Abstract
Hypoglycemia is increasingly recognized as a complication of bariatric surgery. Although medications are often required, medical nutrition therapy remains the key cornerstone for successful prevention of hypoglycemia in patients with post-bariatric hypoglycemia (PBH). We provide suggested approaches to the dietary management of PBH, incorporating data from both the medical literature and extensive clinical experience in an academic referral center for PBH. The overall goal of medical nutrition therapy for PBH is to reduce postprandial surges in glucose, which often trigger surges in insulin secretion and promote subsequent hypoglycemia. Thus, strategies focus on controlled portions of low glycemic index carbohydrates, avoidance of rapidly-absorbed carbohydrates, adjustment of timing of meals and snacks, and attention to personal and cultural barriers to implementation.
Keywords: hypoglycemia, bariatric surgery, medical nutrition therapy
INTRODUCTION
Hypoglycemia is an increasingly recognized complication of bariatric surgery, occurring after both roux-en-Y gastric bypass (RYGB) and vertical sleeve gastrectomy (SG) [1–5]. Post-bariatric hypoglycemia (PBH) typically occurs one to three hours after meals, with increased severity after intake of high glycemic index carbohydrates [6, 7]. In our clinical experience, some patients also experience hypoglycemia with even modest degrees of activity (e.g. household chores, grocery shopping).
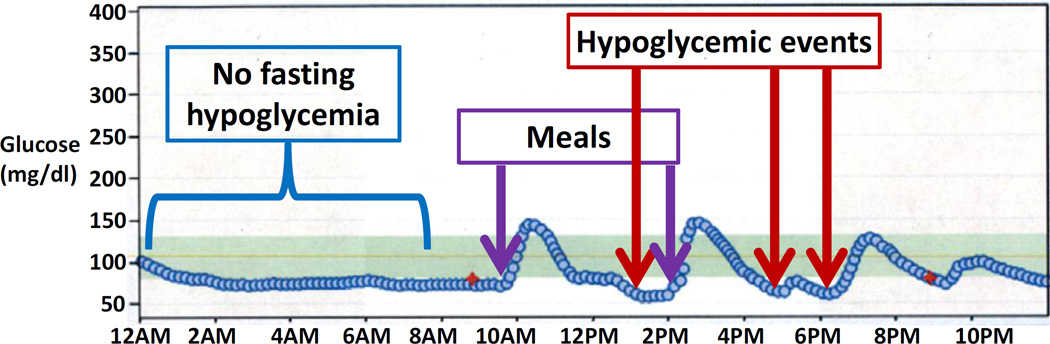
Both insulin-dependent and insulin-independent mechanisms contribute to PBH. After eating, food rapidly enters into the proximal intestine, contributing to rapid rises in blood glucose (within 15–30 minutes), often to quite high levels (e.g. over 200 mg/dl)[7]. Food intake also stimulates excessive secretion of intestinal incretin hormones, including glucagon-like peptide-1 (GLP-1) [7–10]. Both high glucose and incretin hormones trigger excessive insulin secretion, promoting subsequent rapid drops in glucose, with nadir typically between 90 to 180 minutes after eating [11]. Additionally, increased sensitivity of pancreatic β-cells to glucose, reduced insulin clearance, and insulin-independent glucose uptake may also contribute to hypoglycemia [7, 12, 13]. Figure 1 shows a typical postprandial glucose pattern in patient with PBH, with rapid increase in glucose early after the meal (typically within 30 minutes), followed by rapid decline to hypoglycemic range.
Figure 1.
Typical patterns of glucose in the fasting state and after a meal in patients with PBH, as revealed by continuous glucose monitoring. In this patient, glucose levels are stable during the overnight fast.
Recognizing potential symptoms of hypoglycemia in the post-bariatric patient is important to begin prompt diagnostic evaluation and treatment. Symptoms (Table 1) are varied and overlap with other conditions, including anxiety, cardiac arrhythmias, and seizures, sometimes delaying recognition.
Table 1.
Symptoms which should raise concern for PBH.
| Adrenergic Symptoms | Neuroglycopenic Symptoms |
|---|---|
| Tremor | Weakness |
| Palpitations | Difficulty concentrating/thinking |
| Anxiety | Seizure |
| Coma | |
| Cholinergic Symptoms | Unawareness or no symptoms |
| Sweating | Frequent lows |
| Hunger | Drowsiness |
| Paresthesias | Nightmares/bizarre dreams |
| Often nonspecific! | Morning headaches |
The first critical step is to determine whether symptoms are truly linked to hypoglycemia, requiring assessment of plasma glucose at the time of symptoms. This is often challenging, as patients may not be able to safely go to a laboratory for blood testing; a suboptimal but practical alternative is to use a glucometer or diagnostic continuous glucose monitoring. Hypoglycemia is less likely if glucose is normal at the time of symptoms; if so, dumping syndrome or cardiovascular causes should be considered. If low glucose is detected at time of symptoms, then clinicians still need to verify low plasma glucose after a typical stimulus (e.g. provocative meal), and confirm that symptoms resolve with elevation of glucose. If these Whipple’s triad criteria are fulfilled (symptoms, low venous glucose, resolution with elevation in glucose), the diagnosis of hypoglycemia is supported [14].
