumab was not cost‐effective in the US. However, using this model, the cost‐effectiveness of first‐line bevacizumab in many other countries remains unknown. In response to concerns that U.S. payers may not be deriving optimal value for money compared with those in other countries, the objective of this study was to evaluate the cost‐effectiveness of bevacizumab in the first‐line treatment of metastatic colorectal cancer (mCRC) from the perspective of payers across five selected countries, namely the U.S., U.K., Canada, Australia, and Israel.
Materials and Methods
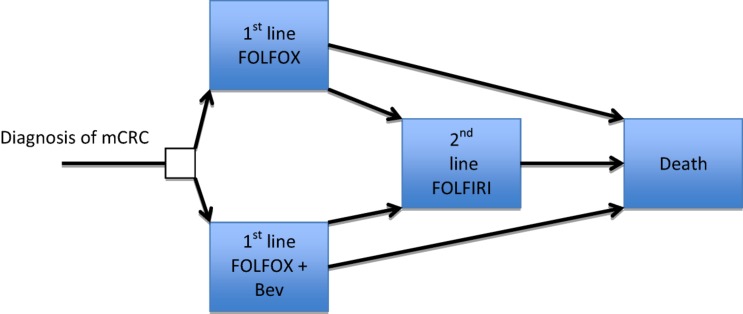
We used a Markov model that was previously developed to analyze the cost‐effectiveness of bevacizumab from the U.S. perspective [10]. In this study, we focused on comparing FOLFOX with or without bevacizumab in patients with newly diagnosed mCRC. The following features of the original model [10] were unchanged: model structure (Fig. 1), mortality and progression risks, adverse event (AE) incidence, AE management strategies, drug doses, health utilities, discounting factors. The model validation was established previously [10].
Figure 1.
Markov model.
Abbreviations: Bev, Bevacizumab; FOLFIRI, 5‐fluourouracil (bolus and continual infusion), leucovorin and irinotecan; FOLFOX, 5‐fluourouracil (bolus and continual infusion), leucovorin and oxaliplatin; mCRC, metastatic colorectal cancer.
Costs
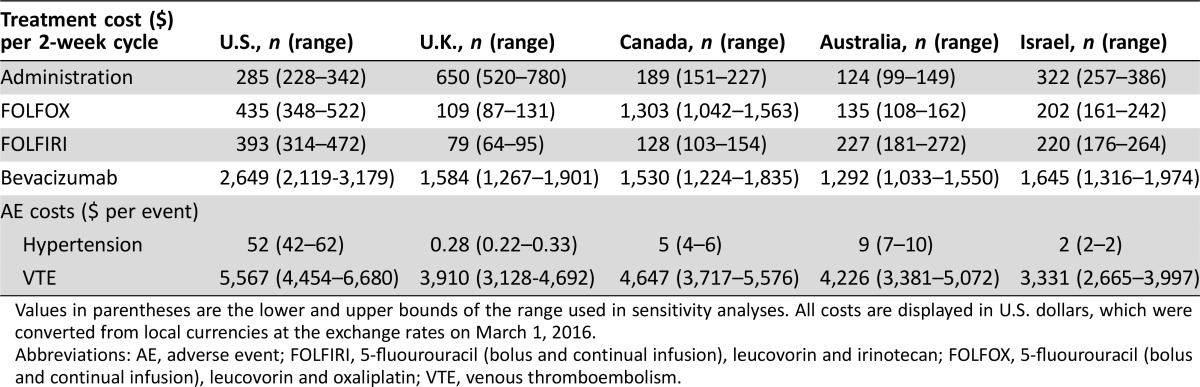
We adjusted all cost estimates for each individual country. All costs were sourced between 2013–2015 and were converted from local currency to U.S. dollars using the exchange rates on March 1, 2016: one U.S. dollar was equivalent to 0.72 U.K. pounds, 1.39 Australian dollars, 1.34 Canadian dollars, and 3.89 Israeli shekels [11]. We did not include sales tax. Details of drug costs are available in Table 1.
