Abstract
Global targets aim to increase the number of people living with HIV (PLWH) who know their status. Using data from Mozambican facility-based HIV testing and counseling (HTC) and a population-based survey, we compared characteristics of PLWH diagnosed in HTC to the general population of PLWH to identify subgroups that are missing from the health system and may be undiagnosed. Male and female PLWH aged 50+ (PPR=0.47, p=0.0001) and with higher HIV knowledge (PPR=0.52, p=0.004) were underrepresented in HTC. A higher proportion of patients diagnosed in health facilities were aged 25–39 (PPR=1.23, p=0.02). Female PLWH with lower economic (PPR=0.70, p=0.04) and educational status (PPR=0.86, p=0.02), and male PLWH aged 18–24 (PPR=0.47, p=0.03) were underrepresented in HTC. Comparing HTC data to population-based data can inform efforts to increase HIV diagnoses and to ensure that all PLWH know their status.
Keywords: HIV testing, undiagnosed HIV, population-based surveys, implementation science
Introduction
UNAIDS 90-90-90 targets propose that 90% of people living with HIV (PLWH) know their status, 90% of people with diagnosed HIV infection receive treatment, and 90% of people receiving treatment have undetectable viral loads by 2020 (UNAIDS, 2014a). While a welcome feature of these targets is that they focus on quality of care across the HIV care continuum, their success hinges on ensuring PLWH know their status. Yet, coverage of diagnosis among PLWH remains low with only 45% of PLWH in sub-Saharan Africa (SSA) estimated to know their status (UNAIDS, 2014b).
Many studies have examined correlates of self-reported HIV testing history in SSA using data from nationally representative population-based surveys (e.g. AIDS Indicator Survey [AIS]) to increase testing uptake in the general population (Godif, Assefa, Alemayehu, & Terefe, 2015; Hutchinson & Mahlalela, 2006; Jean, Anglaret, Moh, Lert, & Dray-Spira, 2012; Ng’ang’a et al., 2014; Peltzer, Matseke, Mzolo, & Majaja, 2009; Ziraba et al., 2011). However, far less is known about correlates of diagnosis (and specifically undiagnosed HIV) among the sub-population of PLWH—information that is critical to meet global targets. While population-based surveys routinely test for HIV and ask participants about their testing history (i.e. have you been tested for HIV in the past 12 months), participants are rarely queried about the results of that test (Johnston et al., 2015). To our knowledge, of more than 45 such surveys completed in the past five years, only four collected specimens for HIV testing and asked participants the result of their most recent HIV test, allowing examination of correlates of undiagnosed HIV among PLWH (Fishel, Barrere, & Kishor, 2014; Kimanga et al., 2014; Ng’ang’a et al., 2014; The Namibia Ministry of Health and Social Services (MoHSS) & ICF International, 2014). In the absence of relevant population-based data, alternative approaches are needed to identify which PLWH are being reached by the health system and, importantly, which are not.
We combined data from a study which recruited participants following HIV diagnosis in health facilities in Mozambique with data from the most recent Mozambican AIS to compare characteristics of PLWH diagnosed in health facilities to the general population of PLWH.
Methods
Mozambique has an HIV prevalence of 11%, approximately 120,000 new infections per year, and 1.6 million PLWH (UNAIDS, 2013). As of 2011, only 26% of females and 14% of males aged 15–49 were tested for HIV in the last 12 months (Instituto Nacional de Estatistica Mozambique & MEASURE DHS/ICF International, 2011). No data are available on the proportion of PLWH with undiagnosed HIV.
The Engage4Health study is testing a package of interventions to improve linkage to and retention in HIV care at health facilities in Maputo City and Inhambane Province. Participants were enrolled from April 2013–June 2015 immediately following diagnosis in facility-based HIV testing and counseling (HTC) services. Eligibility criteria included being ≥18 years old, Portuguese or Xitsua language capacity, not currently pregnant, not planning to leave the community for 12 months, agreeing to be referred to HIV care services at the diagnosing facility, and not having enrolled in HIV care or initiated ART in the prior six months. Eligible individuals who consented to participate in the study completed closed-ended interviews. Ethical clearance for the study was provided by Columbia University and Mozambique’s National Committee for Bioethics in Health. Further study details are available elsewhere (Elul et al., 2014).