Assessment of hypoglycemia patterns in relation to meals, fasting, and activity, magnitude of alcohol consumption, medications, and other medical conditions, is important. PBH typically presents with postprandial hypoglycemia, occurring within 1 to 3 hours after eating. Hypoglycemia which also occurs in the fasting state should raise suspicion for autonomous secretion of insulin by an insulinoma or other hormonal or metabolic disorders [15]. Next, biochemical evaluation should be performed to determine whether hypoglycemia is associated with inappropriately increased insulin levels. Traditional approaches include either overnight outpatient fasting (if history suggests this can be accomplished safely) or prolonged inpatient fasting, with measurements of insulin, C-peptide, β-hydroxybutyrate, and the counterregulatory hormones cortisol and glucagon. In patients with PBH, glucose and insulin levels are normal with fasting, but insulin secretion in excessive in the postprandial state [7].
Once hypoglycemia has been fully evaluated and PBH is diagnosed, medical nutrition therapy (MNT) is the first step to reduce postprandial surges in glucose, as these “spikes” in glucose often trigger excessive insulin secretion and promote subsequent hypoglycemia. While medications may also be needed to control hypoglycemia in some patients (see [11] for more information), MNT remains the cornerstone of therapy.
In this review, we provide suggested approaches to the dietary management of PBH aimed at the practicing bariatric clinician, incorporating data from both the medical literature and extensive clinical experience in an academic referral center for PBH. Strategies focus on controlled portions of low glycemic index carbohydrates, avoidance of rapidly-absorbed carbohydrates, adjustment of timing of meals and snacks, and attention to personal and cultural barriers to implementation.
PREVENTION OF HYPOGLYCEMIA IN POST-BARIATRIC PATIENTS
Once hypoglycemia has been fully evaluated, medical nutrition therapy (MNT) is the cornerstone of treatment to prevent or reduce the frequency and severity of hypoglycemia, even when additional medications are also needed.
In our practice, we have successfully used a 10-point nutrition plan for the prevention of hypoglycemia, while ensuring adequate nutrient intake (Table 2). The overall goal is to reduce the magnitude and rate of the blood glucose rise after eating, reducing stimulus for insulin secretion and subsequent hypoglycemia. Key components include:
Table 2.
10-Point Nutrition Plan for Preventing Hypoglycemia in Post-Bariatric Hypoglycemia.
|
1. Control portions of carbohydrate - 30 grams per meal, 15 grams per snack
Carbohydrate ingestion is a particularly robust stimulus for increasing blood glucose and thus insulin secretion in post-bariatric patients. Some patients with PBH are even more sensitive to carbohydrates, potentially due to excessive intestinal glucose absorption. Thus, limiting carbohydrate reduces the postprandial glucose rise and insulin secretion. This concept was confirmed in one study, which demonstrated that a high carbohydrate (80 grams), low protein (10 grams) meal led to significant hyperinsulinemia and hypoglycemia, while an isocaloric very low carbohydrate (2 grams), high protein meal (25 grams) prevented excessive insulin secretion or postprandial hypoglycemia [16]. While this degree of carbohydrate restriction makes compliance challenging and may not be optimal for the long-term, few studies address the optimal quantity of carbohydrates. In one study, limiting a test meal to 30 grams of solid carbohydrate or 28 grams of liquid low glycemic index supplement was successful in preventing hypoglycemia in patients with PBH [17]. A third study in 10 patients with PBH evaluated the relative carbohydrate composition of a test meal; a high carbohydrate meal containing mostly sucrose provoked postprandial hypoglycemia, while an isocaloric high-carbohydrate meal composed of fructose was associated with lower glycemic peak, lower insulin secretion, and no induction of hypoglycemia [18]. The mechanisms responsible for the differential glycemic effect of fructose is not entirely clear. Fructose can stimulate both incretin and insulin secretion [19], but differential absorption patterns and hepatic metabolism of fructose vs. glucose could contribute to differential prandial glucose patterns [20]. Moreover, an unanswered question is whether the greater effect of fructose to promote hepatic lipogenesis could produce long-term health effects [21]. Nevertheless, some patients utilize pure fructose (available commercially) as a substitute carbohydrate, especially for baking.
In our experience, a gram of carbohydrate can raise blood glucose an average of 3 mg/dl. Thus, a 30 gram carbohydrate meal may increase glucose by ≈100 mg/dl, while a 15 gram carbohydrate snack may increase glucose by ≈50 mg/dl. It is important to recognize that there is substantial variability in glucose “spikes” between individuals, and even from day to day, potentially related to rate of delivery of foods to the intestine, rates of glucose absorption by the proximal intestine, time of day, and other metabolic factors [22]. One recent study demonstrated that carbohydrate restriction to not more than 30 g per meal was effective to reduce the frequency and severity of hypoglycemia episodes [23].
A registered dietitian (RD) can teach patients how to count carbohydrate grams to meet these goals, with the assistance of online tools/apps (e.g., CalorieKing.com, GoMeals.com) and reference databases (e.g., USDA National Nutrient Database for Standard Reference: https://ndb.nal.usda.gov/ndb/search/list). Patients should be counseled that carbohydrates are needed as fuel for the body, so complete elimination is not advised, as this, too, could reduce carbohydrate stores (glycogen) and potentially promote more severe or prolonged hypoglycemia. Rather, limiting quantity and choosing only low glycemic index carbohydrates (#2) is critical. Of course, carbohydrate goals need to be individualized upon review of food diary and glucose patterns.
