Abstract
Background
Lipocalin 2 (Lcn2) is a multifunctional innate immune protein that exhibits antimicrobial activity by the sequestration of bacterial siderophores, regulates iron homeostasis, and augments cellular tolerance to oxidative stress. Studies in the murine model of colitis have demonstrated that Lcn2 deficiency exacerbates colitogenesis, however, the therapeutic potential of Lcn2 supplementation has yet to be elucidated. In light of its potential mucoprotective functions, we herein investigated whether expression of Lcn2 in the probiotic bacterium can be exploited to alleviate experimental colitis.
Methods
Murine Lcn2 was cloned into the pT1NX plasmid and transformed into Lactococcus lactis to generate L. lactis expressing Lcn2 (LactisLcn2) or the empty plasmid (LactisCon). LactisLcn2 was characterized by immunoblot and ELISA, and tested for its antimicrobial efficacy on Escherichia coli. The capacity of LactisLcn2 and LactisCon to withstand adverse conditions were tested using in vitro viability assays. Dextran sodium sulfate colitis model was employed to investigate the colonization ability and therapeutic potential of LactisLcn2 and LactisCon.
Results
Lcn2-derived from LactisLcn2 inhibited the growth of E. coli and reduced the bioactivity of enterobactin (E. coli-derived siderophore) in vitro. LactisLcn2 displayed enhanced tolerance to adverse pH, high concentration of bile acids and oxidative stress in vitro, and survived better in the inflamed gut than LactisCon. Consistent with these features, LactisLcn2 displayed better mucoprotection against intestinal inflammation than LactisCon when administered into mice with DSS-induced acute colitis.
Conclusions
Our findings suggest that Lcn2 expression can be exploited to enhance the survivability of probiotic bacteria during inflammation, which could further improve its efficacy to treat experimental colitis.
Keywords: Siderocalin, Probiotics, Iron, Inflammatory bowel disease
Introduction
Inflammatory bowel diseases (IBD) are idiopathic disorders of the gastrointestinal tract that are characterized by chronic relapsing intestinal inflammation. IBD, such as Crohn's disease (CD) and ulcerative colitis (UC), are associated with the upregulation of many acute-phase proteins (APPs; i.e., C-reactive protein, serum amyloid A, sIL-1Ra). The exploration of the biologic activities of APP has resulted in the development of biological therapeutics to treat IBD1. However, currently, there are no universally acceptable route by which these therapeutics can be administered efficiently. Moreover, the therapeutic potential of one of the most dynamically upregulated APP, Lipocalin 2 (Lcn2; aka Neutrophil Gelatinase-Associated Lipocalin or NGAL, siderocalin, 24p3) is considerably unknown2, 3.
Lcn2 is a small 25 kDa protein that is secreted by a variety of immune and non-immune cells, particularly in response to inflammation2. The levels of circulating and mucosal Lcn2 are reported to increase by log orders of magnitude in various inflammatory conditions including IBD3–9. Previously, we have shown that fecal Lcn2 is a dynamic, sensitive and non-invasive biomarker of gut inflammation3. Among its diverse physiological functions, one well-established role of Lcn2 is to sequester bacterial siderophores, such as enterobactin (Ent) and thus limit the growth of iron-dependent bacteria4, 10–12. In addition, Lcn2 also plays a role in facilitating the hypoferremia of inflammation and suppressing the generation of catalytic iron12. Such mechanisms suggest that the upregulation of Lcn2 during intestinal or systemic inflammation may mediate alterations in the gut microbiota, particularly during IBD13–15. While Lcn2 deficiency has been shown to potentiate gut dysbiosis in mice and increase their susceptibility to colitis16, 17, the therapeutic potential of Lcn2 supplementation has yet to be elucidated.
Numerous therapeutic molecules have been developed to treat experimental colitis model, however, their utility is impeded by the high cost associated with long-term treatment. Such constraint could be potentially circumvented by employing a recombinant lactic acid bacteria to deliver therapeutic biologicals18. The use of bacterial vector would allow for a more cost-effective and direct delivery of therapeutics to the site of inflammation and avoid the risk of systemic side effects. Lactococcus lactis genetically modified to secrete the anti-inflammatory cytokine IL-10 is perhaps the most promising application of this strategy and had been demonstrated in several studies to be effective in treating gastrointestinal inflammation19, 20. Subsequent studies also elucidated the efficacy of various recombinant L. lactis in mitigating experimental colitis, including L. lactis bioengineered to provide mucosal delivery of IL-2721, TGF-β1, elafin, secretory leukocyte protease inhibitor (SLPI)22, and anti-TNF nanobodies23. Despite the feasibility of using L. lactis as a mucosal delivery vector, the efficacy of recombinant L. lactis is still critically impacted by its inherent deficiencies to survive in the inflamed gut. Accordingly, we sought to provide evidence that the fitness of L. lactis could be further modified as an innovative strategy to improve its efficacy to treat experimental colitis.
In this study, we investigated the therapeutic potential of Lcn2 expression in augmenting the persistence and colonization of a probiotic bacterium in the inflamed gut. We demonstrated that ectopic expression of Lcn2 in L. lactis substantially augments its capacity to withstand a spectrum of environmental stressors, including adverse pH, bile acids, and H2O2-induced oxidative stress. We confirmed that L. lactis-secreted Lcn2 is bioactive in sequestering Ent (a bacterial siderophore that is essential for E. coli iron acquisition) and thus able to inhibit the growth of E. coli in vitro. By employing the murine model of dextran sulfate sodium (DSS)-induced acute colitis, we provide evidence that Lcn2 expression confers a survival advantage to L. lactis and allows it to robustly colonize the inflamed gut than L. lactis harboring the empty vector. Further, we demonstrate that Lcn2-expressing L. lactis is more effective than control L. lactis in mediating mucoprotection to colitic mice. Collectively, our results highlight that genetic modification of probiotic bacteria to produce and secrete Lcn2 may offer a feasible strategy to improve the stability, retention, and efficacy of probiotic bacteria in treating experimental colitis.
Materials and Methods
Reagents
Reagent grade dextran sulfate sodium salt (DSS, M.W. 36–50 kDa, Ref = 160110) was purchased from MP Biomedicals (Solon, OH). Duoset ELISA kits for mouse Lcn2 and keratinocyte-derived chemokine CXCL1 (KC), serum amyloid A (SAA) and biotinylated murine Lcn2 antibody were obtained from R&D Systems (Minneapolis, MN). Hydrogen peroxide (H2O2), myeloperoxidase (MPO), hexadecyl trimethyl ammonium bromide, RNAlater®, TRI Reagent®, iron-free enterobactin (Ent), dimethyl sulfoxide, glucose, sodium taurocholate, glycocholate, lithocholate and deoxycholate were procured from Sigma-Aldrich (St. Louis, MO). Guaiacol (2-methoxyphenol) were obtained from Alfa Aesar (Ward Hill, MA). Erythromycin was purchased from Amresco (Solon, OH). The iron atomic absorption (AA) standard was purchased from RICCA Chemical Company. SYBR® Green mix and qScript cDNA synthesis kit were procured from Quanta Biosciences. Mouse recombinant (rec)-Lcn2 (free from endotoxin, siderophore, and iron) was obtained from Cell Signaling. All other chemicals used in the present study were reagent grade and procured from Sigma.
Mice
C57BL/6 WT mice were maintained and bred under specific-pathogen-free conditions in the animal house facility at Pennsylvania State University, PA. Mice were housed in cages (max. 5 mice per cage) containing sawdust bedding and paper tissue nestlets, and fed on chow-control diet ad libitum with unrestricted access to water throughout the study. The cages were kept at 23°C and underwent a 12 h light/dark phase cycle. All animal experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of Pennsylvania State University.
Bacteria
Lactococcus lactis strain MG1363 was grown in M17 broth or agar (1.5% [wt. /vol]) media supplemented with 0.5% glucose and incubated overnight under anaerobic conditions at 30°C. Recombinant L. lactis strains were selected in M17 with 0.5% glucose in the presence of 5.0 μg/ml erythromycin (henceforth described as selective M17). K12 E. coli strain was acquired from the E. coli Genetic Stock Center, Yale University. Ent-overexpressing E.coli isogenic mutant ferrienterobactin permease (ΔfepA) was gifted from Dr. Kathleen Postle, Pennsylvania State University. Non-pathogenic WT K12 E. coli and ΔfepA were grown in LB media (Sigma) containing kanamycin (50 μg/ml) overnight at 37°C. The bacterial colony-forming unit (CFU) were adjusted based on the optical density at 600 nm.
