Abstract
Purpose
To describe the frequency and characteristics of developmental regression in a sample of 50 patients with Phelan McDermid Syndrome (PMS) and investigate the possibility of association between regression, epilepsy, and electroencephalogram (EEG) abnormalities and deletion size.
Methods
The Autism Diagnostic Interview-Revised (ADI-R) was used to evaluate regression in patients with a confirmed diagnosis of PMS. Information on seizure history and EEGs was obtained from medical record review. Deletion size was determined by array CGH.
Results
A history of regression at any age was present in 43% of all patients. Among those exhibiting regression, 67% had onset after the age of 30 months, affecting primarily motor and self-help skills. In 63% of all patients there was a history of seizures and a history of abnormal EEG was also present in 71%. No significant associations were found between regression and seizures or EEG abnormalities. Deletion size was significantly associated with EEG abnormalities, but not with regression or seizures.
Conclusion
This study found a high rate of regression in PMS. In contrast to regression in autism, that often occurs earlier in development and affects language and social skills, we found regression in PMS most frequently has an onset in mid-childhood, affecting motor and self-help skills. We also found high rates of seizures and abnormal EEGs in patients with PMS. However, a history of abnormal EEG and seizures was not associated with an increased risk of regression. Larger deletion sizes were found to be significantly associated with a history of abnormal EEG.
Keywords: Phelan McDermid Syndrome, 22q13.3 deletion syndrome, developmental regression, seizures, electroencephalogram
1. Introduction
Phelan McDermid Syndrome
Phelan McDermid Syndrome (PMS) is a microdeletion syndrome of chromosome 22q13 characterized by developmental delay, impaired speech, intellectual disability, epilepsy, and increased risk of autism spectrum disorders. It has been estimated that up to 84% of children with PMS fulfill criteria for autism spectrum disorders (ASDs)(Soorya et al., 2013). Multiple other organ systems including the cardiac, endocrine, gastrointestinal, immune, ophthalmologic, renal, and respiratory can also be affected(Sarasua et al., 2014; Soorya et al., 2013). The size of the deleted segment in PMS varies from less than 100 kb to more than 9 Mb (Dhar et al., 2010; Sarasua et al., 2011). Based on the shortest deletion, a critical region has been identified that harbors three genes: ACR, RABL2B, and SHANK3(Anderlid et al., 2002). The strongest candidate for the neurobehavioral symptoms observed is SHANK3(Bonaglia et al., 2001). The loss of SHANK3 alone is considered sufficient to cause PMS, although the condition shows variable expressivity.
Regression, the loss of already acquired skills, is a recognized feature of PMS. Most of what is known about regression has largely come from the studies in ASD and Rett Syndrome (RTT). In ASD, the prevalence has been estimated at ~30% (range 15–47%), with onset typically in the second year of life (range 15 to 30 months), and affecting predominantly language and social skills (Baird et al., 2008; Stefanatos, 2008; Tuchman & Rapin, 1997; Zhang, Xu, Liu, Li, & Xu, 2012). In RTT a period of regression referred to as the “rapid destructive phase” that occurs between the first and third years of life is one of the diagnostic criteria for typical RTT (Weaving, Ellaway, Gécz, & Christodoulou, 2005). In PMS, however, the frequency, timing, as well as which specific skills most commonly involved in regression are incompletely defined.
Regression is defined in multiple ways with the majority of studies focusing on language regression. As pointed out by Bradley et al. (2016) previous definitions have frequently utilized the criteria outlined in the Autism Diagnostic Interview—Revised (ADI-R), i.e. the loss of previously acquired developmental skills for a period of at least three months following typical or relatively typical development (Hughes, 2012; Lord, Rutter, & Le Couteur, 1994).
