Abstract
Background
Prior studies have shown that late gadolinium enhancement (LGE) on cardiac magnetic resonance (CMR) and fluorodeoxyglucose (FDG) positron emission tomography (PET) confer incremental risk assessment in patients with cardiac sarcoidosis (CS). However, the incremental prognostic value of the combined use of LGE and FDG compared to either test alone has not been investigated, and this is the aim of the present study.
Methods
Retrospective observational study of 56 symptomatic patients with high clinical suspicion for CS who underwent LGE-CMR and FDG-PET and were followed for the occurrence of death and/or malignant ventricular arrhythmias (VA).
Results
The combination of PET and CMR yielded the following groups: 1) LGE-negative/normal-PET (n=20), 2) LGE-positive/abnormal-FDG (n=20), and 3) LGE-positive/normal FDG (n=16). After a median follow-up of 2.6 years (IQR 1.2–4.1), 16 patients had events (7 deaths, 10 VA). All, but 1, events occurred in patients with LGE. LGE-positive/abnormal-FDG (7 events, HR 10.1 [95% CI 1.2–84]; P=0.03) and LGE-positive/normal-FDG (8 events, HR 13.3 [1.7–107]; P=0.015) patients had comparable risk of events compared to the reference LGE-negative/normal-PET group. In adjusted Cox-regression analysis, presence of LGE (HR 18.1 [1.8–178]; P=0.013) was the only independent predictor of events.
Conclusion
CS patients with LGE alone or in association with FDG were at similar risk of future events, which suggests that outcomes may be driven by the presence of LGE (myocardial fibrosis) and not FDG (inflammation).
1. Introduction
Sarcoidosis is a complex inflammatory condition for which neither the cause nor the cure is known. Recognition of patients with cardiac involvement is of paramount importance since early diagnosis may allow prevention of sudden cardiac death by placement of an implantable cardioverter defibrillator (ICD), and/or slow down the progression of left ventricular (LV) systolic dysfunction by initiation of directed therapy.1,2
However, the clinical diagnosis of cardiac sarcoidosis remains challenging. Endomyocardial biopsy, the natural gold standard, is rarely performed because of its poor sensitivity (due to sampling error) and risks involved with the procedure 3. Consequently, the diagnosis of cardiac sarcoidosis relies mostly on non-invasive modalities. In this sense, cardiac magnetic resonance (CMR) with late gadolinium enhancement (LGE) and F-18 fluorodeoxyglucose (FDG) positron emission tomography (PET) have become the most widely used imaging modalities for the diagnosis of cardiac sarcoidosis.
Previous studies have shown that each technique provides prognostic value beyond standard clinical data.4,5 In patients with suspected cardiac sarcoidosis, the presence of myocardial LGE has been associated with a 9-fold higher rate of major adverse events compared to patients without LGE.4 Similarly, the finding of abnormal myocardial perfusion and FDG uptake on PET identified patients at higher risk of death and/or malignant ventricular arrhythmias.5
However, the literature on the incremental prognostic value of the combined use of LGE-CMR and FDG-PET imaging, or the additive value of either test, during the evaluation process of patients with suspected cardiac sarcoidosis is lacking.
In the present study, we hypothesized that the concomitant presence of FDG (inflammation) and LGE (fibrosis) in the heart would improve risk stratification of patients with symptomatic cardiac sarcoidosis compared to either finding alone.
2. Methods
Study design and patients
We retrospectively reviewed symptomatic patients who had a high clinical suspicion for cardiac sarcoidosis based on their clinical manifestations (suppl. Table 1) and who were referred for both FDG-PET and LGE-CMR as part of their initial work-up between January 2009 and December 2015. We excluded patients with 1) known obstructive coronary artery disease either by history or coronary angiography when available (n=1), 2) biopsy-proven non-ischemic cardiomyopathies other than sarcoidosis (n=1), 3) studies performed more than 12 months apart (n=4), 4) incomplete FDG suppression (n=5), and 5) occurrence of heart transplantation between PET and CMR (n=1). The University of Washington Institutional Review Boards approved this study, including a waiver of consent.
FDG-PET protocol
Glucose suppression protocol
All patients received written instructions to adhere to a specific high fat, high protein, no carbohydrate, no sugar diet for 24 hours and were advised to fast for 12 hours prior to the procedure.
Myocardial perfusion imaging
All patients underwent myocardial perfusion imaging (MPI) at rest with either N-13 ammonia (until January 2014) or Rb-82 (since February 2014) on a Discovery STE PET/CT scanner (GE Healthcare). Individuals were positioned with the help of a CT topogram, and a low-dose CT scan (2.5 mm slice, 120 kVp, 15–20 mA) was acquired for attenuation correction of PET emission data before MPI. Then, either N-13 ammonia (~ 370 MBq [10 mCi]) or Rubidium-82 (1,480–1,850 MBq [40–50 mCi]) was injected as a bolus, and 2-dimensional list-mode PET acquisition was obtained over 15 minutes for N-13 ammonia or 8 minutes for Rb-82 based protocols.
Cardiac FDG imaging
Intravenous unfractionated heparin (50 IU/kg) was administered 10 minutes after Rb-82, or 60 minutes after N-13 ammonia injection.