Table 1. Treatment costs.
Values in parentheses are the lower and upper bounds of the range used in sensitivity analyses. All costs are displayed in U.S. dollars, which were converted from local currencies at the exchange rates on March 1, 2016.
Abbreviations: AE, adverse event; FOLFIRI, 5‐fluourouracil (bolus and continual infusion), leucovorin and irinotecan; FOLFOX, 5‐fluourouracil (bolus and continual infusion), leucovorin and oxaliplatin; VTE, venous thromboembolism.
U.S. Costs
For the U.S. cost estimations, we used the same cost estimates as used in our previous study [10]. In particular, to estimate the unit price of each drug, we used the 2013 average sales price by the Centers for Medicare and Medicaid services [12]. Administration costs and AE costs were calculated according to the Medicare physician fee schedule for 2013. The fees for outpatient physician visits were based on Current Procedure Terminology codes [13].
U.K. Costs
To estimate the unit price for generic drugs, we used the U.K. Department of Health Commercial Medicines Unit electronic Medicines Information Tool [14]. To estimate the unit price for patented drugs, we used the U.K. list price as published in the British National Formulary [15]. This represents the national Drug Tariff arising from negotiation on a 5‐year cycle as part of the Pharmaceuticals Pricing and Reimbursement Scheme. Costs for chemotherapy administration and outpatient physician visits were taken from the National Health Service (NHS) Reference costs, which are published annually on the basis of average costs returned by individual NHS healthcare providers [16].
Canada Costs
To estimate the unit price of drugs, we used the Ontario Drug Benefit Formulary [17] and Sunnybrook Pharmacy Stores Department (Kelvin Chan, personal communication). The costs of chemotherapy supervision were estimated by duration of nursing and pharmacy time as estimated by Cancer Care Ontario [18] and multiplied by their estimated hourly wage [19]. The outpatient physician visits cost was obtained from the Ontario Schedule of Benefits [20]. In Ontario, Canada, there is a differential pricing structure for clinic visits based on the number of prior visits. In order to make appropriate comparisons between countries and not to adjust the overall design of the model, we estimated the price of a single clinic visit as the mean of the first five clinic visits. Although any difference in actual prices would likely have only a tiny impact on the model results, these differences would be accounted for in the subsequent sensitivity analyses.
Australia Costs
To estimate the unit price of each drug, we used the 2015 Pharmaceutical Benefits Scheme prices [21]. This is a federally funded pharmaceutical scheme with nationwide coverage. Administration costs and physician visits were based on the 2015 Medicare Benefits Schedule prices for outpatient health services [22].
Israel Costs
To estimate the unit price of each drug, we first used the list prices as described by the Israeli Ministry of Health [23]. In Israel, it is not possible to obtain actual health care provider's discounted price for drugs. However, based on suggested estimates from experts, we discounted generic drug list prices by 70% and assumed no discount for patented drugs. Administration costs and physician visits were based on the Clalit (Israel's largest health care provider) tariff for services.
Sensitivity Analysis
A series of sensitivity analyses were performed to evaluate the robustness of the model and to address uncertainty in the estimation of variables. Drug costs and physician fees were varied within ±% of their baseline values, and the ranges and distributions of other model parameters were defined the same way as in the prior study [10].
In one‐way sensitivity analyses, we varied the value of one parameter at a time over its defined range and examined the effect on the incremental cost‐effectiveness ratio (ICER). In probabilistic sensitivity analyses (PSA), we ran the model 10,000 times, using Monte Carlo simulation to randomly sample all parameters simultaneously from their stochastic distributions [10].
We performed a structural sensitivity analysis to incorporate the fact that there are alternative estimations of the level of efficacy of bevacizumab.
