Abstract
Impulsivity refers to a tendency to act rapidly without full consideration of consequences. The trait is thought to result from the interaction between high arousal responses to potential rewards and poor self-control. Studies have suggested that impulsivity confers vulnerability to both addiction and obesity. However, results in this area are unclear, perhaps due to the high phenotypic complexity of addictions and obesity. Focusing on impulsivity, the aim of this review is to tackle the putative overlaps between addiction and obesity in four domains: (1) personality research, (2) neurocognitive tasks, (3) brain imaging, and (4) clinical evidence. We suggest that three impulsivity-related domains are particularly relevant for our understanding of similarities between addiction and obesity: lower self-control (high Disinhibition/low Conscientiousness), reward sensitivity (high Extraversion/Positive Emotionality), and negative affect (high Neuroticism/Negative Emotionality). Neurocognitive studies have shown that obesity and addiction are both associated with increased impulsive decision-making and attention bias in response to drug or food cues, respectively. Mirroring this, obesity and different forms of addiction seem to exhibit similar alterations in functional MRI brain activity in response to reward processing and during self-control tasks. Overall, our review provides an integrative approach to understand those facets of obesity that present similarities to addictive behaviors. In addition, we suggest that therapeutic interventions targeting inhibitory control may represent a promising approach for the prevention and/or treatment of obesity.
Keywords: obesity, addiction, impulsivity, brain, personality and neurocognitive characteristics
Introduction
Obesity and addiction are complex and heterogeneous conditions at the intersection of biology and mental health. A bulk of scientific literature has highlighted the importance of neurobiological and neuropsychological factors in the pathophysiology of obesity (Figure 1) (1, 2). More importantly, growing evidence suggests that obesity shares common mechanisms with addiction in terms of neurobiological systems that underlie reward and self-regulation processes (3–5). The goal of this review is to critically assess the putative overlaps between addiction and obesity in four domains: (1) personality research, (2) neurocognitive task, (3) brain imaging, and (4) clinical evidence.
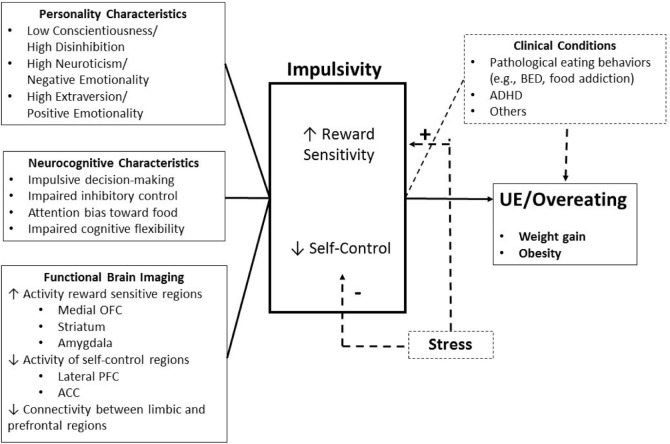
Figure 1.
Brain endophenotype of obesity vulnerability. Personality, cognitive, and functional brain imaging characteristics that increase obesity vulnerability. Uncontrolled eating (UE) results from an interaction of elevated reward sensitivity and poor self-control. OFC, orbitofrontal cortex; PFC, prefrontal cortex; ACC, anterior cingulate cortex; BED, binge-eating disorder; ADHD, attention deficit/hyperactivity disorder; BMI, body mass index.
Brain Mechanisms of Appetite Control and Under Control
Three interconnected brain systems control food intake and eating behavior: (1) the hypothalamus, which responds to internal energy-balance signals, (2) the limbic system [amygdala/hippocampus, insula, orbitofrontal cortex (OFC), and striatum], which is involved in learning and memory and encodes the value or incentive salience of foods, and (3) the cortical (mostly prefrontal) cognitive control system, which enables behavioral self-regulation (6, 7). The normal function of these systems maintains energy homeostasis, enables learning about the nutrient content of foods, and promotes motivation to seek and consume foods as appropriate.
However, individual differences in neurobiological mechanisms involved in the control of food choices and food intake likely explain why some individuals are more susceptible to weight gain than others (8). Indeed, obese individuals may have neurocognitive characteristics that predispose them to overeating upon exposure to favorable environmental or endogenous conditions. One such characteristic is impulsivity. Although many definitions exist (9–14), impulsivity is generally considered as the tendency to act rapidly without full consideration of consequences (15). Sharma et al. (16) recently conducted a meta-analytic principal-components analysis and proposed that impulsivity is a multidimensional construct that includes various distinct psychological components such as disinhibition, neuroticism, extraversion, sensation seeking, inattention, impulsive decision-making, insufficient inhibitory control, and lack of cognitive flexibility (16–19).
Impulsivity is a key component of several neuropsychiatric disorders such as attention deficit/hyperactivity disorder (ADHD), mania, and personality disorders (20, 21). Numerous studies have reported that impulsivity, a personality trait generally observed in individuals with addiction (22–26), may also be associated with high-calorie dietary choices, undercontrolled eating, and the development of obesity (27–31). For instance, individuals characterized by frequent disinhibited behavior and elevated response to potential rewards may be more vulnerable to develop unhealthy weight gain when exposed to the so-called “obesogenic” food-abundant environment (8, 28, 32). Neurobehavioral processes that lead to impulsivity result from the interaction of high arousal response to potential rewards (i.e., reward sensitivity) and poor self-control (i.e., rash spontaneous impulsivity) (14, 28). The reward system is generally thought to encompass projection sites of mesolimbic dopamine neurons, while self-control is dependent on the prefrontal cortex (PFC), especially the lateral PFC, and dorsal anterior cingulate cortex (ACC). Individual differences in impulsivity might constitute a common denominator across obesity and drug addiction. In this regard, several studies have suggested the existence of similarities between addiction and obesity in reward processing (4, 5, 33, 34). In fact, addictive drugs are thought to be addictive by virtue of their actions on neural systems that primarily control appetitive responses to natural rewards such as food (4, 34–36). Dopamine circuitry plays an important role in encoding the reinforcing values of addictive substances (37, 38).
Considering that some neurobehavioral characteristics that confer vulnerability to addiction may also represent risk factors for obesity, this review is aimed at tackling the following question: is the impulsive and poor self-control phenotype identified in drug addiction also present in obesity? The next sections review the evidence in terms of personality, neurocognitive tasks, neuroimaging, and clinical evidence.
Personality Characteristics
Personality traits reflect tendencies for cognitive, emotional, and behavioral responses to events and environments. Traits that capture impulsive tendencies have been associated with unhealthy weight gain and addiction (39). A recent meta-analytic principal component analysis of personality questionnaires identified three distinct impulsivity subdomains (16): (1) Disinhibition versus Constraint/Conscientiousness, (2) Neuroticism/Negative Emotionality, and (3) Extraversion/Positive Emotionality. These dimensions map well to the “Big Five” personality framework (40), the UPPS (Urgency, Perseverance, Premeditation, Sensation Seeking) scale (19), and many other impulsivity conceptualizations (9, 11). Therefore, we use this three-factor decomposition of impulsivity (16) as a base framework to organize evidence that personality-measured impulsivity is associated with addiction and obesity (Table 1).
Table 1.
Summary of the main associations between addiction or obesity and impulsivity measurements.
| Addiction | Obesity | |
|---|---|---|
| Personality characteristics | ||
| High Disinhibition and low Constraint or Conscientiousness | + | + |
| High Neuroticism/Negative Emotionality | + | +, NS |
| High Extraversion/Positive Emotionality | + | +, NS |
| Neurocognitive characteristics | ||
| Impulsive decision-making | + | + |
| Impaired inhibition | +, NS | + |
| Inattention | + | +, NS |
| Impaired set-shifting | +, NS | + |
| Functional brain imaging | ||
| Reward/motivation system | ||
| Medial OFC/VMPFC | ↑ Activity | ↑ Activity |
| Striatum | ↑ Activity | ↑ Activity |
| Amygdala | ↑ Activity | ↑ Activity |
| Self-regulation system | ||
| Lateral PFC | ↓ Activity | ↓ Activity ↓ OFC–PCF connectivity |
| ACC | ↓ Activity | ↓ Activity |
| ↓ ACC–striatum connectivity | ||
+, positive associations; NS, association not significant; ↑, increase; ↓, decrease; OFC, orbitofrontal cortex; VMPFC, ventromedial prefrontal cortex; PFC, prefrontal cortex; ACC, anterior cingulate cortex.
High Disinhibition and Low Constraint/Conscientiousness
The Disinhibition versus Constraint/Conscientiousness factor is comprised of two subfactors associated with behavioral dyscontrol: lack of planning, leading to an inability to refrain from hasty actions, and a lack or perseverance, leading to an inability to maintain self-control in the face of adversity (16). This factor relates to the following measures from commonly used personality scales: lack of perseverance and lack of premeditation from the UPPS, low Conscientiousness from the NEO-Personality Inventory-Revised NEO-PI-R, and motor impulsivity and non-planning impulsivity from the Barratt Impulsiveness Scale (BIS) (16).
Low scores on Conscientiousness have been related to various addictive behaviors (41) including illegal substance abuse (42–44), gambling problems (45), smoking (46–48), and alcohol use (49, 50). Furthermore, lower Conscientiousness increases the risk of relapse after treatment (51). Lack of planning or premeditation assessed using the UPPS scale is also an independent predictor of addiction (52). Thus, the high Disinhibition and low Conscientiousness domain of impulsivity is consistently associated with a higher risk of addiction, supporting the importance of self-control in resisting drug abuse.
