Abstract
Objective:
The reasons why endometriosis is more aggressive and invasive in some patients are unknown. Despite the importance of population-based clinically defined risk factors in the prediction of recurrence, biochemical markers obtained from the patient are more valuable for prediction on an individual basis. Therefore, the discovery of significant potential biomarkers could be useful to clinicians for shedding light on the pathogenesis of endometriosis and in the monitoring recurrence.
Materials and Methods:
This study included 50 patients who underwent surgery for ovarian cysts that were diagnosed as endometrioma. The age of the patients, stage of the endometriosis, diameter and localization of endometriomas, type of surgery, and pre- and postoperative cancer antigen 125 (CA125) levels were compared between patients with and without recurrence. The archived pathology slides were stained with Ki-67 and anti-urocortin antibodies for reevaluation. By comparing the pathology parameters of the patients with and without recurrence, the association between these parameters and recurrence was investigated.
Results:
The median Ki-67 proliferation index of the patients with recurrence (7.5±6.5) was statistically significant compared with that of the patients without recurrence (1±4) (p=0.003). The urocortin epithelial staining intensity and percentage were not found to be statistically significant in comparison. A statistically significant difference was determined between postoperative CA125 median levels of patients without recurrence (10±17.6) and those of patients with recurrence (29.9±18.1) (p=0.003).
Conclusion:
The Ki-67 proliferation index may be useful for predicting prognosis and recurrence risk.
Keywords: Endometriosis, Ki-67 proliferation index, urocortin
Öz
Amaç:
Endometriozisin bazı hastalarda neden daha agresif ve invaziv olduğunun nedenleri bilinmemektedir. Klinik olarak belirlenen risk faktörleri populasyon bazında rekürrens prediksiyonunda değerli olmasına rağmen kişi bazında prediksiyonda hastadan elde edilen biyokimyasal markerlar daha değerlidir. Bu nedenle, rekürrens açısından anlamlı potansiyel biyomarkerların keşfi endomeriozis patogenezine ışık tutabileceği gibi rekürrens takibinde klinisyenlere yardımcı bir parametre olarak kullanılabilir.
Gereç ve Yöntem:
Çalışmamıza over kisti nedeniyle cerrahi geçiren ve patolojik inceleme sonucunda endometrioma tanısı konmuş 50 hasta dahil edildi. Olguların yaş, endometriozis evresi, endometrioma çapı ve lokalizasyonu, cerrahi tipi, preoperatif ve postoperatif CA 125 düzeyleri kaydedildi. Olguların arşiv patoloji preparatları Ki 67 ve anti-urocortin antikor ile boyandı. Patolojik parametreler nüks olan olgular ile olmayanlarda kıyaslanarak bu parametrelerin rekürrens ile ilişkisi araştırıldı.
Bulgular:
Nüks olan olguların Ki67 İndeks medyanı (7,5±6,5) nüks olmayan olguların Ki67 İndeks medyan değerinden (1±4) daha büyük olup, istatistiksel olarak anlamlıydı (p=0,003). Olguların nüks olma durumu ile ürocortin epitel boyanma yoğunluğu ve yüzdesine göre dağılımı ise istatistiksel olarak anlamlı değildi. Nüks olmayan olguların postoperatif CA 125 medyan değeri (10±17,6) nüks olan olguların CA 125 medyan değerinden (29,9±18,1) daha küçük olup bu istatistiksel olarak anlamlıydı (p=0,003).
Sonuç:
Ki67 proliferasyon indeksi prognoz ve rekürrens riskini predikte etmede yardımcı bir belirteç olabilir.
Introduction
Endometriosis, defined as an endometrial tissue and stroma that is ectopically outside the uterus, is a benign condition accompanied by pelvic pain and infertility [1].
Although definitive causes are unknown, multiple genetic, environmental, immunological, angiogenic, and endocrine factors are considered as its etiology. Although endometriosis is evaluated as a benign condition, recent studies have recommended that endometriosis should be evaluated as a neoplastic process due to histopathological, molecular and genomic similarities between endometriosis and cancer. Similar to a neoplastic process, the reasons why endometriosis is more aggressive and invasive in some patients are unknown [1, 2].