The 2009 Mozambique AIS was a nationally representative, population-based survey of adults aged 15–64 (ICF Macro & Instituto Nacional de Estatistica Mozambique, 2010). Participants completed a closed-ended questionnaire, and those who consented to an HIV test had blood collected for subsequent processing at a central lab. To compare the samples, we restricted the AIS sample to individuals who tested HIV-positive, resided in Maputo City or Inhambane Province, and met the Engage4Health eligibility criteria.
Characteristics of individuals testing positive in HTC from the Engage4Health study and PLWH from the general population from AIS were compared. We compared socio-demographic characteristics and theorized determinants of HIV diagnosis available in both datasets including: age, sex, education, marital status, household assets, number of children, recent hospitalizations, and HIV knowledge. Univariate statistics were calculated for each sample and compared using chi-square tests or t-tests. Analyses were further stratified by sex. We assessed how well specific subgroups were represented in the health system by calculating program-to-population ratios (PPR). These ratios compare subgroup proportions of PLWH diagnosed in HTC to the proportion in the general population. Ratios <1 indicate subgroup underrepresentation in the health system relative to the burden of disease in the general population, while ratios >1 suggest subgroup overrepresentation in HTC. Stated differently, ratios <1 highlight subgroups of PLWH which may be unaware of their infection and should be targeted for testing campaigns. Analyses were performed in STATA 13, accounting for the cluster design of both surveys.
Results
There were 2,004 participants in the Engage4Health sample, while in the AIS sample, 287 (19.6%) of the 1,466 individuals testing HIV-positive met the inclusion criteria. Table 1 presents the characteristics of participants in each sample.
Table 1.
Comparison of Engage4Health study sample to AIS study sample
| Engage4Health (N=2004) | AIS (N=287) | p-value | |||
|---|---|---|---|---|---|
|
| |||||
| (N) | (%) | (N) | (%)a | ||
| Age, mean, SD | 34.21 | 0.21 | 36.81 | 0.85 | 0.01* |
| 18–24 | 263 | 13.12 | 41 | 14.29 | 0.50 |
| 25–39 | 1235 | 61.63 | 144 | 50.17 | 0.02* |
| 40–49 | 348 | 17.37 | 54 | 18.82 | 0.86 |
| 50+ | 158 | 7.88 | 48 | 16.72 | 0.0001*** |
| Sex | |||||
| Male | 711 | 35.48 | 92 | 32.58 | |
| Female | 1293 | 64.52 | 195 | 67.42 | 0.39 |
| Level of education | |||||
| None/primary school | 1314 | 56.60 | 199 | 71.94 | 0.14 |
| Secondary school and beyond | 689 | 34.40 | 88 | 28.06 | |
| Marital status | |||||
| Never married | 167 | 8.34 | 26 | 11.45 | 0.33 |
| Married and/or living together | 1119 | 55.87 | 147 | 53.24 | 0.57 |
| Widowed | 181 | 9.04 | 24 | 8.04 | 0.73 |
| Divorced/not living together | 536 | 26.76 | 80 | 27.87 | 0.91 |
| Household does not have electricity | 686 | 34.23 | 127 | 46.86 | 0.09 |
| Household does not have a radio | 623 | 31.09 | 106 | 38.35 | 0.09 |
| Number of children | 2.44 | 0.05 | 2.47 | 0.13 | 0.94 |
| Hospitalized in last 12 months | 111 | 5.54 | 23 | 8.14 | 0.19 |
| Correct HIV knowledge: disagree there is a cure for HIV | 719 | 35.99 | 191 | 69.07 | 0.004** |
=sampling weight proportion;
p<0.05,
p<0.01,
p<0.001
Relative to the general population of PLWH identified in the AIS, older PLWH (≥50 years, PPR=0.47, p=0.0001) and PLWH with higher knowledge of HIV (PPR=0.52, p=0.004) were underrepresented in facility-based HTC (Figure 1). A higher proportion of patients diagnosed in HTC were aged 25–39 (PPR=1.23, p=0.02). Similar to the non-stratified analysis, both older aged male and female PLWH (males: PPR=0.55, p=0.01; females: PPR=0.34, p=0.0001;) and those who correctly believed there is no cure for HIV (males: PPR=0.49, p<0.0001; females: PPR=0.53, p<0.0001) were underrepresented in HTC. For females, PLWH with lower education (PPR=0.86, p=0.02) and residing in households without electricity (PPR=0.70, p=0.04) were underrepresented in facility-based HTC. For males, younger PLWH (18–24 years, PPR=0.47, p=0.03) were underrepresented, while males aged 25–39 were overrepresented (PPR=1.26, p=0.04).