FDG (259–370 MBq [7–10mCi]) was then injected 15 minutes after the administration of heparin and FDG images acquired in 2D list mode for 15 min prior to 10/1/13 and in 3-D list mode for 12 min after 10/1/13.
PET interpretation
Images were interpreted (P.B. and S.E.) in a blinded fashion with respect to CMR and clinical status. MPI was considered abnormal when the 17-American Heart Association summed rest score was 2 or greater. Myocardial FDG uptake was deemed abnormal when focally present to either one LV segment and/or multiple wall segments. A pattern consistent with focal on diffuse FDG uptake was considered incomplete FDG suppression since this pattern cannot reliably differentiate pathologic from partial FDG suppression. Complete suppression of FDG uptake was considered normal. In general, cardiac PET was considered abnormal if either FDG uptake and/or MPI were abnormal, whereas, PET was deemed normal when both myocardial FDG uptake and MPI were within normal limits.
CMR protocol
Scans were performed on a commercially available whole-body scanner using a cardiac coil (Philips 1.5T Achieva) or torso phased array coil (Philips 3T Ingenia; Philips Medical Systems, Best, The Netherlands). Cardiac cine acquisition: retrospective, ECG-gated steady-state free precession segmented cine images in the short axis, two-, three-, and four-chamber views. LGE acquisition: segmented phase-sensitive inversion-recovery gradient-echo turbo fast field echo sequence at end-diastole during a single breath-hold 10 min after injection of 0.2 mmol/kg gadoteridol (ProHance, Bracco) contrast medium. An inversion scout (Look-Locker) sequence was used to select the optimal inversion time for maximal nulling of normal myocardial signal.
Late gadolinium enhancement interpretation
Image processing was performed using a commercially available dedicated workstation (CVI42; Circle Cardiovascular Imaging, Alberta, Canada). Images were interpreted (E.K.) in a blinded fashion with respect to PET and clinical status. The presence of LGE was visually assessed as either present or absent. If present, LGE pattern was classified as: 1) Suggestive of cardiac sarcoid if there was sub-epicardial patchy hyper-enhancement, particularly in the basal or mid ventricular segments of myocardium,6 alone or in combination with sub-endocardial or mesocardial distribution of LGE in the inferior-lateral wall or patchy intramural distribution in the LV.4,7 2) Atypical for sarcoid were LGE patterns, which followed a typical coronary distribution, isolated LGE in the septal insertion sites or a thin linear stripe of mid-myocardial hyper-enhancement.8,9
PET CMR classification
After independent evaluation of PET and CMR images as per above, paired-cases were divided into different sub-groups based on the presence/absence of LGE and abnormal FDG uptake as following: 1) LGE-negative / normal FDG and MPI (normal PET); 2) LGE-positive / abnormal FDG (independent of MPI); 3) LGE-positive / normal FDG (independent of MPI); 4) LGE-negative / abnormal FDG and/or MPI.
Adjudication of clinical events
Medical records were reviewed and the 2006 Japanese Ministry of Health and Welfare (JMHW)10 and 2014 Heart Rhythm Society (HRS) consensus statement11 were blindly applied to all patients by an independent physician (D.R.). Presence of extra-cardiac sarcoidosis was defined as biopsy-confirmed disease (33/37) or clinically suspected based on non-invasive imaging (4/37). Medical records were also reviewed by an independent physician (D.R.) for the occurrence of sustained ventricular tachycardia (VT), ventricular fibrillation (VF), appropriate ICD shock, and all-cause death on follow-up.
Statistical analyses
We analyzed the data using STATA (version 13.1). One-way ANOVA was performed to compare mean values of 3 groups or more. Continuous variables are presented as mean ± SD, except for follow-up time, which is given in median with interquartile range. Association between categorical variables was measured using Fisher’s exact test if variables arranged in a 2×2 table, otherwise, chi2 test was employed and results are presented as percentages. Diagnostic agreement between tests (on a 2×2 table) was measured using Kappa (k) statistic and interpreted according to Landis and Koch as following: < 0 no agreement, 0–0.20 as slight, 0.21–0.40 as fair, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1 as almost perfect agreement.12 Z-test was used to compare two Kappa values for statistical significance. Event-free survival (time to first event) was plotted as Kaplan–Meier curves. Log-rank tests were performed to compare survival curves. Cox proportional hazard models were used for analyzing significant associations with major events. All statistical tests were 2-tailed, and a P value <0.05 was considered statistically significant.
3. Results
The final study cohort consisted of 56 patients who underwent PET and CMR with a median time of 1.3 months (IQR 0.5–4.6) between studies for the evaluation of suspected cardiac sarcoidosis. The most common indications for evaluation were unexplained LV systolic dysfunction, high-degree A-V block, ventricular arrhythmias, dyspnea, and palpitations (Suppl. Table 1).
A total of 36 subjects (64%) had LGE on CMR. The pattern was suggestive of cardiac sarcoidosis in 31 and atypical for sarcoidosis in 5 individuals. PET was abnormal in 29 patients (52%), including 20 with abnormal FDG uptake and 9 with abnormal MPI only.
