Abstract
The existence of a symbiotic relationship between Trichomonas vaginalis and Mycoplasma hominis, which is the first reported example of symbiosis between two obligate human pathogens, has been recently reported by our research group. In this work, we examined the cellular location of M. hominis in respect to T. vaginalis. By using gentamicin protection assays, double immunofluorescence, and confocal microscopy, we obtained strong evidence that M. hominis is located within protozoan cells. 5-Bromodeoxyuridine incorporation assays showed that intracellularly located mycoplasmas actively synthesize DNA. Our results demonstrate that M. hominis has the capability of entering trichomonad cells and of replicating inside the protozoon. These findings suggest that symbiosis might provide the bacteria, during human infection, with the capability to resist to environmental stresses, such as host defense mechanisms and pharmacological therapies.
Trichomonas vaginalis is a parasitic protozoon responsible for trichomoniasis, one of the most common sexually transmitted diseases in humans, estimated to affect at least 200 million people worldwide (37). Studies carried out with large groups of women suffering from vaginitis showed that T. vaginalis is clinically associated with Mycoplasma hominis (18, 35), a bacterium that, like the protozoon, resides exclusively in the human genital tract. The association is strictly species specific, since is not observed with Ureaplasma urealyticum, another Mollicutes species that is a much more common inhabitant of the human genital tract. The clinical association between the two pathogens has been recently explained by the demonstration of a symbiotic relationship between T. vaginalis and M. hominis (27). More than 90% of T. vaginalis clinical isolates from our collection proved to be infected by M. hominis independent of their geographic origin. Our recent work has allowed us to shed light on some aspects of the phenomenon (28).
The presence of endosymbionts in free-living protozoa is frequently described, but it has been never reported in obligate parasitic protozoa. The first example of symbiosis involving a human pathogen was described for Legionella pneumophila, a bacterial pathogen that is responsible for Legionnaire's disease, and Acanthamoeba sp., a free-living opportunistic amoeba (30). The relationship between T. vaginalis and M. hominis is the only one described so far involving two obligate human pathogens. T. vaginalis is responsible for severe vaginitis accompanied by abdominal pain, itching, and foul-smelling discharge (29) and is mainly asymptomatic in men (20). Moreover, trichomoniasis is associated with an enhanced risk of neoplastic transformation in cervical tissues (38) and increased human immunodeficiency virus seroconversion in women (22, 31). The mechanisms by which T. vaginalis exerts its pathogenic effects involve adhesion to host cells (1, 2, 19) and the activity of pH-dependent pore-forming proteins (14, 15) and of cytoskeleton-disrupting proteases (16). M. hominis can be isolated from the genital tracts of both symptomatic and asymptomatic individuals. Nevertheless, there is evidence that the bacterium may play important etiologic roles in genital tract diseases of both men and women (21, 32).
Interestingly, both M. hominis infections and trichomoniasis are associated with several pregnancy and postpartum complications, including preterm delivery and low-birth-weight infants (6, 9, 24, 25). Mycoplasmas are the smallest self-replicating organisms, with a genome of limited size that determines a strong metabolic dependence on host cells. The issue of whether mycoplasmas localize on the surface or within the host cell has long been debated, and intensive investigations of several mycoplasmal species have been carried out (23). In recent years, a lot of strong evidence has shown that numerous pathogenic mycoplasmas can be intracellularly located, which allows bacterial survival over extended periods (4, 10, 33). Intracellular location may protect mycoplasmas against the effects of the host immune response and antibiotics and may account to some extent for the difficulty of eradicating infection.
In this work, we have investigated the location of M. hominis with respect to trichomonad cells. The use of gentamicin protection assays, coupled with confocal microscopy and double-immunofluorescence techniques, showed that M. hominis cells are located and can survive within trichomonad cells. Moreover, 5-bromodeoxyuridine (5-BrdU) incorporation assays have been performed to demonstrate that intracellular M. hominis actively synthesizes DNA.
MATERIALS AND METHODS
Organisms and cultivation.
Four T. vaginalis isolates were included in this study. Three of them were naturally infected with M. hominis (SS14, SS15, and MPM2), and one (SS22) was M. hominis free. The presence or absence of mycoplasmas in each trichomonad strain was assessed by both cultural and molecular methods, as described elsewhere (28). T. vaginalis isolates were cultured by daily passages at 1:16 dilution in fresh Diamond's TYM medium (11) plus 10% fetal bovine serum. Infected and uninfected protozoa, as determined at the moment of isolation from vaginal discharge, were cultured in separate incubators and passaged daily using two different laminar flow hoods. The incubators and culture media were constantly monitored to ensure they were mycoplasma free.
M. hominis cells were isolated from naturally infected T. vaginalis strains as follows: an aliquot of each trichomonad culture was centrifuged at 500 × g for 10 min, and the supernatants were filtered through a 0.45-μm-pore-size filter membrane; aliquots of the filtered supernatant were both inoculated in BEA medium (3) and plated on BE agar and then incubated at 37°C. The isolated bacteria were identified as M. hominis by PCR using specific primers (5). M. hominis isolates from protozoa were named after their originating host, enclosed in brackets, i.e., [ss14] refers to M. hominis originating from the SS14 trichomonad isolate. M. hominis isolate PG21 was kindly provided by G. Christiansen (Department of Medical Microbiology and Immunology, University of Aarhus, Aarhus, Denmark) and was used as a control.