Figure 1.
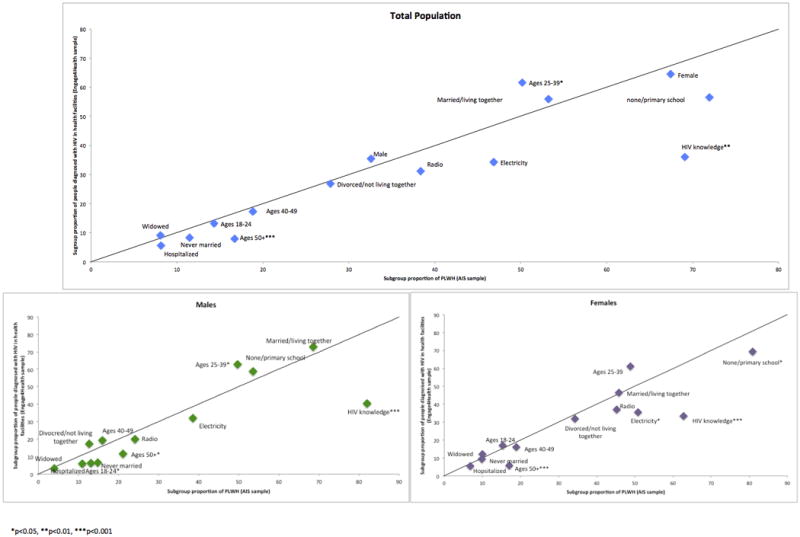
People diagnosed with HIV in health facilities compared to the general population of PLWH: Program-to-population ratios
Discussion
We compared correlates of HIV diagnosis from a facility-based HTC sample to a population-based sample in Mozambique to highlight subgroups of PLWH which are potentially being missed by the health system. We found that older PLWH and those who believed HIV cannot be cured were underrepresented in facility-based HTC. These trends persisted in sex-stratified analyses. Additionally, among females, PLWH of lower socioeconomic status and educational attainment were underrepresented in HTC, highlighting the particular vulnerability of poor HIV-positive women. Among males, those aged 18–24 were missing from HTC. Programs should target these subgroups for HIV testing to increase participation in HTC and community-based testing campaigns.
Similar to the only other published study exploring correlates of undiagnosed HIV (using Kenya AIS data), age emerged as an important facilitator and barrier to HIV diagnosis among PLWH in our study (Ng’ang’a et al., 2014). While in the Kenyan analysis, PLWH aged 15–24 were less likely to have undiagnosed HIV, in our study male PLWH aged 18–24 were found to be underrepresented in facility-based HTC. As the Kenyan analysis was not disaggregated by sex, their findings may be driven by substantial testing of young pregnant women in antenatal care which obscured a pattern of undiagnosed HIV in young men. Low uptake of testing among young men has been consistently noted (Staveteig, Shanxiao, Head, Bradley, & Nybro, 2013). Additionally, we found that both male and female PLWH ≥50 years of age were underrepresented in facility-based HTC, which further buttresses UNAIDS’ efforts to draw attention to this neglected group (UNAIDS, 2014b). Consistent with the Kenya AIS and studies on health-care seeking behaviors generally (Hutchinson & Mahlalela, 2006; Njuki, Kimani, Obare, & Warren, 2014; Peltzer & Matseke, 2014; Ziraba et al., 2011), we found socioeconomic differentials in diagnosis patterns (Ng’ang’a et al., 2014), likely reflecting additional barriers to care for the poor (Kiwanuka et al., 2008). Surprisingly, our analysis suggests that PLWH who correctly believe there is not a cure for HIV (a proxy for correct HIV knowledge) are underrepresented in the health system. Other studies have shown HIV knowledge, measured using composite measures, to be a positive predictor of testing behavior (Jean et al., 2012; Ziraba et al., 2011). It is possible that operationalizing HIV knowledge using a single question inadequately captured HIV knowledge.