Similarly, obesity has consistently been associated with a reduced level of Conscientiousness (28, 53) as measured by the NEO-PI, an association confirmed in a large meta-analysis involving close to 50,000 individuals (54). In a large heterogeneous sample using the BIS, Meule and Blechert (31) found that higher attentional and motor impulsivities were predictive of higher body mass index (BMI) after statistical adjustment for age and sex. However, the effect was small, and non-planning impulsivity was not significantly associated with BMI (31). Finally, studies using the UPPS have also found an association between BMI and lack of perseverance, which is the inability to persist with challenging tasks (55, 56). Furthermore, higher levels of habitual disinhibition, as measured by the Three-Factor Eating Questionnaire, have been associated with body weight gain over time (57). Disinhibition here refers to a tendency to overeat upon exposure to palatable foods or stressful situations, a trait related to consciousness and self-control. In light of these studies, obesity seems to be associated with high Disinhibition and low Conscientiousness. These traits may increase the tendency of an individual to overeat in certain situations and may complicate the maintenance of behaviors associated with body weight reduction in obese individuals (58).
Neuroticism/Negative Emotionality
The factor Neuroticism/Negative Emotionality reflects a tendency to act rashly in response to negative emotions and to experience cravings when in negative mood states (16). It is reflected in neuroticism in the NEO-PI-R, negative urgency in the UPPS, and attentional impulsivity in the BIS (16).
Neuroticism (NEO-PI-R) has been related to various addiction syndromes, including substance abuse (42–44), problem gambling (45), smoking (46–48), and alcohol use (49, 50), and also with increased risk of relapse after treatment (51). Other studies have also reported an association between negative urgency (UPPS) and substance addiction (59–62). In sum, individuals with addictive behavior may engage in drug use as a way of coping with stress and negative emotion.
The relationship between obesity and neuroticism is less evident. While previous reviews have reported a link between the two (28, 53), a recent meta-analysis found no association (54). A possibility for this lack of significant relationship is that body weight is specifically linked only to some facets of negative emotionality. For example, it has been consistently shown that only the impulsiveness subfactor (“N5:Impulsiveness”) of the NEO-PI-R correlates with adiposity (39, 63). Findings from the UPPS support this notion, as negative urgency, a tendency to experience strong impulses during negative affect, has been linked to greater BMI (55, 56). Other factors that could obscure the link between obesity and Neuroticism/Negative Emotionality include the fact that the association may be present only in women and that neuroticism may also predispose to underweight, via a link to eating disorders (64). This could obscure a linear relationship between obesity and neuroticism in population studies. Finally, the link between neuroticism and obesity could be driven by two questions in the Neuroticism scale of the NEO PI-R that specifically target uncontrolled eating (UE) behavior (65, 66).
In summary, the association between the Neuroticism/Negative Emotionality domain and obesity is somewhat less consistent than that with Conscientiousness and Disinhibition. Nonetheless, this personality trait may predispose an individual to overeating in conditions of emotional distress (67), which may lead to adiposity in the long term.
Extraversion/Positive Emotionality
The Extraversion/Positive Emotionality factor refers to sensation seeking and sensitivity to appetitive or rewarding cues (16). Individuals with high Extraversion/Positive Emotionality are sensitive to positive environmental stimuli and more likely to engage in impulsive or reward-seeking behaviors when they experience positive emotions. They are said to seek novel and exciting experiences. Extraversion/Positive Emotionality correlates with the Extraversion domain in the Five-Factor Model of personality and with Sensation Seeking of the UPPS (16). The Sensitivity to Reward portion of the Sensitivity to Punishment and Sensitivity to Reward Questionnaire (SPSR) is a self-report questionnaire that also assesses this dimension (28, 68).
Numerous studies suggest that reward-driven impulsivity represents a risk factor for both drug addiction and overeating by enhancing the motivation to obtain drugs or palatable foods (69, 70). Higher scores in Extraversion have been related to drug addiction (47). A related trait, positive urgency, the tendency to act rapidly in response to positive emotions, was also correlated to substance addiction (59–62). In addition, Sensation Seeking is commonly associated with substance-use disorders and alcohol problems (62). In sum, the literature is consistent in associating the Extraversion/Positive Emotionality domain of impulsivity to addictive disorders.
Some studies have proposed that high BMI is associated with increased levels of Extraversion (28, 53). Higher scores in Extraversion also seem to predict prospective weight gain (after 2 years) (71). However, contradictory findings do exist, with a meta-analysis (54) failing to show a consistent relationship between obesity and Extraversion in longitudinal studies. However, Davis et al. (72) found that reward sensitivity, as assessed by the SPSR, was associated with maladaptive eating behaviors such as preference for high-calorie foods and overeating (72). They suggested that some individuals may have greater reactivity to food cues and that weight management, in these individuals, may represent a continuous struggle in the modern obesity-promoting food environment. Using the SPSR, this group also demonstrated an inverted U-shaped relationship between reward sensitivity and BMI in a sample of subjects covering a large spectrum of adiposity values, suggesting that lean and severely obese subjects were less sensitive to reward than overweight and obese subjects (73). By using the Behavioral Activation Scale, other groups have also provided evidence of a quadratic relationship between BMI and reward sensitivity (74, 75). To explain this curvilinear relationship, Davis and Fox (73) proposed that both hyper- and hyposensitivity to reward could predispose to obesity. The possibility of an inverted U-shape relationship between BMI and Extraversion suggests that differences in the range of sampled BMI across studies might account for the discrepancies in the literature. In addition to this, gender might modulate the correlation between Extraversion and BMI. For women, lower scores in Extraversion seem to relate to higher adiposity (76, 77), while the opposite has been reported in males (76, 78).
Overall, although contradictory findings do exist, the current evidence points in the direction of similar impulsivity profiles in obesity and addictive disorders. Specifically, these two disorders seem to share lower cognitive control (high Disinhibition/low Conscientiousness), and a tendency toward making impulsive decisions in response to positive (high Extraversion/Positive Emotionality) and negative (high Neuroticism/Negative Emotionality) mood states. Figure 2 displays a comprehensive overview of personality differences in obesity and addiction as derived from Ref. (39, 42, 79). This shows that while, on a broad level, obesity seems to be similar to addictive behaviors, there are also differences at the finer level of personality subscales.
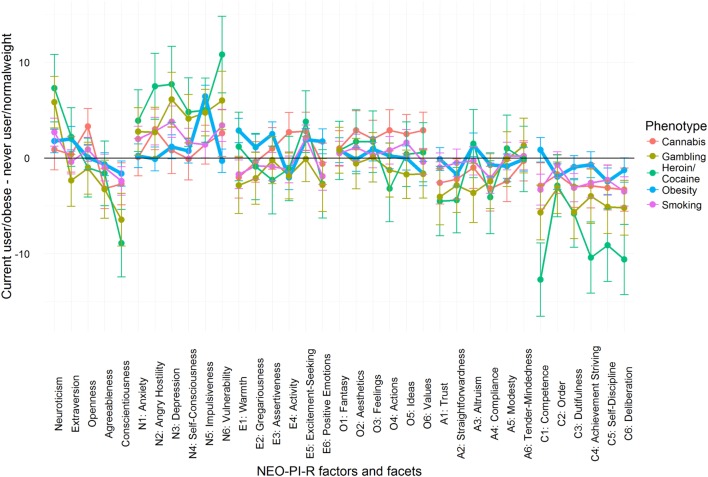
Figure 2.
Personality profiles of obesity and addictive phenotypes according to NEO-personality inventory revised. We present the difference in T-score units between obese minus normal weight group and addiction phenotype group minus control group. On a broad factor level, all phenotypes share higher Neuroticism (high Negative Emotionality) and lower Agreeableness and Conscientiousness (high Disinhibition). However, on a finer facet level, the profiles become less similar. For instance, obesity sets apart from other addictions only peaking at one facet of Neuroticism, and not on all facets of Conscientiousness. Therefore, while there are broad similarities, obesity and addictive phenotypes are not fully similar to each other. Mean scores were obtained from these papers (39, 42, 79).
Neurocognitive Tasks
Laboratory-based neurocognitive tasks can be used to measure inhibitory control or self-regulation. Commonly used examples are the delay discounting task, the stop-signal task (SST), the Go/No-Go task, the Stroop task, and the Wisconsin card sorting task (WCST) (80). These neurocognitive tests assess various dissociable dimensions of impulsivity, including impulsive choice, impulsive responding, and inattention (15, 81). Sharma et al. (16) also performed a meta-analytic principal-components factor analysis of the most commonly used behavioral task measures of impulsivity and they identified four major domains: (1) impulsive decision-making, (2) inattention, (3) inhibition, and (4) shifting. The next sections describe how these four domains of impulsivity are associated with addiction and obesity (Table 1).
Impulsive Decision-Making
Impulsive decision-making (or impulsive choice) refers to a tendency not to delay gratification and to prefer immediately available rewards (16). It is typically tested with the delay discounting task, in which participants must choose between an immediate, smaller monetary sum and a larger, delayed amount (82). A steeper delay discounting rate is associated with a greater preference for immediate rewards, which reflects impulsive decision-making.
Kirby and Petry (83) have demonstrated using a questionnaire version of this task that substance-addicted individuals have higher discounting rates for delayed rewards than controls. Two meta-analyses also provided strong evidence that steeper impulsive discounting rate is associated with the severity and the frequency of addictive behaviors (84, 85). The magnitude of the association was similar between various types of addictive problems (alcohol, gambling, tobacco, cannabis, opiates, and stimulants) (85). The same group also reported a similar relationship in obesity: although results vary, their meta-analysis concluded that obesity is associated with steeper delay discounting of future monetary and food rewards (86). Interestingly, Weygandt et al. (87) recently found that less functional MRI (fMRI) activation of inhibitory-control areas during a delay discounting task is associated with poor weight loss maintenance in the long term. More specifically, obese subjects seem to have greater delay discounting for food compared to other type of rewards. Similarly, substance-addicted subjects have greater delay discounting for drugs compared to other type of rewards (28, 85, 86). Impulsive decision-making in addiction and obesity may explain why some individuals engage in maladaptive behaviors that are immediately rewarding but detrimental in the long run.