Determination of in vitro gentamicin susceptibility.
The MICs of gentamicin for the three M. hominis isolates [ss14], [ss15], and [mpm2] were determined. Mid-log-phase bacteria were incubated in both BEA medium and Diamond medium containing serial dilutions of gentamicin ranging from 250 to 0.125 μg ml−1. All samples were incubated at 37°C for 7 days. Every 24 h, 100 μl of each broth culture was harvested and centrifuged at 20,000 × g for 20 min; the pellet was then washed once in phosphate-buffered saline (PBS) and resuspended in fresh antibiotic-free BEA medium. Bacterial growth was determined by evaluating the induction of color change. The mycoplasma-free T. vaginalis SS-22 isolate was cultivated for 1 week in the presence of the same concentrations of antibiotics to assess the absence of any toxic effect of gentamicin.
Gentamicin protection assay and PCR analysis.
In our experimental model, we used gentamicin at a concentration of 50 μg ml−1, which is bactericidal and has no long-term cytotoxic effect on trichomonad cells. A gentamicin protection assay was carried out, using a modification of the protocol reported by Elsinghorst (13). Mycoplasma-infected T. vaginalis SS14, SS15, and MPM2 were cultivated for 1 month by daily passages at 1:16 dilution in Diamond's TYM supplemented with 50 μg of gentamicin ml−1. Aliquots of each culture were taken at different times (every day the first week and then every 7 days for three more weeks), to assess long-term intracellular survival. The harvested cells were washed three times in PBS in order to remove antibiotics and any residual extracellular mycoplasmas and were then resuspended in BEA. Proper dilutions of the suspension were finally seeded on BEA agar plates and incubated at 37°C for 1 week. After 30 days of coincubation with gentamicin, an aliquot of each trichomonad culture was washed extensively to remove antibiotics and cultured in gentamicin-free Diamond medium for another 2 weeks. Aliquots of protozoa were taken on days 1, 4, 7, and 14 after antibiotic removal, washed, and seeded on solid BEA medium as described above. Reisolated mycoplasmas were finally incubated in the presence of gentamicin to rule out the selection of drug-resistant organisms. The identity of reisolated M. hominis was confirmed by PCR.
An aliquot of each trichomonad sample collected during and after the gentamicin protection assay was subjected to total DNA extraction as described elsewhere (26). Detection of M. hominis-specific DNA by PCR was performed as described previously (5). M. hominis isolates [ss14], [ss15], and [mpm2] were cultured in Diamond medium under the same conditions but without T. vaginalis and were processed like the other samples.
Confocal microscopy.
A volume of 200 μl from a culture of M. hominis-infected trichomonad MPM2 containing 106 organisms/ml was seeded in 24-well plates containing a round 12-mm-diameter coverslip in each well and incubated at 37°C for 5 to 10 min. This time is sufficient to induce slight adhesion to the glass without dramatically modifying the trichomonad shape. The cells were then gently washed with PBS, fixed with 4% paraformaldehyde in PBS for 1 h, and permeabilized in 0.2% Triton X-100 in PBS for 10 min. M. hominis cells were detected by incubating protozoan cells with anti-M. hominis rabbit polyclonal antibodies and then with fluorescein isothyocianate (FITC)-labeled goat anti-rabbit antibodies. Confocal images were acquired using a Microradiance argon confocal laser scanning microscope (Bio-Rad Laboratories Europe Ltd., Hemel Hempstead, United Kingdom) mounted on a Nikon Eclipse 600 upright microscope equipped with a 60×/1.4 oil immersion objective. Optical sections were acquired in sequential mode in 0.5-μm steps, moving from the apical to the basal cell regions. Stacks of images were processed with Lasersharp (Bio-Rad) and Image J (http://rsb.info.nih.gov/ij/) software.
Differential immunofluorescence staining of intracellular versus extracellular mycoplasmas.
The presence of mycoplasmas within trichomonad cells was investigated by double-immunofluorescence microscopy, modifying the procedure previously described (17, 36). T. vaginalis isolates SS14 and SS22 were seeded in 24-well plates containing a round 12-mm-diameter coverslip in each well and incubated at 37°C in Diamond's TYM medium as described above. The cells were then gently washed, and extracellular M. hominis cells were detected by incubating unpermeabilized cells with anti-M. hominis rabbit polyclonal antibodies (kindly provided by G. Christiansen) for 1 h at 4°C and then for 30 min at 4°C with rhodamine-labeled goat anti-rabbit antibodies. The T. vaginalis cells were then fixed with 4% paraformaldehyde in PBS for 1 h, washed, and permeabilized with methanol at −20°C for 10 min to allow intracellular antibody diffusion. The cells were incubated again with the same anti-M. hominis rabbit polyclonal antibodies and then with FITC-labeled goat anti-rabbit antibodies to stain extracellular and intracellular bacteria. The slides were observed with an Olympus BX51 fluorescence microscope equipped with a UplanApo 100×/1.35 objective. Images were acquired with a cooled Magnafire charge-coupled device camera (Optronics, Goleta, Calif.) and processed using Image J software.
