Abstract
Prolonged treatment of tuberculosis (TB) often leads to poor compliance, default and relapse, converting primary TB patients into category II TB (Cat IITB) cases, many of whom may convert to multi-drug resistant TB (MDR-TB). We have evaluated the immunotherapeutic potential of Mycobacterium indicus pranii (MIP) as an adjunct to Anti-Tubercular Treatment (ATT) in Cat II pulmonary TB (PTB) patients in a prospective, randomized, double blind, placebo controlled, multicentric clinical trial. 890 sputum smear positive Cat II PTB patients were randomized to receive either six intra-dermal injections (2 + 4) of heat-killed MIP at a dose of 5 × 108 bacilli or placebo once in 2 weeks for 2 months. Sputum smear and culture examinations were performed at different time points. MIP was safe with no adverse effects. While sputum smear conversion did not show any statistically significant difference, significantly higher number of patients (67.1%) in the MIP group achieved sputum culture conversion at fourth week compared to the placebo (57%) group (p = 0.0002), suggesting a role of MIP in clearance of the bacilli. Since live bacteria are the major contributors for sustained incidence of TB, the potential of MIP in clearance of the bacilli has far reaching implications in controlling the spread of the disease.
Introduction
Tuberculosis (TB) caused by Mycobacterium tuberculosis (M.tb) remains one of the major infectious diseases despite global efforts to contain and treat the disease which includes the Anti-Tubercular Treatment (ATT) through Directly Observed Treatment, Short-course (DOTS) strategy. Although, most of the M.tb infected individuals do not get the disease due to robust immune system, a substantial number progress to full-blown disease as soon as the immune system is compromised resulting in massive bacterial replication1. Despite good cure rate through ATT in primary TB, cases of default, non-compliance & relapse are rampant converting these patients into category II (Cat II) TB. Cat II TB includes those patients who have failed previous TB treatment, relapsed after treatment, or have defaulted during previous treatment2. A significant number of Cat II TB patients have been reported to develop MDR-TB2. Hence, an effective treatment for Cat II TB patients would help in containing the infection and prevent Cat II patients converting into MDR cases.
Immunotherapy as an adjunct to standard ATT could be an important strategy to treat this category of patients. In the present study, we have evaluated the immunotherapeutic potential of Mycobacterium w, renamed as Mycobacterium indicus pranii (MIP) in Cat II Pulmonary TB (Cat II PTB) patients. MIP shares antigens with both M. leprae and M.tb and has been shown to have significant protection against TB in both BCG responder and non-responder strains of mice3–7. MIP has been shown to have both immunotherapeutic and immunoprophylactic effects in multibacillary leprosy patients and their contacts respectively, in both hospital and population based trials8, 9. It induced conversion from lepromin negativity to lepromin positivity in multibacillary leprosy patients suggesting improved immune response to M. leprae antigens10. It also reduced the bacillary load, upgraded the lesions histopathologically, led to complete clearance of granuloma and reduced the duration of multi-drug therapy in leprosy patients8. In a large scale double blind trial for immunoprophylaxis against leprosy, involving 28,948 people in 272 villages of Ghatampur, Kanpur, India, a retrospective analysis after 13 years showed that incidence of TB was significantly reduced in the healthy contacts of leprosy patients vaccinated with MIP as compared to the placebo group11. Also, in a pilot study, addition of MIP to the short course chemotherapy in PTB patients led to earlier sputum conversion by at least 30 days4. Based on these encouraging results, we conducted a multi-centric trial to evaluate the safety and efficacy of MIP as an adjunct to ATT in Cat II PTB patients registered under the Revised National Tuberculosis Control Programme-Directly Observed Treatment, Short-course (RNTCP-DOTS).
Results
A total of 1553 Cat II PTB patients were screened of which 531 were screen failure and remaining 1022 were randomized in 1:1 ratio into the two treatment groups. However, 132 of these, later diagnosed with MDR tuberculosis (resistant to both rifampicin and isoniazid with or without any other drug), were excluded from the final analysis (Fig. 1). Among the 890 eligible patients (MIP Arm = 449, Placebo Arm = 441) that were included in the modified intent to treat (ITT), 642 (72.13%) (MIP Arm = 328, Placebo Arm = 314) patients completed the full course of treatment and were included in per protocol analysis (Fig. 1). 121 patients in the MIP arm (26.94%) and 127 patients in the Placebo arm (28.79%) did not complete the full course of treatment. There were multiple reasons for not completing the treatment which included death, unwillingness to continue in the trial, default in taking the treatment, pregnancy, protocol violations etc. (Supplementary Table 1 and Fig. 1). There was no significant difference in the baseline features of those who did not complete the treatment, in MIP and placebo group except that there were significantly higher number of cases with one drug resistance (p = 0.014) and significantly lower number of cases who had Sputum AFB smear grade of 2+ (p = 0.036) in the MIP group compared with placebo (Supplementary Table 2). 567 (297 in MIP arm and 270 in placebo arm) of the 642 cases who completed the treatment (88.32%), were followed up for two-years and were included in the relapse analysis. 26 patients were lost to follow-up for relapse analysis. There was no significant difference between the two arms in baseline characteristics such as, age, gender, BMI, sputum AFB grade, resistance to drugs, at initiation or radiographic severity (Table 1) in the patients who completed the treatment. MIP was safe and induced self-healing local reaction at the site of injection that subsided shortly.
Figure 1.
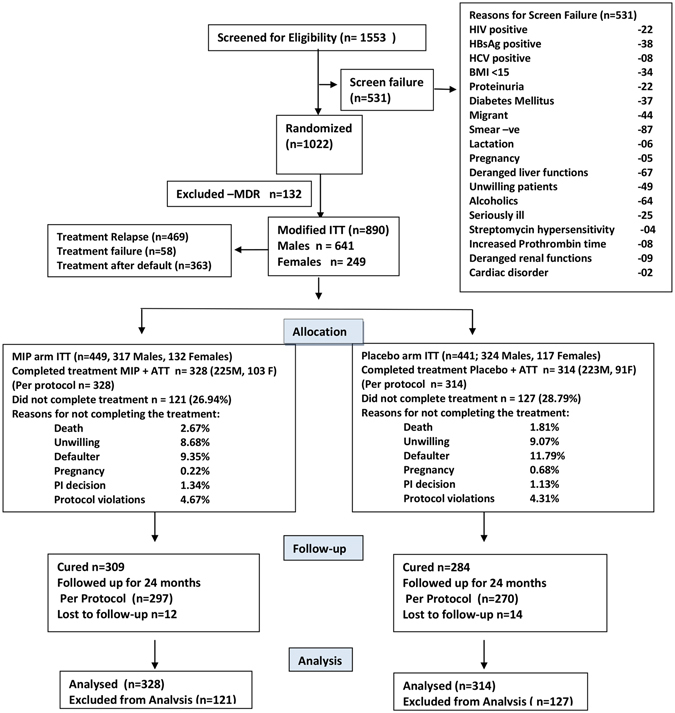
CONSORT Flow chart of Cat II patients screened, randomized, treated and analyzed. All randomized patients excluding MDRs (at baseline) formed the Modified intention to treat (ITT) population whereas all patients who have completed 8/9 months of study treatment (including 12 weeks of intensive phase) formed the per protocol population.
Table 1.
