Abstract
Zika virus (ZIKV) is an arbovirus of the Flavivirus genus, and it has an envelope and a single RNA molecule. In early 2016, the World Health Organization declared ZIKV infection to be an emerging global health threat. The major transmission route of the virus to humans is Aedes mosquitoes. ZIKV can be transmitted between humans by transplacental, perinatal, and sexual routes and via blood and body fluids. ZIKV infection usually results in a mild and self-limiting disease with low-grade fever, conjunctivitis, and periorbital edema. Neurological complications such as Guillain-Barré syndrome, meningoencephalitis, acute disseminated encephalomyelitis, acute myelitis, and transverse myelitis have been reported during ZIKV infection. Intrauterine and congenital ZIKV infections have strong teratogenic effects on the fetus. Intrauterine or congenital ZIKV infection can lead to microcephaly, ocular anomalies (such as macular atrophy, pigment mottling, and optic nerve anomalies), and cardiac anomalies (such as atrial or ventricular septal defect). Calcification in the brain between the cortical and subcortical areas, ventriculomegaly, cerebellar hypoplasia, corpus callosum hypoplasia, cortical/subcortical atrophy, delayed myelination, enlarged cisterna magna, and craniofacial disproportion have been reported as brain development defects. ZIKV infection usually results in a mild disease, and it does not require specific therapy. However, complications of infection during the early period of life are serious. Thus, many drugs have been investigated, and vaccine development studies have been conducted to prevent ZIKV infection. Vector control and personal protection from mosquito-borne transmission are important for decreasing the prevalence of ZIKV infection. In particular, pregnant residents or travelers to endemic areas should be carefully protected against mosquito-borne transmission.
Keywords: Zika virus, Flavivirus, microcephaly, Guillain-Barré Syndrome, aedes, arbovirus
Öz
Zika virus (ZIKV), Flavivirus ailesinden sivrisineklerle bulaşan zarflı ve tek sarmallı RNA’ya sahip bir arbovirusdur. 2016 yılı başında Dünya Sağlık Örgütü (DSÖ) ZIKV enfeksiyonunun giderek önemi artan bir küresel tehdit olduğunu açıklamıştır. Virusun insanlara esas geçiş yolu Aedes tipi sivrisineklerin ısırmasıdır. İnsanlar arasında transplasental, perinatal, cinsel yolla, ayrıca kan ve vücut sıvıları aracılığı ile bulaşır. ZIKV enfeksiyonu genellikle hafif ve kendini sınırlandıran hastalık olup, düşük derecede ateş, konjuktivit ve periorbital ödem ile seyreder. ZIKV enfeksiyonu esnasında Guillain-Barre sendromu, meningoensefalit, akut dissemine ensefalomyelit (ADEM), akut myelit, transvers myelit gibi nörolojik komplikasyonlar rapor edilmiştir. Intrauterin ve konjenital ZIKV enfeksiyonu fetus üzerinde ağır teratojenik etkiye sahiptir. İntrauterin enfeksiyon mikrosefali, oküler anomaliler (maküler atrofi, pigment beneklenmesi ve optik sinir anomalileri gibi) ve kardiyak anomalilere (atrial veya ventriküler septal defekt gibi) yol açabilir. Beyin gelişim defektleri olarak kortikal ve subkortikal alanda kalsifikasyonlar, vetrikülomegali, serebellar hipoplazi, kortikal/subkortikal atrofi, korpus kallosum hipoplazisi, gecikmiş myelin gelişimi, geniş sisterna magna ve kraniofasiyal orantısızlık bildirilmiştir. ZIKV enfeksiyonu genellikle hafiftir ve tedavi gerektirmez. Fakat hayatın erken döneminde geçirilen enfeksiyonun komplikasyonları ciddidir. Bu yüzden ZIKV’e karşı pekçok ilaç araştırılmakta ve aşı geliştirme çalışmaları yürütülmektedir. Vektör kontrolü ve sivrisinek ısırığından korunmak için kişisel koruyucu önlemler ZIKV enfeksiyonunu azaltmak için önemlidir. Özellikle gebeler, endemik bölgede oturanlar ve seyahat edenler sivrisinek ısırığından dikkatice korunmalıdır.
Epidemiology
Virus, history, and geographic distribution
Zika virus (ZIKV) is an enveloped and single RNA virus of the Flavivirus genus. It is an arbovirus transmitted by Aedes mosquitoes. The virus was detected in a monkey in 1947 in Uganda and in humans in 1952 in Uganda and Tanzania [1, 2]. The first outbreaks of ZIKV infection were reported in 2007 from Micronesia, and later, other outbreaks occurred in the French Polynesia, the Pacific Islands, and northern Brazil [2–6]. Later on, the virus rapidly spread to other regions in Brazil and to other Latin American countries. There are two lineages of the virus: African and Asian. The virus in the Americas originated from the Asian lineage [7].
In 2015, the association between ZIKV and microcephaly was reported in northern Brazil [8–11]. From 2015 to 2016, the number of reports on microcephaly increased by up to 20 times and reached 5,400 cases [12, 13]. After the first report suggested an association between Guillain-Barré syndrome (GBS) and ZIKV, health authorities in Brazil became aware of a similar relationship because of the 29.8% increase in the number of GBS cases from 2014 to 2015 [12–14]. A recent report has suggested that the incidence of brain anomalies increased by 20 times during ZIKV outbreaks (between January 2015 and September 2016) in America too [15].