5-BrdU incorporation assay.
Mycoplasma-infected T. vaginalis SS14 was cultivated for 48 h in the presence of 50 μg of gentamicin ml−1 and 30 μg of 5-BrdU ml−1 (12). The cells were then seeded in a 24-well plate containing a round 12-mm-diameter coverslip in each well and incubated at 37°C in the same medium for 5 to 10 min. The cells were then washed with PBS, fixed with 4% paraformaldehyde for 1 h, and permeabilized with 0.2% Triton X-100 for 15 min. Then, cells were treated with 2 N HCl for 30 min in order to denature the DNA, and a borate buffer (0.1 M sodium tetrahydroborate, pH 8.5) was added in order to restore neutral pH. Incorporation of 5-BrdU was finally detected by incubating the cells with mouse monoclonal anti-5-BrdU antibody for 1 h and then with rhodamine-labeled goat anti-mouse antibodies for 1 h. In order to colocalize M. hominis cells, coverslips were subsequently incubated with rabbit polyclonal anti-M. hominis antibodies and then with FITC-conjugated mouse anti-rabbit antibodies.
RESULTS
T. vaginalis can protect M. hominis from the toxic effect of gentamicin.
Gentamicin is a bactericidal antibiotic that is unable to reach the intracellular compartment of eukaryotic cells. Therefore, bacteria adherent to the host cell surface are killed by the drug, while internalized organisms are protected from its effect. The MIC of gentamicin, calculated after 7 days of incubation in BEA medium, was 10 μg ml−1 for all three M. hominis isolates used in our experiments. Gentamicin proved to be bactericidal in BE medium at a concentration of 20 μg ml−1, while determination of M. hominis susceptibility to the drug in Diamond medium showed a bactericidal effect at a concentration of 2 μg ml−1. In order to assess whether M. hominis is associated with the membrane or is able to reside inside T. vaginalis cells, three infected protozoan cultures were treated with gentamicin at a concentration of 50 μg ml−1, which is bactericidal but shows no toxic effects on trichomonad cells. The treated trichomonad cells were seeded on BEA agar at different times throughout the experiment. As shown in Table 1, mycoplasmas were isolated from two out of three M. hominis-infected T. vaginalis cells after up to 1 week of gentamicin treatment. After this period, we were unable to isolate viable mycoplasmas from any gentamicin-treated T. vaginalis organism. Gentamicin treatment was carried out for 30 days, and then the antibiotics were removed and the protozoa were kept in culture in gentamicin-free medium for another 2 weeks. One week after gentamicin removal, bacterial growth could be observed again in strains SS14 and MPM2. M. hominis from T. vaginalis isolate SS15, which grew on BE agar only in the first 2 days of antibiotic treatment, did not grow after gentamicin removal. No selection for gentamicin-resistant organisms was observed in the reisolated bacteria. As a control, M. hominis isolates [ss14], [ss15], and [mpm2] were cultured and processed in the same manner: mycoplasmas were reisolated only during the first day of gentamicin treatment, and no bacterial growth was observed after antibiotic removal. This was not unexpected, since M. hominis is unable to grow in Diamond medium without live T. vaginalis (28).
TABLE 1.
Gentamicin protection assaya
| Treatment period | Day |
T. vaginalis SS14
|
T. vaginalis SS15
|
T. vaginalis MPM2
|
|||
|---|---|---|---|---|---|---|---|
| Bacterial growth | PCR | Bacterial growth | PCR | Bacterial growth | PCR | ||
| Gentamicin treatment | 1 | + | + | + | + | + | + |
| 2 | + | + | + | + | + | + | |
| 3 | + | + | − | + | + | + | |
| 4 | + | + | − | + | + | + | |
| 5 | + | + | − | + | + | + | |
| 6 | + | + | − | − | − | + | |
| 7 | − | + | − | − | − | + | |
| 14 | − | + | − | − | − | + | |
| 21 | − | + | − | − | − | + | |
| 28 | − | + | − | − | − | + | |
| After gentamicin | 1 | − | + | − | − | − | + |
| removal | 4 | − | + | − | − | − | + |
| 7 | + | + | − | − | + | + | |
| 14 | + | + | − | − | + | + | |
Colony growth and specific DNA amplification of M. hominis from three T. vaginalis strains during a gentamicin protection assay and after drug removal. +, positive; −, negative.
At each time point throughout the gentamicin protection experiment, an aliquot of treated trichomonad cells was subjected to PCR analysis to highlight mycoplasmal DNA. Table 1 compares the results obtained by PCR with those related to the isolation of viable mycoplasmas. Interestingly, in T. vaginalis SS15, PCR analysis detected DNA specific for M. hominis only in the first 5 days of gentamicin treatment while the other two strains tested positive throughout the entire experiment. In control M. hominis without trichomonad cells, specific DNA was detectable only in the first 4 days of gentamicin treatment.
M. hominis can localize inside T. vaginalis cells.
In order to further characterize the interaction of M. hominis with T. vaginalis cells, we used indirect immunofluorescence assays to assess the effective location of bacteria and to differentiate internalized from adherent extracellular bacteria. Mycoplasma-infected protozoa were FITC labeled using specific anti-Mycoplasma antibodies and analyzed by confocal laser scanning microscopy.