Distribution of patient characteristics at baseline for Modified intent to treat (ITT, N = 890) and Per Protocol (N = 642) population who completed the treatment.
| Characteristic(s) | Modified ITT Population (N = 890) | Per Protocol Population (N = 642) | ||
|---|---|---|---|---|
| MIP Arm, (N = 449) n (%) | Placebo Arm, (N = 441) n (%) | MIP Arm, (N = 328) n (%) | Placebo Arm, (N = 314) n (%) | |
| Age Group | ||||
| 18–30 years | 249 (55.5%) | 224 (50.8%) | 195 (59%) | 166 (53%) |
| 31–44 years | 133 (29.6%) | 154 (34.9%) | 92(28%) | 106 (34%) |
| ≥45 years | 67 (14.9%) | 63 (14.3%) | 41 (13%) | 42 (13%) |
| Gender | ||||
| Male | 317 (70.6%) | 324 (73.5%) | 225 (69%) | 223 (71%) |
| Female | 132 (29.4%) | 117 (26.5%) | 103 (31%) | 91 (29%) |
| BMI* | ||||
| <18.5 | 441 (98.2%) | 425 (96.4%) | 322 (98%) | 304 (97%) |
| ≥18.5 | 8(1.8%) | 16 (3.6%) | 06 (2%) | 10 (3%) |
| Reason for Inclusion in CAT-II | ||||
| Treatment Failure in CAT-I | 34 (7.6%) | 24 (5.4%) | 23 (7%) | 11 (4%) |
| Treatment after Default | 189 (42.1%) | 174 (39.5%) | 129 (39%) | 125 (40%) |
| Relapse | 226 (50.3%) | 243 (55.1%) | 176 (54%) | 178 (57%) |
| Resistance to Drugs at Treatment Initiation | ||||
| >3 drugs | 0 | 0 | 0 | 0 |
| 2–3 drugs | 41 (9.1%) | 48 (10.9%) | 16 (5%) | 21 (7%) |
| 1 drug | 84 (18.7%) | 60 (13.6%) | 54 (16%) | 44 (14%) |
| Streptomycin | 40 (8.9%) | 44 (10%) | 24 (7%) | 25 (8%) |
| Isoniazid | 78 (17.4%) | 80 (18.1%) | 43 (13%) | 48 (15%) |
| Rifampicin | 15 (3.3%) | 9 (2%) | 08 (2%) | 04 (1%) |
| Ethambutol | 40 (8.9%) | 30 (6.8%) | 18 (5%) | 12 (4%) |
| MDR# | 63 (14%) | 69 (15.6%) | — | — |
| Sputum AFB Smear Grade@ | ||||
| 1+ | 145 (32.3%) | 122 (27.7%) | 104 (32%) | 83 (26%) |
| 2+ | 117 (26.1%) | 127 (28.8%) | 93 (28%) | 87 (28%) |
| 3+ | 168 (37.4%) | 170 (38.5%) | 115 (35%) | 128 (41%) |
| Sc | 19 (4.2%) | 22 (5%) | 16 (5%) | 16 (5%) |
| Chest Radiography at Treatment Initiation | ||||
| Bilateral cavitations | 353 (78.6%) | 336 (76.2%) | 248 (76%) | 233 (74%) |
| Unilateral cavitation | 96 (21.4%) | 105 (23.8%) | 80 (24%) | 81 (26%) |
| No cavitation | — | — | — | — |
| Radiographic Severity of Disease at | ||||
| Initiation$ | 54 (12%) | 57 (12.9%) | 40 (12%) | 40 (13%) |
| Minimal | 306 (68.2%) | 304 (68.9%) | 228 (70%) | 220 (70%) |
| Moderately Advanced | 88 (19.6%) | 80 (18.1%) | 60 (18%) | 54 (17%) |
| Far Advanced | ||||
*BMI- body mass index [weight in kg/ (height in cm)2]; #MDR- Multidrug resistant tuberculosis @WHO sputum grading - > 10 AFB/oil immersion field in at least 20 fields: 3+, 1–10 AFB/oil immersion field in at least 50 fields:2+, 10–99 AFB/ 100 oil immersion field:1+, 1–9 AFB/100 oil immersion field: Sc i.e., scanty);. $Radiographic severity at baseline not available for 1 patient.
Evaluation of the Primary outcome measures
The time of sputum conversion as well as comparison of early sputum conversion between the two groups
The primary outcome measures included time of sputum conversion. So, we studied the time of both sputum smear and sputum culture conversion, after administration of MIP or placebo along with ATT. Subjects who completed the full course of treatment were included in the data analysis and the time point at which the sputum smear or culture conversion took place was noted for each individual in both groups. The median time for sputum smear conversion was 2 weeks in both the groups. 53.35% patients in MIP group and 48.72% patients in placebo group turned sputum smear negative at 2 weeks and this difference was not significant statistically (Table 2). However, the median time for sputum culture conversion was 4 weeks after initiation of therapy in both the groups, suggesting that even when the cases became sputum smear negative, they were still harboring bacilli detectable in culture. Significantly higher number of patients (67.1%) in the MIP group achieved culture conversion at fourth week compared to the placebo (57%) group (p = 0.0002, OR = 1.86, 95% C.I. = 1.31–2.64) suggesting a role for MIP in eradication of detectable bacilli. This trend of significantly higher number of patients showing culture conversion in MIP group was observed at each subsequent visit after fourth week which indicates inability of conventional therapy to provide equivalent culture conversion (Table 2). By the time the follow-up reached 39th week 94.2% of patients (309/328) in the MIP arm showed sputum culture conversion as compared to 89.17% (280/314) in the placebo arm. This difference was still significant even after correction of p value for multiple comparison (p = 0.02, OR = 1.97, 95% C.I.−1.06–3.75) (Table 2), suggesting the sustained effect of MIP in elimination of detectable bacilli.
Table 2.
Sputum-smear and culture conversion time in per protocol group i.e. patients who completed the treatment.
| Week | Smear conversion till (in weeks) | P | Odds Ratio (95% C.I.) | Culture conversion till (in weeks) | P | Odds Ratio (95% C.I.) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIP Arm (N = 328) | Placebo Arm(N = 314) | MIP Arm (N = 328) | Placebo Arm (N = 314) | |||||||||
| Converted (Cumulative numbers) | % | Converted (Cumulative numbers) | % | Converted (Cumulative numbers) | % | Converted (Cumulative numbers) | % | |||||
| 2 | 175 | 53.35 | 153 | 48.7 | 0.24 | 1.2 (0.87–1.66) | 139 | 42.37 | 118 | 37.5 | 0.215 | 1.22 (0.87–1.69) |
| 4 | 234 | 71.34 | 216 | 68.78 | 0.48 | 1.13 (0.79–1.61) | 220 | 67.1 | 179 | 57.0 | 0.0002* | 1.86 (1.31–2.64) |
| 6 | 268 | 81.7 | 242 | 77.1 | 0.146 | 1.33 (0.89–1.98) | 257 | 78.35 | 215 | 68.47 | 0.0046* | 1.67 (1.15–2.41) |
| 8 | 281 | 85.67 | 261 | 83.1 | 0.37 | 1.21 (0.77–1.9) | 286 | 87.1 | 248 | 78.98 | 0.0054* | 1.8 (1.16–2.84) |
| 12 | 298 | 90.85 | 274 | 87.3 | 0.14 | 1.45 (0.85–2.48) | 301 | 91.77 | 269 | 85.67 | 0.014* | 1.86 (1.1–3.22) |
| 16 | 303 | 92.37 | 280 | 89.17 | 0.16 | 1.47 (0.82–2.64) | 303 | 92.37 | 270 | 85.98 | 0.009* | 1.97 (1.14–3.46) |
| 26 | 305 | 92.98 | 283 | 90.13 | 0.19 | 1.45 (0.79–2.67) | 306 | 93.29 | 275 | 87.58 | 0.0136* | 1.97 (1.1–3.58) |
| 35 | 306 | 93.29 | 283 | 90.13 | 0.15 | 1.52 (0.83–2.83) | 306 | 93.29 | 277 | 88.2 | 0.026** | 1.86 (1.03–3.39) |
| 36 | — | — | — | — | 309 | 94.2 | 280 | 89.17 | 0.02* | 1.97 (1.06–3.75) | ||
| 39 | 309 | 94.2 | 284 | 90.4 | 0.073 | 1.72 (0.91–3.3) | 309 | 94.2 | 280 | 89.17 | 0.02* | 1.97 (1.06–3.75) |
| 40 | — | — | — | — | — | 281 | 89.49 | |||||
| Median Time (range) | 2 (2–39) | 2 (2–39) | 4 (2–36) | 4 (2–40) | ||||||||
*P value significant after Boneferroni’s correction. **Corrected p value not significant.
