Abstract
Background
The purpose of this study was to review long-term outcomes of sinonasal adenoid cystic carcinoma (SNACC) and to clarify its association with human papilloma virus (HPV).
Methods
The medical records of 23 patients with SNACC treated with primary surgical resection between 1998 and 2013 were reviewed. Tissue specimens were available for 17 patients. p16 testing was performed using immunohistochemistry and HPV infection was determined using quantitative polymerase chain reaction with primers targeting the E6/E7 region.
Results
Two of the 17 samples showed strong and diffuse p16 staining, while the remaining 15 cases showed p16 positivity isolated to the luminal cells. Only one of the p16 positive cases was positive for HPV. The 5-year local failure, disease-free survival, and overall survival were 51%, 52%, and 62%, respectively.
Conclusions
Local failures are common with advanced SNACC, and the association of HPV with true SNACC is low.
Keywords: adenoid cystic carcinoma, paranasal sinus neoplasms, human papilloma virus, radiotherapy, p16
Introduction
Sinonasal adenoid cystic carcinoma (SNACC) is a rare malignancy characterized by an insidious growth pattern and a tendency for perineural spread along major and minor nerves, resulting in invasion of the skull base as well as intracranial extension.1,2 Therefore, many patients present with advanced disease with involvement of critical structures making treatment difficult and potentially morbid.2 Local recurrence and distant metastasis can occur decades after definitive treatment making some consider adenoid cystic carcinoma (ACC) to be incurable.1,3–5
Infection by high-risk human papilloma virus (HPV) has been identified as a causative agent for squamous cell carcinoma (SCC), particularly in the oropharynx.6 Recently, it has been demonstrated that the incidence is increasing, with estimates that up to 60–70% of oropharynx SCCs are HPV-related.7,8 HPV-related SCC of the oropharynx is associated with a 58% reduction in risk of death when compared with patients with HPV-negative tumors.9 Because of the favorable prognosis of patients with HPV-related oropharynx cancers, there are numerous trials investigating new treatment strategies for HPV-related cancer including the use of alternative chemotherapy agents, induction chemotherapy, and using lower doses of radiation.10,11
The presence of high-risk HPV as a potential causative agent in sinonasal cancer is also being investigated. A recent series suggests that up to 21% of sinonasal carcinomas are HPV-related.12,13 Moreover, patients with sinonasal SCC associated with HPV were found to have improved progression-free (PFS) and overall survival (OS) when compared with their HPV-negative counterparts.13 ACC of the cervix has previously been found to be associated with high risk HPV.14,15 A limited number of studies have investigated the association of high-risk HPV and p16 expression, which is a surrogate marker for the presence of HPV, with SNACC.12,16,17 One study by Bishop et al. identified and characterized an HPV positive sinonasal carcinoma with adenoid cystic-like features.16 In that study, 8 cases, all localized to the sinonasal tract, were found to resemble ACC histologically and were positive for HPV with strong and diffuse p16 immunoreactivity. The purpose of this study was to review our long-term outcomes of patients with SNACC including treatment response, patterns of recurrence, and treatment toxicity. In addition, we sought to further clarify the association of HPV in SNACC.
Materials and Methods
Patient Selection
Following approval from the institutional review board at the Ohio State University James Cancer Hospital, we identified 44 patients with biopsy-confirmed SNACC who were consecutively evaluated for radiation therapy (RT) between 1998 and 2013 using electronic treatment records maintained by the Department of Radiation Oncology. After excluding patients <18 years old and those not treated definitively, 23 patients were left for analysis. Data for the cohort was collected through electronic and paper record review.
Treatment
For each patient, a full medical history and physical examination was completed. Biopsy was obtained as part of the workup for all patients. Imaging of the head/neck and chest was performed as part of the staging with the imaging modality dependent on the time period of treatment. Patients were staged according to the 2010 American Joint Committee on Cancer staging system. The surgical treatment as well as any adjuvant therapy was recorded. RT was delivered either at OSU or at an outside institution. Patients were treated with either 2D, 3D conformal RT, or intensity modulated radiation therapy (IMRT) based on the treatment decade. Several patients were also referred for proton therapy at outside institutions. Intraoperative radiation therapy (IORT) was used at the discretion of the surgeon and radiation oncologist for tumors located adjacent to critical structures or for close surgical margins. All RT was delivered postoperatively with a waiting period between surgery and RT of 4–6 weeks. Patients were evaluated for recurrence by clinical exam and repeat imaging of the primary site.