Net Benefit Calculation
Net Health Benefit (NHB) expresses the ICER on a single scale in units of QALYs [24]. It requires prespecification of a fixed monetary value of a QALY, which can be considered to be the opportunity cost of losing one QALY from a health system. This is equivalent to a back‐calculated cost‐effectiveness threshold. Its specification relies on the decision rule for cost‐effectiveness:
Where ΔE and ΔC are the incremental effects and costs and λ is the cost‐effectiveness threshold. This is rearranged to give:
Having specified NHB, value estimates for the addition of bevacizumab can be based on country‐specific estimates of lambda. Work by Woods et al. has estimated country‐specific lambda values based on income elasticities adjusted for purchasing power parity [25]. Using this, we calculated the country‐specific value of bevacizumab, subject to local pricing, using the value‐metric of incremental NHB per person treated (expressed in QALYs where higher values represent higher value).
Results
Base Case Results
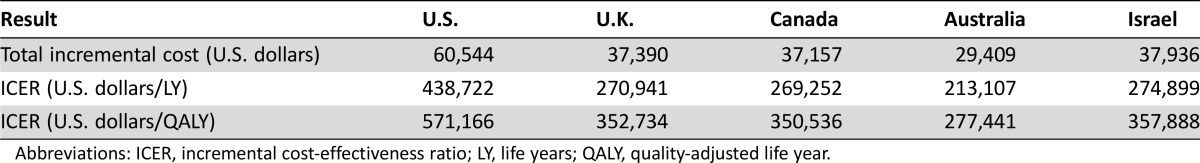
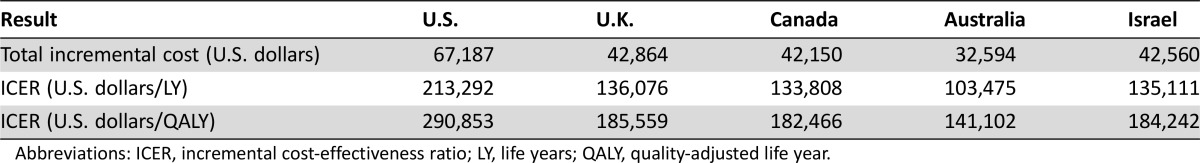
The number of life years (LYs) and QALYs was unchanged from those reported in the previous U.S.‐based study and identical in each country: the addition of bevacizumab to FOLFOX provided an additional benefit of 0.14 LYs or 0.10 QALYs [26]. In the U.S., U.K., Canada, Australia, and Israel, in comparison with the base case results, that addition of bevacizumab to FOLFOX resulted in an additional cost of $571,166, $352,734, $350,536, $277,441, and $357,888 per QALY gained, respectively. Table 2 demonstrates these base case results.
Table 2. Base case results.
Abbreviations: ICER, incremental cost‐effectiveness ratio; LY, life years; QALY, quality‐adjusted life year.
Sensitivity Analyses
The results of univariate sensitivity analyses are presented in the tornado diagrams in the supplemental appendix. For all five countries, the ICER results were the most sensitive to overall survival benefit (i.e., hazard ratio of FOLFOX/bevacizumab arm compared with the FOLFOX arm), drug cost of bevacizumab, and health utility estimates. However, within the range of each parameter, ICERs never decreased below $100,000 per QALY gained. Country‐specific cost parameters were of varying importance between countries.
