Abstract
Aging is the primary risk factor for many neurodegenerative diseases. Thus, understanding the basic biological changes that take place with aging that lead to the brain being less resilient to disease progression of neurodegenerative diseases such as Parkinson’s disease or Alzheimer’s disease or insults to the brain such as stroke or traumatic brain injuries. Clearly this will not cure the disease per se, yet increasing the ability of the brain to respond to injury could improve long term outcomes. The focus of this review is examining changes in microglia with age and possible therapeutic interventions involving the use of polyphenol rich dietary supplements.
Aging is the primary risk factor for neurodegenerative diseases and is associated with increased morbidity and mortality from acute and chronic injuries that lead to cognitive decline. One factor thought to contribute to this loss of resilience is a biological background of elevated inflammation that is characteristic of aged organisms. However the underlying molecular alterations that lead to inflammation and the therapeutic approaches to improve resiliency are not fully understood (Bennet et al., 1996; Michaud et al., 2013; Moll et al., 2014; Niccoli and Partridge 2012). Aging is a complex process that involves cellular senescence, a gradual loss of tissue homeostasis, both of which contribute to reduced organ function. Aging involves multiple mechanisms that lead to diminished organism homeostasis. It is becoming clear that the “environment” of the aged brain as well as the peripheral organs has a profound effect on the function of the brain. These age related changes can compromise the brain’s regenerative capacity in response to the CNS challenges that arise from acute injuries such as stroke or head injuries, or chronic diseases like Parkinson’s Disease and Alzheimer’s Disease. Two major biological processes that characterize this aged “environment” are oxidative stress and inflammation; microglia are one of the primary cell types in the brain that contribute to both oxidative stress and inflammation. Microglia are constantly sensing the environment and responding to numerous signals that indicate the health status of the surrounding neurons and other glial cells. In young brain these responses are appropriately balanced and microglia can effectively protect the CNS from immunologic insults, like invading pathogens, while avoiding the damage associated with sustained activation. In the aged brain microglia have been reported to be in a “primed” state where they have an increased response to pro-inflammatory cytokines such as interleukin (IL)1-β and tumor necrosis factor (TNF) α. In this primed state they also show a blunted response to anti-inflammatory signals such as IL-10 and IL-4 (Fenn et al., 2012; Lee et al., 2013; Norden et al., 2014).
Microglial changes with age
Microglia are continually assessing the microenvironment and can respond to a variety of stimuli by rapidly moving between activation states. These activation states were initially termed M1 or classical pro-inflammatory and M2 or alternative activation. There is an ongoing balance of expression of cytokines from microglia depending on the surrounding signaling molecules. However, it is important to mention that it is becoming clear that microglial phenotype is quite complex. Some researchers have suggested that microglia can be categorized into a further subdivision of phenotypes M2a, M2b and M2c in an attempt to clarify some of these differences, as these have been used to classify macrophage responses to varying stimuli (Wilcock 2012). It has also been shown that even this classification is likely too simple and that at any given time microglia can express markers of many of the subtypes of activation and perhaps we should abandon the dogma of trying to put microglia into a box (Heneka et al., 2015; Morganti et al., 2016). It has been demonstrated that in the aged brain, microglia do not respond to the environment in the same manner as young and there are high levels of IL1β and TNFα and low levels of IL-10 even under basal conditions (Gemma et al., 2005; Gemma et al., 2002; Michaud et al., 2013; Monje et al., 2003). To demonstrate this, Lee et al stimulated microglial activation in the brains of young and old mice (Lee, Ruiz et al. 2013) by treating with cocktails containing either pro-inflammatory compounds (IL1β + IL12) or the anti-inflammatory compounds IL-4 + IL-13. This study not only demonstrated that the aged brain responds more dramatically to the pro-inflammatory cocktail, but it also has an impaired or diminished response to the anti-inflammatory stimuli. This observation has been replicated with isolated microglia and has been termed “priming” (Fenn et al., 2012; Norden et al., 2014). This may be important in terms of response to neurodegenerative diseases such as AD and PD, as arginase 1, one marker of the alternative activation state, is necessary for Aβ plaque reduction. In a recent paper it was demonstrated that arginase-1 positive microglia phagocytose Aβ and if arginase-1 expression was prevented with an IL-4Rα blocking antibody then this impaired the ability of microglia to remove reduce amyloid plaques (Fenn et al., 2014).