Laboratory analysis
Of the 23 patients in the analysis, formalin-fixed and paraffin embedded (FFPE) tissue specimens from the clinical archives were available for 17 of the patients. The pathologist (BJS) selected the paraffin blocks with the largest portion of tumor for this study. Each sample specimen was cut into 4 μm sections and 10 μm curls serially. Four-micrometer sections were prepared for hematoxylin and eosin (H&E) and p16 immunohistochemistry (IHC) staining. Before the 10 μm thickness curls were cut for DNA extraction, one section was cut for H&E staining to confirm the presence of tumor in that section. The 10 μm curls were stored in 2 ml tubes at 4°C preceding DNA processing.
DNA isolation
DNA was extracted from 17 FFPE specimens by deparaffinization with octane, proteinase K digestion, phenol–chloroform extraction, and ethanol precipitation. Briefly, after octane (Sigma-Aldrich, St. Louis, MO, USA) exposure, the sample was washed with 100% ethanol, resuspended in 200 μL of digestion buffer (50 mM Tris–HCl at pH 8.5, 1 mM EDTA, and proteinase K at 0.4 μg/mL), and incubated overnight at 55°C. If tissue remained, 4 μL of proteinase K (20 mg/mL) was added, and digestion was continued for an additional 24–48 hours. Proteinase K solution was inactivated by incubating the tubes at 95°C for 10 minutes. DNA was extracted in phenol–chloroform by use of Qiagen MaXtract tubes (Qiagen, Germantown, MD, USA) and ethanol precipitated in the presence of glycogen (0.02 mg/mL). Pellets were washed once in 70% ethanol, dried in a 37°C heat block, and resuspended in 50 μL of diethylene pyrocarbonate (DEPC)-treated water. DNA quantity and purity (calculated by use of the ratio of the absorbance at 260 nm to that at 280 nm [260/280 ratio]) were measured with the Nanodrop spectrophotometer (Thermo Fisher Scientific, Inc, Wilmington, DE, USA).
Human Papilloma Virus Type-specific Quantitative PCR
HPV viral load in tumor tissue for types 16, 18, 31, 33 and 35 were measured. HPV type-specific PCR was performed using TaqMan Quantitative RT-PCR (qPCR) in ABI’s 7300 real-time PCR systems (Applied Biosystems, Foster City, CA, USA) as previously described.18,19 The primers and probe for amplification were designed to target on the E6 and/or E7 region and were synthesized by Integrated DNA Technologies (Coralville, IA, USA). Probes were labeled with 6-carboxy-fluorescein (FAM) at the 5′ end and with Black Hole Quencher-1 (BHQ1) at the 3′ end. The sequences for primers and probes have been previously published.20 Standard curves for amplification reactions were generated in duplicate by using a fivefold dilution series (from 250,000 to 3.2 copies) of pUC57 vector (GenScript, Piscataway, NJ, USA) containing the complete E6 and E7 region in a background of human placental DNA (5 ng/μL). Each PCR reaction contained 2 × TaqMan universal PCR master mixes (Applied Biosystems, Branchbury, NJ, USA), 0.1 μmol of probe, 0.2 μmol of each primer, and 2 μL of purified tumor DNA or tumor RNA reverse transcribed cDNA. Amplification conditions included one cycle of 2-minute incubation at 50°C (degradation of the uracil containing DNA) and 10-minute incubation at 95°C, followed by 50 cycles of 15 seconds at 95°C and 60 seconds at 60°C. The final dissociation curve analysis used the default program setting.
An estimate of cell numbers in each of the samples was tested by TaqMan real-time PCR targeting on a single-copy human gene on chromosome 7, human endogenous retrovirus 3 (ERV3).21 The 58-bp ERV3 fragment was amplified for 17 samples and the reaction conditions were as previously published.18 Briefly, 2 μL of purified tumor tissue DNA was analyzed. A standard curve was generated in duplicate from a five-fold dilution series (from 150,000 to 1.92 cells) of a diploid human cell line, CCD-18LU (ATCC, Manassas, VA, USA). Results were reported as the number of human diploid genome equivalents of purified genomic DNA from tumor samples that were evaluated for HPV type(s) DNA by quantitative PCR.
