Abstract
Elevated STAT3 activity is a hallmark of many epithelial carcinomas particularly in breast cancers where it is known to contribute to tumor progression through a variety of context-dependent biological responses. However, its role downstream of stress-exposed EGF receptors (EGFR) that are transactivated in endosomes independent of exogenous ligand has not been studied. This review discusses how STAT3 signaling induced by therapeutic stress in EGFR-driven triple-negative breast cancers (TNBC) might override normal epithelial homeostatic mechanisms and provide a survival advantage for tumor cells before they leave the primary tumor and spread to distant sites. Despite continued improvements in breast cancer treatment strategies, TNBC is still associated with poor prognosis and high risk of distant recurrence and death. Understanding EGFR-STAT3 signaling mechanisms regulating the earliest steps of tumor progression is a key to discovery of new targeted therapies against TNBC.
Introduction
Signal Transducer and Activator of Transcription 3 (STAT3) was independently discovered over 20 years ago as an acute-phase response factor that selectively binds to DNA following stimulation with interleukin (IL)-6 or epidermal growth factor (EGF) (1, 2). Both pathways were regulated by STAT3 tyrosine phosphorylation downstream of the intracellular Janus tyrosine kinase (JAK) in the case of IL-6, and intrinsic EGF receptor (EGFR) kinase activity for EGF (3). Since those initial discoveries, STAT3 has been shown to be activated by a variety of additional stimuli, including c-Src family kinases and cell adhesion by fibronectin integrin receptors (3, 4). Once STAT3 has undergone tyrosine phosphorylation, this latent transcription factor forms homodimers, or heterodimers with other STAT family members, capable of nuclear translocation and transcriptional regulation (5). Constitutive STAT3 activation is emerging as a hallmark in diverse solid tumors and hematologic malignancies and is also associated with poor prognosis (6). Given the diversity of upstream STAT3-activating signals, it is important to consider the contribution of the tumor microenvironment to oncogenic STAT3 signaling, particularly in EGFR-driven epithelial carcinomas (3). Most studies documenting oncogenic EGFR-STAT3 signaling assume that this pathway is activated by autocrine growth factors released by tumor cells, or growth factors produced by non-malignant stromal and immune cells in the tumor microenvironment. However, recent evidence suggests that both intrinsic and therapy-induced cellular stressors trigger robust EGFR trafficking and signaling independent of extrinsic ligand (7, 8). Furthermore, these stress-induced EGR responses are associated with a survival advantage and resistance to therapeutics by mechanisms that are incompletely understood (7, 8). The goal of this review is to consider how EGFR signaling that is activated intracellularly independent of exogenous growth factor stimulation at the plasma membrane might contribute to the early evolution of triple-negative breast cancers (TNBCs) by activation of STAT3 signaling.
Triple-negative breast cancer
Breast cancer death rates have been declining steadily since 1990 because of advances in treatment, early detection and imaging, and increased awareness (9). The advent of molecular profiling has led to recognition that breast cancer is a heterogeneous disease with genetically distinct subtypes characterized by differences in tumor biology, prognosis, and response to therapy (10; 11). Thus, breast cancer patients with estrogen receptor or progesterone receptor positive tumors benefit from anti-hormone treatments, while those patients with tumors expressing high levels of the HER2 receptor can be treated with HER2 inhibitors (12, 13). Triple-negative breast cancer (TNBC) is classified as such because this subtype is negative for these three receptors and therefore insensitive to molecularly targeted drugs that have proven to be highly successful for combating other breast cancer subtypes (14–16). However, TNBCs have demonstrated response rates to neoadjuvant chemo-radiotherapies that are usually higher than those of other breast cancer subtypes in several recent trials (17, 18). EGFR is overexpressed, and also a negative prognostic indicator, in ~70% of TNBC patients, suggesting EGFR-targeted therapies that are effective for clinical management of several other EGFR-driven cancers should also be beneficial for this breast cancer subtype (19). Contrary to these expectations, targeted inactivation of EGFR fails to improve TNBC patient survival, and TNBC is still associated with poor prognosis because about one-third of TNBC patients experience recurrence by 2.6 years on average, and nearly all TNBC patients inevitably develop therapeutic resistance (20, 21; 22). Since TNBC accounts for about 1 in 5 breast cancers, and is especially prevalent in African-American and Hispanic women under the age of 40, there is a pressing need to re-evaluate basic research priorities which have chiefly been centered on inhibiting canonical EGFR signaling as a main driver of TNBC growth (19, 23). Although resistance can emerge, pre-clinical studies with BET inhibitors, that displace BET bromodomain proteins such as BRD4 from chromatin leading to inhibition of oncogenic transcriptional programs, have yielded promising results in TNBC cell lines and animal models (24). Drug discovery is also guided by comprehensive analyses of histopathologically well-classified primary tumors to shed light on important signaling pathways as potential new molecular targets for treatment. These types of analyses have implicated roles for several pathways that have been functionally linked to tumor progression, including the tumor suppressor protein p53, phosphatidylinositol-4,5-bisphosphate 3-kinase signaling, ErbB receptor tyrosine kinase signaling, and integrin signaling, at the time of initial TNBC diagnosis (25). However, clonal frequencies of somatic mutations vary more widely in treatment-naïve TNBCs compared to other breast cancer subtypes, raising questions about what drives early clonal expansion in TNBC (25). This review examines possible molecular signaling pathways utilized by emerging TNBC cells to actively respond to chemotherapeutic pressures that may act as stressors on surviving tumor cells. Furthermore, we will consider whether the surviving cells have intrinsic counter-defense measures that may be relatively fitness-neutral in the primary tumor except in the context of chemotherapy where these clones have a positive selective advantage.
