Abstract
Osteoarthritis is a major cause of disability and there is no current pharmaceutical treatment which can prevent the disease or slow its progression. Dietary advice or supplementation is clearly an attractive option since it has low toxicity and ease of implementation on a population level. We have previously demonstrated that sulforaphane, a dietary isothiocyanate derived from its glucosinolate precursor which is found in broccoli, can prevent cartilage destruction in cells, in in vitro and in vivo models of osteoarthritis. As the next phase of this research, we enrolled 40 patients with knee osteoarthritis undergoing total knee replacement into a proof-of-principle trial. Patients were randomised to either a low or high glucosinolate diet for 14 days prior to surgery. We detected ITCs in the synovial fluid of the high glucosinolate group, but not the low glucosinolate group. This was mirrored by an increase in ITCs and specifically sulforaphane in the plasma. Proteomic analysis of synovial fluid showed significantly distinct profiles between groups with 125 differentially expressed proteins. The functional consequence of this diet will now be tested in a clinical trial.
Introduction
Osteoarthritis (OA) of the hip or knee is ranked as 11th of 291 conditions that contribute to global disability; and the consequent years lived with disability (YLDs) are estimated to have risen by 61% from 1990–20101. There are no disease-modifying OA drugs (DMOADs) currently available, and pharmacological interventions provide symptomatic relief only, which is frequently insufficient2, 3.
The National Institute for Health and Care Excellence (NICE) and the American College of Rheumatology both recommend OA management strategies based upon activity and exercise, and weight loss if overweight or obese. A third core treatment area recommended by NICE focuses on information to support self-management4, 5. Within these core treatment areas, dietary and exercise advice have been identified as cost effective approaches to the treatment of OA6.
We and others have shown previously that sulforaphane (1-isothiocyanato-4-methylsulfinylbutane, SFN) is a chondroprotective agent in vitro and in vivo 7–10. SFN is derived from a precursor, glucoraphanin, gained from eating brassicas, particularly broccoli. Sulforaphane has broad biological activity and its role in joint health was recently reviewed11. SFN can repress the expression of key metalloproteinases implicated in OA7, 10, regulate NF-κB and Nrf2 signalling, inhibit production of prostaglandin E2 and nitric oxide in chondrocytes7, 9, 10 and prevent cytokine-induced cartilage destruction in vitro 9, 10. In vivo, SFN reduces cartilage destruction in the ‘destabilisation of the medial meniscus’ (DMM) murine model of OA10 and inhibits synovial hyperplasia in collagen-induced arthritis (CIA)8. Nrf2 knockout mice also showed increased cartilage lesions and oxidative damage in antibody-induced arthritis (AIA)12.
The pharmacokinetics of SFN has been studied, with one report measuring the average peak plasma concentration of SFN (2.2 µM) approximately 2 hours following consumption of 100 g of broccoli florets prepared as a soup. This increased to 7.3 µM following consumption of a high glucoraphanin variety of broccoli13. There is no evidence that normal dietary intake of broccoli is associated with toxicity, even in the long term. SFN has also been shown to accumulate intracellularly via conjugation to glutathione giving millimolar intracellular concentrations in a cell-type dependent manner14. We showed an accumulation of SFN-glutathione (SFN-GSH) in primary chondrocytes of >10-fold and >100-fold in the SW1353 cell line10. There are no publications describing the detection of dietary isothiocyanates (ITCs) in the knee joint.
The aim of this study was firstly to determine whether ITCs could be detected in the synovial fluid of OA patients following a glucosinolate (broccoli)-rich diet and secondly to identify changes in the joint tissues.
Patients and Methods
Participants
Osteoarthritis patients referred for total knee replacement (TKR) to the Norfolk and Norwich University Hospital (NNUH), Norwich, UK, were screened for recruitment onto the study using the eligibility criteria in Table 1. Eligible patients were male or post-menopausal females scheduled for a TKR willing to comply with the dietary intervention. This study was approved by the National Research Ethics Service Committee East of England, Cambridge South, UK (approval number 2012ORTH06L (104-07-12)). All patients gave informed written consent. All methods were performed in accordance with relevant laboratory guidelines and institutional regulations.
Table 1.
Eligibility for study screening criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| Males and post-menopausal females | Those with known allergies to the dietary compounds/foods/commercially available supplements |
| OA patient scheduled for total knee replacement | Those unprepared to adhere to dietary restrictions during the trial. i.e. wash out period, consumption of standardised food/commercial supplement |
| Radiological evidence of some cartilage tissue remaining in the joint to be replaced | Those unwilling to consent to blood sampling or, use of their joint tissues for analysis |
| Normal biochemical, haematological or urinary assessment as determined by clinical assessor. Full blood count | Participation in another dietary intervention trial running concurrently. |
| Willingness to comply with dietary requirements | Those taking other food supplements unless they are prepared to abstain during the trial. |
| Abnormal biochemical, haematological or urinary assessment indicating abnormal renal or liver function. | |
| Smokers or those ceasing <3 mths ago | |
| Pre-menopausal women | |
| Rheumatoid arthritis, inflammation or sepsis | |
| Patients taking warfarin medication |
Patients scheduled for total knee replacement due to osteoarthritis were screened for eligibility to participate in the study. The inclusion and exclusion criteria are shown. Low glucosinolate diet n = 20, high glucosinolate diet n = 20.
Study design
This was a proof-of-principle; randomised controlled trial designed to test whether bioactive molecules derived from broccoli, eaten prior to joint replacement, can be detected in the articular joint and mediate changes.
Patients followed a 7-day washout period, (no consumption of broccoli, cabbage, cauliflower, arugula (rocket), canola/rapeseed, Brussels sprouts, radish, horseradish, kale, turnip root, greens, watercress, pak choi, collard greens, rutabaga (swede) vegetables). The washout diet was either continued (low glucosinolate group) or patients consumed 100 g of a high glucosinolate broccoli15 per day for 14 days prior to TKR (high glucosinolate group). Patients were allocated to a high or low glucosinolate group by block randomisation (blocks of five). The trial nurses screened and recruited patients and were blinded to the pre-allocated study numbers. Throughout the study, patients had telephone access to a trial nurse for advice and received a telephone call to monitor compliance.