Another perspective in impulsive decision-making revolves around the concept of risk sensitivity. Risk sensitivity refers to the individual degree of attraction or aversion to uncertain outcomes (88). A moderate risk-seeking behavior may confer advantages in the discovery of new environments and resources and might lead to experiencing exciting adventures. However, an excessive attraction toward risk may also be associated with adverse consequences and might have a role in the development of drug addiction. In recent years, the concept of risk sensitivity has been used to describe impulsive behavior in addiction and obesity (89, 90). Both addiction and obesity might involve to some extent an approach tendency toward short-term pleasure despite the risk of long-term negative consequences (89, 91). Several studies have suggested the existence of addiction-related alterations in risky choices. For example, compared with healthy controls, participants who binge drink exhibited increased risk-seeking when anticipating large unlikely monetary losses (92). Risky decision-making and higher delay discounting also appear to hamper the maintenance of abstinence following treatment (93).
Relatively few studies have directly examined risk-taking similarities or differences between addiction and obesity to date. One study found that obese individuals with and without binge-eating disorder (BED) made as many risky choices in a monetary task as drug addicts (94).
Inhibition
The inhibition domain refers to the ability to suppress prepotent motor responses (16). Tasks that test inhibition include the Go/No-Go and the SST (80, 82). In the Go/No-Go task, individuals are asked to answer as quickly as possible when a repeated visual stimulus appears (Go signal) but to inhibit their response when a rare stop signal appears (No-Go signal). In the SST task, the stop signal is presented after the Go signal to measure the ability of an individual to stop an already initiated response (95).
Considerable evidence links drug addiction to impaired inhibitory control (96–98). A meta-analysis of 97 studies using the SST or Go/No-Go tasks reported that impaired inhibitory control is generally observed in subjects with heavy substance-use disorders and pathological gambling (99). However, there was lack of evidence for inhibitory deficit in subjects diagnosed with cannabis, opioid, or Internet addiction (99).
Similarly, obesity has been linked to poor inhibitory control. A comprehensive literature review found that obese and overweight individuals have lower inhibitory-control performance in food-specific versions of the SST (100). The authors proposed that the SST may be a good marker to identify individuals at high risk of weight gain or less responsive to weight loss interventions (100). Poor inhibitory control is also associated with higher prospective weight gain (101, 102) and food intake (103). Furthermore, a recent meta-analysis confirmed that obese adults display inhibitory-control deficits compared to lean controls (104). Similar findings have been reported in children and adolescents (104–108). However, Loeber et al. (109) found no significant differences between lean and obese participants in performance during a food-related Go/No-Go task. Furthermore, others did not find an effect of BMI per se on SST performance in response to food, but rather a complex interaction between BMI and impulsivity (110).
Furthermore, Voon et al. (111) used a serial reaction time task adapted from rodent experiments to assess a somewhat different form of motor impulsivity: waiting impulsivity or premature responding. They found that premature responses were significantly higher in addicted individuals (alcohol, smoking, and drugs) but not in obese or BED subjects. Thus, certain forms of motor impulsivity seen in addiction are not present in obesity.
Inattention
The third impulsivity domain considered here refers to the ability to focus attention on specific activities while suppressing the response to distracting stimuli (16). The Stroop task is typically used to measure the inattention domain of impulsivity (16). This task requires participants to identify (usually verbally) the color of a written color word, without reading the word itself. When the word is printed in a color that is incongruent with the word (for example, the word blue printed in green), there is a conflict between word reading and color naming. PFC has been implicated in the performance of the Stroop task (112).
A refinement of this task, the “addiction-Stroop,” in which the distractor stimuli represent the addictive substance of interest, has also been used to assess altered attentional processes associated with addictive behaviors (113). Indeed, there is considerable evidence that individuals with addiction have an attentional bias toward drug-related cues, which may play an important role in drug craving, consumption, and relapse (114). Similarly, some studies have reported that obese individuals may have attentional biases toward food-related cues, which may increase food consumption and weight gain over time (115). Hall et al. (116) found that elevated levels of inattention were predictors of high-calorie snack consumption. Furthermore, a recent study demonstrated that obese individuals are characterized by lower scores on the traditional Stroop task (117). Even though some reviews reported inconsistent associations between attentional bias for food-related cues and obesity (28, 115, 118, 119), we previously concluded in a comprehensive review that the Stroop task seems to be one of the most consistent cognitive control tasks demonstrating replicated associations with obesity and weight-related eating behaviors (28).
Shifting
Behavioral flexibility, or the ability to switch attentional or task set in response to changing rules, has also been linked to impulsivity (16). It is typically evaluated with the WCST (16). During this task, participants are asked to match a response card to one of four category cards based on specific rules (e.g., color, shape, or number) (120). The rules change over time and subjects need to modify their response accordingly. A tendency to fail to switch is called perseveration, and it may reflect a form of impulsivity. Poor cognitive flexibility has been associated with compulsive behaviors (121, 122).
A recent review by Morris and Voon (122) argued that the links between cognitive flexibility assessed using the WCST and addiction are inconsistent. Indeed, some studies reported impaired cognitive flexibility in substance-addicted (123) and non-substance-addicted (gambling, bulimia) individuals (124). However, others found no significant association between performance on the WCST and addiction (125–127). With respect to obesity, a recent study reported impaired performance on the WCST in obese individuals compared to individuals with other eating disorders (128). In addition, a meta-analysis (121) and systematic review (118) both reported impaired WCST performance in obese individuals compared to controls. However, overweight rather than obese individuals were not characterized by set-shifting impairment (121).
Overall, current evidence from neurocognitive tasks is that obese and addicted individuals are both generally characterized by higher impulsive decision-making and attentional bias in response to drug or food cues. In addition, obesity is usually associated with altered cognitive flexibility (set-shifting) assessed with the WCST and poor inhibitory control assessed with the SST.
Neuroimaging
Neuroimaging has been used to investigate functional and anatomical neural correlates of the vulnerability to drug abuse and overeating. Vulnerability to addiction can be considered as resulting from the interaction of increased incentive response to drug cues, propensity for habit formation, poor self-control, and heightened negative emotionality (129, 130). These processes are related to different but interconnected brain systems: (1) the mesolimbic dopamine system, implicated in reward, motivation, and habit formation, which includes the ventral tegmental area, ventral striatum, anterior insula, OFC, amygdala, and hippocampus and (2) cognitive control circuits, implicated in self-regulation, including middle and inferior lateral PFC, ACC, and insula (131). Previous neuroimaging studies have shed light on the role of the mesolimbic system in the pathophysiology of addiction (132–139). Participants with addiction seem to exhibit increased fMRI activation in ventral striatum, amygdala, and medial regions of OFC in response to drug cues (133). In general, these results are consistent with the observation that participants with drug addictions exhibit a heightened attentional or motivational focus toward drug-related stimuli (130).
With regards to cognitive control circuits, adolescents who initiate substance use seem to exhibit reduced blood oxygen level dependent (BOLD) activity in the dorsolateral prefrontal cortex (DLPFC), putamen, and inferior parietal cortex during a Go/No-Go task, suggesting that baseline dysfunction in these areas could predict the initiation of drug use (140, 141). In this vein, theoretical work has highlighted the key role of PFC areas in the endophenotype of addiction vulnerability (112). For instance, participants with addiction seem to exhibit prefrontal dysfunction, implicating the dorsal PFC (dACC and DLPFC) involved in self-control, the ventromedial prefrontal cortex (VMPFC) involved in emotional regulation and salience attribution, as well as the ventrolateral prefrontal cortex and lateral OFC involved in inhibitory or automatic responses (112). It has been proposed that the PFC is involved in addictive behaviors through its capacity to regulate subcortical regions implicated in reward processes (112, 142). For example, the strength of the connectivity between dACC and striatum has been negatively associated with the severity of nicotine addiction (143). PFC dysfunction might be implicated in an endophenotype named impaired response inhibition and salience attribution (112). This endophenotype both increases sensitivity to drug cues and reduces the capacity to inhibit maladaptive behaviors (144). Consistent with these findings, drug craving seems to involve the amygdala, ACC, OFC, and DLPFC (145), suggesting the involvement of both reward-related and inhibitory-control resources.
Numerous brain imaging studies also support the notion that vulnerability to weight gain and overeating may result from the interaction between elevated food reward sensitivity (incentive salience of the cue) and poor inhibitory control. In response to visual food stimuli, participants with obesity exhibit increased activation in the dorsomedial PFC, the ventral striatum, the parahippocampal gyrus, the precentral gyrus, the superior/inferior frontal gyrus (IFG), and the ACC relative to lean subjects (119–121). These brain regions are thought to encode reward responses, incentive salience, motor coordination, and memory. Longitudinal study designs have shown that increased BOLD activity in reward-related areas (i.e., ventral striatum and OFC) predicts weight gain, suggesting a link between heightened reward responsivity and the development of obesity (146, 147). With regards to inhibitory-control circuits, participants with obesity seem to show consistent blunted activity in the DLPFC and insula in response to visual food cues (148), suggesting a reduced engagement of neural resources associated with inhibition, executive control, and interoceptive awareness. Of note, longitudinal studies have reported that increased activation in the DLPFC in response to high-calorie food images is associated with successful voluntary weight loss (149, 150). An interesting possibility is that self-control processes in the DLPFC may downregulate the activity of the VMPFC and thus, modulate eating choices (151). Supporting this model, stronger functional coupling between the DLPFC and the VMPFC has been associated with successful dietary weight loss (102) and healthier dietary decisions (151). Furthermore, other fMRI studies have reported that the regulation of food craving was associated with increased activity in the DLPFC, IFG, and dorsal ACC (152–154).
A few neuroimaging studies in obesity have specifically addressed cognitive control processes by using cued inhibitory-control paradigms. Here, fMRI studies have found negative associations between brain activation in executive-control regions (lateral PFC) and BMI (155–157). Longitudinal studies have reported that activity in the DLPFC during cognitive control tasks seems to predict successful weight loss after treatment (87, 102). Conversely, impairment of cognitive control over appetitive regions may (1) decrease the acquisition of behaviors leading to successful weight loss and (2) enhance the motivation to consume palatable foods, even in the absence of energy requirements (6, 158).