Three case reports suggest that regression occurs, and affects motor and language skills (Cochoy et al., 2015; Figura et al., 2014; Macedoni-Lukšic, Krgovic, Zagradišnik, & Kokalj-Vokac, 2013). Three additional investigations have reported on loss of developmental skills. A survey of parents found that over a third (17/48; 35%) reported a history of loss of skills that were described as having acute onset. However, regression was not specifically defined and there was no description of age of onset or specification of the skills lost (Wilson et al., 2003). Late onset regression after age 30 years has been reported in a few cases; however, the contribution of age-related medical comorbidities to this functional decline is unclear (Bonaglia et al., 2011; Willemsen, Rensen, van Schrojenstein-Lantman de Valk, Hamel, & Kleefstra, 2012). Dhar et al, 2010 found 2 out of 13 participants (15%) experienced regression but assessment methods and characteristics of regression were not described. In a study of 32 participants with PMS, Soorya et al., 2013, used the ADI-R as well as psychiatric evaluation to assess regression. Based on psychiatric evaluation, nine (28%) participants were identified as having loss of skills, most commonly in language; however, these individuals did not meet criteria for language loss as defined by ADI-R.
In five out of nine individuals with loss of language, regression was associated with seizure onset (Soorya et al., 2013). Since seizures have been implicated in other developmental disorders as a possible risk factor for regression the authors speculated as to whether seizure onset could be a factor associated with the timing of regression. Additionally, although findings are mixed (Dhar et al., 2010; Sarasua et al., 2014; Wilson et al., 2003), several studies suggest that deletion size in PMS may be related to phenotype severity, with larger deletions associated with developmental and speech delays, number of dysmorphic features, and medical comorbidities such as epilepsy (Jeffries et al., 2005; Sarasua et al., 2011; Soorya et al., 2013). In this study, we further investigate the frequency and characteristics of developmental regression in a sample of patients with PMS and also investigate the possibility of an association between regression, epilepsy, and electroencephalogram (EEG) abnormalities, and deletion size.
2. Material and Methods
2.1 Patients
Fifty patients diagnosed with PMS were recruited from ongoing research studies, the medical genetics service at Lucile Packard Children’s Hospital, and interest groups for patients and families with genetic disorders associated with autism spectrum manifestations. Participants were recruited through letters and e-mails to patient cohorts from Stanford Hospital and to families known to the Phelan McDermid Syndrome Association. Since PMS is a rare disorder, some patients have participated in other investigations(Soorya et al., 2013).
This study was approved by the Stanford University Institutional Review Board (IRB).
2.2 Medical Record Review
Parents of patients completed a medical history form through the PMS registry, and medical records were obtained. Three clinicians performed medical record reviews (J.B., W.F., G.R) to confirm parents’ reports. Seizure history was considered positive if the patient was reported in the medical record to have at least one seizure. Reports of EEGs were obtained from the hospital or clinic where they were performed, when possible. Other sources of information about EEG report results came from documentation in the medical record. Details of the deletion size were obtained from the medical record for those patients for whom CGH data were available. For those patients for whom the diagnosis was not clear, a microarray (infinium CytoSNP-850K BeadChip) was performed (n=14).
2.3 Instruments
To assess cognitive abilities, subjects were administered either the Stanford-Binet Intelligence Scales, 5th edition (SB5), or, if unable to complete basal scores on the SB5 due to behavioral or cognitive difficulty, the Mullen Scales of Early Learning (MSEL)(Mullen, 1995; Roid, 2003). Non-verbal IQ (NVIQ) was calculated from the Mullen as previously described(Froehlich et al., 2013). These tests were administered by a research assistant trained in both assessments and were completed along with the ADI-R in either the subject’s home or a clinic setting. For subjects not available for testing, IQ tests were obtained from the medical record.
The Autism Diagnostic Interview-Revised (ADI-R) was conducted by a research reliable, trained investigator with the parent or caregiver of the child(Lord et al., 1994). The ADI-R is a standardized, semi-structured interview that uses an algorithm to assess for autism. It is composed of five sections, with questions about communication, social development and play, repetitive and restricted behaviors, and general behavioral problems based on criteria for autism from DSM-IV and ICD-10. Notably, the revised version of the interview includes questions about loss of skills, and these were initially included to distinguish autism from other neurodevelopmental disorders. All behavioral items are defined and then coded by the investigator based on caregiver response. Ultimately, the code assigned on any given item is determined based on the judgement of the investigator as to whether the caregiver’s response meets criteria for that behavioral item. For example, items are coded as 0 if “no definite behavior of the type specified”, as 1 if “behavior of the type specified probably present but the defining criteria not fully met,” and as 2 if “definite abnormal behavior of the type described.”