p16 immunohistochemistry
Samples were also evaluated using immunohistochemistry (IHC) for expression of a surrogate biomarker of HPV E7 oncoprotein function, the cdk inhibitor p16. Automated p16 IHC stain was carried out in the BenchMark XT auto-immunostainer (Ventana, Tucson, AZ, USA). A standardized staining protocol was provided by Ventana for the CINtec p16 Histology kit (MTM Laboratories Inc., Heidelberg, Germany). Briefly, slides were deparaffinized and retrieved at 95°C for 30 minutes. 100 μL of prediluted monoclonal mouse anti-human p16INK4a was placed on the slides and incubated at 37°C for 32 minutes. iView DAB detection kit (Ventana Medical Systems Inc., Tucson, AZ, USA) was used as secondary detection. Slides were then counterstained with hematoxylin and bluing reagent, and mounted under coverslips. All slides were reviewed by a single pathologist (BJS). Slides were considered positive for p16 if immunostaining was present in ≥70% of the tumor cells as previously described.20
Statistical Analysis
Local failure (LF) was defined as the time from the final treatment date to the date of recurrence in the treatment field based on biopsy or imaging. Disease-free survival (DFS) was defined as the time from the final treatment date to the date of disease recurrence either locally or distantly. OS was defined as the time from the date of diagnosis until the date of death. Survival curves were estimated using the method of Kaplan-Meier. The log-rank test was used to compare between the survival curves. All statistical tests were 2-sided and p<0.05 was considered statistically significant. All statistical analyses were performed using SAS 9.4 (SAS, inc; Cary, NC, USA).
Results
Patient Characteristics and Survival Analysis
Patient and tumor characteristics are summarized in Table 1. The median age at presentation was 55 years (range 20–73 years) with the majority of patients being male (65%). The most common presenting symptoms included pain, epistaxis, nasal obstruction, and headaches. Twenty patients (87%) were evaluated for a primary diagnosis while 3 (13%) presented with recurrent disease. The most common primary tumor sites were the maxillary sinus (52%) and nasal cavity (35%) with the majority of patients presenting with clinical T4 disease (57%). All patients were clinically without evidence of distant metastasis on preoperative workup.
Table 1.
Patient Characteristics
| Characteristic | No. of patients (%) |
|---|---|
| Age (years), Median (range) | 55 (20–73) |
| Gender, Female;Male | 8/23 (35);15/23 (65) |
| Presenting diagnosis | |
| Primary Diagnosis | 20/23 (87) |
| Recurrent Disease | 3/23 (13) |
| Presenting symptom | |
| Pain | 8/23 (35) |
| Epistaxis | 7/23 (30) |
| Nasal obstruction | 6/23 (26) |
| Headaches | 5/23 (22) |
| Primary tumor site | |
| Maxillary sinus | 12/23 (52) |
| Nasal cavity | 8/23 (35) |
| Ethmoid sinus | 2/23 (9) |
| Sphenoid sinus | 1/23 (4) |
| Clinical TNM classification | |
| T1 | 1/23 (4) |
| T2 | 1/23 (4) |
| T3 | 8/23 (35) |
| T4 | 13/23 (57) |
| N0 | 22/23 (96) |
| N1 | 1/23 (4) |
| M0 | 23/23 (100) |
| Histology | |
| Tubular | 4/23 (17) |
| Cribriform | 14/23 (61) |
| Solid | 5/23 (22) |
| Perineural Invasion | |
| Yes | 18/23 (78) |
| No | 5/23 (22) |
All patients were treated with primary surgical resection. Total maxillectomy was performed in 9 patients (39%) and partial maxillectomy was performed in 9 patients (39%). Craniofacial resection was required in 4 patients (17%) while endoscopic endonasal resection was completed in 3 patients (13%). Orbital exenteration was required in 1 patient. Final pathology revealed that 5 patients (22%) had solid histology and 14 patients (61%) had cribriform histology. Perineural invasion was present in 18 of the specimens (78%) and bone invasion was observed in 22 cases (96%). R0 resections were accomplished in 8 patients (35%), R1 resections in 14 patients (61%), and 1 patient had an R2 resection.
Eighteen patients received radiation therapy as part of their treatment. Fourteen patients (61%) received postoperative EBRT alone, 1 patient received an IORT boost in addition to postoperative EBRT, and 1 patient received an IORT boost alone. Two patients (9%) went to outside institutions for proton therapy. Five patients (22%) did not receive radiation therapy. Three patients refused adjuvant RT, 1 patient could not complete adjuvant RT because of a prior history of RT to the right orbit for non-Hodgkin’s lymphoma, and 1 patient had early stage disease (T1N0M with negative surgical margins and no evidence of perineural invasion) and adjuvant RT was not indicated. The median EBRT dose was 60 Gy with a range of 40–72 Gy. The median IORT dose was 15 Gy with 1 patient treated with electrons and 1 patient treated with high-dose-rate IORT. Two patients (9%) were treated with concurrent chemotherapy. One patient received weekly cisplatin while the second patient was treated with carboplatin/paclitaxel.