Many investigations have begun to assess the age related shifts in gene and protein expression in isolated microglia in order to understand the molecular underpinnings of the changes in microglial function with age. A recent study using RNAseq comparing isolated microglia to whole brain identified the microglial sensome (Hickman et al., 2013). These authors then examined the microglial sensome in aged cells and a large number of the down regulated genes were related to detecting endogenous ligands whereas those related to the recognition of host defense genes were up-regulated. These findings further suggest that aged microglia do not sense and respond to the microenvironment in the same manner as microglia from young animals. Another recent study using gene microarrays of isolated microglia demonstrated up-regulation of a number of pathways including NFκB related genes and identified Sirt1 epigenetic regulation of IL1β as important in aged microglia (Cho et al., 2015). This study looked at myeloid-specific knockdown of Sirt1 and demonstrated alterations in microglial function. One caveat however is that peripheral macrophages were also altered using this approach, thus the specific role of microglia versus macrophages/monocytes that are known to cross into the brain was not delineated.
A pathway known to regulate microglial priming is the transcription factor nuclear factor erythroid related factor 2 (Nrf2) signaling cascade. Although Nrf2 is normally involved in the cellular response to oxidative injury, when this molecule is knocked out in mice, microglial phenotype shifts towards exaggerated pro-inflammatory responses (Lastres-Becker et al., 2012), precisely reflecting the primed microglial phenotype that is observed with normal aging. Recent evidence linking Nrf2-antioxidant response element (ARE) to microglial function include the critical role of Nrf2-ARE in promoting phagocytosis in microglia/macrophages as shown by a reduction in phagocytosis using a Nrf2 decoy to block Nrf2 actions (Zhao et al., 2014). It is also well established that there is a decline in microglial phagocytosis with age (Norden et al., 2014); again, indicating that alterations in Nrf2 expression can recapitulate the normal age-related changes in glial function.
Impact of inflammation on neural plasticity
The impacts of the changes in microglial function with age are numerous. There is strong evidence for cell non-autonomous effects on stem cell niches, with much of the evidence for this coming from studies using heterochronic parabiosis wherin the circulation of two animals of different ages is combined and early studies in our lab using the technique of in oculo transplantation. These latter experiments excised embryonic CNS tissues and transplanted the grafts into in the anterior chamber of the eye in rats of various ages (Granholm et al., 1987; Willis et al., 2005; Willis et al., 2010). The technique of in oculo transplantation uses fetal brain tissue is grown in the chamber of the eye where it becomes innervated by the blood and nerves from the host retina. In this manner you can study the development of brain tissues, such as the hippocampus in hosts of various ages, thus the environment of the host influences the growth of the brain tissue. When hippocampus is grown in the anterior chamber of aged rats it develops more slowly and does not attain morphology similar to that observed. When aged hosts are used one difference is that there are higher levels of pro-inflammatory cytokines that have a negative impact on the growth of the brain tissues. The “environment” of the aged host can be manipulated by feeding the older rats diets enriched in polyphenols, such as blueberries. This lowers the levels of cytokines and increases the growth rate of the hippocampal tissue. A more organotypic morphology is also observed under these conditions, similar to what is observed when the hippocampal tissue grows in a young host. (Granholm et al., 1987; Willis et al., 2005; Willis et al., 2010). These observations reiterate that the aged environment either lacks some vital factors necessary to perpetuate optimal cellular function or contains negative factors that inhibit proper cellular function.