Characteristics of regression were determined using scores from items 11–28 on the ADI-R. A positive history of regression was identified by coding of a loss of skills on item 11 “loss of language skills after acquisition” or by coding of a “definite” loss of skills on item 20 “loss of skills (for at least 3 months).” Loss of language was specifically evaluated using scores from items 11–19. Language loss was defined as follows: patients had to have at least five different words other than “mama” and “dada” for at least three months with consistent loss of language skills for at least three months. Loss of other skills was specifically evaluated by looking at scores from items 20–28 and includes loss of purposive hand movements (ability to grip/hold objects), motor skills (posture, gait, coordination), self-help skills (feeding, dress, using the bathroom, etc.), constructive or imaginative play (puzzles, games, make-believe etc.), and social engagement and responsiveness (social relatedness, interest, and involvement). Skills were defined as having had to be present and used on a daily basis for at least three months with consistent loss of skills for at least three months. Loss of bladder or bowel control alone did not meet criteria for loss of “other skills” unless it occurred in combination with at least one “other skill.”
2.4 Statistical Analysis
To examine whether seizures, EEG abnormalities, and regression predict one another, we conducted a series of logistic regression models, followed by a logistic regression model in which we included age as a covariate. We subsequently tested whether deletion size predicted each of the outcome variables (seizures, EEG abnormalities, regression) as well as a model that included age as a covariate. Both age and deletion size were standardized to enhance interpretability of the coefficients.
3. Results
3.1 Demographics
Our sample consisted of a total of 50 PMS patients, 25 male and 25 female (Table 1). The patients ranged in age from 4 years – 48 years (mean 12.8, SD 8.7). The majority of the patients (42%) were >6 to12 years old, with 20% between 0 to 6 years old, 22% >12 to18 years old, and 18% over the age of 18 years. Information on ethnicity was available for 22 patients, and the majority of the sample was Caucasian (non-Hispanic) 86% (19/22) with 14% (3/22) Hispanic.
Table 1.
Demographics
| n | % | |
|---|---|---|
| Age (in years) n=50 | ||
| 0–6 | 9 | 20 |
| >6–12 | 21 | 42 |
| >12–18 | 11 | 22 |
| >18 | 9 | 18 |
| Sex (n=50) | ||
| M | 25 | 50 |
| F | 25 | 50 |
| Ethnicity (n=22) | ||
| Caucasian (Non-Hispanic) | 19 | 86 |
| Hispanic | 3 | 14 |
| Other | 0 | 0 |
IQ information was available for 32 PMS patients using the Stanford Binet 5 (n=8) or the Mullen Scores (n=24) either performed (n=27) or obtained from the medical record (n=5) (Table 2). The majority of the sample 59% (19/32) had NVIQ<35, indicating severe to profound intellectual disability, with 28% (9/32) with NVIQ between 35–49, and 9% (3/32) with NVIQ between 50–70. One individual had average NVIQ with a score of 96.
Table 2.