Two patients did not complete radiation therapy despite the recommendation for adjuvant therapy including a patient with a T2 primary who failed locally 26 months after surgical resection. Eleven of 23 patients (48%) developed a local failure, 11 patients (48%) developed a distant failure, and 3 patients developed both local and distant failures. Twelve patients (52%) ultimately died from disease. At a median follow-up of 56 months (range 6–156 months), the 5-year LF, DFS, and OS were 51% (95% CI 26–72%), 52% (95% CI 27–71%), and 62% (95% CI 37–79%). Kaplan-Meier curves for LF, DFS, and OS for the patient cohort are shown in Figure 1. On univariate analysis, African American patients were found to have a significantly lower median OS (155 vs. 32 months, p=0.03) and DFS (80 vs. 31 months, p=0.01) when compared with Caucasian patients.
Figure 1.
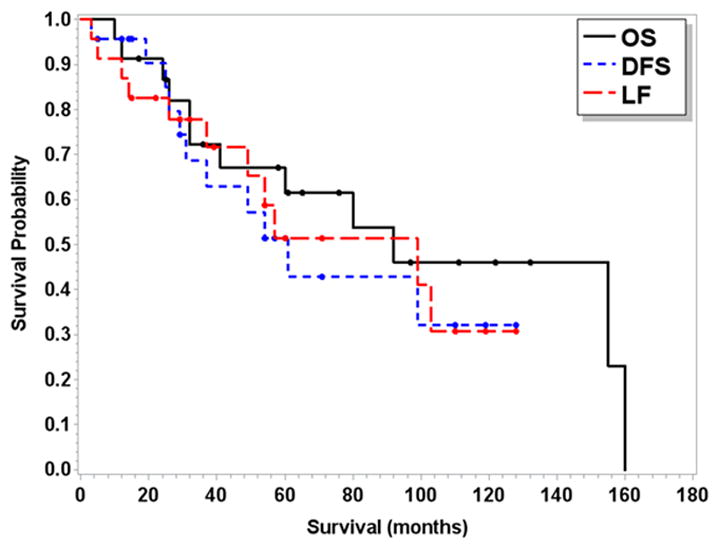
Kaplan-Meier curves for entire patient cohort including local failure (LF), disease-free survival (DFS), and overall survival (OS).
Toxicity from treatment was observed in 39% of patients with 67% of toxicities occurring in long-term survivors more than 12 months from the end of treatment. Toxicities which occurred less than 6 months from the end of treatment included trismus requiring coronoidectomy (2 patients), epidural abscess (1 patient), and dacryocystorhinostomy for nasolacrimal duct obstruction (2 patients). Toxicities which occurred more than 12 months from the completion of treatment included osteoradionecrosis (5 patients), recurrent nasolacrimal duct obstruction (2 patients), chronic nasocutaneous fistula (1 patient), and flap dehiscence requiring revision surgery (1 patient).
Human Papilloma Virus DNA Detection and p16 Immunohistochemical Analysis
Seventeen patient samples were available for p16 IHC and HPV testing. Table 2 summarizes the clinical site and stage of each patient in the cohort with tissue available, the treatment administered, the p16/HPV status, and the clinical outcome. Two of the samples were determined to be p16 positive, while the remaining 15 samples were p16 negative by IHC. Figure 2 shows an example of a p16 positive and negative case. In both the p16 negative and positive cases (Figures 2A and 2C), the tumors had solid histology with perineural invasion present on H & E. Squamous dysplasia of the surface epithelium was not present in any of the cases reviewed consistent with true adenoid cystic carcinoma. The p16 staining in the negative case (Figure 2B) was observed to be isolated to the true luminal cells with all 15 of the p16 negative cases exhibiting a similar staining pattern. In the p16 positive cases, the staining was strong and diffuse nuclear and cytoplasmic staining involving ≥70% of the tumor cells as shown in Figure 2D. One of the p16 positive cases was positive for HPV type 33 using qPCR with a low copy number (9 copies). None of the other cases were positive for HPV types 16, 18, 31, 33 or 35.
Table 2.