Blood was sampled at the end of the washout period (baseline) and collected into K2EDTA. Patients allocated to the high glucosinolate group were given written instructions, a vegetable steamer and a demonstration for preparation of the broccoli. All patients were given a validated 7-day food diary with guidance for completion. A second blood sample was taken on the day of TKR surgery. Synovial fluid, cartilage and fat tissues were obtained at surgery. Synovial fluid was aspirated into a sterile, pre-chilled container immediately prior to surgery, taking care to avoid blood contamination. Cartilage and fat tissues were removed as routine for TKR and immediately placed into separate containers with pre-chilled, sterile phosphate buffered saline (PBS). All tissue samples were transported at 4 °C in sterile conditions and processed or stored within 1 hour. Synovial fluid was centrifuged at 200 g, 5 minutes at 4 °C and the supernatants stored at −80 °C. Undamaged cartilage was dissected at full thickness from the bone, and both cartilage and fat were snap frozen and stored at −80 °C for RNA extraction, or placed directly into culture for in vitro degradation analysis.
Blood plasma and synovial fluid were analysed for ITC levels. Analyses of synovial fluid proteomics, in vitro cartilage degradation, joint tissue gene expression profiling and GST genotyping were performed.
Isothiocyanate detection
Isothiocyanates were detected as described previously13, 15–17.
Isothiocyanate detection: Broccoli
Isothiocyanates were detected as described15, 16. No vicinal dithiol contamination of equipment or materials was detected (data not shown). Control samples contained neither broccoli extracts nor 1,2-benzenedithiole (1,2 BDT). High glucosinolate frozen broccoli (100 g) was steamed for 6 mins and blended (0.5 g/ml) in phosphate buffer (33 mM potassium phosphate, 500 µM ascorbic acid, 1 mM EDTA). The broccoli blend was diluted 1:1.5 in distilled water and glucosinolates were hydrolysed using thioglucosidase, 0.05 U/ml (Sigma T4528) at room temperature, 2 hours. The blend was clarified by centrifugation at 3000 g, 5 min and the supernatant was filtered through Whatman no. 41 filter paper, pre-soaked in phosphate buffer. Extraction was repeated three times and pooled. A cyclocondensation reaction of 1,2 BDT and broccoli extracts to 1,3 benzodithiole-2-thione (1,3-BDT) was performed at 65 °C for 2 hours in the dark: the broccoli extract was diluted 1:9 in 100 mM potassium phosphate buffer, pH8.5 and added 1:1 to 8 mM 1,2 BDT dissolved in 100% v/v methanol. The 1,3-benzodithiole-2-thione product was detected at 365 nm.
Isothiocyanate detection: Plasma
Plasma was deproteinised with a 6% w/v final concentration of polyethylene glycol (PEG) 8000 for 10 mins, at 4 °C, in the dark. Plasma was centrifuged 20 000 g, 5 mins, 4 °C. Supernatant was diluted 1:1 in 100 mM potassium phosphate buffer, pH8.5 with 2x volumes 20 mM 1,2 BDT. Samples were briefly vortexed and then incubated at 65 °C, 2 hours in the dark, followed by centrifugation at 20 000 g for 6 mins, 4 °C. Supernatants were diluted 1:1 in distilled water and purified using solid phase extraction (SPE, C18 Bond Elut, Agilent). The SPE column was activated with 1x vol 100% v/v acetonitrile, 2x vol 25% v/v acetonitile and washed with 2x vols distilled water. Samples were washed x3 using 30% v/v propan-2-ol and eluted with 100% v/v acetonitrile. Reversed phase HPLC was carried out using a Luna® C18 (2) 100 Å, LC Column (5 µm, 150 × 4.60 mm, Phenomenex) at 37 °C using an isocratic mobile phase of 80% v/v methanol, 20% v/v water at a flow rate of 1 ml/min. 1,3 BDT was detected using a SPD-M2OA Prominence HPLC photo diode array detector (Shimadzu) at 365 nm. Sensitivity of the assay in plasma was 6 pmols.
Sulforaphane detection: Plasma
Plasma samples were analysed for SFN and its conjugates using a method that has been described previously [13] with slight modifications. Briefly plasma samples (100 µl) were prepared by adding 20 µl precooled (4 °C) trichloroacetic acid followed by centrifugation at 11 600 g at 4 °C for 10 min. The injection volume was 5 µl, the HPLC column was a Luna (3 µm particle size, 100 × 2 mm from Phenomenex) using mobile phase flow 0.25 ml/min, the mobile phase were consisted of ammonium acetate buffer (13 mmol/L, pH4; solvent A) and acetonitrile plus 0.1% acetic acid (solvent B) in a linear gradient from 5% B to 35% B over 5 min with 6 min re equilibration time. Agilent 6490 Mass spectrometry analysis was performed with the use of electrospray ionization in positive ion mode with nitrogen gas and nitrogen sheath gas temperatures 160 °C and 400 °C, respectively, gas flow 16 l/min, Nebulizer pressure 30 psi, sheath gas flow 12 l/min and capillary voltage 4000 V.
Isothiocyanate detection: Synovial fluid
Synovial fluid was diluted in water if necessary and processed as for plasma with the inclusion of at least 10 mins centrifugation following the deproteinisation step. Sensitivity of the assay in synovial fluid was 3.8 pmols.
Proteomic analysis of synovial fluid
A subset of 18 synovial fluid samples were prepared as described previously18. Briefly, samples were hyaluronidase treated, filtered and total protein quantified using Bradford reagent. Synovial fluid samples containing 6 mg total protein were enriched for low abundance proteins using ProteoMiner™ Small Capacity kit (BioRad) according to the manufacturer’s instructions and analysed by SDS-PAGE.
For LC-MS/MS, samples were trypsin digested and loaded on a 2 hour gradient LC-MS/MS analysis using a NanoAcquity™ ultra-performance LC (Waters) and LTQ-Orbitrap-Velos (Thermo-Fisher Scientific)19. One sample was excluded for technical reasons (blebbing).
Progenesis™ LC-MS software (Waters, Manchester, UK) was used for label-free quantification18. Data were searched using Mascot against the Unihuman Reviewed database. Peptide matches above an identity threshold were adjusted to give a false discovery rate (FDR) of 1% before the protein identifications being re-imported into Progenesis-QI™. Adjusted ANOVA values of p < 0.05 and regulation of >2-fold were regarded as significant. Neopeptides were identified as previously described adjusted to 1% FDR20.
In vitro cartilage degradation
Cartilage was dissected into ~5 mm3 chips and cultured overnight in quadruplicate in Dulbecco’s modified Eagle’s medium (DMEM; GlutaMAX) supplemented with 1,000 IU/ml penicillin, and 100 µg/ml streptomycin at 37 °C, 5% v/v CO2. Culture media were refreshed at day 1 and day 7, with an overall culture of 14 days. Remaining cartilage was papain digested. Culture media and digested cartilage were analysed for glycosaminoglycan and hydroxyproline content as described21, 22.