IQ (Stanford Binet 5, Mullen)
| NVIQ (n=32) | n | % |
|---|---|---|
| <35 | 19 | 59 |
| 35–49 | 9 | 28 |
| 50–70 | 3 | 9 |
| >70 | 1 | 3 |
3.2 Regression
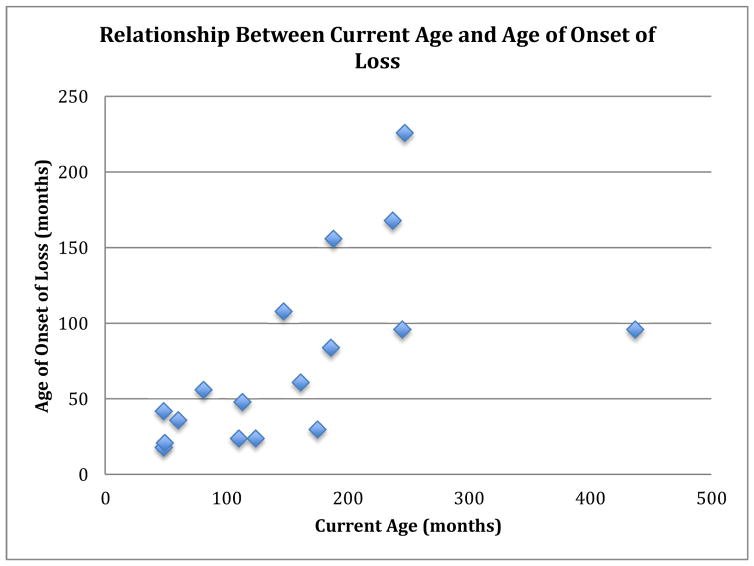
Information about regression was available for 42 of our sample of 50 PMS patients from the ADI-R (Table 3). For the other 8 PMS patients no ADI-R data were available. Parents of 18/42 (43%) of patients reported a history of regression. Of those who reported regression, 67% (12/18) had an age of onset of loss >30 months, 28% (5/18) had an age of onset of loss <30 months, and for one patient there no information was available about age of onset (Figure 1). Average age of onset of loss was around 6 years (mean=76 months, median=56 months, range=18 months to 18 years). Almost twice as many males were noted to have a loss of skills (n=11), versus females (n=7), but gender was not significantly associated with regression of any of these skills. For 6/18 there was a reported temporal association of regression with physical illness, and specifically in 3/18 “impaired consciousness/epileptic attacks” were noted. In a majority 61% (11/18) who lost skills, the loss was still present at the time of assessment. For the remaining 7/18 who regained skills, duration of loss ranged from 1 month to 10 years.
Table 3.
Characteristics of Regression (n=42)
| Motor Skills | n | % |
|---|---|---|
| No loss | 7 | 41 |
| Some loss | 0 | 0 |
| Definite loss | 10 | 59 |
| Self help skills | n | % |
| No loss | 7 | 41 |
| Some loss | 1 | 6 |
| Definite loss | 9 | 53 |
| Language | n | % |
| No loss | 36 | 86 |
| Loss | 6 | 14 |
| Social engagement and responsiveness | n | % |
| No loss | 11 | 65 |
| Some loss | 1 | 6 |
| Definite loss | 6 | 29 |
| Purposive hand movements | n | % |
| No loss | 11 | 65 |
| Some loss | 2 | 6 |
| Definite loss | 5 | 29 |
| Constructive or imaginative play | n | % |
| No loss | 13 | 76 |
| Some loss | 0 | 0 |
| Definite loss | 4 | 24 |
Figure 1.
Relationship Between Current Age and Age of Onset of Loss
Characteristics of regression, including number of participants, percentage, gender breakdown, and average age of onset, are presented by type of skill lost in Table 4. Loss of motor skills was present in 56% (10/18), with average age of loss ~8 years (mean=95 months, median=90 months, range=18 months to 226 months), and equal loss by males (n=5) and females (n=5). Loss of self-help skills was present in 50%(9/18), with average age of loss ~4 years (mean=49 months, median=36 months, range= 18 months to 156 months), and equal loss by males (n=5) and females (n=4). Loss of language was present in 33% (6/18), with average age of loss ~3 years (mean=39 months, median=40 months, range= 24 months to 61 months). More females (n=4) experienced loss of language as compared to males (n=2). Loss of social engagement and responsiveness was present in 33% (6/18), with average age of loss ~5 years (mean=63 months, median=63 months, range= 21 months to 108 months). Twice as many males (n=4) experienced loss of social engagement and responsiveness compared to females (n=2). Loss of purposive hand movements was present in 28% (5/18), with average age of loss ~7 years (mean=80, median= 96, range=21 months to 156 months). Equal numbers of males (n=2) and females (n=3) experienced loss. Lastly, loss of constructive or imaginative play was present in 4/18 (22%), with average age of loss ~4 years (mean=48 months, median=30 months, range= 30 months to 96 months). More females (n=3) experienced loss of constructive or imaginative play as compared to males (n=1). Together our data suggest that the significant majority of PMS patients exhibit motor and/or self-help skills regression, and that this type of regression occurs in mid childhood.
Table 4.