Cure Rate
The cure rate in Cat II PTB patients was evaluated in both the groups after administration of MIP or Placebo as per RNTCP guidelines i.e. sputum smear conversion on at least two occasions, one of which was at the completion of treatment. The frequency of patients cured amongst those who completed the full course of treatment was 94.2% in the MIP group as compared to 90.4% in the placebo group at 39th week after initiation of therapy (Table 2, sputum smear conversion and Supplementary Table 3), however, this difference was not statistically significant (p = 0.073, Odds Ratios (OR) = 1.72, 95% C.I. = 0.91–3.3). 68.8% of all patients (309 out of 449) who were initially inducted for the treatment (ITT) were cured in the MIP group as compared to 64.4% (284 out of 441) in the placebo group and this difference was also not statistically significant (p = 0.162, Supplementary Table 3).
Relapse
Of the 642 patients 593 patients (MIP Arm = 309; Placebo Arm = 284) who were declared cured were eligible for relapse analysis. However, only 567 (MIP Arm = 297; Placebo Arm = 270) cases were analyzed for relapse as the data on relapse was not available for 26 patients. There was no significant difference in the relapse rate between the MIP and placebo groups at 6, 12, 18 and 24 months (Table 3).
Table 3.
Relapse rate (n = 567)‡.
| Interval (in months) | MIP Arm (N = 297) | Placebo Arm (N = 270) | p | Odds Ratio (95% C.I.) | ||
|---|---|---|---|---|---|---|
| Relapsed | % | Relapsed | % | |||
| 6 | 23 | 7.74 | 16 | 5.92 | 0.39 | 1.33 (0.68–2.58) |
| 12 | 6 | 2.02 | 7 | 2.59 | 0.64 | 0.77 (0.25–2.33) |
| 18 | 2 | 0.67 | 2 | 0.74 | 0.65 | 0.9 (0.13–6.5) |
| 24 | 3 | 1.01 | 1 | 0.37 | 0.34 | 2.7 (0.28–26.54) |
‡Total eligible for Relapse Analysis = 593(MIP Arm = 309; Placebo Arm = 284). Among the 593 patients, data on relapse is not available for 26 patients as they were enrolled beyond the project timelines making the numbers available for evaluation of relapse rate as 567.
Adverse Reactions
Cutaneous lesions and local reaction at the site of injection were observed in significantly higher number of patients in the MIP group compared to placebo group, as expected. However, these lesions were self-healing and subsided shortly. Non-vaccine related adverse events were significantly less in the MIP group as compared to placebo (p < 0.000035, Odds Ratio = 0.556) (Tables 4 and 5). The non-vaccine related adverse events included gastrointestinal, respiratory, CVS, skin or tuberculosis related events (Supplementary Table 4).
Table 4.
Vaccine related adverse events.
| Event Term | MIP Arm (N = 449) | Placebo Arm (N = 441) | ||
|---|---|---|---|---|
| Number of patients | Number of events | Number of patients | Number of events | |
| Cutaneous Lesion at Injection Site (Abscess/Blister/Pappule) | 309 | 310 | 10 | 10 |
| Local Reaction at Injection Site (Pain, Swelling, Itching, Redness, Pus Discharge) | 58 | 114 | 8 | 10 |
| Scar at Injection Site | 3 | 3 | — | — |
| Total | 370 | 427 | 18 | 20 |
Table 5.
Non-Vaccine related adverse events.
| Event Term | MIP Arm (N = 449) | Placebo Arm (N = 441) | P value | Odds Ratio (95% C.I.) | ||
|---|---|---|---|---|---|---|
| Number of patients | Number of events | Number of patients | Number of events | |||
| Total | 127 | 159 | 183 | 217 | 0.000035* | 0.556 (0.41–0.74) |
*p value significant after Boneferroni’s correction.
Post-hoc sub-group analysis
Since the cure was not observed in 100% of the patients in either MIP or placebo group, we further investigated whether the base line features of a patient were detrimental for the outcome of the treatments. While we realized during the analysis that sputum culture conversion should be considered as cure rather than sputum smear conversion, we did not deviate from our initial definition of cure i.e. sputum smear conversion on at least two occasions. So, further sub-group analysis was carried out based on sputum smear conversion only. To study the correlation, if any, between the baseline features of the patients and cure in the MIP and placebo groups, an analysis was carried out based on age, gender, BMI, reasons for inclusion, sputum bacillary load, and resistance to drugs at the baseline and radiographic severity (Table 6, Supplementary Table 5).
Table 6.
Sub group analysis of baseline characteristics of cured patients-Per Protocol (those who completed the treatment).