EGFR signaling and trafficking
EGFR is a prototypic receptor tyrosine kinase with key roles in epithelial cells that are among the fastest growing tissues in the body and also the source for epithelial carcinomas accounting for ~90% of all human cancers (26, 27). The importance of EGFR in normal physiology is underscored by phenotypes exhibited in knock-out mice lacking EGFR which usually die immediately after birth because of respiratory failure along with severe deficits in gastrointestinal functions (28). Clinical studies indicate that disturbances in EGFR function, either due to oncogenic mutations or copy number amplification and subsequent EGFR overexpression, have important roles in tumor formation and progression particularly in highly aggressive carcinomas (29). EGFR is activated by ligand binding at the cell surface resulting in autophosphorylation on specific tyrosine residues within the cytoplasmic tail that serve as docking sites for a range of proteins regulating multiple intracellular signaling pathways (30). Ligand-activated EGFRs are rapidly internalized to endosomes that enable signaling from unique intracellular platforms (31, 32). There is also good evidence that endocytosis is important for regulating EGFR-induced transcriptional responses that are elicited primarily by receptors at the cell surface (33). Endocytosis instigates the process of signal termination since receptors are sorted to intraluminal vesicles (ILVs) that are subsequently degraded when these multivesicular endosomes fuse with lysosomes (32, 34). ILV sorting is regulated by the ESCRT (endosomal sorting complexes required for transport) machinery that recognizes ubiquitin moieties attached to EGFR at plasma membrane, and initiates the process of EGFR silencing before receptors are sequestered to ILVs away from their cytosolic substrates (35, 36).
Recent advances have revealed that a wide variety of cellular stresses which activate the p38 MAP kinase (p38MAPK), ranging from common cancer therapies such as irradiation and cisplatin to pro-inflammatory cytokines, induce EGFR internalization and endosomal arrest independent of ligand binding, kinase activity, or ubiquitination (7, 8, 37). Stress-exposed EGFRs are sorted away from the ligand-dependent pathway to a distinct subset of stable multivesicular endosomes by a mechanism involving the WASP and Scar homologue (WASH) complex which regulates actin dynamics (38). These compartments may be precursors to lysosome-related organelles that carry out specialized functions in nearly all cell types (38, 39). Stress-exposed EGFRs are sorted to ILVs by a subset of the ESCRT machinery (see Fig. 1) including the accessory protein Alix that is dispensable in the canonical ligand-induced EGFR trafficking pathway (35, 38). Interestingly, Alix also regulates the back-fusion of ILVs with endosomal limiting membranes which allows stress-exposed EGFRs to escape lysosomal degradation and recycle back to the plasma membrane when p38MAPK activity declines (7, 8, 40). Stress-exposed EGFRs can transduce signals after they have been internalized to endosomes through transactivation (38). Multiple stimuli, including agonists for G-protein–coupled receptors, membrane depolarization agents, and environmental stressors, induce pathways leading to EGFR transactivation by two mechanisms (41). First, activated matrix metalloproteases cleave EGFR ligands such as HB-EGF from membrane-bound precursors that are then released into the extracellular space, bind EGFR, and stimulate canonical receptor dimerization and activation (41). Second, intracellular protein tyrosine kinases such as c-Src family members modify tyrosine residues in the cytosolic domain of EGFR (41). Interestingly, c-Src is activated in endosomes by a mechanism involving the actin cytoskeleton en route to specific plasma membrane domains, supporting a potential role in regulation of EGFR stress signaling from endosomes (42). While ligand shedding probably activates the same signaling cascades as soluble ligands, c-Src-activated EGFRs are phosphorylated on a unique subset of tyrosine residues with markedly distinct physiological outcomes (41). The Alix-dependent EGFR sorting pathway should also favor sustained EGFR signaling by increasing the residence time of receptors that are activated intracellularly on endosomal limiting membranes (40). Understanding c-Src-dependent EGFR pathways that are selectively activated in a stable subset of stress–induced endosomes will provide important insights into how small subpopulations of tumor cells emerge under selective drug pressure.
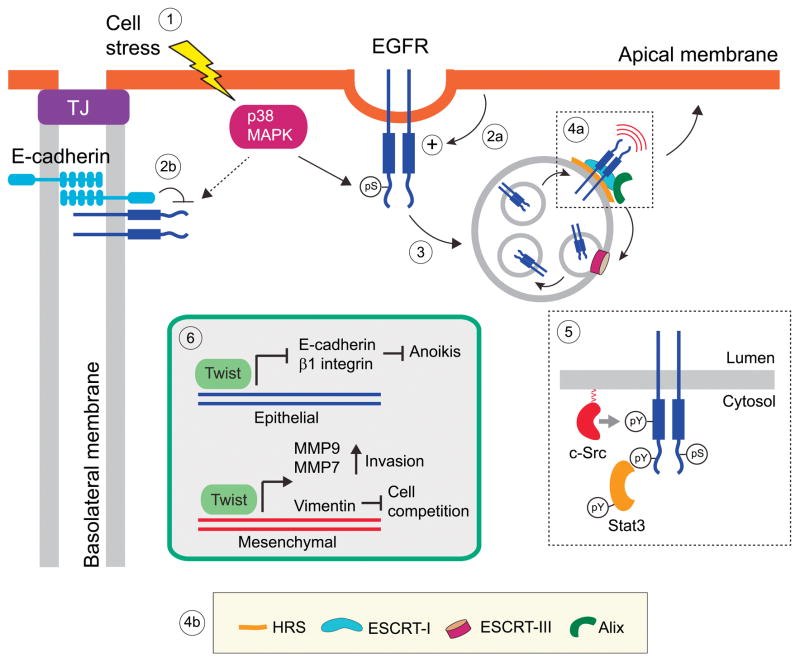
Figure 1. Summary model.
