Abstract
Sound produced by fish spawning aggregations (FSAs) permits the use of passive acoustic methods to identify the timing and location of spawning. However, difficulties in relating sound levels to abundance have impeded the use of passive acoustics to conduct quantitative assessments of biomass. Here we show that models of measured fish sound production versus independently measured fish density can be generated to estimate abundance and biomass from sound levels at FSAs. We compared sound levels produced by spawning Gulf Corvina (Cynoscion othonopterus) with simultaneous measurements of density from active acoustic surveys in the Colorado River Delta, Mexico. During the formation of FSAs, we estimated peak abundance at 1.53 to 1.55 million fish, which equated to a biomass of 2,133 to 2,145 metric tons. Sound levels ranged from 0.02 to 12,738 Pa2, with larger measurements observed on outgoing tides. The relationship between sound levels and densities was variable across the duration of surveys but stabilized during the peak spawning period after high tide to produce a linear relationship. Our results support the use of active acoustic methods to estimate density, abundance, and biomass of fish at FSAs; using appropriately scaled empirical relationships, sound levels can be used to infer these estimates.
Introduction
Quantitative assessments of fish abundance form the basis of fisheries management and conservation strategies1, 2, but the complex migratory patterns, broad spatial distributions, life histories, and population dynamics of many fish species make it difficult to survey entire populations3. Some of these challenges are minimized if surveys are conducted where and when fish form large conspecific spawning aggregations4. Fish spawning aggregations (FSAs) occur at predictable times and locations, permitting surveys to estimate spawning stock abundance, biomass, and length and age distributions of regional populations5, 6.
Efforts to monitor FSAs and manage fishing intensity often rely on fisheries-dependent data (e.g., catch per unit effort) to estimate stock abundance7. However, these data can be insensitive to population declines since fish densities at FSAs may not change proportionally with abundance8, 9, making these methods inefficient for monitoring, assessments, and establishing quotas of total allowable catch. Fisheries-independent data often yield more accurate assessments of stocks10, but methods such as mark-recapture, visual census, and trawls can be invasive, laborious, expensive, and inefficient or ineffective across the diverse environments where FSAs occur11, 12. Therefore, the continued development and expansion of fisheries-independent methods to survey FSAs in challenging habitats are greatly needed to improve the quality of population assessments and manage vulnerable spawning stocks in a sustainable manner6.
The adaptability of active acoustic methods to survey fishes in both deep13 and shallow water habitats14, 15 enables assessments of populations across a spectrum of environments where constraints of depth and low visibility prevent the use of other methods. As a trusted source of fisheries-independent data for resource managers and policy makers16, 17, active acoustic surveys have been used to estimate abundance, biomass, and length and spatial distributions of fish stocks at FSAs13, 18, 19. However, the cost and complexity of collecting and processing data have impeded the widespread application and long-term use of active acoustics for assessments of most aggregate spawning fishes, which are often associated with small-scale fisheries in developing countries6.
Increased recognition of sound production in over 100 families of marine fishes20, 21 has prompted an insurgence of passive acoustic methods to non-invasively and efficiently study and monitor populations22–24. As many fishes produce species-specific calls associated with courtship and reproduction, recordings of sound production have been used to identify the timing and location of spawning in a number of exploited species, such as Atlantic Cod18 (Gadus morhua), Pollock25 (Pollachius pollachius), Haddock26 (Melanogrammus aeglefinus), Nassau Grouper27 (Epinephelus striatus), and numerous species of croakers28, 29 (family Sciaenidae). While some progress has been made to use received sound levels as indices of fish presence, behaviors, and relative abundance at FSAs30–34, difficulties in relating sound production metrics to fish densities have precluded passive acoustic estimations of abundances for use in quantitative population assessments22, 23, 35.
With an understanding of fish calling rates and sound propagation, it is possible to estimate abundance from call occurrences22, 35, 36; however, call rates at FSAs often vary considerably29, 37, 38 and are difficult to characterize in-situ for fishes. Additionally, fish choruses at some FSAs prevent the isolation of individual calls29. A more tractable approach may be to utilize relationships between sound production levels, measured using passive acoustics, with independent measures of fish density estimated with active acoustics22, 35. Ultimately, species-specific models could be used to assess and monitor spawning stock densities, abundances, and biomasses using passive acoustic methods during periods with stable rates of sound production33, 39, 40.
Croakers are an ideal assemblage of fish species to develop and test relationships between sound production and independent measurements of fish density at FSAs, because they produce sounds during spawning activities41, 42 within FSAs that form in estuaries and coastal habitats43, 44 (Table 1). Also, croaker fisheries generate tens of billions of US dollars globally each year45, 46, and among the 166 species of croakers for which information is available on the IUCN Red List, those that are threatened or endangered are aggregate spawners that are also overfished47, 48. Therefore, new methods that demonstrate the use of passive acoustic technologies to estimate density and assess spawning populations of croakers will support the development of monitoring programs that aid adaptive management strategies to prevent stock declines, hasten recovery, and support sustainable harvest of other vulnerable, aggregating species that produce sound during spawning (Table 1).
Table 1.
Families of commercially important fishes that have been documented to produce sounds and form spawning aggregations.
| Family | Common Names | No. Species | Total Landings (tons) |
|---|---|---|---|
| Clupeidae | Herrings, Shads, Sardines, Menhadens | 198 | 9,087,812 |
| Gadidae | Cods and Haddocks | 24 | 6,637,665 |
| Carangidae | Jacks and Pompanos | 146 | 4,315,926 |
| Sciaenidae | Croakers, Drums, and Weakfishes | 283 | 1,908,360 |
| Sparidae | Porgies | 150 | 439,887 |
| Epinephelidae, Serranidae | Groupers and Sea Basses | 537 | 343,236 |
| Lutjanidae | Snappers | 110 | 268,976 |
| Sebastidae, Scorpaenidae | Rockfishes, Rockcods, and Thornyheads | 132, 216 | 265,410 |
| Mullidae | Goatfishes | 87 | 220,604 |
| Haemulidae | Grunts | 133 | 121,682 |
| Ophidiidae | Cusk-eels | 258 | 41,353 |
| Labridae | Wrasses | 520 | 23,464 |
| Ictaluridae | Catfishes | 51 | 12,733 |
| Acanthuridae | Surgeonfishes, tangs, unicornfishes | 82 | 9,785 |
| Scaridae | Parrotfishes | 100 | 5,266 |
| Acipenseridae | Sturgeons | 25 | 422 |
| Batrachoididae | Toadfishes | 83 | 339 |
| Total | 3,135 | 23,702,920 |
The table presents total global landings (tons) reported in 2013 (FAO) for aggregating, sound producing families of fishes. Families are organized by total landings. Sciaenidae, ranking fourth in contributions to landings, supports global economies and is need of new assessment methods to ensure sustained productivity.
In this paper, we developed active and passive acoustic methods to assess the abundance of Gulf Corvina (Cynoscion othonopterus; family Sciaenidae), hereafter Corvina, at its FSA and constructed a model to quantify relationships between sound levels and fish density during spawning. From late February to early June, during the outgoing tide over 2–3 day periods prior to new and full moons43, 49, Corvina migrate from as far south as the Midriff Islands (Fig. 1) and form a FSA at one location in the estuaries of the Colorado River Delta (1–20 m depth) in the Northern Gulf of California, Mexico43, 46. During these brief yet predictable events, the FSA is targeted by a commercial gill net fishery of more than 500 small boats, which harvests several thousand tons of Corvina over the course of a few weeks46. Fishers locate the FSA through knowledge of their predictable migrations and by cueing on the courtship sounds produced by male Corvina during spawning (see Supplementary Fig. S1). The fishery is managed by a quota system in which estimates of catch per unit effort (CPUE) are used to estimate stock abundance and set catch levels for the spawning season. Despite high fishing pressure and uncertain sustainability7, 43 the stock size and status are largely unknown due to the lack of fisheries-independent data50. Therefore, the development of methods to estimate fish density from active acoustic measurements and sound levels would provide accurate estimates of fish abundance and biomass that could inform the quota system by reducing the probability of setting unsustainable harvest limits.
Figure 1.