Prominent support of the cell non-autonomous influences on stem cell vitality has also been generated using the technique of heterochronic parabiosis (Conboy et al., 2005; Villeda et al., 2011). These researchers used this method to surgically conjoin a young and aged animal, allowing the fusion of the two the vascular systems, and exposing each parabiont to the circulating factors of the other. This work demonstrated that the systemic milieu of aged mice reduces function of neural stem cells, hematopoietic stem cells, muscle satellite stem cells, and liver stem cells, (Conboy et al., 2005; Mayack et al., 2010; Villeda et al., 2011). Furthermore, when serum from old rats or mice is used to treat cultures of stem cells from various niches in a model of parabiosis in a dish, the serum from old animals has a negative impact on stem cell proliferation and there are changes in fate determination that recapitulate aging (Mayack et al., 2010; Villeda et al., 2011; Villeda et al., 2014). Several possible factors that are altered in old blood have been identified as possible negative and positive influences on the stem cell niches. For example, Villeda et al. initially suggested that CCL11 (eotaxin) is increased similarly in human and parabiont plasma and may be one of the negative regulators in the aged blood on neurogenesis (Villeda et al., 2011). Another recent paper suggests beta2-microglobulin is also a pro-aging factor (Smith et al., 2015), while several other reports have suggested that growth differentiation protein 11 (GDF-11) is decreased with age. Treatment with GDF-11 has a positive effect on several stem cell niches such as the liver and muscle (Katsimpardi et al., 2014; Sinha et al., 2014).
In addition to circulating factors, one of the main non-autonomous factors impacting the neurogenic niche is influences from surrounding cellular components such as microglia. Several studies have shown that aged microglia negatively impact the stem cell niche in a Nrf2-ARE dependent manner (L'Episcopo et al., 2013; Piccin et al., 2014). Specifically the role of microglia on the aged stem cell niche has become an area of active investigation. A recent study suggests that the cells from the neurogenic niche of aged animals negatively impacts neural stem cells from the subventircular zone ex vivo (Piccin et al., 2014). In contrast, adding neurogenic niche cells from the young animal had a rejuvenating effect on stem cells isolated from the aged niche. Other studies have shown that aged microglia directly impact the niche. Aged microglia grown in culture with young neural progenitors induce senescence in these NPC’s as reflected in decreased proliferation and maturation (L'Episcopo et al., 2013). This influence of the niche was linked to Nrf2 function and treatments that reduced microglial M1 phenotype were associated with increased Nrf2 expression, however a causal link was not established (L'Episcopo et al., 2013). In addition, restoration of the anti-inflammatory response may be just as important as reducing pro-inflammatory responses and this aspect of changes in microglia is less well studied.
Approaches to modulating the systemic milieu and local inflammatory cell influences on the stem cell niche
With the summary of the literature included above, it is established that several cell non autonomous sources have a negative impact on the stem cell niche are present with age. We have examined a number of strategies for modulating the non-autonomous mechanisms. One of the approaches we have used to mitigate these detrimental age-related changes in the CNS is a dietary intervention using polyphenol rich diets. Our group has established that a proprietary combination of natural ingredients termed NT-020 increases proliferation of human CD34+ cells in vitro (Bickford et al., 2006). In this study, we demonstrated that a combination of blueberry, green tea, Vitamin D3 and carnosine (NT-020) was able to increase proliferation of CD34+ human stem cells in a synergistic manner; the NT-020 formula was more effective than any of the individual constituents on their own. In order to determine if this effect observed in cell culture was also present in vivo, we fed young and aged rats with NT-020 for one month and showed an increase in neurogenesis using the proliferation marker Ki67 and the marker of maturing neurons doublecortin. Interestingly, this stimulation of neurogenesis by NT 020 treatment was also associated with a concomitant decrease in the expression of OX-6, which labels MHC II molecules indicative of active microglia in the hippocampus of aged rats. These observations further corroborate the critical interplay between glial function and neural plasticity (Acosta et al., 2010). Subsequent work in a rat model of stroke demonstrated 70% protection from stroke lesion and an increase in stem cells in the area of damage with a dose of 135mg/kg/day (Yasuhara et al., 2008). It is interesting to note that similar studies with blueberry alone used a dose 100x higher and found only 50% protection of brain damage (Wang et al., 2005), thus pointing towards a synergistic effect in vivo as well. Furthermore, we have also published that this polyphenol rich formulation improved cognitive function in healthy aged humans. For example, aged participants (65–85 years old) who took the NT 020 supplement for 2 months benefited from increased processing speed according to two different memory tests. This is especially noteworthy because impaired processing speed can often be detected early on in the aging process and can indicate more substantial and complex cognitive processes (Small et al., 2014).