| Characteristic(s) | All patients in MIP Arm, (N = 328) n (%) | Cured in MIP Arm, (N = 309) n (% of the subgroup) | All patients in Placebo Arm, (N = 314) n (%) | Cured in Placebo Arm, (N = 284) n (% of the subgroup) | p Value | Odds Ratio | 95% Confidence Interval |
|---|---|---|---|---|---|---|---|
| Age Group | |||||||
| 18–30 years | 195 (59%) | 186 (95.4%) | 166 (53%) | 152 (91.6%) | 0.139 | 1.9 | 0.74–5.12 |
| 31–44 years | 92(28%) | 85 (92.4%) | 106 (34%) | 93 (87.7%) | 0.278 | 1.7 | 0.6–5.3 |
| ≥45 years | 41 (13%) | 38 (92.7%) | 42 (13%) | 39 (92.9%) | 0.65 | 0.97 | 0.33–2.9 |
| Gender | |||||||
| Male | 225 (69%) | 211 (93.8%) | 223 (71%) | 199 (89.2%) | 0.085 | 1.8 | 0.87–3.91 |
| Female | 103 (31%) | 98 (95.1%) | 91 (29%) | 85 (93.4%) | 0.42 | 1.38 | 0.34–5.94 |
| BMI | |||||||
| <17.5 | 314 (95.7%) | 295 (93.9%) | 301 (95.8%) | 271 (90.1%) | 0.073 | 1.72 | 0.91–3.31 |
| ≥17.5 | 14 (4.3%) | 14 (100%) | 13 (4.2%) | 13 (100%) | |||
| BMI | |||||||
| <18.5 | 322 (98%) | 303 (94.1%) | 304 (97%) | 274 (90.1%) | 0.065 | 1.75 | 0.93–3.36 |
| ≥18.5 | 06 (2%) | 6 (100%) | 10 (3%) | 10 (100%) | — | ||
| Reason for Inclusion in CAT-II | |||||||
| Treatment Failure in CAT-I | 23 (7%) | 21 (91.3%) | 11 (4%) | 8 (72.7%) | 0.17 | 3.54 | 0.99–12.71 |
| Treatment after Default | 129 (39%) | 122 (94.6%) | 125 (40%) | 115 (92%) | 0.41 | 1.52 | 0.5–4.85 |
| Relapse | 176 (54%) | 166 (94.3%) | 178 (57%) | 161 (90.4%) | 0.17 | 1.75 | 0.73–4.4 |
| Resistance to Drugs at Treatment Initiation | |||||||
| >3 drugs | 0 | 0 | 0 | 0 | 0.046** | 11.0 | 1.34–90.12 |
| 2–3 drugs | 16 (5%) | 16 (100%) | 21 (7%) | 16 (76.2%) | 0.001* | 25.38 | 3.3–194.9 |
| 1 drug | 54 (16%) | 54 (100%) | 44 (14%) | 36 (81.8%) | 0.028** | 6.35 | 1.71–23.59 |
| Streptomycin | 24 (7%) | 23 (95.8%) | 25 (8%) | 18 (72%) | 0.0653 | 3.48 | 1.22–9.88 |
| Isoniazid | 43 (13%) | 41 (95.3%) | 48 (15%) | 40 (83.3%) | 0.333 | 7.2 | 0.64–82.61 |
| Rifampicin | 08 (2%) | 8 (100%) | 04 (1%) | 3 (75%) | 0.152 | 8.8 | 0.96–80.5 |
| Ethambutol | 18 (5%) | 18 (100%) | 12 (4%) | 10 (83.3%) | |||
| Pyrazinamide | ND | ND | |||||
| Sputum AFB Smear Grade | |||||||
| 1+ | 104 (32%) | 99 (95.2%) | 83 (26%) | 78 (93.9%) | 0.713 | 1.26 | 0.28–5.72 |
| 2+ | 93 (28%) | 87 (93.5%) | 87 (28%) | 83 (95.4%) | 0.42 | 0.69 | 0.14–3.07 |
| 3+ | 115 (35%) | 107 (93%) | 128 (41%) | 108 (84.4%) | 0.035** | 2.47 | 0.98–6.77 |
| Sc | 16 (5%) | 16 (100%) | 16 (5%) | 15 (93.8%) | 0.5 | 3.19 | 0.32–32.36 |
| Chest Radiography at Treatment Initiation | |||||||
| Bilateral cavitations | 248 (76%) | 235 (94.8%) | 233 (74%) | 208 (89.3%) | 0.026** | 2.17 | 1.04–4.74 |
| Unilateral cavitations | 80 (24%) | 74 (92.5%) | 81 (26%) | 76 (93.8%) | 0.49 | 0.81 | 0.18–3.34 |
| No cavitations | — | — | — | — | |||
| Radiographic Severity of Disease at Initiation‡ | |||||||
| Minimal | 40 (12%) | 40 (100%) | 40 (13%) | 38 (95%) | 0.25 | 5.25 | 0.9–1.62 |
| Moderately Advanced | 228 (70%) | 214 (93.9%) | 220 (70%) | 201 (91.4%) | 0.31 | 1.44 | 0.21–23.36 |
| Far Advanced | 60 (18%) | 55 (91.7%) | 54 (17%) | 45 (83.3%) | 0.14 | 2.2 | 0.61–8.92 |
‡Radiographic severity at baseline not available for 1 patient; *p value Significant after Boneferroni’s correction. **Corrected p value not significant.
100% of the patients (16/16) who were resistant to two to three drugs were cured in MIP group as compared to 76.2% in the placebo group (16/21) and this difference was statistically significant (p = 0.046, OR = 11.0, 95% C.I. = 1.34–90.12). However, when each drug was analyzed separately, 95.8% of the patients who were resistant to streptomycin responded positively to MIP administration and were cured, compared to 72% in the placebo group (p = 0.028, OR = 6.35, 95% C.I. = 1.71–23.59). Similarly, 100% of the patients (54/54) who were resistant to one drug were cured in the MIP group as compared to 81.8% (36/44) in the placebo group (p = 0.001, OR = 25.38, 95% C.I. = 3.3–194.9).
MIP also had significant effect on patients with high bacillary loads. 93% of the patients (107/115) with a bacillary load of AFB3+ were cured in the MIP group compared to 84.4% (108/128) in the placebo group (p = 0.035, OR = 2.47, 95% C.I. = 0.98–6.77). Similarly 94.8% of the patients (235/248) with bilateral cavities were cured in the MIP group compared to 89.3% (208/233) in the placebo group (p = 0.026, OR 2.17, 95% C.I. = 1.04–4.74). These results highlight the benefits of MIP immunotherapy in the advanced cases of Cat II PTB who had drug resistance / higher bacillary indices / and had bilateral cavitations. However, these p values may not remain significant when corrected for multiple comparisons as the numbers of individuals involved in sub group analysis are small, suggesting that larger cohort of patients with drug resistance, higher bacillary indices and with bilateral cavitations need to be treated with MIP and evaluated before a conclusion can be drawn for its efficacy in ‘worse to treat’ Cat II TB.
Immunological Parameters
PBMCs isolated from 57 blinded samples were studied for immunological parameters. When codes were opened, it was found that MIP group had 27 samples and placebo group had 30 samples.
There was a lot of heterogeneity observed in the samples studied for all the parameters at the base line and throughout the study period at one month, 3 months, 6 months and 12 months. Different assays done to assess the immune responses stimulated by M.tb and MIP antigens at different time points after vaccination / placebo administration did not show any significant differences between the two groups. This could be due to a very significant variance observed in all the groups studied at different time points as was evident from the scatter-graphs of all the assays (data not shown).
However, an important observation was that mean stimulation index on in vitro restimulation of PBMCs with M.tb antigen was consistently higher in the MIP group as compared to placebo group from 1 month till 6 month after the start of therapy. MIP group also showed significantly higher stimulation index (p < 0.047) as compared to healthy controls group at 3 months and 12 months (p < 0.013) when stimulated with M.tb antigen (Supplementary Figure 1). A subgroup analysis based on the initial bacterial index showed that patients with AFB3+ showed better proliferation indices at 1 and 3 months when given MIP as compared to placebo. Further stratification based on AFB3+ along with bilateral cavities showed higher proliferation indices at 1 month after administration of MIP.
The amount of IL-2 secreted after in vitro re stimulation with M.tb antigen showed an increasing trend in the MIP group from Day 0 to 6 month in follow-up samples on M.tb stimulation while no change was seen in the placebo group, however, the difference was not statistically significant (Supplementary Figure 2). Results of lymphocyte proliferation were also reflected in the IFN-γ secretion which increased consistently from Day 0 and almost doubled in 3 months and remained high till 12 month in the MIP group while on the contrary, the amount of secreted IFN-γ showed a decreasing trend in the placebo group from Day 0 onward till 6 month (Supplementary Figure 3). However, due to small number of samples in each group and also because of heterogeneity observed in the immune response of different patients at baseline itself differences observed between study group and placebo group were not statistically significant. Both IL-2 and IFN-γ showed an increase at the first month follow-up, in AFB3+ and AFB3+ along with bilateral cavities patients. Again, the difference was not statistically significant due to small number of cases in this subgroup and huge variance.
Discussion
In spite of the continuous efforts made in reducing the burden of tuberculosis, the global epidemic of TB is growing, both due to untreated cases and rise in MDR-TB12. Prevalence of MDR-TB in Cat II TB patients has been reported to be high in various studies2, 13. Though there are clear WHO guidelines to treat Cat II patients, treatment failure rate reported in different studies range from 4% to 35%14, 15. These facts necessitate additional strategies to boost the immune responses in Cat II patients since they have the potential to become MDR or XDR (extensively drug resistant) TB cases. Addition of immunotherapy to ATT for Cat II PTB may be one such approach as it aims to restore the Th1/Th2 balance by enhancing the Th1 response and suppressing the excessive Th2 response16. Several approaches have been adopted, like use of anti-mycobacterial antibodies, use of mycobacterial antigens for boosting Th1 immune responses, administration of Th1 cytokines etc. (reviewed in detail by Uhlin et al.17). However, none were found suitable for large scale use.
