Abstract
SerpinB3 is a hypoxia- and hypoxia-inducible factor-2α-dependent cystein protease inhibitor that is up-regulated in hepatocellular carcinoma and in parenchymal cells during chronic liver diseases (CLD). SerpinB3 up-regulation in CLD patients has been reported to correlate with the extent of liver fibrosis and the production of transforming growth factor-β1, but the actual role of SerpinB3 in hepatic fibrogenesis is still poorly characterized. In the present study we analyzed the pro-fibrogenic action of SerpinB3 in cell cultures and in two different murine models of liver fibrosis. “In vitro” experiments revealed that SerpinB3 addition to either primary cultures of human activated myofibroblast-like hepatic stellate cells (HSC/MFs) or human stellate cell line (LX2 cells) strongly up-regulated the expression of genes involved in fibrogenesis and promoted oriented migration, but not cell proliferation. Chronic liver injury by CCl4 administration or by feeding a methionine/choline deficient diet to transgenic mice over-expressing human SerpinB3 in hepatocytes confirmed that SerpinB3 over-expression significantly increased the mRNA levels of pro-fibrogenic genes, collagen deposition and αSMA-positive HSC/MFs as compared to wild-type mice, without affecting parenchymal damage. The present study provides for the first time evidence that hepatocyte release of SerpinB3 during CLD can contribute to liver fibrogenesis by acting on HSC/MFs.
Introduction
Liver fibrogenesis is a dynamic and highly integrated process that, irrespective of the etiology, results in a progressive accumulation of extracellular matrix (ECM) components and drives the progression of chronic liver disease (CLD)1–3. A major pro-fibrogenic role is played by hepatic myofibroblasts (MFs)1–9 which mainly originate from a process of activation/trans-differentiation of hepatic stellate cells (HSC) or HSC/MFs8, 9. The persistent activation of these cells is the consequence of a complex interaction between growth factors, cytokines, chemokines, reactive oxygen species (ROS) and other mediators1–7. In the pro-fibrogenic environment these factors are released by- and interact with- several liver cell populations, including damaged hepatocytes, activated inflammatory cells and MFs1–7. Accordingly, the identification and characterization of the mediators involved in sustaining the pro-fibrogenic role of MFs during CLD progression is a critical issue to design novel selective therapeutic strategies and/or to develop diagnostic procedures.
Along these lines, SerpinB3, a member of the family of serine-proteases inhibitors (serpins)10, 11, has recently emerged as a mediator involved in CLD progression12, 13 and in liver carcinogenesis14–18. SerpinB3 expression is regulated by hypoxia through the hypoxia-inducible factor-2α (HIF-2α)19. In normal human and murine livers SerpinB3 is virtually undetectable, but its expression is readily appreciable in a significant percentage of liver biopsies from CLD patients, as well as in hepatocellular carcinoma (HCC)12–18 and hepatoblastomas20. In both neoplastic and non-neoplastic settings, liver SerpinB3 up-regulation correlates with that of transforming growth factor-β1 (TGF-β1), a critical mediator in liver fibrogenesis12, 21. In particular, a study performed in liver biopsies from 94 patients with CLD of different etiology has outlined a significant “in vivo” correlation between TGF-β1 and SerpinB3 expression (protein and mRNA level). The same study also reported “in vitro” data showing that SerpinB3 over-expression in hepatocyte-derived cells also promoted TGF-β1 release, suggesting that SerpinB3 may contribute to up-regulate TGF-β1 production during chronic injury12. The putative pro-fibrogenic action of SerpinB3 has also been evidenced in human idiopathic pulmonary fibrosis22 and in a murine model of lung fibrosis23, 24. Nonetheless, the mechanisms by which SerpinB3 may contribute to liver fibrogenesis are still poorly characterized.
In the present study we investigated the putative pro-fibrogenic role of SerpinB3 first by performing experiments “in vitro” using human HSC/MFs and then “in vivo” taking advantage of two different experimental protocols of chronic liver injury in transgenic mice overexpressing human SerpinB3 in hepatocytes (TG-SB3 mice).
Results
SerpinB3 up-regulates transcription of key pro-fibrogenic genes in human HSC/MFs
The addition of human recombinant SerpinB3 (hrSerpinB3) to cultures of human immortalized activated MF-like cells (LX2 cells) resulted in the up-regulation of the transcripts for key pro-fibrogenic genes, including TGFβ1 (TGFB1), COL1A1, α-SMA, platelet-derived growth factor (PDGF)-B and the related PDGF-β receptor (PDGFBR) subunit, TIMP-1, MMP-1 as well as C-C motif chemokine ligand 2 (CCL2) (Fig. 1a). The activation of these genes was also fully reproduced in primary cultures of human HSC/MFs exposed to hrSerpinB3 (Supplementary Fig. 1a). Furthermore, upon hrSerpinB3 addition, both LX2 cells and primary culture of human HSC/MFs up-regulated the transcription of pro-angiogenic cytokines relevant in sustaining liver fibrogenesis25, 26 such as VEGF-A (VEGFA) and Angiopoietin-1 (Ang-1 or ANGPT1) (Fig. 1b; Supplementary Fig. 2). In HSC/MFs SerpinB3 also stimulated the expression of TIE-2, the gene encoding for the Ang-1 receptor (Supplementary Fig. 2). Interestingly, already 6 hours after hrSerpinB3 addition LX2 cells released VEGF-A protein in the extracellular medium and this effect reached a maximum in the following 24–48 hours (Fig. 1c,d).
Figure 1.