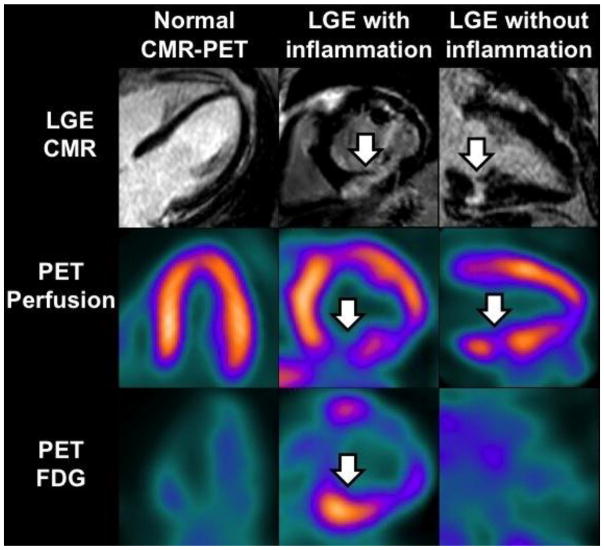
As a result, the combination of PET and CMR yielded the following groups: 1) no evidence of myocardial damage (LGE-negative and normal PET) in 36%, 2) myocardial damage (LGE-positive) with inflammation (abnormal FDG) in 36%, and 3) myocardial damage (LGE-positive) without inflammation (normal FDG) in 28% (Figure 1). We observed no cases where FDG and/or MPI were abnormal and LGE was absent.
Figure 1.
Representative images of the different groups according to PET/CMR findings as following: 1) no evidence of myocardial damage, 2) myocardial damage with inflammation, and 3) myocardial damage without inflammation. Arrows point out the location of LGE, perfusion deficits and abnormal FDG uptake.
Table 1 depicts the cohort baseline characteristics according to their PET/CMR findings. In average, patients were middle-aged with mildly reduced LV systolic function, and 2/3 had known extra-cardiac sarcoidosis. Patients with LGE on CMR in general had lower LVEF, and those with inflammation by FDG were more likely to meet clinical criteria for cardiac sarcoidosis.
Table 1.
Baseline characteristics of patients with suspected cardiac sarcoidosis evaluated with CMR and PET
| Baseline Characteristics | All patients (n=56) | No myocardial damage (n=20) | Myocardial damage with inflammation (n=20) | Myocardial damage without inflammation (n=16) | P value |
|---|---|---|---|---|---|
| Age, years ± SD | 53 ± 12 | 56 ± 10 | 50 ± 10 | 53 ± 14 | 0.3 |
| Males, n (%) | 37 (66%) | 14 (70%) | 12 (60%) | 11 (69%) | 0.8 |
| Hypertension, n (%) | 21 (37%) | 8 (40%) | 6 (30%) | 7 (44%) | 0.7 |
| Diabetes, n (%) | 7 (12%) | 1 (5%) | 3 (15%) | 3 (19%) | 0.4 |
| Taking oral steroids, n (%) | 14 (25%) | 8 (40%) | 4 (20%) | 2 (13%) | 0.14 |
| Known extracardiac sarcoidosis, n (%) | 37 (66%) | 17 (85%) | 12 (60%) | 8 (50%) | 0.07 |
| 2006 JMHW criteria met, n (%) | 16 (29%) | 2 (10%) | 12 (60%) | 2 (13%) | 0.001 |
| 2014 HSR criteria met, n (%) | 20 (36%) | 2 (10%) | 12 (60%) | 6 (38%) | 0.004 |
| Prior high-degree A-V block, n (%) | 9 (16%) | 1 (5%) | 8 (40%) | 0 | 0.001 |
| Prior ventricular arrhythmias, n (%) | 8 (14%) | 0 | 3 (15%) | 5 (31%) | 0.03 |
| CMR | |||||
| LVEF, % ± SD | 49 ± 13 | 53 ±9 | 47 ±15 | 46 ±13 | 0.16 |
| LVEF < 50%, n (%) | 21 (38%) | 5 (25%) | 9 (45%) | 7 (44%) | 0.4 |
| LVEF ≤35%, n (%) | 9 (16%) | 1 (5%) | 5 (25%) | 3 (19%) | 0.2 |
| No LGE, n (%) | 20 (36%) | 20 (100%) | 0 | 0 | |
| LGE suggestive of CS, n (%) | 31 (55%) | 0 | 20 (100%) | 11 (69%) | <0.001 |
| LGE not typical for CS, n (%) | 5 (9%) | 0 | 0 | 5 (31%) | |
| PET | |||||
| Normal PET, n (%) | 27 (48%) | 20 (100%) | 0 | 7 (44%) | |
| Abnormal FDG, n (%) | 20 (36%) | 0 | 20 (100%) | 0 | <0.001 |
| Abnormal MPI only, n (%) | 9 (16%) | 0 | 0 | 9 (56%) | |
JMHW indicates Japanese Ministry of Health and Welfare; HSR, heart rhythm society; A-V, atrial-ventricular
At diagnosis, ventricular arrhythmias occurred only in patients with LGE on CMR independent of inflammation status, whereas high-degree A-V block occurred almost exclusively in patients with inflammation (Table 1). All patients with myocardial damage and inflammation showed an LGE pattern suggestive of cardiac sarcoidosis, whereas, an LGE pattern not typical for sarcoidosis was only seen in patients without inflammation (Table 1, Supplementary Figure 1).