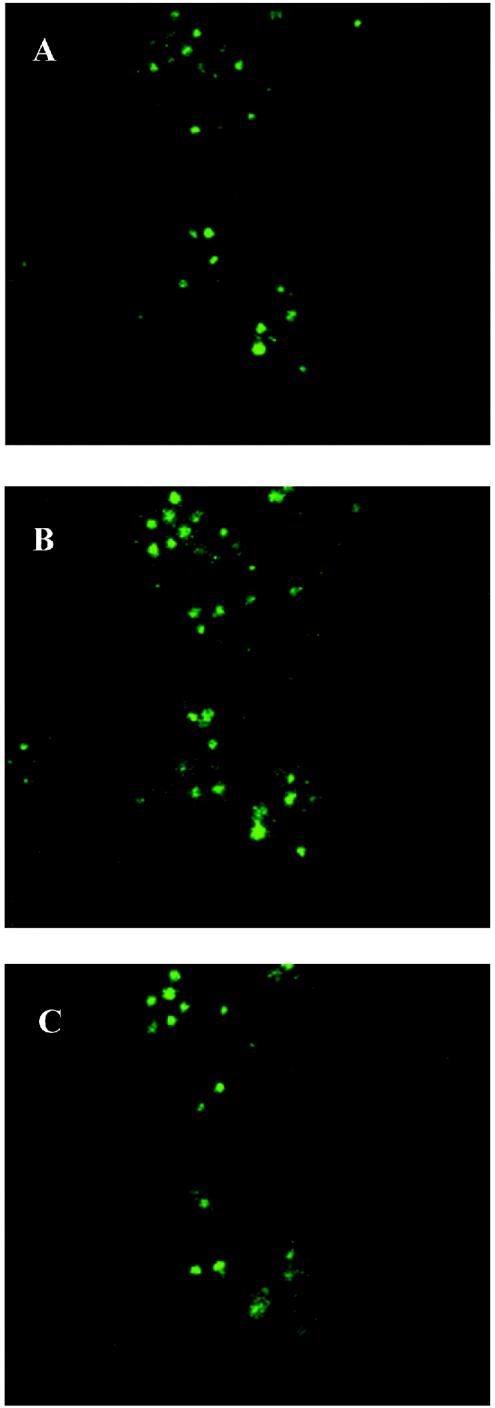
As can be seen in Fig. 1, the distribution of mycoplasmas is not limited to apical (Fig. 1A) and basal (Fig. 1C) sections; bacteria are also detectable in large numbers in the equatorial section (Fig. 1B), suggesting that the bacterium is able to enter the protozoan cell.
FIG. 1.
Confocal laser scanning microscopy; confocal micrographs depicting the interaction of M. hominis with T. vaginalis strain MPM2. Mycoplasmas were anti-M. hominis and FITC labeled. Optical sections were taken from the apical surface (A) of the protozoan cells, moving downward toward the basal surface (C). Section B was taken in the equatorial region of trichomonad cells and shows specific internal fluorescence.
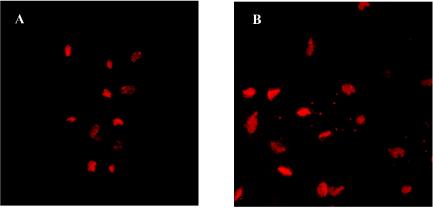
In order to determine whether bacterial growth during and after gentamicin treatment is the consequence of an actual intracellular location, as suggested by confocal microscopy images, we differentially stained impermeable versus permeabilized trichomonad cells with polyclonal antibodies to M. hominis. The results are illustrated in Fig. 2, which shows three micrographs of the same area of a monolayer of T. vaginalis cells infected by M. hominis. Extracellular mycoplasmas (which fluoresce red) were visualized using the rhodamine filter set (Fig. 2B), while FITC filtering (Fig. 2A) revealed both extracellular and intracellular bacteria (which fluoresce green). Figure 2C indicates the localization of extracellular and intracellular mycoplasmas. It is interesting that not all T. vaginalis organisms appear to be infected by mycoplasmas: we previously demonstrated, in different T. vaginalis isolates, a strain-to-strain difference in the multiplicity of infection by M. hominis, and we also noted a remarkable cell-to-cell difference among the strains (28). In fact, specific features of a single trichomonad cell could be responsible for different numbers of infecting bacteria.
FIG. 2.
Double-imunofluorescence micrographs depicting the cellular localization of M. hominis infecting T. vaginalis strain MPM2. Panels A to C represent the same area of a protozoan monolayer immunostained as described in Materials and Methods. (A) FITC fluorescence showing extracellular and intracellular mycoplasmas.(B) Rhodamine fluorescence showing mycoplasmas that are extracellularly located. (C) Superimposed images of panels A and B indicating the localization of extracellular (red) and intracellular (green) mycoplasmas.
No immunofluorescence was detected when the same experiments were performed using T. vaginalis strain SS22, which is naturally M. hominis free (data not shown). These data demonstrate the presence of mycoplasmas within the protozoan cells and confirm the results obtained with the gentamicin protection assay.
Intracellular persistence of M. hominis within T. vaginalis.