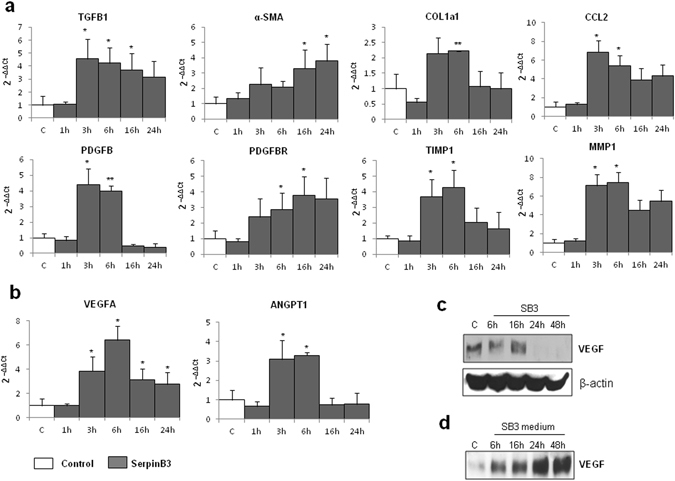
SerpinB3 up-regulates expression of genes involved in liver fibrogenesis in immortalized human HSC (LX2 cells). (a) Analysis by quantitative real-time PCR (Q-PCR) of transcript levels of pro-fibrogenic genes, of CCL2 as well as of (b) VEGF-A or angiopoietin 1 (Ang-1) in human LX2 cells exposed for the indicated time points to 100 ng/ml human recombinant SerpinB3 (SB3). Data are expressed as means ± SEM of three independent experiments (*p < 0.05 or **p < 0.01 vs control values). (c,d) WB Analysis of VEGF-A protein in total extracts (left panel) and immuno-precipitated VEGF-A protein levels released in the extracellular medium collected at the indicated time points. The cropped gels shows in this Fig. have been run under the same experimental conditions.
SerpinB3 induces oriented migration in human HSC/MFs
We next evaluated whether SerpinB3 may modulate proliferation and chemotaxis of activated HSCs. Human LX2 cells as well as human primary HSC/MFs, which responded positively to the potent mitogen and chemoattractant PDGF-BB (used as positive control), failed to show appreciable increase in proliferation when exposed to hrSerpinB3 (Fig. 2a,b). However, both LX2 cells (Fig. 2c) and human HSC/MFs (Fig. 2d) showed oriented migration when exposed to hrSerpinB3 in a modified Boyden’s chamber assay. Additional experiments, designed to investigate the chemoattractant action, revealed that LX2 cells exposed to hrSerpinB3 had features in common with those induced by other chemo-attractants on activated HSCs27. This included very early activation/phosphorylation of c-Jun NH2-terminal kinase isoforms −1 and −2 (JNK1/2) and c-Akt, with no relevant change in activation/phosphorylation of extracellular-regulated kinase isoforms 1 and 2 (ERK1/2) (Fig. 2e). In addition, hrSerpinB3 also promoted HSC/MF generation of intracellular ROS that was evident already after 15 min (Fig. 3b). As previously shown for other chemoattractants27, hrSerpinB3-induced oriented migration was almost abolished by pre-treating HSC/MFs with pharmacological inhibitors of either NADPH-oxidase (apocynin, Diphenyleneiodonium or DPI) or of JNK1/2 and c-Akt (SP600125 or LY294002) (Fig. 3c). Furthermore, apocynin-mediated inhibition of ROS generation by NADPH-oxidase also blocked the activation/phosphorylation of JNK1/2 but not that of c-Akt (Fig. 3d), confirming that the chemotactic action of SerpinB3 mainly involves the same ROS- and JNK1/2-related signals described for other chemoattractants like PDGF-BB, VEGF-A and CCL2 that are active on HSC/MFs27.
Figure 2.
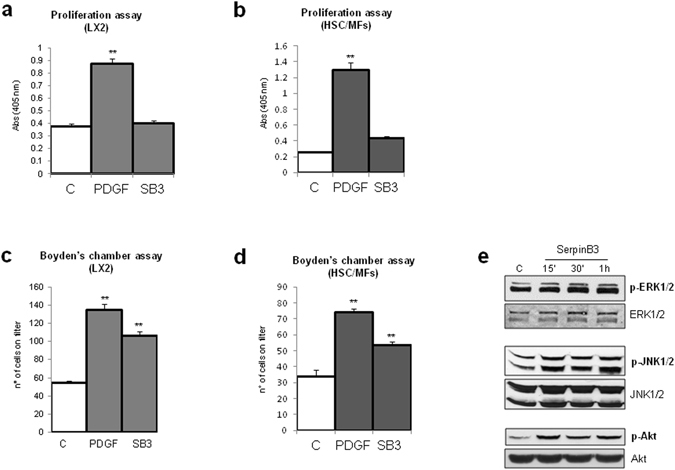
hrSerpinB3 stimulates oriented migration, but not proliferation, in human LX2 cells and human HSC/MFs. (a,b) Confluent and 24 hr starved LX2 cells (a) or HSC/MFs (b) were left untreated or exposed to either hrSerpinB3 (100 ng/ml) or PDGF-BB (10 ng/ml, positive control) for further 48 hrs and then evaluated for proliferation using the crystal violet assay (see Methods). (c,d) Analysis of oriented migration in the modified Boyden’s chamber assay of LX2 cells (c) or HSC/MFs (d) exposed or not to either hrSerpinB3 (100 ng/ml) or PDGF-BB (10 ng/ml, positive control). Data in bar graphs are expressed as means ± SEM of three independent experiments (**p < 0.01 vs control values). (e) Western blot analysis of activation of ERK1/2, JNK1/2 and Akt signaling pathways in LX2 cells exposed to hrSerpinB3 (100 ng/ml). Confluent and 24 hr starved LX2 cells were exposed to SerpinB3 for the indicated time points and activation of pathways was evaluated in total extracts by analysis of levels of phosphorylated versus un-phosphorylated levels of ERK1/2, JNK1/2 and c-Akt. The cropped gels shows in this Fig. have been run under the same experimental conditions.