Construction of recombinant bacterial strains
Murine Lcn2 cDNA (Sino Biological Inc.) was amplified using primers Lcn2-Spe1 5'-GGACTAGTCCTCAGTTGTCAAGCATTGGTCGG-3' and Lcn2-BamH1 5'-CGCGGATCCGCGACCATGGCCCTGAGTGTCA-3'. Amplicon and plasmid pT1NX (LMBP 3498; available from the BCCM/LMBP Plasmid Collection, Ghent University, Belgium (http://bccm.belspo.be/about-us/bccm-lmbp) were then digested by restriction enzymes BamH1 and Spe1 (Roche) to generate compatible ends. Lcn2 transgene was ligated (T4 DNA ligase, Roche) into pT1NX plasmid at BamH1/Spe1 site. Recombinant plasmids were electroporated into competent L. lactis strain MG1363, which was further plated on erythromycin-containing M17 plates for positive selection. Recombinant L. lactis was verified via PCR using primers Lcn2-start 5'GTCCTGGGCCTGGCTCTG3’ and pT1NX-start AGTCATACTTTCTGCTGCAG (targets upstream end of Lcn2 transgene), and Lcn2-stop CTCTGGGCCTCAAGGACG and pT1NX-stop TTTCGGGCTTTGTTAGCAGC (targets downstream end of Lcn2 transgene). Plasmid was further purified from isolated clones, and transgene presence was verified by BamH1/Spe1 digestion and agarose gel electrophoresis.
Characterization of Lcn2 expression in L. lactis
Recombinant Lcn2-expressing L. lactis (LactisLcn2) and L. lactis transformed with empty plasmid (LactisCon) were cultured in selective M17 media as described above for 24 and 48 h. Cultures were centrifuged at 10,000 g for 5 min, and the cell supernatant and pellet were collected. The supernatant was treated with 100 μl of 100% trichloroacetic acid (TCA) and incubated for 10 min on ice to precipitate proteins. The supernatant protein precipitate and cell pellet were resuspended in RIPA buffer (Cell Signaling) containing protease inhibitor cocktail (Roche). Western blotting was performed by standard methods using 4–20% gel (Bio-Rad) and murine Lcn2 polyclonal antibody. The concentration of secreted Lcn2 was quantitated using Lcn2 Duoset ELISA kit according to the manufacturer’s protocol. Cell lysates and culture supernatant of L. lactis transformed with empty plasmid (referred as LactisCon) were used as controls.
Sample preparation, labeling and two-dimensional gel electrophoresis
Protein lysates were obtained from LactisLcn2 and LactisCon. Briefly, LactisLcn2 and LactisCon were pelleted and proteins were extracted by sonication in a lysis buffer (0.5 mM EDTA, 50 mM Tris-HCl, 50 mM NaCl, 1 mM DTT, 5% Glycerol). Protein samples were prepared using 2D-clean-up for subsequent 2D analysis. For 2D-clean-up, one hundred micrograms of proteins were precipitated, purified and cleaned by a 2D-clean-up kit (GE Healthcare) according to manufacturer protocol. Pellets of precipitated proteins were resuspended in 60 μl of rehydration buffer (7 M urea, 2 M thiourea, 4% CHAPS, 25 mM Tris-HCl pH 8.8). Twenty-five micrograms of LactisLcn2 or LactisCon samples were labeled with 200 pmol N-hydroxy-succinimidyl ester of cyanine dye Cy3 and with 200 pmol N-hydroxy-succinimidyl ester of cyanine dye Cy5, respectively (GE Healthcare Life Science, Piscataway, NJ). After quenching with 10 mM lysine, the labeled proteins were mixed. Sample buffer (7 M urea, 4 M thiourea, 4% CHAPS, 2% DTT, 2% IPG buffer, pH 4-11 NL (GE Healthcare Life Science, Piscataway, NJ) and Rehydration Solution (7 M urea, 4 M thiourea, 4% CHAPS, 1% DTT, 1% IPG) were added to a final volume of 350 μl for each gel. First-dimension Iso Electric Focusing (IEF) was performed using 24-cm IPG strips (pH 4–7, GE Healthcare Life Science, Piscataway, NJ) in Ettan IPGphor (GE Healthcare Life Science, Piscataway, NJ). After IEF, the strips were equilibrated, reduced, alkylated and stained by sequential incubation in 1.5% DTT equilibration buffer (50 mM Tris–HCl, pH 8.8), 6 M urea, 30% glycerol, and 2% SDS) and 4.5% iodoacetamide equilibration buffer slightly colored with bromophenol blue for 20 min each. The second-dimension SDS-polyacrylamide gel electrophoresis was conducted on a 10% polyacrylamide gel in the Ettan DALT II system separation unit (GE Healthcare Life Science, Piscataway, NJ) until the tracking dye reached the bottom of the gel. After completion of two-dimensional electrophoresis, gel images were acquired on a Typhoon Trio (GE Healthcare) at appropriate wavelengths for Cy3 and Cy5 dyes and analyzed using the DeCyder image analysis software (v. 7.0, GE Healthcare Life Science, Piscataway, NJ).
Quantification of bacterial growth
Overnight-grown recombinant LactisLcn2 and LactisCon (1x106 CFU) were placed into selective M17 media prepared at different pH (4.0–8.0) or in the presence of primary bile salts (taurocholate or glycocholate; 0–100 mM) or secondary bile salts (lithocholate and deoxycholate; 0–0.1 mM) and then incubated under anaerobic conditions at 30°C for 5 h. Optical density was measured every hour to assess bacterial growth at 600 nm.
Measurement of bacterial viability in the presence of hydrogen peroxide
Overnight-grown recombinant LactisLcn2 and LactisCon (1.0x106 CFU) were incubated with H2O2 (0–10 mM) for 5 h in selective M17 media under aerobic or anaerobic conditions at 30°C. Viable cells were determined by comparing OD600 of H2O2-treated and non-treated in each 1 h time point.
In vitro myeloperoxidase (MPO)-mediated bacterial killing assay
The MPO-mediated bacterial killing assay was performed as described by Atosuo and Lilius24. Briefly, the reaction mixture was prepared by first adding MPO (5.0 μg/ml) in sterile PBS. H2O2 (30 μM) was next added and incubated at room temperature for 5 min. Finally, overnight-grown recombinant LactisLcn2 or LactisCon (1.0x106 CFU) were added to the reaction mixture. After 5 h of incubation at 30°C, the reaction mixture was serially diluted and plated on selective M17 agar plates in triplicates. Plates were incubated overnight at 30°C, and the bacterial CFU were quantified.
Determination of bacterial iron content
The cellular iron content was measured in bacterial lysates from overnight-grown LactisLcn2 and LactisCon cultures 25. Briefly, an equal amount of protein lysates (LactisLcn2 or LactisCon) were mixed with an equivalent volume of solution containing hydrochloric acid (0.1 mg/ml), trichloroacetic acid (1.0 mol/l) and thioglycolic acid (30 ml/l) to precipitate proteins and released protein-bound iron. After centrifugation at room temperature (6,200 g for 15 min), the supernatants were collected and mixed with an equal volume of chromogen solution containing 1.5 M ferrozine and 1.5 M sodium acetate. The optical density of the chromogen was measured at 562 nm. Total iron levels were estimated using a standard curve generated with the iron AA standard (0–5 μg/ml).
MPO inhibition assay
Bacterial cell lysates (LactisLcn2 and LactisCon, 5.0 μg of protein) and Ent (10 μM) were pre-incubated with MPO (100 mU, final concentration) for 5 min. The MPO reaction was initiated by adding final concentrations of 100 mM guaiacol and 6.7x10−3 % H2O2. The change in absorbance at 470 nm was measured over a period of 10 min at 1 min intervals. MPO activity determined via guaiacol method was calculated as previously described26.
E. coli K12 growth inhibition assay
E. coli K12 strain MG1655 (1x106 CFU) were grown in the presence or absence of bacteria lysates (LactisLcn2 or LactisCon, 5.0 μg of protein) for 5 h in LB media at 37°C. Bacterial growth was determined by measuring OD600 every hour.
Chrome Azurol S (CAS) assay
CAS agar plates were prepared according to the protocol as described previously27. Overnight bacterial cultures grown in LB media were placed on CAS agar plates at indicated concentrations in the absence or presence of LactisLcn2 or LactisCon, incubated overnight at 37°C and monitored for orange-coloured halo formation. CAS gives a unique blue color when in complex with iron but turns orange when bacterial siderophores (e.g., Ent) chelates the iron from CAS. The intensity of orange halo formation is directly proportional to the concentration of siderophores secreted by the live bacteria.