Many cellular stress stimuli activate p38MAPK leading to phosphorylation of specific serine residues in the cytoplasmic domain of EGFR (7, 8, 37) [Step 1]. Domain-specific polarity factors could either enhance uptake from the apical membranes [Step 2a], or alternatively inhibit uptake from BL membranes similar to physical interactions between EGFR and E-cadherin that limit ligand-induced EGFR internalization (68) [Step 2b]. These p38MAPK-induced modifications cause EGFR internalization and arrest at nondegradative endosomes, where the receptors have kinase signaling functions independent of exogenous soluble ligands (7, 8) [Step 3]. Stress-exposed EGFRs are sorted to ILVs by a subset of ESCRT components including HRS that enables the recruitment of ESCRT-I, and Alix which carries out multiple functions including cargo recognition, linking ESCRT-I to ESCRT-III necessary for ILV formation, and ILV back-fusion with endosomal limiting membranes (38, 40) [Steps 4a, 4b]. Although it is currently unclear how stress-exposed EGFRs are transactivated in endosomes, the most likely explanation involves c-Src dependent phosphorylation of Tyr-845 in the activation loop of the EGFR kinase domain that also drives robust EGFR-STAT3 signaling (43, 44) [Step 5]. The EGFR-STAT3 pathway triggers a TWIST-dependent EMT transcription program that could conceivably provide positive selection for emerging tumor cells by counteracting anoikis sensitivity, activating matrix metallopeptidases that regulate cell invasiveness, and up-regulating vimentin expression to levels that exceed a critical threshold value that marks viable cells for apical extrusion and death by anoikis (64, 65) [Step 6]. TJ, tight junction.
The c-Src/EGFR/Stat3 signaling axis
The best-established c-Src-phosphorylated residue in EGFR is tyrosine 845 (Tyr845) located in the activation loop of the catalytic kinase domain (43, 44). Tyr845 is highly conserved across the receptor tyrosine kinase family, and EGFR is the exception rather than the rule in that activation-loop phosphorylation regulates the tyrosine kinase domains of most family members but plays no part in EGFR (45). In contrast, EGFR kinase activity is regulated allosterically by formation of asymmetric tyrosine kinase dimers following binding of extracellular ligand (45). Thus c-Src appears to transactivate EGFR by activation loop phosphorylation rather than asymmetric dimer formation. An important consequence of Tyr845 phosphorylation is the ability of the receptor to induce STAT3 activation (44). EGFR Tyr1068 has been reported to be the docking site for the SH2 domain of STAT3 in ligand-stimulated cells (46). However, other EGFR substrates interfere with EGFR-STAT3 signaling, either by competing for the same autophosphorylation docking site (growth factor receptor-bound protein 2 or Grb2) (47), or by inhibiting STAT3 activity (suppressor of cytokine signaling factors SOCS1 and SOCS3) (48). Because of differences in EGFR activation by ligand versus c-Src, the EGFR-STAT3 pathway may be favored when the receptor is activated by the loop phosphorylation mechanism. There are conflicting reports as to whether the Tyr1068 STAT3 docking site is a direct target for c-Src kinase activity, or if c-Src-dependent Tyr845 phosphorylation leads to EGFR activation and the subsequent autophosphorylation at EGFR Tyr1068 (49–51). It is also possible that stress-exposed EGFRs are protected from protein tyrosine phosphatases that maintain basal EGFR tyrosine kinase activity (52). In any event, STAT3 likely has critical roles in EGFR responses activated downstream of c-Src in stress induced endosomes.
Homeostatic growth control
Homeostasis of epithelial tissues plays critical roles in organ morphogenesis, cell differentiation, and tissue regeneration, and mechanisms that disrupt the balance between cell division and cell loss are emerging as critical factors in cancer initiation (54). Epithelial cells are held together through several types of interactions including tight junctions that delineate the apical side facing the lumen and the basolateral (BL) side providing connections with extracellular matrix (ECM) and other cells in epithelial tissues (53). Epithelia maintain constant cell numbers because cell proliferation and cell death are highly coordinated through the process of “cell extrusion” (54). Epithelial cell crowding causes viable cells to be extruded into luminal spaces where they succumb to cell death by anoikis, a form of programmed cell death occurring in anchorage-dependent cells when they detach from neighboring cells (55–58). Although many details are still incomplete, the molecular pathway that regulates apical cell extrusion is beginning to be understood. Cells that are destined for extrusion produce and secrete the bioactive lipid sphingosine-1-phosphate (S1P), which then binds to its cognate receptor S1PR2 leading to activation of Rho-Rho kinase signaling in the surrounding cells (54). This causes acto-myosin rings to form that squeeze the S1P-secreting cell out of the monolayer into the apical lumen without disrupting epithelial barrier function (54). While the S1P-S1PR2 pathway regulates growth homeostasis in epithelial cells that are genetically identical, this same mechanism has recently been implicated in a highly conserved process called cell competition whereby cells that would ordinarily survive in a homogenous environment are eliminated by surrounding cells of higher fitness (59). Cell competition is gaining experimental support in epithelial tissues where normal cells sense and actively eliminate neighboring transformed cells by cell death-independent apical extrusion followed by anoikis (60). Thus, normal epithelial appear to be equipped with inherent anti-tumorigenic properties independent of the immune system (60). If cell competition is a tumor suppressor mechanism, then is the lack of competition tumor promoting? Tissue culture studies have shown that transformed cells which subvert this innate epithelial defense mechanism form dynamic basal protrusions that could potentially invade the basal matrix and metastasize to distant tissues if they also acquire resistance to anoikis-induced cell death and enhanced pro-invasive signals by mechanisms that are still largely unknown (56). Understanding how chemotherapy-induced c-Src/EGFR/STAT3 responses provide a selective advantage during cell competition will provide important targets for potential therapeutic strategies in the treatment of TNBC.