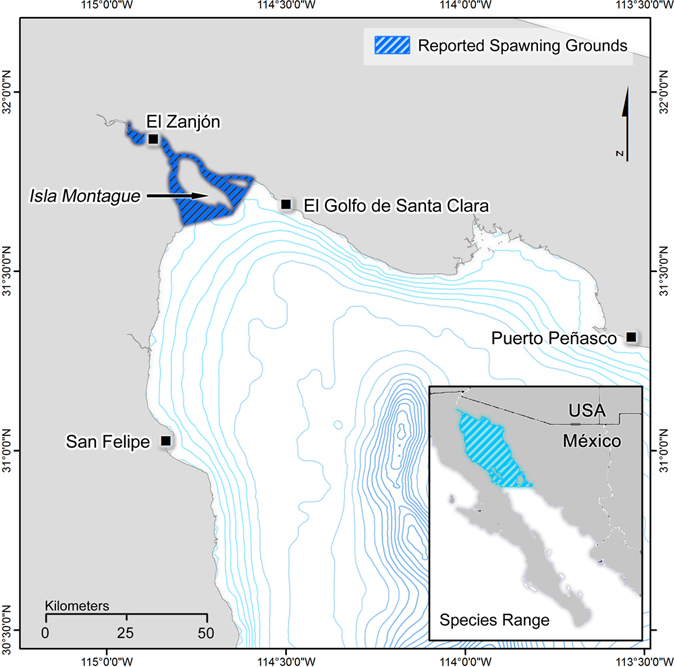
Species range and spawning grounds of Gulf Corvina (Cynoscion othonopterus). The species range extends from the Midriff Islands to the northern limit of the Gulf of California (inset map). During the months of February to early June, the reproductive population migrates northward to the only reported spawning grounds in the Colorado River Delta. The map is adapted by permission from Macmillan Publishers Ltd: [SCIENTIFIC REPORTS] (Erisman et al.43), copyright 2012.
We used active acoustic methods to measure density, lengths, abundance, biomass, and spatial extent of spawning fish and passive acoustic methods to measure the spatial distribution of sound levels attributable to fish sound production across eight surveys in March and April 2014. We then compared independent measures of fish density and sound levels at the FSA site to test the hypothesis that sound levels would increase linearly with density (H1, Fig. 2), resulting in a relationship with strong predictive power. While the potential for a weaker relationship that becomes asymptotic and insensitive at high densities exists (H2, Fig. 2), we expected that the careful selection of a sound level metric that is proportional to acoustic power would negate this possibility. We also acknowledged that a predictive relationship may not exist (H0, Fig. 2) due to the inherent variability in fish sound production rates at FSAs. However, we anticipated that a significant relationship would be observed if a period of stable sound production rate could be identified, resulting in a model to estimate fish density from sound production levels.
Figure 2.
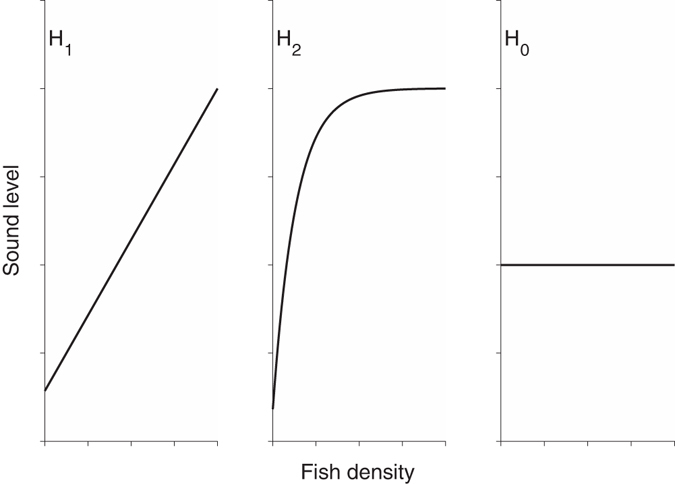
Hypotheses of the relationship between fish sound production levels and density. (H1) A strong, predictive linear relationship with increases in sound levels as a function of density, (H2) a weaker non-linear relationship where sound levels fail to respond to high densities, and (H0) a non-predictive relationship with changes in density not affecting sound levels.
Results
Fish distribution, abundance, and biomass
Over the four days of active acoustic surveys, fish distributions varied between incoming and outgoing tides with higher densities extending progressively farther into the delta on the outgoing tide during spawning (Fig. 3). Differences in the density distributions mapped during the incoming and outgoing tides revealed that the aggregation moved slower than the survey progressed, mitigating the potential to survey individuals more than once. On the incoming tides of 27 March and 27 April 2014 and the outgoing tides of 28 March, 27 April, and 28 April 2014, we surveyed the entire aggregation present in the northeastern channel of the delta as evident from lower densities at the start and endpoints of surveys. Among these days, the aggregation was distributed over a linear distance of 8 to 25 km with densities greater than 5 fish/1000 m3. On the incoming tides on 28 March and 28 April and outgoing tide on 27 March 2014, the surveys did not sample the entire aggregation due to a miscalculation of the southern extent of the aggregation, absence of large Corvina in the channel, and degraded survey conditions, respectively. We observed large spawning events on all days except for 28 April 2014 when an imminent new moon prompted the aggregation to disperse from the reproductive grounds.
Figure 3.
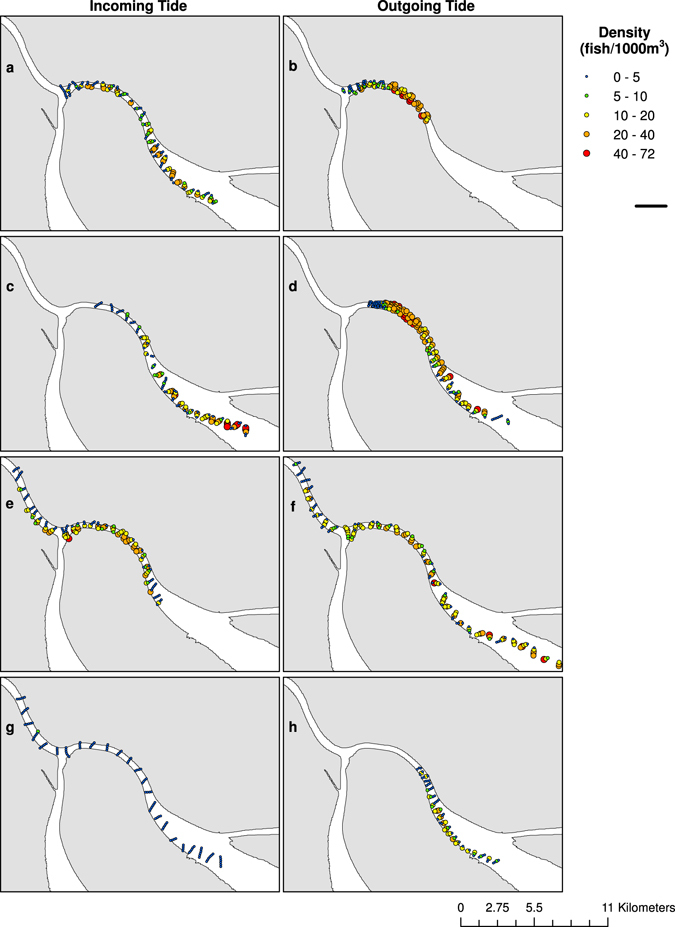
Spatial distribution of Gulf Corvina (Cynoscion othonopterus) densities (fish/1000 m3). Mean fish densities per every 150-m survey length in the spawning grounds of the northeastern channel of the Colorado River Delta on the incoming and outgoing tides on (a,b) 27 March 2014, (c,d) 28 March 2014, (e,f) 27 April 2014, and (g,h) 28 April 2014. All survey data are depicted, including 150-m survey lengths with densities of 0 fish/1000 m3. Collection of active acoustic data on the outgoing tide of 27 March 2014 (b) ended prior to the termination of passive acoustic data collection due to deteriorated survey conditions. Maps were generated using the ArcMap extension of ArcGIS version 10.2.2 (http://www.esri.com/, ESRI, USA).
Mean densities (±S.E.) of Corvina ranged from 0.43 ± 0.07 to 15.97 ± 2.04 fish/1000 m3, which corresponded to an estimated 76,052 ± 12,380 to 1,551,729 ± 177,691 fish (±S.E.) across surveys (Table 2). Mean survey densities were higher on the outgoing tides in comparison to incoming tides, corresponding to the known timing of peak spawning activity, but were not significantly different (one-tailed t-test, p = 0.073). We estimated the abundance of Corvina in the northeastern spawning grounds at 1.53 million ± 196,443 and 1.55 million ± 177,691 fish (±S.E.) on the outgoing tides of 28 March and 27 April 2014 when we surveyed the entire aggregation in the northeastern channel. During the presence of FSAs, we observed fish of total lengths (L) 21–100 cm, as estimated from the following Kirchoff-ray Mode (KRM) model-derived relationship between mean lateral-aspect 120-kHz target strength (TS) and Corvina L (cm):
| 1 |
Table 2.