2. Choose low glycemic index carbohydrates
Low glycemic index carbohydrates are digested relatively slowly. We have found that low glycemic index carbohydrates slow the postprandial rise in glucose, resulting in fewer postprandial glucose “spikes” and, therefore, less postprandial hypoglycemia. Table 3 provides a list of low glycemic foods that have proved successful in limiting postprandial hypoglycemia for many of our patients. An extensive glycemic index table may be accessed at www.glycemicindex.com. In general, carbohydrates which are highly processed are not low glycemic index. As noted above, one study demonstrated that substitution of fructose for sucrose could reduce hypoglycemia [18]; the long-term effects of increasing fructose intake in this context remain unknown. Again, responses to specific foods vary considerably, so initial advice needs to be modified for each patient after reviewing glucose patterns.
Table 3.
Low Glycemic Index Carbohydrates (CHOOSE)
|
Some patients find that avoiding high glycemic index carbohydrates, especially pasta and bread, is very challenging. Patients might consider substituting pasta made from 100% whole wheat or protein-supplemented pasta in carefully measured, small quantities. Pasta-like products made from thinly-sliced vegetables (e.g. zucchini, spaghetti squash) may be helpful. Asian noodles made from glucomannan can provide desired pasta flavor/texture, but it is important that these noodles be consumed with plenty of fluid, and not at bedtime, to avoid risk of obstruction [24]. Some patients find that enyzme-enriched high fiber wheat products (e.g. carbalose) are useful as a substitute for regular flour when baking. High doses of pectin (e.g. 14.5 gram), a complex plant-based polysaccharide, have been shown to reduce postprandial hypoglycemia in post-gastric surgery patients when added to meals containing carbohydrates; however, quantities sufficient to prevent hypoglycemia are often poorly tolerated, and lower doses are ineffective [25, 26].
3. Avoid high glycemic index carbohydrates
High glycemic index carbohydrates are digested relatively quickly, contributing to rapid Increases in blood glucose, further stimulation of excessive secretion of insulin postprandially, and more postprandial hypoglycemia as compared with low glycemic index carbohydrates. Table 4 provides a list of high glycemic index carbohydrates that typically exacerbate postprandial hypoglycemia in our patients.
Table 4.
High Glycemic Index Carbohydrates (AVOID)
|
4. Include heart-healthy fats in each meal and snack
Fat can reduce gastric emptying reduce postprandial “spikes” in glucose in patients with type 1 diabetes [27]. However, the magnitude of this effect is reduced in patients who have had gastric bypass, since emptying of foods from the pouch to the roux limb occurs very rapidly. While fat intake before a meal prolonged overall intestinal transit time, glucose excursions were not markedly affected by a fat preload in a recent study [28]. Nevertheless, fats are also beneficial as they are a source of calories which do not typically trigger insulin secretion/hypoglycemia independently, and thus may be considered a more “safe” food [22]. While any fat can produce this result, we recommend heart-healthy fats (Table 5). While many patients have difficulty overcoming the ingrained notion that fats are high-calorie foods which should be avoided at all cost, emphasizing that healthy fats are not only required for good health but also can serve as a substitute calorie source to compensate for the reduction in carbohydrates may be helpful.
Table 5.
Heart-Healthy Fats
|
5. Emphasize adequate protein intake
A general rule of thumb is that post-bariatric patients should consume about 30 grams of protein at each meal [29, 30]. The American Society of Metabolic and Bariatric Surgery (ASMBS) recommends at least 60–80 grams per day [31], while other guidelines suggest 1.5–2.1 g/kg of ideal body weight or 0.91 g/kg actual weight [32]. We typically begin with 0.9 protein/kg actual body weight to estimate protein needs. Preferred protein sources are those with high biological value such as meat, chicken, egg whites, fish, and milk, and high quality non-animal proteins such as soy.
Excessive protein intake is not desirable, as it may displace other nutrients in the diet. In some patients, excessive liquid protein intake may also contribute to hypoglycemia, as proteins also robustly stimulate insulin secretion [28]. If the patient consumes protein shakes, the shake should be moderately low in carbohydrate and include a high quality source of protein. 100% whey protein isolate powder is preferred, but soy protein powder is an option. According to the Obesity Action Collation (OAC), whey protein isolates have lower lactose and more protein compared with whey protein concentrates [33]. We recommend that the shake contain 20–30 grams protein, 15 grams carbohydrate, and about 10 grams of fat per serving. Protein can be blended with low-fat or skim milk, low glycemic index fruits or ice, and optional flavor extracts. Natural nut butters (containing no added sugar or corn syrup) can be added as a source of healthy fat, and uncooked cornstarch added to provide very complex, slowly metabolized carbohydrate.
Given that dietary strategies for PBH center on limiting carbohydrates to minimize postprandial glycemic surges, it is clear that protein and healthy fats must make up the difference for a weight maintenance meal plan. This is a very individualized and iterative process. We begin with limiting carbohydrates to a suggested cap of 30 g per meal and 15 g per snack [23], regardless of body weight. Protein and healthy fats will make up the balance to achieve protein and calorie goals. Recommendations for protein are typically 0.9 gram per kg of actual body weight. We typically initiate fat intake goals using a ratio of 2 grams of fat for every 3 g of carbohydrate. These calculations typically yield a caloric distribution of ≈ 30% carb, 45–50% fat, and 20–25% protein, but differs according to patient weight (in which case protein percentage will be higher) and glucose patterns in response to initial dietary plan.