Recurrent lesions may originate from residual structures or de novo cells. Knowledge of the risk factors for recurrence provides the definitions of subgroups for controlling the disease. Potentially significant biomarkers in terms of recurrence form the basis of targeted treatments. Despite the importance of clinically defined risk factors in the prediction of recurrence in a population, biochemical markers obtained from the patient are more valuable for prediction on an individual basis. Therefore, research is being conducted regarding noninvasive, cheap, and easily applicable biomarkers [1, 2].
Endometriosis begins with invasion, gains autonomy, and progresses with proliferation, finally damaging the target organ and exhibiting tumor-like features. Endometriosis shows features similar to neoplasia, which has recently increased interest in the Ki-67 monoclonal antibody.
Ki-67 is a non-histone nuclear protein found in all phases of the cell cycle, other than the G0 phase, and comprises two molecules of 345- and 395-kD weight; its gene is located on chromosome 10. Ki-67 is a nuclear protein observed in proliferating cells. Because it is a protein that shows the morphological features of cell proliferation well, it is often used in the mitotic index and for tumor grading [3]. In immunohistochemical evaluations, the cell percentage that shows positive nuclear staining for Ki-67 indicates the proliferation index. In aggressive tumors, this rate is high. In several system tumors (breast, lungs, esophagus, kidney and prostate cancer, malignant melanoma, non-Hodgkin lymphoma, glial tumors, etc.), a high Ki-67 rate has been shown to be a factor in poor prognosis [4]. In a study by Park et al. [5], endometrial cell proliferation in patients with endometriosis was determined using the Ki-67 proliferation index and was found to be higher than that in patients without endometriosis.
Urocortin is a member of the cortico-releasing factor protein family. Cortico-releasing hormone (CRH) and urocortin 1, 2, and 3 are primarily identified in the central nervous system and are neuropeptides with endocrine, immune, and vasoactive effects [6–8]. While urocortin reduces serum TNF-α and IL-1B levels, it increases IL-6 secretion. Urocortin is a potent mediator in mast cell degranulation and increases vascular permeability [9].
Endometriosis is a sex steroid hormone-dependent condition characterized by inflammation and neoangiogenesis. Neuroendocrine cells are observed in eutopic endometria of females with endometriosis [10]. CRH and urocortin, neuropeptides related to inflammation, vascularization, and apoptosis, are considered to play a role in the pathogenesis of endometriosis. Previous studies have shown that urocortin plays a role in the physiology of endometriosis by inducing endometrial cell differentiation, stimulating cell-adhesion molecule expression, and affecting the immune system and vascular endothelial tonus [11, 12]. Therefore, urocortin, believed to play a role in the pathogenesis of endometriosis, is being researched as a promising biomarker for determining recurrence in high-risk patients.
This study aimed to compare these markers in patients with and without recurrence of endometrioma and investigate their use as a predictive marker for recurrence.
Materials and Methods
The study included 50 patients who underwent laparoscopy or laparotomy with conservative or semiconservative surgery for ovarian cysts that were diagnosed as endometrioma after pathology examination between 2008 and 2013. The Local Ethics Committee approved the study. Clinical, surgical, and pathological records of the patients were retrospectively analyzed. Each patient was interviewed by telephone, and follow-up examinations were performed. Informed consent was obtained from all participants.
Patients were excluded if interviews could not be conducted, all records were not available, follow-up was performed in <6 months, hysterectomy was performed along with cystectomy or salpingo-oophorectomy, or patients with malignancy. For each patient, we recorded age, stage of endometriosis, diameter and localization of endometrioma, type of surgery, and preand postoperative cancer antigen 125 (CA125) levels. The presence of a typical cyst appearance of ≥3 cm on transvaginal sonography after two consecutive menstrual cycles at follow-up was accepted as recurrence.
The archived pathology preparates stained with hematoxylin-eosin were obtained for reevaluation, and the slides were again examined under a light microscope. For each case, the clearest block of endometrioma tissue was selected for immunohistochemical analysis. Patients were excluded if epithelial tissues could not be observed on the slide. By comparing the pathology parameters of the patients with and without recurrence, the association between these parameters and recurrence was investigated.