Estimates of density and abundance of mature Gulf Corvina (Cyonscion othonopterus) per survey.
| Tide | Volume (m3) | Density (fish/1000 m3) | S.E. (fish/1000 m3) | C.V. (%) | 95% C.I. (fish/1000 m3) | Abundance (fish) | S.E. (fish) | 95% C.I. (fish) |
|---|---|---|---|---|---|---|---|---|
| 27 March 2014 | ||||||||
| Incoming | 91,966,129 | 7.47 | 0.99 | 13.3 | 5.86–9.95 | 686,987 | 91,046 | 538,922–915,063 |
| Outgoing | 49,576,934 | 13.04 | 1.87 | 14.3 | 9.32–16.66 | 646,483 | 97,709 | 462,057–825,952 |
| 28 March 2014 | ||||||||
| Incoming | 111,125,728 | 11.73 | 1.86 | 15.9 | 8.29–15.62 | 1,303,505 | 206,694 | 921,232–1,735,784 |
| Outgoing | 96,295,547 | 15.97 | 2.04 | 12.8 | 12.13–20.16 | 1,537,840 | 196,443 | 1,168,065–1,941,318 |
| 27 April 2014 | ||||||||
| Incoming | 132,894,113 | 11.00 | 1.21 | 11.1 | 8.60–13.37 | 1,461,835 | 160,802 | 1,142,889–1,776,794 |
| Outgoing | 153,181,587 | 10.13 | 1.16 | 11.5 | 8.18–12.98 | 1,551,729 | 177,691 | 1,253,025–1,988,297 |
| 28 April 2014 | ||||||||
| Incoming | 176,863,975 | 0.43 | 0.07 | 15.3 | 0.32–0.59 | 76,052 | 12,380 | 56,596–104,350 |
| Outgoing | 64,708,929 | 8.07 | 1.11 | 13.8 | 5.99–10.41 | 522,201 | 71,827 | 387,606–673,620 |
S.E. = standard error; C.V. = coefficient of variation (%); 95% C.I. = 95% confidence interval.
The results of the KRM model are detailed in Supplementary Fig. S2. The mean L (±95% C.I.) of the aggregated fish ranged from 38.677 ± 2.761 cm to 53.402 ± 1.407 cm among surveys, corresponding to mean weights (W) of 0.578–1.464 kg (Table 3). Biomass varied across days and tides depending on extent of area surveyed; however, on the outgoing tides of 28 March and 27 April 2014, estimated biomass was 2,145 ± 537 and 2,133 ± 480 metric tons (±95% C.I.), respectively (Table 3).
Table 3.
Mean total length, weight, and estimated biomass of mature Gulf Corvina (Cynoscion othonopterus) per survey.
| Tide | Length (cm) | S.E. (cm) | Weight (kg) | Biomass (kg) | S.E. (kg) | 95% C.I. (kg) |
|---|---|---|---|---|---|---|
| 27 March 2014 | ||||||
| Incoming | 53.112 | 0.520 | 1.441 | 989,948 | 131,555 | 732,100–1,247,797 |
| Outgoing | 52.846 | 0.525 | 1.421 | 918,652 | 132,055 | 659,824–1,177,480 |
| 28 March 2014 | ||||||
| Incoming | 51.442 | 0.568 | 1.315 | 1,714,109 | 272,461 | 1,180,086–2,248,132 |
| Outgoing | 52.512 | 0.338 | 1.395 | 2,145,287 | 274,386 | 1,607,491–2,683,082 |
| 27 April 2014 | ||||||
| Incoming | 52.798 | 0.407 | 1.417 | 2,071,420 | 228,415 | 1,623,727–2,519,114 |
| Outgoing | 52.249 | 0.447 | 1.375 | 2,133,627 | 245,006 | 1,653,417–2,613,838 |
| 28 April 2014 | ||||||
| Incoming | 38.677 | 1.409 | 0.578 | 43,958 | 7,333 | 29,586–58,331 |
| Outgoing | 53.402 | 0.718 | 1.464 | 764,502 | 105,656 | 557,417–971,588 |
S.E. = standard error; 95% C.I. = 95% confidence interval. Mean total length on the incoming tide of 28 April 2014 reflects the absence of high abundances of large Gulf Corvina within the survey area.
Fish sound production and relationship to density
We recorded Corvina sound production associated with spawning during all survey days. We did not observe sound production by any other fish species in our recordings. Measurements of mean square pressure amplitude (p 2) over the frequency band of Corvina chorusing (251–498 Hz, see Supplementary Fig. S1) ranged from 0.02 to 12,738 Pa2 among surveys but varied across the spawning grounds and tidal periods (Fig. 4). Values of mean p 2 per survey were significantly higher on the outgoing tides (one-tailed t-test, p < 0.001), coinciding with spawning; mean p 2 (±95% C.I.) ranged from 1.56 ± 1.9 Pa2 on the incoming tide of 28 April to 4430 ± 1004.0 Pa2 on the outgoing tide of 27 April 2014. Spatial differences in p 2 showed similarities to measured differences in fish density across the spawning grounds during the outgoing tides but not during the incoming tides (Figs 3 and 4). We observed elevated sound levels (p 2 > 800 pa2) attributable to dense aggregations and spawning activity over distances of 6.5 to 25 km on the outgoing tides of 27 and 28 March, and 27 and 28 April 2014.
Figure 4.
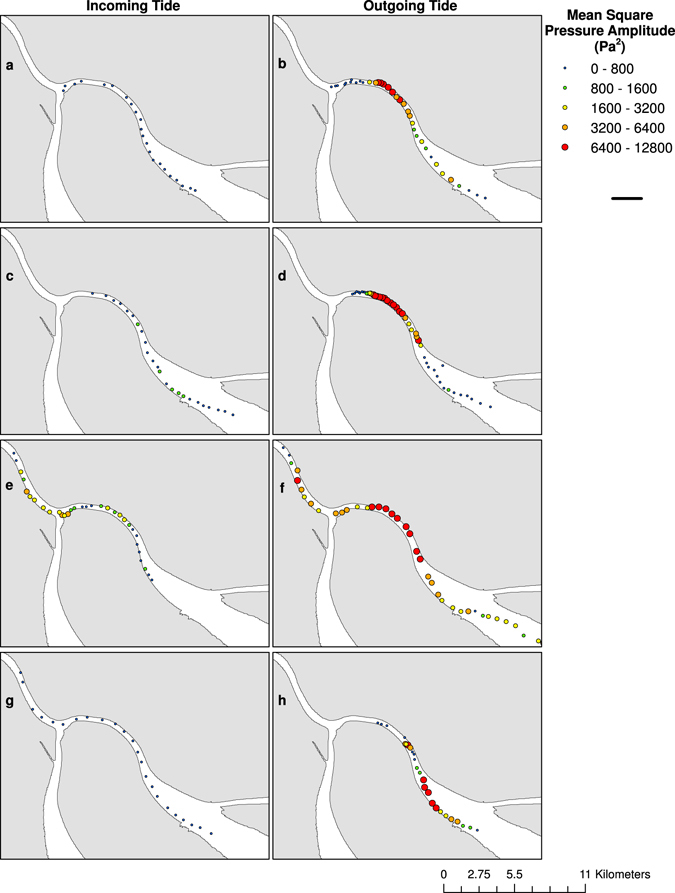
Spatial distribution of mean square pressure amplitude (p 2; Pa2; 251 Hz–498 Hz). Sound levels over the frequency band of Gulf Corvina (Cynoscion othonopterus) chorusing in the spawning grounds of the northeastern channel of the Colorado River Delta on the incoming and outgoing tides on (a,b) 27 March 2014, (c,d) 28 March 2014, (e,f) 27 April 2014, and (g,h) 28 April 2014. Collection of passive acoustic data on the outgoing tide of 27 March 2014 (b) continued after the conclusion of the active acoustic survey. Maps were generated using the ArcMap extension of ArcGIS version 10.2.2 (http://www.esri.com/, ESRI, USA).