Polyphenol rich diets effects on the system milieu with age
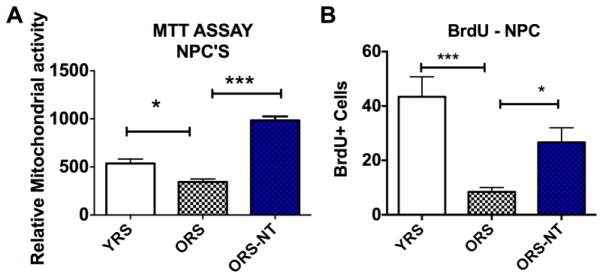
In a recent paper we used an in vitro approach designed to mimic parabiosis, where we examined the effect of serum from young and aged rats on proliferation of MSC and NPC’s in culture (Bickford et al., 2015). Furthermore, this study also assessed additional experimental groups that had been treated with a polyphenol rich diet. Serum was collected from both young rats (4 months) and aged rats (21 months) with or without supplementation with NT-020 (135mg/kg) for one month. NPC’s were grown in proliferation media for 24 hours prior to addition of 10% rat serum for 48 hours. Figure 1A illustrates that treatment with old rat serum reduces the stem cell proliferation, as measured by the MTT assay. This assay correlates the formation of the colored, intracellular formazan product with the cell population. In contrast, the treatment with serum from old rats that had ingested NT-020 supplemented diet stimulated significantly more cell growth than that of serum collected from the old rats fed the standard diet. (One-way ANOVA F (2,12)=65.45 followed by Sidak’s multiple comparison test * p<0.05; ** p<0.001). The MTT assay is an indirect measure of cell proliferation, as the tetrazolium regent is reduced in a mitochondrial dependent process and could be influenced by treatments that increase mitochondrial function. Therefore, we also assessed cell proliferation by quantifying BrdU incorporation after application of the various serum conditions in order to corroborate the MTT results. A similar effect is observed in Figure 1B when examining BrdU uptake after 48 hours treatment with 10% serum. There is a significant negative effect of old rat serum on the number of dividing stem cells, however, this is reversed with the serum from old rats fed NT-020 (One-way ANOVA F (2,17)=13.64, followed by Sidak’s multiple comparison test *** p<0.001 and * p<0.05). -These trends were also replicated in similar experiments using mesenchymal stem cells (MSC’s) (Bickford et al., 2015).
Figure 1.
Bargraph showing that treatment of NPC’s with old rat serum reduces the MTT signal (1A), whereas the serum from old rats treated with NT-020 shows MTT significantly higher than that found with old rat serum (One-way ANOVA F (2,12)=65.45 followed by Sidak’s multiple comparison test * p<0.05; ** p<0.001). In 1B there is a significant negative effect of old rat serum on the number of dividing stem cells measured with BrDU, and this is reversed with the serum from old rats fed NT-020 (One-way ANOVA F (2,17)=13.64, followed by Sidak’s multiple comparison test *** p<0.001 and * p<0.05).