Figure 3.
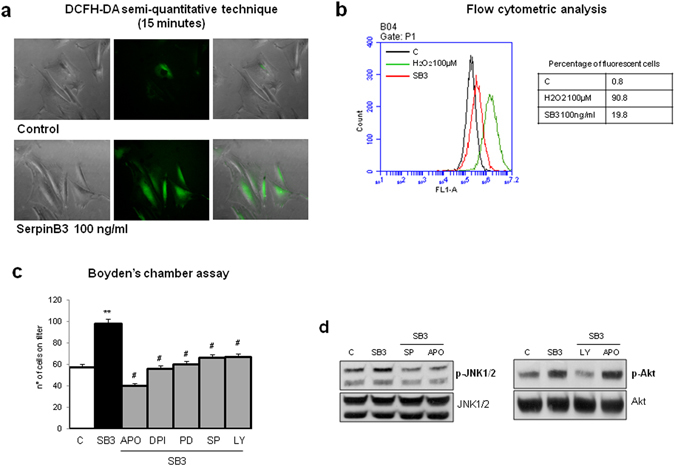
hrSerpinB3-dependent induction of oriented migration relies on intracellular generation of ROS and specific signaling pathways. (a,b) Confluent and 24 hr starved LX2 cells were left untreated or exposed to either hrSerpinB3 (100 ng/ml) or PDGF-BB (10 ng/ml, positive control) and then evaluated for early (i.e., 15 minutes) intracellular generation of ROS as shown by DCFH-DA fluorescence (a) or flow cytometry analysis (b). (c,d) Analysis of oriented migration in the modified Boyden’s chamber assay (c) or Western blot analysis of activation of JNK1/2 and Akt signaling pathways (d) of control LX2 cells or LX2 cells exposed to hrSerpinB3 (100 ng/ml) in the presence of pharmacological inhibitors of JNK1/2 (SP, SP600125,) and Akt (LY, LY294002) or in the presence of apocynin (APO) or diphenylen-iodonium (DPI), all added 30 min before addition of hrSerpinB3. Data in bar graphs are expressed as means ± SEM of three independent experiments (*p < 0.05 or **p < 0.01 vs control values, #p < 0.05 vs related stimulus). Images from Western blot analysis or from evaluation of ROS generation are representative from at least three experiments performed. The cropped gels shows in this Fig. have been run under the same experimental conditions.
Transgenic mice over-expressing human SerpinB3 in murine hepatocytes develop more liver fibrosis than wild type littermates
As mentioned above, SerpinB3 is virtually undetectable in either human or murine livers, but it is produced in response to liver hypoxia by hepatocytes or hepatic cancer cells in a significant fraction of patients with cirrhosis or hepatocellular carcinoma19. In order to mechanistically investigate whether SerpinB3 may operate as a pro-fibrogenic mediator we employed previously characterized transgenic mice that overexpress human SerpinB3 (TG-SB3 mice) in hepatocytes and in lung epithelial cells24. The livers of naïve SerpinB3 TG-mice had normal morphology and hepatocytes of naïve SerpinB3 TG-mice as well as of related wild type mice did not differ for the expression of albumin, cytokeratin 18, α-fetoprotein or the ability to store glycogen and were all negative for cytokeratin 7 expression (Supplementary Figure 3). SerpinB3 expression was evident in parenchymal cells (Fig. 4a) and Western blot analysis confirmed the exclusive presence of human SerpinB3 in TG animals (Fig. 4b). This pattern corresponded to that seen in CLD patients where SerpinB3 immunostaining was mainly detectable in hepatocytes (Fig. 4c). In human specimens from HCV cirrhotic patients (METAVIR F4) SerpinB3-positive cells were usually detectable in hypoxic areas, as shown by the concomitant expression of HIF2α and VEGF-A (Fig. 4d).
Figure 4.
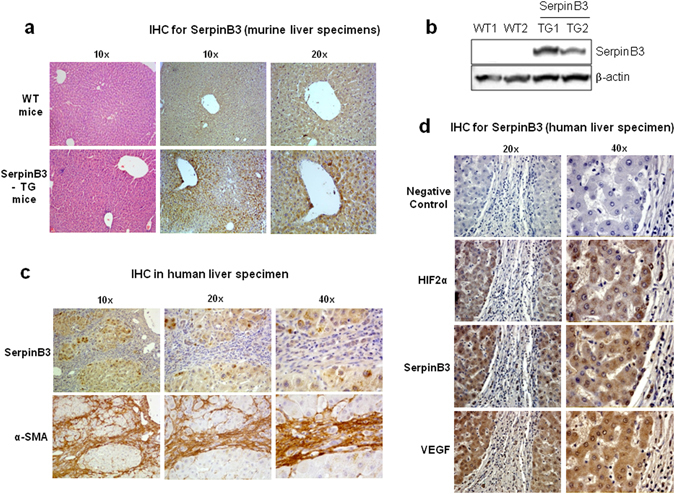
SerpinB3 overexpression in TG mice and human liver specimens from HCV patients. (a) Liver morphology (hematoxylin/eosin staining) and immunohistochemistry (IHC) for SerpinB3 expression in the liver of control WT and TG mice. Original magnification as indicated. (b) Western blot analysis of SerpinB3 protein levels performed on total liver extracts obtained from either WT and TG mice. Equal loading was monitored by re-blotting membranes for β-actin. The cropped gels shows in this Fig. have been run under the same experimental conditions. Immunohistochemistry analysis for (c) SerpinB3 and α-smooth muscle actin (α-SMA) or (d) for HIF2α, SerpinB3, and VEGF-A in liver specimens from HCV infected patients (METAVIR F4).