DSS-induced colitis model
Female WT mice (6–8 weeks old, n=6) were administered 1.5% DSS in drinking water to induce acute colitis as described previously28. Briefly, mice were treated with one cycle of 1.5% DSS (MP Biological) in drinking water for 7 days followed by regular water for 4 days. Induction of colonic inflammation was confirmed via fecal occult blood, diarrhea and loss in body weight. For the therapeutic study, mice were administered with LactisLcn2 or LactisCon (1.0x108 CFU/mouse) via oral gavage once on the following days upon withdrawal of DSS treatment. After 4 days of bacteria administration, mice were euthanized and severity of colitis was analyzed.
Euthanasia and blood collection
At the termination of the experiment, mice were euthanized via CO2 asphyxiation and analyzed for standard colitis parameters as described previously 29. Blood was collected at the time of euthanasia in BD microtainer® (Becton, Dickinson, Franklin Lakes, NJ), via retro-orbital plexus. Hemolysis-free sera were obtained after centrifugation and stored at −80°C until further used.
Bacterial quantification in fecal samples, cecal content, and colonic mucosa
Fecal samples from LactisLcn2 or LactisCon-treated colitic mice were collected at 48, 72 and 96 h post-inoculation. Cecal content and colonic tissue were collected on the day of euthanasia and homogenized in sterile PBS (100 mg/ml). The fecal, cecal and colon homogenates were centrifuged at 200 rpm for 1 min and plated (100 μl) on selective M17 agar plates. Plates were incubated overnight at 30°C and bacterial CFU were quantified.
Colonic MPO assay
MPO assay was performed according to the previously described method26. Briefly, frozen or fresh colon tissue (50 mg) was homogenized in 1 mL of 50 mM potassium phosphate buffer (pH 6.0) containing 0.5% hexadecyl trimethyl ammonium bromide, freeze-thawed 3x, sonicated, centrifuged (10000 g, 4°C) and the clear supernatants were collected. The reaction was initiated by adding final concentrations of 50 mM guaiacol and 0.002% H2O2 to the clear supernatant prepared in a 96-well plate (Corning). The change in absorbance at 470 nm was measured over a period of 10 min at 1-min intervals. One unit of MPO activity is defined as the amount that increases absorbance at 470 nm by OD of 1.0 per min at 25°C, calculated from the initial rate of reaction.
Enzyme-linked immunosorbent assay (ELISA)
Serum KC and SAA were measured by ELISA kits according to the manufacturer’s protocols. Frozen or freshly collected feces were reconstituted (100 mg/mL) in PBS containing 0.1% Tween 20, vortexed (30 min, room temperature) and centrifuged (10,000 g, 4°C) to collect clear supernatants for fecal Lcn2 quantification as described previously3.
Quantitative RT-PCR
Mouse distal colons were collected in RNAlater®. Total colonic RNA was extracted using TRI Reagent® as per manufacturer's protocol. Purified RNA was used to synthesize cDNA for qRT-PCR using SYBR® Green mix according to the manufacturer's protocol. qRT-PCR was performed in StepOnePlusTM real-time PCR instrument (Life Technologies, Grand Island, NY). Sequences of primers used for qRT-PCR were (sense and antisense respectively): KC 5’-TTGTGCGAAAAGAAGTGCAG-3’, and reverse, 5’-TACAAACACAGCCTCCCACA-3’; TNFα 5’-ACTCCAGGCGGTGCCTATGT-3’ and 5’-AGTGTGAGGGTCTGGGCCAT-3’; iNOS 5’-TTTGCTTCCATGCTAATGC-GAAAG-3’, and 5’-GCTCTGTTGAGGTCTAAAGGCTCCG-3’; 36B4 5’-TCCAGGCTTTGGGCATCA and 5’-CTTTATTCAGCTGCACATCACTCAGA-3’. Thermal profile for the reaction was: initial denaturation at 95°C for 3 min and 40 cycles of denaturation (95°C for 15 s) and annealing and extension (60°C for 1 min). Relative fold difference between groups was calculated using comparative Ct (2−− −Ct) method. Results obtained were normalized with the housekeeping 36B4 gene.
Histology
Swiss rolls of colons were fixed in 10% buffered formalin (Fisher Scientific) overnight and then stored in 70% ethanol. Paraffin embedding tissue sections (5 microns) were stained with hematoxylin & eosin (H&E) using standard protocols at the Animal Diagnostics Laboratories, Pennsylvania State University. To detect the presence of acidic mucin in the goblet cells, alcian blue staining (pH 2.5) was performed in 5 μm section of a Swiss roll made from the colon using Alcian blue (pH 2.5) stain kit from Vector laboratories, USA. Histological scoring for colonic inflammation was graded as described previously30, 31 based on intestinal lesions, inflammation, mucosa, and percentage of affected area.
Statistical analysis
All experimental results were reproduced in at least three independent experiments performed in triplicate. All values in the results are expressed as mean ± SEM. Statistical significance between two groups was analyzed using unpaired, two-tailed t-test. Data from more than two groups was compared using a one-way analysis of variance (ANOVA) followed by Dunnett’s post hoc test (when to compare the mean of each column with the mean control column) or Tukey’s multiple comparison tests (when to compare the mean of each column with the mean of every other column). p< 0.05 was considered as statistically significant. All statistical analyses were performed with the GraphPad Prism 7.0 program (GraphPad, Inc, La Jolla, CA).
Results
Generation L. lactis producing Lipocalin 2
Overexpression of Lcn2 has been demonstrated to enhance cellular resistance to oxidative stress and apoptosis in various mammalian cell lines32–36. To investigate whether Lcn2 could be exploited to augment the stability of probiotic bacteria, we have engineered recombinant L. lactis strain expressing either the murine Lcn2 (LactisLcn2) or the empty plasmid as control (LactisCon). The pT1NX plasmid employed herein is a well-characterized secretion vector for L. lactis. Accordingly, we inserted the Lcn2 gene into the pT1NX plasmid at the BamH1/Spe1 site downstream of the P1 promoter and USP45 signal sequence (Fig. 1A). Construction of Lcn2 under the P1 promoter allows for constitutive expression, whereas USP45 encodes for the sequence signal to drive the secretion of Lcn2 from L. lactis. In addition, pT1NX plasmid contains the erythromycin resistance gene, which allowed positive selection for LactisLcn2 and LactisCon.
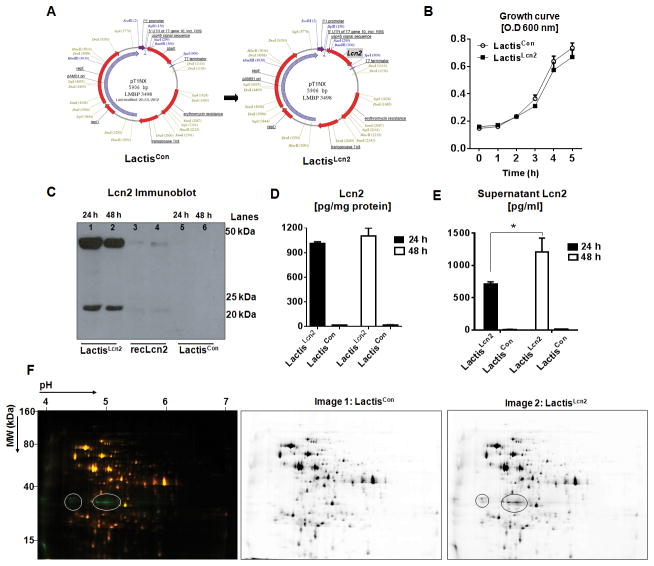
Figure 1. Schematic representation and characterization of Lcn2 expressing L. lactis.
(A) Murine Lcn2 gene was cloned into pT1NX plasmid at BamH1/Spe1 sites downstream of P1 promoter and USP45 signal sequence to allow for constitutive expression and secretion, respectively. (B) Line graphs represent the growth curve for the recombinant LactisLcn2 and LactisCon cultured in M17 media supplemented with erythromycin. LactisLcn2 and LactisCon were cultured for 24 and 48 h, and Lcn2 levels were assayed in the cell lysates by (C) immunoblotting and by (D) ELISA, and (E) culture supernatant by ELISA. Mouse recombinant (rec)-Lcn2 was used as a positive control for the Lcn2 immunoblot. (F) Composite overlay representative 2D-DIGE image (left) and individual images (right) showing protein accumulation in LactisCon (Cy5, red fluorescence) and LactisLcn2 (Cy3, green fluorescence) lysates. Green spots represent proteins up-regulated in LactisLcn2 and yellow spots represent proteins that are not deregulated. Circles indicate the protein bands predicted to be Lcn2. All experiments were performed in triplicates and are representative of three independent experiments. Results are expressed as mean ± SEM; *p<0.05.