Polyphenol rich diets reduce microglial pro-inflammatory activation, increase neurogenesis, and improve learning in aged rats
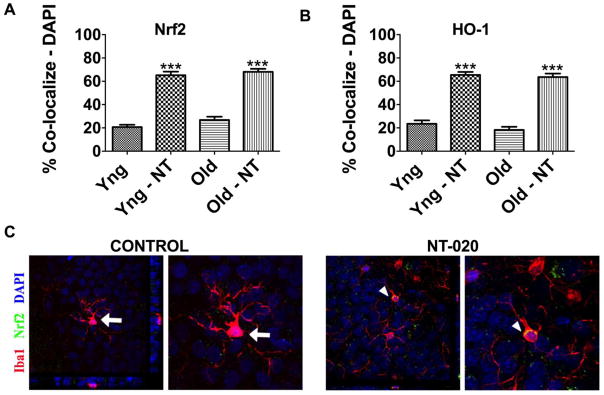
Polyphenols are potent modulators of inflammation in the aged brain and are thus a potential therapeutic approach to increase the resiliency of the aged brain to insults and improve homeostasis. Work from our lab and others support the role of polyphenols and other components of complex botanical mixtures to increase Nrf2 as a main mechanism of action (Bhullar and Rupasinghe 2013; Shah et al., 2010; Turan et al., 2012). Data from our lab shows that a NT-020, a polyphenol rich supplement, increases Nrf2 translocation to the nucleus in microglia and other cells in the dentate gyrus and the subventricular zone of young and aged rats (Flowers et al., 2015). As shown in Figure 2c using a confocal microscope to visualize co-localization of Nrf2 in the nucleus, the green (Nrf2) is observed in the nucleus of microglia (red IBA1 positive cells) only the microglia from the aged rat on the NT-020 diet show co-localization. The percentage of Nrf2 positive cells in the nucleus was calculated and shown in the bargraph (Figure 2A), translocation to the nucleus in the polyphenol rich diet group was observed in both young and aged rats. This same technique was used to examine neurons, glia and newborn neurons in the neurogenic niche of the hippocampus with similar results. Additionally, this treatment increases expression of heme-oxygenase 1 (HO-1), one of the antioxidant response enzymes that is transcribed following Nrf2 transcription factor activation (Figure 2B).
Figure 2.
Bargraphs showing numbers of cells with nuclear-localization of Nrf2 (A) or HO-1 (B). Below are confocal images (C) of microglia from aged control or NT-020 fed rats. Confocal microscopy was used to examine the nuclear localization of Nrf2. Cellular markers were used to determine specific localization in microglia with IBA-1. Here are shown a few examples of the confocal images demonstrating nuclear (DAPI, blue) co-localization of Nrf-2 (green) IBA-1 (red) positive cells in the NT-020 treated aged rats. Z-stacks (1 micron) were taken and rotated in 2 dimensions as shown in the side panels of the figures on the left of each subpanel for control and NT-020 treated rats. Higher power images are inserted to the right of each image focusing on the cell at the center of the Z-stack rotations. Only cells with clear co-labeling from all 3 views were counted. Cells shown for control (large arrows) clearly show no co-localization. Cells shown in NT-020 panel (arrowheads) demonstrate nuclear co-localization.
Activation of the Nrf2-ARE may be one mechanism by which these polyphenol rich diets reduce the production of pro-inflammatory cytokines in the aged rats and modulate microglial function, rendering the “environment” of the aged brain more conducive to neural plasticity, neurogenesis, and cognitive function. In a second cohort of rats in this study, we examined gene expression of inflammation related genes in the young and aged rats with and without the NT-020 diet. As has been shown previously, aged animals had higher gene expression of pro-inflammatory genes, and this was reversed by the NT-020 treatment (Flowers et al., 2015).
Polyphenols hold great promise in the realm of neuroprotection. NT-020 is a proof of concept that polyphenolic rich mixtures are capable of modulating several age relevant targets simultaneously, and improve the aging “environment” as well as improve cognition with minimal risk of side effects. We have discussed two recent papers from our group that have shown that NT-020 a polyphenol rich supplement, can reduce the effect of the systemic milieu on stem cell proliferation (Bickford et al., 2015) and to modulate transcription factors involved in response to stress response pathways (Nrf2) (Flowers et al., 2015) and stem cell proliferation as WNT gene expression pathways were also upregulated by NT-020 in that study. Restoring redox balance with polyphenolic rich supplements represents one strategy to reduce microglial priming and thus enhance neural plasticity in the aged brain. If this approach targeted to diseases of aging such as Alzheimer’s disease or Parkinson’s disease it is not likely to address the primary cause of the neurodegenerative disease. Rather these approaches strive to alter the “environment” where the disease is unfolding and restoring the resilience lost with age.
