Abstract
The role of the Escherichia coli iron-regulated gene homologue adhesin (Iha) in the pathogenesis of urinary tract infections (UTIs) is unknown. We performed a series of complementary analyses to confirm or refute the hypothesis that Iha is a virulence factor in uropathogenic E. coli. Fecal E. coli isolates exhibited significantly lower prevalences of iha (range, 14 to 22%) than did clinical isolates from cases of pediatric cystitis or pyelonephritis, adult pyelonephritis or urosepsis, or bacteremia (range, 38 to 74%). Recombinant Iha from E. coli pyelonephritis isolate CFT073 conferred upon nonadherent E. coli ORN172 the ability to adhere to cultured T-24 human uroepithelial cells. In a well-established mouse model of ascending UTI, CFT073 and its derivative UPEC76 (a pap [P fimbriae] mutant version of strain CFT073) each significantly outcompeted their respective iha deletion mutants in CBA/J mice 48 h after bladder challenge (P < 0.03 for urine, both kidneys, and bladders of both constructs, except for bladders of mice challenged with UPEC76 and its deletion mutant, where P = 0.11). These data suggest that IhaCFT073 is a virulence factor and might be a target for anti-UTI interventions.
Extraintestinal pathogenic Escherichia coli cells produce diverse factors (6, 13, 16) that allow them to overcome or subvert host defenses and to colonize, injure, and invade host cells or tissues. Delineation of the roles of these factors could lead to measures to prevent or attenuate infections caused by the organisms that express them (28, 29).
iha encodes the IrgA homologue adhesin (Iha), an outer membrane protein (OMP) first characterized in E. coli O157:H7, which confers adherence to nonadherent laboratory strains (41). iha occurs frequently among uropathogenic E. coli strains (3, 16-18, 22, 23, 25, 27). Its designation is derived from the similarity of Iha to IrgA (9), which is postulated to play a role in the colonization of mice experimentally infected with Vibrio cholerae (10).
ihaO157:H7 and open reading frame R4 in pathogenicity-associated island 1 (PAI-1) of the well-studied E. coli pyelonephritogenic strain CFT073 (serotype O6:K2:H1) (12) differ by five synonymous nucleotide changes and one nonsynonymous nucleotide change. Strain UPEC76, a derivative of CFT073 in which both pyelonephritis-associated pap (pilus associated with pyelonephritis; P fimbriae) operons, in separate PAIs, have been inactivated by deletions, colonizes the urinary tract in a mouse model of ascending urinary tract infection (UTI) as well as does its parent (32). This finding suggests that CFT073 requires non-pap loci for virulence, with iha being one such candidate critical locus. Also, the virulence of a Proteus mirabilis strain, in which irgA was identified by signature-tagged mutagenesis, was attenuated in a mouse model of ascending UTI (4).
Because Iha's status as a virulence factor has been incompletely elucidated, we performed complementary studies to test the hypotheses that iha+ extraintestinal pathogenic E. coli iha is epidemiologically associated with human disease, that cloned iha confers the ability to adhere to uroepithelial cells, and that IhaCFT073 is needed for complete urovirulence in mice challenged with strain CFT073 or UPEC76.
MATERIALS AND METHODS
Bacteria and plasmids used.
E. coli strains CFT073, UPEC76 (32), and 86-24 (serotype O157:H7) (41) have been previously described. Ampicillin and nalidixic acid concentrations in media were 200 and 20 μg ml−1, respectively. Cloned PCR products were transformed into E. coli strains DH5α (36) and ORN172, a laboratory E. coli strain devoid of all known adherence mechanisms including type 1 pili (46). Nalidixic acid-resistant CFT073 (CFT073nalR) was derived by plating ca. 1010 CFT073 cells on Luria-Bertani (LB) agar containing nalidixic acid and selecting a spontaneously resistant mutant. E. coli SM10(λpir) (38) was transformed with, and then was the donor for, suicide plasmid constructs. E. coli strain B171 (O111:NM) (33) was used as a positive adherence control. pSK+Iha-O157:H7, formerly pIha, is pSK+ (Stratagene, La Jolla, Calif.) containing cloned ihaO157:H7 (41). To clone and express IhaCFT073, we amplified CFT073 DNA with primers A (5′GGGGATCCAATTCTGGCATGCCGAGGCAGTGC3′) and B (5′GGTCTAGATTCTCGTTGCCACTGTTCCGCCAGG3′) (41), containing engineered 5′ BamHI and XbaI sites, respectively. This amplicon, consisting of ihaCFT073 and 141 and 80 bp 5′ and 3′ to its termini, respectively, was digested with BamHI and XbaI and cloned into the corresponding sites in pSK+, resulting in pSK+Iha-CFT073. It was then sequenced bidirectionally.
Deletion of ihaCFT073 from CFT073 and UPEC76.
To create an in-frame, unmarked, isogenic deletion of iha in strains CFT073 and UPEC76, we cloned an in-frame deletion construct of the target gene into suicide plasmid pCVD442 (5). We used inverse PCR (42) to obtain a candidate DNA sequence (not shown) 5′ to ihaCFT073. We then used primer C (5′GCAGAGCTCCCTTGCAAGAGGGCGTCGAGC3′) and 5′GCTATGGATCCGGCTGAAAATCCGAGACAGGG3′ to produce a 704-bp amplicon that includes the 5′ terminus of ihaCFT073 and 630 bp of the 5′ noncoding region. This amplicon was sequentially cloned into, and excised from, the pGem-TEasy vector and pSK+ with SacI and BamHI. Next, the cloned 558-bp amplicon that spans the ihaO157:H7 3′ terminus (41), which differs from the corresponding region in CFT073 by only 3 nucleotides in the downstream noncoding region of the gene, was excised from pSK+ with BamHI and XhoI and ligated to the SacI-BamHI fragment spanning the 5′ ihaCFT073 terminus. This fusion, representing a central truncation of ihaCFT073, was then cloned into and excised from the pSK+ SacI and XhoI sites and finally inserted into the SacI and XhoI sites of pCVD442 (5), producing pCVD442ΔihaCFT073. Though the 5′ end of primer C imperfectly represents the iha upstream region, the deletion mutant is, as intended, identical to its parent at that site (sequence data not shown).