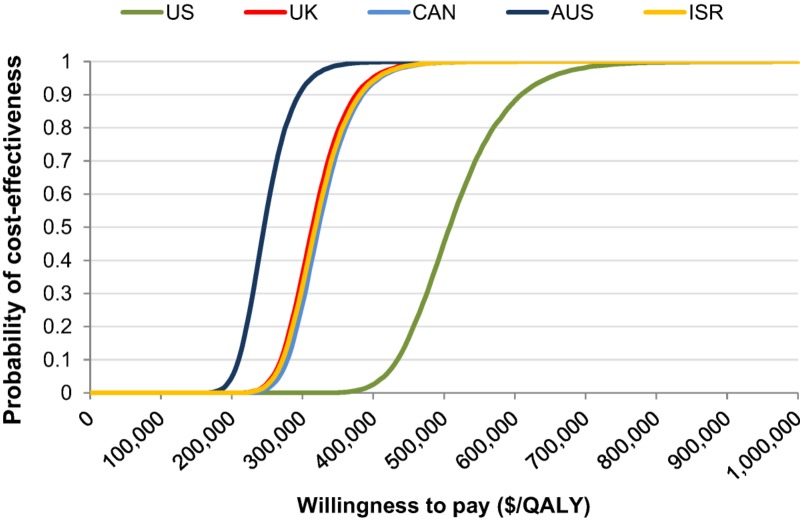
The results of the PSA are demonstrated by the cost‐effectiveness acceptability curves for varying willingness to pay values (Fig. 2). These demonstrated that there was a 0% likelihood of bevacizumab being cost‐effective in any country at a willingness to pay (WTP) threshold of $150,000/QALY. For a 90% likelihood of bevacizumab being cost‐effective in the U.S., the U.K., Canada, Australia, and Israel, the WTP thresholds would be $610,000/QALY, $380,000/QALY, $385,000/QALY, $295,000/QALY, and $385,000/QALY, respectively.
Figure 2.
Cost‐effectiveness acceptability curves in U.S. dollars.
Abbreviations: AUS, Australia; CAN, Canada; ISR, Israel; QALY, quality‐adjusted life years; UK, United Kingdom; US, United States.
Country‐Specific Issues and Subsequent Sensitivity Analyses
During the development of the parameters for each country, we noted some country‐specific differences related to drug prices and coverage. At the time of data collection, oxaliplatin was no longer patented in all countries evaluated except for Canada. In the U.S., the U.K., Australia, and Israel, a 2‐week course of FOLFOX costs $435, $109, $135, and $202, respectively. In Canada, in 2015, it cost $1,303. However, the patent expired in 2016. We therefore did a secondary analysis, replacing the current price of oxaliplatin with the current price of irinotecan, as the prices for these generic drugs are similar in most countries. In this way, we performed a sensitivity analysis of the model using a projected 2016 price for oxaliplatin in Canada. With this analysis, we found that this change in drug price had only a small impact on the overall value of bevacizumab. The reason for this is likely due to duration of first‐line therapy in different arms of the model.
Structural Sensitivity Analysis Adjusting for Improved Estimate of the Efficacy of Bevacizumab
In the NO19699 study, the hazard ratio (HR) for progression free survival (PFS) was 0.83, and the HR for overall survival (OS) was 0.89. However, some alternative studies demonstrate a higher level of survival benefit when bevacizumab is added to a chemotherapy backbone [8]. To incorporate this uncertainty, we adjusted the HR in the model for both PFS and OS to 0.75 and reran the model to produce base case results, which are presented in Table 3. With this improved estimate of efficacy, the ICERs were lower but did not fall below $140,000 per QALY in all countries analyzed.
Table 3. Structural sensitivity analysis with HR for progression‐free survival adjusted from 0.83 to 0.75 and HR for overall survival adjusted from 0.89 to 0.75.
Abbreviations: ICER, incremental cost‐effectiveness ratio; LY, life years; QALY, quality‐adjusted life year.
Country‐Specific Value Estimates
Expressed as NHB, the country specific estimates of the value of adding bevacizumab are as follows: U.S. −2.4 to −1.4 QALYs; U.K. −1.9 to −1.9 QALYs; Canada −1.7 to −1.3 QALYs; Australia −1.3 to −1.0 QALYs; Israel −2.4 to −2.1 QALYs. This approach suggests that country‐specific prices result in Australia obtaining best value for money and Israel likely the worst, taking into account the country‐specific opportunity cost of investment in the new technology.