Immunohistochemical staining and evaluation
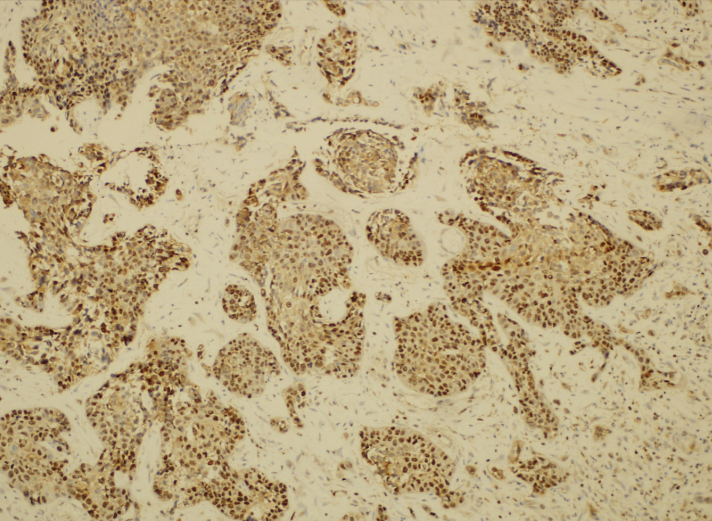
New slices 4–5-μm-thick sections were cut from the selected paraffin blocks and placed on polylysine-coated slides; these tissue samples were then kept at 58°C in an incubator for 30 min. The tissues were then subjected to antigen-retrieval at 95°C for 20 minutes on a Dako PT Link Envision Flex-Target retrieval solution (50 ×) low pH liquid. The slides were then removed from the unit and incubated for 10 min in Envision Flex Washing buffer (20×). In the Dako Autostainer Link 48 automatic immunohistochemical staining device, sections were stained with Ki-67 (Clone MIB-1, Zeta Corporation, USA) antibodies and anti-urocortin antibodies (Code no. ab58459; DakoCytomation, Denmark), which were prepared by dilution to 1/75. The slides were then passed through a series of alcohol-xylene for 2 min each. Tonsil tissues were used as a positive control for Ki-67 and pulmonary carcinoma tissues were used for urocortin (Figure 1).
Figure 1.
Urocortin staining pattern of lung carcinoma tissues used as the positive control.
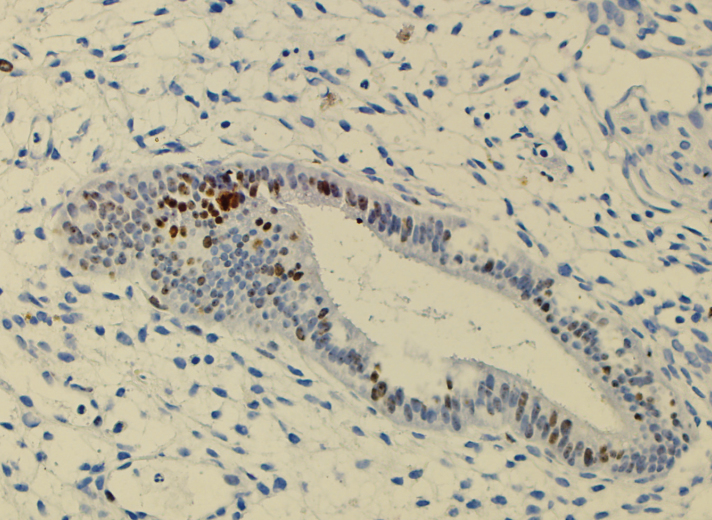
Ki67 proliferation index
The Ki67 proliferation index was calculated by counting cells showing nuclear staining in 500 epithelial cells using an ocular micrometer (Figure 2).
Figure 2.
Ki-67 staining pattern of endometrioma case (case 34) (H&E, ×100 HPF).