For evaluating the actual impact of SerpinB3 on the evolution of hepatic fibrosis TG-SB3 mice and corresponding WT littermates were exposed to chronic liver injury. To this aim we used two different experimental murine models: (i) chronic CCl4 administration, which results in post-necrotic bridging fibrosis resembling pan-lobular/parenchymal fibrosis found in the clinical conditions in which SerpinB3 expression has been best characterized2, 28, 29; (ii) mice fed with the MCD diet, that leads to pericellular/perisinusoidal fibrosis similar to that seen in human NAFLD/NASH2, 28, 29. Naive TG-SB3 mice had plasma SerpinB3 levels around 2 ng/mL (1.8 ± 0.6 ng/mL) that were enhanced by 5–10 fold in response to chronic liver injury. In particular, CCl4-treated animals showed circulating SerpinB3 twice as higher than those receiving the MCD diet (21.5 ± 6.7 ng/mL vs 10.5 ± 5.4 ng/mL; p < 0.001). In the transgenic animals SerpinB3 over-production did not influence hepatocellular damage, as liver histology and the circulating levels of alanine (ALT) and aspartate (AST) aminotransferases were comparable to those in wild type mice (Fig. 5). On the other hand, TG-SB3 mice receiving CCl4 or the MCD diet showed a higher hepatic expression of inflammatory markers TNF-α and CD11b (Fig. 5) than the relative wild type animals. In both models of chronic liver injury, collagen Sirius Red staining revealed that the overexpression of SerpinB3 resulted in a marked increase in the extension of fibrosis. In a similar manner the number of α-SMA positive HSC/MFs was significantly higher in TG-SB3 mice exposed to CCl4 or the MCD diet than in similarly treated wild-type animals (Figs 6 and 7). Consistently, Q-PCR analysis for a set of genes involved in fibrogenesis demonstrated that in both the experimental models the hepatic transcripts for collagen 1A1 (Col1a1), tissue inhibitor of metalloproteases type 1 (Timp1) and TGFβ1 (Tgfb1) were significantly enhanced in TG-SB3 (Figs 6 and 7). Among TG-SB3 mice receiving CCl4 and the MCD diet we also observed a significant positive correlation (r = 0.74; p = 0.04) between the individual levels of circulating SerpinB3 and those of liver collagen 1A1 transcripts. The mRNAs for heme-oxygenase 1 (Hmox-1) and matrix-metalloprotease type 2 (Mmp2) were also elevated in TG-SB3 animals receiving the MCD diet, but not in those exposed to CCl4 (Figs 6 and 7). Altogether, these data provide in vitro and in vivo evidence supporting the implication of SerpinB3 in the processes leading to CLD progression to fibrosis.
Figure 5.
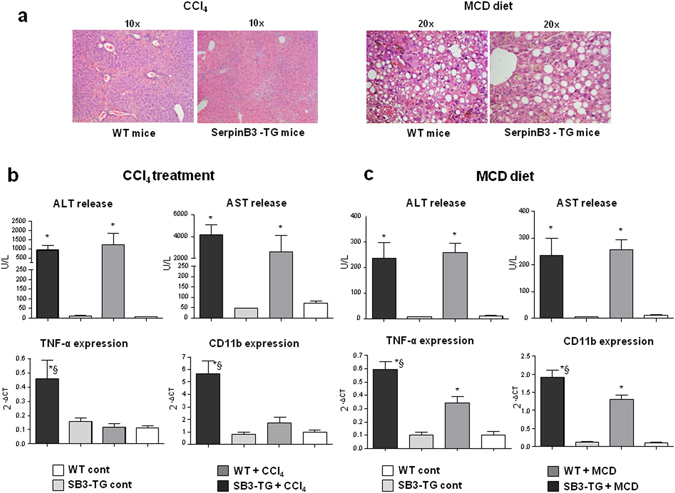
Parenchymal injury and inflammatory markers in SerpinB3 transgenic (SB3-TG) mice and wild type (WT) mice following chronic (10 weeks) CCl4 administration or 4 weeks feeding with a methionine/choline deficient (MCD) diet. (a) Liver morphology in mice exposed to CCl4 or the MCD diet was evaluated by hematoxylin/eosin staining (magnification 10X). Parenchymal injury, estimated by measuring the circulating levels of alanine (ALT) and aspartate (AST) aminotransferases and liver expression of inflammatory markers TNF-α and CD11c, evaluated by quantitative real-time PCR (Q-PCR), are reported in CCl4 treated mice (b) and in MCD fed mice (c). Data in graphs are expressed as means ± SD (n = 6 mice for each experimental group) (*p < 0.01 vs the relative control mice; §p < 0.05 vs WT-mice receiving CCl4 or the MCD diet).
Figure 6.
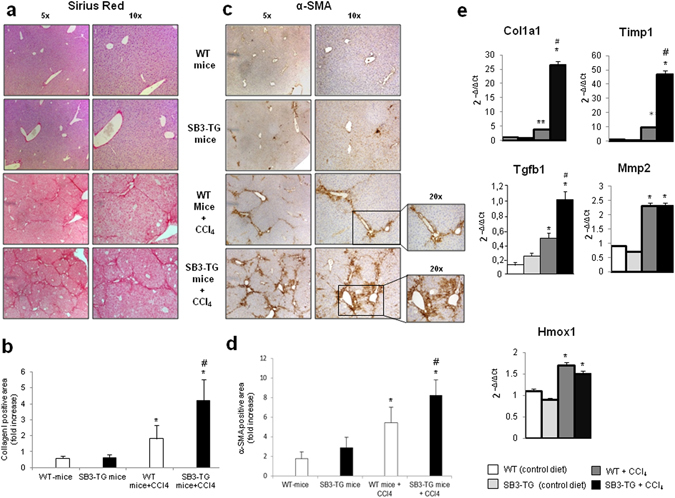
Liver fibrosis “in vivo” in SerpinB3 transgenic (TG) mice vs wild type (WT) mice exposed to chronic CCl4 administration. (a,c) Following chronic exposure to CCl4 (10 weeks) liver fibrosis was morphologically evaluated by Sirius red staining (a) or by immunohistochemistry for α-SMA (c). Original magnification as indicated. (b,d) ImageJ software analysis was performed for both Sirius red staining (b) and α-SMA immunohistochemistry analysis (d) to evaluate the amount of fibrosis. (e) Analysis by quantitative real-time PCR (Q-PCR) of SerpinB3 transcript levels of pro-fibrogenic genes in the different experimental groups. Data in graphs are expressed as means ± SEM (n = 6 mice for any experimental group) (*p < 0.05 vs relative control mice; #p < 0.05 vs WT-mice treated with CCl4).