Discussion
Understanding the value of cancer therapies requires a clear understanding of both cost and benefit. While it may be acceptable to assume that clinical benefit is generalizable between countries, given the large differences in drug costs between countries, it is necessary to use country‐specific cost parameters in order to estimate country‐specific value. In this study, we used a cost‐effectiveness model to provide assessments of cost‐effectiveness in multiple countries. At a WTP threshold of $150,000/QALY, we demonstrated that bevacizumab does not appear to be cost‐effective in any of the five countries analyzed for the first‐line management of metastatic colorectal cancer. However, we also demonstrated that the cost per QALY of this drug varies markedly between countries. With ICERs between $278,000–$358,000 per QALY gained, bevacizumab is substantially more cost‐effective in the U.K., Australia, Israel, and Canada than in the U.S., where it costs $571,000 per QALY. Perhaps more importantly, when considering the differences in purchasing power and also the differing impact on the health systems of budgetary reallocation, we demonstrate that bevacizumab fails to meet a value threshold in any country, with an estimated net health loss between one and two QALYs per patient across the countries studied.
The impact of global variation in drug pricing on cost‐effectiveness is economically significant. The countries with predominately government funded health services (Australia, Israel, Canada, and the U.K.) may be able to negotiate better drug prices with pharmaceutical companies than countries with a predominately private health system such as the U.S. This underscores the need for more transparency of drug prices and negotiations globally.
We compared the cost‐effectiveness of one health intervention across different countries (or health care settings) with the same model structure while adjusting the costs by the country‐specific prices and maintaining other variables constant. However, we did note that there are specific circumstances that may require special sensitivity analyses due to differences in drug coverage between countries. Specifically, we noted that termination of patents of specific drugs can vary between countries, thus influencing costs. Specifically, the patent expiration for oxaliplatin was variable between countries. When using a single model to analyze cost‐effectiveness in different countries, one must pay careful attention to such issues, as they can influence the model results.
As with any economic model, there were many assumptions that influenced the overall results. We assumed that survival benefits, AE incidence, and utilities were standard between countries. We did not incorporate drug wastage, although this may be significant [26]. We assumed that management strategies of AEs were standard between countries; however, there may be some variability. Data for age of diagnosis of colorectal cancer were taken from the Surveillance Epidemiology and End Results Program, which is based on the U.S. cancer population. However, it is unlikely that there are significant differences between countries for age of diagnosis of colon cancer. We did not include taxes in drug costs for any country, as tax rates and criteria are different between countries.
Understanding the true clinical benefit of bevacizumab continues to be a great challenge. This model and the original U.S.‐based model were based on survival data from the NO19699 trial, which was the largest randomized trial analyzing the benefit of bevacizumab [7]. However, one could challenge the applicability of the results due to the failure to continue with maintenance therapy after initial induction with bevacizumab. In this trial, there was an approximate 6‐week overall survival benefit. Our structural sensitivity analysis with improved estimates of efficacy lowered the ICERs in all countries, but not below conventional thresholds.
There is significant debate regarding appropriate “willingness to pay” thresholds for cost‐effectiveness. In the U.K. the threshold is 30,000 pounds per QALY (approximately $43,000/QALY). In the U.S., the cost‐effectiveness of dialysis ($50,000/QALY) was originally estimated to be an appropriate threshold [27]. However, subsequent health care cost increases may mean that this is now an underestimate. An alternative proposal by the World Health Organization suggested that any intervention costing less than three times the gross domestic product per capita per disability‐adjusted LY averted represents an appropriate threshold [28]. A prior analysis of bevacizumab performed in the U.K. demonstrated a different level of cost‐effectiveness, although the exact reasons are unclear due to the commercial‐in‐confidence model parameters and results that are a necessary component of the Patient Access Scheme offered by Roche to the U.K. health service [29]. There are many ways that cost‐effectiveness could be improved—the most obvious way would be to reduce the price. However, in an age of increasingly personalized medicine, the need for an effective biomarker is of paramount importance. This would undoubtedly improve cost‐effectiveness.