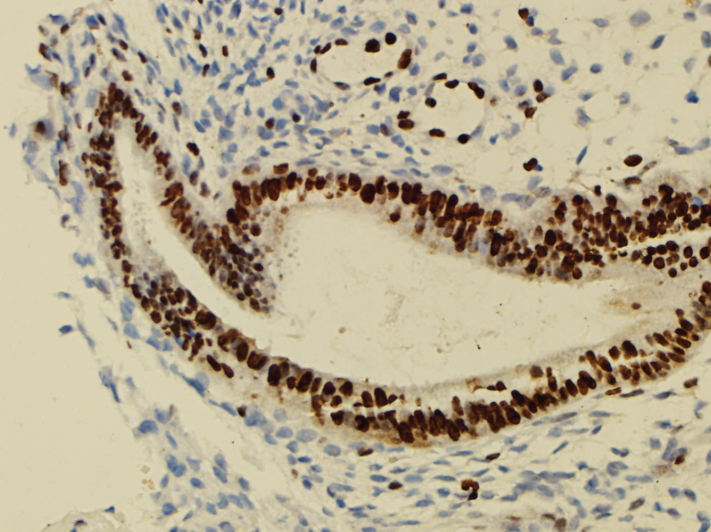
Urocortin
Nuclear staining in the endometriotic gland epithelial cells was accepted as positive for urocortin. Cells (lymphocytes, endothelial cells, etc.) other than epithelial cells showing staining were not included in the evaluation. All endometriotic foci were detected on a light microscope (×10), and the ratio of the stained epithelial cells to the whole gland epithelium at ×20 magnification and intensity of the staining of the epithelial cells at high magnification (×40) were semiquantitatively and subjectively determined (Figure 3).
Figure 3.
Urocortin staining pattern of endometriotic glandular epithelium.
Statistical analysis
The Statistical Package for the Social Sciences 21.0 (IBM Corp.; Armonk, NY, USA) and MedCalc 9 (Acacialaan 22, B-8400 Ostend, Belgium) software programs were used analyses of data. Parametric methods were used in the analysis of variables with normal distribution, and nonparametric methods were used in the analysis of variables without normal distribution. For comparing two independent groups, independent sample t-test and Mann-Whitney U test were used, and for comparing dependent paired data, McNemar’s test was used. Quantitative data were expressed as mean±standard deviation and median±interquartile range values. Categorical data were expressed as number (n) and percentage (%). Data were examined with a 95% confidence interval, and a p value of <0.05 was accepted to be statistically significant.
Results
The study included 50 patients, 24 (48%) with recurrence and 26 (52%) without recurrence. In patients with recurrence, the mean time to recurrence was 11.1±10.7 months (median, 6.5 months; range, 1–48 months). The mean cyst size was 6.8±2.4 cm (median, 6 cm; range, 3–15 cm). The mean Ki-67 proliferation index was 5.18%±4.28% (median, 4%, range, 1%–15%). The mean urocortin epithelial staining percentage was 12.3%±18.03% (median, 5%; range, 1%–90%). When the distribution of the patients was evaluated according to the epithelial staining intensity, mild staining was observed in 30 (60%) of 50 patients and strong staining was observed in 20 (40%). Demographic data of patients are shown in Table 1.
Table 1.
Demographic data of the patients
| Recurrence | p | ||
|---|---|---|---|
|
| |||
| Negative (n=26) | Positive (n=24) | ||
| Stage (n) | |||
| Early stage (stage I–II) | 17 (65.4) | 15 (62.5) | 0.831 |
| Advanced stage (stage III–IV) | 9 (34.6) | 9 (37.5) | |
| Lesion side (n | |||
| Right | 9 (34.6) | 7 (29.2) | 0.365 |
| Left | 11 (42.3) | 10 (41.7) | |
| Bilateral | 6 (23.1) | 7 (29.2) | |
| Postoperative medical treatment (n) | |||
| Oral contraceptive | 12 (85.7) | 8 (80%) | 0.385 |
| GnRH analog | 2 (14.3) | 2 (20%) | |
| Surgery type (n) | |||
| Cystectomy | 21 (80.8) | 18 (75) | 0.237 |
| Salpingo-oophorectomy | 5 (19.2) | 6 (25) | |
| Laparoscopy/laparotomy (n) | |||
| Laparotomy | 21 (80.8) | 19 (79.2) | 0.272 |
| Laparoscopy | 5 (19.2) | 5 (20.8) | |
| Age (years) | 35.2±6.5 | 32.1±6.7 | 0.106 |
| Cyst size (cm) | 6±4 | 7±4 | 0.317 |
GnRH: gonadotropin-releasing hormone
Data are given as mean±standard deviations or frequencies (percentages within parentheses).
The median Ki-67 proliferation index of the patients with recurrence (7.5±6.5) was statistically significant compared with that of the patients without recurrence (1±4) (p=0.003). Distribution of the recurrence status of the patients according to the urocortin epithelial staining intensity and percentage was not found to be statistically significant (Table 2).