Figure 7.
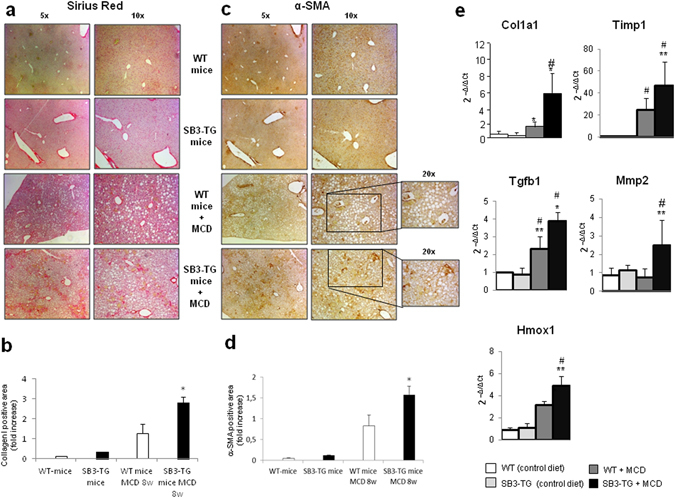
Liver fibrosis “in vivo” in SerpinB3 transgenic (SB3-TG) mice vs wild type (WT) mice fed with MCD diet. (a,c) Following MCD diet (8 weeks) liver fibrosis was morphologically evaluated by Sirius red staining (a) or by immunohistochemistry for α-SMA (c). Original magnification as indicated. (b,d) ImageJ software analysis was performed for both Sirius red staining (b) and α-SMA immunohistochemistry analysis (d) to evaluate the amount of fibrosis. (e) Analysis by quantitative real-time PCR (Q-PCR) of SerpinB3 transcript levels of pro-fibrogenic genes in the different experimental groups. Data in graphs are expressed as means ± SEM (n = 6 mice for any experimental group) (*p < 0.05 or **p < 0.01 vs relative control mice; #p < 0.05 vs WT-mice fed with MCD diet). Col1a1: collagen 1A1; Timp1: tissue inhibitor of metalloproteases type 1; Tgfb1: TGFβ1; Hmox-1: heme-oxygenase 1; Mmp2: matrix-metalloprotease type 2.
Discussion
SerpinB3 is virtually undetectable in normal human livers, but several studies have evidenced the presence of SerpinB3 in liver biopsies from patients with CLD, mostly chronic HCV infection, where it has been associated to CLD progression and liver carcinogenesis13–21. In fact, in CLD patients SerpinB3 up-regulation correlates with TGF-β1 expression and the extent of hepatic fibrosis12. Furthermore, experiments using hepatocyte-derived cell lines over-expressing SerpinB3 have evidenced its role in promoting TGF-β1 production, suggesting SerpinB3 as a putative pro-fibrogenic mediator. More recently, SerpinB3 expression has been reported to be up-regulated by hypoxia through a HIF-2α-dependent and ROS-modulated mechanism19. This is a potentially critical issue, since in the last decade liver hypoxia and angiogenesis have been suggested to parallel or even drive the fibrogenic progression of CLDs6, 25, 26, 29–31. Literature data relating SerpinB3 with advanced cirrhosis and hepatocellular carcinoma have also evidenced that, beside enhancing TGF-β1 expression, SerpinB3 can induce epithelial-mesenchymal transition (EMT) of hepatoma cancer cells, resulting in their increased invasiveness and proliferation12, 17, 19, 21. Indeed, in HCC patients high levels of SerpinB3 were significantly associated with early tumour recurrence and poor prognosis21. However, the involvement of EMT in liver fibrogenesis has been recently challenged, with most researchers suggesting a minor, if any, pro-fibrogenic role for EMT and supporting the concept that MFs originate mainly from activation/trans-differentiation of HSC4, 6, 8, 9, 32. These considerations have led us to explore the hypothesis that SerpinB3 might directly affect the behaviour of MF-like cells.
Our in vitro experiments show that hrSerpinB3 can directly act on activated HSCs by both up-regulating the transcription of critical genes involved in fibrogenesis and stimulating their oriented migration. In these settings, the capacity of the cells to readily respond to hrSerpinB suggests that SerpinB3 may exert its action on a receptor-mediated basis. Furthermore, at difference of other mediators involved in fibrogenesis, SerpinB3 action on human activated HSCs seems quite selective and does not involves cell proliferation. Unfortunately, at present a specific receptor for SerpinB3 has not yet been characterized. Whatever might be the receptor involved, in human HSC/MFs SerpinB3 promotes NADPH-oxidase-dependent generation of intracellular ROS and ROS-related activation of JNK1/2 signalling pathways. These intracellular events have been implicated in both up-regulation of ECM synthesis/remodelling by MFs as well as in the induction of their oriented migration1–6. The latter is a relevant issue for these cells in order to align with nascent and more mature fibrotic septa. On this respect, it is noteworthy that the signal pathways leading to SerpinB3-induced oriented migration are identical to those characterized in human HSC/MFs migrating in response to chemo-attractant polypeptides (PDGF-BB, CCL2 and VEGF) or hypoxic conditions27, 30.