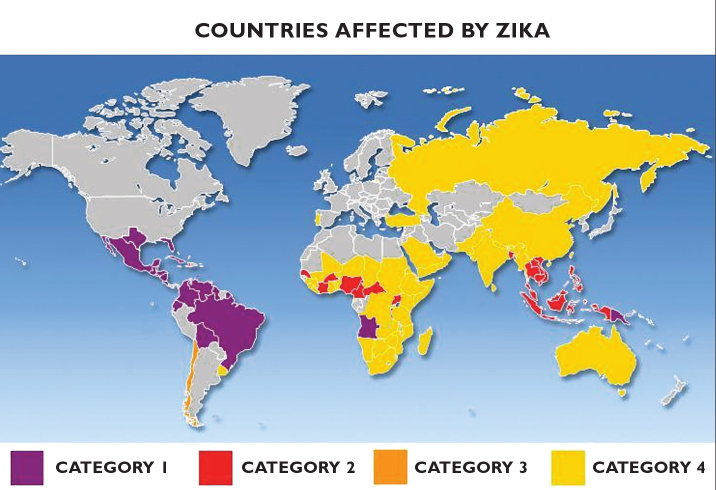
In early 2016, the World Health Organization (WHO) declared ZIKV infection to be an emerging global health threat [16]. Viral distribution has been continued to different geographical areas, and case reports are being published almost every day on infection due to travel to endemic areas. Thus, ZIKV has spread worldwide by both travelers and mosquitoes, and so far travelers WHO describes geographic risk assessment for Zika virus transmission (Figure 1) [3, 17, 18].
Figure 1.
Countries affected by ZIKV. Countries with a reported outbreak from 2015 onwards (Category 1), countries with possible endemic transmission or evidence of local mosquito-borne ZIKV infections in 2016 (Category 2), countries with evidence of local mosquito-borne ZIKV infections in or before 2015, but without documentation of cases in 2016 (Category 3), area with an established competent vector but no known documented past or current transmission (Category 4). ZIKV: Zika virus
Transmission
To date, ZIKV has been isolated from nonhuman primates and mosquitoes as well as humans [19]. The major transmission route of the virus to humans is mosquitoes, and Aedes aegypti is the main vector of ZIKV.
The virus can be transmitted between humans via transplacental, perinatal, sexual, and blood-borne routes [3, 20–23]. The virus has been isolated from blood, urine, saliva, and semen, and person-to-person transmission is possible because the virus can spread through these body fluids [24]. During the outbreak in the French Polynesia, Polymerase Chain Reaction (PCR) positivity for ZIKV was found in 3% of the sera of blood donations [3]. Additionally, the virus can persist in vaginal secretions and semen for up to 115 days [17, 24–27].
Recently, ZIKV RNA was found in the breast milk of three mothers with ZIKV infection, and two of their newborns were diagnosed with ZIKV infection. Although perinatal transmission is possible in these cases, the role of breastfeeding was not clear [28].
Pathogenesis
Zika virus enters cells by endocytosis, and it is seen in the nucleus. Similar to other flaviviruses, ZIKV replicates in dendritic cells and spreads in blood. ZIKV infection is self-limiting [17].
The absolute risks of microcephaly in live births in Brazil in 2012 and 2014 have been reported to be 10% and 50%, respectively, and the relative risk has been estimated to be 1.98 in 10,000 live births. The risk of microcephaly in infants whose mothers have ZIKV infection during pregnancy has been reported 18-127-times greater than others, and the association between microcephaly and ZIKV has been reported in numerous studies [8, 11, 29–31].
Zika virus can be transmitted through transplacental routes and can lead to fetal infection [10]. The virus was seen in the brain tissue of fetuses [32]. Similar to other congenital infections such as rubella, ZIKV infections are more teratogenic in the first trimester and neurological complications such as microcephaly develop during this period. The impact on the fetus continues with or without microcephaly, low fetal weight, and ophthalmic and cardiac anomalies.
Zika virus IgM antibodies have been detected in the cerebrospinal fluid in a microcephalic infant as evidence of ZIKV being the causative agent of microcephaly [33, 34]. Hughes et al. [35] found persistent infection in immature neuronal tissues. Bhatnagar et al. [36] reported PCR positivity for ZIKV in 62% of the brain tissue samples of microcephalic fetuses and in placental/fetal tissues of 52 pregnant women. They demonstrated persistent viral replication in brain and placental tissues, which resulted in the loss of pregnancy in the first or second trimester. Although the virus was detected in brain and placental tissues, it was not detected in other tissues such as the liver, spleen, and kidney. Thus, they suggested that this provides evidence for the association between the virus and microcephaly. In contrast, Chan et al. [37] found both viral replication and cytopathic effects in neuronal, placental, hepatic, colonic, retinal, pulmonary, and muscular cell lines. They found viral replication but not cytopathic effects in renal, testicular, and prostate cells.
In experimental studies, viral surface glycoprotein E has been found to interact with neural cell surface receptors such as AXL. Brault et al. [38] found that ZIKV is a tropism to neural stem cells, impairing cell cycle progression and inhibiting apoptosis at the early stages of infection. McGrath et al. [39] showed that the virus can impair neuronal differentiation. Recently, Smith et al. [40] showed that ZIKV causes acute and subacute encephalitis/encephalomyelitis, neuronal cell death, and astrogliosis.
In their experimental study, Cugola et al. [41] reported that ZIKV causes birth defects. They demonstrated that after the virus crosses the placenta, it infects cortical cells and causes cell death and impairs neurodevelopment.
In a recent study, Vermillion et al. [42] investigated the intrauterine pathogenesis of ZIKV in a mouse model. They found the viral antigen in placental trophoblast and endothelial cells and in fetal brain endothelial, glial, and neural progenitor cells. They showed the ability of ZIKV to infect fetal and placental tissues and to reduce fetal viability through placental inflammation and dysfunction. ZIKV may lead to a thinner fetal brain cortex due to increased microglial activity.