Table 2.
Evaluation of patients’ recurrence status with Ki67 proliferation index and urocortin epithelial staining
| Recurrence | p | ||
|---|---|---|---|
|
| |||
| Positive median±IQR (n=24) | Negative median±IQR (n=26) | ||
| Ki 67 proliferation index | 7.5±6.5 | 1±4 | 0.003 |
| Urocortin epithelial staining percentage (%) | 5±21.5 | 2±9 | 0.355 |
| Urocortin epithelial staining intensity | |||
| Weak | 13 (54.2) | 17 (65.4) | 0.565 |
| Strong | 11 (45.8) | 9 (34.6) | |
IQR: interquartile range
Data are given as mean±standard deviations or frequencies (percentages within parentheses)
When the recurrence status of the patients was evaluated according to the pre- and postoperative CA125 levels, the median preoperative CA125 levels (29.55±78) of the patients without recurrence were found to be similar to the median CA125 levels (40±83.2) of the patients with recurrence (p=0.620). A statistically significant difference was determined between the postoperative CA125 median levels of the patients without recurrence (10±17.6) and those of the patients with recurrence (29.9±18.1) (p=0.003) (Table 3).
Table 3.
Comparison of pre- and postoperative CA125 levels of patients with the state of relapsing
| CA125 levels (U/mL) | Recurrence | p* | |
|---|---|---|---|
|
| |||
| Negative (n=26) Median±IQR | Positive (n=24) Median±IQR | ||
| Preoperative | 29.55±78 | 40±83.2 | 0.620 |
| Postoperative | 10±17.6 | 29.9±18.1 | 0.003 |
IQR: interquartile range; CA125: cancer antigen 125
Mann-Whitney U test (exact)
No statistically significant difference was determined between the median value of the Ki-67 proliferation index of early stage (grade I–II) patients with and without recurrence (p=0.066).
A statistically significant difference was determined between the median value of the Ki-67 proliferation index of the advanced stage (grade III–IV) patients with and without recurrence (p=0.013) (Table 4).
Table 4.
Distribution of Ki67 proliferation index values of patients based on the recurrence status and staging
| Stage | Recurrence | p* | |
|---|---|---|---|
|
| |||
| Negative (n=26) Ki67 proliferation index (median±IQR) | Positive (n=24) Ki67 proliferation index (median±IQR) | ||
| Early stage (stage I–II) | 3±6 | 8±5 | 0.066 |
| Advanced stage (stage III–IV) | 1±1 | 7±7 | 0.013 |
IQR: interquartile range
Mann-Whitney U test (exact)
Discussion
Endometriosis is a chronic inflammatory process with a broad clinical spectrum. Similar to a neoplastic process, the reason why endometriosis is more aggressive and invasive in some patients is unknown. Depending on the criteria considered, the recurrence rate varies from 6% to 67% [2]. Which of the several different reasons for recurrence is more predictive is uncertain and remains debatable. Several studies have been conducted to define the risk factors of recurrence of ovarian endometrioma after surgery.
Ki-67 is an antibody that can display the proliferative cells in the tumor. When the Ki-67 proliferation rate is higher, tumors have a tendency to have a more aggressive course with vascular invasion, proliferation, and metastasis, which are often observed in these patients [3]. Recent studies have emphasized that endometriosis demonstrates tumor-like behavior [1, 2], and interest has increased regarding the association between Ki-67 antibodies and endometriosis.
A total of 45 patients with stage 3 and 4 endometriosis were evaluated by Toki et al. [13] in a research of the association between serum CA125 levels and endometriotic epithelial cell proliferation. Serum CA125-19.9 levels were compared with the Ki67 index of endometriotic cells and CA125 -19.9 staining degree in pathology specimens. There was a correlation between serum CA19.9 levels and tissue staining but no correlation with the Ki-67 proliferation index. In contrast, no strong correlation was observed between serum CA125 levels and tissue staining, but a correlation was determined with the Ki-67 proliferation index. This finding showed that active proliferative endometriotic cells secreted more CA125 and that CA125 was a valuable marker for predicting recurrence of endometriosis, particularly in grade 3 and 4 patients, and this result was consistent with the findings reported by Nagamani et al. [14] and Fedele et al. [15].