Comparisons between p 2 and densities (ρ, fish/1000 m−3) resulted in different relationships dependent on the timing of measurements in relation to high tide (Fig. 5, ANCOVA, p < 0.001). The slopes of hourly regressions from 3 hours before high tide to high tide and from 2 to 5 hours after high tide were not significantly different (multiple comparisons, Tukey-Kramer, p > 0.52); however, these time periods were unsuitable to construct a model between p 2 and ρ due to the lack of good line fits and decoupling of changes in sound levels with density. From high tide until two hours after high tide, the slopes of regressions were homogeneous (multiple comparisons, Tukey-Kramer, p > 0.99) and significantly different from all other hours (multiple comparisons, Tukey-Kramer, p < 0.001), indicative of stationary call rates of Corvina that are additive in p 2 as a function of density during the two-hour window (Fig. 6). The modeled relationship between ρ and p 2 from high tide to two hours after high tide (Fig. 6) resulted in a method to estimate fish density from measured sound production (F1,68 = 216, p < 0.001) with the following equation:
| 2 |
Figure 5.
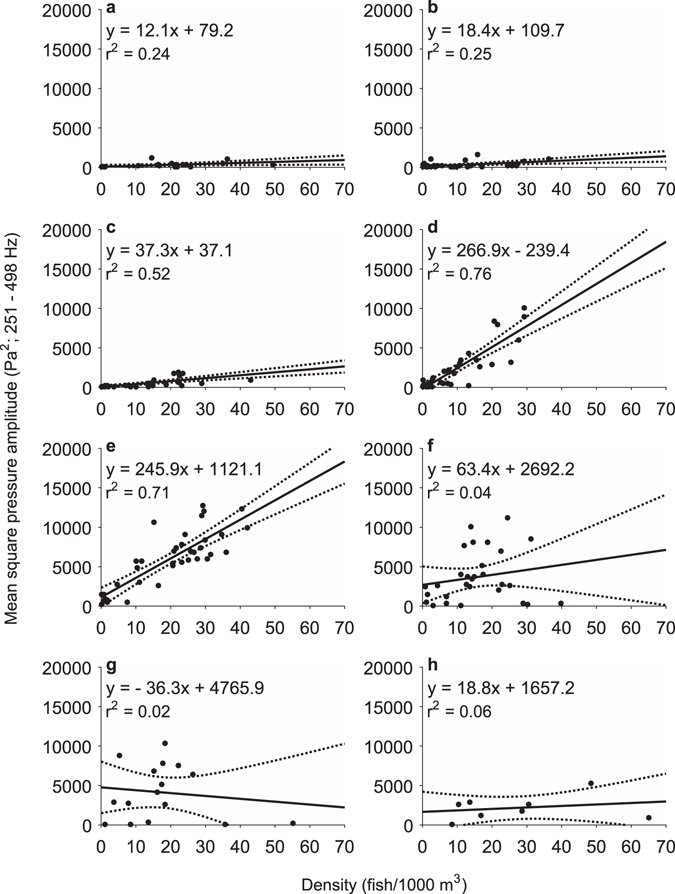
Relationship between sound levels and fish density over one-hour periods. Regressions of mean square pressure amplitude (p 2; Pa2; 251–498 Hz) versus fish density (ρ; fish/1000 m3) from (a) 3–2 hours before high tide, (b) 2–1 hours before high tide, (c) 1–0 hours before high tide, (d) 0–1 hours after high tide, (e) 1–2 hours after high tide, (f) 2–3 hours after high tide, (g) 3–4 hours after high tide, and (h) 4–5 hours after high tide. Dotted lines indicate 95% confidence intervals. Densities are mean densities per 150-m survey length that were nearest in space and time to sound measurements. See Supplementary Fig. S3 for hourly relationships using mean density per 300-m survey length.
Figure 6.
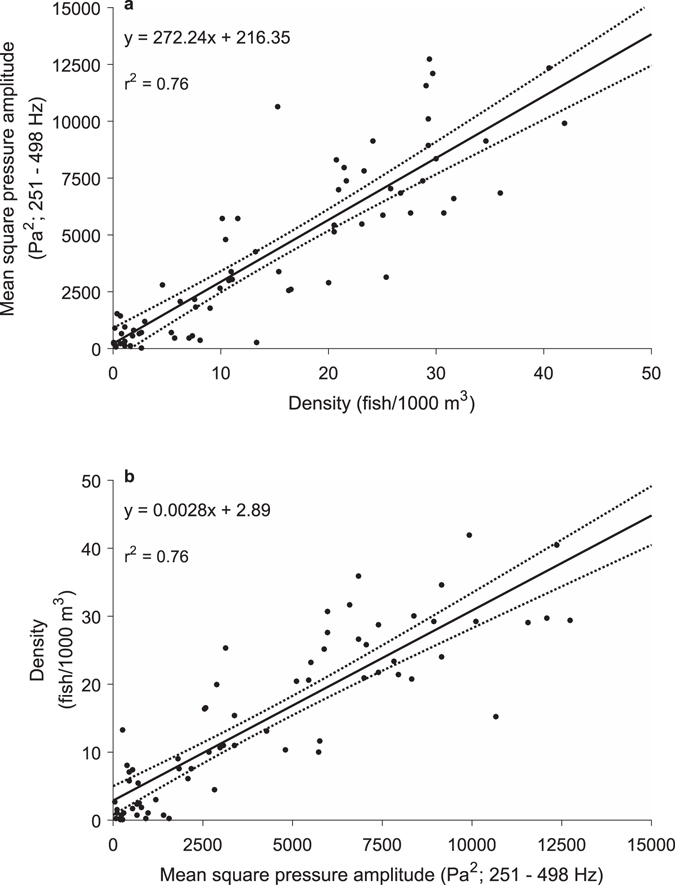
Relationship between sound levels and fish density during the peak spawning period. (a) Mean square pressure amplitude (p 2; Pa2; 251–498 Hz) as a function of density (ρ; fish/1000 m3) for measurements during the first two hours after high tide and (b) the modeled relationship generated for estimating ρ from future measurements of p 2, (F1,68 = 216, p < 0.001). Dotted lines indicate 95% confidence intervals. Densities are mean densities per 150-m survey length that were nearest in space and time to sound measurements. See Supplementary Fig. S4 for the modeled relationships using mean density per 300-m survey length.
This result did not refute our hypothesis that a relationship exists for a period of stable sound production (H1, Fig. 2). Equation (2) opens the possibility to estimate ρ, a fundamental measurement for population assessments, across different spatial scales (see Supplementary Figs S3 and S4) and thus abundance and biomass from future measurements of p 2 during the two hours after high tide.
Discussion
The results of this study demonstrate that sound levels may be used to estimate the densities of soniferous fishes at FSAs, which provides an alternative means to assess their abundance, biomass, and spatial distribution. The selection of p 2 as an additive metric of Corvina sound production coupled with the identification of a two-hour period of stable sound production as a function of density resulted in the generation of a predictive relationship. While several methods are currently available to provide independent measurements fish density12 for comparison to sound levels, here we corroborate and further develop the use of active acoustic data to not only provide measures of density in a challenging shallow water environment but also to estimate abundance, mean length, biomass, and spatial distribution of fish. Our results support the continued use of passive acoustic methods to determine the spatio-temporal distributions of spawning activity and relative abundance27, 29, 31, 34, 37. More importantly, they show that when these methods are calibrated to the spatial and temporal dynamics of spawning activity of the study system, the difficulties of relating sound levels to actual fish densities can be overcome to provide a new fisheries-independent method to assess FSAs.
The selection and validity of different methods to estimate density for comparison to passive acoustic measurements are often challenged and limited by resource and equipment availability, environmental conditions, and spatial and reproductive dynamics of target species12; thus, different FSAs pose unique challenges that need to be considered. For example, aspects of the Corvina FSA present a series of obstacles that have thus far impeded fisheries-independent assessments: the aggregation forms in a remote shallow water environment (1–20 m) with near-zero visibility and large tidal ranges (7 m over a 6 hour period), which inhibits visual surveys; spawning takes place within a no-take biosphere reserve thereby discouraging invasive trawl sampling; and heavy fishing mortality during stock migrations and some spawning events within the protected reserve conflicts with mark-recapture, telemetry, and extractive methodologies. In this study, the versatility of active acoustic methods overcame these challenges to elucidate spawning and stock dynamics of Corvina and produce measurements of fish density, abundance, and biomass in a non-invasive manner.