Clinical Features and Differential Diagnosis
The incubation period of ZIKV infection is 3–12 days [2, 26, 43]. ZIKV infection usually results in a mild and self-limiting disease [26]. It is estimated that 80% of cases may be asymptomatic [17]. The symptoms and signs of the disease are low-grade fever (15 days), pruritic maculopapular rash (lasting 4–6 days), conjunctival hyperemia, headache, sore throat, retro-ocular pain, joint pain, back pain, myalgia, and distal edema on the limb and desquamation on hands and feet, as well as lymphadenopathies [2, 5, 14, 26]. Transient hearing loss and genitourinary symptoms such as hematospermia and hypotension were reported to be rare [44]. The symptoms last for one week on average, and arthralgia may last two weeks [45]. Conjunctivitis and periorbital edema are more frequent symptoms than in other Flaviviridea infections such as dengue [46, 47]. The hospitalization rate is low and fatality is extremely rare [3]. Fatal outcomes are only seen in cases with present immunosuppression, alcoholism, or sickle cell anemia and in infants [44].
Guillain-Barré syndrome might develop after ZIKV infection as an immunological complication. There are many pieces of evidence for the close relationship between the GBS and ZIKV infection [4, 12]. Some other neurologic complications such as meningoencephalitis, acute disseminated encephalomyelitis, acute myelitis, and transverse myelitis have been reported during ZIKV infection [12, 48–50].
Rubella, measles, adenovirus, enterovirus, parvovirus, and other Flavivirus infections such as dengue and chikungunya should be considered as differential diagnosis. Malaria, rickettsia, leptospirosis, and group A beta hemolytic Streptococcus infection should also be kept in mind considering the similarity of some features [43].
Congenital and Intrauterine Zika Virus Infection
The most frequent clinical symptoms in infants with ZIKV infection are irritability, convulsion, clonus, crying, pyramidal and extrapyramidal symptoms, epilepsy, dysphagia, and persistent primitive reflex [51]. Positive examination findings were reported to be poor head growth and negative z-score, biparietal depression, prominent occiput, excess nuchal skin, congenital club-foot, arthrogryposis, cleft lip, or cleft palate [51]. Microcephaly may be present or develop within 5 months after birth in infants with congenital ZIKV infection [52]. Kleber et al. [8] reported that febrile illness with rash in the first trimester was associated with microcephaly. França et al. [9] found that the presence of rash in the third trimester of pregnancy was associated with fetal brain anomalies despite normal head size.
Congenital anomalies were reported in 5% of infants from 972 completed pregnancies out of 1,297 pregnancies with ZIKV infection. The anomaly rate was similar in infants from symptomatic and asymptomatic pregnancies. The ratio of congenital anomaly was 15% in infants whose exposure of the virus occurred during the first trimester. Microcephaly was found in 75% of infants with brain anomalies from mothers with confirmed ZIKV infection [53].
Ocular pathologies were found bilaterally in 34% of the cases. The most frequently reported were macular atrophy, pigment mottling (63%), optic nerve anomalies (such as hypoplasia with double ring sign) (11%), lacunar maculopathy (6.9%), foveal reflex loss, and increased cup-disk ratio [31, 43, 54, 55]. Additionally, Yepez et al. [54] recently reported congenital glaucoma, corneal clouding, buphthalmos, increased intraocular pressure, and the clinical triad of epiphora, blepharospasm, and photophobia in infants with microcephaly.
A recent study demonstrated that 13% of 103 intrauterine cases with ZIKV infection had cardiac anomalies such as ostium secundum atrial septal defect (in 5 of 14 cases), small apical ventricular septal defect (in 8 cases), and large ventricular septal defect (in 1 case) [56].
The clinical features of congenital Zika syndrome have been summarized in Table 1.
Table 1.
Clinical Features of ZIKV* infection
| ZIKV Infection | Complications of ZIKV Infection | Congenital Zika Syndrome |
|---|---|---|
| Low-grade fever Pruritic maculopapular rash Periorbital edema Conjunctival hyperemia Headache Sore throat Retro-ocular pain Joint pain Back pain Myalgia Desquamation on hands and feet Transient hearing loss Lymphadenopathies |
Guillain-Barré syndrome Meningoencephalitis Acute disseminated encephalomyelitis (ADEM) Acute myelitis Transverse myelitis |
Irritability, convulsion, clonus, crying, epilepsy, dysphagia, pyramidal and extrapyramidal symptoms, and persistent primitive reflex Brain anomalies: Microcephaly, brain calcification, ventriculomegaly, cerebellar hypoplasia, cortical and subcortical corpus callosum hypoplasia, delayed myelination, enlarged cisterna magna Ocular pathologies: Macular atrophy, pigment mottling, optic nerve anomalies, lacunar maculopathy (6.9%), foveal reflex loss, congenital glaucoma, epiphora, blepharospasm, photophobia, corneal clouding, buphthalmos, increased intraocular pressure Cardiac anomalies: Atrial or ventricular septal defect |
ZIKV: Zika virus; ADEM: acute disseminated encephalomyelitis
Laboratory Features and Diagnosis
Parallel to other viral infections, lymphopenia, neutropenia, thrombocytopenia, and mild elevated liver enzymes and inflammation markers such as CRP were noted as laboratory features of ZIKV infection.