Conclusion
Understanding the value of cancer therapies is crucial, and we need a robust tool to understand this, as policy makers must make difficult decisions regarding drug coverage. Economic models are flexible and can be used, with caution, across international borders. While the challenges of implementing a global value‐based price are undoubtedly large in a complex global market, further development of this work may form an essential part of efforts to achieve price and value equity between countries as part of the international effort to combat the escalating financial crisis in cancer care.
Acknowledgments
C.F. received the Burroughs Wellcome Fund Innovation in Regulatory Science Award, in support of this work.
See http://www.TheOncologist.com for supplemental material available online.
Author Contributions
Conception/design: Daniel A. Goldstein, Qiushi Chen, Turgay Ayer, Kelvin K. W. Chan, Kiran Virik, Ariel Hammerman, Baruch Brenner, Christopher R. Flowers, Peter S. Hall
Provision of study material or patients: Daniel A. Goldstein, Qiushi Chen, Turgay Ayer, Kelvin K. W. Chan, Kiran Virik, Ariel Hammerman, Baruch Brenner, Christopher R. Flowers, Peter S. Hall
Collection and/or assembly of data: Daniel A. Goldstein, Qiushi Chen, Turgay Ayer, Kelvin K. W. Chan, Kiran Virik, Ariel Hammerman, Baruch Brenner, Christopher R. Flowers, Peter S. Hall
Data analysis and interpretation: Daniel A. Goldstein, Qiushi Chen, Turgay Ayer, Kelvin K. W. Chan, Kiran Virik, Baruch Brenner, Christopher R. Flowers, Peter S. Hall
Manuscript writing: Daniel A. Goldstein, Qiushi Chen, Turgay Ayer, Kelvin K. W. Chan, Kiran Virik, Ariel Hammerman, Baruch Brenner, Christopher R. Flowers, Peter S. Hall
Final approval of manuscript: Daniel A. Goldstein, Qiushi Chen, Turgay Ayer, Kelvin K. W. Chan, Kiran Virik, Ariel Hammerman, Baruch Brenner, Christopher R. Flowers, Peter S. Hall
Disclosures
Peter S. Hall: Roche (RF); Christopher R. Flowers: Gilead, Bayer, Celgene (C/A), Millennium, Takecka, Pharmacycles, TG Therapeutic, Gilead, Genentech/Roche (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supplementary Information
References
- 1. Schnipper LE, Davidson NE, Wollins DS et al. American Society of Clinical Oncology Statement: A conceptual framework to assess the value of cancer treatment options. J Clin Oncol 2015;33:2563–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cherny NI, Sullivan R, Dafni U et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti‐cancer therapies: The European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO‐MCBS). Ann Oncol 2015;26:1547–1573. [DOI] [PubMed] [Google Scholar]
- 3. Vogler S, Vitry A, Babar ZU. Cancer drugs in 16 European countries, Australia, and New Zealand: A cross‐country price comparison study. Lancet Oncol 2016;17:39–47. [DOI] [PubMed] [Google Scholar]
- 4. Yabroff KR, Borowski L, Lipscomb J. Economic studies in colorectal cancer: Challenges in measuring and comparing costs. J Natl Cancer Inst Monogr 2013;2013:62–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goldberg RM, Sargent DJ, Morton RF et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol 2004;22:23–30. [DOI] [PubMed] [Google Scholar]
- 6. Hochster HS, Hart LL, Ramanathan RK et al. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first‐line treatment of metastatic colorectal cancer: Results of the TREE Study. J Clin Oncol 2008;26:3523–3529. [DOI] [PubMed] [Google Scholar]
- 7. Saltz LB, Clarke S, Díaz‐Rubio E et al. Bevacizumab in combination with oxaliplatin‐based chemotherapy as first‐line therapy in metastatic colorectal cancer: A randomized phase III study. J Clin Oncol 2008;26:2013–2019. [DOI] [PubMed] [Google Scholar]
- 8. Hurwitz H, Fehrenbacher L, Novotny W et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335–2342. [DOI] [PubMed] [Google Scholar]
- 9. Tournigand C, André T, Achille E et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol 2004;22:229–237. [DOI] [PubMed] [Google Scholar]
- 10. Goldstein DA, Chen Q, Ayer T et al. First‐ and second‐line bevacizumab in addition to chemotherapy for metastatic colorectal cancer: A United States‐based cost‐effectiveness analysis. J Clin Oncol 2015;33:1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Current and Historical Rate Tables. Available at http://www.xe.com/currencytables/?from=USD& date=2016-03-01. Accessed March 19, 2016.