Our active acoustic surveys generated the first fisheries-independent estimate of Corvina density, abundance, mean length, biomass, and spatial distribution of mature fish across four days of spawning in 2014 that may supplement existing catch per unit effort data to develop more robust and comprehensive stock assessments for the fishery. Although the active acoustic survey results elucidated the dynamics of the aggregation and the fishery, they were difficult to conduct due to sound propagation in shallow water environments, low insonified volumes, and potential vessel avoidance by fish51, 52. These challenges were mitigated by orienting the acoustic beam axis nominally 10° below horizontal to detect Corvina up to 50 m away from the vessel and removing the first 10 m range of acoustic data that exhibited low detection probability, respectively. As opposed to echo-integration16, fish were detected as tracks with multiple, spatially coherent TS measurements, permitting the calculation of density, abundance, and TS distributions of aggregated Corvina. The selection of Corvina-specific detection parameters and a lack of bycatch in the fishery50 provide credence that our estimates were not influenced by the presence of additional species. KRM modeling provided an equation to relate mean lateral-aspect TS to fish length for Corvina, thus enabling the estimation of mean fish lengths, weights, and total biomass.
While this study observed the formation of multiple FSAs comprised of over 1 million fish, these data do not implicitly provide an estimate of the total spawning population. To assess the total abundance and biomass of spawning populations using active or passive acoustics, the spatio-temporal dimensions and reproductive dynamics of FSAs need to be known. For example, previous efforts that have tracked fishing intensity at the FSA have shown that the Corvina likely aggregate in both channels of the Colorado River Delta43. As we only focused our efforts in the northeastern channel due to logistical limitations, only a portion of the spawning grounds were sampled. Additionally, FSAs of Corvina form prior to the new and full moons in the months of February to June, but it remains unknown whether the entire reproductive population participates in all spawning events with fixed residency times, if individuals spawn only once or multiple times, if mean length and weight across all spawning events are constant, and what are the effects of fishing mortality during migrations to the delta. However, with the future documentation of these dynamics, active and passive acoustic estimates can be calibrated using a hierarchical model to determine the total abundance and biomass of the spawning population53.
Fish sound production rates at FSAs are difficult to measure and highly variable, often influenced by the timing of spawning activity29, 37, 38, resulting in temporally inconsistent relationships between sound levels and abundance, as observed in this study. Consequently, the use of call numbers as predictors of fish abundance35, 36 has proven challenging for researchers22, 23. For many species, sound production associated with courtship and spawning is only present in males26, 41, thus without a knowledge of sex ratios, the use of call rates and occurrences to estimate abundance and density may only be applicable to males, assuming factors affecting sound production rates are understood. However, it has been argued that the call rates of courting males may be influenced by the presence of females, where call levels may be indicative of both male and female density33 but these relationships have not been fully investigated. At some FSAs high abundances of fish and sound production prevent the detection of individual calls altogether, as in this study, requiring measurements of ambient sound pressure levels31, 54 and the designation of indices to infer aggregation sizes30, 32.
Given these challenges and uncertainties, we compared fish sound production to independent measurements of density, which may be a more feasible manner to model their relationship at FSAs22, 35. Previous works have exemplified the potential of this method through comparisons of sound production indices with CPUE of simultaneous trawls39, densities of early stage eggs31, and asynchronous fish densities estimated with active acoustics40 and visual census33. In this study, we compared p 2 over the bandwidth of Corvina chorusing to fish density estimated simultaneously with active acoustics. We selected p 2 over other measurements, such as root-mean-square sound pressure level (dB), because the summed power from incoherent sounds from multiple calling fish is proportional to p 2. By comparing the relationship of p 2 with density over several one-hour periods, we were able to model the time-evolving relationship and identify patterns of similarity. The insensitivity of sound levels as a function of density prior to high tide and beyond two hours after high tide suggested inconsistencies in fish chorusing rates and a lack of coordinated courtship behaviors outside the peak spawning period. From high tide to two hours after high tide we observed a stable positive relationship, where increases in density corresponded linearly to increases in p 2, indicating a two-hour period of homogeneous sound production rates during which density can be estimated from passive acoustic measurements. Importantly, comparisons of regression slopes provided an approach to identify periods of stationary sound production, thereby negating the need to estimate calling and chorusing rates of males and sex ratios in a challenging environment. However, this strategy assumed that sex ratios remained stable across measurements, which has been estimated at 1:1 from fisheries landings49.
As with most assessment methods, the application of the model developed in this study for population monitoring and fishery management is limited by the inherent uncertainty of density estimates from sound levels, warranting confidence intervals and the potential for future improvement with additional data and possible sources of variance, such as tidal current strength. However, this model presents an opportunity to assess Corvina density and calculate abundance with confidence intervals, during the two hours after high tide, using passive acoustic survey techniques. With this relationship, sound production measurements can be used to monitor the spatio-temporal distribution of density and abundance and quickly detect potential impacts of fishing activities on stock size and spawning activity. Estimates of abundance can also be used to calculate biomass if fish lengths at the FSA site are known through independent sampling. Lengths may also be estimated using passive acoustics through analyses of the fundamental frequencies of sounds55.
Our results support the application of both active and passive acoustics to conduct surveys of FSAs that could provide needed information to resource managers, policymakers, conservationists, and fishing communities. We highlight the growing potential to develop comparative models between sound production and density of fishes using accurate independent measurements of fish density, but we acknowledge the future importance of testing their robustness against more complex acoustic modeling approaches with additional covariates that affect sound propagation, such as water depth, bathymetry, and boundary conditions35. Although our methods were applied to Corvina, they may be further developed and adapted to other commercially important species of soniferous fishes that form FSAs (Table 1), such as members of the cod, grouper, and croaker families25, 29, 34, based on knowledge of study species and systems and the appropriate spatial and temporal scales for predicting abundance from sound levels35. Active acoustic surveys can be designed to provide estimates of density, abundance, and biomass at FSAs; however, other independent sources of density may be viable, such as visual census, if conditions permit. The inclusion of fixed, long-term acoustic recorders in future efforts at FSAs will enable entire spawning grounds to be assessed simultaneously over multiple spawning events, providing a cost effective means to capture ambient sound and estimate abundance across a suite of habitats and economic environments. The continuation, improvement, and expansion of these methods across multiple species will validate passive acoustics as a frontier tool for the management and conservation of fish populations.
Methods
All methods were conducted in accordance with approved guidelines and regulations. The use of deceased fish from the fishery for TS modeling and collection of acoustic data were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of California San Diego under IACUC protocol S13240 and at the University of Texas at Austin under IACUC protocol AUP-2015-00162.
Survey design
Prior to the new moons in March and April 2014, we simultaneously conducted eight active and passive acoustic surveys in the northeastern channel of the Colorado River Delta from two small (8 m long) fishing vessels. As there are only two days of peak spawning per FSA event (2–3 days before new and full moons)43, we focused our efforts on these days to assess the aggregation and minimize daily variation. We surveyed the spawning grounds of Corvina twice daily, on the incoming and outgoing tide, progressing in the direction of tidal current (see Supplementary Fig. S5 and Table S1). Active acoustic surveys of Corvina were comprised of semi-randomized parallel transects across the delta channel spaced, on average, 0.5 km apart with deviations attributable to tidal flow and avoidance of surface gill nets. From a second vessel, we conducted passive acoustic surveys of Corvina sound production in synchrony with the timing and location of each active acoustic transect (see Supplementary Fig. S5), and the independent measurements were compared to estimate the spatial distribution of sound levels attributable to spawning Corvina and to model the relationship between acoustic measurements of density and sound production.
Active acoustic sampling
We conducted active acoustic sampling with a 120-kHz echosounder (ES60, Simrad-Kongsberg, Norway) configured with a split-beam transducer (ES120-F; Simrad-Kongsberg, Norway) having a nominal 9° beam width. Prior to sampling, we calibrated the complete system using the standard sphere method56, 57 and a 38.1 mm diameter sphere constructed of tungsten carbide with 6% cobalt binder material (Bal-tecTM, Micro Surface Engineering, Inc., USA). For each survey, we collected water temperature and salinity profiles using a handheld CTD (Castaway®-CTD, SonTek/Xylem, Inc., USA) to measure sound speeds, calculate absorption coefficients, and calibrate data during processing. Throughout the surveys, the echosounder operated with a pulse duration of 256 µs, a ping rate of 0.25 s per transmission, and a transmit power of 200 W. The transducer was positioned off the port side of the vessel with the face nominally 0.53 m below the sea surface. As the survey area ranged in depth from 1 to 20 m, the beam axis was oriented nominally 10° below horizontal to increase the insonified volume, limit reverberation from the water surface, and increase the observational range. Surveys were performed at an average speed of 6 knots, and received power and split-beam phase data, indexed by time and geographic location, were sampled and stored every 64 µs.