We verified that the growth curves of LactisLcn2 and LactisCon were comparable, which indicate that the expression of Lcn2 did not impede the growth of L. lactis (Fig. 1B). Immunoblot of LactisLcn2 cell lysate confirmed the expression of Lcn2 as ~25 and 50 kDa bands (Fig. 1C) which corresponded to the monomeric and dimeric forms of Lcn237, 38, respectively. While the levels of intracellular Lcn2 in LactisLcn2 remained unchanged from 24 to 48 h of culture (Fig 1D), they were higher in the culture supernatant at 48 h than at 24 h (Fig 1E), suggesting that Lcn2 accumulated extracellularly over time upon secretion, or released from lysed bacteria. The appearance of Lcn2 band from LactisLcn2 with molecular weight lesser than 25 kDa could be due to the lack of post-translational modification, such as glycosylation, in bacteria. The analysis of LactisLcn2 and LactisCon cell lysates, respectively labeled with Cy5 and Cy3 by 2D differential gel electrophoresis, demonstrated that the ectopic expression of Lcn2 did not alter the overall proteome expression in LactisLcn2 (Fig 1F). The loss of the 50 kDa band in the 2D gel could be due to the use of DTT and iodoacetamide, which are strong reducing and alkylating agents, respectively, that reduce and prevent the formation of disulfide bonds in the sample preparation. In comparison, the 50 kDa band observed in the immunoblot could be potentially explained by the use of β-mercaptoethanol, which is relatively less efficient in ensuring complete removal of protein dimers.
Recombinant Lcn2 expressed by L. lactis is bioactive
Among its well-characterized functions, Lcn2 is known to sequester Ent from E. coli, thus preventing their acquisition of iron and inhibiting their growth. Hence, we first investigated the bioactivity of LactisLcn2-derived Lcn2 by assessing its antimicrobial and Ent-sequestering properties. Indeed, the cell lysates from LactisLcn2, but not LactisCon, significantly inhibited the growth of E. coli K12 (MG1655; Lcn2-sensitive strain, Fig. 2A). We next assessed the ability of Ent to inhibit neutrophil myeloperoxidase (MPO) in the presence of LactisLcn2 cell lysate. Previously, we have shown that Ent can inhibit the MPO activity by interfering the peroxidase-catalyzed reaction; Ent interferes with compound I [oxoiron, Fe(IV)O] and reverts the enzyme back to its native ferric [Fe(III)] state26. Consistent with our previous report26, 10 μM of Ent inhibited MPO activity by approximately 60% (Fig. 2B). However, the addition of cell lysate from LactisLcn2, but not LactisCon, rescued the MPO activity from Ent-mediated inhibition (~10% inhibition; Fig. 2B), thus implicating that LactisLcn2-derived Lcn2 is bioactive in sequestering Ent and neutralize its bioactivity.
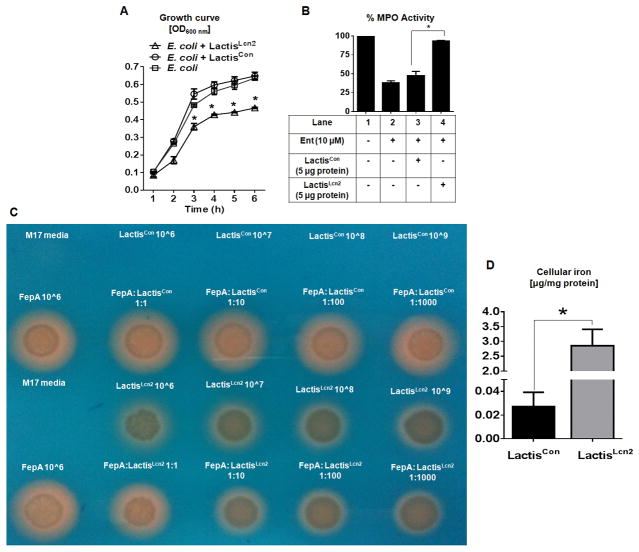
Figure 2. Lcn2 produced by LactisLcn2 is bioactive.
(A) Line graph represents the growth curve for E. coli cultured in the presence of cell lysates (5 μg of protein) from LactisLcn2, LactisCon or vehicle control. Myeloperoxidase (MPO, 100 mU) were pre-incubated with enterobactin (Ent, 10 μM) and cell lysates (5 μg of protein) from LactisLcn2 or LactisCon for 5 min before the assay was initiated. (B) Bar graph represents % MPO activity. (C) Orange halo formation indicates siderophore production by ΔfepA E. coli mutant on CAS plate (cultured overnight, 37ºC) in the presence of LactisLcn2, LactisCon or vehicle control at the indicated CFU concentration/ratio. (D) The cellular iron levels were measured in the cell lysates from LactisLcn2 and LactisCon. All experiments were performed in triplicates and are representative of three independent experiments. Results are expressed as mean ± SEM. *p < 0.05.
To verify these findings, we co-cultured the recombinant L. lactis with Ent-overproducing E. coli (ΔfepA mutant; K12) on chrome azurol S (CAS) media that detects siderophore production. The formation of orange halo due to iron-chelating Ent from ΔfepA E. coli was not affected when co-cultured with the control LactisCon. However, the presence of LactisLcn2 dose-dependently inhibited the size of the halo formed by ΔfepA E. coli, which further confirms the bioactivity of LactisLcn2-derived Lcn2 (Fig. 2C).
Intriguingly, we observed that LactisLcn2 on CAS media could also form a halo, indicative of iron-binding property even though L. lactis is not known to produce any siderophore39, 40 (Fig. 2C). This observation correlates with the approximately 100-fold increase in intracellular iron levels in LactisLcn2 compared with LactisCon (Fig 2D). Since LactisLcn2 lacks the mechanism to uptake the secreted Lcn2, therefore, it is unlikely that Lcn2 could facilitate iron intake. Instead, we envisioned that the cytosolic Lcn2 might have trapped the iron in the cell, thus resulting in the accumulation of cellular iron. The ability of LactisLcn2 to sequester and possibly deplete iron bioavailability may further explain their capability to inhibit the growth of E. coli in vitro.
Lcn2 expressing L. lactis displays enhanced tolerance to environmental stressors
The efficacy of probiotic bacteria can be significantly mitigated by deficiencies to withstand the harsh environmental stressors in the gut, especially the inflammatory environment encountered during IBD. Several studies have reported that Lcn2 overexpression in various mammalian cell lines could enhance their cellular resistance to oxidative stress34, 35. Hence, we sought to investigate whether expression of Lcn2 would confer any advantage to LactisLcn2 against adverse conditions. Alteration in extracellular pH, for instance, could induce cellular stress and impede bacterial growth41. While we observed LactisCon to be sensitive to pH changes, we noted that LactisLcn2 could grow more robustly over a range of physiological pH levels (pH 5–8; Fig. 3A) in vitro. The growth advantage of LactisLcn2 over LactisCon at adverse pH could be possibly due to reduced pH-mediated stress in the presence of Lcn2.
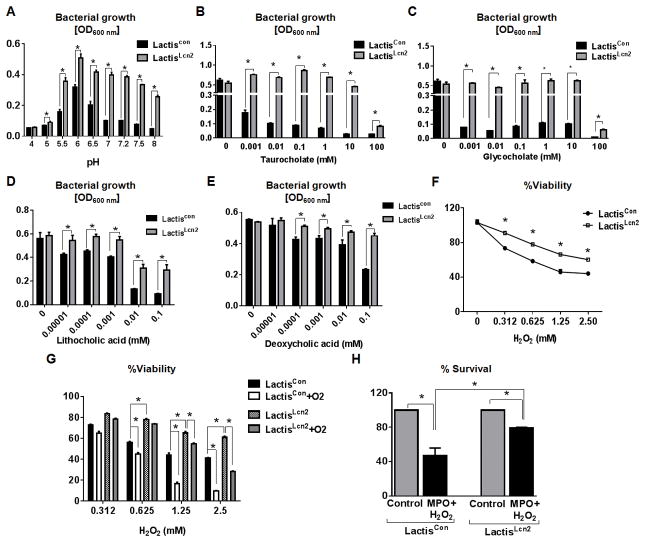
Figure 3. Lcn2 expressing L. lactis (LactisLcn2) exhibits augmented resistance to various stressors.