CFT073nalR or UPEC76 was mated separately with E. coli SM10(λpir) transformed with pCVD442ΔihaCFT073 on LB agar at 37°C. Transconjugants were selected by plating mated bacteria on LB agar containing ampicillin and nalidixic acid. The resulting presumed merodiploids were grown (37°C in LB broth [36]), plated onto LB agar containing 5% sucrose but no NaCl, and incubated (30°C). Sucrose-resistant, ampicillin-susceptible colonies from these matings were designated CFT073ΔihaCFT073 and UPEC76ΔihaCFT073, respectively.
Deletion mutant characterizations.
Genomic DNA from the presumptive mutants and their parents was digested with BstXI, electrophoresed, transferred to a MagnaCharge nylon membrane (GE Osmonics, Minnetonka, Minn.), and probed with ihaO157:H7. A 371-bp amplicon produced by primers A and B from each candidate deletion mutant was cloned into pSK+ and sequenced. The deletion mutants were serotyped by Flemming Scheutz, digested with XbaI, and analyzed by pulsed-field gel electrophoresis, and tested by PCR for 11 (iha, papA, papC, sfa/focDE, focG, fimH, iutA, fyuA, iroN, hlyD, and malX) (11, 26) putative or demonstrated urovirulence loci that are known to be present in the parent plus, specifically, the F7-1 and F7-2 papA alleles (21, 22). The deletion mutants and their parents were also evaluated for mannose-sensitive agglutination of guinea pig erythrocytes and baker's yeast and for mannose-resistant agglutination of human erythrocytes after overnight growth in static broth or on agar plates to indicate expression of type 1 or P fimbriae, respectively (24), and for differences in growth rates (compared to parents) over 18 h in LB broth shaken at 37°C. Finally, the mutants were hybridized with digoxigenin-labeled pCVD442 to confirm suicide plasmid loss.
Anti-Iha antibodies.
The PolyQuik protocol (Zymed Laboratories, South San Francisco, Calif.) and affinity purification produced lapine polyclonal antibodies against an Iha epitope. The resulting affinity-purified antibodies (designated anti-Iha antibodies) do not detect an externally directed Iha epitope (41).
OMP analyses.
OMPs were prepared (1) from bacteria grown overnight at 37°C in 100 ml of LB broth or Dulbecco's minimal essential medium (D-5030; Sigma, St. Louis, Mo.) containing 0.45% glycerol, 25 mM HEPES, 10 mM sodium pyruvate, 4.5 mN sodium hydroxide, 4 mM l-glutamine, and 44 mM sodium bicarbonate (DMEM). Protein concentrations were determined (protein assay kit; Bio-Rad, Hercules, Calif.). OMPs (2 μg) in loading buffer were electrophoretically separated (sodium dodecyl sulfate-10% polyacrylamide) and transferred to membranes (Immobilon-P; Millipore, Billerica, Maine) or Coomassie stained to confirm equal loading of analyte. The membranes to which the proteins were transferred were blocked overnight in antibody buffer (phosphate-buffered saline with 0.05% [vol/vol] Tween 20) containing 5% nonfat dried milk and 0.02% sodium azide) and washed once with antibody buffer. The membranes were then incubated overnight with anti-Iha antibodies diluted 1:2,000 in antibody buffer, washed three times with antibody buffer, incubated for 30 min with affinity-purified goat anti-rabbit immunoglobulin G (heavy plus light chains) peroxidase conjugate (Boehringer Mannheim, Indianapolis, Ind.) diluted 1:2,000 in antibody buffer, and washed again three times with antibody buffer. Membranes were incubated at 4°C or room temperature (blocking or all subsequent steps, respectively). Bound antibodies were detected with the use of the SuperSignal Western blotting (Pierce, Rockford, Ill.).
Adherence assay.
T-24 human bladder epithelial cells (30), after growth to confluence at 37°C in 5% CO2 in plastic flasks in culture medium (McCoy's 5a medium; Sigma; M-4892) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum, were trypsinized, pelleted, resuspended in the above medium, and then added to wells (in 2-ml volumes) of a six-well plastic flat-bottom cell culture plate (Costar 3516; Corning, Corning, N.Y.) containing sterile plastic 25-mm-diameter coverslips. When cells were ca. 75% confluent, the medium was replaced with fresh medium containing mannose (0.5%) and ampicillin, and adherence assays were performed.
E. coli strain B171 and E. coli ORN172 containing pSK+Iha-CFT073 or pSK+ were grown in LB broth with ampicillin at 37°C without shaking, before assessment in the adherence assay. Cells and bacteria were incubated for 1 (wild type and wild-type derivatives) or 2 h (ORN172 transformants) at 37°C in 5% CO2, after which they were washed eight times with sterile phosphate-buffered saline, fixed (100% methanol, 1 min), stained (0.5% crystal violet, 15 min), and thoroughly washed with water to remove background stain. Coverslips were then air dried and mounted on glass slides, and adherent organisms were enumerated by a microscopist unaware of the identity of the organisms in each well. The test organisms were concurrently assessed in duplicate on each of multiple days. Five fields were randomly selected for analysis from each of two duplicate wells per test organism. The adherent organisms were counted separately for each cell within each selected field.