Characteristics of Regression (n=18)
| ADI-R Skill Loss | n | % | Males | Females | Mean age of loss (mo.) | Median age of loss (mo.) |
|---|---|---|---|---|---|---|
| Motor skills | 10 | 56 | 5 | 5 | 95 | 90 |
| Self-help skills | 9 | 50 | 5 | 4 | 49 | 36 |
| Language | 6 | 33 | 2 | 4 | 39 | 40 |
| Social engagement and responsiveness | 6 | 33 | 4 | 2 | 63 | 63 |
| Purposive hand movements | 5 | 28 | 2 | 3 | 80 | 96 |
| Constructive or imaginative play | 4 | 22 | 1 | 3 | 48 | 30 |
3.3 Seizures and EEG and their Relationship to Regression
Information about seizure history was available for 35 of our sample of 50 PMS patients (Table 5). ADI-R regression data was available for 27 of these 35 PMS patients and seizure data from the eight patients with missing ADI-R data. Of the 35 patients with seizure history information, a history of seizures was present in 63% (22/35) and absent in 37% (13/35). An equal number of males and females had a history of seizures. For these patients there was information available about EEGs, either through presence of the EEG report or indicated in the medical record that an EEG was conducted, and if conducted what the findings were. EEG was abnormal in 71% (25/35) of patients and within normal limits in 29% (10/35). 13 males and 12 females had an abnormal EEG. Of those with seizures (n=22), 20 had an abnormal EEG and 2 had an EEG within normal limits.
Table 5.
Medical Record Review
| Seizure History (n=35) | n | % |
|---|---|---|
| Negative | 13 | 37 |
| Positive | 22 | 63 |
| EEG (n=35) | ||
| Normal | 10 | 29 |
| Abnormal | 25 | 71 |
In terms of bivariate predictions, neither seizures nor abnormal EEG predicted regression (p ≥ .223), but abnormal EEG significantly predicted seizures, such that presence of abnormal EEG increased the odds of having a seizure by 30 (OR = 30, p = 0.002), which remained significant even after controlling for age (OR = 24.661, p = 0.005).
3.4 Genotype Phenotype Correlations
We hypothesized that deletion size would be associated with EEG abnormalities, seizures and regression, as some studies have reported an association between larger deletions and a more severe phenotype. Deletion size did not significantly predict regression or seizures (n=34 and n=32 resp). However, deletion size significantly predicted abnormal EEG (n=32), such that a one standard deviation unit increase in deletion size increased the odds of having an abnormal EEG nearly threefold (OR = 2.90, p = 0.039), and there was a trend towards significance for the effect after controlling for age (p = 0.062).
4. Discussion
Our study found that 43% of patients with PMS experienced regression, which is even greater than the highest rate (35%) previously reported in the literature (Dhar et al., 2010; Soorya et al., 2013; Wilson et al., 2003). Further, we found that onset of regression was around mid-childhood, on average six years of age (mean=76 months), which is much later than is typically observed in other neurodevelopment disorders, such as ASD and Rett syndrome. We observed a trend towards an increased frequency of regression in males, however, this did not reach statistical significance. Motor skills and self-help skills were the most common skills that were lost, and for the majority of patients (61%), lost skills were not regained. For loss of language skills, the average onset was around 39 months and both genders were equally affected. Our data suggest that the significant majority of PMS patients exhibit motor and self-help skills regression, and that this type of regression occurs in PMS later than in other neurodevelopmental disorders, typically in mid-childhood.
One other study has used the ADI-R to systematically study regression to further characterize both loss of language and non-language or “other skills” in PMS (Soorya et al., 2013). Similar to the present study, the majority of participants had IQ<35, indicating profound intellectual disability. Soorya et al 2013 found a lower rate of loss of “other skills” (23%), with mostly hand movements (5/7) and social engagement (4/7) affected, and fewer reports of loss of other motor skills (3/7), self-help (3/7), and play (2/7), whereas the present study found higher rates of loss of motor and self-help skills, and lower rates of loss of hand movements, social engagement, and play (see Table 6 for comparison of regression characteristics). Soorya et al 2013 also report a higher rate of loss of language (28%) overall, and associated with seizures in 5 out of 9 patients, whereas in the present study, we found only 1 out of 6 (17%) patients with loss of language further described as “loss of consciousness/epileptic.” The authors note that when they used stricter criteria they did not observe any regression of language. They suggest this may reflect the poor language ability in many patients with PMS, which results in insufficient language skills in the child for regression to occur. This may have also accounted for our own observation of a limited number of PMS patients who exhibited language regression. Discrepancies in the results between the present study and Soorya et al 2013 could be influenced by differences in the age of the patients examined. In particular, the older average age of patients in the present study may have contributed to our finding of a higher rate of regression.