Equal amount of LactisLcn2 or LactisCon (1 x 106 CFU) were cultured at the indicated (A) pH, or in the presence of (B) taurocholate, (C) glycocholate, (D) lithocholic acid and (E) deoxycholic acid at the indicated concentration. LactisLcn2 or LactisCon were subjected to the indicated concentration of (F) H2O2, (G) H2O2 with aeration, and (H) myeloperoxidase (MPO)-mediated bacterial killing. All experiments were performed in triplicates and are representative of three independent experiments. Results are expressed as mean ± SEM. *p < 0.05.
Aside from pH changes, bacteria traversing the gastrointestinal tract could be exposed to primary bile salts (i.e. taurocholate and glycocholate) that are toxic to most bacteria 42. Indeed, 0.001 mM of taurocholate and glycocholate were sufficient to significantly inhibit the growth of LactisCon in vitro (Fig. 3B, C). In comparison, LactisLcn2 could better tolerate high concentrations of taurocholate and glycocholate up to 10 mM, and only displayed reduced growth in the presence of 100 mM of taurocholate and glycocholate in vitro (Fig. 3B, C). While most of the primary bile acids are reabsorbed by the small intestine, a fraction of these bile acids are metabolized by intestinal bacteria into secondary bile acids, such as deoxycholate and lithocholate 43 which could inhibit bacterial growth. Interestingly, we observed that LactisLcn2 could also better withstand the presence of lithocholate and deoxycholate at concentrations ranging from 0.001 to 0.1 mM in vitro, when compared with LactisCon (Fig. 3D, E).
In the context of experimental colitis, many probiotics and gut bacteria would be exposed to reactive oxygen species (ROS) generated by immune and non-immune cells in the inflamed gut. To assess whether Lcn2 expression in L. lactis exerts antioxidative effects, we next challenged LactisLcn2 and LactisCon with a range of oxidizing conditions. The viability of both LactisLcn2 and LactisCon decreased dose-dependently with increasing H2O2 concentrations, yet LactisLcn2 consistently survived better than LactisCon (Fig. 3F). When the assay was performed with 1.25 mM H2O2 under aerobic conditions (Fig. 3G), we observed that the viability of LactisCon decreased from 40% (without aeration) to 20% (with aeration). This outcome is expected, since the presence of O2 in the aerated culture generates ROS, such as H2O2, OH−, and O2−, which are unfavorable for L. lactis. However, 1.25 mM H2O2-treated LactisLcn2 was able to better withstand aeration with a slight decrease in viability from 65% to 55% (Fig. 3G). When the H2O2 concentration was increased to 2.5 mM with aeration, the viability of LactisLcn2 decreased to 30%, whereas LactisCon displayed only 10% viability (Fig. 3G). Next, we tested the extent to which LactisLcn2 and LactisCon can survive bacterial killing by neutrophil MPO, an oxidative enzyme which uses H2O2 and halide ions to generate hypochlorous acid 44. As anticipated, LactisLcn2 displayed a higher capacity to remain viable (80%) than LactisCon (40%), even when exposed to MPO-H2O2 mediated antimicrobial activity (Fig. 3H).
Lcn2 expression allows L. lactis to colonize and survive better in the inflamed gut
To assess the survivability of LactisLcn2 in the inflamed gut, we first induced colitis in 8 weeks old female WT mice (n=6) via administration of low dose of DSS (1.5%) in drinking water for 7 days. The induction of acute colitis was confirmed by the presence of occult blood and body weight loss. DSS treatment was withdrawn on day 7, and mice were maintained on regular drinking water. On the next day, the mice were orally administered with LactisLcn2 or LactisCon (1.0x108 CFU/mouse), and fecal shedding of these bacteria was monitored. Interestingly, the fecal shedding of LactisLcn2 was more robust and persisted up to 96 h post-inoculation when compared to LactisCon (Fig. 4A). To verify the colonization capacity of LactisLcn2 and LactisCon in the inflamed gut, we next measured their bacterial load in the cecal content and colonic tissue. Our results showed that LactisLcn2 can colonize in the colonic mucosa, in a more efficient manner than LactisCon (Fig. 4B). LactisLcn2 also colonized more robustly than LactisCon in the ceca (Fig 4C). We next recovered the LactisLcn2 from colitic mice and confirmed that it retained its Lcn2 expression (Fig. 4D). We next evaluated their tolerance to H2O2 exposure in order to assess whether LactisLcn2 recovered from colitic mice retained their oxidant-resistant phenotype. Consistent with our previous findings, the recovered-LactisLcn2 exhibited greater resistance to H2O2 in vitro than the recovered-LactisCon (Fig. 4E).
Figure 4. Lcn2 expression provides a survival advantage to LactisLcn2 in the inflamed gut.
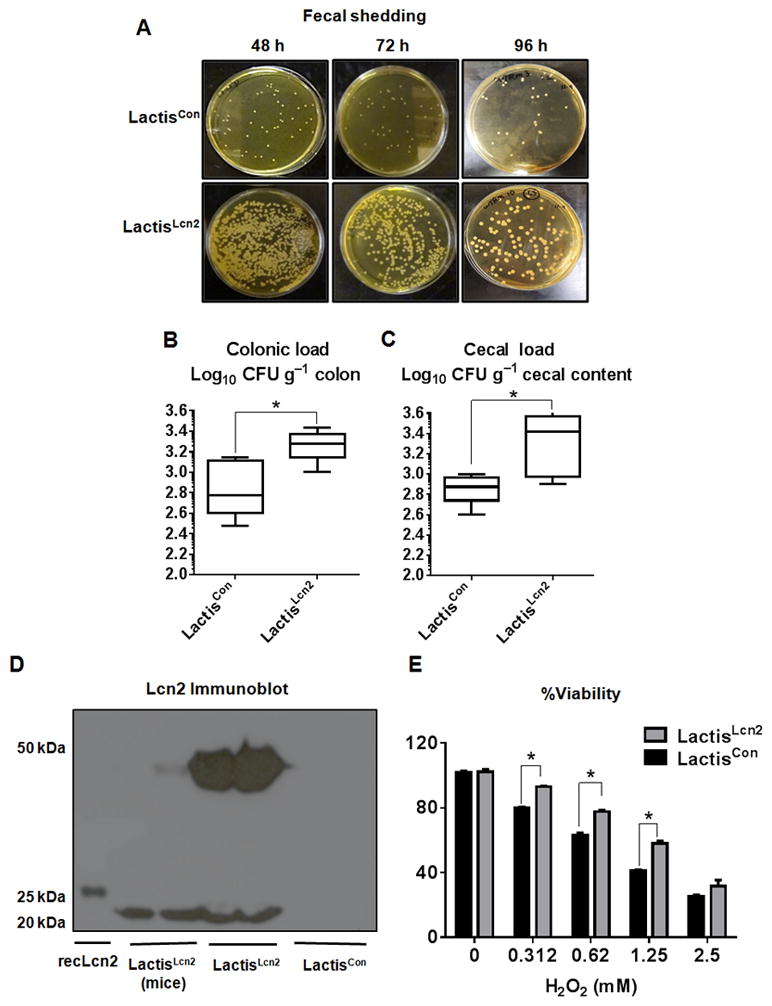
DSS-induced colitic female WT mice (n=6) were orally administered with LactisLcn2 or LactisCon. (A) Fecal shedding collected at 48, 72 and 96 h post-inoculation were plated on selective M17 plates containing erythromycin. Bacterial load in the (B) colonic mucosa and (C) cecal content were measured on selective M17 plates. (D) Lcn2 expression in the cell lysates of LactisLcn2 recovered from colitic mice feces (96 h, DSS+LactisLcn2) was assayed by immunoblotting. Mouse recombinant (rec)-Lcn2 and the parent stock of LactisLcn2 were used as positive controls for the Lcn2 immunoblot. (E) LactisLcn2 or LactisCon recovered from colitic mice were subjected to the indicated concentration of H2O2 in vitro. All experiments were performed in triplicates and are representative of three independent experiments. Results are expressed as mean ± SEM. *p < 0.05.