Gene expression analysis
Cartilage (200 mg) was crushed under liquid nitrogen to a fine powder and total RNA was extracted using TRIzol® reagent (Invitrogen) and RNeasy mini kit (Qiagen), as described23, 24.
Approximately 100 mg of fat tissue was homogenised in 600 µl RLT buffer (Qiagen) using the TissueLyser LT (Qiagen) and spherical ball bearings. Total RNA was extracted using the RNeasy mini kit (Qiagen) according to the manufacturer’s instructions.
RNA was quantified using the NanoDrop 2000 spectrophotometer (Thermo Scientific) and the quality assessed using the Experion RNA StdSens analysis kit (Bio-Rad). cDNA was synthesised using 500 ng of RNA and Superscript II reverse transcriptase (Invitrogen) with random hexamers according to the manufacturer’s instructions.
Relative quantification of gene expression was measured using the 7900HT Fast Real Time PCR System (Applied Biosystems) and a custom designed Taqman Low Density Array (TLDA). Genes were selected by analysis of microarray data from SFN treated SW1353 cells, primary human articular chondrocytes and human articular cartilage explants (data not shown). Assay details are included in Supplementary Table S1. Cycling conditions were 50 °C, 20 s, 95 °C, 10 min, 95 °C, 15 s, and 60 °C, 1 min × 40 cycles. Data were analysed using DataAssist Software v3.01 (Life Technologies), normalised to 18 S rRNA and expressed as 2−ΔCt.
Biomarkers
Plasma samples were analysed for Coll 2-1, (a peptide from the triple helix of type II collagen) using ELISA25, and hsCRP (high sensitivity C-reactive protein) using a particle-enhanced immunoturbidimetric assay. Analyses were performed by the Bioanalytical Facility (BAF) at the University of East Anglia. Synovial fluid samples were analysed for cytokine protein expression using the Cytometric Bead Array Flex Set (BD Biosciences) and was performed by Flow Cytometry Services, University of East Anglia.
Diet diary
All patients completed a validated 7-day food diary26. Dietary data was analysed using WISP V4.0 (Tinuviel Software).
Genotyping
DNA was extracted from plasma using QIAamp DNA Blood Mini Kit (Qiagen) according to manufacturer’s instructions. Copy number variation for members of the glutathione S-transferase superfamily, (GSTP1, GSTM1 and GSTT1) was detected using TaqMan® Copy Number Assays (Life Technologies cat: Hs01541162_cn, Hs02575461_cn, Hs00010004) and analysed using CopyCaller® software v2.0 (Life Technologies).
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Statistical testing
High and low glucosinolate groups were compared using unpaired t-test (two tailed) and when applied, false discovery rate (FDR) was calculated using the Benjamini-Hochberg FDR method. It was noted that within the plasma samples of the low glucosinolate group, all except five samples of the post intervention samples had undetectable levels of SFN. Undetected points were treated as zero for the comparisons of plasma ITCs or SFN, and synovial fluid ITC. Since ITCs were not detected in the low glucosinolate group of the synovial fluid samples, no statistical testing was performed. Differences observed between groups remains the same and conclusions are also identifical using ‘limit of detection’ values instead of zero. All data was analysed using GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego California USA, unless otherwise stated in the methods. Graphpad Prism calculated descriptive statistics to include confidence intervals and normality testing (D’Agostino & Pearson omnibus normality test). Data with non-Gaussian distribution were transformed prior to further statistical testing. A p value < 0.05 was considered significant.
Ethics approval
This study protocol was approved by the NRES Committee East of England, Cambridge South, UK.
Results
Patients and baseline characteristics
A total of 268 patients were screened for eligibility where 143 (53%) were eligible, of which 40 patients (15%) were recruited. A flow diagram to show the process of recruitment and parallel randomisation of the trial is shown in Fig. 1, and more details are given in Supplementary Table S2. Drug use was not recorded. Patient characteristics were males n = 17, (low glucosinolate 7, high glucosinolate 10,), females n = 20 (low glucosinolate 13, high glucosinolate 7). Low glucosinolate group median age 71 (range 50–89), high glucosinolate group median age 73 (range 58–84).
Figure 1.
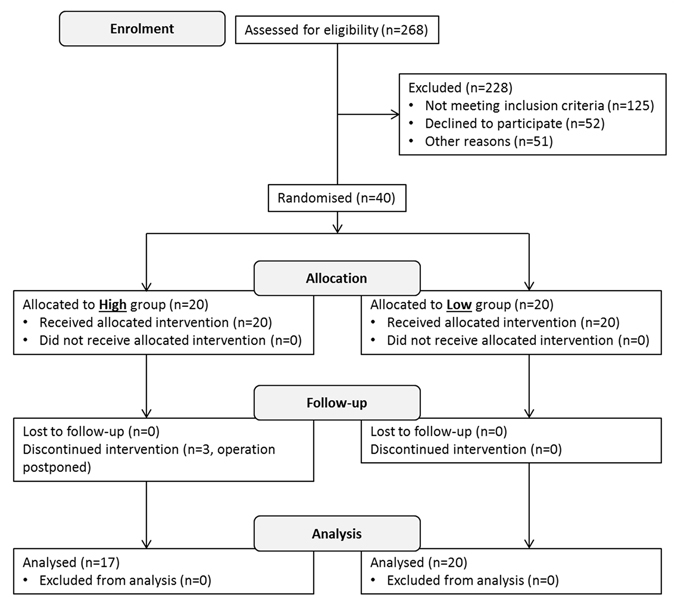
A flow diagram to show the process of enrolment and parallel randomisation for the primary outcome.
Isothiocyanate levels in plasma and synovial fluid
Total ITCs measured in the high glucosinolate broccoli were 1.8 µmols g−1, so patients consumed 180 µmols per day. Patients in the high glucosinolate group attained greater increases in plasma total ITC concentrations between baseline and post-intervention: high glucosinolate group (mean fold change: 2.20, 95% CI: 1.45 to 2.92) compared to the low glucosinolate group (mean fold change: 0.90, 95% CI: 0.68 to 1.12), p < 0.0001 (Table 2). This confirmed compliance with the intervention. Sulforaphane was also significantly increased in the plasma of the high glucosinolate group (mean change: 0.32, 95% CI: 0.20 to 0.44) compared to low glucosinolate (mean change: 0.02, 95% CI: −0.01 to 0.04), p < 0.0001 (Table 2); since SFN in many post-intervention samples in the low glucosinolate group was undetected and therefore treated as zero, fold change cannot be calculated. Importantly, ITCs were detected in the synovial fluids of the high glucosinolate group (mean 496.6 nM, 95% CI: 419.1 to 574.2) but not in the low glucosinolate group (Table 2). Mean total ITCs and SFN measured in the plasma of the high glucosinolate group (post intervention) were 2.22 µM, 95% CI: 1.19 to 3.24 and 0.32 µM, 95% CI: 0.20 to 0.44 respectively) (Table 2). Data do not segregate on the basis of gender.