Clinical Presentation, Treatment, p16/HPV Status, and Outcome for Patients with Available Tumor Tissue
| Site | TNM classification | Grade | Therapy | p16 status | p16 intensity | % of p16+ tumor cells | HPV status | Local failure | Distant failure | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|
| Maxillary sinus | T4N0M0 | Cribriform | S+RT | − | 2 | 30 | − | Yes | Yes | DOD |
| Maxillary sinus | T4N0M0 | Cribriform | S+IORT+RT | + | 3 | 70 | − | Yes | Yes | M |
| Maxillary sinus | T4N0M0 | Cribriform | S | − | 2 | 60 | − | No | Yes | DOD |
| Maxillary sinus | T4N0M0 | Cribriform | S+RT | − | 3 | 20 | − | Yes | No | DOD |
| Maxillary sinus | T4N0M0 | Solid | S+RT | − | 3 | 50 | − | No | No | NED |
| Maxillary sinus | T4N0M0 | Cribriform | S+RT | − | 2 | 40 | − | Yes | Yes | DOD |
| Maxillary sinus | T3N0M0 | Cribriform | S | − | 2 | 10 | − | Yes | Yes | DOD |
| Maxillary sinus | T3N0M0 | Solid | S+IORT | − | 3 | 20 | − | No | Yes | DOD |
| Maxillary sinus | T3N0M0 | Cribriform | S+RT | − | 2 | 30 | − | Yes | Yes | M |
| Nasal cavity | T4N0M0 | Cribriform | S+RT | − | 2 | 50 | − | No | No | NED |
| Nasal cavity | T4N0M0 | Solid | S+RT | − | 2 | 10 | − | Yes | Yes | DOD |
| Nasal cavity | T3N0M0 | Cribriform | S+CRT | − | 3 | 50 | − | No | No | NED |
| Nasal cavity | T3N0M0 | Cribriform | S+RT | − | 2 | 10 | − | No | No | NED |
| Nasal cavity | T2N0M0 | Solid | S | + | 3 | 100 | + | Yes | Yes | DOD |
| Nasal cavity | T1N0M0 | Cribriform | S | − | 3 | 60 | − | No | No | NED |
| Sphenoid sinus | T4N0M0 | Cribriform | S+RT | − | 3 | 40 | − | No | Yes | DOD |
| Ethmoid sinus | T3N0M0 | Tubular | S+RT | − | 3 | 50 | − | Yes | No | DOD |
Abbreviations: CRT=concurrent chemoradiation; DOD=dead of disease; IORT=intraoperative radiation therapy; M=metastatic disease; NED=no evidence of disease; RT=external beam radiation therapy; S=surgery
Figure 2.
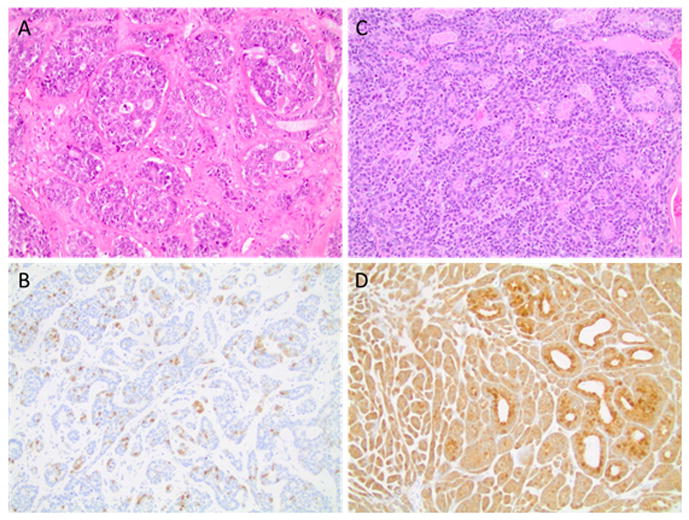
Hematoxylin and eosin and p16 immunohistochemistry staining of a p16 negative (panels A, B) and a p16 positive (panels C, D) sinonasal adenoid cystic carcinoma case. Note, the case shown in C, D was also positive for HPV 33. All magnifications x 20.