6. Space meals/snacks 3–4 hours apart
A small meal or snack will likely be completely digested in 3–4 hours. We recommend a healthy meal/snack (following the above guidelines) every 3–4 hours, especially if the patient engages in physical activity.
7. Avoid consuming liquids with meals and chew foods slowly and thoroughly
Hypoglycemia is sometimes a component of the “dumping syndrome” and can follow both gastric bypass and sleeve gastrectomy [34]. Indeed, hormonal responses in patients with “dumping” are similar to those in patients with severe post-bypass hypoglycemia, including rapid surges in glucose and incretin and insulin secretion [35, 36]. Dietary management of the dumping syndrome includes recommendations to avoid ingesting liquids with meals, in order to attenuate rapid delivery of nutrients to the intestine, glucose absorption, and increased insulin secretion [37]. Thus, water or non-carbonated low calorie caffeine-free beverages should be separated from meals by at least 30–60 minutes. Eating slowly, over 30–60 minutes, with thorough chewing of small bites of food, is a challenging goal but can also be helpful to reduce dumping symptoms [36].
8. Avoid alcohol
During metabolism of alcohol by the liver, the production of glucose by the liver is reduced, increasing the risk for hypoglycemia. Alcohol intake can also compromise B vitamin absorption, such as B1 and B12, and is thus undesirable in bariatric patients already at risk for vitamin deficiency.
9. Avoid caffeine
Among caffeine-sensitive individuals, caffeine can rapidly increase blood glucose via increased hepatic glucose production and decreased glucose uptake into skeletal muscle; in some studies, caffeine increased insulin levels [38]. While the net impact is uncertain for any specific patient, we advise patients to attempt a trial of caffeine reduction to assess impact on hypoglycemia.
10. Maintain post-bariatric vitamin and mineral intake
Patients with PBH typically present for evaluation of hypoglycemia several years postoperatively. A frequent misconception is that strict attention to vitamin and mineral supplementation is no longer needed. While we are unaware of any evidence that vitamin deficiencies contribute to frequency or severity of hypoglycemia, evaluation of vitamin and mineral status is critical for any post-bariatric patient. This may be especially important for in patients with hypoglycemia who may be attempting to self-treat hypoglycemia by limiting specific foods. Recommendations by the ASMBS include: multivitamins with minerals, oral or sublingual B12, iron, B complex vitamins, calcium citrate, and vitamin D (Table 6). Repeated emphasis on the importance of vitamins and mineral supplements is often helpful.
Table 6.
Recommended vitamin supplementation after bariatric surgery (17)
| Supplement | Recommendations for the Post- Bariatric Surgery Patient |
|---|---|
Multivitamin-multi-mineral:
|
200% of daily value (2 per day) |
Vitamin B12:
|
1000 µg/month intramuscularly Sublingual/oral tablet dose: 350–500 µg/day |
Calcium Citrate and Vitamin D3
|
1500–2000 mg/day Calcium Citrate 1500–2000 IU/day Vit D3 (with dose adjustments guided by laboratory assessment of vitamin D levels) |
Iron
|
18–27 mg/day elemental iron |
B complex vitamins
|
Vitamin B-50 complex 1 per day |
KEY COMPONENTS FOR SUCCESSFUL MNT EDUCATION
Clinical experience in working with patients with PBH has revealed several key components for successful MNT.
Emphasize food as friend rather than foe
Changing food habits is difficult. Typically, PBH patients have spent years, if not decades, trying to lose weight. They have tried multiple diets which promised weight loss if only they would eat this instead of that, and received meal plans from weight loss centers or dieticians. Typically they have gotten the message that protein foods are “good” and carbohydrate and fat are “bad”. With the onset of hypoglycemia, many patients are advised to eat frequently (e.g. every 2 hours or with symptoms). Not surprisingly, changing messages and resulting weight gain can lead to frustration.
Thus, to be successful, we need to partner with patients to convince them that the 10-point nutrition plan can help them with their problem, i.e. that food can be their friend instead of their enemy. We usually suggest trying the meal plan for a week to assess impact on reducing frequency of hypoglycemia. If not, they can abandon it without incurring either side effects or significant expense. If the plan helps them, they will gain relief and may even begin to lose at least some of the weight they may have recently regained.
Careful dietary assessment is essential!
Individuals vary significantly in their response to foods, requiring detailed and personalized assessment of response to different foods/food combinations.
This is best accomplished with the aid of a food diary, which includes records of hypoglycemic events and the foods consumed during several hours before each hypoglycemic event. Pay particular attention to foods/food combinations and time of day that are repeatedly associated with hypoglycemia. It is helpful to use food models to assess portion sizes and elicit preparation methods. Do not assume that low glycemic index foods are unrelated to low blood glucoses, as responses differ from person to person.
Careful dietary assessment can help our patients discover which foods/meals work for them and which don’t. Emphasize that instructions are general, and need to be modified as experience is gained.