Table 2.
Isothiocyanate levels in synovial fluid and plasma.
| Outcome | Low glucosinolate Mean (95%CI) | High glucosinolate Mean (95%CI) | p-value |
|---|---|---|---|
| ITC synovial fluid (nM) | n.d | 496.6 (419.10 to 574.20) | — |
| ITC plasma (fold change) | 0.90 (0.68 to 1.12) | 2.20 (1.45 to 2.92) | <0.0001 |
| SFN plasma (change) | 0.02 (−0.01 to 0.04) | 0.32 (0.2 to 0.44) | <0.0001 |
| ITC plasma (µM) baseline | 1.71 (1.10 to 2.33) | 1.17 (0.69 to 1.64) | — |
| ITC plasma (µM) post intervention | 1.36 (0.86 to 1.87) | 2.22 (1.19 to 3.24) | — |
| SFN plasma (µM) baseline | n.d | 0.004 (−0.004 to 0.012 | — |
| SFN plasma (µM) post intervention | 0.02 (−0.005 to 0.04) | 0.32 (0.20 to 0.44) | — |
Total isothiocyanates (ITCs) measured in synovial fluid and plasma, and the specific metabolite sulforaphane (SFN) measured in plasma from low and high glucosinolate groups. Fold change: post intervention/baseline, Change: post intervention – baseline, low glucosinolate n = 20, high glucosinolate n = 17, n.d – not detected. Mean values with 95% confidence intervals and p-values are given.
Proteomics
Peptides identified by LC-MS/MS in the synovial fluid samples (low glucosinolate n = 8, high glucosinolate n = 9) were analysed using Progenesis™ QI software (Waters, Manchester, UK). 382 quantifiable proteins were identified, 348 with >1 peptide. One hundred and twenty-five proteins were identified with >1 peptide and differentially expressed between high and low glucosinolate diets (p < 0.05, 1% FDR). An FDR of 5% revealed 29 differentially expressed proteins identified by >1 peptide with a minimum fold change of >2. In the high glucosinolate group 18 proteins had decreased expression whilst 11 proteins were increased compared to the low glucosinolate group. Twelve proteins are commonly found in plasma, 10 of which were decreased in the high glucosinolate group (Table 3). Aside from common plasma proteins, the top four differentially expressed proteins, were neuroblastoma suppressor of tumorigenicity 1 (NBL1), histidine protein methyltransferase 1 homolog (METTL18), calreticulin (CALR) and SWI/SNF complex subunit SMARCC1 (SMARCC1) each p < 0.0001.
Table 3.
Differentially expressed proteins identified in synovial fluid.
| Description | Accession | Peptide count | Unique peptides | q-value | Mean FC (H/L) | Ref |
|---|---|---|---|---|---|---|
| Neuroblastoma suppressor of tumorigenicity 1 (NBL1) | P41271 | 2 | 2 | 1.26E-07 | 0.33 | 34 |
| Histidine protein methyltransferase 1 homolog (METTL18) | O95568 | 2 | 1 | 2.50E-06 | 3.29 | |
| Calreticulin (CALR) | P27797 | 2 | 2 | 1.37E-05 | 0.28 | |
| SWI/SNF complex subunit SMARCC1 (SMARCC1) | Q92922;Q9UL12 | 2 | 1 | 2.17E-05 | 0.31 | |
| Collagen alpha-1(VI) chain (COL6A1) | P12109 | 3 | 2 | 8.15E-05 | 0.47 | 33, 34 |
| Adenylyl cyclase-associated protein 1 (CAP1) | Q01518 | 2 | 1 | 1.41E-04 | 1.0E-06 | |
| Kinesin-like protein KIF26B (KIF26B) | Q2KJY2 | 3 | 1 | 1.08E-03 | 15.12 | |
| Tubulin alpha-1B chain (TUBA1B) | P68363;A6NHL2;P68366;Q13748;Q71U36 | 4 | 4 | 2.75E-03 | 2.27 | |
| Adiponectin (ADIPOQ) | Q15848 | 2 | 2 | 3.90E-03 | 0.19 | 34 |
| Integrin beta-like protein 1 (ITGBL1) | O95965 | 3 | 3 | 5.22E-03 | 0.39 | |
| Thrombospondin-3 (THBS3) | P49746 | 3 | 1 | 6.51E-03 | 5.85 | 34 |
| Coronin-1A (CORO1A) | P31146 | 8 | 8 | 6.69E-03 | 3.47 | |
| Golgin subfamily B member 1 (GOLGB1) | Q14789 | 3 | 2 | 7.33E-03 | 3.92 | |
| Collagen alpha-1(V) chain (COL5A1) | P20908 | 3 | 2 | 1.03E-02 | 4.78 | 34 |
| Neutrophil defensin 1 (DEFA1) | P59665 | 3 | 3 | 1.53E-02 | 3.03 | |
| Ganglioside GM2 activator (GM2A) | P17900 | 2 | 2 | 3.00E-02 | 3.28 | 34 |
| Tumor necrosis factor-inducible gene 6 protein (TNFAIP6) | P98066 | 5 | 5 | 3.19E-02 | 0.43 | 34 |
| Common plasma proteins | ||||||
| Apolipoprotein A-II (APOA2) | P02652 | 8 | 7 | 3.82E-09 | 0.34 | 33, 35 |
| Alpha-1-antitrypsin (SERPINA1) | P01009 | 17 | 17 | 1.52E-07 | 0.33 | 34, 35 |
| Vitamin K-dependent protein Z (PROZ) | P22891 | 9 | 9 | 1.85E-06 | 0.32 | 34 |
| Plasma protease C1 inhibitor (SERPING1) | P05155 | 7 | 5 | 7.53E-06 | 0.41 | 34, 35 |
| Haemopexin (HPX) | P02790 | 3 | 3 | 3.87E-05 | 0.39 | 33, 35 |
| Apolipoprotein C-I (APOC1) | P02654 | 2 | 2 | 2.79E-04 | 0.18 | 33 |
| Ig heavy chain V-III region VH26 | P01764;P01768 | 3 | 1 | 4.35E-04 | 0.29 | |
| Ig kappa chain V-II region Cum | P01614;P01617 | 3 | 1 | 4.15E-03 | 2.14 | |
| Ig kappa chain V-II region RPMI 6410 | P06310;P01615;P01616 | 3 | 2 | 6.26E-03 | 0.34 | |
| Complement factor B (CFB) | P00751 | 5 | 5 | 8.16E-03 | 0.47 | 34, 35 |
| Fibrinogen gamma chain (FGG) | P02679 | 28 | 27 | 1.14E-02 | 0.50 | 33–36 |
| Ig mu heavy chain disease protein | P04220 | 25 | 2 | 1.62E-02 | 5.16 | |
Differentially expressed proteins in synovial fluid identified by LC-MS/MS. The table shows proteins of interest and common plasma proteins with significantly different levels of expression in low and high glucosinolate groups. Proteins were identified with >1 peptide, >2 fold change and controlled for 5% FDR. The number of peptides identified for each protein and the number of unique peptides for each protein are indicated. Proteins previously identified in the synovial fluid are referenced. Low glucosinolate n = 8, high glucosinolate n = 9. q-values are shown, significance level is q < 0.05. Mean fold change (FC) is given, calculated as peptide abundance in high/low glucosinolate group.