Together, the aforementioned studies suggest that participants with obesity and patients with addictions present similar functional alterations in frontal regions and in mesocorticolimbic circuits. However, to date few neuroimaging studies have directly compared the impact of obesity and various types of addictions on brain activation. This last point is especially relevant, since food and drug cues seem to activate similar brain regions involved in reward processes, such as striatum, amygdala, OFC, and insula (135). A previous meta-analysis observed that participants with obesity and subjects with different forms of substance addiction exhibited similar heightened BOLD activity in the amygdala and ventral striatum in response to the relevant cues (food in obesity and drugs in addiction) (159).
Overall, current fMRI studies provide evidence for the existence of shared neural mechanisms associated with obesity and different forms of addiction. Poor inhibitory control in combination with increased reward sensitivity and attention to cues (foods or drugs) may be relevant for both obesity and addictive disorders.
Clinical Evidence
Binge-Eating Disorder
Binge-eating disorder (BED) is an eating disorder characterized by recurrent episodes of consumption of larger than normal amounts of food in short periods of time (160). These binges are associated with a sense of loss of control and subsequent distress and culpability. Many studies report that individuals with BED display increased impulsivity, altered reward sensitivity, and altered attentional and memory biases to food-related stimuli (161, 162). For example, individuals with BED have steeper delay discounting of rewards (163) and lower activation in the PFC regions during inhibitory-control tasks (164, 165), suggesting that impulsivity may be importantly related to BED. BED presents phenotypic similarities with substance-use disorders (166). Indeed, substance-use disorders and BED are both characterized by loss of control over consumption, and chronic overconsumption despite negative consequences (167).
The observation that BED shares behavioral and neural underpinnings with substance-use disorders has led to the use of the expression “food addiction,” specifically with respect to individuals who meet BED diagnostic criteria, but also more generally as an explanation for obesity. The model hypothesizes that hyper-palatable foods may lead to an addictive response in vulnerable and high-risk individuals (168, 169). Individual variations in “food addiction” can be operationalized by means of scales such as the Yale Food Addiction Scale (YFAS) (166, 170, 171) or the YFAS 2.0 (a revised version adapted for the DSM-5 criteria for substance-related and addictive disorders) (172). However, the model of “food addiction” in humans remains controversial (173–177). The main criticism is that the model is based mostly on animal studies and that the type and quantity of food that characterize “food addiction” are imprecise (173, 174, 177). Furthermore, animals rarely exhibit addition-like behaviors toward sugar; these behaviors only occur when access to sugar is intermittent, and not because of some neurochemical effect of sugar (177). This failure in characterizing what constitutes an addictive agent in foods has led to some theorists to advocate in favor of referring to the phenomenon as “eating addiction” instead (178). We have proposed the term “UE” (65). In addition, even though “food addiction” scores are positively correlated with several measures of adiposity (179), not all individuals with obesity or BED exhibit “food addiction,” and conversely, some individuals displaying “food addiction” are not obese (174, 180). Davis (171) suggests that “food addiction” constitutes the last stage of an overeating spectrum (65) and may represent an extreme subtype of BED. In a similar vein, BED has been strongly associated with obesity; however, BED can also occur in individuals with a wide spectrum of body weight (181). As suggested by previous studies, obese individuals with BED seem to represent a specific and possibly rare subtype of obesity (166, 182). Nonetheless, while the lines between BED, “food addiction,” and obesity are ill-defined, these conditions seem to share common characteristics including impulsivity and reward dysfunction.
Attention Deficit/Hyperactivity Disorder
Attention deficit/hyperactivity disorder is a neurodevelopmental disorder characterized by inattention, hyperactivity, and impulsivity (160). Neuroimaging studies have suggested a link between ADHD and dysfunction in frontostriatal circuits. For instance, anatomical studies have observed that participants with ADHD exhibit cortical thinning in the PFC, associated with inhibitory-control deficits (183, 184). A frequent comorbidity of ADHD is substance-use disorders (185–187). For example, a longitudinal study found that children and adolescents with ADHD are at higher risk of substance-use disorders and tobacco smoking after a 10-year follow-up period (188).
There is also growing evidence of a link between ADHD and obesity. However, this relationship remains controversial (189, 190). A recent meta-analytic report found a significant association between obesity and ADHD in both children and adults after controlling for possible confounding factors (e.g., gender, study design, country, and study quality) (190). Conversely, another recent meta-analysis reported that the strength of the association between ADHD and obesity was weak. Nevertheless, the effect size increases with age suggesting that the association is stronger in adults than children (189). Two longitudinal studies found that individuals with ADHD are at higher risk of obesity than controls (191, 192). A recent systematic review found that the strength of the association between ADHD and disordered-eating behavior was moderate (193). Furthermore, genetic correlations were found between ADHD, BMI, and smoking (194). To explain the link between ADHD and obesity, researchers have hypothesized that these two disorders exhibit common neurocognitive features, such as impulsivity and inattention (195). Davis et al. (196) also suggested that individuals with ADHD may be more inattentive to their internal signals of hunger and satiety, which may lead to subsequent overeating. Interestingly, the pharmacological treatment of ADHD with dopaminomimetics may facilitate weight control by modulating satiety signals and eating behaviors (197). Overall, ADHD appears to be associated with both addiction and obesity and with the neural endophenotypes that predispose to both, namely, self-control deficits and impulsivity.
Stress or Emotion Dysregulation
Stress is a ubiquitous risk factor across several psychiatric disorders, and it has important implications for our current understanding of addiction and obesity (198, 199). Studies have shown associations between stress and drug craving (200, 201). Chronic exposure to life stressors also predisposes to the transition from casual drug use to substance abuse (202), and it seems to increase the risk of relapse among abstinent users (202). Stress is one of the central elements of the model of addiction proposed by Koob and Le Moal (203). According to this framework, addiction can be conceived as a continuous process of hedonic and homeostasis dysregulation (204). The spiraling distress cycle describes how continued drug use along with failures in self-regulation can cause chronic dysregulation of the reward system. As the drug use escalates, patients reach a pathological state that is characterized by increased negative affect and distress, which are particularly pronounced after drug withdrawal. The model hypothesizes that this aversive emotional state constitutes a powerful motivator for drug-seeking, since patients at severe stages of drug addiction will consume drugs to find relief from distress (203).
With regards to obesity, mounting evidence suggests that stress can modify eating patterns (198, 205). Negative mood states or chronic stress increase subjective appetite or food cravings, selective attention toward food, and individual preferences for high-calorie snacks (e.g., sweets and chocolate) (206–209). Increments in food seeking and food consumption during emotionally demanding situations might relate to the fact that eating a so-called “comfort food” promotes improvements in negative affect (210, 211), in line with the model of Koob and Le Moal. The relationship between stress and food intake, however, presents remarkable interindividual variations. Indeed, stress can be associated with both augmented and diminished appetite (205), with around 30% of the population experiencing increases in appetite, 48% appetite suppression, and the rest no change (212). Studies have suggested that obesity constitutes a crucial predictor of increases in food intake during stress. For instance, while work stress has been associated with weight gain in male participants with elevated BMI, the same psychological stressor leads to weight loss in lean participants (213). Finally, individuals with obesity seem to suffer a higher numbers of adverse life events and chronic stressors compared to lean individuals (198).
Stress acts on brain areas involved in both sides of appetite regulation: the reward/motivation system and the inhibitory-control pathways. For example, Tryon et al. (214) found that in response to high-calorie food pictures, women characterized by higher chronic stress have increased activation in brain regions involved in reward and motivation as well as reduced activation in prefrontal regions. These women also demonstrated greater consumption of high-calorie foods after the scanning session. In a similar vein, Maier et al. (215) compared the neural responses between participants assigned to a laboratory stressor versus those assigned to a neutral condition during a food choice task. Subjects assigned to the stressor put greater value on the taste of the food items presented. Paralleling this, bilateral amygdala and right nucleus accumbens reflected the relative taste value of chosen options more strongly in stressed compared to control participants. The authors interpreted these findings as suggesting that acute stress may increase the rewarding attributes of food stimuli (215). Furthermore, Jastreboff et al. (216) observed that obese individuals exhibit increased activation in striatal, insular, and hypothalamic regions in response to stress and favorite-palatable food cues compared to lean individuals. These increased corticolimbic-striatal activations in response to food cues and stress were also positively associated with food craving ratings, suggesting that some individuals may be at higher risk to consume high-calorie foods during stressful periods (216). On the basis of the theoretical model proposed by Sinha and Jastreboff (198), highly palatable food cues in combination with chronic stress exposure could modulate emotions, metabolic responses (e.g., glucose and energy-balance hormones), and stress-responsive hormones (e.g., adrenocorticotrophin cortisol) that influence brain regions involved in reward, motivation, self-control, and decision-making. Thus, stress sensitivity likely interacts with reward systems to promote either drug use or overeating (or both) in vulnerable individuals (217).
Conclusion
Evidence of Non-Overlap
Despite the similarities exposed here there is also evidence that obesity and other addictive behaviors differ and may only overlap partially (218). While some studies have observed higher rates of addictive disorders in obese populations (219, 220), others have reported a lack of significant relationships between addiction and obesity (221–224). Methodological aspects (224) as well as the remarkable intrinsic complexity and heterogeneity associated with obesity and addiction (225) might help to explain the discrepancies observed between studies. Multiple factors (e.g., impulsivity and depressive symptoms) might interact with obesity/eating behavior in complex ways that are difficult to account for in studies with relatively small sample sizes. These factors may explain conflicting studies in the literature. Furthermore, an interesting possibility is that some subtypes of obesity might be at higher risk for developing addictive behavior (33). For instance, some post-bariatric surgery patients seem to exhibit increased rates of addictive problems (226–228). This phenomenon is commonly referred to as “cross addiction” or “addiction transfer.”