Diagnosis is made by viral culture, PCR, detected specific antigen, antibody (IgM, IgG) with ELISA, or a plaque reduction neutralization test. Acute samples should be tested in 3–10 days. Samples should be stored at 2–8°C if tested in the first 48 hours, at −10°C to −20°C if tested between 48 h and 7 days, and at −70°C if tested later than 7 days [57] (Table 2).
Table 2.
Diagnostic methods and samples in ZIKV infection
| Samples | PCR | IgM by ELISA/IFA |
|---|---|---|
| Serum, CSF | 7 days after symptom onset (may be positive up to the 10th day) | ≥5th day after symptom onset |
| Saliva | First 3–5 days after symptom onset | |
| Urine, semen | 7 days after symptom onset | |
| Placenta, umbilical cord, amniotic fluid | During delivery | |
| Fetal samples | 2 days after delivery |
ZIKV: Zika virus; CSF: cerebrospinal fluid; PCR: polymerase chain reaction; ELISA: enzyme-linked immunosorbent assay; IFA: indirect fluorescent antibody
IgM might be negative during the acute phase, so antigen and PCR might be useful at this phase [44, 58]. After 7 days, the onset of viremia symptoms decreases, so serological methods are preferred at this period. Neutralizing antibody occurs later after the end of the first week, usually after the 4th day. Thus IgM might be negative in the first week. IgM positivity lasts for 2–12 weeks [57]. False-positive results were detected by serological assay in people vaccinated with yellow fever and Japanese encephalitis virus. Additionally, cross reactivity in other Flavivirus infections such as dengue are a major problem for ZIKV serological test, so the usefulness of these tests is limited [2, 44]. When the ZIKV neutralizing antibody titer is more 4 times the titer of antibodies against dengue, ZIKV diagnosis is confirmed; if it is less than 4 times the titer of antibodies against dengue, the result is interpreted as intermediate [57].
The diagnosis of congenital infection was made with PCR positivity in fetal samples such as blood, cerebrospinal fluid, cord blood, amniotic fluid, and placenta [57]. For cases in which ZIKV infection occurred in the early period of pregnancy, PCR results were found to be negative because the virus was not present. In this situation, IgM screening in infant samples by ELISA is recommended [57].
Radiologic Image Findings of Congenital Zika Syndrome
Abnormal brain development, microcephaly (85%), brain calcification between the cortical and subcortical areas, ventriculomegaly, cerebellar hypoplasia, cortical (mostly the frontal lobe)/subcortical atrophy, craniofacial disproportion (95.8%), corpus callosum hypoplasia, delayed myelination, and enlarged cisterna magna have been reported as brain development defects [51, 59]. These findings were symmetric in 75% of the patients [59].
Treatment
Zika virus infection usually results in a mild disease, and it does not require specific therapy. Bed rest, drinking fluids, and fever and pain therapy are sufficient. If a patient shows severe symptoms, he/she should be admitted to a hospital.
A new drug that is active against ZIKV is needed. The US National Institute of Health (NIH) has a program that aims to develop a broad-spectrum antiviral drug against all flaviviruses such as dengue and yellow fever [12]. Sixty antiviral drugs were investigated, and 15 of them were found to be active against ZIKV [60]. Recently, it has been suggested that sofosbuvir, which is used in HCV treatment, is used as a second-line drug for ZIKV [61]. Nucleoside inhibitors are under investigation [62].
In an experimental model, Type 1 interferons have been successfully used to treat ZIKV-disseminated infection, including orchitis, in an immunosuppressed mouse [63].
Prevention
Vector control
Both vector control and personal protection are essential to prevent ZIKV infection. Aerial spraying of insecticides should be carried out 400 m around a city or relevant locations [63, 64]. The number of adult and larval mosquitoes can be reduced by repeated aerial sprays [65]. Breeding sites of mosquitoes such as construction sites or junk car lots should be removed [66].
Other vector control strategies consist of biological control of Aedes by Wolbachia bacteria to reduce the number of larvae by modifying genes and sterile insect technologies. However, the long-term results of these are not still clear [12].
Personal protection
People living in and traveling to endemic areas should be informed about both the ZIKV infection and protective measures. In particular, pregnant women should be protected. Personal protection from the transmission of ZIKV should aim to prevent mosquito bites. For such protection, it is necessary to wear pale-colored clothes, to avoid sleeping outside, to close doors and windows or use physical barriers, and to use repellents (such as N,N-diethyl-meta-toluamide: DEET) [66]. DEET (20% DEET for pregnant women and 10% DEET for children under 2 years), picaridine, IR3535, oil of lemon eucalyptus, para-menthane-diol, and 2-undecanone have been approved by the Environmental Protection Agency to be safe for use in pregnant and breast-feeding women [66]. Lemon eucalyptus and para-menthane-diol should not be used in children younger than 3 years. No insecticide should be used for those younger than 2 months [65].
People living in or traveling to endemic areas should cover their arms and legs with dressing. Additionally, people travelling back from endemic to non-endemic areas should use repellents for 2–3 weeks to prevent the transmission of ZIKV to non-infected mosquitoes [66]. To prevent the sexual transmission of ZIKV, condom usage is recommended [65].
UVA light inactivation and amotosalen (a photochemical treatment using a psoralen)-inactivation processes may reduce the ZIKV load in plasma, so these techniques can be used to prevent virus transmission through blood trans-fusion [43].
Vaccine
A vaccine is an important and emergent requirement for the prevention of ZIKV. There are many researchers who have been conducting studies for developing a vaccine [67]. The NIH is investigating a DNA vaccine, and 18 companies are conducting various studies on live, live attenuated, and inactivated vaccines. Kim et al. [68] have successfully developed an adenoviral-based vaccine and a subunit vaccine against the envelope (E) antigen. They suggested that the ZIKV E antigen vaccine is a promising candidate [68]. Larocca et al. [69] have developed a vaccine that is an inactive subunit E antigen plasmid DNA vaccine, and they found that single-dose immunization provides protection against ZIKV. Shan et al. [70] have reported a safe and effective live attenuated vaccine.