Kahyaoglu et al. [16] compared the Ki67 proliferation index in eutopic and ectopic endometrium and found an increase in the proliferation index as the endometriosis stage increased, in a study of 38 patients with endometriosis and 21 with control group. Furthermore, a correlation between the proliferation index and CA125 levels existed. It was reported that cells with increased proliferative potential gained autonomy in the same way as tumor cells, and thus, endometriosis damaged surrounding tissues, impaired anatomy, and adhesions. The Ki-67 proliferation index may be a useful marker for demonstrating this association and for predicting prognosis, and recurrence risk.
Unlike these studies, Scotti et al. [17] suggested in their study investigating the proliferative and invasive character of endometriosis using Ki67 and E-cadherin markers that epithelial cells in endometriotic lesions exhibit an invasive character with a tendency to differentiate rather than being hyperproliferative.
In this study, a positive correlation was observed between postoperative serum CA125 levels, Ki-67 proliferation index, and recurrence. However, contrary to the study by Kahyaoglu et al. [16], no correlation was observed between postoperative CA125 levels and the Ki-67 proliferation index (p=0.731). The increase in the Ki-67 proliferation index was also related to a more advanced stage of the disease.
Cortico-releasing hormone and urocortin expression in the endometrium of healthy women were higher in the secretory endometrium than in the proliferative phase, whereas in the eutopic endometrium of the women with endometriosis, there was no difference [18]. It is believed that CRH and urocortin have a role in stromal cell decidualization, and a synergistic effect is shown with progesterone. The typical phenotype of endometria in females with endometriosis is impaired decidualization with a tendency to implant tissue and survive outside the uterus. The impaired CRH/urocortin pathway of the eutopic endometrium may be responsible for this situation and may also partially explain spontaneous abortion and low fertility rates related to endometriosis [11, 18].
In addition to impaired decidualization, in patients with endometriosis, there is an increased adhesion capacity in endometrial cells that facilitates implantation on the peritoneal and ovarian surfaces. Urocortin stimulates the secretion of matrix-metalloproteinase 9 in placental cells, but it is not clear if the same adhesion molecules affect endometrial cells [19]. Determining this status would be beneficial for clarifying the role of urocortin in the pathogenesis of endometriosis.
In a study by Florio et al. [20], patients with ovarian endometrioma were compared with control patients with benign, non-endometriotic ovarian cysts. The preoperative plasma urocortin and CA125 levels were examined, and the levels in the cyst and peritoneal fluids were investigated. The urocortin levels within the cyst were higher than those in the peritoneal fluid and reached approximately three times the plasma level. Supporting these findings, immunohistochemical examination revealed intense and diffuse urocortin staining in the epithelial cells of the endometriotic gland. In 88% of patients, plasma urocortin was determined with 90% specificity, and in only 65% of patients, plasma CA125 was determined with the same specificity. With these findings, it was reported that urocortin, expressed and secreted in endometriotic tissues, could be used as a specific and sensitive marker in the differentiation of endometrioma from benign ovarian cysts. In contrast to that study, Tokmak et al. [21] compared urocortin and CA125 levels in patients with endometrioma and benign ovarian cysts and reported that urocortin was not effective in the differentiation of endometrioma from benign ovarian cysts and was not superior to CA125 in the diagnosis of endometrioma.
Whatever role urocortin is assumed to have in the pathogenesis of endometriosis, urocortin is found in increased levels in endometriotic tissues and is thought to provide the potential for a greater tendency for adhesion and implantation in these tissues. Therefore, in patients with recurrent endometriosis, a high urocortin level is an anticipated finding in both plasma and tissue immunohistochemical examination. In this study, urocortin staining was examined in the epithelial cells of the endometriotic gland, but no significant difference was determined with respect to staining percentage or intensity between patients with and without recurrence. This could be related to the small sample size or the staining techniques of this study. Therefore, to be able to better understand the association between urocortin and recurrence risk, further studies conducted with larger samples are required.
In conclusion, there is ongoing research for determining appropriate biomarkers for early diagnosis, risk factors, and recurrence patterns of endometriosis. Identifying the risk factors may be helpful in determining high-risk groups that require more aggressive treatment and closer monitoring and in providing personalized treatment. The discovery of significant potential biomarkers could be of use to clinicians both in shedding light on the pathogenesis of endometriosis and in monitoring recurrence.