Passive acoustic sampling
We recorded ambient sound at a single random position along each active acoustic transect (see Supplementary Fig. S5), using a calibrated Tascam DR-680 Portable Multitrack Recorder (TEAC Corporation, Japan) and a single HTI-96-MIN hydrophone (High Tech, Inc., USA; sensitivity = −192.0 dBV/µPa). The location of a recording along each transect was dictated by ability of the vessel to position itself along the next transect at the same time as the active acoustic vessel passed by. Files were digitized with 24-bit resolution at a sampling rate of 192 kHz and stored (.wav format) on a secure digital high capacity (SDHC) memory card. At each sampling location, the vessel engine was turned off, and 60 s of ambient sound was recorded with the hydrophone lowered 2 m below the hull of the vessel as the vessel drifted across the path of the portion of the active acoustic transect recently completed.
Specimen collection and target strength modeling
To identify Corvina in the active acoustic dataset and estimate mean L and W, we collected five individuals from the local fishery and used measurements of their shapes and L values (27.4–75.3 cm) to parameterize the Kirchoff-ray Mode model58 (KRM) and estimate their target strength (TS) values at 120 kHz. The range of L approximated the size range (L = [21, 100 cm]) of mature Corvina at the FSA46, 49, 50. Each specimen was photographed, weighed, measured, and stored on ice before radiography. We radiographed fish ventrally and laterally at a distance of 116 mm with 48 kV at 15 mAs using a MinXray HF100+ mobile X-ray unit (MinXray, Inc., USA). Fish bodies and swim bladders were traced from X-ray images and used to parameterize the KRM. We estimated the average lateral-aspect TS for each length by summing the backscattering cross sections across incident angles of 65° to 115° (90° equals broadside), dividing by 180°, then converting to decibels58. The relationship between TS at 120 kHz and Corvina L (cm) was determined by a nonlinear least squares fit of the following equation:
Active acoustic data analysis
Active acoustic data were calibrated, visualized, and processed using commercial software (Echoview V5.4, Echoview Software Pty Ltd, Australia). We identified seabed echoes using an automatic detection algorithm and manual editing, and excluded them from further analyses. Echoes within the near-field range (0.51 m), corresponding to a beam-axis depth of 0.62 m, were also removed before further processing. We visually examined echograms to identify and remove regions of noise, propeller wash, bubbles, and along-shore segments of the surveys, resulting in a series of noise reduced across-channel transects for each survey. We identified individual targets attributable to Corvina using a single target detection operator (Split Beam Method 2, Echoview Software Pty Ltd, Australia) and TS and angular-position operands. A conservative, minimum-TS threshold of −46.5 dB was chosen, based on the mean KRM-TS versus L (see Supplementary Fig. S2), to include measures of all sizes of mature individuals potentially within the spawning aggregation49, 50. We refined the parameters of single target detection after the completion of sensitivity analyses (see Supplementary Table S2). To identify individual fish from multiple single targets, fish tracks were detected using a tracking algorithm (Alpha-Beta, Echoview Software Pty Ltd, Australia) parameterized with limits on range, alongships- and athwartships-angles, and time. We required a minimum of two single targets to generate a track and adjusted additional parameters for proficiency based on vessel speed and transducer orientation (see Supplementary Table S2).
We gridded fish track detections into 1-m range bins and exported with summed wedge volume59 (m3) per bin to estimate the probability density function (PDF) of fish density versus distance from the transducer face and depth. Regions of data corresponding to ranges less than 10 m (2.3 m depth) from the transducer were excluded from further analyses, as a result of exhibiting a non-stationary density PDF attributed to low beam volume, fish avoidance of the vessel, or both16, 52; beyond 10 m range (2.3 m depth) fish densities were stationary and homogenous. We separately partitioned the remaining data (range 10 m to the seabed) into 150-m and 300-m survey lengths and complete across-channel transects and exported them with summed wedge volume, total number of fish tracks, and mean geographic position and time per partition. We estimated fish density (fish/1000 m3) for each 150-m and 300-m survey length and complete across-channel transect by dividing the total number of fish tracks by summed wedge volume. The spatial distributions of fish densities were visualized in geographic information system software (ArcMap, Esri, USA).
Mean fish density per survey was calculated by a transect-volume weighted average of across-channel transect densities. Standard errors, coefficients of variation, and 95% confidence intervals (95% C.I.) of survey densities were estimated from bootstrap resampling (n = 10,000) of mean density of across-channel transect densities16, 17. We estimated fish abundance per survey by multiplying mean fish density by total survey volume as determined from bathymetric data and time-evolving tidal height. Standard errors and 95% C.I. of abundance were estimated by multiplying the bootstrap-estimated values for density by the volume of surveys. Autocorrelation analysis was conducted to ensure statistical independence between across-channel transect densities and the unbiased estimation of variance17. However, for systematic surveys with strong spatial trends in fish densities (e.g., aggregated or correlated), variance can be biased high, potentially warranting post-stratification of data to mitigate variance inflation17, 60; this potential bias was not evaluated in this study. We tested that mean density on incoming tides was lower than outgoing tides with a one tailed t-test (α = 0.05) after the data were tested for homoscedasticity with Levene’s test (α = 0.05) and normality with an Anderson-Darling test (α = 0.05).
To estimate mean fish length and biomass for each survey, we isolated mean TS of fish tracks with at least three consecutive measures to increase the probability of detections with multiple incidence angles. We converted mean TS of fish tracks to L using the derived KRM-TS versus L equation, and mean L per survey was calculated. We converted mean L to mean weight using a length-to-weight relationship previously estimated for Corvina49, 50 and multiplied by total abundance to estimate spawning stock biomass per survey. Variances of mean L and abundance were propagated and summed in quadrature as fractional uncertainties to estimate the standard errors and 95% C.I. of biomass.
Passive acoustic data analysis
We visually and audibly inspected recordings of ambient sound and extracted 20-s segments free of nearby boat noise and operational disturbances. For each 20-s recording, the mean square pressure amplitude (p 2; Pa2) over the 251–498 Hz band was measured by integrating the pressure spectral density (µPa2/Hz; Hanning window; 16384-point FFT) across the peak frequency bandwidth of Corvina chorusing (see Supplementary Fig. S1), thereby limiting contributions from nearby vessels. We plotted p 2 at each sampling location in geographic information system software (ArcMap, Esri, USA) to visualize the spatial and temporal distribution of sound production and their relation to fish densities. We tested that mean p 2 on the incoming tide was lower than on outgoing tides with one-tailed t-test (α = 0.05) after the data were tested for homoscedasticity with Levene’s test (α = 0.05) and normality with an Anderson-Darling test (α = 0.05).
Comparison of active and passive acoustic data
Fish densities per 150-m and 300-m survey length (ρ; fish/1000 m3) that were nearest in space and time to each passive acoustic recording station were compared to p 2 measurements separately. Measurements of ρ and p 2 that were not coupled in space and time were excluded from comparisons. We generated and examined plots of p 2 vs. ρ as a function of time in relation to high tide to test our hypotheses that a relationship with either strong (H1, Fig. 2) or weak (H2, Fig. 2) predictive power exists among measurements. We compared p 2 and ρ over one hour periods (3 hrs before high tide – 5 hrs after high tide) to observe the evolving relationship between sound production and fish density. We generated regressions between p 2 vs. ρ for each hour using generalized linear model (GLM) regressions. We compared regression slopes using analysis of covariance (ANCOVA, α = 0.05) to test for homogeneity and through multiple comparisons (Tukey-Kramer Method, α = 0.05) to identify the time period with a stable relationship between p 2 and ρ. We combined hour periods with homogeneous regression slopes to test our hypotheses and construct a model (GLM) to estimate ρ from p 2. Results from complementary analyses using densities over 150-m and 300-m survey lengths were compared and tested for homogeneity using analysis of covariance (ANCOVA, α = 0.05) and found to not be significantly different; thus, comparative results are only presented for modeled relationships between p 2 and ρ per 150-m survey length (see Supplementary Figs S3 and S4 for a detailed description of 300-m results).