Continuous glucose monitoring systems (CGMS), while not perfect, can improve the utility of a comprehensive dietary assessment. CGMS record interstitial fluid glucose levels every five minutes. If properly calibrated, CGMS-measured interstitial glucose closely approximates blood glucose levels at steady-state, but may lag behind blood glucose when glucose is changing rapidly, as in the postprandial state [39]. With CGMS, a patient’s glucose response to food can be immediately and precisely captured and then correlated with the patient’s food diary. CGMS is particularly useful for detecting hypoglycemia overnight, when the patient may not be aware of hypoglycemia. Thus, foods and other provocative factors can be identified.
BARRIERS TO SUCCESSFUL MEDICAL NUTRITION THERAPY
In our experience, the barriers to dietary change can be formidable, including:
1. Suboptimal nutrition knowledge
Unfortunately, many patients who have embarked on a bariatric surgical program have limited nutrition background and prior professional counseling. Many enter the program with high anxiety, unrealistic goals for weight loss and postoperative body changes, and inadequate understanding of the long-term dietary changes and attention to nutrition which are needed for successful weight loss and health. Despite robust preoperative counseling, many patients who present with hypoglycemia (typically 2–3 years postoperatively), may require a refresher of basic nutrition concepts as well as specific counseling about medical nutrition therapy tailored to avoidance of hypoglycemia.
2. Food Habits
Suboptimal food habits, either longstanding or learned in the early postoperative period, are often deleterious for the patient with hypoglycemia. For example, frequent eating outside the home or takeout meals, skipping meals, drinking with meals, drinking sweetened beverages, alcohol intake, and a diet high in added sugar are common prior to surgery. Moreover, disordered eating/eating disorders, such as binge eating, emotional eating, and bulimia may have been present before surgery and persist to some extent after surgery [40], making dietary changes more challenging. After surgery, patients sometimes forget to separate beverages from foods, or may form a new habit of using liquid protein supplements as a major source of food.
3. Food traditions and cultural considerations
The food traditions of many cultures include the daily use of high glycemic index carbohydrates, such as corn meal, white rice, or bread/pasta made with refined flour. While these foods can trigger dumping syndrome and/or PBH, it is often difficult to completely eliminate a food that is a staple in their culture. Preserving a patient’s most cherished food tradition may increase compliance for dietary changes and reduce stress.
Taste and texture define the palatability of food, so we aim to preserve these qualities as much as possible. We encourage our patients to substitute low glycemic index I carbohydrates for their traditional high glycemic carbohydrates, but to prepare them in traditional fashion by using similar spices and condiments. For example, suggesting that patients use barley instead of white rice to make rice and beans using similar ethnic spices, instead of eliminating the grain accompanying beans entirely, may make low glycemic index I carbohydrates more acceptable.
4. Burnout with post-bariatric medical care
With time, bariatric patients are less likely to maintain follow-up care. This may be due, in part, to their perception that they can manage on their own. Additionally, costs of recurrent medical visits and copays, transportation to visits, and unreimbursed medication and vitamin costs are a challenge to nearly all patients. In our experience, these issues may collectively pose a barrier to nutrition therapy, both initial and followup visits, as well as costs associated with selecting fresh foods.
5. The need for convenience
Patients may rely on dining out/takeout, using microwaveable meals, ready-made bars or protein shakes, or simply skip meals because it is convenient. Planning meals in a structured manner in order to prevent hypoglycemia is often challenging, but may become easier with practice. Providing patients with easy-to-prepare meal ideas or even daily menus can be key to engaging patients in the low glycemic index meal preparation process, and often requires several visits with nutrition staff.
6. Lack of family support
Regrettably, some patients do not have the support of their family and friends. In some cases, family/friends can even undermine the patient’s efforts to follow their nutrition plan. It may be helpful to meet with both patient and family members to suggest supportive strategies. In extreme cases, referral to a behavioral medicine provider may be considered.
7. Guilt
It is important to reinforce that PBH is not the patient’s fault. While it is true that hypoglycemia occurs in response to food intake, the metabolic and hormonal factors which initiate hypoglycemia are not under direct control of the patient. Rather, making good food choices can lessen the frequency and severity of hypoglycemia.
8. Patient as family food preparer
The majority of bariatric patients are women, who often continue to serve as the primary meal providers for their families. It is often difficult for patients to prepare food for the family and a separate meal to meet their needs. Identifying low glycemic index carbohydrates which are acceptable to family and patients alike is key. Some patients find that baking with alternative flours and sweeteners, such as carbalose and fructose, will allow them to prepare “treats” that are satisfying to all family members. Emphasizing that changing the content of family meals can yield improved health and body weight for patient, spouse and children alike, may provide an extra incentive to make meal changes.
OTHER COMPONENTS OF EDUCATION TO IMPROVE SAFETY IN THE PATIENT WITH HYPOGLYCEMIA
Acute treatment of hypoglycemia
When post-bariatric patients experience hypoglycemia, we recommend immediate treatment with glucose tablets or gels to optimize safety. We recommend treatment if the glucose is under 70 mg/dL according to the “rule of 15”: consume 15 grams of glucose, such as 4 glucose tablets or 1 tube of glucose gel, then wait 15 minutes to recheck blood glucose. If glucose is not at least 80 mg/dl, repeat treatment with 15 grams of glucose. Blood glucose less than 50 mg/dL typically requires treatment with 30 grams of glucose. Glucose tablets need to be chewed and swallowed. Gels can be swallowed, but for faster glucose absorption, glucose gels can be held between gum and cheek for absorption via the buccal mucosa. Liquid glucose may be preferred by some patients, but it is typically much more expensive than tablets or gels. Use of glucose, rather than sucrose-containing treatments, is especially important for patients who are being treated with acarbose, as acarbose will slow digestion of non-glucose carbohydrates.