Neopeptides
Neopeptides identified in synovial fluid unique to high and low glucosinolate groups were identified for aggrecan, biglycan, decorin, fibromodulin, cartilage oligomeric matrix protein and a number of collagens.
There was no correlation between diet and neopeptide abundance, (an indicator of proteolysis), in synovial fluid (see Supplementary Table S3). This holds true whether considering neopeptides unique or common to either intervention.
In vitro cartilage degradation
No significant differences were observed for in vitro rates of cartilage degradation (glycosaminoglycan and hydroxyproline loss) between the high and low glucosinolate interventions (Table 4).
Table 4.
In vitro cartilage degradation, biomarkers and cytokine profile.
| Outcome | Low glucosinolate Mean (95%CI) | High glucosinolate Mean (95%CI) | p-value |
|---|---|---|---|
| Cartilage matrix degradation | |||
| n | 19 | 15 | |
| Glycosaminoglycan (% loss) | 23.72 (19.58 to 27.87) | 25.68 (21.21 to 30.14) | 0.50 |
| Hydroxyproline (% loss) | 0.71 (0.47 to 0.96) | 0.77 (0.50 to 1.03) | 0.65 |
| Biomarkers | |||
| n | 20 | 17 | |
| hsCRP (fold change) | −5.48 (−16.02 to 5.05) | −5.49 (−26.90 to 15.93) | 0.33 |
| Col2-1 (fold change) | 4.41 (−1.02 to 9.83) | 13.21 (−11.85 to 38.27) | 0.44 |
| Cytokine protein expression | |||
| n | 19 | 17 | |
| Interleukin 6 (pg/ml) | 84.54 (64.36 to 104.7) | 70.42 (53.08 to 87.76) | 0.25 |
| Interleukin 8 (pg/ml) | 46.62 (30.00 to 63.23) | 36.50 (26.94 to 46.07) | 0.45 |
| Interleukin 1 (pg/ml) | 36.80 (36.59 to 37.00) | 36.98 (36.72 to 37.25) | 0.25 |
| Tumor necrosis factor (pg/ml) | 39.03 (38.70 to 39.37) | 39.36 (39.13 to 39.60) | 0.10 |
| Chemokine (C-C Motif) ligand 5 (pg/ml) | 54.04 (43.39 to 64.69) | 66.80 (31.05 to 102.6) | 0.68 |
In vitro degradation of cartilage aggrecan and collagen were measured by glycosaminoglycan and hydroxyproline release into culture media. Remaining cartilage was digested and percentage degradation calculated. High sensitivity C reactive protein (hsCRP) and collagen type II (col 2-1) biomarkers for inflammation and collagen degradation were measured in plasma (mean fold change given). The protein expression levels of cytokines were profiled in synovial fluid. Mean values and 95% confidence intervals are shown. P-values are given and the significance level was p < 0.05.
Gene Expression
Gene expression was measured by TLDA in cartilage (low glucosinolate n = 20, high glucosinolate n = 17) and fat (low glucosinolate n = 13, high glucosinolate n = 10) tissues. Adjustment for multiple testing by controlling the false discovery rate (5%) generated no significant differences between groups for either tissue (Supplementary Table S4).
However, unadjusted data showed that in cartilage HERC5 (HECT and RLD Domain Containing E3 Ubiquitin Protein Ligase 5) (p = 0.009) and LUM (lumican) (p = 0.05) were decreased in the high glucosinolate group. IL-1β (interleukin 1β) (p = 0.01) and IGFBP4 (Insulin-Like Growth Factor Binding Protein 4) (p = 0.03) were increased in the high glucosinolate group. Taking large fold changes into account CCL7 (Chemokine (C-C Motif) Ligand 7) (p = 0.06) and SFRP1 (Secreted Frizzled-Related Protein 1) (p = 0.06) showed tendencies to decreased and increased expression respectively in the high glucosinolate group. In fat tissue CXCL10 (Chemokine (C-X-C Motif) Ligand 10) (p = 0.03) was decreased in the high glucosinolate group whilst IRX3 (Iroquois Homeobox 3) (p = 0.06) was increased.
Plasma Biomarkers – hsCRP and Coll 2-1
The 14-day intervention did not alter levels of either hsCRP (with many samples undetected) or Coll 2-1 in plasma, or protein cytokine expression of IL-6, IL-8, IL-1, TNF, CCL5, in synovial fluid (Table 4).
Diet Diaries
Nutrient intakes were largely similar between groups however, a significant increased intake of vitamin C (p = 9.96 × 10−06) was observed following adjustment for multiple testing using a 1% FDR (Supplementary Table S5).
Genotyping
The overall frequency of GSTT1 copy number was measured at 3 (8.1%) 0 copies, 27 (73%) 2 copies, and 7 (18.9%) 4 copies. The overall frequency of GSTM1 copy number was measured at: 17 (45.9%) 0 copies, 18 (48.6%) 2 copies, and 2 (5.4%) 4 copies. The overall frequency of GSTP1 copy number was measured at: 18 (48.6%) 2 copies, 7 (18.9%) 3 copies, 10 (27%) 4 copies and 1 (2.7%) 5 copies. Predicted copy numbers were similar between the high and low glucosinolate groups and are detailed in Supplementary Table S6. No correlation was observed between genotype and any outcome measure.