Lcn2-expressing L. lactis accelerate mice recovery from DSS-induced acute colitis
Having established that LactisLcn2 can survive and colonize the inflamed gut, we next investigated the therapeutic potential of LactisLcn2 in attenuating intestinal inflammation in comparison to LactisCon. First, we verified that the sole inoculation of LactisLcn2 and LactisCon into healthy mice did not result in diarrhea, colomegaly or splenomegaly (data not shown). Next, we performed DSS-induced colitis with 8 weeks old female WT (n=6), as described above. After confirming the onset of colitis on day 7, DSS was withdrawn, and mice were orally administered with LactisLcn2 or LactisCon (1.0x108 CFU/mouse) therapeutically. Mice were monitored for body weight for another 4 days and then euthanized. We did not observe any significant difference in body weight gain/loss in LactisLcn2 or LactisCon-treated mice when compared to DSS-alone given mice (Fig. 5A). However, the treatment with LactisLcn2, but not LactisCon, successfully mitigated the splenomegaly, colon shortening, and colonic MPO activity that are associated with DSS-induced acute colitis (Fig. 5B–F). Given that LactisLcn2 was administered after DSS-induced colon shortening had already occurred, it is thus possible that LactisLcn2 treatment may either prevent further shortening of the colon or promote the recovery, including restoration of colon length. In comparison to LactisLcn2 treated mice, the mice receiving LactisCon displayed only modest amelioration of colitis in all parameters analyzed. Histological (H&E) analysis indicated that the colitis in DSS+LactisLcn2 treated mice was less severe, when compared to DSS-alone and DSS+ LactisCon treated mice (Fig. 5G,I). Similarly, the number of mature goblet cells containing large mucosal granules, as evident with Alcian blue staining, were markedly increased in DSS+ LactisLcn2 treated mice compared with the DSS-alone and DSS+LactisCon treated mice (Fig. 5H).
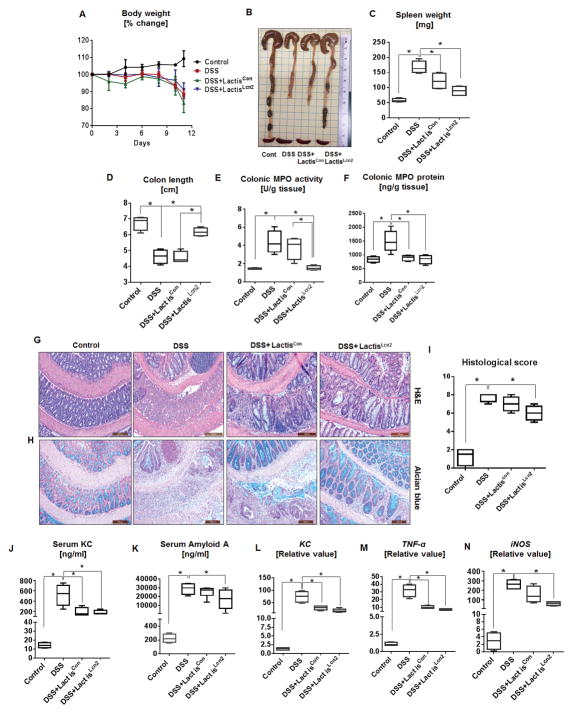
Figure 5. Lcn2 expressing L. lactis protects against DSS-induced mucosal injury.
Eight weeks old female BL6 WT mice (n=6) were given 1.5% DSS in drinking water for 7 days. After cessation of DSS on day 7, mice were maintained on regular water. On day 8 onwards, mice were orally administered with LactisLcn2 or LactisCon (1x108 CFU/mouse) once daily. The following parameters were analyzed (A) body weight, (B) gross colon, (C) spleen weight, (D) colon length, (E) colonic MPO activity and (F) colonic MPO protein level. Histology images of (G) H&E-stained and (H) Alcian blue-stained colons. (I) Histological scores of H&E-stained colons. In vitro assays were performed in triplicates and are representative of two independent mouse experiments. The perceptive markers of intestinal inflammation: (J) serum KC, and (K) SAA were measured by ELISA. The qRT-PCR analysis was used to measure the mRNA expression of colonic (L) KC, (M) TNF-α and (N) iNOS. Values are represented as fold change normalized to 36B4 housekeeping gene and compared to the control group. In vitro assays were performed in triplicates and are representative of two independent mouse experiments. Results are expressed as mean ± SEM. *p<0.05.
To further assess the effect of LactisLcn2 on colitis, we evaluated a panel of systemic and colonic markers that correlate with the severity of inflammation. Colitic mice therapeutically treated with either LactisLcn2 or LactisCon displayed a significant decrease in levels of serum KC (Fig. 5J). The levels of SAA were also substantially reduced in LactisLcn2 treated mice compared with LactisCon treated mice (Fig. 5K). Furthermore, the colonic expression of inflammatory genes such as KC, tumor necrosis factor alpha (TNFα) and inducible nitric oxide synthase (iNOS) was reduced in both LactisLcn2 and LactisCon-treated mice, although their downregulation were slightly more pronounced in the LactisLcn2 given mice (Fig. 5L, M & N).
Discussion
Intestinal inflammation is accompanied with systemic hypoferremia as an acute phase response to deplete circulating iron and prevent iron-induced oxidative stress. Such state of iron deficiency substantially impacts the composition of gut bacteria since iron is an essential micronutrient for almost all microorganisms, except Borrelia species and most lactic acid bacteria 45, 46. During this iron-deprived state, the opportunistic pathogen E. coli produce Ent, a siderophore, to acquire the iron from the host and thereby possess survival advantage in the inflamed gut. On the flip side, the host relies on the innate immune protein Lcn2 to sequester Ent and curtail the overgrowth of E. coli. In comparison, L. lactis are largely unaffected by the antimicrobial activity of Lcn2 since L. lactis neither require iron nor express any siderophore39, 40. However, the inability of L. lactis to respond to iron status in the gut microenvironment would allow it to be rapidly out-competed by E. coli and other iron-responsive bacteria in the inflamed gut 47. In this study, we have engineered L. lactis to express Lcn2 (LactisLcn2) as an innovative strategy to augment the fitness and efficacy of L. lactis in mitigating gut inflammation.
Given its small size (25 kDa) and the lack of post-translational modification requirement, Lcn2 presents a feasible candidate to be expressed in bacteria. We considered the food-grade L. lactis as the ideal vector strain since it is Gram-positive (endotoxin-free), non-pathogenic, non-invasive, and is a transient colonizer having probiotic properties. Most importantly, the lack of iron requirement and siderophore expression in L. lactis 39, 40 ensures that recombinant Lcn2 would be secreted in its active form that is free from iron and siderophore. We noted that LactisLcn2 is capable of producing both the monomeric and dimeric forms of Lcn2, albeit the dimeric form was noticeably more abundant within the cell lysates. Although the functional differences between monomeric and dimeric forms of Lcn2 are yet to be clarified37, 38, our in vitro assays nonetheless confirmed that LactisLcn2-derived Lcn2 is indeed bioactive in inhibiting the growth of E. coli and mitigating Ent bioactivity. Rather intriguingly, the expression of Lcn2 seemed to confer L. lactis with the ability to sequester iron from its microenvironment. Lcn2 alone, however, does not bind iron directly but requires siderophores as co-factors to form a ‘Lcn2-siderophore-iron’ ternary complex. The accumulation of iron in L. lactis hence implies the involvement of an unknown siderophore, either generated by the bacterium or acquired from the media, which need to be further investigated for its role in facilitating iron chelation by Lcn2 in LactisLcn2. Nevertheless, the ability of Lcn2 to augment iron-responses of L. lactis may be beneficial in allowing it to compete better with other iron-dependent bacteria in the gut 47.
Aside from its antimicrobial functions, Lcn2 overexpression has been demonstrated to enhance cellular resistance to oxidative stress and iron-induced toxicity in various mammalian cell lines32–36, 48, 49. As anticipated, the expression of Lcn2 substantially increased the capacity of LactisLcn2 to tolerate adverse conditions, including extreme pH, exposure to high concentrations of bile acids, oxidizing environments and MPO-mediated bacterial killing in vitro. The cytoprotective properties of Lcn2 are not well-understood, although several studies had associated its beneficial effects to its ability to modulate the labile iron pool and to exert anti-oxidative properties32–36, 48, 49. The changes in extracellular pH, for instance, could cause a fraction of lactic acid to become protonated, which enters and accumulates in the bacteria, thus triggering stress41. In this regard, we surmise that the growth advantage of LactisLcn2 over LactisCon at adverse pH could be possibly due to the mitigation of pH-mediated stress in the presence of Lcn2 acting as an antioxidant. We also presumed that similar antioxidant properties of Lcn2 may also increase the capacity of LactisLcn2 to tolerate the adverse oxidizing conditions tested in this study. Additionally, the survival of LactisLcn2, in the large intestine, was more in the DSS given mice than LactisCon. More importantly, LactisLcn2 administration substantially reduced the severity of DSS-induced colitis in mice when compared to LactisCon, suggesting that the increased fitness of LactisLcn2 in colonizing the inflamed gut with a higher retention rate could, in part, contribute to the better amelioration of colitis. These findings provide evidence that successful colonization and fitness of recombinant probiotic bacteria in the inflamed gut is an important consideration when optimizing probiotics regimen.