While this treatment protocol is designed to rapidly increase the patient’s glucose level, thus improving safety, rapid spikes in blood glucose may also trigger later hypoglycemia in PBH patients. If this is a consistent pattern, a lower initial glucose treatment dose e.g. 8–12 g, may be suggested, with careful testing to ensure adequate treatment of hypoglycemia. In addition, we recommend that patients eat a low glycemic index snack after treating hypoglycemia to avoid repeated hypoglycemia.
Glucagon
Glucagon is used to treat severe hypoglycemia with neuroglycopenia. If blood glucose falls below 30–40 mg/dl, the patient’s cognitive function may be temporarily impaired and the patient may be unable to self-treat hypoglycemia. Glucagon injected subcutaneously stimulates glucose release from the liver, thus raising glucose. Others who live/work with the patient should be taught to mix and inject glucagon for emergency use. Glucagon should be kept immediately available for use wherever the patient lives/works.
Activity counseling
Hypoglycemia in patients with PBH is often exacerbated by physical activity. In our experience, this appears to be especially true for patients whose carbohydrate intake is severely limited. Physical activity increases insulin sensitivity and causes muscle uptake of glucose to fuel muscular activity. If liver glycogen stores are compromised, hypoglycemia may result.
We routinely counsel patients with PBH to check their blood glucose to ensure that it is at least 80 mg/dl, and to consume a low glycemic index snack (15 g low glycemic index carbohydrate and 5–8 g fat) before physical activity. Since the effect of activity can persist for many hours, we also recommend more frequent monitoring after activity.
CGM as tool for hypoglycemia prevention
CGM devices not only measure interstitial glucose, but can also alert patients when their glucose levels are trending towards pre-set low or high levels or changing at a rapid rate, as often occurs when hypoglycemia is developing after a meal. For example, we often recommend that the patient ingest glucose (e.g. 8–12 grams) when glucose is dropping rapidly (as guided by alarm indicating rapid rate of decline greater than 2–3 mg/dl/min), even before the sensor glucose is overtly low. This allows patients to take action to prevent hypoglycemia, rather than merely to react to hypoglycemia after it occurs.
Use of cornstarch
Uncooked cornstarch is a very low glycemic index carbohydrate which has been shown to stabilize blood glucose levels and has been used to prevent hypoglycemia in individuals with type 1 diabetes [41]. Cornstarch can be added to protein shakes, yogurt or milk. It is the “active ingredient” in several commercial products, including Extend Nutrition products (bars, shakes, crisps) (www.extendbar.com) and UCAN powders. While the impact of cornstarch on glycemia in PBH has not been formally studied, we have anecdotally observed improved stability of blood glucose levels, particularly when consumed at bedtime to reduce nocturnal hypoglycemia.
Severity of illness
If dietary measures are insufficient to keep the patient safe, the patient should be counseled to see an endocrinologist for further diagnostic workup and possible treatment with medications.
Considerations for preoperative evaluation
With increasing recognition of the relatively high prevalence of PBH, we suggest that previous hypoglycemia should be queried during the preoperative history. One study demonstrated that patients who subsequently develop hypoglycemia after bariatric surgery have a lower glucose value during preoperative testing, still within the normal range [42, 43]. However, if a patient has signs or symptoms of hypoglycemia preoperatively (Table 1), we would recommend a full medical evaluation to guide decision-making about surgery and choice of procedure based on metabolic profiles. Until ongoing research identifies markers of individuals at high risk for hypoglycemia, this approach is unlikely to uncover all high-risk individuals.
SUMMARY AND CONCLUSIONS
Dietary modification is an essential first step in management of post-bariatric hypoglycemia. Guided by data from both available studies as well as long-term clinical experience, our recommendations focus on intake of controlled portions of low glycemic index carbohydrates, avoidance of rapidly-absorbed carbohydrates, choice of heart-healthy fats and ample protein, avoidance of alcohol and liquids with meals, and adjustment of meals and snack timing. It is important to recognize that this approach may not be sufficient in isolation, and individualization is usually required after assessment of initial response to dietary modification. Moreover, even with strict compliance, additional medical treatment aimed at reducing glucose spikes and stimulus for insulin secretion may be required. Additional studies of components of medical nutrition therapy will be helpful to further refine our management of this often-challenging clinical syndrome.
Acknowledgments
We gratefully acknowledge grant support from NIH R44 DK107114, NIH T32 DK007260, and P30 DK036836, and guidance and support from Jo-Anne Rizzotto.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
The authors acknowledge investigator-initiated grant support not related to this manuscript from Medimmune, MetaboDx, and Janssen, a collaboration with Xeris Pharmaceuticals (NIH SBIR grant), and consulting fees from Eiger Pharmaceuticals and Biomedical Insight.