Limitations of the present review should be acknowledged. Obesity results from a chronic positive imbalance between energy intake and energy expenditure. Almost all studies in obesity and impulsivity presented here describe obese participants in terms of the BMI (kg/m2). While the BMI is an indicator of total adiposity, an important disadvantage is that it might not necessarily be associated with addictive-like eating patterns. In this vein, it is thus crucial to include a description of the participants in terms of their eating behavior or their UE patterns. Furthermore, clinical conditions that often present in comorbidity with obesity, such as BED or ADHD are not systematically evaluated and excluded in all the studies included in the present review. This point constitutes an important limitation that might obscure or inflate the overlap between addiction and obesity.
Concluding Sentences
Addiction and obesity are health problems with high phenotypic complexity. Growing evidence from personality, cognitive neuroscience, and brain imaging studies suggest that the combination of reduced cognitive control and, to a lesser extent, increased reward sensitivity is a risk factor for the development and maintenance of both syndromes. This is especially true in the domain of cognitive control (Figure 2) as measured by the Conscientiousness versus Disinhibition factors on personality questionnaires, by cognitive tasks of executive function, or by diminished recruitment of areas associated with cognitive control, such as the lateral PFC, in fMRI studies. Individuals characterized by high food drive and high cognitive control might better control their body weight in an environment rich in palatable foods.
The present review provides a comprehensive view of impulsivity-related alterations in obesity and addiction, covering results from the personality, neurocognitive, neuroimaging, and clinical fields. The conclusions of the review have the potential to inform clinical approaches aimed at the prevention or treatment of obesity. Diminished self-control is a predictor of poorer treatment outcomes in substance abuse disorders (51) and might also be one in obesity treatment. The findings of the present review might, as such, be of interest to cognitive behavioral therapists aiming to foster impulse control strategies in participants with obesity. Specific inhibitory-control interventions may also represent a promising approach for the prevention of obesity in individuals with poor self-control and high reward sensitivity.
Author Contributions
AM: design and conception of the manuscript; wrote the manuscript; and gave final approval. UV and IG: wrote and critically revised the manuscript; gave final approval. AD: design and conception of the manuscript; wrote and critically revised the manuscript; study supervision and responsible for funding; and gave final approval.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was supported by operating funds from the Canadian Institutes of Health Research to AD. AM is the recipient of a postdoctoral fellowship from Canadian Institutes of Health Research.
References
- 1.O’Rahilly S, Farooqi IS. Human obesity: a heritable neurobehavioral disorder that is highly sensitive to environmental conditions. Diabetes (2008) 57(11):2905–10. 10.2337/db08-0210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Volkow ND, O’Brien CP. Issues for DSM-V: should obesity be included as a brain disorder? Am J Psychiatry (2007) 164(5):708–10. 10.1176/ajp.2007.164.5.708 [DOI] [PubMed] [Google Scholar]
- 3.Frascella J, Potenza MN, Brown LL, Childress AR. Carving addiction at a new joint? Shared brain vulnerabilities open the way for non-substance addictions. Ann N Y Acad Sci (2010) 1187:294–315. 10.1111/j.1749-6632.2009.05420.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Volkow ND, Wise RA. How can drug addiction help us understand obesity? Nat Neurosci (2005) 8(5):555–60. 10.1038/nn1452 [DOI] [PubMed] [Google Scholar]
- 5.Volkow ND, Baler RD. NOW vs LATER brain circuits: implications for obesity and addiction. Trends Neurosci (2015) 38(6):345–52. 10.1016/j.tins.2015.04.002 [DOI] [PubMed] [Google Scholar]
- 6.Dagher A. Functional brain imaging of appetite. Trends Endocrinol Metab (2012) 23(5):250–60. 10.1016/j.tem.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 7.Rangel A. Regulation of dietary choice by the decision-making circuitry. Nat Neurosci (2013) 16(12):1717–24. 10.1038/nn.3561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boswell RG, Kober H. Food cue reactivity and craving predict eating and weight gain: a meta-analytic review. Obes Rev (2016) 17(2):159–77. 10.1111/obr.12354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray JA. The psychophysiological basis of introversion-extraversion. Behav Res Ther (1970) 8(3):249–66. 10.1016/0005-7967(70)90069-0 [DOI] [PubMed] [Google Scholar]
- 10.Eysenck SB, Eysenck HJ. The place of impulsiveness in a dimensional system of personality description. Br J Soc Clin Psychol (1977) 16(1):57–68. 10.1111/j.2044-8260.1977.tb01003.x [DOI] [PubMed] [Google Scholar]
- 11.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clin Psychol (1995) 51(6):768–74. [DOI] [PubMed] [Google Scholar]
- 12.Zuckerman M. Behavioral Expressions and Biosocial Bases of Sensation Seeking. New York: Cambridge University Press; (1994). [Google Scholar]
- 13.Cloninger CR. A systematic method for clinical description and classification of personality variants. A proposal. Arch Gen Psychiatry (1987) 44(6):573–88. 10.1001/archpsyc.1987.01800180093014 [DOI] [PubMed] [Google Scholar]
- 14.Dawe S, Loxton NJ. The role of impulsivity in the development of substance use and eating disorders. Neurosci Biobehav Rev (2004) 28(3):343–51. 10.1016/j.neubiorev.2004.03.007 [DOI] [PubMed] [Google Scholar]
- 15.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron (2011) 69(4):680–94. 10.1016/j.neuron.2011.01.020 [DOI] [PubMed] [Google Scholar]
- 16.Sharma L, Markon KE, Clark LA. Toward a theory of distinct types of “impulsive” behaviors: a meta-analysis of self-report and behavioral measures. Psychol Bull (2014) 140(2):374–408. 10.1037/a0034418 [DOI] [PubMed] [Google Scholar]
- 17.Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn Sci (2012) 16(1):81–91. 10.1016/j.tics.2011.11.009 [DOI] [PubMed] [Google Scholar]
- 18.Franken IHA, van Strien JW, Nijs I, Muris P. Impulsivity is associated with behavioral decision-making deficits. Psychiatry Res (2008) 158(2):155–63. 10.1016/j.psychres.2007.06.002 [DOI] [PubMed] [Google Scholar]
- 19.Whiteside S, Lynam D. The Five Factor Model and impulsivity: using a structural model of personality to understand impulsivity. Pers Individ Dif (2001) 4:669–89. 10.1016/S0191-8869(00)00064-7 [DOI] [Google Scholar]
- 20.Grant JE, Potenza MN, editors. The Oxford Handbook of Impulse Control Disorders. 1st ed Oxford University Press; (2011). Available from: http://www.oxfordhandbooks.com/view/10.1093/oxfordhb/9780195389715.001.0001/oxfordhb-9780195389715 [Google Scholar]
- 21.Chamberlain SR, Sahakian BJ. The neuropsychiatry of impulsivity. Curr Opin Psychiatry (2007) 20(3):255–61. 10.1097/YCO.0b013e3280ba4989 [DOI] [PubMed] [Google Scholar]
- 22.Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) (2008) 200(1):1–26. 10.1007/s00213-008-1173-0 [DOI] [PubMed] [Google Scholar]
- 23.Potenza MN, Taylor JR. Found in translation: understanding impulsivity and related constructs through integrative preclinical and clinical research. Biol Psychiatry (2009) 66(8):714–6. 10.1016/j.biopsych.2009.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verdejo-García A, Bechara A. A somatic marker theory of addiction. Neuropharmacology (2009) 56(Suppl 1):48–62. 10.1016/j.neuropharm.2008.07.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine taking. Science (2008) 320(5881):1352–5. 10.1126/science.1158136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brewer JA, Potenza MN. The neurobiology and genetics of impulse control disorders: relationships to drug addictions. Biochem Pharmacol (2008) 75(1):63–75. 10.1016/j.bcp.2007.06.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis C. Psychobiological traits in the risk profile for overeating and weight gain. Int J Obes (2005) 2009(33 Suppl 2):S49–53. 10.1038/ijo.2009.72 [DOI] [PubMed] [Google Scholar]
- 28.Vainik U, Dagher A, Dubé L, Fellows LK. Neurobehavioural correlates of body mass index and eating behaviours in adults: a systematic review. Neurosci Biobehav Rev (2013) 37(3):279–99. 10.1016/j.neubiorev.2012.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guerrieri R, Nederkoorn C, Jansen A. The interaction between impulsivity and a varied food environment: its influence on food intake and overweight. Int J Obes (Lond) (2008) 32(4):708–14. 10.1038/sj.ijo.0803770 [DOI] [PubMed] [Google Scholar]
- 30.Guerrieri R, Nederkoorn C, Stankiewicz K, Alberts H, Geschwind N, Martijn C, et al. The influence of trait and induced state impulsivity on food intake in normal-weight healthy women. Appetite (2007) 49(1):66–73. 10.1016/j.appet.2006.11.008 [DOI] [PubMed] [Google Scholar]