Molecular epidemiological analyses.
iha was sought in E. coli from the feces of healthy volunteers and noninfected hospitalized subjects and from E. coli recovered from the urine, blood, or cerebrospinal fluid of infected patients by PCR with internal primers D (5′CTGGCGGAGGCTCTGAGATCA3′) and E (5′TCCTTAAGCTCCCGCGGCTGA3′) and/or by dot blot hybridization with an internal digoxigenin-labeled iha probe (synthesized with primers D and E) (22). Isolates were tested in duplicate, with independently prepared boiled lysates, for the presence of iha. Strains CFT073 and K-12 were the positive and negative controls, respectively (22, 25).
Mouse models of UTI.
In an extensively evaluated ascending atraumatic model of UTI, 6- to 10-week-old CBA/J mice were anesthetized and inoculated via the urethra with 1.0 μl of the test bacterial suspension/g of body weight−1, under conditions that avoided vesicoureteral reflux (14, 15, 35). Challenge bacteria were grown in shaking, overnight LB broth cultures at 37°C, pelleted, and resuspended in LB broth before challenge. After a standard 2-day period, mice were euthanized, and aseptically harvested urine and bladder and kidney homogenates were cultured quantitatively on agar. Dual-strain challenges were used to compare the colonizing abilities of CFT073 and of UPEC76 to those of their respective iha deletion mutants. Intra-animal competition assessments were used in these studies because they minimize the impact of mouse-to-mouse variation and maximize the ability to identify differences among test strains. The challenge inocula contained ca. 2 × 109 total CFU, which is standard in our use of this model and which is within the range used by others (7, 32, 43) in various mouse models of UTI.
Because we used unmarked mutations in competition experiments, neither CFT073 nor UPEC76 could be easily phenotypically differentiated from their respective ihaCFT073 mutants in the postmortem cultures in mouse UTI models. Therefore, we tested colonies from each mouse culture, randomly selected to preclude systematic bias favoring one test strain over the other, by using dot blot hybridization with an internal iha probe or PCR with internal iha primers D and E (25) to determine the relative proportions of recovered organisms that contained an intact copy of iha. Each isolate was analyzed in duplicate, with the corresponding challenge strains used as controls. Colonies from the inoculum suspension cultures were similarly analyzed to define the relative abundances of the two test strains as administered to the mice, and this proportion (the input ratio) was used to adjust the postmortem quantitative culture results (the output ratio) from the mouse infection experiments to obtain the competitive index (CI). Between 10 and 64 colonies per culture (i.e., from inoculum suspensions and postmortem samples) were so assessed. In addition, to exclude contamination, one putative representative of each test strain from each positive culture from the mouse experiments was compared with the actual test strain by random amplified polymorphic DNA analysis (22, 44).
Statistics.
Differences in proportions were tested for significance by Fisher's exact test for unpaired comparisons or McNemar's test for paired comparisons. Differences involving continuous variables were assessed for significance by the Mann-Whitney U test and the Wilcoxon rank sum tests for unpaired and paired comparisons, respectively. The threshold for statistical significance was a P value <0.05, but selected values <0.10 are noted in Table 2 to demonstrate trends.
TABLE 2.
Comparative urovirulences of CFT073 and UPEC76 versus their respective ΔihaCFT073 derivatives in the mouse model of ascending UTI (competition experiments)
| Parent | Site cultured | Total no.c | No. (%)a with:
|
Pd | No. (%)b with:
|
Pd | ||
|---|---|---|---|---|---|---|---|---|
| Parent > ΔihaCFT073 mutant | ΔihaCFT073 mutant > parent | Parent only | ΔihaCFT073 mutant only | |||||
| CFT073nalR | Urine | 19 | 12 (63) | 2 (11) | <0.02 | 8 (42) | 0 (0) | <0.01 |
| Bladder | 19 | 16 (84) | 3 (16) | <0.01 | 2 (11) | 0 (0) | ||
| Right kidney | 19 | 11 (58) | 1 (5) | <0.01 | 2 (11) | 0 (0) | ||
| Left kidney | 19 | 11 (58) | 1 (5) | <0.01 | 4 (21) | 0 (0) | ||
| UPEC76 | Urine | 24c | 17 (71) | 5 (21) | 0.017 | 7 (29) | 1 (4) | 0.07 |
| Bladder | 30 | 18 (60) | 11 (37) | 6 (20) | 0 (0) | 0.03 | ||
| Right kidney | 30 | 19 (63) | 7 (23) | 0.03 | 8 (27) | 3 (10) | ||
| Left kidney | 30 | 16 (53) | 8 (27) | 9 (30) | 5 (17) | |||
Percentages do not sum to 100% in all instances because some cultures (included in denominators) yielded no growth. Comparative prevalence of the competing strains was assessed based on the CI, i.e., after adjusting the output ratio from that culture for the input ratio from the same experiment.
Percentages do not sum to 100% because some cultures yielded no growth or growth of both competing strains.
Urine was unavailable for six mice from the UPEC76 experiments.
P values (by McNemar's test; two tailed) are provided where P < 0.10.
Nucleotide sequence accession number.
The nucleotide sequence of the region upstream of ihaCFT073 in CFT073 has been deposited in the GenBank database under accession number AF401752.
RESULTS
Molecular epidemiology of iha.
To determine the epidemiological association of iha with specific extraintestinal infection syndromes, iha was sought among 286 fecal E. coli isolates and 839 clinical isolates from diverse human populations (Table 1). Fecal isolates consistently exhibited a lower prevalence of iha (range, 14 to 22%) than did clinical isolates from all infection syndromes except cystitis in women and neonatal meningitis. The highest prevalences of iha (50 to 74%) occurred among cystitis and pyelonephritis isolates from children and pyelonephritis and urosepsis isolates from adults. Intermediate prevalences (38 to 39%) that still significantly exceeded control frequencies were observed among bacteremia isolates from diverse sources. These differences demonstrate that Iha fulfills the first of the molecular restatement of Koch's postulates, which requires that the property of interest be epidemiologically associated with disease (8).