An alternative approach is to use an immunomodulator which can induce multifaceted immune response and eliminate the tubercle bacilli with minimal side effects. Therapeutic and prophylactic effects of M. vaccae have been evaluated in a few studies. While M. vaccae was effective in preventing TB in PPD strong positive individuals, its immunotherapeutic effects were inconclusive18–20. While several new prophylactic vaccines are being evaluated to either replace BCG and/or as booster to BCG21–25, there is a dearth of studies on immunotherapeutic intervention along with ATT in TB patients. Immunotherapeutic potential of whole bacteria as well as individual specific antigens like hsp6526, Ag85B27, MPT-64, MPT-8328, DNA-acr, DNA-sod, Ag85C29 etc. have been evaluated in animal models of tuberculosis and had shown some promise but these subunit vaccines have their limitations for mass scale use in humans. Another candidate named RUTI that consists of liposome encapsulated detoxified bacterial fragments of M. tuberculosis, has undergone phase I and II trials on healthy participants and latently infected TB patients for safety, tolerability and antigenicity30, 31. However, there are very few studies on immunotherapy for treatment failure or category II TB patients.
A study reported that 62% of the treatment failure TB patients (n = 48) became sputum negative in one month when an oral vaccine called V-5 Immunitor (V5) was given to them32. However, in our study 91.3% (21/23) of the category I treatment failure patients were cured in the MIP group as compared to 72.7% (8/11) cases in placebo group (Table 4). Also, there is a serious limitation of this oral immunotherapy as the pill V-5 preparation is derived from pooled blood of HBV- and HCV-positive donors with chemical and heat inactivation32. Such formulation may not be practical for large scale use and has ethical issues due to the potential risk of developing HBV or HCV infections.
Our study is the first large scale, multi-centric trial on Cat II PTB where an immunomodulator MIP was given as an adjunct therapy along with standard ATT. Significantly higher number of patients in the MIP group showing sputum culture conversion as early as 4 weeks after initiation of therapy, suggests a role for MIP in elimination of detectable bacilli which may help in shortening the duration of treatment. While sputum smear conversion was envisaged as the primary read out for cure as per RNTCP guidelines, our data suggests that culture conversion may be a better marker for cure. At four weeks, while 68.78% of the cases in placebo group showed sputum smear conversion, only 57% of them showed sputum culture conversion (Table 2), suggesting that significantly higher number of cases (11.78%) in the placebo group were still harboring bacilli (p = 0.002, OR = 1.66, 95% C.I. = 1.19–2.3). On the other hand, in the MIP group, 71.34% of cases showed sputum smear conversion and 67.1% showed sputum culture conversion at fourth week and this difference was not significant statistically suggesting that most of the sputum smear negative cases had also become sputum culture negative in this group. Based on these data one can speculate that sputum smear conversion may actually be giving a false negative signal and one should trust sputum culture conversion to evaluate cure rate since the acid test for clearance of the detectable bacilli would be culture negativity. Support for this also comes from recent studies where sputum culture conversion has been considered as the only widely accepted marker of sterilizing activity and efficacy and used for monitoring therapy in patients with pulmonary TB which is crucial to avoid relapses12, 33–35. Time to culture conversion which effectively shows mycobacterial elimination, has been used in several trials to evaluate the efficacies of drugs like moxifloxacin, ciprofloxacin etc. in tuberculosis35–38. Perrin et al. also suggested that early or faster culture conversion could indeed be a better surrogate marker for effectiveness of new drug as compared to culture negativity at 2-month38.
Our post-hoc analysis showed a significant difference in cure rate in difficult to treat patients (with sputum AFB smear grade of 3+ and those with bilateral cavitations) in the MIP arm, which would definitely help in lowering the burden of TB. The immunological parameters also showed higher proliferation indices, IL-2 and IFN-gamma secretion in these cases given MIP compared to placebo. While calculating the sample size we had hoped to get at least 10% improvement over and above ATT, however, we saw 24% improvement in cases that were resistant to two to three drugs, where 100% of the patients were cured in the MIP group compared to only 76.2% in the placebo group. These advanced cases, otherwise, have poor prognosis and significant improvement in them provides rationale for inclusion of MIP in RNTCP. However, larger studies are needed to substantiate the findings of the present study.
MIP has been shown to have both immunotherapeutic as well as immunoprophylactic effects in multibacillary leprosy patients8, 9. While we could not correlate the immune responses with cure due to small sample size, MIP therapy as an adjunct to the chemotherapy in guinea pig model of tuberculosis accelerated bacterial killing through an increase in early protective Th1 immune response in the lungs of guinea pigs. This was followed by a balanced inflammatory and suppressive immune response in later part of the treatment suggesting that MIP induced immunomodulation is involved in accelerated bacterial clearance and reduction in the lung pathology7. Recent comparative genomic analysis of MIP has attributed its immunomodulatory properties to its high antigenic potential39.
Prophylactic potential of MIP against M.tb was earlier demonstrated in a retrospective population based double blind study in Ghatampur, India9, 11. However, the present study shows its clinical benefit as an immunotherapeutic agent for difficult to treat tuberculosis. At the time of initiation of the present study in 2005 the success rate of Cat II treatment in India was 68% compared to the success rate of 86% in newly sputum positive patients treated with Cat I treatment regimen. The retreatment outcomes for default and failed Cat I treatment varied from 48.8% to 55.8% in different studies40. However, in the present study, 94.2% of the cases in MIP group and 90.4% of the cases in the placebo group were cured at 39th week. Higher cure even in the placebo group could be due to lower number of subjects enrolled with treatment failure (4%), treatment after default (40%) and removal of MDR patients from the study. Thus, this may be an effect of controlled conditions which is reflected in improved success rate.
Merits of the present study are that it is a large scale, multi-centric trial done meticulously and methodically with a good sample size. It is also the first study from India that has followed up patients after completion of treatment under Cat II regimen of the RNTCP. Shortcomings of the study are high number of cases (27.86%) who did not complete the treatment due to various reasons and heterogeneity in response rate across the study centers. Further, although there was slightly higher percentage of relapse in MIP group, the difference was not statistically significant from placebo group. The other drawback is small number of cases in the post-hoc subgroup analysis, suggesting that a much larger cohort needs to be evaluated for the efficacy of MIP in different sub-groups. Although the study was designed to be double blind, the cutaneous lesions and local reaction at the site of injection could have revealed the identity of the treatment to the doctors administering MIP or placebo. However, coded samples were sent to the laboratories for sputum smear and culture conversion and the laboratory staff involved in these analyses were totally blind to the treatment given to the patients. In spite of these short comings, our study clearly demonstrates superiority of MIP- immunomodulator in inducing early culture negativity and improving the cure rate of Cat II PTB patients with parameters that would include them in ‘difficult to treat’ category (like resistant to two or three drugs, and/or very high bacillary loads and/or bilateral cavitations) where current therapy is suboptimal and adjunct immunotherapy could assist in bacillary clearance. More studies in such cases would help strengthen the findings of the present study and justify inclusion of MIP in the RNTCP.