Diagnostic agreement between PET and CMR
The agreement between PET and CMR abnormalities was substantial (k 0.75 [95% CI 0.49–1.00]) if PET was classified as abnormal under the basis of either abnormal FDG uptake and/or MPI. In contrast, if only FDG uptake was considered abnormal, then the agreement between PET and CMR was moderate (k 0.47 [95% CI 0.25–0.69]). Concordance between FDG-PET and CMR was not affected by the inclusion of patients taking steroids since the agreement between both modalities did not change significantly after excluding such patients from the analyses (Supplementary Table 2). Agreement between MPI and LGE was found to be substantial as well (k 0.68 [95% CI 0.43–0.93]).
Major adverse events
Patients were followed for a median of 2.6 years (IQR 1.2–4.1). During this time period, 30 patients (54%) underwent ICD/pacemaker placement, and a total of 16 patients had major adverse events as follows: 10 subjects experienced VT/VF (including 4 patients who previously presented with VT/VF), and 7 patients died, including one patient who also had VT on follow-up (Table 2).
Table 2.
Univariate analysis of patients experiencing death, and/or sustained ventricular arrhythmias during follow-up*
| Characteristics | Group without Events (n=40) | Group with Events (n=16) | Hazard Ratio [95% CI] | P value |
|---|---|---|---|---|
| Age, years ± SD | 54 ± 11 | 51 ± 13 | 0.98 [0.9–1.0] | 0.5 |
| Males, n (%) | 24 (60%) | 13 (81%) | 2.1 [0.6–7.2] | 0.3 |
| Prior use of steroids, n (%) | 12 (30%) | 2 (12%) | 0.4 [0.09–1.2] | 0.24 |
| Extracardiac sarcoidosis, n (%) | 28 (70%) | 9 (56%) | 0.8 [0.3–2.1] | 0.6 |
| 2006 JMHW criteria met, n (%) | 11 (28%) | 5 (31%) | 1.5 [0.5–4.5] | 0.4 |
| 2014 HSR criteria met, n (%) | 12 (30%) | 8 (50%) | 2.8 [1.0–7.6] | 0.045 |
| VT/VF at presentation, n (%) | 4 (10%) | 4 (25%) | 2.1 [0.7–6.7] | 0.2 |
| CMR | ||||
| LVEF, % ± SD | 50 ± 10 | 46 ± 18 | 0.97 [0.9–1.0] | 0.16 |
| LVEF ≤ 35%, n (%) | 4 (10%) | 5 (31%) | 2.9 [1.0–8.4] | 0.049 |
| LGE present, n (%) | 21 (53%) | 15 (94%) | 11.7 [1.5–90] | 0.018 |
| PET | ||||
| Abnormal FDG | 13 (32%) | 7 (44%) | 3.3 [0.9–11] | 0.06 |
| Abnormal MPI only | 4 (10%) | 5 (31%) | 3.4 [0.9–13] | 0.07 |
| Normal PET | 23 (58%) | 4 (25%) | Reference | |
| Any PET abnormality (FDG and/or perfusion), n (%) | 17 (42%) | 12 (75%) | 3.3 [1.1–10] | 0.039 |
| PET/CMR | ||||
| Myocardial damage with inflammation | 13 (32%) | 7 (44%) | 10.1 [1.2–84] | 0.032 |
| Myocardial damage without inflammation | 8 (20%) | 8 (50%) | 13.3 [1.7–107] | 0.015 |
| No myocardial damage | 19 (48%) | 1 (6%) | Reference | |
Median follow-up 2.6 years (IQR 1.2–4.1)
JMHW indicates Japanese Ministry of Health and Welfare; HSR, heart rhythm society
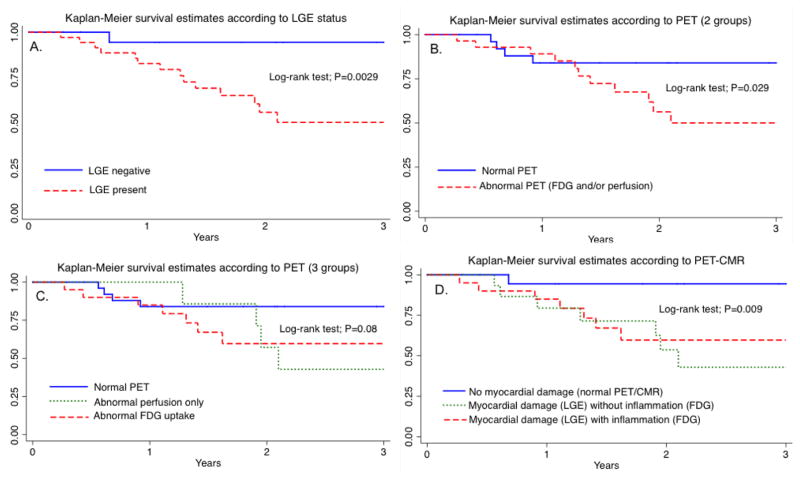
On univariate (unadjusted) analysis, LVEF less than 35% (HR 2.9 [95% CI 1.0–8.4]; P=0.049), clinical diagnosis of cardiac sarcoidosis (HR 2.8 [1.0–7.6]; P=0.045), any PET abnormality (HR 3.3 [1.1–10]; P=0.039), and presence of myocardial LGE (HR 11.7 [1.5–90]; P=0.018) were significant predictors of future major adverse events. When PET and CMR findings were combined, the proportion of future events in patients with evidence of myocardial damage and inflammation was similar to those with myocardial damage without inflammation (Table 2). Both sub-groups showed significantly higher event rates compared to patients without myocardial damage. Figure 2 summarizes the survival curves according to LGE, PET and the combination of PET/CMR for all patients with suspected cardiac sarcoidosis.