TABLE 1.
Prevalence of iha among fecal and clinical isolates of E. coli from diverse host populations
| Group no. | Host population (sample source) and/or syndrome | iha proportion (%) |
P: (by Fisher's exact test) for comparison with group no.:
|
Referenceb for:
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | Isolates | iha data | |||
| 1 | Healthy women (fecal) | 13/77 (17) | |||||||||||||||
| 2 | Healthy children (fecal) | 9/46 (20) | — | ||||||||||||||
| 3 | Healthy adults (fecal) | 20/92 (22) | — | — | |||||||||||||
| 4 | Hospitalized veterans (fecal) | 10/71 (14) | — | — | — | 36a | 36a | ||||||||||
| 5 | Pediatric cystitis | 26/39 (74) | *** | *** | *** | *** | 19 | ||||||||||
| 6 | Pediatric cystitis | 22/44 (50) | *** | ** | *** | *** | — | 2 | |||||||||
| 7 | Pediatric pyelonephritis | 25/48 (52) | *** | *** | *** | *** | — | — | 2 | ||||||||
| 8 | Women, cystitis | 12/74 (16) | — | — | — | — | *** | *** | *** | 22 | 22 | ||||||
| 9 | Women, cystitis | 22/82 (27) | — | — | — | — | *** | *** | *** | — | |||||||
| 10 | Women, pyelonephritis | 97/170 (57) | *** | *** | *** | *** | — | — | — | *** | *** | 42 | |||||
| 11 | Adults, urosepsis | 37/66 (56) | *** | *** | *** | *** | — | — | — | *** | *** | — | 26 | 26 | |||
| 12 | Adults, bacteremia | 69/182 (38) | *** | * | ** | *** | *** | — | — | *** | — | *** | ** | 36a | 36a | ||
| 13 | Veterans, bacteremia | 25/64 (39) | *** | * | * | ** | ** | — | — | ** | — | * | — | — | |||
| 14 | Neonatal meningitis | 13/70 (19) | — | — | — | — | *** | *** | *** | — | — | *** | *** | ** | ** | 24a | 24a |
P value symbols: —, P > 0.05; *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
If sources for these collections were not this study.
Structure of ihaCFT073 and its upstream region.
IhaCFT073 has a deduced molecular size of 78 kDa and contains 696 amino acids. IhaCFT073 and IhaO157:H7 differ by only one amino acid (an Asp469→Asn469 alteration), but the two iha genes differ by 6 nucleotides. Upstream of the iha ATG start codon, CFT073 and E. coli O157:H7 are 98.8 and 34.8% identical from position −1 to −489 and positions −490 through −630, respectively, consistent with CFT073's extensive chromosomal mosaicism (45).
Role of IhaCFT073 in adherence of E. coli to uroepithelial cells.
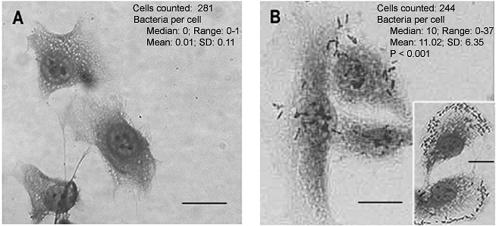
pSK+Iha-CFT073 conferred on E. coli ORN172 the ability to adhere to cultured T-24 uroepithelial cells, whereas pSK+ did not (Fig. 1). The adherence pattern observed for E. coli ORN172 transformed with pSK+Iha-CFT073 was diffuse, and there was interfield variability. This adherence pattern of Iha recombinants was also observed on HEp-2 cells (data not shown). Strain B171 displayed its characteristic localized adherence to cells (data not shown). Assessments of the cellular adherence of CFT073 or UPEC76, their iha deletion mutants, or their deletion mutants transformed with pSK+Iha-CFT073 and pSK+ were precluded by the considerable cytotoxicity of these strains (31), even after only 1 h of incubation (data not shown).
FIG. 1.
Adherence of laboratory E. coli to T-84 uroepithelial cells, with and without cloned Iha. Shown is E. coli ORN172 transformed with pSK+ (A) or pSK+Iha-CRT073 (B). Panel B demonstrates the typical adherence of E. coli ORN172 transformed with pSK+Iha-CFT073 to cells, but the adherence intensity is variable, with some cells quite densely covered (B, inset). Numbers are values associated with cellular adherence for the corresponding recombinant strains. The P value in panel B is relative to the vector control (Mann-Whitney U test). Bars, 20 μm.
Comparisons of CFT073 to CFT073ΔihaCFT073, and of UPEC76 to UPEC76ΔihaCFT073.
Compared with their respective parents, CFT073ΔihaCFT073 and UPEC76ΔihaCFT073 exhibit the same growth characteristics, colony morphology, O:K:H serotype, XbaI pulsed-field gel electrophoresis profile, baker’s yeast and erythrocyte agglutination patterns, and extended virulence genotype (except, of course, for ihaCFT073). By Southern hybridization, 4.7-kb BstXI fragments detected by the iha probe in CFT073 and UPEC76 were not evident in their respective ΔihaCFT073 mutants. Most importantly, sequencing across the target deletion site demonstrated a 1,939-bp deletion in CFT073ΔihaCFT073 and UPEC76ΔihaCFT073, with the intended insertion of GATC. This resulted in the in-frame replacement of the 647 amino acids between Glu25 and Gln673 with a Gly and a Ser.
OMP analysis.
Anti-Iha antibodies detected abundant 76-kDa antigen, as well as probable breakdown products, in OMPs from E. coli ORN172 transformed with pSK+Iha-O157:H7 or pSK+Iha-CFT073 (Fig. 2A). OMPs from strains CFT073 and UPEC76 produced less intensely immunoreactive 76-kDa Iha (Fig. 2A). As expected, OMPs from iha deletion mutants UPEC76ΔihaCFT073 and CFT073ΔihaCFT073 did not exhibit Iha antigen (Fig. 2B and C). Iha was not detectable when CFT073 was grown in LB broth but was detectable when it was grown in DMEM (Fig. 2D).