Adverse events
There was one serious adverse event of a fractured humerus considered unrelated to the intervention. In terms of minor events, one participant reported bloating (high glucosinolate group), although the participant continued with the intervention.
Discussion
Our results provide the first evidence that following broccoli intake, the bioactive constituent ITCs reach the synovial fluid at concentrations with biological impact on the articular joint tissues, and alter the synovial fluid protein profile. This study clearly demonstrates that a dietary bioactive with chondroprotective properties can penetrate the knee in osteoarthritis. These data support our previous mouse model work, where we showed that a SFN-rich diet can provide chondroprotection in a mouse model of OA, though SFN and its metabolites were not measured directly in the joint.
Mean total ITC levels were 4.47-fold lower in the synovial fluid than the plasma. The concentration of small molecules in synovial fluid often reflects that of plasma since permeability into synovial fluid occurs through free diffusion from the endothelium of the synovial microvasculature and surrounding interstitium. The synovial lining has no basement membrane and therefore small molecule diffusion is limited only by the volume of intercellular space27, however, many non-steroidal anti-inflammatory drugs also show lower concentrations in synovial fluid than plasma28, 29. This reflects the kinetics of elimination of the drug, repeat administration and also protein binding. In this study we did not standardize the timing of broccoli intake compared to sampling of blood or synovial fluid. It is also possible that the diet modifies synovial fluid volume which was not monitored in this study, though this is unlikely to be significant. Plasma levels for ITCs and SFN were higher than expected given the limited available published data. In the current study the timing of the broccoli meal during the day was not controlled. This and possible differences in the rate of metabolism by each patient’s unique microbiome30 may explain the greater variation observed for plasma ITC levels in the high glucosinolate group. Twelve of the 29 synovial fluid proteins with significant difference between groups are commonly found in plasma, and 10 of these were decreased in the high glucosinolate group. In the inflamed joint, changes in the permeability of the synovium have been described, whereby increases in protein and solute concentration within the joint cavity have been observed31, 32. Resolution of aberrant synovial permeability by the high glucosinolate diet may offer one possible explanation for a reduction in the number of plasma proteins observed in this group, though this remains to be confirmed. Furthermore, a number of the proteins highlighted in our study (table 3) have been reported in human OA synovial fluid previously33–36.
Common plasma proteins aside, there were a number of interesting proteins decreased in abundance with the high glucosinolate diet including NBL1, CALR, SMARCC1 and type VI collagen. NBL1 is an antagonist of bone morphogenetic proteins (BMPs), key in cartilage homeostasis. Increased gene expression of NBL1 in cartilage is reported in mouse and rat models of OA and in human OA cartilage37–40. Calreticulin has functions outside of calcium regulation41. Calreticulin is increased in the plasma and synovial fluid of RA patients and this correlated with swollen joint count and disease activity score42. SMARCC1 is a regulator of gene transcription through chromatin remodelling, and is found in exosomes in cancer with no reported function in the joint43–45. Type VI collagen is a structural component of the perichondral matrix within articular cartilage. It has been reported in synovial fluid, with no significant difference between OA and control33. Methyltransferase-like 18 (METTL18) was significantly increased in abundance in the high glucosinolate group. It is a putative methyltransferase, and interacts with molecular chaperones46. Individual neopeptides were not reproducibly identified across all samples and this technology requires further development to be conclusive.
Gene expression in either cartilage or fat was not significantly different between groups when data were adjusted for multiple testing. Genes shown to be significant in unadjusted data are therefore of uncertain importance.
The collagen degradation biomarker Coll 2-1 has previously been shown to decrease after supplementation with a form of curcumin47, however only at the 85 day time point and not at the earlier 14 day time point.
Validated diet diaries showed that patients consumed similar levels of energy, fat, protein and fibre during the intervention. The high glucosinolate group consumed significantly higher levels of vitamin C, likely due to the high vitamin C content of broccoli. We therefore cannot rule out the contribution of vitamin C to the effects observed in the study. Future studies involving broccoli intake should include a control for vitamin C levels.
The interaction of GST genotype and ITC metabolism measured as rate of urinary secretion has been reported previously with differing associations13, 48–50. It has been suggested that specific GST null genotypes may confer a protective effect through a lowered rate of metabolism that extends tissue exposure time. Our cohort showed no associations of genotype with study outcomes though numbers in each subgroup were small (data not shown).
Limitations
Our study was a proof-of-principle study and limitations of the trial design were the short intervention time and unspecified timing of broccoli intake. These limitations and/or the use of end-stage OA patients whose tissue gene expression maybe beyond modification by dietary intervention, may have contributed to the small changes in gene expression observed. Similarly, cartilage degradation rates in vitro were likely affected by rapid metabolism of dietary SFN in these cultures. The link between ITCs in the joint and changes in the synovial fluid may be indirect and this requires further investigation.
Conclusion
This is the first human study to show that increased broccoli intake results in ITC uptake into the joint, with concomitant changes in the joint. Coupled with our earlier data showing efficacy of SFN in both in vitro and in vivo laboratory models of OA, this supports the need for an appropriately designed clinical trial to determine the impact of dietary SFN in OA.
Electronic supplementary material
Acknowledgements
We are very grateful to Dr. Charles Brearley (University of East Anglia) for his expertise in HPLC methodology, Mr James Anderson for his help analysing the neopeptide dataset, Dr. Darren Sexton and Dr. Andy Goldson (University of East Anglia) for their flow cytometry work, Amy Jennings (University of East Anglia) for her nutrition analysis advice, Szumin Yu and Jennifer Diniz (University of East Anglia) for their contributions inputting nutrition data and Tracey Potter (NNUH Orthopaedics Research Department) for lending her efficient coordination skills. We would also like to make special thanks to the Spire Norwich Hospital and all the staff who enabled this study, without which this work would not have been possible. This work was funded by the BBSRC Diet and Health Research Industry Club grant BB/I006060/1 and Action Arthritis. NIHR supported Clinical Research Nurses. Dr Peffers is funded by a Wellcome Trust Intermediate Clinical Fellowship (WT107471MA).
Author Contributions
Contributors I.M.C., R.K.D., S.T.D., Y.P.B., and A.C. were involved in the conception and design of the study, acquisition of data, analysis and interpretation of data; drafting and revising of manuscript. Contributors S.G., O.J., A.B., S.B., L.W., M.T., R.F.M., S.S., M.P. and P.C. were involved in the acquisition, analysis and interpretation of data. Contributors M.T., S.S., M.P., P.C. were involved in the drafting of the manuscript and all contributors were involved in the revision and final approval for publication.