Highlights.
Aging leads to reduced resilience of the brain that underlies neurodegenerative disease prevalence.
Altered function of microglia is one source of reduced resilience in the aging brain.
Polyphenol rich dietary supplements can increase resilience in the aged brain.
Acknowledgments
This work was supported by NIH grants R01AG044919 (PCB), and VA grant I01BX003421 (PCB). This work was supported by the Veterans Administration. Content does not represent the views of the Department of Veterans Affairs nor the US Government.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature cited
- Acosta S, Jernberg J, Sanberg CD, Sanberg PR, Small BJ, Gemma C, Bickford PC. NT-020, a natural therapeutic approach to optimize spatial memory performance and increase neural progenitor cell proliferation and decrease inflammation in the aged rat. Rejuvenation research. 2010;13:581–588. doi: 10.1089/rej.2009.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennet DA, Beckett LA, Murray AM, Shannon KM, Goetz CG, Pilgram DM, Evans DA. Prevalence of Parkinsonian signs and associated mortality in a community population of older people. The New England Journal of Medicine. 1996;334:71–76. doi: 10.1056/NEJM199601113340202. [DOI] [PubMed] [Google Scholar]
- Bhullar KS, Rupasinghe HP. Polyphenols: multipotent therapeutic agents in neurodegenerative diseases. Oxidative medicine and cellular longevity. 2013;2013:891748. doi: 10.1155/2013/891748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford PC, Kaneko Y, Grimmig B, Pappas C, Small B, Sanberg CD, Sanberg PR, Tan J, Douglas Shytle R. Nutraceutical intervention reverses the negative effects of blood from aged rats on stem cells. Age (Dordr) 2015;37:103. doi: 10.1007/s11357-015-9840-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickford PC, Tan J, Shytle RD, Sanberg CD, El-Badri N, Sanberg PR. Nutraceuticals synergistically promote proliferation of human stem cells. Stem Cells Dev. 2006;15:118–123. doi: 10.1089/scd.2006.15.118. [DOI] [PubMed] [Google Scholar]
- Cho SH, Chen JA, Sayed F, Ward ME, Gao F, Nguyen TA, Krabbe G, Sohn PD, Lo I, Minami S, Devidze N, Zhou Y, Coppola G, Gan L. SIRT1 deficiency in microglia contributes to cognitive decline in aging and neurodegeneration via epigenetic regulation of IL-1beta. J Neurosci. 2015;35:807–818. doi: 10.1523/JNEUROSCI.2939-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- Fenn AM, Hall JC, Gensel JC, Popovich PG, Godbout JP. IL-4 signaling drives a unique arginase+/IL-1beta+ microglia phenotype and recruits macrophages to the inflammatory CNS: consequences of age-related deficits in IL-4Ralpha after traumatic spinal cord injury. J Neurosci. 2014;34:8904–8917. doi: 10.1523/JNEUROSCI.1146-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Henry CJ, Huang Y, Dugan A, Godbout JP. Lipopolysaccharide-induced interleukin (IL)-4 receptor-alpha expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain, behavior, and immunity. 2012;26:766–777. doi: 10.1016/j.bbi.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers A, Lee JY, Acosta S, Hudson C, Small B, Sanberg CD, Bickford PC. NT-020 treatment reduces inflammation and augments Nrf-2 and Wnt signaling in aged rats. J Neuroinflammation. 2015;12:174. doi: 10.1186/s12974-015-0395-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gemma C, Fister M, Hudson C, Bickford PC. Improvement of memory for context by inhibition of caspase-1 in aged rats. EurJNeurosci. 2005;22:1751–1756. doi: 10.1111/j.1460-9568.2005.04334.x. [DOI] [PubMed] [Google Scholar]