FIG. 2.
OMPs of E. coli ORN172, with and without recombinant IhaCFT073, and wild-type and derivative E. coli. (A) Immunoblots of OMPs from strain ORN172 transformed with pSK+ (lane 1), pSK+Iha-O157:H7 (lane 2), pSK+Iha-CFT073 (lane 3), E. coli CFT073 (lane 4), UPEC76 (lane 5), and E. coli O157:H7 (lane 6). (B to D) Immunoblots of OMPs from CFT073 (B, lane 1), CFT073Δiha-CFT073 (B, lane 2), UPEC76 (C, lane 1), UPEC76Δiha-CFT073 (B, lane 2), UPEC76 (C, lane 1), UPEC76Δiha-CFT073 (C, lane 2), and CFT073 grown in LB broth (D, lane 2) or DMEM (D, lane 2). The single immunoreactive bands in panels B to D represent the 76-kDa Iha antigen.
In vivo challenge experiments.
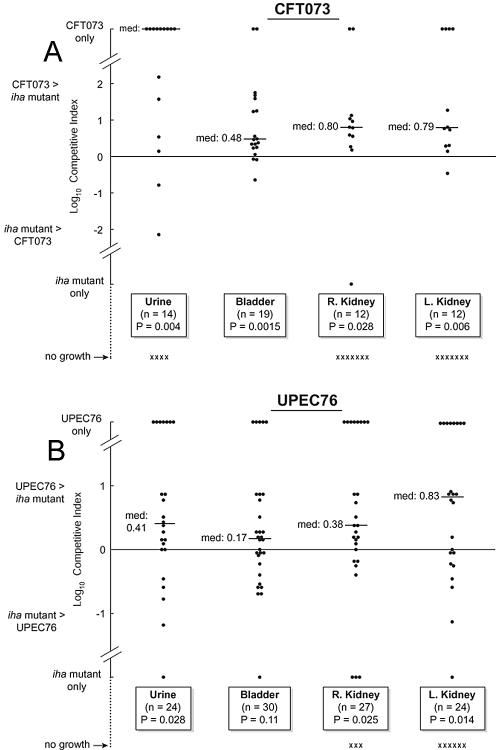
CBA/J mice were challenged via the urethra with mixtures of CFT073 and CFT073ΔihaCFT073 or mixtures of UPEC76 and UPEC76ΔihaCFT073, in dual-strain competition model experiments. Urine, bladders, and kidneys harvested 48 h after inoculation uniformly showed greater overall intensity and prevalence of colonization with CFT073 or UPEC76 than with these strains' respective ΔihaCFT073 mutants, as summarized for categorical outcomes in Table 2 and as illustrated for absolute log10 CI values in Fig. 3. Of note, many cultures yielded only the parent strain, indicating that the mutants were present at levels below the detection threshold or were lost in vivo. These data establish the contribution of ihaCFT073 to bladder and kidney colonization in mice challenged with a urovirulent E. coli strain, with or without an intact pap operon.
FIG. 3.
Comparative urovirulences in mice of CFT073 and UPEC76 versus those of their respective ΔihaCFT073 mutants. Mice were challenged via the urethra with 20 μl containing ca. 2 × 109 CFU of a mixture of CFT073 and CFT073Δiha-CFT073 (A) or UPEC76 and UPECΔihaCFT073 (B). CIs were calculated as the parent/deletion mutant ratios, normalized to the corresponding input ratio. X, no growth; horizontal bars, median values.
DISCUSSION
The impaired abilities to colonize the mouse urinary tract displayed by isogenic, in-frame, ihaCFT073 deletion mutants in two different E. coli backgrounds provide strong experimental support for the hypothesis that Iha is a urovirulence factor. That IhaCFT073 conferred the ability to adhere to T-24 cells is particularly interesting, because E. coli adherence to these cells of human bladder origin has been reported to be independent of known UTI-associated adhesins, such as type 1, P, or S fimbriae, or afimbrial adhesin I (30). These assay results also demonstrate that the single amino acid difference between IhaCFT073 and IhaO157:H7 does not negate the ability of the cloned protein to confer cellular adherence capability to nonadherent laboratory E. coli. This phenotype, though anticipated, warranted confirmation because of the known ability of single amino acid polymorphisms to alter the receptor binding characteristics of the pathogenetically important E. coli adhesin, FimH (39). Whether Iha augments adherence directly or indirectly via effects on other bacterial components and, if it acts directly, whether this phenotype involves receptor-specific or nonspecific binding to the host cell await further assessment.
Additionally, our work confirms the localization of Iha to the cell envelope of another iha+ strain and demonstrates that its presence in the cell envelope of CFT073, like the presence of the Iha homologue in the cell envelope of locus of enterocyte effacement-negative E. coli O91:H− strain 4797/97 (37), depends on the growth medium of the organism, with expression being suppressed in LB broth. Indeed, this diminished expression in LB broth might have actually led to an underattribution of the role of Iha in the mouse colonization experiments, because the challenge inocula were prepared in LB broth, which would tend to diminish the expression of Iha in the parent at the time of inoculation.