Figure 2.
Kaplan-Meier curves show that survival-free of major events is lower in patients with evidence of LGE on CMR (A), abnormal PET (B and C), or myocardial damage with or without inflammation on PET/CMR (D).
In a multivariate cox-regression analysis that included abnormal PET (HR 1.7 [0.4–6.5]), LVEF < 35% (HR 2.6 [0.7–9.3]), and HSR diagnosis of cardiac sarcoidosis (HR 2.1 [0.7–6.7]), presence of myocardial LGE (HR 18.1 [1.8–178]; P=0.013) was the only independent predictor of future events (Suppl. Table 3).
4. Discussion
In the present study we found that the presence of myocardial LGE, a marker of myocardial damage, appears to be a better discriminator or predictor of events compared to FDG. In addition, we observed a reasonably good diagnostic agreement between PET and CMR in the evaluation of patients with suspected cardiac sarcoidosis.
Accurate detection of patients with cardiac sarcoidosis is clinically important because of the high morbidity and mortality associated with this diagnosis.1,13,14 FDG-PET and LGE-CMR have emerged as the two most commonly employed non-invasive imaging tests for diagnosing cardiac sarcoidosis. FDG-PET identifies active sarcoid lesions under the premise that activated macrophages and other inflammatory cells are FDG-avid, whereas, LGE-CMR localizes to areas of focally expanded extracellular space from fibrosis and scarring associated with both active and inactive sarcoid infiltration, relative to normal myocardium, thus allowing for its visualization on delayed images (usually 10 minutes after injection) 15.
The diagnosis of cardiac sarcoidosis can be challenging since the sole presence of LGE may not be diagnostic by itself, and the location and pattern of LGE may be more relevant for diagnostic accuracy. In addition, LGE is limited in distinguishing active from treated, inactive or burned-out sarcoidosis. Similarly, the hallmark of active cardiac sarcoidosis on PET is focally increased FDG uptake, however, abnormal perfusion (Fig 1, group 3), in the absence of FDG uptake may also represent sarcoid involvement, especially in cases of inactive or “burned out” sarcoidosis or if the patient has been previously treated with steroids.
However, within these known diagnostic limitations, the overall agreement of PET and CMR in our study appeared to be reasonably good in the evaluation of patients with suspected cardiac sarcoidosis. We also found the two tests to be rather complementary, allowing classification of patients into 3 distinct groups that informed prognosis: 1) No myocardial damage: patients in this group were unlikely to have cardiac involvement, despite the fact that 85% of these patients had known extra-cardiac sarcoidosis. They also had the best prognosis and highest negative predictive value for future events; 2) Myocardial damage with inflammation: this is the group most likely to correspond to active cardiac sarcoidosis. Yet, documented extracardiac sarcoidosis was absent in 40% of these patients, suggesting the presence of isolated cardiac sarcoidosis (at least in some of them), a condition that is increasingly recognized, and is undiagnosed using current guidelines. 16,17,18 Patients in this group had significantly higher events compared to those without myocardial damage; 3) Myocardial damage without inflammation: this group represents more of a diagnostic conundrum since LGE is a common feature among many non-ischemic cardiomyopathies, and thus lack of co-existing myocardial inflammation certainly reduces the accuracy of this diagnosis. However, absence of FDG uptake could also indicate “burned-out” or previously treated sarcoidosis. Furthermore, 50% of the patients in this group had extracardiac sarcoidosis, which strongly supports the diagnosis of cardiac sarcoidosis in at least those patients. From a prognostic perspective, adverse events were significantly higher compared to those without myocardial damage; but were comparable to patients with LGE and co-existing inflammation.
Over a follow-up period of more than 2 years, all events but one occurred in patients with LGE on CMR, emphasizing the fact that the presence of myocardial LGE is an ominous risk factor for ventricular arrhythmias and death independent of the underlying etiology.19,20 In our analysis, myocardial LGE was shown to carry the highest risk for the occurrence of major events. This is in accordance with prior CMR-based studies in patients with known extracardiac sarcoidosis where myocardial LGE was a strong predictor of death and ventricular arrhythmic events.4,7
Conversely, FDG was not found in our study to be an independent risk factor for adverse outcomes after adjusting for LGE. Previously, Blankstein et al reported that the presence of abnormal MPI and FDG uptake on cardiac PET identified patients at higher risk of death or ventricular tachycardia after adjusting for LVEF and clinical criteria.5 In the aforementioned study, the authors followed 118 patients with suspected cardiac sarcoidosis for a median of 1.5 years after cardiac PET for the occurrence of all-cause death or sustained VT. Patients were divided into 3 groups according to PET findings as following: 1) normal MPI and FDG (n=47), 2) abnormal MPI or FDG (n=37), and 3) abnormal MPI and FDG (n=34). A total of 31 patients (26%) experienced events with an annualized event rate of 7.3% (reference), 18.4% (HR 2.55 [0.94–6.92]; P=0.065), and 31.9% (HR 3.94 [1.50–10.31]) for groups 1, 2 and 3 respectively. In a multivariate analysis, abnormal MPI and FDG remained as an independent predictor of events.