The intracellular persistence of M. hominis was studied by specific PCR analysis throughout 30 days of gentamicin treatment of mycoplasma-infected T. vaginalis cultures. As shown in Table 1, we observed specific mycoplasmal-DNA amplification throughout the whole gentamicin treatment for two out of three isolates analyzed. In fact, specific DNA for M. hominis was detected in trichomonad strain SS15 only in the first 5 days of antibiotic treatment. This result is consistent with the data obtained by cultivating the same samples in M. hominis-specific medium. The persistence of DNA specific for M. hominis throughout the long-term gentamicin treatment, together with the reisolation of living bacteria after drug removal, strongly indicates active multiplication of mycoplasmas inside the protozoan cell. In fact, the daily dilution for routine culturing of infected T. vaginalis is ∼1:16, corresponding, at the end of the 30-day treatment, to a dilution of 1/1630. Hence, since M. hominis is unable to grow in Diamond medium alone, the total bacterial load would have been eliminated in a few days without multiplication within T. vaginalis.
Intracellular M. hominis is able to synthesize DNA.
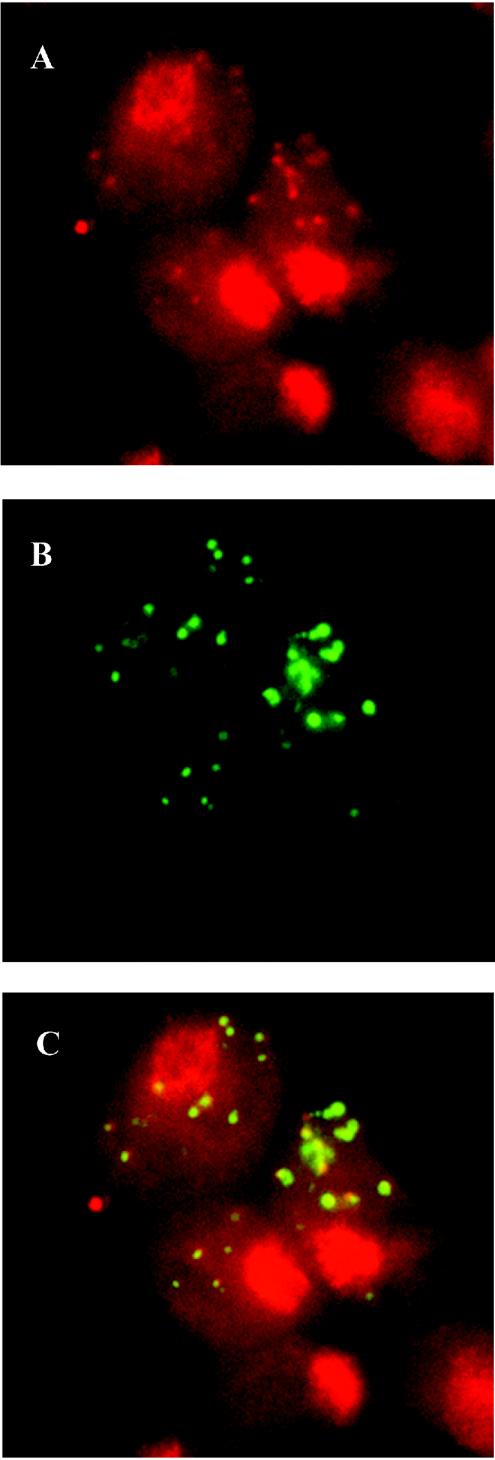
To confirm the hypothesis of intracellular multiplication of M. hominis, we performed a 5-BrdU incorporation assay of DNA from Mycoplasma-infected protozoan cells. T. vaginalis is an ancient protozoon lacking mitochondria and therefore does not possess any extranuclear DNA (8). Consequently, any 5-BrdU incorporation in a location other than nuclear should be due to the presence of another actively replicating organism. Experiments were performed under gentamicin pressure to eliminate any extracellular or membrane-associated M. hominis. Incorporation of 5-BrdU was detected using specific rhodamine-conjugated anti-5-BrdU antibodies. Micrographs show a high number of fluorescent spots in the cytoplasm of trichomonad strain SS14 (Fig. 3A) and no fluorescence in the cytoplasm of strain SS22 (Fig. 3B), which is not parasitized by mycoplasmas. These results indicate that M. hominis cells located within T. vaginalis actively synthesize DNA. In order to confirm that the extranuclear 5-BrdU incorporation observed in trichomonad strain SS14 is actually due to the presence of intracellular M. hominis, we performed a colocalization experiment using specific anti-mycoplasma antibodies. A 5-BrdU incorporation experiment was performed as described above using T. vaginalis SS14 cells, and protozoa were subsequently incubated with anti-M. hominis and FITC-conjugated antibodies. Figure 4 shows three micrographs of the same area of a protozoan monolayer treated as described above. The red extranuclear spots indicate active DNA synthesis by organisms other than T. vaginalis, and their identity with intracellular M. hominis was confirmed by their colocalization with anti-M. hominis antibodies (Fig. 4C).
FIG. 3.
5-BrdU incorporation assay; detection of 5-BrdU incorporation by M. hominis located within T. vaginalis cells. (A) Mycoplasma-free T. vaginalis isolate SS22; (B) M. hominis-infected T. vaginalis SS14 after 48 h of incubation with 5-BrdU and gentamicin. Incorporation has been highlighted using anti-5-BrdU antibodies. DNA biosynthesis is detectable in T. vaginalis strain nuclei and in the cytoplasm of Mycoplasma-infected strain SS14.