The evolving role of STAT3 during breast cancer tumor progression
EGFR-induced STAT3 activity has a well-established role in the growth of primary human tumors that express high levels of EGFR including TNBC (3). The JAK2/STAT3 signaling pathway regulates a cell population with stem-like features and tumor-initiating capacity in Claudin-low models of human breast cancer (61). Furthermore, STAT3 activity may underlie intrinsic resistance to broad-based chemotherapies and selective drugs that target oncogene-addiction pathways (62). STAT3 is activated downstream of cell adhesion to one of the most common and highly expressed transcripts in breast cancer, namely fibronectin, independent of EGFR, and this novel STAT3 activation pathway is necessary for successful metastatic colonization in a TNBC animal model (63). However, the idea that stress-induced EGFR-STAT3 signaling from endosomes facilitates positive selection of tumor cells has not been explored. It is already known that aberrant STAT3 activity induces expression of the transcription factor Twist which is a master regulator of epithelial-mesenchymal transition (EMT), an essential developmental program that initiates invasive and metastatic behavior if it becomes reactivated in localized epithelial carcinomas (46). How might EGFR-STAT3-TWIST signaling integrate cell stress with positive selection responses in emerging tumor cells? First, a stress-induced EGFR-STAT3 pathway could enhance survival signaling since TWIST down-regulates proteins such as E-cadherin and β1-integrin that control anoikis sensitivity (64). Second, TWIST up-regulates expression of matrix metallopeptidases MMP9 and MMP7 that play essential roles in local proteolysis of ECM supporting a role for endosomal EGFR signaling in cell invasion (64, 65). In addition, STAT3 has a number of non-nuclear functions including the ability to regulate microtubule dynamics which could lead to enhanced tumor cell motility (66). But, could a stress-induced EGFR-STAT3-TWIST pathway also protect cells with EGFR polarity defects from elimination by targeting key intermediate filament proteins involved in cell competition (60)? Interestingly, TWIST up-regulates expression of the intermediate filament protein vimentin, which has an essential role in the ability of normal epithelial cells to out-compete transformed cells by providing physical force for apical extrusion (60, 65). In fact, in vitro studies have shown that overexpression of vimentin in normal epithelial cells at the contact surface with transformed cells is essential for cell competition (60). Thus, tumor cells with up-regulated vimentin expression in response to EGFR-STAT3 stress signaling may exceed a critical threshold level that marks a particular cell for elimination compared to its neighbors leading to basal extrusion and increased metastatic potential, representing a novel tumor promoting mechanism. However, in order for stress-induced EGFR-STAT3 signaling to be involved in positive selection of emerging tumor cells, this pathway must be tightly regulated in normal epithelial cells.
Cell polarity and EGFR stress signaling
Disruption of cell polarity is a hallmark of epithelial carcinomas. However, rather than being a by-product of abnormal accumulation of tumor cell masses, it is increasingly evident that aberrant cell-polarity mechanisms have a causal role in tumorigenesis (67). E-cadherin mediated cell adhesion is known to negatively regulate the ligand-dependent activation of multiple receptor tyrosine kinases including EGFR, which forms a physical complex with E-cadherin that significantly reduces receptor mobility on BL membranes in high density epithelial cell cultures (68). Contact-dependent inhibition of EGFR mobility also impairs internalization of ligand-activated EGFRs, and presumably receptors that are internalized downstream of cell stressors that induce p38MAPK (69). Selective deregulation of EGFR polarity could therefore sensitize cells to EGFR-STAT3 stress responses by uncoupling EGFR function from selective physical constraints on BL membranes. Alternatively, BL sorting could sequester EGFR away from polarity factors that up-regulate EGFR stress signaling which are selectively enriched on apical membranes. BL EGFR sorting appears to be regulated by sequential interactions between sorting signals in EGFR and the Sec24B subunit of COP II coatamer in endoplasmic reticulum and the clathrin adaptor AP-1B complex in endosomes in mature epithelia (70, 71). Although EGFR BL sorting signals are not affected by any known oncogenic mutations, EGFR polarity could be disrupted by aberrant expression of the BL sorting machinery in pre-malignant cells. The epithelial-specific μ1B subunit of AP-1B appears to be a target for methylation silencing in a subset of women with familial breast cancer (72). Loss of μ1B expression has also been implicated in the tumorigenic phenotype of certain colon cell lines and a subset of colon cancer patients (73). Similar to other BL proteins EGFR may require an unidentified, cargo-specific AP-1B co-factor that is aberrantly expressed in a subset of tumor cells (74). EGFR sorting could be disrupted at the level of the endoplasmic reticulum since Sec24 isoforms are known substrates for several protein kinases frequently misregulated in cancer that could alter their ability to assemble into COP II coatamer or release EGFR cargo (75). Another factor that could influence stress-induced EGFR signaling is the intracellular distribution of c-Src which is enriched in apical membranes and apically-derived endosomes in polarized epithelial cells (76). Experimentally-introduced mutations that inactivate the AP-1B and Sec24B sorting signals in EGFR cause receptor missorting to apical membranes without affecting overall epithelial cell architecture (70, 71). This suggests cell polarity defects that disrupt BL EGFR sorting could induce EGFR-STAT3 responses which select for emerging tumor cells before loss of cell polarity associated with the later stages of tumor progression or the onset of EMT and metastasis, and also contribute to chemoresistance.
Conclusions and future directions
The next breakthroughs in cancer treatment will be made by integrating risk factors and processes across multiple fields of cancer cell biology particularly the role of cell polarity in tumor suppression. Several key concepts discussed in this review, and summarized in Fig. 1, should now be rigorously tested. First, is EGFR stress signaling under negative regulation by BL sorting, and if so how might tumor cells gain a selective advantage by disrupting sorting machinery? Second, do therapeutic pressures contribute to chemoresistance by sensitizing cells to EGFR stress signaling? Third, how is EGFR stress signaling regulated in endosomes, and could this pathway be down-regulated therapeutically by “tricking” the stable population of multivesicular endosomes that mediate EGFR stress responses into a lysosome maturation program? Fourth, how do cells with aberrant EGFR polarity avoid elimination by surrounding normal epithelial cells through STAT3 signaling, and are other endosomal EGFR signaling pathways involved? Finally, is restoration of epithelial cell competition a viable target for cancer therapy?