In agreement with Blankstein’s data, we did observe in our study a similar risk of future events in patients with abnormal FDG and/or MPI (HR 3.3 [1.1–10]] or abnormal FDG alone (HR 3.3 [0.9–11]). However, PET in general and FDG in particular did not hold significance as independent predictors of death and/or arrhythmic ventricular events after adjusting for LGE. The following observations may help explain this phenomena: 1) our study revealed that myocardial LGE was invariably present in all patients with abnormal FDG uptake and/or perfusion defects on PET; 2) there were 7 patients in our cohort (12%) with evidence of myocardial LGE despite normal PET and 3 of them eventually experienced adverse outcomes; 3) the risk of future events was comparable between LGE-positive patients with or without abnormal FDG uptake; and 4) prior electrophysiologic studies have demonstrated that the most common mechanism of VT in cardiac sarcoidosis is re-entry caused by myocardial scar,21,22 and that VT inducibility does not appear to be associated with disease activity.23 Everything considered these findings support the evidence that arrhythmic ventricular events are mostly driven by the presence of myocardial LGE, a strong marker of fibrosis/scar, and not PET.
However, we would like to emphasize that these preliminary results should not undermine the important clinical role that cardiac PET plays in the management of these patients. Unlike LGE-CMR, FDG-PET is a powerful tool for the initial evaluation and follow-up of inflammation as a marker of therapy response in patients with cardiac sarcoidosis.2 At present, corticosteroids remain the mainstay therapy in patients with cardiac sarcoidosis but are usually restricted to patients with active disease based on FDG-PET. There is emerging data that nearly half of patients treated with steroids can regain normal A-V conduction, and/or have significantly less progression of LV systolic dysfunction compared with untreated individuals.2,24–26 In contrast, the role of steroids in the management of ventricular tachyarrhythmias is more controversial since some studies have suggested benefit (especially in early stages),27 while others have failed to show improvement in VT burden in patients treated with steroids compared to standard anti-arrhythmic therapy alone.28,29
For instance, it is the standard in our practice that most patients with high enough suspicion of cardiac sarcoidosis undergo LGE-CMR first (unless contra-indicated) followed by cardiac PET since we find both modalities to be complementary in the initial evaluation of cardiac sarcoidosis. Specifically, cardiac PET serves a two-fold purpose: to confirm this challenging diagnosis, and to help guide immunosuppressive therapy. Consequently, the broader clinical applications of FDG-PET in the management of patients with cardiac sarcoidosis should not be misinterpreted in light of our study results.
Finally, as an ongoing effort to minimize radiation exposure, one could potentially consider the use of an FDG only (without MPI) PET protocol when performed after LGE-CMR. Resting myocardial perfusion defects are the result of underlying fibrosis in cardiac sarcoidosis, a substrate that is better detected and delineated by LGE given the superior spatial resolution of CMR compared to PET. However, while this may be a reasonable approach for the initial evaluation and risk-stratification, it may not be a feasible option for follow-up studies since most patients will eventually undergo ICD placement and, thereby, not be candidates for follow-up CMR anymore. In such cases, MPI will still be required because not only does it serve as a fibrosis map, but also provides LV function assessment. In any event, this is an interesting concept that requires future investigation.
Study Limitations
Our study is subject to a number of limitations that deserve discussion. First, this is a retrospective single center study lacking relevant information such as a gold standard for diagnosis, control group for comparison, and systematic assessment of therapy. Moreover, presence of extracardiac sarcoidosis was not an inclusion requirement, thus, it is possible that some cases may represent cardiomyopathies other than sarcoidosis. Second, some of the patients were treated with corticosteroids at the time of obtaining the PET scans, however, the number was small and the overall agreement and outcomes results did not change after exclusion.
Another important limitation is pre-selection bias. Our study population consisted of a highly select cohort of symptomatic patients presenting in many cases with advanced or late cardiac manifestations of sarcoidosis including heart block, ventricular tachyarrhythmias, and LV systolic dysfunction. This preselection bias may likely help explain the high event rate (29%) seen in our relatively small sample. This also means that the superior prognostic value of LGE over PET applies to populations with similar clinical characteristics, and not necessarily to asymptomatic patients with systemic sarcoidosis.
We would also like to acknowledge the use of two different PET radiotracers (N-13 ammonia and Rb-82) for MPI and two different acquisition protocols (2-D and 3-D mode) for cardiac FDG scanning in our study. While this might be viewed as a potential limitation, prior studies have shown comparable performance of both radiotracers30 and acquisition methods31 in clinical practice, therefore, their possible impact on the final study results is likely minimal.
Finally, our study sample was small, and therefore our idea of exploring the correlation of imaging findings with outcomes in this small retrospective study deserves caution in the interpretation of our results. And even though, the observed findings might still be valid, they should be taken carefully as preliminary and will require further validation. Future studies should also focus on identification of risk predictors at earlier disease stages, including novel inflammatory markers,32 and investigate the role of immunosuppressive therapy to alter outcomes. This will help clarify some of the relevant aspects of the disease evolution and deepen our understanding of this challenging condition.