Discussion
SNACC is an insidious tumor that often presents at an advanced stage making treatment difficult. The propensity of ACC for perineural invasion also presents a challenge for obtaining negative surgical margins. Lupinetti et al. published one of the largest single institutional series on SNACC, consisting of 105 patients.2 The majority of patients were T3/T4 (76.7%) and the local recurrence rate and distant metastasis rates were 30% and 38%, respectively. The 5-year OS and disease-specific survival rates were 62.9% and 70.9%, respectively. A systematic review consisting of both individual and aggregate SNACC patient data reported a 5-year survival rate of 62.5%.22 A retrospective cohort study using the United States National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) registry identified a total of 412 cases between 1973 and 2009. The 5-year OS rate was 68.8% with African Americans having inferior survival compared to other races, which is consistent with our findings. As in all SEER studies, limited information about the clinical characteristics of these patients was available including tumor stage and treatment performed. The cohort reported in the present study consisted of patients with almost exclusive T3/T4 (92%) tumors with a high percentage of perineural invasion (78%) and R1/R2 resections (65%). Our LF rate was 50% and the 5-year OS rate was 62%. While the LF rate in our cohort was higher likely due to the higher proportion of patients with advanced disease, the OS of our patients was consistent with that reported in the literature.
With a long median follow-up time, we were able to report on late toxicity in long-term survivors. Patients tolerated treatment well with 2 cases of postoperative infections occurring within 4 months of treatment completion. The majority of treatment toxicity (67% of recorded events) occurred 12 months or more from the completion of treatment, demonstrating the late sequelae of treatment. Lupinetti et al. reported an overall treatment complication rate of 15.2% with a surgical complication rate of 6.7%. The complications listed were similar to those reported in our series and included osteoradionecrosis, trismus, cerebrospinal fluid leak, and pneumocephalus.
The overexpression of p16 in head and neck ACC has been reported in prior studies.23,24 Based on these prior reports of p16 expression in ACC of the head and neck and reports of HPV positivity in ACC of the uterine cervix,14,15 Boland et al. hypothesized that p16 expression in cases of ACC of the head and neck may be associated with HPV infection.17 Twenty-seven cases of ACC of the head and neck were tested for p16 expression using IHC and HPV using in situ hybridization (ISH). Two cases of high grade ACC demonstrated a high percentage of p16 positive cells (76–100% of tumor cells positive) and both were positive for high-risk HPV 16 by ISH. Of note, both cases were in the nasal cavity with none of the other head and neck sites positive for HPV. The clinical presentation and outcomes of the two positive HPV cases were also consistent with ACC. Both cases occurred in middle-aged men in the nasal cavity and both developed either local or distant disease following definitive treatment.
Bishop et al. performed p16 IHC analysis and HPV ISH on 161 sinonasal tract carcinomas and identified 4 carcinomas, which were histologically similar to the solid variant of ACC and exhibited strong and diffuse p16 staining.12 HPV 31/33 was detected in 2 of the cases and the other 2 were positive for HPV type 16. In 3 of the 4 cases, squamous cell carcinoma in situ of the overlying epithelium was noted. In a follow-up study by Bishop et al., 8 cases of HPV-related carcinomas isolated to the sinonasal tract were identified with features similar to ACC.16 IHC staining for p16 showed strong and diffuse immunoreactivity in all of the cases. HPV 33 was identified in 6 cases and type 35 was present in 1 case based on qPCR. Despite similar histologic appearances, Bishop et al. concluded that the HPV-related carcinomas were not true ACCs based on their predilection for the sinonasal tract, lack of MYB-NFIB gene fusion, and presence of surface dysplasia not present in ACC. In addition, only 2 cases were found to have perineural invasion which is generally observed in 40–50% of cases.2,25 The pattern of p16 staining discovered in the HPV-related ACC-like carcinoma also was not typical for ACC as p16 expression is generally isolated to the luminal cells in true ACC.26
The results of the present series demonstrate that the association with HPV in true ACC is low. Although we did not test for MYB-NFIB gene fusion, all of our cases were reviewed by a single pathologist (BJS), and no evidence of squamous dysplasia of the surface epithelium was noted, which is consistent with true ACC. The clinical presentation, patterns of recurrence, and tumor control outcomes were also consistent with ACC. In our cohort of 17 patients where p16 IHC and qPCR for HPV was performed, we found 2 cases with strong and diffuse p16 staining with the remaining 15 cases demonstrating p16 expression isolated to the luminal cells, which is typically observed in conventional ACC. One of the p16 positive cases was also positive for HPV 33 with a low copy number (9) detected. In addition to the finding of HPV-related ACC, Bishop et al. also found that 12% of true ACCs exhibited positive p16 staining with none of the cases positive for HPV based on ISH.12 We also found that 12% of true ACCs were p16 positive. The single HPV positive case for type 33 had a low copy number, so it is possible that HPV in this case is simply a co-infection as opposed to playing a role in carcinogenesis. Interestingly, 6 of the 8 HPV-related adenoid cystic-like carcinomas found by Bishop et al. were also positive for HPV 3316 while 17% of all sinonasal carcinomas were associated with HPV 16.12
Although there were a limited number of cases because of the rarity of this tumor, the patients that were p16 positive did not have a superior outcome when compared to their p16 negative counterparts. Both patients developed metastatic disease with the HPV 33 positive patient ultimately dying from disease. The other p16 positive patient is currently alive with metastatic disease. Due to the limited number of p16/HPV positive cases of SNACC and HPV-related adenoid cystic-like carcinoma, an assessment of how p16/HPV impacts tumor control and patient outcomes cannot be performed. However, a retrospective study investigating sinonasal tract SCC demonstrated an HPV association rate of 20% with HPV type 16 identified in 92% of the cases. A better prognosis was observed in the HPV positive group as there was a statistically significant improvement in 5-year PFS and OS when compared to the HPV negative cohort.