Competing Interests
The broccoli with elevated glucoraphanin is the subject of patents filed by Plant Bioscience Limited (PBL), the technology transfer company of the John Innes Centre. R.F. Mithen is an inventor named on these patents. PBL has licensed rights under these patents to Seminis Vegetable Seeds Inc. The John Innes Centre and RFM may receive payments from commercialization as specified by the Biotechnology and Biological Sciences Research Council Rewards to Inventors policy. None of the other authors have competing interests to declare.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-03629-5
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cross M, et al. The global burden of hip and knee osteoarthritis: estimates from the Global Burden of Disease 2010 study. Annals of the Rheumatic Diseases. 2014;73:1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 2.Smith, S. OiANation 2012 (2012).
- 3.Conaghan PG, et al. Inadequate pain relief and large functional loss among patients with knee osteoarthritis: evidence from a prospective multinational longitudinal study of osteoarthritis real-world therapies. Rheumatology. 2015;54:270–277. doi: 10.1093/rheumatology/keu332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hochberg MC, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012;64:465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 5.Conaghan. Vol. Clinical guideline CG177 (ed National Institute for Health and Care Excellence) (National Clinical Guideline Centre, 2014).
- 6.Sevick MA, Miller GD, Loeser RF, Williamson JD, Messier SP. Cost-effectiveness of exercise and diet in overweight and obese adults with knee osteoarthritis. Medicine and science in sports and exercise. 2009;41:1167–1174. doi: 10.1249/MSS.0b013e318197ece7. [DOI] [PubMed] [Google Scholar]
- 7.Kim HA, Yeo Y, Kim WU, Kim S. Phase 2 enzyme inducer sulphoraphane blocks matrix metalloproteinase production in articular chondrocytes. Rheumatology (Oxford) 2009;48:932–938. doi: 10.1093/rheumatology/kep132. [DOI] [PubMed] [Google Scholar]
- 8.Kong JS, et al. Inhibition of synovial hyperplasia, rheumatoid T cell activation, and experimental arthritis in mice by sulforaphane, a naturally occurring isothiocyanate. Arthritis Rheum. 2009;62:159–170. doi: 10.1002/art.25017. [DOI] [PubMed] [Google Scholar]
- 9.Kim H-A, et al. Phase 2 enzyme inducer sulphoraphane blocks prostaglandin and nitric oxide synthesis in human articular chondrocytes and inhibits cartilage matrix degradation. Rheumatology. 2012;51:1006–1016. doi: 10.1093/rheumatology/ker525. [DOI] [PubMed] [Google Scholar]
- 10.Davidson RK, et al. Sulforaphane represses matrix-degrading proteases and protects cartilage from destruction in vitro and in vivo. Arthritis Rheum. 2013;65:3130–3140. doi: 10.1002/art.38133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berenbaum F. Does broccoli protect from osteoarthritis? Joint Bone Spine. 2014;81:284–286. doi: 10.1016/j.jbspin.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Wruck CJ, et al. Role of oxidative stress in rheumatoid arthritis: insights from the Nrf2-knockout mice. Annals of the Rheumatic Diseases. 2011;70:844–850. doi: 10.1136/ard.2010.132720. [DOI] [PubMed] [Google Scholar]
- 13.Gasper AV, et al. Glutathione S-transferase M1 polymorphism and metabolism of sulforaphane from standard and high-glucosinolate broccoli. Am J Clin Nutr. 2005;82:1283–1291. doi: 10.1093/ajcn/82.6.1283. [DOI] [PubMed] [Google Scholar]
- 14.Ye L, Zhang Y. Total intracellular accumulation levels of dietary isothiocyanates determine their activity in elevation of cellular glutathione and induction of Phase 2 detoxification enzymes. Carcinogenesis. 2001;22:1987–1992. doi: 10.1093/carcin/22.12.1987. [DOI] [PubMed] [Google Scholar]
- 15.Armah CN, et al. A diet rich in high-glucoraphanin broccoli interacts with genotype to reduce discordance in plasma metabolite profiles by modulating mitochondrial function. Am J Clin Nutr. 2013;98:712–722. doi: 10.3945/ajcn.113.065235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Cho C-G, Posner GH, Talalay P. Spectroscopic quantitation of organic isothiocyanates by cyclocondensation with vicinal dithiols. Analytical Biochemistry. 1992;205:100–107. doi: 10.1016/0003-2697(92)90585-U. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Wade KL, Prestera T, Talalay P. Quantitative Determination of Isothiocyanates, Dithiocarbamates, Carbon Disulfide, and Related Thiocarbonyl Compounds by Cyclocondensation with 1,2-Benzenedithiol. Analytical Biochemistry. 1996;239:160–167. doi: 10.1006/abio.1996.0311. [DOI] [PubMed] [Google Scholar]
- 18.Peffers MJ, McDermott B, Clegg PD, Riggs CM. Comprehensive protein profiling of synovial fluid in osteoarthritis following protein equalization. Osteoarthritis and Cartilage. 2015;23:1204–1213. doi: 10.1016/j.joca.2015.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peffers MJ, et al. Proteomic Analysis Reveals Age-related Changes in Tendon Matrix Composition, with Age- and Injury-specific Matrix Fragmentation. Journal of Biological Chemistry. 2014;289:25867–25878. doi: 10.1074/jbc.M114.566554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peffers MJ, Thornton DJ, Clegg PD. Characterization of neopeptides in equine articular cartilage degradation. J Orthop Res. 2016;34:106–120. doi: 10.1002/jor.22963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Billington CJ. Cartilage proteoglycan release assay. Methods Mol Biol. 2001;151:451–456. doi: 10.1385/1-59259-046-2:451. [DOI] [PubMed] [Google Scholar]
- 22.Shingleton WD, Ellis AJ, Rowan AD, Cawston TE. Retinoic acid combines with interleukin-1 to promote the degradation of collagen from bovine nasal cartilage: matrix metalloproteinases-1 and -13 are involved in cartilage collagen breakdown. J Cell Biochem. 2000;79:519–531. doi: 10.1002/1097-4644(20001215)79:4<519::AID-JCB10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 23.Price JS, et al. The role of chondrocyte senescence in osteoarthritis. Aging Cell. 2002;1:57–65. doi: 10.1046/j.1474-9728.2002.00008.x. [DOI] [PubMed] [Google Scholar]