- Gemma C, Mesches MH, Sepesi B, Choo K, Holmes DB, Bickford PC. Diets enriched in foods with high antioxidant activity reverse age-induced decreases in cerebellar beta-adrenergic function and increases in proinflammatory cytokines. JNeurosci. 2002;22:6114–6120. doi: 10.1523/JNEUROSCI.22-14-06114.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granholm AC, Gerhardt GA, Eriksdotter-Nilsson M, Bickford-Wimer PC, Palmer MR, Seiger A, Olson L, Hoffer BJ. Age-related changes in cerebellar noradrenergic pre- and postsynaptic mechanisms: intrinsic vs extrinsic determinants evaluated with brain grafts in oculo. Brain Res. 1987;423:71–78. doi: 10.1016/0006-8993(87)90826-2. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP. Neuroinflammation in Alzheimer's disease. The Lancet Neurology. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, El Khoury J. The microglial sensome revealed by direct RNA sequencing. Nat Neurosci. 2013;16:1896–1905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL. Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science. 2014;344:630–634. doi: 10.1126/science.1251141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- L'Episcopo F, Tirolo C, Testa N, Caniglia S, Morale MC, Impagnatiello F, Pluchino S, Marchetti B. Aging-induced Nrf2-ARE pathway disruption in the subventricular zone drives neurogenic impairment in parkinsonian mice via PI3K-Wnt/beta-catenin dysregulation. J Neurosci. 2013;33:1462–1485. doi: 10.1523/JNEUROSCI.3206-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastres-Becker I, Ulusoy A, Innamorato NG, Sahin G, Rábano A, Kirik D, Cuadrado A. α-Synuclein expression and Nrf2 deficiency cooperate to aggravate protein aggregation, neuronal death and inflammation in early-stage Parkinson's disease. Human Molecular Genetics. 2012;21:3173–3192. doi: 10.1093/hmg/dds143. [DOI] [PubMed] [Google Scholar]
- Lee DC, Ruiz CR, Lebson L, Selenica ML, Rizer J, Hunt JB, Jr, Rojiani R, Reid P, Kammath S, Nash K, Dickey CA, Gordon M, Morgan D. Aging enhances classical activation but mitigates alternative activation in the central nervous system. Neurobiol Aging. 2013;34:1610–1620. doi: 10.1016/j.neurobiolaging.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayack SR, Shadrach JL, Kim FS, Wagers AJ. Systemic signals regulate ageing and rejuvenation of blood stem cell niches. Nature. 2010;463:495–500. doi: 10.1038/nature08749. [DOI] [PubMed] [Google Scholar]
- Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F. Proinflammatory cytokines, aging, and age-related diseases. Journal of the American Medical Directors Association. 2013;14:877–882. doi: 10.1016/j.jamda.2013.05.009. [DOI] [PubMed] [Google Scholar]
- Moll L, El-Ami T, Cohen E. Selective manipulation of aging: a novel strategy for the treatment of neurodegenerative disorders. Swiss medical weekly. 2014;144:w13917. doi: 10.4414/smw.2014.13917. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Morganti JM, Riparip LK, Rosi S. Call Off the Dog(ma): M1/M2 Polarization Is Concurrent following Traumatic Brain Injury. PloS one. 2016;11:e0148001. doi: 10.1371/journal.pone.0148001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niccoli T, Partridge L. Ageing as a risk factor for disease. Current biology : CB. 2012;22:R741–752. doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Norden DM, Muccigrosso MM, Godbout JP. Microglial Priming and Enhanced Reactivity to Secondary Insult in Aging, and Traumatic CNS injury, and Neurodegenerative Disease. Neuropharmacology. 2014 doi: 10.1016/j.neuropharm.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccin D, Tufford A, Morshead CM. Neural stem and progenitor cells in the aged subependyma are activated by the young niche. Neurobiol Aging. 2014;35:1669–1679. doi: 10.1016/j.neurobiolaging.2014.01.026. [DOI] [PubMed] [Google Scholar]