FIG. 4.
Colocalization of anti-5-BrdU and anti-M. hominis antibodies. The micrographs show the colocalization of BrdU incorporation and M. hominis in T. vaginalis cells. (A) Incorporation of 5-BrdU (red spots) detected by specific antibodies. (B) The same field showing T. vaginalis-associated M. hominis detected by anti-M. hominis antibodies (green spots). (C) Superimposition of images A and B demonstrating the exact colocalization of the two fluorescent signals.
DISCUSSION
In this report, we provide experimental evidence of the localization and multiplication of M. hominis within T. vaginalis cells. The presence of mycoplasmas within T. vaginalis cells was demonstrated by several approaches. By gentamicin protection assay, we showed that M. hominis associated with T. vaginalis is able to survive under drug pressure for at least 1 month. Since cell cultures were passaged every day at a 1:16 dilution with fresh medium supplemented with gentamicin, the persistence of mycoplasmal DNA after 30 days clearly indicates that M. hominis not only resists the bactericidal effect of the antibiotic but also multiplies within T. vaginalis cells. This finding was further corroborated by the reisolation of M. hominis cells from T. vaginalis cultures after gentamicin removal. Interestingly, one out of three M. hominis strains was not able to resist gentamicin, as demonstrated by the inability of PCR to reveal mycoplasmal DNA after the fifth day of gentamicin treatment and by the failure of bacterial reisolation after drug removal. This result indicates that not all M. hominis isolates can be considered intracellular microorganisms, suggesting the existence of isolate-to-isolate differences in the ability to invade host cells and survive intracellularly. Alternatively, different capabilities of T. vaginalis isolates to sustain the intracellular growth of bacteria could be hypothesized. The intracellular location of mycoplasmas was further confirmed by double immunofluorescence. Differential labeling of extracellular and intracellular mycoplasmas in fact represents an accurate and reliable method to investigate the cellular location of bacterial cells (17, 36).
Internalized bacteria decrease significantly in the long period during the gentamicin protection assay and are detectable only by PCR after the first week of treatment. This suggests either that replication of M. hominis occurs mainly in an extracellular location or that there is a concomitant release of bacteria into the extracellular compartment. The question of the capability of M. hominis to actively multiply inside T. vaginalis cells has been addressed in this study.
T. vaginalis is an amitochondriate protist, and the absence of cytoplasmic DNA has been definitively demonstrated (8). By using specific antibodies, we highlighted the incorporation of 5-BrdU in the trichomonad cytoplasm, which could be due only to DNA synthesis by mycoplasmas. The use of anti-mycoplasma antibodies that precisely colocalized with the 5-BrdU-positive spots further confirmed these findings.
The intracellular localization of M. hominis in mammalian cells has been reported by other authors (33). The persistence of mycoplasmas in the T. vaginalis cytoplasm as well indicates that the bacteria are able to evolve specific strategies to resist killing mechanisms and to adapt to intracellular environments. The presence of endosymbionts is frequently reported in free-living protozoa that are able to establish symbiotic relationships with algae, archaea, or bacteria. In these cases, protozoa can act as microbial reservoirs, protecting microorganisms from environmental stresses. On the other hand, pathogenic microorganisms have only rarely been described as symbionts, and the role of protozoa as vectors for the transmission of human diseases has received little attention. Interest increased after the finding that symbiotic relationships between protozoa and bacteria could exert a strong influence on the pathogenesis of one or both microorganisms (7).
The ability of M. hominis to invade, survive, and multiply within the T. vaginalis cytoplasm represents an important defense mechanism for these bacteria during human infection. It is interesting to speculate that entry into protozoan cells may provide a protected niche for M. hominis survival, which may help to explain the ability of the bacterium to persist in the adverse environment of the vaginal tract (34) despite antimicrobial therapy and host immunoresponse.
The close relationship between M. hominis and T. vaginalis is confirmed by clinical studies: in a group of 804 women, van Belkun and colleagues observed that “in 79% of all samples positive for T. vaginalis, M. hominis could be detected, compared to only 6% in control samples” (35). These results are in perfect agreement with those recently obtained by our group for >200 symptomatic women (unpublished data). Since both microorganisms are obligate human parasites unable to survive freely in the environment, the stable and long-lasting symbiosis must be established within the infected human genital tract. The results presented in this work suggest a role for the protozoon in transmitting M. hominis infection to a new host in vivo.
The relationship between T. vaginalis and its prokaryotic symbiont remains largely unknown. In particular, the physiological and nutritional interchanges occurring between M. hominis and its protozoan host should be explored, as well as the possibility that T. vaginalis derives some advantage from the presence of intracellular bacteria. Finally, the relationship between T. vaginalis and M. hominis could represent an interesting model to better understand basic biological mechanisms of microbial symbiosis and the origin of intracellular organelles.
Acknowledgments
This work was supported by the Ministero dell'Istruzione, dell'Università e della Ricerca of Italy (PRIN 2003) and by the University of Sassari (Progetto di ricerca sul 60%, Centro di Eccellenza sulla Biodiversità).