Epithelial homeostatic growth control mechanisms have largely been studied in epithelial cell tissue culture models that form a simple epithelium composed of a single layer of polarized cells. However, most epithelial tissues are highly complex structures undergoing constant remodeling during normal physiology, and it is unclear how mechanisms elucidated in a simple epithelium apply in these settings. The human mammary gland develops post-natally in response to hormonal cues and undergoes repeated expansion and renewal during the lifetime of the female (77). Even though tumor cells accumulate in a closed lumen without dying in breast ductal carcinoma in situ, they rarely invade and metastasize (77). However, oncogenic events that influence the direction of cell extrusion to occur basally could enhance the ability of these tumor cells to initiate metastasis and migrate to other sites in the body (56). The epithelium of the mammary gland is composed of two main epithelial cell lineages: luminal cells that surround a central lumen, and myoepithelial cells located basally adjacent to the basement membrane (77). Breast cancers can be categorized into either ‘basal’ or ‘luminal’ sub-types based on their molecular similarity to the basal or luminal progenitor cells of the normal mammary gland (78). Until recently, it was thought that breast cancers with a basal-like molecular signature including most TNBCs arise from a basal progenitor cell (79). However, the cell of origin for basal-like breast cancers has recently been challenged by studies indicating that luminal progenitor cells might be able to give rise to breast carcinomas with a basal molecular signature through a process of dedifferentiation (80). This type of bidirectional plasticity raises two important questions. First, are transformed luminal epithelial cells that avoid elimination by cell competition the cell of origin for basal-like TNBC? Second, does EGFR-STAT3 stress signaling regulate luminal progenitor dedifferentiation to a basal-like phenotype by activating an EMT plasticity program controlling mammary gland development or tissue remodeling? These considerations represent an important, and largely unexplored, new perspective for design of novel therapeutic strategies to eradicate TNBC and other types of EGFR-STAT3 driven cancers.
Acknowledgments
Research support was provided in part by the National Institutes of Health (GM081498) to C.R.C.; a pilot project grant from development funds of the Case Comprehensive Cancer Center Support Grant (CA043703) to C.R.C.; and a National Institutes of Health training grant (T32 HL007653) to N.B.
Abbreviations
- BL
basolateral
- ECM
extracellular matrix
- EGF
epidermal growth factor
- EGFR
EGF receptor
- ESCRT
endosomal sorting complexes required for transport
- IL
interkeukin
- ILV
intraluminal vesicle
- JAK
Janus tyrosine kinase
- p38MAPK
p38 MAP kinase
- S1P
sphingosine-1-phosphate
- STAT3
Signal Transducer and Activator of Transcription 3
- TNBC
triple-negative breast cancer
- WASH
WASP and Scar homologue
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- 1.Akira S, Nishio Y, Inoue M, Wang XJ, We S, Matsusaka T, Yoshida K, Sudo T, Naruto M, Kishimoto T. Molecular cloning of APRF, a novel IFN-stimulated gene factor 3 p91-related transcription factor involved in the gp130-mediated signaling pathway. Cell. 1994;77:63–71. doi: 10.1016/0092-8674(94)90235-6. [DOI] [PubMed] [Google Scholar]
- 2.Zhong Z, Wen Z, Darnell J. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- 3.Wendt M, Balanis N, Carlin C, Schiemann W. STAT3 and epithelial-mesenchymal transitions in carcinomas. JAKSTAT. 2014;3:e28975. doi: 10.4161/jkst.28975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim BH, Yi EH, Ye SK. Signal transducer and activator of transcription 3 as a therapeutic target for cancer and the tumor microenvironment. Archives of Pharmacal Research. 2016;39:1085–1099. doi: 10.1007/s12272-016-0795-8. [DOI] [PubMed] [Google Scholar]
- 5.Levy DE, Darnell JE. STATs: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 6.Darnell J. Validating Stat3 in cancer therapy. Nat Med. 2005;11:595–596. doi: 10.1038/nm0605-595. [DOI] [PubMed] [Google Scholar]
- 7.Tan X, Lambert PF, Rapraeger AC, Anderson RA. Stress-induced EGFR trafficking: mechanisms, functions, and therapeutic implications. Trends Cell Biol. 2016;26:352–366. doi: 10.1016/j.tcb.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomas A, Futter CE. Stress reveals new destination for EGF receptor. Cell Cycle. 2015;14:3343–3344. doi: 10.1080/15384101.2015.1093432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson HD, Fu R, Cantor A, Pappas M, Daeges M, Humphrey L. Effectiveness of breast cancer screening: Systematic review and meta-analysis to update the 2009 u.s. preventive services task force recommendation. Annals Internal Med. 2016;164:244–255. doi: 10.7326/M15-0969. [DOI] [PubMed] [Google Scholar]
- 10.Sotiriou C, Neo S, McShane L, Korn E, Long P, Jazaeri A, Martiat P, Fox S, Harris A, Liu E. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci U S A. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Network TCGA. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ross J, Linette G, Stec J, Clark E, Ayers M, Leschly N, Symmans W, Hortobagyi G, Pusztai L. Breast cancer biomarkers and molecular medicine. Expert Rev Mol Diagn. 2003;3:573–585. doi: 10.1586/14737159.3.5.573. [DOI] [PubMed] [Google Scholar]