Methods
Study design and population
The study was a prospective, randomized, double blind, placebo controlled, multi-centric clinical trial. Patients aged between 18 to 60 years who were sputum smear positive and candidates for Cat II TB treatment under the RNTCP - DOTS were included in the trial after obtaining informed written consent. Cat II PTB patients included those with treatment after default (TAD), treatment failure (TF), and treatment relapse (TR). The study was approved by the Drugs Controller General of India (DCGI) in addition to the Institutional Ethics Committee at each site and was conducted adopting the ethical principles stated in the latest version of Helsinki Declaration as well as the applicable guidelines for good clinical practice (GCP). Ethical approvals were obtained from Institutional Ethics Committee of All India Institute of Medical Sciences, New Delhi, Ethical Committee of Central JALMA Institute of Leprosy, Agra, UP, Ethical Committee of Lala Ram Sarup Institute of Tuberculosis and Respiratory diseases, New Delhi, Ethics Committee of Bhagwan Mahavir Medical Research Centre, Hyderabad, Institutional Ethics Committee, NHL Municipal Medical College, Ahemadabad, Gujrat, Institutional Ethics Committee, Tubeculosis Research Centre (ICMR), Chennai, and Ethics Committee of SMS Medical College & Attached Hospitals, Jaipur. An independent Data Safety Monitoring Board (DSMB) was constituted to look at the safety aspects of the trial. The clinical trial was registered at the US National Institutes of health ClinicalTrials.gov Identifier: NCT00265226 available at their website (https://clinicaltrials.gov/ct2/show/NCT00265226).
Exclusion criteria included patients with extra-pulmonary tuberculosis, immunodeficiency, significant organ dysfunction (cardiac, renal or hepatic), co-infected with Hepatitis B or C virus, receiving cytotoxic therapy and pregnant or lactating women. Additional exclusion criteria were known hypersensitivity to ATT, seizure disorder, abnormal hematologic function and patients on oral and systemic steroids continuously for more than seven days, a week prior to inclusion. Patients with other serious or uncontrolled concurrent illness or with history of alcohol and drug abuse were also excluded. Eligible patients who consented to participate in the study were enrolled from 7th March 2005 to February 2009. Primary completion date for data collection for primary outcome was December 31st, 2010 and follow up for 2 years after the completion of treatment finished in March 2011. The calculation of sample size was done assuming at least 10% difference in the sputum conversion rate at the end of active treatment as well as in the rate of relapse at 6, 12, 18 and 24 months of follow up period in the group getting MIP as an adjunct to ATT as compared to placebo. At 95% confidence interval and at power of 90, anticipating about 30% loss to follow up, at least 510 patients were required per group. The sample size was calculated as described in supplementary materials.
Study protocol, Randomization and treatment plan
Eligible patients who consented to participate in the study were enrolled from 7th March 2005 to February 2009 at seven centers in different parts of India, involved in tuberculosis research and treatment. 1553 patients were screened for exclusion and inclusion criteria and 1022 of them were included in the trial (Fig. 1). A randomization list was generated by an independent statistician using center-wise stratification with fixed sizes of blocks using Ralloc software. For every subject labeling of the vials was done as numbers (which had combination of centre number and the serial number). Double blinding was ensured by assigning unique numbers to each trial patient. Neither the investigators nor the patients were aware about the intervention they had received. The codes were kept with a person who had no conflict of interest with the trial. Codes were opened after final analysis duly approved by the trial DSMB.
Patients were randomized to receive either six intradermal injections of MIP at a dose of 5 × 108 heat-killed bacilli in 0.1 ml normal saline or placebo (normal saline without bacilli) once in 2 weeks over 2 months. The schedule of administration of MIP/placebo was two simultaneous injections initially in the deltoid regions of both upper arms followed by single injection on alternate deltoid every two weeks, along with the standard chemotherapy, as per RNTCP guidelines. For the intensive phase, the patients received Streptomycin 750 mg (intramuscular injection) along with Isoniazid 600 mg + Rifampicin 450 mg + Ethambutol 1200 mg and Pyrazinamide 1500 mg (SHRZE i.e., S – Streptomycin, H- Isoniazid, R – Rifampicin, E – Ethambutol, Z – Pyrazinamide) orally three times in a week for two months followed by the same treatment but without Streptomycin i.e., HRZE for one month (Intensive Phase). If the patient was found to be sputum positive at the end of three months, the same treatment i.e., HRZE was continued for one more month. During the Intensive phase, the patients were administered the drugs during their visit at the clinic/hospital only. The intensive phase was followed by the continuous phase wherein the patients were administered Isoniazid 600 mg + Rifampicin 450 mg + Ethambutol 1200 mg three times in a week for five months. During the continuous phase the patients were dispensed medication on weekly basis from the study center.
Since the time of sputum conversion as well as the early sputum conversion between the 2 groups were to be compared, we performed both Sputum smear and sputum culture examinations at baseline and at weeks 2, 4, 6, 8, 12, 16, 26, 35, 36 and 39. In patients where the sputum smear was still positive at 3 months after initiation of the therapy, intensive phase was extended for one more month and further sputum examination was performed at 4, 6, 9 months of the treatment. Drug sensitivity was performed at baseline and at 3rd/4th month. The patients were followed up at months 6, 12, 18 and 24 after the completion of the therapy.
Primary Outcome Measures as defined in the registered trial NCT00265226 were as follows
The time of sputum conversion as well as the early sputum conversion between the 2 groups.
The cure rate to be evaluated as the primary parameter of efficacy.
The relapse in patients of category II tuberculosis to be compared in both the groups at an interval of 6, 12, 18 and 24 months after the completion of the therapy.
Recording of any clinical adverse reactions at anytime during the study for assessment of safety.
Study definitions
Cured
Initially sputum smear positive patient who had completed treatment and had negative sputum smears, on at least two occasions, one of which was at the completion of treatment.
Speed of Sputum Smear/Culture Conversion
The time-point at which first smear/culture turned negative for initially smear/culture positive patient who had completed treatment.
Relapse
A patient declared cured of TB but found to be bacteriologically positive during the 24 months follow-up period.
Definition of ITT and Per Protocol Population
All randomized patients excluding MDRs (at baseline) formed the Modified intention to treat (ITT) population whereas all patients who had completed 8/9 months of study treatment (including 12 weeks of intensive phase) formed the per protocol population.
Sputum Smear Examination
Sputum smears were examined by Ziehl-Neelsen Staining (ZN) method for Acid-fast bacilli (AFB) using carbolfuchsin and methylene blue, as per the standards laid down under RNTCP guidelines. A grading of 3+ was assigned for more than 10 AFB per oil immersion field in at least 20 fields whereas 2+ and 1+ was assigned for 1–10 AFB per oil immersion field in at least 50 fields and 10–99 AFB per 100 oil immersion fields in at least 100 fields, respectively. A grading of scanty was assigned for 1–9 AFB per 100 oil immersion fields in at least 200 fields whereas negative was assigned for no AFB in 100 oil immersion fields in at least 100 fields.
Sputum Culture Examination
AFB culture and sensitivity test were done using the Gold Standard Isolation Techniques: Lowenstein Jensen’s medium, radiometric or fluorescence method in different laboratories, as described in supplementary materials.
Immunological parameters
To Study and compare the immunomodulatory effect of MIP in the study group as compared to placebo group immunological markers were studied in the peripheral blood of small representative group. Blood samples from 57 category II patients and 36 healthy controls were studied for immunological parameters. Blinded blood samples were collected at AIIMS at baseline, 1, 3, 6 and 12 month after treatment. Memory recall response was studied by lympho proliferation induced by M.tb and MIP sonicates using thymidine uptake assay. IFN-gamma, IL-12 and IL-4 production were studied using ELISPOT assays, Intracellular cytokines in CD4+ and CD8+ T cell compartments were determined by FACS after stimulation of PBMCs culture with sonicates of M.tb and MIP. The secreted cytokines were studied in the culture supernatants, which were stored frozen and assayed together using multiplex Cytometric Bead array system (human Th1/Th2 CBA kit) of BD Bioscience (USA) which included IL-2, IL-4, IL-5, IL-10, TNF-α, and IFN-γ. Unpaired t-test with Welch’s correction was used to analyze the significance of differences between the two groups with Graph-pad prism 4 software (San Diego, CA, USA).