Beside the chemotactic action, the exposure of HSC/MFs or LX2 cells to hrSerpinB3 also results in an increased transcription of genes relevant for the fibrogenic progression of CLD along with VEGF-A, Angiopoietin-1 and CCL2, suggesting that SerpinB3 may combine pro-fibrogenic and pro-angiogenic activities. Of interest, the pro-fibrogenic genes stimulated “in vitro” by hrSerpinB3 are also up-regulated in response to chronic liver injury in transgenic mice over-expressing human SerpinB3. Accordingly, by using SerpinB3 transgenic mice we observed that the induction of chronic liver injury by repeated exposure to CCl4, or the development of steatohepatitis in mice fed the MCD diet leads to a massive stimulation in SerpinB3 production by the hepatocytes and a very significant increase in ECM deposition. This later effect was associated with an increase in the number of α-SMA-positive cells (i.e., MF-like cells) as well as in an enhanced transcription of several pro-fibrogenic genes, including collagen 1A1, TGFβ1, α-SMA, PDGF-B and TIMP1 as compared to WT-mice. This scenario resembles that previously outlined for bleomycin-induced lung fibrosis, in which a direct correlation between SerpinB3 expression, TGFβ1 levels and lung fibrosis was reported24. The enhanced deposition of extracellular matrix observed in SerpinB3 transgenic mice exposed to chronic liver injury is independent from the extent of parenchymal injury supporting in vitro data concerning direct stimulation of MF-like cells by SerpinB3. Nonetheless, we have observed that hepatic damage in SerpinB3 transgenic mice associates with an enhanced expression of inflammatory markers. At present, it is still unclear whether SerpinB3 can directly sustain the activation of liver inflammatory cells but since chronic inflammation is one of the driving force for the progression of hepatic fibrosis, we cannot exclude that a pro-inflammatory action of SerpinB3 might also contribute to the dramatic increase in liver collagen deposition observed in our experimental settings. By contrast, we do not have in vitro or in vivo evidence that other non-parenchymal cells, in particular macrophages, may express and release SerpinB3.
A previous study has shown that SerpinB3 expression in the liver is modulated by hypoxia through HIF2α-dependent mechanisms19. Based on the knowledge that hypoxic conditions have a pro-fibrogenic and pro-angiogenic role during the progression of CLD1–6, 25, 26, 29–34, our study points on the possible implication of SerpinB3 among the mediators responsible for the pro-fibrogenic action of hepatic hypoxia.
In conclusion, the present study provides for the first time evidence indicating that SerpinB3 produced by liver parenchymal cells can contribute to the fibrogenic progression of CLDs by stimulating the responses of HSC/MFs.
Materials and Methods
In vitro experiments with activated, MF-like, hepatic stellate cells or HepG2 cells
Human LX2 cells, a model of immortalized and activated, MF-like, human HSC, were kindly provided by Prof. Scott L. Friedman and were cultured in Dulbecco’s modified Eagle’s medium (Sigma Aldrich Spa, Milan, Italy), supplemented with 10% fetal calf serum and 1% antibiotics. In some experiments we also used human HSC that were isolated from surgical wedge sections of at least three different human livers not suitable for transplantation as previously described35. These HSCs, kindly provided by Prof. Fabio Marra, were cultured as previously described27 and used between passages 4 and 7 when showing a phenotype of fully activated, MF-like HSCs (HSC/MFs), plated to obtain the desired sub-confluence level and then left for 24 hrs in serum-free Iscove’s medium to have cells at the lowest level of spontaneous proliferation. In in vitro experiments LX2 cells or HSC/MFs were exposed to human recombinant SerpinB3 (hrSerpinB3, 100 ng/ml), obtained as previously described17.
Detection of intracellular generation of ROS
Detection of ROS generation in cultured cells was performed by either the conversion of 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA, 1 μM) into the corresponding fluorescent derivative or by combining DCFH-DA technique and flow cytometric analysis as previously described19, 27. More details are available in the Supplementary Material section.
Quantitative real-time PCR (Q-PCR)
RNA extraction, complementary DNA synthesis, quantitative real-time PCR (Q-PCR) reactions were performed as previously described19, 21. mRNA levels were measured by Q-PCR, using the SYBR® green method as described21. More details and oligonucleotide sequences of primers used for Q-PCR are available in the Supplementary Material section.
Proliferation and Chemotaxis
Proliferation of human LX2 cells or primary HSC/MFs was evaluated by crystal violet proliferation assay by seeding cells in a 96-well plate at a density of 1.5 × 103 cells per well. The cells were incubated in serum-free medium (SFM) for 24 hrs at 100 ng/ml hrSerpinB3 concentration. At the desired time, the medium was removed, and the cells were washed twice with phosphate-buffered saline, fixed with 11% glutaraldehyde; after fixation, cells were washed with H2O bd and then stained with 0.1% (w/v) crystal violet solution for 10 min. After washing with water, the crystal violet was solubilized with 50 μl of 10% acetic acid solution, and absorbance was measured at 595–650 nm using a microplate reader (SpectraMAX M3; Molecular Devices, Sunnyvale, CA, USA). Chemotaxis of human LX2 cells or HSC/MFs was evaluated by performing the modified Boyden’s chamber assay as previously described27, 30.