- 31.Meule A, Blechert J. Trait impulsivity and body mass index: a cross-sectional investigation in 3073 individuals reveals positive, but very small relationships. Health Psychol Open (2016) 3(2):2055102916659164. 10.1177/2055102916659164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bryant EJ, King NA, Blundell JE. Disinhibition: its effects on appetite and weight regulation. Obes Rev (2008) 9(5):409–19. 10.1111/j.1467-789X.2007.00426.x [DOI] [PubMed] [Google Scholar]
- 33.Davis C, Curtis C, Levitan RD, Carter JC, Kaplan AS, Kennedy JL. Evidence that “food addiction” is a valid phenotype of obesity. Appetite (2011) 57(3):711–7. 10.1016/j.appet.2011.08.017 [DOI] [PubMed] [Google Scholar]
- 34.Wise RA, Spindler J, deWit H, Gerberg GJ. Neuroleptic-induced “anhedonia” in rats: pimozide blocks reward quality of food. Science (1978) 201(4352):262–4. 10.1126/science.566469 [DOI] [PubMed] [Google Scholar]
- 35.Davis C, Carter JC. Compulsive overeating as an addiction disorder. A review of theory and evidence. Appetite (2009) 53(1):1–8. 10.1016/j.appet.2009.05.018 [DOI] [PubMed] [Google Scholar]
- 36.Kenny PJ. Common cellular and molecular mechanisms in obesity and drug addiction. Nat Rev Neurosci (2011) 12(11):638–51. 10.1038/nrn3105 [DOI] [PubMed] [Google Scholar]
- 37.Wise RA. The neurobiology of craving: implications for the understanding and treatment of addiction. J Abnorm Psychol (1988) 97(2):118–32. 10.1037/0021-843X.97.2.118 [DOI] [PubMed] [Google Scholar]
- 38.Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res (2002) 137(1–2):3–25. 10.1016/S0166-4328(02)00282-6 [DOI] [PubMed] [Google Scholar]
- 39.Sutin AR, Ferrucci L, Zonderman AB, Terracciano A. Personality and obesity across the adult life span. J Pers Soc Psychol (2011) 101(3):579–92. 10.1037/a0024286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.John OP, Srivastava S, editors. The big five trait taxonomy: history, measurement, and theoretical perspectives. Handbook of Personality: Theory and Research. 2nd ed (1999). p. 102–138. [Google Scholar]
- 41.Bogg T, Roberts BW. Conscientiousness and health-related behaviors: a meta-analysis of the leading behavioral contributors to mortality. Psychol Bull (2004) 130(6):887–919. 10.1037/0033-2909.130.6.887 [DOI] [PubMed] [Google Scholar]
- 42.Terracciano A, Löckenhoff CE, Crum RM, Bienvenu OJ, Costa PT. Five-Factor Model personality profiles of drug users. BMC Psychiatry (2008) 8:22. 10.1186/1471-244X-8-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kotov R, Gamez W, Schmidt F, Watson D. Linking “big” personality traits to anxiety, depressive, and substance use disorders: a meta-analysis. Psychol Bull (2010) 136(5):768–821. 10.1037/a0020327 [DOI] [PubMed] [Google Scholar]
- 44.Ruiz MA, Pincus AL, Schinka JA. Externalizing pathology and the five-factor model: a meta-analysis of personality traits associated with antisocial personality disorder, substance use disorder, and their co-occurrence. J Pers Disord (2008) 22(4):365–88. 10.1521/pedi.2008.22.4.365 [DOI] [PubMed] [Google Scholar]
- 45.Brunborg GS, Hanss D, Mentzoni RA, Molde H, Pallesen S. Problem gambling and the five-factor model of personality: a large population-based study. Addiction (2016) 111(8):1428–35. 10.1111/add.13388 [DOI] [PubMed] [Google Scholar]
- 46.Malouff JM, Thorsteinsson EB, Schutte NS. The five-factor model of personality and smoking: a meta-analysis. J Drug Educ (2006) 36(1):47–58. 10.2190/9EP8-17P8-EKG7-66AD [DOI] [PubMed] [Google Scholar]
- 47.Hakulinen C, Hintsanen M, Munafò MR, Virtanen M, Kivimäki M, Batty GD, et al. Personality and smoking: individual-participant meta-analysis of nine cohort studies. Addiction (2015) 110(11):1844–52. 10.1111/add.13079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Terracciano A, Costa PT. Smoking and the five-factor model of personality. Addiction (2004) 99(4):472–81. 10.1111/j.1360-0443.2004.00687.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malouff JM, Thorsteinsson EB, Rooke SE, Schutte NS. Alcohol involvement and the Five-Factor model of personality: a meta-analysis. J Drug Educ (2007) 37(3):277–94. 10.2190/DE.37.3.d [DOI] [PubMed] [Google Scholar]
- 50.Ruiz MA, Pincus AL, Dickinson KA. NEO PI-R predictors of alcohol use and alcohol-related problems. J Pers Assess (2003) 81(3):226–36. 10.1207/S15327752JPA8103_05 [DOI] [PubMed] [Google Scholar]
- 51.Bottlender M, Soyka M. Impact of different personality dimensions (NEO Five-Factor Inventory) on the outcome of alcohol-dependent patients 6 and 12 months after treatment. Psychiatry Res (2005) 136(1):61–7. 10.1016/j.psychres.2004.07.013 [DOI] [PubMed] [Google Scholar]
- 52.Torres A, Catena A, Megías A, Maldonado A, Cándido A, Verdejo-García A, et al. Emotional and non-emotional pathways to impulsive behavior and addiction. Front Hum Neurosci (2013) 7:43. 10.3389/fnhum.2013.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gerlach G, Herpertz S, Loeber S. Personality traits and obesity: a systematic review. Obes Rev (2015) 16(1):32–63. 10.1111/obr.12235 [DOI] [PubMed] [Google Scholar]
- 54.Jokela M, Hintsanen M, Hakulinen C, Batty GD, Nabi H, Singh-Manoux A, et al. Association of personality with the development and persistence of obesity: a meta-analysis based on individual-participant data. Obes Rev (2013) 14(4):315–23. 10.1111/obr.12007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphy CM, Stojek MK, MacKillop J. Interrelationships among impulsive personality traits, food addiction, and body mass index. Appetite (2014) 73:45–50. 10.1016/j.appet.2013.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mobbs O, Crépin C, Thiéry C, Golay A, Van der Linden M. Obesity and the four facets of impulsivity. Patient Educ Couns (2010) 79(3):372–7. 10.1016/j.pec.2010.03.003 [DOI] [PubMed] [Google Scholar]
- 57.Hays NP, Roberts SB. Aspects of eating behaviors “disinhibition” and “restraint” are related to weight gain and BMI in women. Obesity (Silver Spring) (2008) 16(1):52–8. 10.1038/oby.2007.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sullivan S, Cloninger CR, Przybeck TR, Klein S. Personality characteristics in obesity and relationship with successful weight loss. Int J Obes (Lond) (2007) 31(4):669–74. 10.1038/sj.ijo.0803464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Smith GT, Fischer S, Cyders MA, Annus AM, Spillane NS, McCarthy DM. On the validity and utility of discriminating among impulsivity-like traits. Assessment (2007) 14(2):155–70. 10.1177/1073191106295527 [DOI] [PubMed] [Google Scholar]
- 60.Whiteside SP, Lynam DR. Understanding the role of impulsivity and externalizing psychopathology in alcohol abuse: application of the UPPS impulsive behavior scale. Exp Clin Psychopharmacol (2003) 11(3):210–7. 10.1037/1064-1297.11.3.210 [DOI] [PubMed] [Google Scholar]
- 61.Verdejo-García A, Lawrence AJ, Clark L. Impulsivity as a vulnerability marker for substance-use disorders: review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci Biobehav Rev (2008) 32(4):777–810. 10.1016/j.neubiorev.2007.11.003 [DOI] [PubMed] [Google Scholar]
- 62.Mitchell MR, Potenza MN. Addictions and personality traits: impulsivity and related constructs. Curr Behav Neurosci Rep (2014) 1(1):1–12. 10.1007/s40473-013-0001-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Terracciano A, Sutin AR, McCrae RR, Deiana B, Ferrucci L, Schlessinger D, et al. Facets of personality linked to underweight and overweight. Psychosom Med (2009) 71(6):682–9. 10.1097/PSY.0b013e3181a2925b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sutin AR, Terracciano A. Five-Factor Model personality traits and the objective and subjective experience of body weight. J Pers (2016) 84(1):102–12. 10.1111/jopy.12143 [DOI] [PubMed] [Google Scholar]
- 65.Vainik U, Neseliler S, Konstabel K, Fellows LK, Dagher A. Eating traits questionnaires as a continuum of a single concept. Uncontrolled eating. Appetite (2015) 90:229–39. 10.1016/j.appet.2015.03.004 [DOI] [PubMed] [Google Scholar]
- 66.Vainik U, Mõttus R, Allik J, Esko T, Realo A. Are trait–outcome associations caused by scales or particular items? Example analysis of personality facets and BMI. Eur J Pers (2015) 29(6):622–34. 10.1002/per.2009 [DOI] [Google Scholar]
- 67.Emery RL, King KM, Fischer SF, Davis KR. The moderating role of negative urgency on the prospective association between dietary restraint and binge eating. Appetite (2013) 71:113–9. 10.1016/j.appet.2013.08.001 [DOI] [PubMed] [Google Scholar]
- 68.Torrubia R, Āvila C, Moltó J, Caseras X. The sensitivity to punishment and sensitivity to reward questionnaire (SPSRQ) as a measure of Gray’s anxiety and impulsivity dimensions. Pers Individ Dif (2001) 31(6):837–62. 10.1016/S0191-8869(00)00183-5 [DOI] [Google Scholar]
- 69.Kreek MJ, Nielsen DA, Butelman ER, LaForge KS. Genetic influences on impulsivity, risk taking, stress responsivity and vulnerability to drug abuse and addiction. Nat Neurosci (2005) 8(11):1450–7. 10.1038/nn1583 [DOI] [PubMed] [Google Scholar]
- 70.Dagher A. The neurobiology of appetite: hunger as addiction. Int J Obes (2009) 33(S2):S30–3. 10.1038/ijo.2009.69 [DOI] [PubMed] [Google Scholar]
- 71.Magee C, Heaven P. Big five personality factors, obesity and 2-year weight gain in Australian adults. Fac Health Behav Sci (2011) 3:332–5. 10.1016/j.jrp.2011.02.009 [DOI] [Google Scholar]
- 72.Davis C, Patte K, Levitan R, Reid C, Tweed S, Curtis C. From motivation to behaviour: a model of reward sensitivity, overeating, and food preferences in the risk profile for obesity. Appetite (2007) 48(1):12–9. 10.1016/j.appet.2006.05.016 [DOI] [PubMed] [Google Scholar]