Statistical analysis
The data analysis was performed using Stata 9.2 program for windows. The primary efficacy end point was taken as per protocol principle and included patients who had completed 8/9 months of active treatment. In addition, a modified intent-to-treat (ITT) efficacy analysis was also performed. However, for safety analysis all patients were included as per the ICH-GCP guidelines. Chi-square test was used to compare baseline characteristics and cure rates between the two groups. Fisher’s exact test was used whenever the numbers were ≤5 in any group and one-tail analysis was used to calculate the p values. In such cases Odds ratios were calculated using Woolf’s method with Haldane’s modification41. p value of <0.05 was considered as statistically significant. Boneferroni’s correction was done by multiplying the p value by the number of comparisons done for each feature.
Electronic supplementary material
Acknowledgements
The study was funded by Department of Biotechnology grant number BT/PR/4526/Med/14/534/2004, Ministry of Science & Technology, New Delhi, India and Cadila Pharmaceuticals Ltd., Ahmadabad, India. We thank all the members of Data Safety Monitoring Board (Prof. K. Ramachandran, Dr. V.K. Vijayan, Dr. K.R. Sundaram, Dr. R.M. Pandey, Dr. Subhash Verma), patients, paramedics, hospitals, RNTCP centers, funding agency and experts (Prof. V.M. Katoch, Prof. N.K. Mehra, Prof. G.P. Talwar, Prof. Seyed E. Hasnain, Dr. Subrata Sinha, Dr. Kameshwar Prasad, Dr. Shreemanta K. Parida, Dr. Vineeta Bal and Dr. Sita Naik) for their invaluable contribution towards the study. Authors would like to thank Dr. Neeraj Nischal for critically going through the manuscript and giving valuable suggestions. Further we would like to acknowledge the contribution made by Dr. Rajeshwari Ramachandran, Dr. Vijay Arora, Late Dr. Adeep Bagati and Dr. Bhaswat Chakraborty, during the early phase of the study. A special tribute is offered to late Shri I.A. Modi, Chairman, Cadila Pharmaceuticals, Ahmadabad.
Author Contributions
B.D., S.K.S., R.R., B.K. Participated in designing of study protocol. B.D. supervised the conduct of the study including progress, DSMB meetings, supervising and funding of monitoring activities, collection of study protocols and final analysis of the data. B.K. contributed in designing, printing of study protocol, CRF’s and preparation and supply of MIP and placebo for the study. S.K.S., K.K., R.S., R.B., N.K.J., N.P., K.J.R.M., N.S., P.K.S., A.K., U.S., S.K., A.S., J.N.B., K.R., D.S.C., S.S., M.W., P.K., S.T., N.D., N.Ja., N.Jo., S.R.R.P. and S.G., were involved in the clinical study, recruitment, treatment and follow up of patients, bacteriological as well as laboratory tests of subjects, D.S.T. as well as assessment of immunological parameters. D.K.M., S.A., S.B. and R.R. were involved in immunological studies. SGu was involved in monitoring the study and collecting data from all centers. S.Gu., B.D. and R.R. were involved in final analysis of the data. S.K.S., B.K., B.D., K.K., S.B. and R.R. were involved in writing the manuscript.
Competing Interests
Funding agency was involved in designing of study protocol, management of the conduct of the study including progress, DSMB meetings, supervising and funding of monitoring activities, collection of study protocols and final analysis of the data. Dr. Bindu Dey was an Adviser in Department of Biotechnology, the funding agency. Bakulesh Khamar is the Executive Director, Research, at Cadila Pharmaceuticals Ltd., Ahmadabad, India, company which has been licensed to manufacture MIP. However, he was not involved in clinical study directly. Sanjay Gupta of Catalyst Clinical Services Pvt. Ltd., New Delhi, India was involved in collection of data from various clinical sites, collating and analysis of the data. However, there were no potential competing financial interests as such for the other authors involved in carrying out the project.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-03514-1
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Surendra K. Sharma, Email: surensk@gmail.com
Rajni Rani, Email: rajni@nii.ac.in.
References
- 1.Andersen P. Tuberculosis vaccines - an update. Nat Rev Microbiol. 2007;5:484–7. doi: 10.1038/nrmicro1703. [DOI] [PubMed] [Google Scholar]
- 2.Sharma SK, et al. Prevalence of multidrug-resistant tuberculosis among category II pulmonary tuberculosis patients. Indian J Med Res. 2011;133:312–5. [PMC free article] [PubMed] [Google Scholar]
- 3.Singh IG, Mukherjee R, Talwar GP. Resistance to intravenous inoculation of Mycobacterium tuberculosis H37Rv in mice of different inbred strains following immunization with a leprosy vaccine based on Mycobacterium w. Vaccine. 1991;9:10–4. doi: 10.1016/0264-410X(91)90309-T. [DOI] [PubMed] [Google Scholar]
- 4.Patel N, Trapathi SB. Improved cure rates in pulmonary tuberculosis category II (retreatment) with mycobacterium w. J Indian Med Assoc. 2003;101:680–682. [PubMed] [Google Scholar]
- 5.Faujdar J, et al. Mycobacterium indicus pranii as stand-alone or adjunct immunotherapeutic in treatment of experimental animal tuberculosis. Indian J Med Res. 2011;134:696–703. doi: 10.4103/0971-5916.90999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gupta A, et al. Protective efficacy of Mycobacterium indicus pranii against tuberculosis and underlying local lung immune responses in guinea pig model. Vaccine. 2012;30:6198–209. doi: 10.1016/j.vaccine.2012.07.061. [DOI] [PubMed] [Google Scholar]
- 7.Gupta A, et al. Efficacy of Mycobacterium indicus pranii immunotherapy as an adjunct to chemotherapy for tuberculosis and underlying immune responses in the lung. PLoS One. 2012;7:e39215. doi: 10.1371/journal.pone.0039215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sharma P, et al. Mycobacterium w vaccine, a useful adjuvant to multidrug therapy in multibacillary leprosy: a report on hospital based immunotherapeutic clinical trials with a follow-up of 1-7 years after treatment. Lepr Rev. 2000;71:179–92. doi: 10.5935/0305-7518.20000020. [DOI] [PubMed] [Google Scholar]
- 9.Sharma P, et al. Immunoprophylactic effects of the anti-leprosy Mw vaccine in household contacts of leprosy patients: clinical field trials with a follow up of 8-10 years. Lepr Rev. 2005;76:127–43. [PubMed] [Google Scholar]
- 10.Sharma P, et al. Induction of lepromin positivity and immunoprophylaxis in household contacts of multibacillary leprosy patients: a pilot study with a candidate vaccine, Mycobacterium w. Int J Lepr Other Mycobact Dis. 2000;68:136–42. [PubMed] [Google Scholar]
- 11.Katoch K, et al. Potential of Mw as a prophylactic vaccine against pulmonary tuberculosis. Vaccine. 2008;26:1228–34. doi: 10.1016/j.vaccine.2007.12.025. [DOI] [PubMed] [Google Scholar]