Experimental fibrosis in SerpinB3 transgenic mice and related wild type mice
The two protocols for experimental fibrosis employed in this study were performed by taking advantage of a model of C57Bl/6 transgenic (TG) mice, fully characterized in previous studies23, 24, that overexpress human SerpinB3 in the liver and lungs. Full details of the experimental protocols on TG-mice and age-matched C57Bl/6 wild type (WT) mice, receiving either i) chronic administration of carbon tetrachloride (CCl4, Sigma-Aldrich, Milano, Italy) for 10 weeks as described by Wang et al.36 or ii) the methionine- and choline-deficient (MCD) diet up to 8 weeks (as a model of NAFLD/NASH-related fibrosis)37 are described in the Supplementary Material section. Experiments and protocols, approved by the Animal Ethical Committee of University of Padua and by the Animal Investigation Committee of the Italian Ministry of Health, were performed in accordance with the Helsinki convention and national guidelines and regulations for animal experiments provided by Italian Ministry of Health.
Serological analysis
ALT and AST were determined in serum by laboratory routine assays. Circulating SerpinB3 was evaluated by enzyme-linked immunozymatic assay (SCCA-ELISA, Xeptagen, Marghera, VE, Italy) using biotinylated rabbit anti-human SerpinB3 antibody as previously described15.
Immunohistochemistry, Sirius Red staining and histo-morphometric analysis
Paraffin liver sections of cirrhotic specimens derived from patients with HCV-related liver cirrhosis (METAVIR F4) were employed. The use of human material conforms to the ethical guidelines of the 1975 Declaration of Helsinki and was approved for this study by the University of Torino Bioethical Committee. Immunostaining procedure19, 27 and Sirius Red staining38 were as previously described, as detailed in the Supplementary Material section. Quantification of fibrosis in the murine liver was performed by histo-morphometric analysis using a digital camera and a bright field microscope to collect images that were then analyzed by employing the ImageJ software.
Statistical analysis
The data presented are means ± SEM or SD and were obtained from at least three independent experiments. Luminograms and morphological images are representative of at least three experiments with similar results. Statistical analysis for these experiments was performed by Student’s t-test or one way ANOVA with Kruskal-Wallis correction for multiple analysis. Significance was taken at p < 0.05.
Electronic supplementary material
Acknowledgements
The authors are deeply grateful to Prof. Fabio Marra (Dept. Experimental and Clinical Medicine, University of Florence, Firenze, Italy) for providing primary human activated- and myofibroblast like, hepatic stellate cells (HSC/MFs). This work obtained financial support by: (a) the Italian Ministero dell’Università e della Ricerca (MIUR, Rome: i) PRIN Project 2006067527 - MP and ii) FIRB Project Prot. RBLA03S4SP_005 - PP); (b) the Fondazione CRT (Torino); (c) the Associazione Italiana per la Ricerca sul Cancro (AIRC); (d) the University of Padova (Project No. CPDA110795 - PP); (e) the University of Torino (Fondo di Ateneo ex 60% - EN, MP).
Author Contributions
E.N., G.V., P.P. and M.P. conceived the study; E.N., G.V., C.T., S.C., C.P., C.B., A.B., S.Q., E.M., C.B., A.M., E.D. and S.S. carried out experiments and were involved in data acquisition and analysis; E.N., G.M., M.P.L., E.A., M.P. and P.P. supervised experiments, were involved in data interpretation and manuscript editing. All Authors were involved in writing the paper and had final approval of the submitted manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Erica Novo and Gianmarco Villano contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-03744-3
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Friedman S. Mechanisms of hepatic fibrogenesis. Gastroenterology. 2008;134:1655–1669. doi: 10.1053/j.gastro.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parola M, Marra F, Pinzani M. Myofibroblast - like cells and liver fibrogenesis: emerging concepts in a rapidly moving scenario. Mol Aspects Med. 2008;29:58–66. doi: 10.1016/j.mam.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 3.Wells RG. The portal fibroblast: not just a poor man’s stellate cell. Gastroenterology. 2014;147:41–47. doi: 10.1053/j.gastro.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang DY, Friedman S. Fibrosis-dependent mechanisms of hepatocarcinogenesis. Hepatology. 2012;56:769–775. doi: 10.1002/hep.25670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosselli M, MacNaughtan J, Jalan R, Pinzani M. Beyond scoring: a modern interpretation of disease progression in chronic liver disease. Gut. 2013;62:1234–1241. doi: 10.1136/gutjnl-2012-302826. [DOI] [PubMed] [Google Scholar]
- 6.Novo E, et al. Cellular and molecular mechanisms in liver fibrogenesis. Arch Biochem Biophys. 2014;548:20–37. doi: 10.1016/j.abb.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 7.Marra F, Tacke F. Roles for chemokines in liver disease. Gastroenterology. 2014;147:577–594. doi: 10.1053/j.gastro.2014.06.043. [DOI] [PubMed] [Google Scholar]
- 8.Mederacke I, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wells RG, Schwabe R. Origin and function of myofibroblasts in the liver. Semin Liver Dis. 2015;35:97–106. doi: 10.1055/s-0035-1550061. [DOI] [PubMed] [Google Scholar]
- 10.Silverman GA, et al. The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. J Biol Chem. 2001;276:33293–33296. doi: 10.1074/jbc.R100016200. [DOI] [PubMed] [Google Scholar]
- 11.Gettins PG. Serpin structure, mechanism, and function. Chem Rev. 2002;102:4751–4804. doi: 10.1021/cr010170+. [DOI] [PubMed] [Google Scholar]