- 13.Nam B, Kim S, Han H, Kwon Y, Lee K, Kim T, Ro J. Breast cancer subtypes and survival in patients with brain metastases. Breast Cancer Res. 2008;10:R20. doi: 10.1186/bcr1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carey L, Dees E, Sawyer L, Gatti L, Moore D, Collichio F, Ollila D, Sartor C, Graham M, Perou C. The triple negative paradox: primary tumor chemosensitivity of breast cancer subtypes. Clin Cancer Res. 2007;13:2329–2334. doi: 10.1158/1078-0432.CCR-06-1109. [DOI] [PubMed] [Google Scholar]
- 15.Schmadeka R, Harmon BE, Singh M. Triple-negative breast carcinoma: Current and emerging concepts. Am J Clin Pathology. 2014;141:462–477. doi: 10.1309/AJCPQN8GZ8SILKGN. [DOI] [PubMed] [Google Scholar]
- 16.de Ruijter T, Veeck J, de Hoon J, van Engeland M, Tjan-Heijnen V. Characteristics of triple-negative breast cancer. J Cancer Res Clin Oncology. 2010;137:183–192. doi: 10.1007/s00432-010-0957-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isakoff SJ. Triple negative breast cancer: Role of specific chemotherapy agents. Cancer J. 2011;16:53–61. doi: 10.1097/PPO.0b013e3181d24ff7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Laurentiis M, Cianniello D, Caputo R, Stanzione B, Arpino G, Cinieri S, Lorusso V, De Placido S. Treatment of triple negative breast cancer (TNBC): current options and future perspectives. Cancer Treatment Rev. 2010;36:S80–S86. doi: 10.1016/S0305-7372(10)70025-6. [DOI] [PubMed] [Google Scholar]
- 19.Ueno NT, Zhang D. Targeting EGFR in triple negative breast cancer. J Cancer. 2011;2:324–328. doi: 10.7150/jca.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berrada N, Delaloge S, Andra F. Treatment of triple-negative metastatic breast cancer: toward individualized targeted treatments or chemosensitization? Annals Oncology. 2010;21:vii30–vii35. doi: 10.1093/annonc/mdq279. [DOI] [PubMed] [Google Scholar]
- 21.Haffty B, Yang Q, Reiss M, Kearney T, Higgins S, Weidhaas J, Harris L, Hait W, Toppmeyer D. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J Clin Oncol. 2006;24:5652–5657. doi: 10.1200/JCO.2006.06.5664. [DOI] [PubMed] [Google Scholar]
- 22.Hudis CA, Gianni L. Triple-negative breast cancer: An unmet medical need. The Oncologist. 2011;16:1–11. doi: 10.1634/theoncologist.2011-S1-01. [DOI] [PubMed] [Google Scholar]
- 23.Boyle P. Triple-negative breast cancer: epidemiological considerations and recommendations. Annals Oncology. 2012;23:vi7–vi12. doi: 10.1093/annonc/mds187. [DOI] [PubMed] [Google Scholar]
- 24.Shu S, Lin CY, He HH, Witwicki RM, Tabassum DP, Roberts JM, Janiszewska M, Jin Huh S, Liang Y, Ryan J, Doherty E, Mohammed H, Guo H, Stover DG, Ekram MB, Peluffo G, Brown J, DeSantos C, Krop IE, Dillon D, McKeown M, Ott C, Qi J, Ni M, Rao PK, Duarte M, Wu S-Y, Chiang C-M, Anders L, Young RA, Winer EP, Letai A, Barry WT, Carroll JS, Long HW, Brown M, Shirley Liu X, Meyer CA, Bradner JE, Polyak K. Response and resistance to BET bromodomain inhibitors in triple-negative breast cancer. Nature. 2016;529:413–417. doi: 10.1038/nature16508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, Turashvili G, Ding J, Tse K, Haffari G, Bashashati A, Prentice LM, Khattra J, Burleigh A, Yap D, Bernard V, McPherson A, Shumansky K, Crisan A, Giuliany R, Heravi-Moussavi A, Rosner J, Lai D, Birol I, Varhol R, Tam A, Dhalla N, Zeng T, Ma K, Chan SK, Griffith M, Moradian A, Cheng SWG, Morin GB, Watson P, Gelmon K, Chia S, Chin SF, Curtis C, Rueda OM, Pharoah PD, Damaraju S, Mackey J, Hoon K, Harkins T, Tadigotla V, Sigaroudinia M, Gascard P, Tlsty T, Costello JF, Meyer IM, Eaves CJ, Wasserman WW, Jones S, Huntsman D, Hirst M, Caldas C, Marra MA, Aparicio S. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–399. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hanahan D, Weinberg Robert A. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Linggi B, Carpenter G. ErbB receptors: new insights on mechanisms and biology. Trends Cell Biol. 2006;16:649–656. doi: 10.1016/j.tcb.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 28.Threadgill DW. Targeted disruption of mouse EGF receptor: effect of genetic background on mutant phenotype. Science. 1995;269:230–234. doi: 10.1126/science.7618084. [DOI] [PubMed] [Google Scholar]
- 29.Yarden Y. The EGFR family and its ligands in human cancer. Signalling mechanisms and therapeutic opportunities. Eur J Cancer. 2001;37(Suppl 4):S3–S8. doi: 10.1016/s0959-8049(01)00230-1. [DOI] [PubMed] [Google Scholar]
- 30.Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 2000;103:211–225. doi: 10.1016/s0092-8674(00)00114-8. [DOI] [PubMed] [Google Scholar]
- 31.von Zastrow M, Sorkin A. Signaling on the endocytic pathway. Curr Opin Cell Biol. 2007;19:436–445. doi: 10.1016/j.ceb.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brankatschk B, Wichert SP, Johnson SD, Schaad O, Rossner MJ, Gruenberg J. Regulation of the EGF transcriptional response by endocytic sorting. Sci Signal. 2012;5:ra21. doi: 10.1126/scisignal.2002351. [DOI] [PubMed] [Google Scholar]
- 34.Wiley HS, Burke PM. Regulation of receptor tyrosine kinase signaling by endocytic trafficking. Traffic. 2001;2:12–18. doi: 10.1034/j.1600-0854.2001.020103.x. [DOI] [PubMed] [Google Scholar]