Footnotes
Ethics Committee Approval: Ethics committee approval was received for this study from the ethics committee of Izmir Katip Çelebi University (Decision Date: 08.04.2013/Decision No: 87).
Informed Consent: Written informed consent was obtained from patients who participated in this study.
Peer-review: Externally peer-reviewed.
Author contributions: Concept - S.E.Y., I.O., S.K.; Design - SE.Y., I.O., S.K.; Supervision - S.K., H.A.; Resource - Y.Y., H.S.S., M.D.C.; Materials - S.E.Y., Y.Y., H.A.; Data Collection and/or Processing - I.O., S.E.Y., H.S.S.; Analysis and/or Interpretation - S.E.Y., Y.Y., M.D.C.; Literature Search - S.E.Y., H.A.; Writing - SE.Y.; Critical Reviews - S.E.Y., I.O., Y.Y., H.S.S., M.D.C., H.A., S.K.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: This research was supported by İzmir Katip Çelebi University Scientific Research Projects Foundation (Project No: 2013-T1-TSBP-01).
References
- 1.Bhanoori M, Arvind Babu K, Pavankumar Reddy NG, et al. The vascular endothelial growth factor (VEGF) +405G>C 5′-untranslated region polymorphism and increased risk of endometriosis in South Indian women: a case control study. Hum Reprod. 2005;20:1844–9. doi: 10.1093/humrep/deh852. https://doi.org/10.1093/humrep/deh852. [DOI] [PubMed] [Google Scholar]
- 2.Kim SH, Choi YM, Choung SH, Jun JK, Kim JG, Moon SY. Vascular endothelial growth factor gene +405 C/G polymorphism is associated with susceptibility to advanced stage endometriosis. Hum Reprod. 2005;20:2904–8. doi: 10.1093/humrep/dei146. https://doi.org/10.1093/humrep/dei146. [DOI] [PubMed] [Google Scholar]
- 3.Huisman MA, De Heer E, Grote JJ. Cholesteatoma epithelium is characterized by increased expression of Ki-67, p53 and p21, with minimal apoptosis. Acta Otolaryngol. 2003;123:377–82. doi: 10.1080/00016480310001376. https://doi.org/10.1080/00016480310001376. [DOI] [PubMed] [Google Scholar]
- 4.Dahmoun M, Boman K, Cajander S, Westin P, Backström T. Apoptosis, proliferation, and sex hormone receptors in superficial parts of human endometrium at the end of the secretory phase. J Clin Endocrinol Metab. 1999;84:1737–43. doi: 10.1210/jcem.84.5.5706. https://doi.org/10.1210/jcem.84.5.5706. [DOI] [PubMed] [Google Scholar]
- 5.Park JS, Lee JH, Kim M, Chang HJ, Hwang KJ, Chang KH. Endometrium from women with endometriosis shows increased proliferation activity. Fertil Steril. 2009;92:1246–9. doi: 10.1016/j.fertnstert.2009.04.025. https://doi.org/10.1016/j.fertnstert.2009.04.025. [DOI] [PubMed] [Google Scholar]
- 6.Vaughan J, Donaldson C, Bittencourt J, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–92. doi: 10.1038/378287a0. https://doi.org/10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 7.Reyes TM, Lewis K, Perrin MH, et al. Urocortin II: a member of the corticotropin-releasing factor (CRF) neuropeptide family that is selectively bound by type 2 CRF receptors. Proc Natl Acad Sci U S A. 2001;27:2843–8. doi: 10.1073/pnas.051626398. https://doi.org/10.1073/pnas.051626398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis K, Li C, Perrin MH, et al. Identification of urocortin III, an additional member of the corticotropin-releasing factor (CRF) family with high affinity for the CRF2 receptor. Proc Natl Acad Sci U S A. 2001;19:7570–5. doi: 10.1073/pnas.121165198. https://doi.org/10.1073/pnas.121165198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agnello D, Bertini R, Sacco S, Meazza C, Villa P, Ghezzi P. Corticosteroid-independent inhibition of tumor necrosis factor production by the neuropeptide urocortin. Am J Physiol. 1998;275:E757–62. doi: 10.1152/ajpendo.1998.275.5.E757. [DOI] [PubMed] [Google Scholar]