- 73.Davis C, Fox J. Sensitivity to reward and body mass index (BMI): evidence for a non-linear relationship. Appetite (2008) 50(1):43–9. 10.1016/j.appet.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 74.Dietrich A, Federbusch M, Grellmann C, Villringer A, Horstmann A. Body weight status, eating behavior, sensitivity to reward/punishment, and gender: relationships and interdependencies. Front Psychol (2014) 5:1073. 10.3389/fpsyg.2014.01073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verbeken S, Braet C, Lammertyn J, Goossens L, Moens E. How is reward sensitivity related to bodyweight in children? Appetite (2012) 58(2):478–83. 10.1016/j.appet.2011.11.018 [DOI] [PubMed] [Google Scholar]
- 76.Faith MS, Flint J, Fairburn CG, Goodwin GM, Allison DB. Gender differences in the relationship between personality dimensions and relative body weight. Obes Res (2001) 9(10):647–50. 10.1038/oby.2001.86 [DOI] [PubMed] [Google Scholar]
- 77.Davis C, Cerullo D. Fat distribution in young women: associations and interactions with behavioural, physical and psychological factors. Psychol Health Med (1996) 1(2):159–67. 10.1080/13548509608400015 [DOI] [Google Scholar]
- 78.Brummett BH, Babyak MA, Williams RB, Barefoot JC, Costa PT, Siegler IC. NEO personality domains and gender predict levels and trends in body mass index over 14 years during midlife. J Res Personal (2006) 40(3):222–36. 10.1016/j.jrp.2004.12.002 [DOI] [Google Scholar]
- 79.Bagby RM, Vachon DD, Bulmash EL, Toneatto T, Quilty LC, Costa PT. Pathological gambling and the five-factor model of personality. Pers Individ Dif (2007) 43(4):873–80. 10.1016/j.paid.2007.02.011 [DOI] [Google Scholar]
- 80.Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev (2009) 33(5):631–46. 10.1016/j.neubiorev.2008.08.016 [DOI] [PubMed] [Google Scholar]
- 81.Hamilton KR, Mitchell MR, Wing VC, Balodis IM, Bickel WK, Fillmore M, et al. Choice impulsivity: definitions, measurement issues, and clinical implications. Personal Disord (2015) 6(2):182–98. 10.1037/per0000099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.MacKillop J, Weafer J, Gray JC, Oshri A, Palmer A, de Wit H. The latent structure of impulsivity: impulsive choice, impulsive action, and impulsive personality traits. Psychopharmacology (Berl) (2016) 233(18):3361–70. 10.1007/s00213-016-4372-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction (2004) 99(4):461–71. 10.1111/j.1360-0443.2003.00669.x [DOI] [PubMed] [Google Scholar]
- 84.MacKillop J, Amlung MT, Few LR, Ray LA, Sweet LH, Munafò MR. Delayed reward discounting and addictive behavior: a meta-analysis. Psychopharmacology (Berl) (2011) 216(3):305–21. 10.1007/s00213-011-2229-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Amlung M, Vedelago L, Acker J, Balodis I, MacKillop J. Steep delay discounting and addictive behavior: a meta-analysis of continuous associations. Addiction (2016). 10.1111/add.13535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Amlung M, Petker T, Jackson J, Balodis I, MacKillop J. Steep discounting of delayed monetary and food rewards in obesity: a meta-analysis. Psychol Med (2016) 46(11):2423–34. 10.1017/S0033291716000866 [DOI] [PubMed] [Google Scholar]
- 87.Weygandt M, Mai K, Dommes E, Ritter K, Leupelt V, Spranger J, et al. Impulse control in the dorsolateral prefrontal cortex counteracts post-diet weight regain in obesity. Neuroimage (2015) 109:318–27. 10.1016/j.neuroimage.2014.12.073 [DOI] [PubMed] [Google Scholar]
- 88.Platt ML, Watson KK, Hayden BY, Shepherd SV, Klein JT. Neuroeconomics: implications for understanding the neurobiology of addiction. 2nd ed In: Kuhn CM, Koob GF, editors. Advances in the Neuroscience of Addiction. Boca Raton, FL: CRC Press/Taylor & Francis; (2010). (Frontiers in Neuroscience). Available from: http://www.ncbi.nlm.nih.gov/books/NBK53362/ [PubMed] [Google Scholar]
- 89.Weinstein SM, Mermelstein R, Shiffman S, Flay B. Mood variability and cigarette smoking escalation among adolescents. Psychol Addict Behav (2008) 22(4):504–13. 10.1037/0893-164X.22.4.504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Brogan A, Hevey D, O’Callaghan G, Yoder R, O’Shea D. Impaired decision making among morbidly obese adults. J Psychosom Res (2011) 70(2):189–96. 10.1016/j.jpsychores.2010.07.012 [DOI] [PubMed] [Google Scholar]
- 91.Leehr EJ, Krohmer K, Schag K, Dresler T, Zipfel S, Giel KE. Emotion regulation model in binge eating disorder and obesity – a systematic review. Neurosci Biobehav Rev (2015) 49:125–34. 10.1016/j.neubiorev.2014.12.008 [DOI] [PubMed] [Google Scholar]
- 92.Worbe Y, Irvine M, Lange I, Kundu P, Howell NA, Harrison NA, et al. Neuronal correlates of risk-seeking attitudes to anticipated losses in binge drinkers. Biol Psychiatry (2014) 76(9):717–24. 10.1016/j.biopsych.2013.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stevens L, Verdejo-García A, Goudriaan AE, Roeyers H, Dom G, Vanderplasschen W. Impulsivity as a vulnerability factor for poor addiction treatment outcomes: a review of neurocognitive findings among individuals with substance use disorders. J Subst Abuse Treat (2014) 47(1):58–72. 10.1016/j.jsat.2014.01.008 [DOI] [PubMed] [Google Scholar]
- 94.Voon V, Morris LS, Irvine MA, Ruck C, Worbe Y, Derbyshire K, et al. Risk-taking in disorders of natural and drug rewards: neural correlates and effects of probability, valence, and magnitude. Neuropsychopharmacology (2015) 40(4):804–12. 10.1038/npp.2014.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: a model and a method. J Exp Psychol Hum Percept Perform (1984) 10(2):276–91. 10.1037/0096-1523.10.2.276 [DOI] [PubMed] [Google Scholar]
- 96.Kaufman JN, Ross TJ, Stein EA, Garavan H. Cingulate hypoactivity in cocaine users during a GO-NOGO task as revealed by event-related functional magnetic resonance imaging. J Neurosci (2003) 23(21):7839–43. 23/21/7839 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hester R, Garavan H. Executive dysfunction in cocaine addiction: evidence for discordant frontal, cingulate, and cerebellar activity. J Neurosci (2004) 24(49):11017–22. 10.1523/JNEUROSCI.3321-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fu L, Bi G, Zou Z, Wang Y, Ye E, Ma L, et al. Impaired response inhibition function in abstinent heroin dependents: an fMRI study. Neurosci Lett (2008) 438(3):322–6. 10.1016/j.neulet.2008.04.033 [DOI] [PubMed] [Google Scholar]
- 99.Smith JL, Mattick RP, Jamadar SD, Iredale JM. Deficits in behavioural inhibition in substance abuse and addiction: a meta-analysis. Drug Alcohol Depend (2014) 145:1–33. 10.1016/j.drugalcdep.2014.08.009 [DOI] [PubMed] [Google Scholar]
- 100.Bartholdy S, Dalton B, O’Daly OG, Campbell IC, Schmidt U. A systematic review of the relationship between eating, weight and inhibitory control using the stop signal task. Neurosci Biobehav Rev (2016) 64:35–62. 10.1016/j.neubiorev.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 101.Kulendran M, Vlaev I, Sugden C, King D, Ashrafian H, Gately P, et al. Neuropsychological assessment as a predictor of weight loss in obese adolescents. Int J Obes (2014) 38(4):507–12. 10.1038/ijo.2013.198 [DOI] [PubMed] [Google Scholar]
- 102.Weygandt M, Mai K, Dommes E, Leupelt V, Hackmack K, Kahnt T, et al. The role of neural impulse control mechanisms for dietary success in obesity. Neuroimage (2013) 83:669–78. 10.1016/j.neuroimage.2013.07.028 [DOI] [PubMed] [Google Scholar]
- 103.Appelhans BM, Woolf K, Pagoto SL, Schneider KL, Whited MC, Liebman R. Inhibiting food reward: delay discounting, food reward sensitivity, and palatable food intake in overweight and obese women. Obesity (Silver Spring) (2011) 19(11):2175–82. 10.1038/oby.2011.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lavagnino L, Arnone D, Cao B, Soares JC, Selvaraj S. Inhibitory control in obesity and binge eating disorder: a systematic and meta-analysis of neurocognitive and neuroimaging studies. Neurosci Biobehav Rev (2016) 68:714–26. 10.1016/j.neubiorev.2016.06.041 [DOI] [PubMed] [Google Scholar]
- 105.Reinert KRS, Po’e EK, Barkin SL. The relationship between executive function and obesity in children and adolescents: a systematic literature review. J Obes (2013) 2013:820956. 10.1155/2013/820956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Miller AL, Lee HJ, Lumeng JC. Obesity-associated biomarkers and executive function in children. Pediatr Res (2015) 77(1–2):143–7. 10.1038/pr.2014.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Liang J, Matheson BE, Kaye WH, Boutelle KN. Neurocognitive correlates of obesity and obesity-related behaviors in children and adolescents. Int J Obes (2014) 38(4):494–506. 10.1038/ijo.2013.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Carnell S, Benson L, Pryor K, Driggin E. Appetitive traits from infancy to adolescence: using behavioral and neural measures to investigate obesity risk. Physiol Behav (2013) 121:79–88. 10.1016/j.physbeh.2013.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Loeber S, Grosshans M, Korucuoglu O, Vollmert C, Vollstädt-Klein S, Schneider S, et al. Impairment of inhibitory control in response to food-associated cues and attentional bias of obese participants and normal-weight controls. Int J Obes (2012) 36(10):1334–9. 10.1038/ijo.2011.184 [DOI] [PubMed] [Google Scholar]