Table 6.
Regression characteristics in comparison to Soorya et al 2013 study; n/r=not reported
| Variable | Current Study (n=42) | Soorya et al 2013 (n=32) |
|---|---|---|
| Mean age (median) of all patients (years) | 12.5 (9.9) | 8.8 (6.5) |
| Mean age (median) of patients with regression (years) | 12.7 (11.3) | n/r (n/r) |
| Mean age (median) of patients without regression (years) | 12.3 (9.1) | n/r (n/r) |
| NVIQ <35 of all patients (%) | 59.4 | 76.7 |
| Mean NVIQ (median) of all patients | 28.6 (19.9) | 25.2 (19.4) |
| Mean NVIQ (median) patients with regression | 19.4 (19) | n/r |
| Mean NVIQ (median) patients without regression | 37.8 (42) | n/r |
| Mean (median) age of onset of skills regression (years) | 6.3 (4.7) | n/r |
| Loss of skills | 18/42 | 9/32 |
| (%) | 43 | 28 |
| Purposive hand movements | 5/18 | 5/7 |
| Motor | 10/18 | 3/7 |
| Self help | 9/18 | 3/7 |
| Constructive or imaginative play | 4/18 | 2/7 |
| Social engagement and responsiveness | 6/18 | 4/7 |
| language | 6/18 | 9/32 |
Our study and other studies of regression in neurodevelopmental disorders suggest that different neurodevelopmental disorders may have distinct or characteristic patterns of regression. In PMS, we found regression to occur mainly in mid childhood, affecting dominantly motor and self-help skills, with a smaller number of children exhibiting language regression at an earlier age. The regression phenotype of ASD has been described to have an early onset (around age 2 years), affects language and social skills, and does not have a clear association with seizures(Baird et al., 2008; Stefanatos, 2008; Tuchman & Rapin, 1997; Zhang et al., 2012). In Rett Syndrome caused by mutations in the MECP2 gene, regression has been reported to have an earlier onset (ages 1–3 years), affects language and hand skills, and is associated with seizures(Amir et al., 1999). In MECP2 duplication syndrome, however, regression has an onset between 2–7 years, affects both language and motor skills, and has an association with seizures(Peters et al., 2013). It may be that the relationship of seizures to regression also varies according to the type of disorder, but future, longitudinal prospective studies of PMS and other neurodevelopmental disorders are required to more fully characterize this relationship.
Finally, we hypothesized that larger deletion size would be associated with a more severe phenotype, and while we found that larger deletion sizes were significantly associated with a history of abnormal EEG, we did not find a statistically significant association with seizures. Regression was also not significantly associated with deletion size.
Several limitations of this study should be considered. Because our PMS sample was predominantly Caucasian, it is uncertain whether these results can be generalized to other ethnicities. As this was a retrospective study, the prevalence of seizures and EEG abnormalities might have been underestimated. We did attempt to follow up on missing data by directly contacting parents. However, we were not able to track down all missing data or follow participants over time to detect new cases of seizures, abnormal EEGs, or regression. Our sample may not have been large enough to yield sufficient power to detect all significant effects, for example the lack of association of larger deletion size and seizures may have reflected limited power to detect this association. Indeed, given the small sample size, and the fact that median scores for age of loss were lower than mean values, our findings should be interpreted cautiously. However, we note that even the median age of regression, 4.7 years, is older than is typically observed for other neurodevelopmental disorders. Future, larger, prospective, longitudinal investigations should be conducted to confirm our results.