Electronic supplementary material
Supplementary Information for Rowell et al. Estimating fish abundance at spawning aggregations from courtship sound levels
Acknowledgements
We are grateful for the assistance of C. López-Sagástegui, S. E. Freeman, J. Montañez Rivera, B. Legare, A. Montes Zamudio, J. Cabrera Rivera, E. Cabrera Rivera, Y. Flores, and M. Moreno-Báez for their efforts in helping with fieldwork, data collection, and project logistics. We are indebted to the Comisión Nacional de Acuacultura y Pesca (CONAPESCA) and the Comisión Nacional de Áreas Naturales Protegidas (CONANP) for their willingness to support and provide us with the necessary permits to conduct research inside the Upper Gulf of California and Colorado River Delta Biosphere Reserve. Additionally, we would like to express our gratitude to T. S. Sessions, J. P. Zwolinski, J. S. Renfree, and S. A. Mau at the NOAA/National Marine Fisheries Service and the Southwest Fisheries Science Center and G. L. D’Spain, B. X. Semmens, P. A. Hastings, J. B. Shurin, G. B. Deane, and M. J. Buckingham at the University of California San Diego and Scripps Institution of Oceanography for their intellectual support. The project was funded in part by grants to J.R.H. and B.E.E from the NOAA Advanced Acoustics Program and grants to O.A.O. and B.E.E. from the Walton Family Foundation and the David and Lucile Packard Foundation.
Author Contributions
T.J.R., B.E.E., and D.A.D. designed the study, performed analyses, and wrote the manuscript; T.J.R., B.E.E., D.A.D., and J.J.C. collected data; B.E.E., D.A.D., O.A., and J.R.H. supervised the project and assisted in analytical methods. All authors reviewed and edited the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-03383-8
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hilborn, R. & Walters, C. J. Quantitative Fisheries Stock Assessment: Choice, Dynamics and Uncertainty (eds Hilborn, R. & Walters, C. J.) 1–570 (Springer, 1992).
- 2.IUCN. Guidelines for Application of IUCN Red List Criteria at Regional and National Levels: version 4.0. (Gland, 2012).
- 3.Lynch, P. D., Graves, J. E. & Latour, R. J. Challenges in the assessment and management of highly migratory bycatch species: a case study of the Atlantic marlins In Sustainable Fisheries: Multi-level Approaches to a Global Problem (eds Taylor, W. W., Lynch, A. J. & Schechter, M. G.) 197–225 (American Fisheries Society Press, 2011).
- 4.Molloy PP, Anticamara JA, Rist JL, Vincent ACJ. Frugal conservation: what does it take to detect changes in fish populations? Biol. Cons. 2010;143:2532–2542. doi: 10.1016/j.biocon.2010.06.020. [DOI] [Google Scholar]
- 5.Heppell SA, et al. Documenting recovery of a spawning aggregation through size frequency analysis from underwater laser calipers measurements. Biol. Cons. 2012;155:119–127. doi: 10.1016/j.biocon.2012.06.002. [DOI] [Google Scholar]
- 6.Erisman B, et al. Fish spawning aggregations: where well-placed management actions can yield big benefits for fisheries and conservation. Fish Fish. 2017;18:128–144. doi: 10.1111/faf.12132. [DOI] [Google Scholar]
- 7.Ruelas-Peña JH, Valdez-Muñoz C, Aragón-Noriega EA. La pesquería de la corvina golfina y las acciones de manejo en el Alto Golfo de California, México. Lat. Am. J. Aquat. Res. 2013;41:498–505. [Google Scholar]
- 8.Rose GA, Kulka DW. Hyperaggregation of fish and fisheries: how catch-per-unit-effort increased as the northern cod (Gadus morhua) declined. Can. J. Fish. Aquat. Sci. 1999;56:118–127. doi: 10.1139/f99-207. [DOI] [Google Scholar]
- 9.Erisman BE, et al. The illusion of plenty: hyperstability masks collapses in two recreational fisheries that target fish spawning aggregations. Can. J. Fish. Aquat. Sci. 2011;68:1705–1716. doi: 10.1139/f2011-090. [DOI] [Google Scholar]
- 10.Harley SJ, Myers RA, Dunn A. Is catch-per-unit-effort proportional to abundance? Can. J. Fish. Aquat. Sci. 2001;58:1760–1772. doi: 10.1139/f01-112. [DOI] [Google Scholar]
- 11.Rotherham D, Underwood AJ, Chapman MG, Gray CA. A strategy for developing scientific sampling tools for fishery-independent surveys of estuarine fish in New South Wales, Australia. ICES J. Mar. Sci. 2007;64:1512–1516. doi: 10.1093/icesjms/fsm096. [DOI] [Google Scholar]
- 12.Colin, P. L. Studying and monitoring aggregating species In Reef Fish Spawning Aggregations: Biology, Research and Management (eds Sadovy de Mitcheson, Y. & Colin, P. L.) 285–330 (Springer, 2012).
- 13.Bull B, Doonan I, Tracey D, Hart A. Diel variation in spawning orange roughy (Hoplostethus atlanticus, Trachichthyidae) abundance over a seamount feature on the north‐west Chatham Rise. New Zeal. J. Mar. Fresh. 2010;35:435–444. doi: 10.1080/00288330.2001.9517013. [DOI] [Google Scholar]
- 14.Thorne RE. Review: experiences with shallow water acoustics. Fish. Res. 1998;35:137–141. doi: 10.1016/S0165-7836(98)00068-X. [DOI] [Google Scholar]
- 15.Boswell KM, Wilson MP, Wilson CA. Hydroacoustics as a tool for assessing fish biomass and size distribution associated with discrete shallow water estuarine habitats in Louisiana. Estuar. Coast. 2007;30:607–617. doi: 10.1007/BF02841958. [DOI] [Google Scholar]
- 16.Simmonds, J. & MacLennan, D. Fisheries Acoustics: Theory and Practice (eds Simmonds, J. & MacLennan, D.) 1–437 (Blackwell Science Ltd, 2005).
- 17.Demer DA, et al. Prediction and confirmation of seasonal migration of Pacific sardine (Sardinops sagax) in the California Current Ecosystem. Fish. Bull. 2012;110:52–70. [Google Scholar]
- 18.Fudge SB, Rose GA. Passive- and active-acoustic properties of a spawning Atlantic cod (Gadus morhua) aggregation. ICES J. Mar. Sci. 2009;66:1259–1263. doi: 10.1093/icesjms/fsp097. [DOI] [Google Scholar]
- 19.Costa B, Taylor JC, Kracker L, Battista T, Pittman S. Mapping reef fish and the seascape: using acoustics and spatial modeling to guide coastal management. PLoS ONE. 2014;9:e85555. doi: 10.1371/journal.pone.0085555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fish, M. P. & Mowbray, W. H. Sounds of Western North Atlantic Fishes. 1–207 (Johns Hopkins University Press, 1970).
- 21.Lobel, P. S., Kaatz, I. M. & Rice, A. N. Acoustical behavior of coral reef fishes In Reproduction and Sexuality in Marine Fishes: Patterns and Processes (ed. Cole, K. S.) 307–387 (University of California Press, 2010).
- 22.Rountree RA, et al. Listening to Fish. Fisheries. 2006;31:433–446. doi: 10.1577/1548-8446(2006)31[433:LTF]2.0.CO;2. [DOI] [Google Scholar]
- 23.Luczkovich JJ, Mann DA, Rountree RA. Passive acoustics as a tool in fisheries science. T. Am. Fish. Soc. 2008;137:533–541. doi: 10.1577/T06-258.1. [DOI] [Google Scholar]
- 24.Mann, D. A., Hawkins, A. D. & Jech, J. M. Active and passive acoustics to locate and study fish In Fish Bioacoustics (eds Webb, J. F., Popper, A. N. & Fay, R. R.) 279–309 (Springer, 2008).