Studies concerning the development of vaccines should progress faster in order to meet public needs [12].
Conclusion
Zika virus is an important public health problem. In recent years, ZIKV has spread throughout a wide geographic area, and it affects large numbers of people. Public health authorities have to estimate the future direction of this global threat. .They should be monitored spread of ZIKV in order to prepare for new outbreaks and economical burden of the disease. Rapid diagnostic and screening tests and vaccine and drug development studies must be encouraged. Public education of both residents of the endemic area and travelers must be continuous.
Footnotes
Peer-review: Externally peer-reviewed.
Author contributions: Concept - Z.O., E.T.; Design -Z.O., E.T.; Supervision - Z.O., E.T.; Resource - Z.O., E.T.; Materials - Z.O., E.T.; Data Collection and/or Processing - Z.O., E.T.; Analysis and /or Interpretation - Z.O., E.T.; Literature Search - Z.O., E.T.; Writing - Z.O., E.T.; Critical Reviews - Z.O., E.T.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Dick GW, Kitchen SF, Haddow AJ. Zika virus. I. Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–20. doi: 10.1016/0035-9203(52)90042-4. https://doi.org/10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 2.Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N Engl J Med. 2009;360:2536–43. doi: 10.1056/NEJMoa0805715. https://doi.org/10.1056/NEJMoa0805715. [DOI] [PubMed] [Google Scholar]
- 3.Musso D, Nhan T, Robin E, et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill. 2014:19. doi: 10.2807/1560-7917.es2014.19.14.20761. https://doi.org/10.2807/1560-7917.es2014.19.14.20761. [DOI] [PubMed] [Google Scholar]
- 4.Cao-Lormeau VM, Roche C, Teissier A, et al. Zika virus, French polynesia, South pacific, 2013. Emerg Infect Dis. 2014;20:1085–6. doi: 10.3201/eid2006.140138. https://doi.org/10.3201/eid2006.140138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zanluca C, Melo VC, Mosimann AL, Santos GI, Santos CN, Luz K. First report of autochthonous transmission of Zika virus in Brazil. Mem Inst Oswaldo Cruz. 2015;110:569–72. doi: 10.1590/0074-02760150192. https://doi.org/10.1590/0074-02760150192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization; Zika Virus Microcephaly Guillain-Barré Syndrome. Situation Report. 3 November 2016. Available at: http://apps.who.int/iris/bitstream/10665/250724/1/zikasitrep3Nov16-eng.pdf. [Google Scholar]
- 7.Haddow AD, Nasar F, Guzman H, et al. Genetic Characterization of Spondweni and Zika Viruses and Susceptibility of Geographically Distinct Strains of Aedes aegypti, Aedes albopictus and Culex quinquefasciatus (Diptera: Culicidae) to Spondweni Virus. PLoS Negl Trop Dis. 2016;10:e0005083. doi: 10.1371/journal.pntd.0005083. https://doi.org/10.1371/journal.pntd.0005083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kleber de Oliveira W, Cortez-Escalante J, De Oliveira WT, et al. Increase in reported prevalence of microcephaly in infants born to women living in areas with confirmed Zika virus transmission during the first trimester of pregnancy - Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:242–7. doi: 10.15585/mmwr.mm6509e2. https://doi.org/10.15585/mmwr.mm6509e2. [DOI] [PubMed] [Google Scholar]
- 9.França GV, Schuler-Faccini L, Oliveira WK, et al. Congenital Zika virus syndrome in Brazil: a case series of the first 1501 livebirths with complete investigation. Lancet. 2016;388:891–7. doi: 10.1016/S0140-6736(16)30902-3. https://doi.org/10.1016/S0140-6736(16)30902-3. [DOI] [PubMed] [Google Scholar]
- 10.Calvet G, Aguiar RS, Melo AS, et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis. 2016;16:653–60. doi: 10.1016/S1473-3099(16)00095-5. https://doi.org/10.1016/S1473-3099(16)00095-5. [DOI] [PubMed] [Google Scholar]
- 11.Schuler-Faccini L, Ribeiro EM, Feitosa IM, et al. Possible association between Zika virus infection and microcephaly - Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:59–62. doi: 10.15585/mmwr.mm6503e2. https://doi.org/10.15585/mmwr.mm6503e2er. [DOI] [PubMed] [Google Scholar]
- 12.Possas C, Brasil P, Marzochi MC, et al. Zika puzzle in Brazil: peculiar conditions of viral introduction and dissemination - A Review. Mem Inst Oswaldo Cruz. 2017;112:319–327. doi: 10.1590/0074-02760160510. https://doi.org/10.1590/0074-02760160510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oehler E, Watrin L, Larre P, et al. Zika virus infection complicated by Guillain-Barre syndrome--case report, French Polynesia, December 2013. Euro Surveill. 2014:19. doi: 10.2807/1560-7917.es2014.19.9.20720. https://doi.org/10.2807/1560-7917.es2014.19.9.20720. [DOI] [PubMed] [Google Scholar]