- 12.ASP drug pricing files. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice/2013ASPFiles.html. Accessed September 23, 2016.
- 13.Medicare Physician Fee Schedule. 2013.
- 14.Commercial Medicines Unit electronic Medicines Information Tool (eMIT). Available at http://www.gov.uk/government/collections/commercial-medicines-unit-cmu. Accessed March 18, 2016.
- 15.UpToDate. Available at www.uptodate.com. Accessed December 18, 2015.
- 16.NHS Reference Costs 2013 to 2014. Available at https://www.gov.uk/government/publications/nhs-reference-costs-2013-to-2014. Accessed March 19, 2016.
- 17.ODB Formulary. Available at https://www.formulary.health.gov.on.ca/formulary/. Accessed September 22, 2015.
- 18.Cancer Care Ontario Drug Formulary. Available at http://www.cancercare.on.ca/toolbox/drugformulary/. Accessed 22 September 2015.
- 19.Government of Canada Job Bank. Available at http://www.jobbank.gc.ca/wage-outlook_search-eng.do?reportOption=wage. Accessed September 22, 2015.
- 20.Schedule of Benefits for Physician Services under the Health Insurance Act. Available at http://www.health.gov.on.ca/english/providers/program/ohip/sob/physserv/physserv_mn.html. Accessed September 22, 2015.
- 21.The Pharmaceutical Benefits Scheme, 2015. Available at http://www.pbs.gov.au/browse/medicine-listing. Accessed April 17, 2017.
- 22.Australian Government Department of Health and Ageing. Medicare Benefits Schedule Book. Available at http://www.mbsonline.gov.au. Accessed March 31, 2016.
- 23.Ministry of Health of Israel. Available at http://www.health.gov.il/Subjects/Finance/DrugPrice/Pages/default.aspx. Accessed December 1, 2015.
- 24. Stinnett AA, Mullahy J. Net health benefits: A new framework for the analysis of uncertainty in cost‐effectiveness analysis. Med Decis Making 1998;18(suppl 2):S68–S80. [DOI] [PubMed] [Google Scholar]
- 25. Woods B, Revill P, Sculpher M et al. Country‐level cost‐effectiveness thresholds: Initial estimates and the need for further research. York, U.K: University of York, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lien K, Cheung MC, Chan KK. Adjusting for drug wastage in economic evaluations of new therapies for hematologic malignancies: A systematic review. J Oncol Pract 2016;12:e369–e379. [DOI] [PubMed] [Google Scholar]
- 27. Neumann PJ, Cohen JT, Weinstein MC. Updating cost‐effectiveness–The curious resilience of the $50,000‐per‐QALY threshold. N Engl J Med 2014;371:796–797. [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization . Macroeconomics and health: Investing in health for economic development. Report of the Commission on Macroeconomics and Health. Geneva, Switzerland: World Health Organization,2001.
- 29.NICE. Bevacizumab in combination with oxaliplatin and either Fluorouracil plus folinic acid or capecitabine for the treatment of metastatic colorectal cancer. NICE, December 15, 2010.