REFERENCES
- 1.Goldfine AB, Patti ME. How common is hypoglycemia after gastric bypass? Obesity (Silver Spring) 2016;24(6):1210–1211. doi: 10.1002/oby.21520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen JB, et al. Prevalence, severity, and predictors of symptoms of dumping and hypoglycemia after Roux-en-Y gastric bypass. Surg Obes Relat Dis. 2016;12(8):1562–1568. doi: 10.1016/j.soard.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Ritz P, et al. Hypoglycaemia after gastric bypass: mechanisms and treatment. Diabetes Obes Metab. 2016;18(3):217–223. doi: 10.1111/dom.12592. [DOI] [PubMed] [Google Scholar]
- 4.Malik S, et al. Recognition and management of hyperinsulinemic hypoglycemia after bariatric surgery. Obes Res Clin Pract. 2016;10(1):1–14. doi: 10.1016/j.orcp.2015.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vilarrasa N, et al. Hyperinsulinemic Hypoglycemia after Bariatric Surgery: Diagnosis and Management Experience from a Spanish Multicenter Registry. Obes Facts. 2016;9(1):41–51. doi: 10.1159/000442764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sarwar H, et al. Hypoglycemia after Roux-en-Y gastric bypass: the BOLD experience. Obes.Surg. 2014;24(7):1120–1124. doi: 10.1007/s11695-014-1260-8. [DOI] [PubMed] [Google Scholar]
- 7.Goldfine AB, et al. Patients with neuroglycopenia after gastric bypass surgery have exaggerated incretin and insulin secretory responses to a mixed meal. J.Clin.Endocrinol.Metab. 2007;92(12):4678–4685. doi: 10.1210/jc.2007-0918. [DOI] [PubMed] [Google Scholar]
- 8.Seeley RJ, Chambers AP, Sandoval DA. The role of gut adaptation in the potent effects of multiple bariatric surgeries on obesity and diabetes. Cell Metab. 2015;21(3):369–378. doi: 10.1016/j.cmet.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salehi M, Prigeon RL, D’Alessio DA. Gastric bypass surgery enhances glucagon-like Peptide 1-stimulated postprandial insulin secretion in humans. Diabetes. 2011;60(9):2308–2314. doi: 10.2337/db11-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Craig CM, et al. Critical role for GLP-1 in symptomatic post-bariatric hypoglycaemia. Diabetologia. 2016 doi: 10.1007/s00125-016-4179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patti ME, Goldfine AB. The rollercoaster of post-bariatric hypoglycaemia. Lancet Diabetes Endocrinol. 2016;4(2):94–96. doi: 10.1016/S2213-8587(15)00460-X. [DOI] [PubMed] [Google Scholar]
- 12.Salehi M, Gastaldelli A, D’Alessio DA. Altered islet function and insulin clearance cause hyperinsulinemia in gastric bypass patients with symptoms of postprandial hypoglycemia. J Clin Endocrinol Metab. 2014;99(6):2008–2017. doi: 10.1210/jc.2013-2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patti ME, Li P, Goldfine AB. Insulin response to oral stimuli and glucose effectiveness increased in neuroglycopenia following gastric bypass. Obesity (Silver.Spring) 2015 doi: 10.1002/oby.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cryer PE, et al. Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2009;94(3):709–728. doi: 10.1210/jc.2008-1410. [DOI] [PubMed] [Google Scholar]
- 15.Mulla CM, et al. Insulinoma After Bariatric Surgery: Diagnostic Dilemma and Therapeutic Approaches. Obes Surg. 2016;26(4):874–881. doi: 10.1007/s11695-016-2092-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kellogg TA, et al. Postgastric bypass hyperinsulinemic hypoglycemia syndrome: characterization and response to a modified diet. Surg Obes Relat Dis. 2008;4(4):492–499. doi: 10.1016/j.soard.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Botros N, et al. Effect of carbohydrate restriction in patients with hyperinsulinemic hypoglycemia after Roux-en-Y gastric bypass. Obes Surg. 2014;24(11):1850–1855. doi: 10.1007/s11695-014-1319-6. [DOI] [PubMed] [Google Scholar]
- 18.Bantle AE, Wang Q, Bantle JP. Post-Gastric Bypass Hyperinsulinemic Hypoglycemia: Fructose is a Carbohydrate Which Can Be Safely Consumed. J Clin Endocrinol Metab. 2015;100(8):3097–3102. doi: 10.1210/jc.2015-1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seino Y, et al. Carbohydrate-induced secretion of glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1. J Diabetes Investig. 2016;7(Suppl 1):27–32. doi: 10.1111/jdi.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim MS, et al. ChREBP regulates fructose-induced glucose production independently of insulin signaling. J Clin Invest. 2016;126(11):4372–4386. doi: 10.1172/JCI81993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herman MA, Samuel VT. The Sweet Path to Metabolic Demise: Fructose and Lipid Synthesis. Trends Endocrinol Metab. 2016;27(10):719–730. doi: 10.1016/j.tem.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanaire H, et al. High glycemic variability assessed by continuous glucose monitoring after surgical treatment of obesity by gastric bypass. Diabetes Technol Ther. 2011;13(6):625–630. doi: 10.1089/dia.2010.0203. [DOI] [PubMed] [Google Scholar]