D.D. is grateful to Gunna Christiansen (University of Aarhus, Aarhus, Denmark) for introducing him to the field of microplasmology.
Editor: W. A. Petri, Jr.
REFERENCES
- 1.Addis, M. F., P. Rappelli, and P. L. Fiori. 2000. Host and tissue specificity of Trichomonas vaginalis is not mediated by its known adhesion proteins. Infect. Immun. 68:4358-4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alderete, J. F., and E. Pearlman. 1984. Pathogenic Trichomonas vaginalis cytotoxicity to cell culture monolayers. Br. J. Vener. Dis. 60:99-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andersen, H., S. Birkelund, G. Christiansen, and E. A. Freundt. 1987. Electrophoretic analysis of proteins from Mycoplasma hominis strains detected by SDS-PAGE, two-dimensional gel electrophoresis and immunoblotting. J. Gen. Microbiol. 133:181-191. [DOI] [PubMed] [Google Scholar]
- 4.Baseman, J. B., M. Lange, N. L. Criscimagna, J. A. Giron, and C. A. Thomas. 1995. Interplay between mycoplasmas and host target cells. Microb. Pathog. 19:105-116. [DOI] [PubMed] [Google Scholar]
- 5.Blanchard, A., A. Yanez, K. Dybvyg, H. L. Watson, G. Griffiths, and G. H. Cassel. 1993. Evaluation of intraspecies genetic variation within the 16S rRNA gene of M. hominis and detection by PCR. J. Clin. Microbiol. 31:1358-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassell, G. H., K. B. Waites, and D. T. Crouse. 1991. Perinatal mycoplasmal infections. Clin. Perinatol. 18:241-262. [PubMed] [Google Scholar]
- 7.Cirillo, J. D., S. L. Cirillo, L. Yan, L. E. Bermudez, S. Falkow, and L. S. Tompkins. 1999. Intracellular growth in Acanthamoeba castellanii affects monocyte entry mechanisms and enhances virulence of Legionella pneumophila. Infect. Immun. 67:4427-4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clemens, D. L., and P. J. Johnson. 2000. Failure to detect DNA in hydrogenosomes of Trichomonas vaginalis by nick translation and immunomicroscopy. Mol. Biochem. Parasitol. 106:307-313. [DOI] [PubMed] [Google Scholar]
- 9.Cotch, M. F., J. G. Pastorek, R. P. Nugent, S. L. Hillier, R. S. Gibbs, D. H. Martin, D. A. Eschenbach, R. Edelman, J. C. Carey, J. A. Regan, M. A. Krohn, M. A. Klebanoff, A. V. Rao, and G. G. Rhoads. 1997. Trichomonas vaginalis associated with low birth weight and pre-term delivery. Sex. Transm. Dis. 24:353-360. [DOI] [PubMed] [Google Scholar]
- 10.Dallo, S. F., and J. B. Baseman. 2000. Intracellular DNA replication and long-term survival of pathogenic mycoplasmas. Microb. Pathog. 29:301-309. [DOI] [PubMed] [Google Scholar]
- 11.Diamond, L. S. 1957. The establishment of various trichomonads of animals and man in axenic cultures. J. Parasitol. 43:488-490. [PubMed] [Google Scholar]
- 12.Dolbeare, F. 1995. Bromodeoxyuridine: a diagnostic tool in biology and medicine, part I: historical perspectives, histochemical methods and cell kinetics. Histochem. J. 27:339-369. [PubMed] [Google Scholar]
- 13.Elsinghorst, E. A. 1994. Measurement of invasion by gentamicin resistance. Methods Enzymol. 236:405-420. [DOI] [PubMed] [Google Scholar]
- 14.Fiori, P. L., P. Rappelli, A. M. Rocchigiani, and P. Cappuccinelli. 1993. Trichomonas vaginalis haemolysis: evidence of functional pore formation on red cell membranes. FEMS Microbiol. Lett. 109:13-18. [DOI] [PubMed] [Google Scholar]
- 15.Fiori, P. L., P. Rappelli, M. F. Addis, A. Sechi, and P. Cappuccinelli. 1996. Trichomonas vaginalis haemolysis: pH regulates a contact-independent mechanism based on pore-forming proteins. Microb. Pathog. 20:109-118. [DOI] [PubMed] [Google Scholar]
- 16.Fiori, P. L., P. Rappelli, M. F. Addis, F. Mannu, and P. Cappuccinelli. 1997. Contact-dependent disruption of the host cell membrane skeleton induced by Trichomonas vaginalis. Infect. Immun. 65:5142-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heesemann, J., and R. Laufs. 1985. Double immunofluorescence microscopic technique for accurate differentiation of extracellularly and intracellularly located bacteria in cell culture. J. Clin. Microbiol. 22:168-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koch, A., A. Bilina, L. Teodorowicz, and A. Stary. 1997. Mycoplasma hominis and Ureaplasma urealyticum in patients with sexually transmitted diseases. Wien. Klin. Wochenschr. 109:584-589. [PubMed] [Google Scholar]
- 19.Krieger, J. K., J. I. Ravdin, and M. F. Rein. 1985. Contact-dependent cytopathogenic mechanism of Trichomonas vaginalis. Infect. Immun. 50:778-786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krieger, J. N. 1990. Epidemiology and clinical manifestations of urogenital trichomoniasis in men, p. 235-245. In B. M. Honigberg (ed.), Trichomonads parasitic in humans. Springer-Verlag, New York, N.Y.