5. Conclusions
In summary, the combined use of PET and CMR yields important diagnostic and prognostic information. While, PET and CMR demonstrated reasonably good diagnostic agreement and appeared to be complementary during the initial evaluation of patients with cardiac sarcoidosis, the presence of myocardial LGE (a marker of fibrosis) alone or in combination with FDG (a marker of inflammation) was found to be the strongest predictor of death and ventricular arrhythmic events among patients who presented with clinical, often advanced, manifestations of cardiac sarcoidosis. Within the limitations of our study, FDG-PET showed no additional benefit in risk stratification for this population. Further studies will be required to validate our preliminary findings.
Supplementary Material
Footnotes
All authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation
Conflict of interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kron J, Sauer W, Schuller J, et al. Efficacy and safety of implantable cardiac defibrillators for treatment of ventricular arrhythmias in patients with cardiac sarcoidosis. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2013;15(3):347–354. doi: 10.1093/europace/eus316. [DOI] [PubMed] [Google Scholar]
- 2.Osborne MT, Hulten EA, Singh A, et al. Reduction in (1)(8)F-fluorodeoxyglucose uptake on serial cardiac positron emission tomography is associated with improved left ventricular ejection fraction in patients with cardiac sarcoidosis. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2014;21(1):166–174. doi: 10.1007/s12350-013-9828-6. [DOI] [PubMed] [Google Scholar]
- 3.Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153–2165. doi: 10.1056/NEJMra071714. [DOI] [PubMed] [Google Scholar]
- 4.Patel MR, Cawley PJ, Heitner JF, et al. Detection of myocardial damage in patients with sarcoidosis. Circulation. 2009;120(20):1969–1977. doi: 10.1161/CIRCULATIONAHA.109.851352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blankstein R, Osborne M, Naya M, et al. Cardiac positron emission tomography enhances prognostic assessments of patients with suspected cardiac sarcoidosis. J Am Coll Cardiol. 2014;63(4):329–336. doi: 10.1016/j.jacc.2013.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ichinose A, Otani H, Oikawa M, et al. MRI of cardiac sarcoidosis: basal and subepicardial localization of myocardial lesions and their effect on left ventricular function. AJR. American journal of roentgenology. 2008;191(3):862–869. doi: 10.2214/AJR.07.3089. [DOI] [PubMed] [Google Scholar]
- 7.Greulich S, Deluigi CC, Gloekler S, et al. CMR imaging predicts death and other adverse events in suspected cardiac sarcoidosis. JACC. Cardiovascular imaging. 2013;6(4):501–511. doi: 10.1016/j.jcmg.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Vignaux O, Dhote R, Duboc D, et al. Clinical significance of myocardial magnetic resonance abnormalities in patients with sarcoidosis: a 1-year follow-up study. Chest. 2002;122(6):1895–1901. doi: 10.1378/chest.122.6.1895. [DOI] [PubMed] [Google Scholar]
- 9.Vignaux O, Dhote R, Duboc D, et al. Detection of myocardial involvement in patients with sarcoidosis applying T2-weighted, contrast-enhanced, and cine magnetic resonance imaging: initial results of a prospective study. Journal of computer assisted tomography. 2002;26(5):762–767. doi: 10.1097/00004728-200209000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Soejima K, Yada H. The work-up and management of patients with apparent or subclinical cardiac sarcoidosis: with emphasis on the associated heart rhythm abnormalities. Journal of cardiovascular electrophysiology. 2009;20(5):578–583. doi: 10.1111/j.1540-8167.2008.01417.x. [DOI] [PubMed] [Google Scholar]
- 11.Birnie DH, Sauer WH, Bogun F, et al. HRS expert consensus statement on the diagnosis and management of arrhythmias associated with cardiac sarcoidosis. Heart rhythm : the official journal of the Heart Rhythm Society. 2014;11(7):1305–1323. doi: 10.1016/j.hrthm.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 12.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174. [PubMed] [Google Scholar]
- 13.Betensky BP, Tschabrunn CM, Zado ES, et al. Long-term follow-up of patients with cardiac sarcoidosis and implantable cardioverter-defibrillators. Heart rhythm : the official journal of the Heart Rhythm Society. 2012;9(6):884–891. doi: 10.1016/j.hrthm.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Mehta D, Mori N, Goldbarg SH, Lubitz S, Wisnivesky JP, Teirstein A. Primary prevention of sudden cardiac death in silent cardiac sarcoidosis: role of programmed ventricular stimulation. Circulation. Arrhythmia and electrophysiology. 2011;4(1):43–48. doi: 10.1161/CIRCEP.110.958322. [DOI] [PubMed] [Google Scholar]
- 15.Kim RJ, Chen EL, Lima JA, Judd RM. Myocardial Gd-DTPA kinetics determine MRI contrast enhancement and reflect the extent and severity of myocardial injury after acute reperfused infarction. Circulation. 1996;94(12):3318–3326. doi: 10.1161/01.cir.94.12.3318. [DOI] [PubMed] [Google Scholar]