This is a retrospective study, as such it is subject to selection bias which is present in all retrospective reviews. As this is a rare tumor, we were limited in the ability to perform univariate/multivariate statistical analysis due to the small sample size. This study includes patients treated over a long time period during which changes in the management of SNACC have evolved including more advanced surgical and radiation techniques. Finally, our toxicity data is entirely taken from the patient chart and does not include physician-graded toxicity or patient-reported outcomes, which would be more ideal for this patient population.
In conclusion, SNACC generally presents at an advanced stage making treatment difficult and potentially morbid. While surgical resection and adjuvant RT remains the therapy of choice, local failures are common and the survival at 5 years is anticipated to be 60%. Based on our results, the association of HPV with true SNACC is low.
Acknowledgments
Funding sources: This research was supported in part by the John F. & Ann I. Wolfe Research Fund and grant P30 CA16058, National Cancer Institute, Bethesda, MD.
The authors acknowledge the input of Amit Agrawal, MD, Matthew O. Old, MD, Theodoros N. Teknos, MD, James W. Rocco, MD, PhD, and Enver Ozer, MD from the Department of Otolaryngology at The Ohio State University, Columbus, Ohio.
Footnotes
This paper was presented at the 2016 Multidisciplinary Head and Neck Cancer Symposium.
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Fordice J, Kershaw C, El-Naggar A, Goepfert H. Adenoid cystic carcinoma of the head and neck: predictors of morbidity and mortality. Arch Otolaryngol Head Neck Surg. 1999;125:149–152. doi: 10.1001/archotol.125.2.149. [DOI] [PubMed] [Google Scholar]
- 2.Lupinetti AD, Roberts DB, Williams MD, et al. Sinonasal adenoid cystic carcinoma: the M. D. Anderson Cancer Center experience. Cancer. 2007;110:2726–2731. doi: 10.1002/cncr.23096. [DOI] [PubMed] [Google Scholar]
- 3.Chilla R, Schroth R, Eysholdt U, Droese M. Adenoid cystic carcinoma of the head and neck. Controllable and uncontrollable factors in treatment and prognosis. ORL J Otorhinolaryngol Relat Spec. 1980;42:346–367. doi: 10.1159/000275515. [DOI] [PubMed] [Google Scholar]
- 4.Dal Maso M, Lippi L. Adenoid cystic carcinoma of the head and neck: a clinical study of 37 cases. Laryngoscope. 1985;95:177–181. [PubMed] [Google Scholar]
- 5.Issing PR, Hemmanouil I, Stover T, et al. Adenoid cystic carcinoma of the skull base. Skull Base Surg. 1999;9:271–275. doi: 10.1055/s-2008-1058137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 7.Adelstein DJ, Ridge JA, Gillison ML, et al. Head Neck; Head and neck squamous cell cancer and the human papillomavirus: summary of a National Cancer Institute State of the Science Meeting; November 9–10, 2008; Washington, D.C. 2009. pp. 1393–1422. [DOI] [PubMed] [Google Scholar]
- 8.Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29:4294–4301. doi: 10.1200/JCO.2011.36.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mirghani H, Amen F, Blanchard P, et al. Treatment de-escalation in HPV-positive oropharyngeal carcinoma: Ongoing trials, critical issues and perspectives. Int J Cancer. 2014 doi: 10.1002/ijc.28847. [DOI] [PubMed] [Google Scholar]