- 35.Raiborg C, Malerod L, Pedersen NM, Stenmark H. Differential functions of Hrs and ESCRT proteins in endocytic membrane trafficking. Exp Cell Res. 2008;314:801–813. doi: 10.1016/j.yexcr.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 36.Hurley JH, Emr SD. The ESCRT complexes: structure and mechanism of a membrane-trafficking network. Annu Rev Biophys Biomol Struct. 2006;35:277–298. doi: 10.1146/annurev.biophys.35.040405.102126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zwang Y, Yarden Y. p38 MAP kinase mediates stress-induced internalization of EGFR: implications for cancer chemotherapy. EMBO J. 2006;25:4195–4206. doi: 10.1038/sj.emboj.7601297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomas A, Vaughan SO, Burgoyne T, Sorkin A, Hartley JA, Hochhauser D, Futter CE. WASH and Tsg101/ALIX-dependent diversion of stress-internalized EGFR from the canonical endocytic pathway. Nat Commun. 2015;6:7324. doi: 10.1038/ncomms8324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marks MS, Heijnen HFG, Raposo G. Lysosome-related organelles: unusual compartments become mainstream. Curr Opin Cell Biol. 2013;25:495–505. doi: 10.1016/j.ceb.2013.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bissig C, Gruenberg J. ALIX and the multivesicular endosome: ALIX in Wonderland. Trends Cell Biol. 2013;24:19–25. doi: 10.1016/j.tcb.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Wang Z. Transactivation of epidermal growth factor receptor by G protein-coupled receptors: Recent progress, challenges and future research. Int J Mol Sci. 2016;17:95. doi: 10.3390/ijms17010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sandilands E, Cans C, Fincham VJ, Brunton VG, Mellor H, Prendergast GC, Norman JC, Superti-Furga G, Frame MC. RhoB and actin polymerization coordinate Src activation with endosome-mediated delivery to the membrane. Dev Cell. 2004;7:855–869. doi: 10.1016/j.devcel.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 43.Biscardi JS, Maa MC, Tice DA, Cox ME, Leu TH, Parsons SJ. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J Biol Chem. 1999;274:8335–8343. doi: 10.1074/jbc.274.12.8335. [DOI] [PubMed] [Google Scholar]
- 44.Boerner JL, Biscardi JS, Silva CM, Parsons SJ. Transactivating agonists of the EGF receptor require Tyr 845 phosphorylation for induction of DNA synthesis. Mol Carcinogenesis. 2005;44:262–273. doi: 10.1002/mc.20138. [DOI] [PubMed] [Google Scholar]
- 45.Lemmon MA, Schlessinger J, Ferguson KM. The EGFR family: Not so prototypical receptor tyrosine kinases. CSH Perspectives Biol. 2014;6:a020768. doi: 10.1101/cshperspect.a020768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lo HW, Hsu SC, Xia W, Cao X, Shih JY, Wei Y, Abbruzzese JL, Hortobagyi GN, Hung MC. Epidermal growth factor receptor cooperates with signal transducer and activator of transcription 3 to induce epithelial-mesenchymal transition in cancer cells via up-regulation of TWIST gene expression. Cancer Research. 2007;67:9066–9076. doi: 10.1158/0008-5472.CAN-07-0575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang T, Ma J, Cao X. Grb2 regulates Stat3 activation negatively in epidermal growth factor signalling. Biochem J. 2003;376:457–464. doi: 10.1042/BJ20030668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xia L, Wang L, Chung AS, Ivanov SS, Ling MY, Dragoi AM, Platt A, Gilmer TM, Fu XY, Chin YE. Identification of both positive and negative domains within the epidermal growth factor receptor COOH-terminal region for signal transducer and activator of transcription (STAT) activation. J Biol Chem. 2002;277:30716–30723. doi: 10.1074/jbc.M202823200. [DOI] [PubMed] [Google Scholar]
- 49.Samet J, Dewar B, Wu W, Graves L. Mechanisms of Zn2+-induced signal initiation through the epidermal growth factor receptor. Toxicol Appl Pharmacol. 2003;191:86–93. doi: 10.1016/s0041-008x(03)00219-9. [DOI] [PubMed] [Google Scholar]
- 50.Amos S, Martin P, Polar G, Parsons S, Hussaini I. Phorbol 12-myristate 13-acetate induces epidermal growth factor receptor transactivation via protein kinase Cd/c-Src pathways in glioblastoma cells. J Biol Chem. 2005;280:7729–7738. doi: 10.1074/jbc.M409056200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wu W, Wages PA, Devlin RB, Diaz-Sanchez D, Peden DB, Samet JM. Src-mediated EGF receptor activation regulates ozone-induced interleukin 8 expression in human bronchial epithelial cells. Environ Health Perspect. 2015;123:ehp.1307379. doi: 10.1289/ehp.1307379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tal TL, Graves LM, Silbajoris R, Bromberg PA, Wu W, Samet JM. Inhibition of protein tyrosine phosphatase activity mediates epidermal growth factor receptor signaling in human airway epithelial cells exposed to Zn2+ Toxicology and Applied Pharmacology. 2006;214:16–23. doi: 10.1016/j.taap.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez-Boulan E, Nelson WJ. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989;245:718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- 54.Eisenhoffer GT, Rosenblatt J. Bringing balance by force: live cell extrusion controls epithelial cell numbers. Trends Cell Biol. 2013;23:185–192. doi: 10.1016/j.tcb.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Buchheit CL, Weigel KJ, Schafer ZT. Cancer cell survival during detachment from the ECM: multiple barriers to tumour progression. Nat Rev Cancer. 2014;14:632–641. doi: 10.1038/nrc3789. [DOI] [PubMed] [Google Scholar]
- 56.Slattum GM, Rosenblatt J. Tumour cell invasion: an emerging role for basal epithelial cell extrusion. Nat Rev Cancer. 2014;14:495–501. doi: 10.1038/nrc3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Slattum G, Gu Y, Sabbadini R, Rosenblatt J. Autophagy in oncogenic K-Ras promotes basal extrusion of epithelial cells by degrading S1P. Current Biol. 2014;24:19–28. doi: 10.1016/j.cub.2013.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta - Mol Cell Res. 2013;1833:3481–3498. doi: 10.1016/j.bbamcr.2013.06.026. [DOI] [PubMed] [Google Scholar]
- 59.Yamamoto S, Yako Y, Fujioka Y, Kajita M, Kameyama T, Kon S, Ishikawa S, Ohba Y, Ohno Y, Kihara A, Fujita Y. A role of the sphingosine-1-phosphate (S1P)-S1P receptor 2 pathway in epithelial defense against cancer (EDAC) Mol Biol Cell. 2016;27:491–499. doi: 10.1091/mbc.E15-03-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kajita M, Fujita Y. EDAC: Epithelial defence against cancer - cell competition between normal and transformed epithelial cells in mammals. J Biochem. 2015;158:15–23. doi: 10.1093/jb/mvv050. [DOI] [PubMed] [Google Scholar]