- 21.Ladefoged, S. A. 2000. Molecular dissection of Mycoplasma hominis. APMIS Suppl. 97:1-45. [PubMed] [Google Scholar]
- 22.Laga, M., A. Manoka, M. Kivuvu, B. Malele, M. Tuliza, and N. Nzila. 1993. Nonulcerative sexually transmitted diseases as risk factors for HIV-1 transmission in women: results from a cohort study. AIDS 7:95-102. [DOI] [PubMed] [Google Scholar]
- 23.Marshall, A., B. Afshar, J. Pacy, D. Pitcher, and R. Miles. 1998. Intracellular location of mycoplasmas. Microbiology 144:3240-3241. [DOI] [PubMed] [Google Scholar]
- 24.Paul, V. K., U. Gupta, M. Singh, V. L. Nag, D. Takkar, and M. K. Bhan. 1998. Association of genital mycoplasma colonization with low birth weight. Int. J. Gynaecol. Obstet. 63:109-114. [DOI] [PubMed] [Google Scholar]
- 25.Platt, R., J. S. L. Lin, J. W. Warren, B. Bosner, K. C. Edelin, and W. M. McCormack. 1980. Infection with Mycoplasma hominis in postpartum fever. Lancet ii:1217-1221. [DOI] [PubMed]
- 26.Rappelli, P., A. M. Rocchigiani, G. Erre, M. M. Colombo, P. Cappuccinelli, and P. L. Fiori. 1995. Sequence of cDNA coding for a 65 kDa adhesive protein for the specific detection of Trichomonas vaginalis by PCR. FEMS Microbiol. Lett. 129:21-26. [DOI] [PubMed] [Google Scholar]
- 27.Rappelli, P., M. F. Addis, F. Carta, and P. L. Fiori. 1998. Mycoplasma hominis parasitism of Trichomonas vaginalis. Lancet 352:1286. [DOI] [PubMed] [Google Scholar]
- 28.Rappelli, P., F. Carta, G. Delogu, M. F. Addis, D. Dessì, P. Cappuccinelli, and P. L. Fiori. 2001. Mycoplasma hominis and Trichomonas vaginalis symbiosis: multiplicity of infection and transmissibility to human cells. Arch. Microbiol. 175:70-74. [DOI] [PubMed] [Google Scholar]
- 29.Rein, M. F. 1990. Clinical manifestations of urogenital trichomoniasis in women, p. 225-234. In B. M. Honigberg (ed.), Trichomonads parasitic in humans. Springer-Verlag, New York, N.Y.
- 30.Rowbotham, T. J. 1980. Preliminary report on the pathogenicity of Legionella pneumophila for freshwater and soil amoebae. J. Clin. Pathol. 33:1179-1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutton, M. Y., M. Sternberg, M. Nsuami, F. Behets, A. M. Nelson, and M. E. St Louis. 1999. Trichomoniasis in pregnant human immunodeficiency virus-infected and human immunodeficiency virus-uninfected Congolese women: prevalence, risk factors, and association with low birth weight. Am. J. Obstet. Gynecol. 181:656-662. [DOI] [PubMed] [Google Scholar]
- 32.Taylor-Robinson, D., and W. M. McCormack. 1980. The genital mycoplasmas. N. Engl. J. Med. 302:1003-1010. [DOI] [PubMed] [Google Scholar]
- 33.Taylor-Robinson, D., H. A. Davies, P. Sarathchandra, and P. M. Furr. 1991. Intracellular location of mycoplasmas in cultured cells demonstrated by immunocytochemistry and electron microscopy. Int. J. Exp. Pathol. 72:705-714. [PMC free article] [PubMed] [Google Scholar]
- 34.Valore, E. V., C. H. Park, S. L. Igreti, and T. Ganz. 2000. Antimicrobial components of vaginal fluid. Am. J. Obstet. Gynecol. 187:561-568. [DOI] [PubMed] [Google Scholar]
- 35.van Belkun, A., C. van der Schee, W. I. van der Meijden, H. Verbrugh, and H. J. Sluiters. 2001. A clinical study on the association of Trichomonas vaginalis and Mycoplasma hominis infections in women attending a sexually transmitted disease (STD) outpatient clinic. FEMS Immunol. Med. Microbiol. 32:27-32. [DOI] [PubMed] [Google Scholar]
- 36.Winner, F., R. Rosengarten, and C. Citti. 2000. In vitro cell invasion of Mycoplasma gallisepticum. Infect. Immun. 68:4238-4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.World Health Organization. 2001. Trichomoniasis, p. 26-27. In Global prevalence and incidence of selected curable sexually transmitted infections. World Health Organization, Geneva, Switzerland.
- 38.Yap, E. H., T. H. Ho, Y. C. Chan, T. W. Thong, G. C. Ng, L. C. Ho, and M. Singh. 1995. Serum antibodies to Trichomonas vaginalis in invasive cervical cancer patients. Genitourin. Med. 71:402-404. [DOI] [PMC free article] [PubMed] [Google Scholar]