- 11.Bhatia A, Burtness B. Human Papillomavirus-Associated Oropharyngeal Cancer: Defining Risk Groups and Clinical Trials. J Clin Oncol. 2015;33:3243–3250. doi: 10.1200/JCO.2015.61.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bishop JA, Guo TW, Smith DF, et al. Human papillomavirus-related carcinomas of the sinonasal tract. Am J Surg Pathol. 2013;37:185–192. doi: 10.1097/PAS.0b013e3182698673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alos L, Moyano S, Nadal A, et al. Human papillomaviruses are identified in a subgroup of sinonasal squamous cell carcinomas with favorable outcome. Cancer. 2009;115:2701–2709. doi: 10.1002/cncr.24309. [DOI] [PubMed] [Google Scholar]
- 14.Daponte A, Grayson W, Moisuc D, Ebrahim S, Guidozzi F. Adenoid cystic carcinoma stage Ib1 treated with radical surgery displaying human papilloma virus 33 (HPV 33): immunoelectron microscopy and review. Gynecol Oncol. 2003;90:673–676. doi: 10.1016/s0090-8258(03)00403-7. [DOI] [PubMed] [Google Scholar]
- 15.Grayson W, Taylor L, Cooper K. Detection of integrated high risk human papillomavirus in adenoid cystic carcinoma of the uterine cervix. J Clin Pathol. 1996;49:805–809. doi: 10.1136/jcp.49.10.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bishop JA, Ogawa T, Stelow EB, et al. Human papillomavirus-related carcinoma with adenoid cystic-like features: a peculiar variant of head and neck cancer restricted to the sinonasal tract. Am J Surg Pathol. 2013;37:836–844. doi: 10.1097/PAS.0b013e31827b1cd6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boland JM, McPhail ED, Garcia JJ, Lewis JE, Schembri-Wismayer DJ. Detection of human papilloma virus and p16 expression in high-grade adenoid cystic carcinoma of the head and neck. Mod Pathol. 2012;25:529–536. doi: 10.1038/modpathol.2011.186. [DOI] [PubMed] [Google Scholar]
- 18.Gillison ML, D’Souza G, Westra W, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 19.Gravitt PE, Burk RD, Lorincz A, et al. A comparison between real-time polymerase chain reaction and hybrid capture 2 for human papillomavirus DNA quantitation. Cancer Epidemiol Biomarkers Prev. 2003;12:477–484. [PubMed] [Google Scholar]
- 20.Jordan RC, Lingen MW, Perez-Ordonez B, et al. Validation of methods for oropharyngeal cancer HPV status determination in US cooperative group trials. Am J Surg Pathol. 2012;36:945–954. doi: 10.1097/PAS.0b013e318253a2d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan CC, Miley W, Waters D. A quantification of human cells using an ERV-3 real time PCR assay. J Virol Methods. 2001;91:109–117. doi: 10.1016/s0166-0934(00)00244-5. [DOI] [PubMed] [Google Scholar]
- 22.Husain Q, Kanumuri VV, Svider PF, et al. Sinonasal adenoid cystic carcinoma: systematic review of survival and treatment strategies. Otolaryngol Head Neck Surg. 2013;148:29–39. doi: 10.1177/0194599812464020. [DOI] [PubMed] [Google Scholar]
- 23.Nishimine M, Nakamura M, Kishi M, et al. Alterations of p14ARF and p16INK4a genes in salivary gland carcinomas. Oncol Rep. 2003;10:555–560. [PubMed] [Google Scholar]
- 24.Maruya S, Kurotaki H, Shimoyama N, Kaimori M, Shinkawa H, Yagihashi S. Expression of p16 protein and hypermethylation status of its promoter gene in adenoid cystic carcinoma of the head and neck. ORL J Otorhinolaryngol Relat Spec. 2003;65:26–32. doi: 10.1159/000068658. [DOI] [PubMed] [Google Scholar]
- 25.Thompson LD, Penner C, Ho NJ, et al. Sinonasal tract and nasopharyngeal adenoid cystic carcinoma: a clinicopathologic and immunophenotypic study of 86 cases. Head Neck Pathol. 2014;8:88–109. doi: 10.1007/s12105-013-0487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis JS, Jr, Westra WH, Thompson LD, et al. The sinonasal tract: another potential “hot spot” for carcinomas with transcriptionally-active human papillomavirus. Head Neck Pathol. 2014;8:241–249. doi: 10.1007/s12105-013-0514-4. [DOI] [PMC free article] [PubMed] [Google Scholar]