- 14.Watrin L, Ghawché F, Larre P, Neau JP, Mathis S, Fournier E. Guillain-Barre‘ Syndrome (42 Cases) occurring during a Zika virus outbreak in French polynesia. Medicine (Baltimore) 2016;95:e3257. doi: 10.1097/MD.0000000000003257. https://doi.org/10.1097/MD.0000000000003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cragan JD, Mai CT, Petersen EE, et al. Baseline prevalence of birth defects associated with congenital zika virus infection - Massachusetts, North Carolina, and Atlanta, Georgia, 2013–2014. MMWR Morb Mortal Wkly Rep. 2017;66:219–22. doi: 10.15585/mmwr.mm6608a4. https://doi.org/10.15585/mmwr.mm6608a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization WHO statement on the first meeting of the International Health Regulations (2005) (IHR 2005) Emergency Committee on Zika virus and observed increase in neurological disorders and neonatal malformations;; 2016. Available at: http://www.who.int/mediacentre/news/statements/2016/1st-emergency-committee-zika/en/. [Google Scholar]
- 17.Sharma A, Lal SK. Zika Virus: Transmission, detection, control, and prevention. Front Microbiol. 2017;8:110. doi: 10.3389/fmicb.2017.00110. https://doi.org/10.3389/fmicb.2017.00110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gaskell KM, Houlihan C, Nastouli E, Checkley AM. Persistent Zika Virus detection in semen in a traveler returning to the United Kingdom from Brazil, 2016. Emerg Infect Dis. 2017;23:137–9. doi: 10.3201/eid2301.161300. https://doi.org/10.3201/eid2301.161300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li XF, Dong HL, Huang XY, et al. Characterization of a 2016 Clinical Isolate of Zika Virus in Non-human Primates. EBioMedicine. 2016;12:170–7. doi: 10.1016/j.ebiom.2016.09.022. https://doi.org/10.1016/j.ebiom.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marano G, Pupella S, Vaglio S, Liumbruno GM, Grazzini G. Zika virus and the never-ending story of emerging pathogens and transfusion medicine. Blood Transfus. 2016;14:95–100. doi: 10.2450/2015.0066-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected Female-to-Male Sexual Transmission of Zika Virus - New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:716–7. doi: 10.15585/mmwr.mm6528e2. https://doi.org/10.15585/mmwr.mm6528e2. [DOI] [PubMed] [Google Scholar]
- 22.Frank C, Cadar D, Schlaphof A, et al. Sexual transmission of Zika virus in Germany, April 2016. Euro Surveill. 2016:21. doi: 10.2807/1560-7917.ES.2016.21.23.30252. https://doi.org/10.2807/1560-7917.es.2016.21.23.30252. [DOI] [PubMed] [Google Scholar]
- 23.Hills SL, Russell K, Hennessey M, et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission - Continental United States, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:215–6. doi: 10.15585/mmwr.mm6508e2. https://doi.org/10.15585/mmwr.mm6508e2er. [DOI] [PubMed] [Google Scholar]
- 24.Bonaldo MC, Ribeiro IP, Lima NS, et al. Isolation of infective Zika virus from urine and saliva of patients in Brazil. PLoS Negl Trop Dis. 2016;10:e0004816. doi: 10.1371/journal.pntd.0004816. https://doi.org/10.1371/journal.pntd.0004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mansuy JM, Suberbielle E, Chapuy-Regaud S, et al. Zika virus in semen and spermatozoa. Lancet Infect Dis. 2016;16:1106–7. doi: 10.1016/S1473-3099(16)30336-X. https://doi.org/10.1016/S1473-3099(16)30336-X. [DOI] [PubMed] [Google Scholar]
- 26.Murray KO, Gorchakov R, Carlson AR, et al. Prolonged detection of Zika virus in vaginal secretions and whole blood. Emerg Infect Dis. 2017;23:99–101. doi: 10.3201/eid2301.161394. https://doi.org/10.3201/eid2301.161394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Atkinson B, Thorburn F, Petridou C, et al. Presence and persistence of Zika virus RNA in semen, United Kingdom, 2016. Emerg Infect Dis. 2017;23:611–5. doi: 10.3201/eid2304.161692. https://doi.org/10.3201/eid2304.161692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Colt S, Garcia-Casal MN, Peña-Rosas JP, et al. Transmission of Zika virus through breast milk and other breastfeeding-related bodily-fluids: A systematic review. PLoS Negl Trop Dis. 2017;11:e0005528. doi: 10.1371/journal.pntd.0005528. https://doi.org/10.1371/journal.pntd.0005528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jaenisch T, Rosenberger KD, Brito C, Brady O, Brasil P, Marques ET. Risk of microcephaly after Zika virus infection in Brazil, 2015 to 2016. Bull World Health Organ. 2017;95:191–8. doi: 10.2471/BLT.16.178608. https://doi.org/10.2471/BLT.16.178608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu J, Huang DY, Ma JT, Ma YH, Hu YF. Available evidence of association between Zika virus and microcephaly. Chin Med J (Engl) 2016;129:2347–56. doi: 10.4103/0366-6999.190672. https://doi.org/10.4103/0366-6999.190672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ventura CV, Maia M, Ventura BV, et al. Ophthalmological findings in infants with microcephaly and presumable intra-uterus Zika virus infection. Arq Bras Oftalmol. 2016;79:1–3. doi: 10.5935/0004-2749.20160002. https://doi.org/10.5935/0004-2749.20160002. [DOI] [PubMed] [Google Scholar]
- 32.Martines RB, Bhatnagar J, Keating MK, et al. Notes from the Field: Evidence of Zika virus infection in brain and placental tissues from two congenitally infected newborns and two fetal losses--Brazil, 2015. MMWR Morb Mortal Wkly Rep. 2016;65:159–60. doi: 10.15585/mmwr.mm6506e1. https://doi.org/10.15585/mmwr.mm6506e1. [DOI] [PubMed] [Google Scholar]