- 61.Wei W, Tweardy DJ, Zhang M, Zhang X, Landua J, Petrovic I, Bu W, Roarty K, Hilsenbeck SG, Rosen JM, Lewis MT. STAT3 signaling is activated preferentially in tumor-initiating cells in Claudin-Low models of human breast cancer. Stem Cells. 2014;32:2571–2582. doi: 10.1002/stem.1752. [DOI] [PubMed] [Google Scholar]
- 62.Lee HJ, Zhuang G, Cao Y, Du P, Kim HJ, Settleman J. Drug resistance via feedback activation of Stat3 in oncogene-addicted cancer cells. Cancer Cell. 2014;26:207–221. doi: 10.1016/j.ccr.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 63.Balanis N, Wendt MK, Schiemann BJ, Wang Z, Schiemann WP, Carlin CR. Epithelial to mesenchymal transition promotes breast cancer progression via a fibronectin-dependent STAT3 signaling pathway. J Biol Chem. 2013;288:17954–17967. doi: 10.1074/jbc.M113.475277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hardy K, Booth B, Hendrix M, Salomon D, Strizzi L. ErbB/EGF signaling and EMT in mammary development and breast cancer. J Mamm Gland Biol Neoplasia. 2010;15:191–199. doi: 10.1007/s10911-010-9172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Banerjee K, Resat H. Constitutive activation of STAT3 in breast cancer cells: A review. Int J Cancer. 2016;138:2570–2578. doi: 10.1002/ijc.29923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ng DCH, Lin BH, Lim CP, Huang G, Zhang T, Poli V, Cao X. Stat3 regulates microtubules by antagonizing the depolymerization activity of stathmin. J Cell Biol. 2006;172:245–257. doi: 10.1083/jcb.200503021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tanos B, Rodriguez-Boulan E. The epithelial polarity program: machineries involved and their hijacking by cancer. Oncogene. 2008;27:6939–6957. doi: 10.1038/onc.2008.345. [DOI] [PubMed] [Google Scholar]
- 68.Qian X, Karpova T, Sheppard AM, McNally J, Lowy DR. E-cadherin-mediated adhesion inhibits ligand-dependent activation of diverse receptor tyrosine kinases. EMBO J. 2004;23:1739–1748. doi: 10.1038/sj.emboj.7600136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chiasson-MacKenzie C, Morris ZS, Baca Q, Morris B, Coker JK, Mirchev R, Jensen AE, Carey T, Stott SL, Golan DE, McClatchey AI. NF2/Merlin mediates contact-dependent inhibition of EGFR mobility and internalization via cortical actomyosin. J Cell Biol. 2015;211:391–405. doi: 10.1083/jcb.201503081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.He C, Hobert M, Friend L, Carlin C. The epidermal growth factor receptor juxtamembrane domain has multiple basolateral plasma membrane localization determinants, Including a dominant signal with a polyproline core. J Biol Chem. 2002;277:38284–38293. doi: 10.1074/jbc.M104646200. [DOI] [PubMed] [Google Scholar]
- 71.Ryan S, Verghese S, Cianciola NL, Cotton CU, Carlin CR. Autosomal recessive polycystic kidney disease epithelial cell model reveals multiple basolateral epidermal growth factor receptor sorting pathways. Mol Biol Cell. 2010;21:2732–2745. doi: 10.1091/mbc.E09-12-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vasilatos SN, Broadwater G, Barry WT, Baker JC, Lem S, Dietze EC, Bean GR, Bryson AD, Pilie PG, Goldenberg V, Skaar D, Paisie C, Torres-Hernandez A, Grant TL, Wilke LG, Ibarra-Drendall C, Ostrander JH, D’Amato NC, Zalles C, Jirtle R, Weaver VM, Seewaldt VL. CpG island tumor suppressor promoter methylation in non-BRCA-associated early mammary carcinogenesis. Cancer Epidemiology Biomarkers Prevention. 2009;18:901–914. doi: 10.1158/1055-9965.EPI-08-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mimura M, Masuda A, Nishiumi S, Kawakami K, Fujishima Y, Yoshie T, Mizuno S, Miki I, Ohno H, Hase K, Minamoto T, Azuma T, Yoshida M. AP1B plays an important role in intestinal tumorigenesis with the truncating mutation of an APC gene. Int J Cancer. 2011;130:1011–1020. doi: 10.1002/ijc.26131. [DOI] [PubMed] [Google Scholar]
- 74.Fölsch H. The building blocks for basolateral vesicles in polarized epithelial cells. Trends Cell Biol. 2005;15:222–228. doi: 10.1016/j.tcb.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 75.Conibear E. Vesicle transport: Springing the TRAPP. Curr Biol. 21:R506–R508. doi: 10.1016/j.cub.2011.05.045. [DOI] [PubMed] [Google Scholar]
- 76.de Diesbach P, Medts T, Carpentier S, D’Auria L, Van Der Smissen P, Platek A, Mettlen M, Caplanusi A, van den Hove M-F, Tyteca D, Courtoy PJ. Differential subcellular membrane recruitment of Src may specify its downstream signalling. Exp Cell Res. 2008;314:1465–1479. doi: 10.1016/j.yexcr.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 77.Inman JL, Robertson C, Mott JD, Bissell MJ. Mammary gland development: cell fate specification, stem cells and the microenvironment. Development. 2015;142:1028–1042. doi: 10.1242/dev.087643. [DOI] [PubMed] [Google Scholar]
- 78.Stadler ZK, Come SE. Review of gene-expression profiling and its clinical use in breast cancer. Crit Rev Oncol/Hematol. 2009;69:1–11. doi: 10.1016/j.critrevonc.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 79.Badve S, Dabbs DJ, Schnitt SJ, Baehner FL, Decker T, Eusebi V, Fox SB, Ichihara S, Jacquemier J, Lakhani SR, Palacios J, Rakha EA, Richardson AL, Schmitt FC, Tan PH, Tse GM, Weigelt B, Ellis IO, Reis-Filho JS. Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Mod Pathol. 2011;24:157–167. doi: 10.1038/modpathol.2010.200. [DOI] [PubMed] [Google Scholar]
- 80.Molyneux G, Geyer FC, Magnay FA, McCarthy A, Kendrick H, Natrajan R, MacKay A, Grigoriadis A, Tutt A, Ashworth A, Reis-Filho JS, Smalley MJ. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell. 2010;7:403–417. doi: 10.1016/j.stem.2010.07.010. [DOI] [PubMed] [Google Scholar]