- Shah ZA, Li RC, Ahmad AS, Kensler TW, Yamamoto M, Biswal S, Dore S. The flavanol (−)-epicatechin prevents stroke damage through the Nrf2/HO1 pathway. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2010;30:1951–1961. doi: 10.1038/jcbfm.2010.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha M, Jang YC, Oh J, Khong D, Wu EY, Manohar R, Miller C, Regalado SG, Loffredo FS, Pancoast JR, Hirshman MF, Lebowitz J, Shadrach JL, Cerletti M, Kim MJ, Serwold T, Goodyear LJ, Rosner B, Lee RT, Wagers AJ. Restoring systemic GDF11 levels reverses age-related dysfunction in mouse skeletal muscle. Science. 2014;344:649–652. doi: 10.1126/science.1251152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small BJ, Rawson KS, Martin C, Eisel SL, Sanberg CD, McEvoy CL, Sanberg PR, Shytle RD, Tan J, Bickford PC. Nutraceutical intervention improves older adults' cognitive functioning. Rejuvenation research. 2014;17:27–32. doi: 10.1089/rej.2013.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LK, He Y, Park JS, Bieri G, Snethlage CE, Lin K, Gontier G, Wabl R, Plambeck KE, Udeochu J, Wheatley EG, Bouchard J, Eggel A, Narasimha R, Grant JL, Luo J, Wyss-Coray T, Villeda SA. beta2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat Med. 2015 doi: 10.1038/nm.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan B, Tuncay E, Vassort G. Resveratrol and diabetic cardiac function: focus on recent in vitro and in vivo studies. Journal of bioenergetics and biomembranes. 2012;44:281–296. doi: 10.1007/s10863-012-9429-0. [DOI] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Despres S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, Wabl R, Udeochu J, Wheatley EG, Zou B, Simmons DA, Xie XS, Longo FM, Wyss-Coray T. Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice. Nat Med. 2014;20:659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Chang CF, Chou J, Chen HL, Deng X, Harvey BK, Cadet JL, Bickford PC. Dietary supplementation with blueberries, spinach, or spirulina reduces ischemic brain damage. ExpNeurol. 2005;193:75–84. doi: 10.1016/j.expneurol.2004.12.014. [DOI] [PubMed] [Google Scholar]
- Wilcock DM. A changing perspective on the role of neuroinflammation in Alzheimer's disease. Int J Alzheimers Dis. 2012;2012:495243. doi: 10.1155/2012/495243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis L, Bickford P, Zaman V, Moore A, Granholm AC. Blueberry extract enhances survival of intraocular hippocampal transplants. Cell Transplant. 2005;14:213–223. doi: 10.3727/000000005783983142. [DOI] [PubMed] [Google Scholar]
- Willis LM, Freeman L, Bickford PC, Quintero EM, Umphlet CD, Moore AB, Goetzl L, Granholm AC. Blueberry supplementation attenuates microglial activation in hippocampal intraocular grafts to aged hosts. Glia. 2010;58:679–690. doi: 10.1002/glia.20954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuhara T, Hara K, Maki M, Masuda T, Sanberg CD, Sanberg PR, Bickford PC, Borlongan CV. Dietary supplementation exerts neuroprotective effects in ischemic stroke model. RejuvenationRes. 2008;11:201–214. doi: 10.1089/rej.2007.0608. [DOI] [PubMed] [Google Scholar]
- Zhao X, Sun G, Ting SM, Song S, Zhang J, Edwards NJ, Aronowski J. Cleaning up after ICH: the role of Nrf2 in modulating microglia function and hematoma clearance. J Neurochem. 2014 doi: 10.1111/jnc.12974. [DOI] [PMC free article] [PubMed] [Google Scholar]