- 33.Cordeiro MT, Brito CA, Pena LJ, et al. Results of a Zika Virus (ZIKV) Immunoglobulin M-specific diagnostic assay are highly correlated with detection of neutralizing anti-ZIKV antibodies in neonates with congenital disease. J Infect Dis. 2016;214:1897–1904. doi: 10.1093/infdis/jiw477. https://doi.org/10.1093/infdis/jiw477. [DOI] [PubMed] [Google Scholar]
- 34.Meneses JD, Ishigami AC, de Mello LM, Albuquerque LL, Brito CA, Tenório Cordeiro M, Pena LJ. Lessons learned at the epicenter of Brazil’s congenital Zika epidemic: evidence from 87 confirmed cases. Clin Infect Dis. 2017;64:1302–8. doi: 10.1093/cid/cix166. https://doi.org/10.1093/cid/cix166. [DOI] [PubMed] [Google Scholar]
- 35.Hughes BW, Addanki KC, Sriskanda AN, McLean E, Bagasra O. Infectivity of immature neurons to Zika virus: a link to congenital Zika syndrome. EBioMedicine. 2016;10:65–70. doi: 10.1016/j.ebiom.2016.06.026. https://doi.org/10.1016/j.ebiom.2016.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhatnagar J, Rabeneck DB, Martines RB, et al. Zika virus RNA replication and persistence in brain and placental tissue. Emerg Infect Dis. 2017;23:405–14. doi: 10.3201/eid2303.161499. https://doi.org/10.3201/eid2303.161499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chan JF, Yip CC, Tsang JO, et al. Differential cell line susceptibility to the emerging Zika virus: implications for disease pathogenesis, non-vector-borne human transmission and animal reservoirs. Emerg Microbes Infect. 2016;5:e93. doi: 10.1038/emi.2016.99. https://doi.org/10.1038/emi.2016.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brault JB, Khou C, Basset J, et al. Comparative analysis between flaviviruses reveals specific neural stem cell tropism for zika virus in the mouse developing neocortex. EBioMedicine. 2016;10:71–6. doi: 10.1016/j.ebiom.2016.07.018. https://doi.org/10.1016/j.ebiom.2016.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGrath EL, Rossi SL, Gao J, et al. differential responses of human fetal brain neural stem cells to Zika virus infection. Stem Cell Reports. 2017;8:715–27. doi: 10.1016/j.stemcr.2017.01.008. https://doi.org/10.1016/j.stemcr.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith DR, Hollidge B, Daye S, et al. Neuropathogenesis of Zika virus ina highly susceptible immunocompetent mouse model afterantibody blockade of type I interferon. PLoS Negl Trop Dis. 2017;11:e0005296. doi: 10.1371/journal.pntd.0005296. https://doi.org/10.1371/journal.pntd.0005296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cugola FR, Fernandes IR, Russo FB, et al. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534:267–71. doi: 10.1038/nature18296. https://doi.org/10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vermillion MS, Lei J, Shabi Y, et al. Intrauterine Zika virus infection of pregnant immunocompetent mice models transplacental transmission and adverse perinatal outcomes. Nat Commun. 2017;8:14575. doi: 10.1038/ncomms14575. https://doi.org/10.1038/ncomms14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Passi D, Sharma S, Dutta SR, Ahmed M. Zika Virus Diseases - The new face of an ancient enemy as global public health emergency (2016): Brief review and recent updates. Int J Prev Med. 2017;8:6. doi: 10.4103/2008-7802.199641. https://doi.org/10.4103/2008-7802.199641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Plourde AR, Bloch EM. A literature review of Zika virus. Emerg Infect Dis. 2016;22:1185–92. doi: 10.3201/eid2207.151990. https://doi.org/10.3201/eid2207.151990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Paixão ES, Barreto F, Teixeira Mda G, Costa Mda C, Rodrigues LC. History, epidemiology, and clinical manifestations of Zika: A systematic review. Am J Public Health. 2016;106:606–12. doi: 10.2105/AJPH.2016.303112. https://doi.org/10.2105/AJPH.2016.303112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ioos S, Mallet HP, Leparc Goffart I, Gauthier V, Cardoso T, Herida M. Current Zika virus epidemiology and recent epidemics. Med Mal Infect. 2014;44:302–7. doi: 10.1016/j.medmal.2014.04.008. https://doi.org/10.1016/j.medmal.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Waggoner JJ, Pinsky BA. Zika Virus: Diagnostics for an Emerging Pandemic Threat. J Clin Microbiol. 2016;54:860–7. doi: 10.1128/JCM.00279-16. https://doi.org/10.1128/JCM.00279-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zare Mehrjardi M, Carteaux G, Poretti A, et al. Neuroimaging findings of postnatally acquired Zika virus infection: a pictorial essay. Jpn J Radiol. 2017 Apr 26; doi: 10.1007/s11604-017-0641-z. DOI: https://doi.org/10.1007/s11604-017-0641-z. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 49.Carteaux G, Maquart M, Bedet A, et al. Zika virus associated with meningoencephalitis. N Engl J Med. 2016;374:1595–6. doi: 10.1056/NEJMc1602964. https://doi.org/10.1056/NEJMc1602964. [DOI] [PubMed] [Google Scholar]
- 50.Mécharles S, Herrmann C, Poullain P, et al. Acute myelitis due to Zika virus infection. Lancet. 2016;387:1481. doi: 10.1016/S0140-6736(16)00644-9. https://doi.org/10.1016/S0140-6736(16)00644-9. [DOI] [PubMed] [Google Scholar]