In summary, we found high rates of regression, seizures and abnormal EEG in a sample of PMS patients. This study specifically investigated the frequency and characteristics of developmental regression in this population, providing information about gender, age of onset, specific skills lost, and association of loss with seizures. We found that in PMS loss of skills most frequently occurred in mid-childhood and largely affected motor skills and self-help skills. Our finding that up to 43% of PMS participants experienced regression is of potential significance for those who care for patients with PMS, as providers might consider screening or monitoring for possible loss of skills in mid-childhood. Clinician determination of the presence of skill loss could also prompt referral for genetic testing to determine whether PMS is present. It will be important to develop and apply interventions that may be helpful in maintaining skills in later childhood or regaining them after regression.
Acknowledgments
The Phelan-McDermid Syndrome Foundation is recognized for assistance in subject recruitment. This study was also supported by funding from National Institute of Mental Health (NIMH) grant R33MH087898 (to J.F.H.)
We would like to thank the participants and their families. The Phelan-McDermid Syndrome Foundation is recognized for assistance in subject recruitment. This study was also supported by funding from National Institute of Mental Health (NIMH) grant R33MH087898 (to J.F.H.)
Footnotes
Conflicts of interest: none
Contributors
GWR participated in the data collection, statistical analysis, and interpreting data. SB, AU, and CP participated in the data collection. WFS participated in the study design, data collection, and interpreting data. JB, RO, and JH participated in the study design and interpreting data. JJ conducted additional analysis as requested by reviewers. All authors contributed to the drafting and revising of the manuscript and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23(2):185–188. doi: 10.1038/13810. [DOI] [PubMed] [Google Scholar]
- Anderlid BM, Schoumans J, Annerén G, Tapia-Paez I, Dumanski J, Blennow E, Nordenskjöld M. FISH-mapping of a 100-kb terminal 22q13 deletion. Hum Genet. 2002;110(5):439–443. doi: 10.1007/s00439-002-0713-7. [DOI] [PubMed] [Google Scholar]
- Baird G, Charman T, Pickles A, Chandler S, Loucas T, Meldrum D, … Simonoff E. Regression, developmental trajectory and associated problems in disorders in the autism spectrum: the SNAP study. J Autism Dev Disord. 2008;38(10):1827–1836. doi: 10.1007/s10803-008-0571-9. [DOI] [PubMed] [Google Scholar]
- Bonaglia MC, Giorda R, Beri S, De Agostini C, Novara F, Fichera M, … Zuffardi O. Molecular mechanisms generating and stabilizing terminal 22q13 deletions in 44 subjects with Phelan/McDermid syndrome. PLoS Genet. 2011;7(7):e1002173. doi: 10.1371/journal.pgen.1002173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonaglia MC, Giorda R, Borgatti R, Felisari G, Gagliardi C, Selicorni A, Zuffardi O. Disruption of the ProSAP2 gene in a t(12;22)(q24.1;q13.3) is associated with the 22q13.3 deletion syndrome. Am J Hum Genet. 2001;69(2):261–268. doi: 10.1086/321293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochoy DM, Kolevzon A, Kajiwara Y, Schoen M, Pascual-Lucas M, Lurie S, … Schmeisser MJ. Phenotypic and functional analysis of SHANK3 stop mutations identified in individuals with ASD and/or ID. Mol Autism. 2015;6:23. doi: 10.1186/s13229-015-0020-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar SU, del Gaudio D, German JR, Peters SU, Ou Z, Bader PI, … Sahoo T. 22q13.3 deletion syndrome: clinical and molecular analysis using array CGH. Am J Med Genet A. 2010;152A(3):573–581. doi: 10.1002/ajmg.a.33253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figura MG, Coppola A, Bottitta M, Calabrese G, Grillo L, Luciano D, … Elia M. Seizures and EEG pattern in the 22q13.3 deletion syndrome: clinical report of six Italian cases. Seizure. 2014;23(9):774–779. doi: 10.1016/j.seizure.2014.06.008. [DOI] [PubMed] [Google Scholar]