- 12.Zumla A, George A, Sharma V, Herbert N, Baroness Masham of I. WHO’s 2013 global report on tuberculosis: successes, threats, and opportunities. Lancet. 2013;382:1765–7. doi: 10.1016/S0140-6736(13)62078-4. [DOI] [PubMed] [Google Scholar]
- 13.Tabarsi P, et al. Revised Category II regimen as an alternative strategy for retreatment of Category I regimen failure and irregular treatment cases. Am J Ther. 2011;18:343–9. doi: 10.1097/MJT.0b013e3181dd60ec. [DOI] [PubMed] [Google Scholar]
- 14.Spinaci S. Treatment failure and MDR-TB. Int J Tuberc Lung Dis. 1999;3:365. [PubMed] [Google Scholar]
- 15.Goble M, et al. Treatment of 171 patients with pulmonary tuberculosis resistant to isoniazid and rifampin. N Engl J Med. 1993;328:527–32. doi: 10.1056/NEJM199302253280802. [DOI] [PubMed] [Google Scholar]
- 16.Guo S, Zhao J. Immunotherapy for tuberculosis: what’s the better choice? Front Biosci (Landmark Ed) 2012;17:2684–90. doi: 10.2741/4079. [DOI] [PubMed] [Google Scholar]
- 17.Uhlin M, Andersson J, Zumla A, Maeurer M. Adjunct immunotherapies for tuberculosis. J Infect Dis. 2012;205(Suppl 2):S325–34. doi: 10.1093/infdis/jis197. [DOI] [PubMed] [Google Scholar]
- 18.de Bruyn, G. & Garner, P. Mycobacterium vaccae immunotherapy for treating tuberculosis. Cochrane Database Syst Rev, CD001166 (2003). [DOI] [PubMed]
- 19.Stanford JL, Stanford CA. Immunotherapy of tuberculosis with Mycobacterium vaccae NCTC 11659. Immunobiology. 1994;191:555–63. doi: 10.1016/S0171-2985(11)80462-6. [DOI] [PubMed] [Google Scholar]
- 20.Yang XY, Chen QF, Cui XH, Yu Y, Li YP. Mycobacterium vaccae vaccine to prevent tuberculosis in high risk people: a meta-analysis. J Infect. 2010;60:320–30. doi: 10.1016/j.jinf.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Horwitz MA, Harth G. A new vaccine against tuberculosis affords greater survival after challenge than the current vaccine in the guinea pig model of pulmonary tuberculosis. Infect Immun. 2003;71:1672–9. doi: 10.1128/IAI.71.4.1672-1679.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grode L, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. J Clin Invest. 2005;115:2472–9. doi: 10.1172/JCI24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Agger EM, et al. Protective immunity to tuberculosis with Ag85B-ESAT-6 in a synthetic cationic adjuvant system IC31. Vaccine. 2006;24:5452–60. doi: 10.1016/j.vaccine.2006.03.072. [DOI] [PubMed] [Google Scholar]
- 24.Dietrich J, et al. Mucosal administration of Ag85B-ESAT-6 protects against infection with Mycobacterium tuberculosis and boosts prior bacillus Calmette-Guerin immunity. J Immunol. 2006;177:6353–60. doi: 10.4049/jimmunol.177.9.6353. [DOI] [PubMed] [Google Scholar]
- 25.Dietrich J, et al. Exchanging ESAT6 with TB10.4 in an Ag85B fusion molecule-based tuberculosis subunit vaccine: efficient protection and ESAT6-based sensitive monitoring of vaccine efficacy. J Immunol. 2005;174:6332–9. doi: 10.4049/jimmunol.174.10.6332. [DOI] [PubMed] [Google Scholar]
- 26.Silva CL, et al. Immunotherapy with plasmid DNA encoding mycobacterial hsp65 in association with chemotherapy is a more rapid and efficient form of treatment for tuberculosis in mice. Gene Ther. 2005;12:281–7. doi: 10.1038/sj.gt.3302418. [DOI] [PubMed] [Google Scholar]
- 27.Sheikh JA, Khuller GK, Verma I. Immunotherapeutic role of Ag85B as an adjunct to antituberculous chemotherapy. J Immune Based Ther Vaccines. 2011;9:4. doi: 10.1186/1476-8518-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu DH, Hu XD, Cai H. Efficient tuberculosis treatment in mice using chemotherapy and immunotherapy with the combined DNA vaccine encoding Ag85B, MPT-64 and MPT-83. Gene Ther. 2008;15:652–9. doi: 10.1038/gt.2008.13. [DOI] [PubMed] [Google Scholar]
- 29.Chauhan P, Jain R, Dey B, Tyagi AK. Adjunctive immunotherapy with alpha-crystallin based DNA vaccination reduces Tuberculosis chemotherapy period in chronically infected mice. Sci Rep. 2013;3:1821. doi: 10.1038/srep01821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nell AS, et al. Safety, tolerability, and immunogenicity of the novel antituberculous vaccine RUTI: randomized, placebo-controlled phase II clinical trial in patients with latent tuberculosis infection. PLoS One. 2014;9:e89612. doi: 10.1371/journal.pone.0089612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vilaplana C, et al. Double-blind, randomized, placebo-controlled Phase I Clinical Trial of the therapeutical antituberculous vaccine RUTI. Vaccine. 2009;28:1106–16. doi: 10.1016/j.vaccine.2009.09.134. [DOI] [PubMed] [Google Scholar]
- 32.Arjanova OV, et al. Adjunct oral immunotherapy in patients with re-treated, multidrug-resistant or HIV-coinfected TB. Immunotherapy. 2011;3:181–91. doi: 10.2217/imt.10.96. [DOI] [PubMed] [Google Scholar]
- 33.Mitchison DA. Assessment of new sterilizing drugs for treating pulmonary tuberculosis by culture at 2 months. Am Rev Respir Dis. 1993;147:1062–3. doi: 10.1164/ajrccm/147.4.1062. [DOI] [PubMed] [Google Scholar]
- 34.Kurbatova EV, et al. Sputum culture conversion as a prognostic marker for end-of-treatment outcome in patients with multidrug-resistant tuberculosis: a secondary analysis of data from two observational cohort studies. Lancet Respir Med. 2015;3:201–9. doi: 10.1016/S2213-2600(15)00036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velayutham BV, et al. Sputum culture conversion with moxifloxacin-containing regimens in the treatment of patients with newly diagnosed sputum-positive pulmonary tuberculosis in South India. Clin Infect Dis. 2014;59:e142–9. doi: 10.1093/cid/ciu550. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy N, et al. Randomized controlled trial of a drug regimen that includes ciprofloxacin for the treatment of pulmonary tuberculosis. Clin Infect Dis. 1996;22:827–33. doi: 10.1093/clinids/22.5.827. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy N, et al. Early bactericidal and sterilizing activities of ciprofloxacin in pulmonary tuberculosis. Am Rev Respir Dis. 1993;148:1547–51. doi: 10.1164/ajrccm/148.6_Pt_1.1547. [DOI] [PubMed] [Google Scholar]
- 38.Perrin FM, Lipman MC, McHugh TD, Gillespie SH. Biomarkers of treatment response in clinical trials of novel antituberculosis agents. Lancet Infect Dis. 2007;7:481–90. doi: 10.1016/S1473-3099(07)70112-3. [DOI] [PubMed] [Google Scholar]
- 39.Saini V, et al. Massive gene acquisitions in Mycobacterium indicus pranii provide a perspective on mycobacterial evolution. Nucleic Acids Res. 2012;40:10832–50. doi: 10.1093/nar/gks793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Azhar GS. DOTS for TB relapse in India: A systematic review. Lung India. 2012;29:147–53. doi: 10.4103/0970-2113.95320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haldane JB. The estimation and significance of the logarithm of a ratio of frequencies. Ann Hum Genet. 1956;20:309–11. doi: 10.1111/j.1469-1809.1955.tb01285.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