- 24.Davidson RK, et al. Expression profiling of metalloproteinases and their inhibitors in synovium and cartilage. Arthritis Res Ther. 2006;8:R124. doi: 10.1186/ar2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Henrotin Y, et al. Type II collagen peptides for measuring cartilage degradation. Biorheology. 2004;41:543–547. [PubMed] [Google Scholar]
- 26.Bingham SA, et al. Nutritional methods in the European Prospective Investigation of Cancer in Norfolk. Public health nutrition. 2001;4:847–858. doi: 10.1079/PHN2000102. [DOI] [PubMed] [Google Scholar]
- 27.Levick JR. Synovial-Fluid Exchange - a Case of Flow through Fibrous Mats. News Physiol Sci. 1989;4:198–202. [Google Scholar]
- 28.Netter P, Bannwarth B, Royer-Morrot MJ. Recent findings on the pharmacokinetics of non-steroidal anti-inflammatory drugs in synovial fluid. Clinical pharmacokinetics. 1989;17:145–162. doi: 10.2165/00003088-198917030-00002. [DOI] [PubMed] [Google Scholar]
- 29.Hunter RP, et al. Plasma pharmacokinetics and synovial fluid concentrations after oral administration of single and multiple doses of celecoxib in greyhounds. Am J Vet Res. 2005;66:1441–1445. doi: 10.2460/ajvr.2005.66.1441. [DOI] [PubMed] [Google Scholar]
- 30.Angelino D, et al. Myrosinase-dependent and—independent formation and control of isothiocyanate products of glucosinolate hydrolysis. Frontiers in Plant Science. 2015;6:831. doi: 10.3389/fpls.2015.00831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallis WJ, Simkin PA, Nelp WB, Foster DM. Intraarticular volume and clearance in human synovial effusions. Arthritis Rheum. 1985;28:441–449. doi: 10.1002/art.1780280413. [DOI] [PubMed] [Google Scholar]
- 32.Pitsillides AA, Will RK, Bayliss MT, Edwards JC. Circulating and synovial fluid hyaluronan levels. Effects of intraarticular corticosteroid on the concentration and the rate of turnover. Arthritis Rheum. 1994;37:1030–1038. doi: 10.1002/art.1780370708. [DOI] [PubMed] [Google Scholar]
- 33.Gobezie R, et al. High abundance synovial fluid proteome: distinct profiles in health and osteoarthritis. Arthritis Research & Therapy. 2007;9:R36. doi: 10.1186/ar2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balakrishnan L, et al. Proteomic analysis of human osteoarthritis synovial fluid. Clin Proteomics. 2014;11:6. doi: 10.1186/1559-0275-11-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sohn DH, et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res Ther. 2012;14:R7. doi: 10.1186/ar3555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kamphorst JJ, et al. Profiling of Endogenous Peptides in Human Synovial Fluid by NanoLC–MS: Method Validation and Peptide Identification. Journal of Proteome Research. 2007;6:4388–4396. doi: 10.1021/pr0704534. [DOI] [PubMed] [Google Scholar]
- 37.Aigner T, et al. Large-scale gene expression profiling reveals major pathogenetic pathways of cartilage degeneration in osteoarthritis. Arthritis Rheum. 2006;54:3533–3544. doi: 10.1002/art.22174. [DOI] [PubMed] [Google Scholar]
- 38.Appleton CT, Pitelka V, Henry J, Beier F. Global analyses of gene expression in early experimental osteoarthritis. Arthritis Rheum. 2007;56:1854–1868. doi: 10.1002/art.22711. [DOI] [PubMed] [Google Scholar]
- 39.Wei T, et al. Analysis of early changes in the articular cartilage transcriptisome in the rat meniscal tear model of osteoarthritis: pathway comparisons with the rat anterior cruciate transection model and with human osteoarthritic cartilage. Osteoarthritis and Cartilage. 2010;18:992–1000. doi: 10.1016/j.joca.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Gardiner MD, et al. Transcriptional analysis of micro-dissected articular cartilage in post-traumatic murine osteoarthritis. Osteoarthritis Cartilage. 2015;23:616–628. doi: 10.1016/j.joca.2014.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gold LI, et al. Calreticulin: non-endoplasmic reticulum functions in physiology and disease. The FASEB Journal. 2010;24:665–683. doi: 10.1096/fj.09-145482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarr JM, et al. Extracellular calreticulin is present in the joints of patients with rheumatoid arthritis and inhibits FasL (CD95L)–mediated apoptosis of T cells. Arthritis & Rheumatism. 2010;62:2919–2929. doi: 10.1002/art.27602. [DOI] [PubMed] [Google Scholar]
- 43.Skog J, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liang B, et al. Characterization and proteomic analysis of ovarian cancer-derived exosomes. Journal of Proteomics. 2013;80:171–182. doi: 10.1016/j.jprot.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 45.Skogberg G, et al. Characterization of Human Thymic Exosomes. PLoS ONE. 2013;8:e67554. doi: 10.1371/journal.pone.0067554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cloutier P, Lavallée-Adam M, Faubert D, Blanchette M, Coulombe B. A Newly Uncovered Group of Distantly Related Lysine Methyltransferases Preferentially Interact with Molecular Chaperones to Regulate Their Activity. PLoS Genetics. 2013;9:e1003210. doi: 10.1371/journal.pgen.1003210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henrotin Y, et al. Decrease of a specific biomarker of collagen degradation in osteoarthritis, Coll2-1, by treatment with highly bioavailable curcumin during an exploratory clinical trial. BMC Complementary and Alternative Medicine. 2014;14:159. doi: 10.1186/1472-6882-14-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.London SJ, et al. Isothiocyanates, glutathione S-transferase M1 and T1 polymorphisms, and lung-cancer risk: a prospective study of men in Shanghai, China. Lancet. 2000;356:724–729. doi: 10.1016/S0140-6736(00)02631-3. [DOI] [PubMed] [Google Scholar]
- 49.Steck SE, Gammon MD, Hebert JR, Wall DE, Zeisel SH. GSTM1, GSTT1, GSTP1, and GSTA1 polymorphisms and urinary isothiocyanate metabolites following broccoli consumption in humans. J Nutr. 2007;137:904–909. doi: 10.1093/jn/137.4.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Egner PA, et al. Bioavailability of Sulforaphane from two broccoli sprout beverages: results of a short-term, cross-over clinical trial in Qidong, China. Cancer prevention research. 2011;4:384–395. doi: 10.1158/1940-6207.CAPR-10-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.