Our study has important strengths. First, we examined correlates of HIV diagnosis among PLWH, as opposed to correlates of HIV testing, thus providing insights into which subgroups of PLWH may be undiagnosed and/or underrepresented in the health system. Second, our study relied on biological samples to determine HIV status, and thus was not subject to social desirability bias (Fishel et al., 2014). A few limitations should also be noted. The AIS sample may include PLWH with known HIV status, in addition to those with undiagnosed HIV. However, a sensitivity analysis restricting the AIS sample of PLWH to those who had never been tested for HIV revealed similar findings. Our comparison was limited by the variables available in both datasets. For example, we could not explore the role of alcohol use, stigma, and sexual behaviors, which have been associated with HIV testing in the general population (Hutchinson & Mahlalela, 2006; Ziraba et al., 2011). Additionally, the facility-based sample was recruited from HTC where non-pregnant adults tend to self-refer for testing. Other testing points likely capture PLWH with different characteristics. Further, the facility-based sample was derived from an implementation science study and thus may not represent the larger population testing positive at health facilities. Lastly, there was a time lag between the two samples. It is possible that characteristics of PLWH in Mozambique changed over time, and secular trends account for some of the observed differences.
Ensuring that PLWH know their status is the first step in the HIV continuum of care. In the absence of relevant population-based data on undiagnosed HIV, comparing characteristics of individuals diagnosed with HIV in testing programs to those PLWH identified in population-based surveys can highlight populations which might be unaware of their infection and should be targeted in HIV testing campaigns.
Acknowledgments
Funding: This study was funded by United States Agency for International Development (USAID), USAID Award Number: AID-OAA-A-12-00027 and the National Institute of Allergy & Infectious Diseases of the National Institutes of Health under award number T32AI114398 (SK). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of USAID or the National Institutes of Health.
References
- Elul B, Lahuerta M, Abacassamo F, Lamb MR, Ahoua L, McNairy ML, Jani I. A combination strategy for enhancing linkage to and retention in HIV care among adults newly diagnosed with HIV in Mozambique: study protocol for a site-randomized implementation science study. BMC Infectious Diseases. 2014;14(1):549. doi: 10.1186/s12879-014-0549-5. http://doi.org/10.1186/s12879-014-0549-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishel JD, Barrere B, Kishor S. Validity of data on self-reported HIV status in Malawi and Uganda and implications for measurement of ARV coverage. Rockville, Maryland: 2014. (DHS Methodological Reports No. 10). Retrieved from http://dhsprogram.com/pubs/pdf/MR10/MR10.pdf. [Google Scholar]
- Godif M, Assefa H, Alemayehu M, Terefe W. Factors Associated with HIV Counseling and Testing among Males and Females in Ethiopia: Evidence from Ethiopian Demographic and Health Survey Data. Journal of AIDS & Clinical Research. 2015;6(3):1–11. http://doi.org/10.4172/2155-6113.1000429. [Google Scholar]
- Hutchinson PL, Mahlalela X. Utilization of voluntary counseling and testing services in the Eastern Cape, South Africa. AIDS Care. 2006;18(5):446–455. doi: 10.1080/09540120500213511. http://doi.org/10.1080/09540120500213511. [DOI] [PubMed] [Google Scholar]
- ICF Macro & Instituto Nacional de Estatistica Mozambique. Insida 2009 2010 [Google Scholar]
- Instituto Nacional de Estatistica Mozambique, & MEASURE DHS/ICF International. Inquerito Démografico de Sáude Moçambique (DHS 2011) 2011:412. [Google Scholar]