- 16.Kandolin R, Lehtonen J, Airaksinen J, et al. Cardiac sarcoidosis: epidemiology, characteristics, and outcome over 25 years in a nationwide study. Circulation. 2015;131(7):624–632. doi: 10.1161/CIRCULATIONAHA.114.011522. [DOI] [PubMed] [Google Scholar]
- 17.Okura Y, Dec GW, Hare JM, et al. A clinical and histopathologic comparison of cardiac sarcoidosis and idiopathic giant cell myocarditis. J Am Coll Cardiol. 2003;41(2):322–329. doi: 10.1016/s0735-1097(02)02715-8. [DOI] [PubMed] [Google Scholar]
- 18.Sperry BW, Oldan J, Hachamovitch R, Tamarappoo BK. Insights into biopsy-proven cardiac sarcoidosis in patients with heart failure. J Heart Lung Transplant. 2016;35(3):392–393. doi: 10.1016/j.healun.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuruvilla S, Adenaw N, Katwal AB, Lipinski MJ, Kramer CM, Salerno M. Late gadolinium enhancement on cardiac magnetic resonance predicts adverse cardiovascular outcomes in nonischemic cardiomyopathy: a systematic review and meta-analysis. Circulation. Cardiovascular imaging. 2014;7(2):250–258. doi: 10.1161/CIRCIMAGING.113.001144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scott PA, Rosengarten JA, Curzen NP, Morgan JM. Late gadolinium enhancement cardiac magnetic resonance imaging for the prediction of ventricular tachyarrhythmic events: a meta-analysis. European journal of heart failure. 2013;15(9):1019–1027. doi: 10.1093/eurjhf/hft053. [DOI] [PubMed] [Google Scholar]
- 21.Furushima H, Chinushi M, Sugiura H, Kasai H, Washizuka T, Aizawa Y. Ventricular tachyarrhythmia associated with cardiac sarcoidosis: its mechanisms and outcome. Clin Cardiol. 2004;27(4):217–222. doi: 10.1002/clc.4960270409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar S, Barbhaiya C, Nagashima K, et al. Ventricular tachycardia in cardiac sarcoidosis: characterization of ventricular substrate and outcomes of catheter ablation. Circulation. Arrhythmia and electrophysiology. 2015;8(1):87–93. doi: 10.1161/CIRCEP.114.002145. [DOI] [PubMed] [Google Scholar]
- 23.Mezaki T, Chinushi M, Washizuka T, et al. Discrepancy between inducibility of ventricular tachycardia and activity of cardiac sarcoidosis. Requirement of defibrillator implantation for the inactive stage of cardiac sarcoidosis. Intern Med. 2001;40(8):731–735. doi: 10.2169/internalmedicine.40.731. [DOI] [PubMed] [Google Scholar]
- 24.Kato Y, Morimoto S, Uemura A, Hiramitsu S, Ito T, Hishida H. Efficacy of corticosteroids in sarcoidosis presenting with atrioventricular block. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG / World Association of Sarcoidosis and Other Granulomatous Disorders. 2003;20(2):133–137. [PubMed] [Google Scholar]
- 25.Yodogawa K, Seino Y, Shiomura R, et al. Recovery of atrioventricular block following steroid therapy in patients with cardiac sarcoidosis. J Cardiol. 2013;62(5):320–325. doi: 10.1016/j.jjcc.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Chiu CZ, Nakatani S, Zhang G, et al. Prevention of left ventricular remodeling by long-term corticosteroid therapy in patients with cardiac sarcoidosis. Am J Cardiol. 2005;95(1):143–146. doi: 10.1016/j.amjcard.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 27.Yodogawa K, Seino Y, Ohara T, Takayama H, Katoh T, Mizuno K. Effect of corticosteroid therapy on ventricular arrhythmias in patients with cardiac sarcoidosis. Ann Noninvasive Electrocardiol. 2011;16(2):140–147. doi: 10.1111/j.1542-474X.2011.00418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Banba K, Kusano KF, Nakamura K, et al. Relationship between arrhythmogenesis and disease activity in cardiac sarcoidosis. Heart rhythm : the official journal of the Heart Rhythm Society. 2007;4(10):1292–1299. doi: 10.1016/j.hrthm.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 29.Mohsen A, Jimenez A, Hood RE, et al. Cardiac sarcoidosis: electrophysiological outcomes on long-term follow-up and the role of the implantable cardioverter-defibrillator. Journal of cardiovascular electrophysiology. 2014;25(2):171–176. doi: 10.1111/jce.12302. [DOI] [PubMed] [Google Scholar]
- 30.El Fakhri G, Kardan A, Sitek A, et al. Reproducibility and accuracy of quantitative myocardial blood flow assessment with (82)Rb PET: comparison with (13)N-ammonia PET. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2009;50(7):1062–1071. doi: 10.2967/jnumed.104.007831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strobel K, Rudy M, Treyer V, Veit-Haibach P, Burger C, Hany TF. Objective and subjective comparison of standard 2-D and fully 3-D reconstructed data on a PET/CT system. Nuclear medicine communications. 2007;28(7):555–559. doi: 10.1097/MNM.0b013e328194f1e3. [DOI] [PubMed] [Google Scholar]
- 32.Rothkrantz-Kos S, van Dieijen-Visser MP, Mulder PG, Drent M. Potential usefulness of inflammatory markers to monitor respiratory functional impairment in sarcoidosis. Clin Chem. 2003;49(9):1510–1517. doi: 10.1373/49.9.1510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.