- 10.Florio P, Arcuri F, Ciarmela P, et al. Identification of urocortin mRNA and peptide in the human endometrium. J Endocrinol. 2002;173:R9–14. doi: 10.1677/joe.0.173r009. https://doi.org/10.1677/joe.0.173R009. [DOI] [PubMed] [Google Scholar]
- 11.Torricelli M, De Falco G, Florio P, et al. Secretory endometrium highly expresses urocortin messenger RNA and peptide: possible role in the decidualization process. Hum Reprod. 2007;22:92–6. doi: 10.1093/humrep/del331. https://doi.org/10.1093/humrep/del331. [DOI] [PubMed] [Google Scholar]
- 12.Hao Z, Huang Y, Cleman J, et al. Urocortin2 inhibits tumor growth via effects on vascularization and cell proliferation. Proc Natl Acad Sci U S A. 2008;105:3939–44. doi: 10.1073/pnas.0712366105. https://doi.org/10.1073/pnas.0712366105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toki T, Kubota J, Lu X, Nakayama K. Immunohistochemical analysis of CA125, CA19-9, and Ki-67 in stage III or IV endometriosis: positive correlation between serum CA125 level and endometriotic epithelial cell proliferation. Acta Obstet Gynecol Scand. 2000;79:771–6. https://doi.org/10.1034/j.1600-0412.2000.079009771.x. [PubMed] [Google Scholar]
- 14.Nagamani M, Kelver ME, Smith ER. CA 125 levels in monitoring therapy for endometriosis and in prediction of recurrence. Int J Fertil. 1992;37:227–31. [PubMed] [Google Scholar]
- 15.Fedele L, Arcaini L, Vercellini P, Bianchi S, Candiani GB. Serum CA 125 measurements in the diagnosis of endometriosis recurrence. Obstet Gynecol. 1988;72:19–22. [PubMed] [Google Scholar]
- 16.Kahyaoglu I, Kahyaoglu S, Moraloglu O, Zergeroglu S, Sut N, Batioglu S. Comparison of Ki-67 proliferative index between eutopic and ectopic endometrium: a case control study. Taiwan J Obstet Gynecol. 2012;51:393–6. doi: 10.1016/j.tjog.2012.07.013. https://doi.org/10.1016/j.tjog.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 17.Scotti S, Regidor PA, Schindler AE, Winterhager E. Reduced proliferation and cell adhesion in endometriosis. Mol Hum Reprod. 2000;6:610–7. doi: 10.1093/molehr/6.7.610. https://doi.org/10.1093/molehr/6.7.610. [DOI] [PubMed] [Google Scholar]
- 18.Florio P, Torres PB, Torricelli M, Toti P, Vale W, Petraglia F. Human endometrium expresses urocortin II and III messenger RNA and peptides. Fertil Steril. 2006;86:1766–70. doi: 10.1016/j.fertnstert.2006.05.041. https://doi.org/10.1016/j.fertnstert.2006.05.041. [DOI] [PubMed] [Google Scholar]
- 19.Novembri R, Borges LE, Carrarelli P, et al. Impaired CRH and urocortin expression and function in eutopic endometrium of women with endometriosis. J Clin Endocrinol Metab. 2011;96:1145–50. doi: 10.1210/jc.2010-2263. https://doi.org/10.1210/jc.2010-2263. [DOI] [PubMed] [Google Scholar]
- 20.Florio P, Reis FM, Torres PB, et al. Plasma urocortin levels in the diagnosis of ovarian endometriosis. Obstet Gynecol. 2007;110:594–600. doi: 10.1097/01.AOG.0000278572.86019.ae. https://doi.org/10.1097/01.AOG.0000278572.86019.ae. [DOI] [PubMed] [Google Scholar]
- 21.Tokmak A, Ugur M, Tonguc E, Var T, Moraloğlu O, Ozaksit G. The value of urocortin and Ca-125 in the diagnosis of endometrioma. Arch Gynecol Obstet. 2011;283:1075–9. doi: 10.1007/s00404-010-1505-2. https://doi.org/10.1007/s00404-010-1505-2. [DOI] [PubMed] [Google Scholar]