Lcn2 primarily controls the gut bacterial overgrowth by limiting its iron uptake and also mediates facilitation of the hypoferremia during inflammation 12. Additionally, Lcn2 serve as an indispensable factor for proper neutrophil migration, adhesion, and function50. In our recent study16, 38 as well as others17, 51, Lcn2 has been demonstrated to mediate an essential role in maintaining gut homeostasis, whereas its deficiency increases their susceptibility to colitis in mice. Considering that endogenous Lcn2 are significantly upregulated during IBD2, 3, it is, therefore, unlikely that the delivery of additional Lcn2 via L. lactis per se could adequately account for the therapeutic properties of LactisLcn2. Our findings suggested that the beneficial effects of LactisLcn2 could be potentially explained, at least in part, by the augmented survival or colonization ability of the probiotic L. lactis that express Lcn2. Nevertheless, we could not rule out the possibility that exogenous Lcn2 may also provide mucoprotective effects to the host via: (i) mitigating gut dysbiosis such as preventing the bloom of Enterobacteriaceae (e.g., E. coli)4, 10, (ii) restricting luminal iron and the labile iron pool12, thus attenuating iron-induced oxidative stress, (iii) serving as an epithelial survival factor 52 and promote wound healing53, 54. Further prolonged survival may also enhance the generation of L. lactis derived soluble, effector molecules, which can diminish gut inflammation.
Most probiotics tested in clinical trials failed to improve human IBD55, despite their efficacy in mitigating the gut inflammation in experimental animal models. The lack of success in the clinical setting may be explained by the inherent deficiency of these probiotics to survive against the host inflammatory responses. Such notion coincides with the consideration that many candidate probiotics are selected from bacteria that transiently colonizes the gut, and thus may be rapidly depleted in the inflamed gut. In the present study, we demonstrated that Lcn2 expression could be engineered into L. lactis to enhance its survivability, colonization, and capacity to tolerate the harsh environments in the inflamed gut. Overall, our findings highlight the potential for Lcn2 expression or co-expression alongside other therapeutic molecules as an innovative strategy to further stabilize probiotic bacteria that are currently studied to mitigate intestinal inflammation and allow for better management of experimental colitis.
Acknowledgments
Funding: This work was supported by a grant from the National Institutes of Health R01 (DK097865) to MV-K. BSY is supported by NIH T32 (T32AI074551) grant. VS and EV are supported by CCFA s Research Fellowship Award. BC is supported by CCFA s Career Development Award.
Abbreviations
- APP
acute-phase protein
- DSS
Dextran sodium sulfate
- Ent
Enterobactin
- IBD
Inflammatory bowel diseases
- L. lactis
Lactococcus lactis
- Lcn2
Lipocalin 2
- LactisLcn2
Lipocalin 2 expressing L. lactis
- LactisCon
L. lactis transformed with empty plasmid
- MPO
Myeloperoxidase
- ROS
Reactive oxygen species
Footnotes
Conflict of interest: The authors declared no conflict of interest.
Authors contributions: P.S. designed and performed the experiments, analyzed the data and co-wrote the paper; B.C. designed and generated the recombinant bacteria, performed the experiments, analyzed the data; B.S.Y. performed experiments and co-wrote the paper; E.V. designed, performed, and analyzed the experiment with 2D gel electrophoresis; X.X. performed animal experiments; V.S. contributed to the experimental design and analysis, M.J.K. performed histologic analysis in a blinded fashion and analyzed the data, M.V-K. conceptually developed the project, designed the experiments, analyzed the data, and co-wrote the manuscript.
References
- 1.Vermeire S, Van Assche G, Rutgeerts P. Laboratory markers in IBD: useful, magic, or unnecessary toys? Gut. 2006;55:426–431. doi: 10.1136/gut.2005.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakraborty S, Kaur S, Guha S, et al. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim Biophys Acta. 2012;1826:129–169. doi: 10.1016/j.bbcan.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chassaing B, Srinivasan G, Delgado MA, et al. Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One. 2012;7:e44328. doi: 10.1371/journal.pone.0044328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flo TH, Smith KD, Sato S, et al. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- 5.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 6.Vijay-Kumar M, Sanders CJ, Taylor RT, et al. Deletion of TLR5 results in spontaneous colitis in mice. J Clin Invest. 2007;117:3909–3921. doi: 10.1172/JCI33084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vijay-Kumar M, Wu H, Aitken J, et al. Activation of toll-like receptor 3 protects against DSS-induced acute colitis. Inflamm Bowel Dis. 2007;13:856–864. doi: 10.1002/ibd.20142. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen BS, Borregaard N, Bundgaard JR, et al. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996;38:414–420. doi: 10.1136/gut.38.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oikonomou KA, Kapsoritakis AN, Theodoridou C, et al. Neutrophil gelatinase-associated lipocalin (NGAL) in inflammatory bowel disease: association with pathophysiology of inflammation, established markers, and disease activity. J Gastroenterol. 2012;47:519–530. doi: 10.1007/s00535-011-0516-5. [DOI] [PubMed] [Google Scholar]
- 10.Goetz DH, Holmes MA, Borregaard N, et al. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10:1033–1043. doi: 10.1016/s1097-2765(02)00708-6. [DOI] [PubMed] [Google Scholar]
- 11.Sia AK, Allred BE, Raymond KN. Siderocalins: Siderophore binding proteins evolved for primary pathogen host defense. Curr Opin Chem Biol. 2013;17:150–157. doi: 10.1016/j.cbpa.2012.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao X, Yeoh BS, Saha P, et al. Lipocalin 2 alleviates iron toxicity by facilitating hypoferremia of inflammation and limiting catalytic iron generation. Biometals. 2016;29:451–465. doi: 10.1007/s10534-016-9925-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sartor RB. Key questions to guide a better understanding of host-commensal microbiota interactions in intestinal inflammation. Mucosal Immunol. 2011;4:127–132. doi: 10.1038/mi.2010.87. [DOI] [PubMed] [Google Scholar]
- 14.Nagalingam NA, Kao JY, Young VB. Microbial ecology of the murine gut associated with the development of dextran sodium sulfate-induced colitis. Inflamm Bowel Dis. 2011;17:917–926. doi: 10.1002/ibd.21462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Werner T, Wagner SJ, Martinez I, et al. Depletion of luminal iron alters the gut microbiota and prevents Crohn's disease-like ileitis. Gut. 2011;60:325–333. doi: 10.1136/gut.2010.216929. [DOI] [PubMed] [Google Scholar]
- 16.Singh V, Yeoh BS, Chassaing B, et al. Microbiota-inducible Innate Immune, Siderophore Binding Protein Lipocalin 2 is Critical for Intestinal Homeostasis. Cell Mol Gastroenterol Hepatol. 2016;2:482–498. e486. doi: 10.1016/j.jcmgh.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moschen AR, Gerner RR, Wang J, et al. Lipocalin 2 Protects from Inflammation and Tumorigenesis Associated with Gut Microbiota Alterations. Cell Host Microbe. 2016;19:455–469. doi: 10.1016/j.chom.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 18.Bermudez-Humaran LG, Kharrat P, Chatel JM, et al. Lactococci and lactobacilli as mucosal delivery vectors for therapeutic proteins and DNA vaccines. Microb Cell Fact. 2011;10(Suppl 1):S4. doi: 10.1186/1475-2859-10-S1-S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.LeBlanc JG, Aubry C, Cortes-Perez NG, et al. Mucosal targeting of therapeutic molecules using genetically modified lactic acid bacteria: an update. FEMS Microbiol Lett. 2013;344:1–9. doi: 10.1111/1574-6968.12159. [DOI] [PubMed] [Google Scholar]