- Froehlich W, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, … Hallmayer J. Head circumferences in twins with and without Autism Spectrum Disorders. J Autism Dev Disord. 2013;43(9):2026–2037. doi: 10.1007/s10803-012-1751-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes V. Scientists track adult regression in autism-related syndrome. 2012 https://spectrumnews.org/news/scientists-track-adult-regression-in-autism-related-syndrome/
- Jeffries AR, Curran S, Elmslie F, Sharma A, Wenger S, Hummel M, Powell J. Molecular and phenotypic characterization of ring chromosome 22. Am J Med Genet A. 2005;137(2):139–147. doi: 10.1002/ajmg.a.30780. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism Diagnostic Interview-Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Macedoni-Lukšic M, Krgovic D, Zagradišnik B, Kokalj-Vokac N. Deletion of the last exon of SHANK3 gene produces the full Phelan-McDermid phenotype: a case report. Gene. 2013;524(2):386–389. doi: 10.1016/j.gene.2013.03.141. [DOI] [PubMed] [Google Scholar]
- Mullen EM. Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Services, Inc; 1995. [Google Scholar]
- Peters SU, Hundley RJ, Wilson AK, Carvalho CM, Lupski JR, Ramocki MB. Brief report: regression timing and associated features in MECP2 duplication syndrome. J Autism Dev Disord. 2013;43(10):2484–2490. doi: 10.1007/s10803-013-1796-9. [DOI] [PubMed] [Google Scholar]
- Roid GH. Technical Manual. 5. Itasca, IL: Riverside Publishing; 2003. Stanford Binet’s Intelligence Scales. [Google Scholar]
- Sarasua SM, Dwivedi A, Boccuto L, Chen CF, Sharp JL, Rollins JD, … DuPont BR. 22q13.2q13.32 genomic regions associated with severity of speech delay, developmental delay, and physical features in Phelan-McDermid syndrome. Genet Med. 2014;16(4):318–328. doi: 10.1038/gim.2013.144. [DOI] [PubMed] [Google Scholar]
- Sarasua SM, Dwivedi A, Boccuto L, Rollins JD, Chen CF, Rogers RC, … Collins JS. Association between deletion size and important phenotypes expands the genomic region of interest in Phelan-McDermid syndrome (22q13 deletion syndrome) J Med Genet. 2011;48(11):761–766. doi: 10.1136/jmedgenet-2011-100225. [DOI] [PubMed] [Google Scholar]
- Soorya L, Kolevzon A, Zweifach J, Lim T, Dobry Y, Schwartz L, … Buxbaum JD. Prospective investigation of autism and genotype-phenotype correlations in 22q13 deletion syndrome and SHANK3 deficiency. Mol Autism. 2013;4(1):18. doi: 10.1186/2040-2392-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanatos GA. Regression in autistic spectrum disorders. Neuropsychol Rev. 2008;18(4):305–319. doi: 10.1007/s11065-008-9073-y. [DOI] [PubMed] [Google Scholar]
- Tuchman RF, Rapin I. Regression in pervasive developmental disorders: seizures and epileptiform electroencephalogram correlates. Pediatrics. 1997;99(4):560–566. doi: 10.1542/peds.99.4.560. [DOI] [PubMed] [Google Scholar]
- Weaving LS, Ellaway CJ, Gécz J, Christodoulou J. Rett syndrome: clinical review and genetic update. J Med Genet. 2005;42(1):1–7. doi: 10.1136/jmg.2004.027730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willemsen MH, Rensen JH, van Schrojenstein-Lantman de Valk HM, Hamel BC, Kleefstra T. Adult Phenotypes in Angelman- and Rett-Like Syndromes. Mol Syndromol. 2012;2(3–5):217–234. doi: 10.1159/000335661. 000335661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson HL, Wong AC, Shaw SR, Tse WY, Stapleton GA, Phelan MC, … McDermid HE. Molecular characterisation of the 22q13 deletion syndrome supports the role of haploinsufficiency of SHANK3/PROSAP2 in the major neurological symptoms. J Med Genet. 2003;40(8):575–584. doi: 10.1136/jmg.40.8.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xu Q, Liu J, Li SC, Xu X. Risk factors for autistic regression: results of an ambispective cohort study. J Child Neurol. 2012;27(8):975–981. doi: 10.1177/0883073811430163. [DOI] [PubMed] [Google Scholar]