The mutations that produced the colonization deficiencies of CFT073 and UPEC76 were each in-frame deletions of ihaCFT073. Such deletions are quite unlikely to exert polar effects on downstream genes, although we cannot exclude completely the possibility that occult cis-acting effects of the deletion reduced colonization ability. Additionally, the deletion mutants were indistinguishable from the parent with respect to a broad panel of characteristics analyzed other than ihaCFT073 and antigenic IhaCFT073. Moreover, two independent deletions of ihaCFT073 in two different organisms produced the same diminished-colonization phenotype, to similar extents, without perturbing other analyzed loci. Recent data demonstrate the fairly frequent occurrence of secondary mutations in extraintestinal pathogenic E. coli using pCVD442 (20); therefore, it is important to generate such confirmatory results to increase confidence that the altered phenotype actually was caused by the intended mutation. The extensive methods that we used to exclude unanticipated secondary mutations in the iha mutants exceed those customarily used for this purpose in studies of this sort and were the same as those that uncovered otherwise-occult secondary mutations in the recent study; their use increases confidence that such alterations were absent from the present mutants.
Although inactivating papG in E. coli pyelonephritis strain DS17 (O6:K5) by introducing a premature stop codon reduced the ability of this strain to cause ascending pyelonephritis in monkeys (34), inactivation of both pap operons in CFT073 did not diminish CFT073's ability to colonize the mouse urinary tract (32). Likewise, inactivating the putative virulence factor sat (secreted autotransporter toxin) in CFT073 had no impact on in vivo colonization ability (32). Thus, iha joins tonB, iutA, chuA, and fimH as the only known putative urovirulence genes, the mutation of which in the CFT073 background significantly attenuates urovirulence (43). Moreover, ihaCFT073 is the first such gene for which an unmarked, in-frame deletion was used to generate the evaluated CFT073 mutant.
Finally, our epidemiological data provide novel molecular evidence that iha is statistically significantly more frequent among diverse groups of extraintestinal E. coli pathogens than among fecal control isolates. The absence of a categorical association of iha with all infection syndromes and host groups suggests differing pathogenetic mechanisms across clinical and epidemiological settings, a worthy topic for future investigations.
In summary, IhaCFT073's demonstrated pathogenetic importance in a mouse model of UTI complements (i) epidemiological data associating iha with recurrent or invasive UTI and diverse-source bacteremia and (ii) in vitro uroepithelial adherence data. However, even though ihaCFT073 mutants exhibited impaired in vivo colonization in two different bacterial hosts and although this colonization deficit is plausibly attributable to iha's adherence-conferring function, Iha CFT073 has not yet been proven to serve as an adhesin in wild-type pathogens. Nonetheless, our data suggest that Iha deserves further scrutiny as a molecule to exploit for prevention or treatment of human UTIs.
Acknowledgments
This material is based on work supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs (J.R.J.), and National Institutes of Health grants AI47499 (P.I.T.), AI43363 (H.L.T.M.), and DK47504 (J.R.J.).
We thank Jennifer L. Bradley for assistance with the preparation of the manuscript, David Prentiss for preparing the mouse data figures, and Adam Stell for expert technical assistance.
Editor: J. T. Barbieri
REFERENCES
- 1.Achtman, M., A. Mercer, B. Kusecek, A. Pohl, M. Heuzenroeder, W. Aaronson, A. Sutton, and R. P. Silver. 1983. Six widespread bacterial clones among Escherichia coli K-1 isolates. Infect. Immun. 39:315-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arthur, M., C. E. Johnson, R. H. Rubin, R. D. Arbeit, C. Campanelli, C. Kim, S. Steinbach, M. Agarwal, R. Wilkinson, and R. Goldstein. 1989. Molecular epidemiology of adhesin and hemolysin virulence factors among uropathogenic Escherichia coli. Infect. Immun. 57:303-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bauer, R. J., L. Zhang, B. Foxman, A. Siitonen, M. E. Jantunen, H. Saxen, and C. F. Marrs. 2002. Molecular epidemiology of 3 putative virulence genes for Escherichia coli urinary tract infection—usp, iha, and iroN (E. coli). J. Infect. Dis. 185:1521-1524. [DOI] [PubMed] [Google Scholar]
- 4.Burall, L. S., J. M. Harro, X. Li, C. V. Lockatell, S. D. Himpsl, J. R. Hebel, D. E. Johnson, and H. L. Mobley. 2004. Proteus mirabilis genes that contribute to the pathogenesis of urinary tract infection: identification of 25 signature-tagged mutants attenuated at least 100-fold. Infect. Immun. 72:2922-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donnenberg, M. S., and R. A. Welch. 1996. Virulence determinants of uropathogenic Escherichia coli, p. 135-174. In H. L. T. Mobley and J. W. Warren (ed.), Urinary tract infections: molecular pathogenesis and clinical management. ASM Press, Washington, D.C.