- 23.Meijeren JV, et al. Evaluation of carbohydrate restriction as primary treatment for post-gastric bypass hypoglycemia. Surg Obes Relat Dis. 2016 doi: 10.1016/j.soard.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 24.Kneepkens CM, Fernandes J, Vonk RJ. Dumping syndrome in children. Diagnosis and effect of glucomannan on glucose tolerance and absorption. Acta Paediatr Scand. 1988;77(2):279–286. doi: 10.1111/j.1651-2227.1988.tb10643.x. [DOI] [PubMed] [Google Scholar]
- 25.Speth PA, Jansen JB, Lamers CB. Effect of acarbose, pectin, a combination of acarbose with pectin, and placebo on postprandial reactive hypoglycaemia after gastric surgery. Gut. 1983;24(9):798–802. doi: 10.1136/gut.24.9.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jenkins DJ, et al. Effect of dietary fiber on complications of gastric surgery: prevention of postprandial hypoglycemia by pectin. Gastroenterology. 1977;73(2):215–217. [PubMed] [Google Scholar]
- 27.Bell KJ, et al. Impact of fat, protein, and glycemic index on postprandial glucose control in type 1 diabetes: implications for intensive diabetes management in the continuous glucose monitoring era. Diabetes Care. 2015;38(6):1008–1015. doi: 10.2337/dc15-0100. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen NQ, et al. Effects of Fat and Protein Preloads on Pouch Emptying, Intestinal Transit, Glycaemia, Gut Hormones, Glucose Absorption, Blood Pressure and Gastrointestinal Symptoms After Roux-en-Y Gastric Bypass. Obes Surg. 2016;26(1):77–84. doi: 10.1007/s11695-015-1722-7. [DOI] [PubMed] [Google Scholar]
- 29.Heber D, et al. Endocrine and nutritional management of the post-bariatric surgery patient: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2010;95(11):4823–4843. doi: 10.1210/jc.2009-2128. [DOI] [PubMed] [Google Scholar]
- 30.Moize VL, et al. Nutritional pyramid for post-gastric bypass patients. Obes Surg. 2010;20(8):1133–1141. doi: 10.1007/s11695-010-0160-9. [DOI] [PubMed] [Google Scholar]
- 31.Allied Health Sciences Section Ad Hoc Nutrition, C et al. ASMBS Allied Health Nutritional Guidelines for the Surgical Weight Loss Patient. Surg Obes Relat Dis. 2008;4(5 Suppl):S73–S108. doi: 10.1016/j.soard.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 32.Faria SL, et al. Dietary protein intake and bariatric surgery patients: a review. Obes Surg. 2011;21(11):1798–1805. doi: 10.1007/s11695-011-0441-y. [DOI] [PubMed] [Google Scholar]
- 33.Kimberly Mahoney MRDLDN. Protein Supplements & Weight-loss Surgery. 2016 [Google Scholar]
- 34.Tzovaras G, et al. Symptoms suggestive of dumping syndrome after provocation in patients after laparoscopic sleeve gastrectomy. Obes Surg. 2012;22(1):23–28. doi: 10.1007/s11695-011-0461-7. [DOI] [PubMed] [Google Scholar]
- 35.Tack J, et al. Pathophysiology, diagnosis and management of postoperative dumping syndrome. Nat.Rev.Gastroenterol.Hepatol. 2009 doi: 10.1038/nrgastro.2009.148. [DOI] [PubMed] [Google Scholar]
- 36.Gebhard B, et al. Postprandial GLP-1, norepinephrine, and reactive hypoglycemia in dumping syndrome. Dig.Dis.Sci. 2001;46(9):1915–1923. doi: 10.1023/a:1010635131228. [DOI] [PubMed] [Google Scholar]
- 37.van Beek AP, et al. Dumping syndrome after esophageal, gastric or bariatric surgery: pathophysiology, diagnosis, and management. Obes Rev. 2017;18(1):68–85. doi: 10.1111/obr.12467. [DOI] [PubMed] [Google Scholar]
- 38.Zaharieva DP, Riddell MC. Caffeine and glucose homeostasis during rest and exercise in diabetes mellitus. Appl Physiol Nutr Metab. 2013;38(8):813–822. doi: 10.1139/apnm-2012-0471. [DOI] [PubMed] [Google Scholar]
- 39.Pleus S, et al. Rate-of-Change Dependence of the Performance of Two CGM Systems During Induced Glucose Swings. J Diabetes Sci Technol. 2015;9(4):801–807. doi: 10.1177/1932296815578716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Opozda M, Chur-Hansen A, Wittert G. Changes in problematic and disordered eating after gastric bypass, adjustable gastric banding and vertical sleeve gastrectomy: a systematic review of pre-post studies. Obes Rev. 2016;17(8):770–792. doi: 10.1111/obr.12425. [DOI] [PubMed] [Google Scholar]
- 41.Kaufman FR, Halvorson M, Kaufman ND. Evaluation of a snack bar containing uncooked cornstarch in subjects with diabetes. Diabetes Res Clin Pract. 1997;35(1):27–33. doi: 10.1016/s0168-8227(96)01360-5. [DOI] [PubMed] [Google Scholar]
- 42.Pigeyre M, et al. Increased risk of OGTT-induced hypoglycemia after gastric bypass in severely obese patients with normal glucose tolerance. Surg.Obes Relat Dis. 2015;11(3):573–577. doi: 10.1016/j.soard.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 43.Nannipieri M, et al. Risk Factors for Spontaneously Self-Reported Postprandial Hypoglycemia After Bariatric Surgery. J Clin Endocrinol Metab. 2016;101(10):3600–3607. doi: 10.1210/jc.2016-1143. [DOI] [PubMed] [Google Scholar]