- 25.Wilson LJ, Burrows MT, Hastie GD, Wilson B. Temporal variation and characterization of grunt sounds produced by Atlantic cod Gadus morhua and pollack Pollachius pollachius during the spawning season. J. Fish Biol. 2014;84:1014–1030. doi: 10.1111/jfb.12342. [DOI] [PubMed] [Google Scholar]
- 26.Hawkins AD, Amorim M. Spawning sounds of the male haddock. Melanogrammus aeglefinus. Environ. Biol. Fish. 2000;59:29–41. doi: 10.1023/A:1007615517287. [DOI] [Google Scholar]
- 27.Schärer MT, Rowell TJ, Nemeth MI, Appeldoorn RS. Sound production associated with reproductive behavior of Nassau grouper Epinephelus striatus at spawning aggregations. Endang. Species. Res. 2012;19:29–38. doi: 10.3354/esr00457. [DOI] [Google Scholar]
- 28.Luczkovich JJ, Pullinger RC, Johnson SE, Sprague MW. Identifying sciaenid critical spawning habitats by the use of passive acoustics. T. Am. Fish. Soc. 2008;137:576–605. doi: 10.1577/T05-290.1. [DOI] [Google Scholar]
- 29.Locascio JV, Mann DA. Diel and seasonal timing of sound production by black drum (Pogonias cromis) Fish. Bull. 2011;109:327–338. [Google Scholar]
- 30.Saucier MH, Baltz DM. Spawning site selection by spotted seatrout, Cynoscion nebulosus, and black drum, Pogonias cromis, in Louisiana. Environ. Biol. Fish. 1993;36:257–272. doi: 10.1007/BF00001722. [DOI] [Google Scholar]
- 31.Luczkovich JJ, Sprague MW, Johnson SE, Pullinger RC. Deliminating spawning areas of weakfish Cynoscion regalis (family Sciaenidae) in Pamlico Sound, North Carolina using passive hydroacoustic surveys. Bioacoustics. 1999;10:143–160. doi: 10.1080/09524622.1999.9753427. [DOI] [Google Scholar]
- 32.Walters S, Lowerre-Barbieri S, Bickford J, Mann D. Using a passive acoustic survey to identify spotted seatrout spawning sites and associated habitat in Tampa Bay, Florida. T. Am. Fish. Soc. 2009;138:88–98. doi: 10.1577/T07-106.1. [DOI] [Google Scholar]
- 33.Rowell TJ, et al. Sound production as an indicator of red hind density at a spawning aggregation. Mar. Ecol. Prog. Ser. 2012;462:241–250. doi: 10.3354/meps09839. [DOI] [Google Scholar]
- 34.Rowell TJ, Nemeth RS, Schärer MT, Appeldoorn RS. Fish sound production and acoustic telemetry reveal behaviors and spatial patterns associated with spawning aggregations of two Caribbean groupers. Mar. Ecol. Prog. Ser. 2015;518:239–254. doi: 10.3354/meps11060. [DOI] [Google Scholar]
- 35.Gannon DP. Passive acoustic techniques in fisheries science: a review and prospectus. T. Am. Fish. Soc. 2008;137:638–656. doi: 10.1577/T04-142.1. [DOI] [Google Scholar]
- 36.Marques TA, et al. Estimating animal population density using passive acoustics. Biol. Rev. 2013;88:287–309. doi: 10.1111/brv.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schärer MT, et al. Sound production and reproductive behavior of Yellowfin Grouper, Mycteroperca venenosa (Serranidae) at a spawning aggregation. Copeia. 2012;2012:135–144. doi: 10.1643/CE-10-151. [DOI] [Google Scholar]
- 38.Schärer MT, Nemeth MI, Rowell TJ, Appeldoorn RS. Sounds associated with the reproductive behavior of the black grouper (Mycteroperca bonaci) Mar. Biol. 2014;161:141–147. doi: 10.1007/s00227-013-2324-3. [DOI] [Google Scholar]
- 39.Gannon DP, Gannon JG. Assessing trends in the density of Atlantic croaker (Micropogonias undulatus): a comparison of passive acoustic and trawl methods. Fish. Bull. 2009;108:106–116. [Google Scholar]
- 40.Širović A, Cutter GR, Butler JL, Demer DA. Rockfish sounds and their potential use for population monitoring in the Southern California Bight. ICES J. Mar. Sci. 2009;66:981–990. doi: 10.1093/icesjms/fsp064. [DOI] [Google Scholar]
- 41.Ramcharitar J, Gannon DP, Popper AN. Bioacoustics of fishes of the family Sciaenidae (Croakers and Drums) T. Am. Fish. Soc. 2006;135:1409–1431. doi: 10.1577/T05-207.1. [DOI] [Google Scholar]
- 42.Mok HK, Yu HY, Ueng JP, Wei RC. Characterization of sounds of the blackspotted croaker Protonibea diacanthus (Sciaenidae) and localization of its spawning sites in estuarine coastal waters of Taiwan. Zool. Stud. 2009;48:325–333. [Google Scholar]
- 43.Erisman B, et al. Spatio-temporal dynamics of a fish spawning aggregation and its fishery in the Gulf of California. Sci. Rep. 2012;2:284. doi: 10.1038/srep00284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lowerre-Barbieri SK, Walters Burnsed SL, Bickford JW. Assessing reproductive behavior important to fisheries management: a case study with red drum, Sciaenops ocellatus. Ecol. Appl. 2016;26:979–995. doi: 10.1890/15-0497. [DOI] [PubMed] [Google Scholar]
- 45.Chao NL, et al. A popular and potentially sustainable fishery resource under pressure–extinction risk and conservation of Brazilian Sciaenidae (Teleostei: Perciformes) Global Ecology and Conservation. 2015;4:117–126. doi: 10.1016/j.gecco.2015.06.002. [DOI] [Google Scholar]
- 46.Erisman B, et al. A comparison of fishing activities between two coastal communities within a biosphere reserve in the Upper Gulf of California. Fish. Res. 2015;164:254–265. doi: 10.1016/j.fishres.2014.12.011. [DOI] [Google Scholar]
- 47.IUCN. The IUCN Red List of Threatened Species: version 2016-3 (IUCN, 2016).
- 48.Sadovy de Mitcheson YS. Mainstreaming fish spawning aggregations into fishery management calls for a precautionary approach. BioScience. 2016;66:295–306. doi: 10.1093/biosci/biw013. [DOI] [Google Scholar]
- 49.Gherard KE, Erisman BE, Aburto-Oropeza O, Rowell K, Allen LG. Fishery-dependent estimates of growth, development, and reproduction in Gulf Corvina (Cynoscion othonopterus) Bull. South. Calif. Acad. Sci. 2013;112:1–18. [Google Scholar]
- 50.Erisman BE, Apel AM, MacCall AD, Roman MJ, Fujita R. The influence of gear selectivity and spawning behavior on a data-poor assessment of a spawning aggregation fishery. Fish. Res. 2014;159:75–87. doi: 10.1016/j.fishres.2014.05.013. [DOI] [Google Scholar]
- 51.Kubecka J, Wittingerova M. Horizontal beaming as a crucial component of acoustic fish stock assessment in freshwater reservoirs. Fish. Res. 1998;35:99–106. doi: 10.1016/S0165-7836(98)00064-2. [DOI] [Google Scholar]
- 52.Draštík V, Kubečka J. Fish avoidance of acoustic survey boat in shallow waters. Fish. Res. 2005;72:219–228. doi: 10.1016/j.fishres.2004.10.017. [DOI] [Google Scholar]
- 53.Harley SJ, Myers RA, Field CA. Hierarchical models improve abundance estimates: Spawning biomass of hoki in Cook Strait, New Zealand. Ecol. Appl. 2004;14:1479–1494. doi: 10.1890/03-5078. [DOI] [Google Scholar]
- 54.Locascio JV, Burghart S, Mann DA. Quantitative and temporal relationships of egg production and sound production by black drum Pogonias cromis. J. Fish Biol. 2012;81:1175–1191. doi: 10.1111/j.1095-8649.2012.03376.x. [DOI] [PubMed] [Google Scholar]
- 55.Krahforst CS, Luczkovich JJ, Sprague MW. Are passive acoustics an appropriate method to accurately represent a soniferous Atlantic croaker (Micropogonias undulatus) population? J. Acoust. Soc. Am. 2010;127:1826–1826. doi: 10.1121/1.3384231. [DOI] [Google Scholar]
- 56.Foote, K. G., Knudsen, F. R., Vestnes, G., MacLennan, D. N. & Simmonds, E. J. Calibration of Acoustic Instruments for Fish Density Estimation: a Practical Guide. ICES Cooperative Research Report No. 144 (1987).
- 57.Demer, D. A. et al. Calibration of Acoustic Instruments. ICES Cooperative Research Report No. 326 (2015).
- 58.Clay CS, Horne JK. Acoustic models of fish - the Atlantic cod (Gadus morhua) J. Acoust. Soc. Am. 1994;96:1661–1668. doi: 10.1121/1.410245. [DOI] [Google Scholar]
- 59.Kieser R, Mulligan TJ. Analysis of Echo Counting Data: A Model. Can. J. Fish. Aquat. Sci. 1984;41:451–458. doi: 10.1139/f84-054. [DOI] [Google Scholar]
- 60.Fewster RM, et al. Estimating the encounter rate variance in distance sampling. Biometrics. 2009;65:225–236. doi: 10.1111/j.1541-0420.2008.01018.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information for Rowell et al. Estimating fish abundance at spawning aggregations from courtship sound levels