- 12.Turato C, et al. SERPINB3 modulates TGF-β expression in chronic liver disease. Lab Invest. 2010;90:1016–1023. doi: 10.1038/labinvest.2010.55. [DOI] [PubMed] [Google Scholar]
- 13.Biasiolo A, et al. Monitoring SCCA-IgM complexes in serum predicts liver disease progression in patients with chronic hepatitis. J Viral Hep. 2008;15:246–249. doi: 10.1111/j.1365-2893.2007.00935.x. [DOI] [PubMed] [Google Scholar]
- 14.Pontisso P, et al. Overexpression of squamous cell carcinoma antigen variants in hepatocellular carcinoma. Br J Cancer. 2004;90:833–837. doi: 10.1038/sj.bjc.6601543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beneduce L, et al. Squamous cell carcinoma antigen immunoglobulin M complexes as novel biomarkers for hepatocellular carcinoma. Cancer. 2005;103:2558–2565. doi: 10.1002/cncr.21106. [DOI] [PubMed] [Google Scholar]
- 16.Guido M, et al. Squamous cell carcinoma antigen in human liver carcinogenesis. J Clin Pathol. 2008;61:445–447. doi: 10.1136/jcp.2007.051383. [DOI] [PubMed] [Google Scholar]
- 17.Quarta S, et al. SERPINB3 induces epithelial-mesenchymal transition. J Pathol. 2010;221:343–356. doi: 10.1002/path.2708. [DOI] [PubMed] [Google Scholar]
- 18.Pontisso P. Role of SERPINB3 in hepatocellular carcinoma. Ann Hepatol. 2014;13:722–727. [PubMed] [Google Scholar]
- 19.Cannito S, et al. Hypoxia up-regulates SERPINB3 through HIF-2α in liver cancer cells. Oncotarget. 2015;6:2206–2221. doi: 10.18632/oncotarget.2943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turato C, et al. Over-expression of SERPINB3 in hepatoblastoma: a possible insight into the genesis of this tumour? Eur J Cancer. 2012;48:1219–1226. doi: 10.1016/j.ejca.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Turato C, et al. SERPINB3 is associated with TGF-β1 and cytoplasmic β-catenin expression in hepatocellular carcinomas with poor prognosis. Br J Cancer. 2014;110:2708–2715. doi: 10.1038/bjc.2014.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calabrese F, et al. Overexpression of squamous cell carcinoma antigen in idiopathic pulmonary fibrosis: clinicopathological correlations. Thorax. 2008;63:795–802. doi: 10.1136/thx.2007.088583. [DOI] [PubMed] [Google Scholar]
- 23.Villano G, et al. Role of squamous cell carcinoma antigen-1 on liver cells after partial hepatectomy in transgenic mice. Int J Mol Med. 2010;25:137–143. [PubMed] [Google Scholar]
- 24.Lunardi F, et al. Overexpression of SERPIN B3 promotes epithelial proliferation and lung fibrosis in mice. Lab Invest. 2011;91:945–954. doi: 10.1038/labinvest.2011.1. [DOI] [PubMed] [Google Scholar]
- 25.Fernández M, et al. Angiogenesis in liver diseases. J Hepatol. 2009;50:604–620. doi: 10.1016/j.jhep.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 26.Rosmorduc O, Housset C. Hypoxia: a link between fibrogenesis, angiogenesis, and carcinogenesis in liver disease. Semin Liver Dis. 2010;30:258–270. doi: 10.1055/s-0030-1255355. [DOI] [PubMed] [Google Scholar]
- 27.Novo E, et al. Intracellular reactive oxygen species are required for directional migration of resident and bone marrow-derived hepatic pro-fibrogenic cells. J Hepatol. 2011;54:964–974. doi: 10.1016/j.jhep.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 28.Starkel P, Leclerq IA. Animal models for the study of liver fibrosis. Best Practice & Research Clinical Gastroenterology. 2011;25:319–333. doi: 10.1016/j.bpg.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 29.Popov Y, Schuppan D. Targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology. 2009;50:1294–1306. doi: 10.1002/hep.23123. [DOI] [PubMed] [Google Scholar]
- 30.Novo E, et al. The biphasic nature of hypoxia-induced directional migration of activated human hepatic stellate cells. J Pathol. 2012;226:588–597. doi: 10.1002/path.3005. [DOI] [PubMed] [Google Scholar]
- 31.Nath B, Szabo G. Hypoxia and hypoxia inducible factors: diverse roles in liver diseases. Hepatology. 2012;55:622–633. doi: 10.1002/hep.25497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie G, Diehl AM. Evidence for and against epithelial-to-mesenchymal transition in the liver. Am J Physiol Gastrointest Liver Physiol. 2013;305:G881–890. doi: 10.1152/ajpgi.00289.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aleffi S, et al. Proangiogenic cytokines as hypoxia-dependent factors stimulating migration of human hepatic stellate cells. Hepatology. 2005;42:1339–1348. doi: 10.1002/hep.20965. [DOI] [PubMed] [Google Scholar]
- 34.Cannito S, et al. Hypoxia, hypoxia-inducible factors and fibrogenesis in chronic liver diseases. Histol Histopathol. 2014;29:33–44. doi: 10.14670/HH-29.33. [DOI] [PubMed] [Google Scholar]
- 35.Casini A, et al. Regulation of extracellular matrix synthesis by transforming growth factor beta 1 in human fat-storing cells. Gastroenterology. 1993;105:245–253. doi: 10.1016/0016-5085(93)90033-9. [DOI] [PubMed] [Google Scholar]
- 36.Wang L, et al. Effects of retinoic acid on the development of liver fibrosis produced by carbon tetrachloride in mice. Biochimica et Biophysica Acta. 2007;1772:66–71. doi: 10.1016/j.bbadis.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 37.Locatelli I, et al. Endogenous annexin A1 is a novel protective determinant in nonalcoholic steatohepatitis in mice. Hepatology. 2014;60:531–544. doi: 10.1002/hep.27141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galastri S, et al. Lack of CC chemokine ligand 2 differentially affects inflammation and fibrosis according to the genetic background in a murine model of steatohepatitis. Clin Sci. 2012;123:459–471. doi: 10.1042/CS20110515. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