- 7.Eden, C. S., R. Freter, L. Hagberg, R. Hull, S. Hull, H. Leffler, and G. Schoolnik. 1982. Inhibition of experimental ascending urinary tract infection by an epithelial cell-surface receptor analogue. Nature 298:560-562. [DOI] [PubMed] [Google Scholar]
- 8.Falkow, S. 1988. Molecular Koch's postulates applied to microbial pathogenicity. Rev. Infect. Dis. 10:S274-S276. [DOI] [PubMed] [Google Scholar]
- 9.Goldberg, M. B., S. A. Boyko, J. R. Butterton, J. A. Stoebner, S. M. Payne, and S. B. Calderwood. 1992. Characterization of a Vibrio cholerae virulence factor homologous to the family of TonB-dependent proteins. Mol Microbiol. 6:2407-2418. [DOI] [PubMed] [Google Scholar]
- 10.Goldberg, M. B., V. J. DiRita, and S. B. Calderwood. 1990. Identification of an iron-regulated virulence determinant in Vibrio cholerae, using TnphoA mutagenesis. Infect. Immun. 58:55-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gunther, N. W., IV, J. A. Snyder, V. Lockatell, I. Blomfield, D. E. Johnson, and H. L. T. Mobley. 2002. Assessment of virulence of uropathogenic Escherichia coli type 1 fimbrial mutants in which the invertible element is phase-locked on or off. Infect. Immun. 70:3344-3354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guyer, D. M., J. S. Kao, and H. L. Mobley. 1998. Genomic analysis of a pathogenicity island in uropathogenic Escherichia coli CFT073: distribution of homologous sequences among isolates from patients with pyelonephritis, cystitis, and catheter-associated bacteriuria and from fecal samples. Infect. Immun. 66:4411-4417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson, J. R. 1991. Virulence factors in Escherichia coli urinary tract infection. Clin. Microbiol. Rev. 4:80-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson, J. R., T. Berggren, and J. C. Manivel. 1992. Histopathologic-microbiologic correlates of invasiveness in a mouse model of ascending unobstructed urinary tract infection. J. Infect. Dis. 165:299-305. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, J. R., and J. J. Brown. 1996. Defining inoculation conditions for the mouse model of ascending urinary tract infection that avoid immediate vesicoureteral reflux yet produce renal and bladder infection. J. Infect. Dis. 173:746-749. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, J. R., P. Delavari, M. Kuskowski, and A. L. Stell. 2001. Phylogenetic distribution of extraintestinal virulence-associated traits in Escherichia coli. J. Infect. Dis. 183:78-88. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, J. R., P. Delavari, and T. T. O'Bryan. 2001. Escherichia coli O18:K1:H7 isolates from patients with acute cystitis and neonatal meningitis exhibit common phylogenetic origins and virulence factor profiles. J. Infect. Dis. 183:425-434. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, J. R., P. Delavari, A. L. Stell, T. S. Whittam, U. Carlino, and T. A. Russo. 2001. Molecular comparison of extraintestinal Escherichia coli isolates of the same electrophoretic lineages from humans and domestic animals. J. Infect. Dis. 183:154-159. [DOI] [PubMed] [Google Scholar]
- 19.Johnson, J. R., C. E. Johnson, and J. N. Maslow. 1999. Clinical and bacteriologic correlates of the papG alleles among Escherichia coli strains from children with acute cystitis. Pediatr. Infect. Dis. J. 18:446-451. [DOI] [PubMed] [Google Scholar]
- 20.Johnson, J. R., H. A. Lockman, K. Owens, S. Jelacic, and P. I. Tarr. 2003. High-frequency secondary mutations after suicide-driven allelic exchange mutagenesis in extraintestinal pathogenic Escherichia coli. J. Bacteriol. 185:5301-5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson, J. R., and J. C. Manivel. 1991. Vesicoureteral reflux induces renal trauma in a mouse model of ascending, unobstructed pyelonephritis. J. Urol. 145:1306-1311. [DOI] [PubMed] [Google Scholar]
- 22.Johnson, J. R., T. T. O'Bryan, P. Delavari, M. Kushkowski, A. Stapleton, U. Carlino, and T. A. Russo. 2001. Clonal relationships and extended virulence genotypes among Escherichia coli isolates from women with a first or recurrent episode of cystitis. J. Infect. Dis. 183:1508-1517. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, J. R., T. T. O'Bryan, M. Kuskowski, and J. N. Maslow. 2001. Ongoing horizontal and vertical transmission of virulence genes and papA alleles among Escherichia coli blood isolates from patients with diverse-source bacteremia. Infect. Immun. 69:5363-5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson, J. R., I. Orskov, F. Orskov, P. Goullet, B. Picard, S. L. Moseley, P. L. Roberts, and W. E. Stamm. 1994. O, K, and H antigens predict virulence factors, carboxylesterase B pattern, antimicrobial resistance, and host compromise among Escherichia coli strains causing urosepsis. J. Infect. Dis. 169:119-126. [DOI] [PubMed] [Google Scholar]
- 24a.Johnson, J. R., E. Oswald, T. T. O’Bryan, M. A. Kuskowski, and L. Spanjaard. 2002. Phylogenetic distribution of virulence-associated genes among Escherichia coli isolates associated with neonatal bacterial meningitis in The Netherlands. J. Infect. Dis. 185:774-784. [DOI] [PubMed] [Google Scholar]
- 25.Johnson, J. R., T. A. Russo, P. I. Tarr, U. Carlino, S. S. Bilge, J. C. Vary, Jr., and A. L. Stell. 2000. Molecular epidemiological and phylogenetic associations of two novel putative virulence genes, iha and iroN(E. coli), among Escherichia coli isolates from patients with urosepsis. Infect. Immun. 68:3040-3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, J. R., A. L. Stell, F. Scheutz, T. T. O'Bryan, T. A. Russo, U. B. Carlino, C. Fasching, J. Kavle, L. Van Dijk, and W. Gaastra. 2000. Analysis of the F antigen-specific papA alleles of extraintestinal pathogenic Escherichia coli using a novel multiplex PCR-based assay. Infect. Immun. 68:1587-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanamaru, S., H. Kurazono, S. Ishitoya, A. Terai, T. Habuchi, M. Nakano, O. Ogawa, and S. Yamamoto. 2003. Distribution and genetic association of putative uropathogenic virulence factors iroN, iha, kpsMT, ompT and usp in Escherichia coli isolated from urinary tract infections in Japan. J. Urol. 170:2490-2493. [DOI] [PubMed] [Google Scholar]