- 51.Moura da Silva AA, Ganz JS, Sousa PD, et al. Early growth and neurologic outcomes of infants with probable congenital zika virus syndrome. Emerg Infect Dis. 2016;22:1953–6. doi: 10.3201/eid2211.160956. https://doi.org/10.3201/eid2211.160956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Friedrich MJ. WHO Calls Off Global Zika Emergency. JAMA. 2017;317:246. doi: 10.1001/jama.2016.20447. https://doi.org/10.1001/jama.2016.19034. [DOI] [PubMed] [Google Scholar]
- 53.Reynolds MR, Jones AM, Petersen EE, et al. Vital Signs: Update on Zika Virus-Associated Birth Defects and Evaluation of All U.S. Infants with Congenital Zika Virus Exposure - U.S. Zika Pregnancy Registry, 2016. MMWR Morb Mortal Wkly Rep. 2017;66:366–73. doi: 10.15585/mmwr.mm6613e1. https://doi.org/10.15585/mmwr.mm6613e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yepez JB, Murati FA, Pettito M, et al. Ophthalmic manifestations of congenital Zika syndrome in Colombia and Venezuela. JAMA Ophthalmol. 2017 doi: 10.1001/jamaophthalmol.2017.0561. https://doi.org/10.1001/jamaophthalmol.2017.0561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ventura LO, Ventura CV, Lawrence L, et al. Visual impairment in children with congenital Zika syndrome. J AAPOS. 2017 doi: 10.1016/j.jaapos.2017.04.003. https://doi.org/10.1016/j.jaapos.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Cavalcanti DD, Alves LV, Furtado GJ, et al. Echocardiographic findings in infants with presumed congenital Zika syndrome: Retrospective case series study. PLoS One. 2017;12:e0175065. doi: 10.1371/journal.pone.0175065. https://doi.org/10.1371/journal.pone.0175065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Demir T, Kılıc S. Zika virus: a new arboviral public health problem. Folia Microbiol. 2016;61:523–7. doi: 10.1007/s12223-016-0467-6. https://doi.org/10.1007/s12223-016-0467-6. [DOI] [PubMed] [Google Scholar]
- 58.Aziz H, Zia A, Anwer A, Aziz M, Fatima S, Faheem M. Zika virus: Global health challenge, threat and current situation. J Med Virol. 2017;89:943–51. doi: 10.1002/jmv.24731. https://doi.org/10.1002/jmv.24731. [DOI] [PubMed] [Google Scholar]
- 59.de Fatima Vasco Aragao M, van der Linden V, Brainer-Lima AM, et al. Clinical features and neuroimaging (CT and MRI) findings in presumed Zika virus related congenital infection and microcephaly: retrospective case series study. BMJ. 2016;353:i1901. doi: 10.1136/bmj.i1901. https://doi.org/10.1136/bmj.i1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zika Virus Treatment. Available at: www.niaid.nih.gov/diseases-conditions/zika-treatment.
- 61.Sacramento CQ, de Melo GR, de Freitas CS, et al. Corrigendum: The clinically approved antiviral drug sofosbuvir inhibits Zika virus replication. Sci Rep. 2017;7:46772. doi: 10.1038/srep46772. https://doi.org/10.1038/srep40920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eyer L, Nencka R, Huvarová I, et al. Nucleoside Inhibitors of Zika Virus. J Infect Dis. 2016;214:707–11. doi: 10.1093/infdis/jiw226. https://doi.org/10.1093/infdis/jiw226. [DOI] [PubMed] [Google Scholar]
- 63.Chan JF, Zhang AJ, Chan CC, et al. Zika virus infection in dexamethasone immunosuppressed mice demonstrating disseminated infection with multiorgan involvement including orchitis effectively treated by recombinant type I interferons. EBioMedicine. 2016;14:112–22. doi: 10.1016/j.ebiom.2016.11.017. https://doi.org/10.1016/j.ebiom.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zika virus and complications: Questions and answers. Available at: http://www.who.int/features/qa/zika/en/.
- 65. Zika Virus Clinical Guidance. Available at: https://www.cdc.gov/zika/hc-providers/clinical-guidance.html.
- 66.Chan JF, Choi GK, Yip CC, Cheng VC, Yuen KY. Zika fever and congenital Zika syndrome: An unexpected emerging arboviral disease. J Infect. 2016;72(5):507–24. doi: 10.1016/j.jinf.2016.02.011. https://doi.org/10.1016/j.jinf.2016.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vannice KS, Giersing BK, Kaslow DC, et al. Meeting Report: WHO consultation on considerations for regulatory expectations of Zika virus vaccines for use during an emergency. Vaccine. 2016 doi: 10.1016/j.vaccine.2016.10.034. https://doi.org/10.1016/j.vaccine.2016.10.034. [DOI] [PubMed] [Google Scholar]
- 68.Kim E, Erdos G, Huang S, Kenniston T, Falo LD, Jr, Gambotto A. Preventative vaccines for zika virus outbreak: preliminary evaluation. EBioMedicine. 2016;13:315–320. doi: 10.1016/j.ebiom.2016.09.028. https://doi.org/10.1016/j.ebiom.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Larocca RA, Abbink P, Peron JP, et al. Vaccine protection against Zika virus from Brazil. Nature. 2016;536:474–8. doi: 10.1038/nature18952. https://doi.org/10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shan C, Muruato AE, Nunes BTD, et al. A live-attenuated Zika virus vaccine candidate induces sterilizing immunity in mouse models. Nat Med. 2017 Apr 10; doi: 10.1038/nm.4322. doi: https://doi.org/10.1038/nm.4322. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]