- Jean K, Anglaret X, Moh R, Lert F, Dray-Spira R. Barriers to HIV testing in Côte d’ivoire: The role of individual characteristics and testing modalities. PLoS ONE. 2012;7(7):12–18. doi: 10.1371/journal.pone.0041353. http://doi.org/10.1371/journal.pone.0041353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LG, Mcfarland W, Sabin L, Prybylski D, Sabin K, Baral S, Raymond HF. Measuring self-reported HIV status in bio-behavioural surveys. WHO Bulletin. 2015 doi: 10.2471/BLT.15.153064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS) Global Report: UNAIDS report on the global AIDS epidemic 2013. Geneva, Switzerland: UNAIDS; 2013. [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90 An ambitious treatment target to help end the AIDS epidemic. Geneva, Switzerland: UNAIDS; 2014a. [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS) The Gap Report. Geneva, Switzerland: UNAIDS; 2014b. [Google Scholar]
- Kimanga DO, Ogola S, Umuro M, Ng’ang’a A, Kimondo L, Murithi P, Kim AA. Prevalence and Incidence of HIV Infection, Trends, and Risk Factors Among Persons Aged 15–64 Years in Kenya: Results From a Nationally Representative Study. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2014;66:S13–S26. doi: 10.1097/QAI.0000000000000124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiwanuka SN, Ekirapa EK, Peterson S, Okui O, Rahman MH, Peters D, Pariyo GW. Access to and utilisation of health services for the poor in Uganda: a systematic review of available evidence. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2008;102(11):1067–74. doi: 10.1016/j.trstmh.2008.04.023. http://doi.org/10.1016/j.trstmh.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Ng’ang’a A, Waruiru W, Ngare C, Ssempijja V, Gachuki T, Njoroge I, Kim AA. The status of HIV testing and counseling in Kenya: results from a nationally representative population-based survey. Journal of Acquired Immune Deficiency Syndromes. 2014;66(Suppl 1):S27–36. doi: 10.1097/QAI.0000000000000102. http://doi.org/10.1097/QAI.0000000000000102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njuki R, Kimani J, Obare F, Warren C. Using verbal and social autopsies to explore health-seeking behaviour among HIV-positive women in Kenya: A retrospective study. BMC Women’s Health. 2014;14(1):1–11. doi: 10.1186/1472-6874-14-77. http://doi.org/10.1186/1472-6874-14-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltzer K, Matseke G. Determinants of HIV testing among young people aged 18 – 24 years in South Africa. African Health Sciences. 2014;13(4):1012–1020. doi: 10.4314/ahs.v13i4.22. Retrieved from http://www.ajol.info/index.php/ahs/article/view/100856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltzer K, Matseke G, Mzolo T, Majaja M. Determinants of knowledge of HIV status in South Africa: results from a population-based HIV survey. BMC Public Health. 2009;9:174. doi: 10.1186/1471-2458-9-174. http://doi.org/10.1186/1471-2458-9-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staveteig S, Shanxiao W, Head SK, Bradley SE, Nybro E. (DHS Comoparative Reports No. 30).Demographic Patterns of HIV Testing Uptake in Sub-Saharan Africa. 2013 Apr;:1–81. [Google Scholar]
- The Namibia Ministry of Health and Social Services (MoHSS), & ICF International. The Namibia demographic and health survey 2013. Windhoek, Namibia and Rockville, Maryland, USA: 2014. Retrieved from https://dhsprogram.com/pubs/pdf/FR298/FR298.pdf. [Google Scholar]
- Ziraba AK, Madise NJ, Kimani JK, Oti S, Mgomella G, Matilu M, Ezeh A. Determinants for HIV testing and counselling in Nairobi urban informal settlements. BMC Public Health. 2011;11(1):663. doi: 10.1186/1471-2458-11-663. http://doi.org/10.1186/1471-2458-11-663. [DOI] [PMC free article] [PubMed] [Google Scholar]