- 28.Langermann, S., R. Mollby, J. E. Burlein, S. R. Palaszynski, C. G. Auguste, A. DeFusco, R. Strouse, M. A. Schenerman, S. J. Hultgren, J. S. Pinkner, J. Winberg, L. Guldevall, M. Soderhall, K. Ishikawa, S. Normark, and S. Koenig. 2000. Vaccination with FimH adhesin protects cynomolgus monkeys from colonization and infection by uropathogenic Escherichia coli. J. Infect. Dis. 181:774-778. [DOI] [PubMed] [Google Scholar]
- 29.Lund, B., F. Lindberg, B. I. Marklund, and S. Normark. 1988. Tip proteins of pili associated with pyelonephritis: new candidates for vaccine development. Vaccine 6:110-112. [DOI] [PubMed] [Google Scholar]
- 30.Miyazaki, J., W. Ba-Thein, T. Kumao, M. O. Yasuoka, H. Akaza, and H. Hayashi. 2002. Type 1, P and S fimbriae, and afimbrial adhesin I are not essential for uropathogenic Escherichia coli to adhere to and invade bladder epithelial cells. FEMS Immunol. Med. Microbiol. 33:23-26. [DOI] [PubMed] [Google Scholar]
- 31.Mobley, H. L., D. M. Green, A. L. Trifillis, D. E. Johnson, G. R. Chippendale, C. V. Lockatell, B. D. Jones, and J. W. Warren. 1990. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect. Immun. 58:1281-1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mobley, H. L., K. G. Jarvis, J. P. Elwood, D. I. Whittle, C. V. Lockatell, R. G. Russell, D. E. Johnson, M. S. Donnenberg, and J. W. Warren. 1993. Isogenic P-fimbrial deletion mutants of pyelonephritogenic Escherichia coli: the role of alpha Gal(1-4) beta Gal binding in virulence of a wild-type strain. Mol. Microbiol. 10:143-155. [DOI] [PubMed] [Google Scholar]
- 33.Riley, L. W., L. N. Junio, L. B. Libaek, and G. K. Schoolnik. 1987. Plasmid-encoded expression of lipopolysaccharide O-antigenic polysaccharide in enteropathogenic Escherichia coli. Infect. Immun. 55:2052-2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roberts, J. A., B. I. Marklund, D. Ilver, D. Haslam, M. B. Kaack, G. Baskin, M. Louis, R. Mollby, J. Winberg, and S. Normark. 1994. The Gal(alpha 1-4)Gal-specific tip adhesin of Escherichia coli P-fimbriae is needed for pyelonephritis to occur in the normal urinary tract. Proc. Natl. Acad. Sci. USA 91:11889-11893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Russo, T. A., C. D. McFadden, U. B. Carlino-MacDonald, J. M. Beanan, T. J. Barnard, and J. R. Johnson. 2002. IroN functions as a siderophore receptor and is a urovirulence factor in an extraintestinal pathogenic isolate of Escherichia coli. Infect. Immun. 70:7156-7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 36a.Sannes, M. R., M. A. Kuskowski, K. Owens, A. Gajewski, and J. R. Johnson. 2004. Virulence factor profiles and phylogenetic background of Escherichia coli isolates from veterans with bacteremia and uninfected control subjects. J. Infect. Dis. 190:2121-2128. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt, H., W. L. Zhang, U. Hemmrich, S. Jelacic, W. Brunder, P. I. Tarr, U. Dobrindt, J. Hacker, and H. Karch. 2001. Identification and characterization of a novel genomic island integrated at selC in locus of enterocyte effacement-negative, Shiga toxin-producing Escherichia coli. Infect. Immun. 69:6863-6873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simon, R., U. Priefer, and A. Puhler. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. BioTechniques. 1:784-791. [Google Scholar]
- 39.Sokurenko, E. V., V. Chesnokova, D. E. Dykhuizen, I. Ofek, X. R. Wu, K. A. Krogfelt, C. Struve, M. A. Schembri, and D. L. Hasty. 1998. Pathogenic adaptation of Escherichia coli by natural variation of the FimH adhesin. Proc. Natl. Acad. Sci. USA 95:8922-8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Talan, D. A., W. E. Stamm, T. M. Hooton, G. J. Moran, T. Burke, A. Iravani, J. Reuning-Scherer, L. Faulkner, and D. Church. 2000. Comparison of ciprofloxacin (7 days) and trimethoprim-sulfamethoxazole (14 days) for acute uncomplicated pyelonephritis in women. JAMA 283:1583-1590. [DOI] [PubMed] [Google Scholar]
- 41.Tarr, P. I., S. S. Bilge, J. C. Vary, Jr., S. Jelacic, R. L. Habeeb, T. R. Ward, M. R. Baylor, and T. E. Besser. 2000. Iha: a novel Escherichia coli O157:H7 adherence-conferring molecule encoded on a recently acquired chromosomal island of conserved structure. Infect. Immun. 68:1400-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tarr, P. I., L. M. Schoening, Y. L. Yea, T. R. Ward, S. Jelacic, and T. S. Whittam. 2000. Acquisition of the rfb-gnd cluster in evolution of Escherichia coli O55 and O157. J. Bacteriol. 182:6183-6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Torres, A. G., P. Redford, R. A. Welch, and S. M. Payne. 2001. TonB-dependent systems of uropathogenic Escherichia coli: aerobactin and heme transport and TonB are required for virulence in the mouse. Infect. Immun. 69:6179-6185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, G., T. S. Whittam, C. M. Berg, and D. E. Berg. 1993. RAPD (arbitrary primer) PCR is more sensitive than multilocus enzyme electrophoresis for distinguishing related bacterial strains. Nucleic Acids Res. 21:5930-5933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Welch, R. A., V. Burland, G. Plunkett III, P. Redford, P. Roesch, D. Rasko, E. L. Buckles, S. R. Liou, A. Boutin, J. Hackett, D. Stroud, G. F. Mayhew, D. J. Rose, S. Zhou, D. C. Schwartz, N. T. Perna, H. L. Mobley, M. S. Donnenberg, and F. R. Blattner. 2002. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl. Acad. Sci. USA 99:17020-17024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woodall, L. D., P. W. Russell, S. L. Harris, and P. E. Orndorff. 1993. Rapid, synchronous, and stable induction of type 1 piliation in Escherichia coli by using a chromosomal lacUV5 promoter. J. Bacteriol. 175:2770-2778. [DOI] [PMC free article] [PubMed] [Google Scholar]