- 20.Steidler L, Hans W, Schotte L, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–1355. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 21.Hanson ML, Hixon JA, Li W, et al. Oral delivery of IL-27 recombinant bacteria attenuates immune colitis in mice. Gastroenterology. 2014;146:210–221. e213. doi: 10.1053/j.gastro.2013.09.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bermudez-Humaran LG, Motta JP, Aubry C, et al. Serine protease inhibitors protect better than IL-10 and TGF-beta anti-inflammatory cytokines against mouse colitis when delivered by recombinant lactococci. Microb Cell Fact. 2015;14:26. doi: 10.1186/s12934-015-0198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vandenbroucke K, de Haard H, Beirnaert E, et al. Orally administered L. lactis secreting an anti-TNF Nanobody demonstrate efficacy in chronic colitis. Mucosal Immunol. 2010;3:49–56. doi: 10.1038/mi.2009.116. [DOI] [PubMed] [Google Scholar]
- 24.Atosuo JT, Lilius EM. The real-time-based assessment of the microbial killing by the antimicrobial compounds of neutrophils. ScientificWorldJournal. 2011;11:2382–2390. doi: 10.1100/2011/376278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walmsley TA, George PM, Fowler RT. Colorimetric measurement of iron in plasma samples anticoagulated with EDTA. J Clin Pathol. 1992;45:151–154. doi: 10.1136/jcp.45.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singh V, Yeoh BS, Xiao X, et al. Interplay between enterobactin, myeloperoxidase and lipocalin 2 regulates E. coli survival in the inflamed gut. Nat Commun. 2015;6:7113. doi: 10.1038/ncomms8113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SK, Keasling JD. A propionate-inducible expression system for enteric bacteria. Appl Environ Microbiol. 2005;71:6856–6862. doi: 10.1128/AEM.71.11.6856-6862.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chassaing B, Aitken JD, Malleshappa M, et al. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104(Unit 15):25. doi: 10.1002/0471142735.im1525s104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh V, Yeoh BS, Carvalho F, et al. Proneness of TLR5 deficient mice to develop colitis is microbiota dependent. Gut Microbes. 2015;6:279–283. doi: 10.1080/19490976.2015.1060390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cooper HS, Murthy SN, Shah RS, et al. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–249. [PubMed] [Google Scholar]
- 31.Arroyo A, Modriansky M, Serinkan FB, et al. NADPH oxidase-dependent oxidation and externalization of phosphatidylserine during apoptosis in Me2SO-differentiated HL-60 cells. Role in phagocytic clearance. J Biol Chem. 2002;277:49965–49975. doi: 10.1074/jbc.M204513200. [DOI] [PubMed] [Google Scholar]
- 32.Cao Z, Yu W, Li W, et al. Oxidative Damage and Mitochondrial Injuries Are Induced by Various Irrigation Pressures in Rabbit Models of Mild and Severe Hydronephrosis. PLoS One. 2015;10:e0127143. doi: 10.1371/journal.pone.0127143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ebrahimi-Kalan A, Soleimani Rad J, Kafami L, et al. MS14 down-regulates lipocalin2 expression in spinal cord tissue in an animal model of multiple sclerosis in female C57BL/6. Iran Biomed J. 2014;18:196–202. doi: 10.6091/ibj.1375.2014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Halabian R, Tehrani HA, Jahanian-Najafabadi A, et al. Lipocalin-2-mediated upregulation of various antioxidants and growth factors protects bone marrow-derived mesenchymal stem cells against unfavorable microenvironments. Cell Stress Chaperones. 2013;18:785–800. doi: 10.1007/s12192-013-0430-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miyamoto T, Kashima H, Yamada Y, et al. Lipocalin 2 Enhances Migration and Resistance against Cisplatin in Endometrial Carcinoma Cells. PLoS One. 2016;11:e0155220. doi: 10.1371/journal.pone.0155220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macedo-Marquez A, Vazquez-Acevedo M, Ongay-Larios L, et al. Overexpression of a monomeric form of the bovine odorant-binding protein protects Escherichia coli from chemical-induced oxidative stress. Free Radic Res. 2014;48:814–822. doi: 10.3109/10715762.2014.910867. [DOI] [PubMed] [Google Scholar]
- 37.Martensson J, Xu S, Bell M, et al. Immunoassays distinguishing between HNL/NGAL released in urine from kidney epithelial cells and neutrophils. Clin Chim Acta. 2012;413:1661–1667. doi: 10.1016/j.cca.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 38.Saha P, Singh V, Xiao X, et al. Data on importance of hematopoietic cell derived Lipocalin 2 against gut inflammation. Data Brief. 2016;8:812–816. doi: 10.1016/j.dib.2016.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pandey A, Bringel F, Meyer JM. Iron Requirement and Search for Siderophores in Lactic-Acid Bacteria. Applied Microbiology and Biotechnology. 1994;40:735–739. [Google Scholar]
- 40.Bruyneel B, Vandewoestyne M, Verstraete W. Lactic-Acid Bacteria - Microorganisms Able to Grow in the Absence of Available Iron and Copper. Biotechnology Letters. 1989;11:401–406. [Google Scholar]
- 41.Madsen SM, Arnau J, Vrang A, et al. Molecular characterization of the pH-inducible and growth phase-dependent promoter P170 of Lactococcus lactis. Mol Microbiol. 1999;32:75–87. doi: 10.1046/j.1365-2958.1999.01326.x. [DOI] [PubMed] [Google Scholar]
- 42.Yokota A, Veenstra M, Kurdi P, et al. Cholate resistance in Lactococcus lactis is mediated by an ATP-dependent multispecific organic anion transporter. J Bacteriol. 2000;182:5196–5201. doi: 10.1128/jb.182.18.5196-5201.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 44.Klebanoff SJ, Kettle AJ, Rosen H, et al. Myeloperoxidase: a front-line defender against phagocytosed microorganisms. J Leukoc Biol. 2013;93:185–198. doi: 10.1189/jlb.0712349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weinberg ED. The Lactobacillus anomaly: total iron abstinence. Perspect Biol Med. 1997;40:578–583. doi: 10.1353/pbm.1997.0072. [DOI] [PubMed] [Google Scholar]
- 46.Posey JE, Gherardini FC. Lack of a role for iron in the Lyme disease pathogen. Science. 2000;288:1651–1653. doi: 10.1126/science.288.5471.1651. [DOI] [PubMed] [Google Scholar]
- 47.Bailey JR, Probert CS, Cogan TA. Identification and characterisation of an iron-responsive candidate probiotic. PLoS One. 2011;6:e26507. doi: 10.1371/journal.pone.0026507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bahmani B, Roudkenar MH, Halabian R, et al. Lipocalin 2 decreases senescence of bone marrow-derived mesenchymal stem cells under sub-lethal doses of oxidative stress. Cell Stress Chaperones. 2014;19:685–693. doi: 10.1007/s12192-014-0496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu L, Hittelman W, Lu T, et al. NGAL decreases E-cadherin-mediated cell-cell adhesion and increases cell motility and invasion through Rac1 in colon carcinoma cells. Lab Invest. 2009;89:531–548. doi: 10.1038/labinvest.2009.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schroll A, Eller K, Feistritzer C, et al. Lipocalin-2 ameliorates granulocyte functionality. Eur J Immunol. 2012;42:3346–3357. doi: 10.1002/eji.201142351. [DOI] [PubMed] [Google Scholar]
- 51.Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56. doi: 10.1016/0003-2697(87)90612-9. [DOI] [PubMed] [Google Scholar]
- 52.Tong Z, Wu X, Ovcharenko D, et al. Neutrophil gelatinase-associated lipocalin as a survival factor. Biochem J. 2005;391:441–448. doi: 10.1042/BJ20051020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miao Q, Ku AT, Nishino Y, et al. Tcf3 promotes cell migration and wound repair through regulation of lipocalin 2. Nat Commun. 2014;5:4088. doi: 10.1038/ncomms5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Playford RJ, Belo A, Poulsom R, et al. Effects of mouse and human lipocalin homologues 24p3/lcn2 and neutrophil gelatinase-associated lipocalin on gastrointestinal mucosal integrity and repair. Gastroenterology. 2006;131:809–817. doi: 10.1053/j.gastro.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 55.Jonkers D, Penders J, Masclee A, et al. Probiotics in the management of inflammatory bowel disease: a systematic review of intervention studies in adult patients. Drugs. 2012;72:803–823. doi: 10.2165/11632710-000000000-00000. [DOI] [PubMed] [Google Scholar]