- 110.Mühlberg C, Mathar D, Villringer A, Horstmann A, Neumann J. Stopping at the sight of food – how gender and obesity impact on response inhibition. Appetite (2016) 107:663–76. 10.1016/j.appet.2016.08.121 [DOI] [PubMed] [Google Scholar]
- 111.Voon V, Irvine MA, Derbyshire K, Worbe Y, Lange I, Abbott S, et al. Measuring “waiting” impulsivity in substance addictions and binge eating disorder in a novel analogue of rodent serial reaction time task. Biol Psychiatry (2014) 75(2):148–55. 10.1016/j.biopsych.2013.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Goldstein RZ, Volkow ND. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci (2011) 12(11):652–69. 10.1038/nrn3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cox WM, Fadardi JS, Pothos EM. The addiction-Stroop test: theoretical considerations and procedural recommendations. Psychol Bull (2006) 132(3):443–76. 10.1037/0033-2909.132.3.443 [DOI] [PubMed] [Google Scholar]
- 114.Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend (2008) 97(1–2):1–20. 10.1016/j.drugalcdep.2008.03.030 [DOI] [PubMed] [Google Scholar]
- 115.Nijs IMT, Franken IHA, Muris P. Food-related Stroop interference in obese and normal-weight individuals: behavioral and electrophysiological indices. Eat Behav (2010) 11(4):258–65. 10.1016/j.eatbeh.2010.07.002 [DOI] [PubMed] [Google Scholar]
- 116.Hall PA, Lowe C, Vincent C. Executive control resources and snack food consumption in the presence of restraining versus facilitating cues. J Behav Med (2014) 37(4):587–94. 10.1007/s10865-013-9528-3 [DOI] [PubMed] [Google Scholar]
- 117.Wu X, Nussbaum MA, Madigan ML. Executive function and measures of fall risk among people with obesity. Percept Mot Skills (2016) 122(3):825–39. 10.1177/0031512516646158 [DOI] [PubMed] [Google Scholar]
- 118.Fitzpatrick S, Gilbert S, Serpell L. Systematic review: are overweight and obese individuals impaired on behavioural tasks of executive functioning? Neuropsychol Rev (2013) 23(2):138–56. 10.1007/s11065-013-9224-7 [DOI] [PubMed] [Google Scholar]
- 119.Werthmann J, Jansen A, Roefs A. Worry or craving? A selective review of evidence for food-related attention biases in obese individuals, eating-disorder patients, restrained eaters and healthy samples. Proc Nutr Soc (2015) 74(2):99–114. 10.1017/S0029665114001451 [DOI] [PubMed] [Google Scholar]
- 120.Berg EA. A simple objective technique for measuring flexibility in thinking. J Gen Psychol (1948) 39:15–22. 10.1080/00221309.1948.9918159 [DOI] [PubMed] [Google Scholar]
- 121.Wu M, Brockmeyer T, Hartmann M, Skunde M, Herzog W, Friederich H-C. Set-shifting ability across the spectrum of eating disorders and in overweight and obesity: a systematic review and meta-analysis. Psychol Med (2014) 44(16):3365–85. 10.1017/S0033291714000294 [DOI] [PubMed] [Google Scholar]
- 122.Morris LS, Voon V. Dimensionality of cognitions in behavioral addiction. Curr Behav Neurosci Rep (2016) 3:49–57. 10.1007/s40473-016-0068-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Woicik PA, Urban C, Alia-Klein N, Henry A, Maloney T, Telang F, et al. A pattern of perseveration in cocaine addiction may reveal neurocognitive processes implicit in the Wisconsin Card Sorting Test. Neuropsychologia (2011) 49(7):1660–9. 10.1016/j.neuropsychologia.2011.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Alvarez-Moya EM, Jiménez-Murcia S, Moragas L, Gómez-Peña M, Aymamí MN, Ochoa C, et al. Executive functioning among female pathological gambling and bulimia nervosa patients: preliminary findings. J Int Neuropsychol Soc (2009) 15(2):302–6. 10.1017/S1355617709090377 [DOI] [PubMed] [Google Scholar]
- 125.Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia (2000) 38(8):1180–7. 10.1016/S0028-3932(99)00158-X [DOI] [PubMed] [Google Scholar]
- 126.Nowakowska K, Jabłkowska K, Borkowska A. [Cognitive dysfunctions in patients with alcohol dependence]. Psychiatr Pol (2007) 41(5):693–702. [PubMed] [Google Scholar]
- 127.Boog M, Höppener P, Vande Wetering BJM, Goudriaan AE, Boog MC, Franken IH. Cognitive inflexibility in gamblers is primarily present in reward-related decision making. Front Hum Neurosci (2014) 8:569. 10.3389/fnhum.2014.00569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Perpiñá C, Segura M, Sánchez-Reales S. Cognitive flexibility and decision-making in eating disorders and obesity. Eat Weight Disord (2016). 10.1007/s40519-016-0331-3 [DOI] [PubMed] [Google Scholar]
- 129.Dagher A. Alcohol and the paradox of self-control. Biol Psychiatry (2014) 76(9):674–5. 10.1016/j.biopsych.2014.08.019 [DOI] [PubMed] [Google Scholar]
- 130.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry (2016) 3(8):760–73. 10.1016/S2215-0366(16)00104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Garavan H, Weierstall K. The neurobiology of reward and cognitive control systems and their role in incentivizing health behavior. Prev Med (2012) 55(Suppl):S17–23. 10.1016/j.ypmed.2012.05.018 [DOI] [PubMed] [Google Scholar]
- 132.Kühn S, Gallinat J. Common biology of craving across legal and illegal drugs – a quantitative meta-analysis of cue-reactivity brain response. Eur J Neurosci (2011) 33(7):1318–26. 10.1111/j.1460-9568.2010.07590.x [DOI] [PubMed] [Google Scholar]
- 133.Chase HW, Eickhoff SB, Laird AR, Hogarth L. The neural basis of drug stimulus processing and craving: an activation likelihood estimation meta-analysis. Biol Psychiatry (2011) 70(8):785–93. 10.1016/j.biopsych.2011.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol (2013) 18(1):121–33. 10.1111/j.1369-1600.2012.00464.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tang DW, Fellows LK, Small DM, Dagher A. Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiol Behav (2012) 106(3):317–24. 10.1016/j.physbeh.2012.03.009 [DOI] [PubMed] [Google Scholar]
- 136.Hanlon CA, Dowdle LT, Naselaris T, Canterberry M, Cortese BM. Visual cortex activation to drug cues: a meta-analysis of functional neuroimaging papers in addiction and substance abuse literature. Drug Alcohol Depend (2014) 143:206–12. 10.1016/j.drugalcdep.2014.07.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, et al. Neural substrates of smoking cue reactivity: a meta-analysis of fMRI studies. Neuroimage (2012) 60(1):252–62. 10.1016/j.neuroimage.2011.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Noori HR, Cosa Linan A, Spanagel R. Largely overlapping neuronal substrates of reactivity to drug, gambling, food and sexual cues: a comprehensive meta-analysis. Eur Neuropsychopharmacol (2016) 26(9):1419–30. 10.1016/j.euroneuro.2016.06.013 [DOI] [PubMed] [Google Scholar]
- 139.Meng Y, Deng W, Wang H, Guo W, Li T. The prefrontal dysfunction in individuals with Internet gaming disorder: a meta-analysis of functional magnetic resonance imaging studies. Addict Biol (2015) 20(4):799–808. 10.1111/adb.12154 [DOI] [PubMed] [Google Scholar]
- 140.Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend (2011) 119(3):216–23. 10.1016/j.drugalcdep.2011.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wetherill RR, Squeglia LM, Yang TT, Tapert SF. A longitudinal examination of adolescent response inhibition: neural differences before and after the initiation of heavy drinking. Psychopharmacology (Berl) (2013) 230(4):663–71. 10.1007/s00213-013-3198-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Tang Y-Y, Posner MI, Rothbart MK, Volkow ND. Circuitry of self-control and its role in reducing addiction. Trends Cogn Sci (2015) 19(8):439–44. 10.1016/j.tics.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 143.Hong LE, Gu H, Yang Y, Ross TJ, Salmeron BJ, Buchholz B, et al. Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry (2009) 66(4):431–41. 10.1001/archgenpsychiatry.2009.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry (2002) 159(10):1642–52. 10.1176/appi.ajp.159.10.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wilson SJ, Sayette MA, Fiez JA. Prefrontal responses to drug cues: a neurocognitive analysis. Nat Neurosci (2004) 7(3):211–4. 10.1038/nn1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. J Neurosci (2012) 32(16):5549–52. 10.1523/JNEUROSCI.5958-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Stice E, Burger KS, Yokum S. Reward region responsivity predicts future weight gain and moderating effects of the TaqIA allele. J Neurosci (2015) 35(28):10316–24. 10.1523/JNEUROSCI.3607-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brooks SJ, Cedernaes J, Schiöth HB. Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PLoS One (2013) 8(4):e60393. 10.1371/journal.pone.0060393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Goldman RL, Canterberry M, Borckardt JJ, Madan A, Byrne TK, George MS, et al. Executive control circuitry differentiates degree of success in weight loss following gastric-bypass surgery. Obesity (Silver Spring) (2013) 21(11):2189–96. 10.1002/oby.20575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jensen CD, Kirwan CB. Functional brain response to food images in successful adolescent weight losers compared with normal-weight and overweight controls. Obesity (Silver Spring) (2015) 23(3):630–6. 10.1002/oby.21004 [DOI] [PubMed] [Google Scholar]
- 151.Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science (2009) 324(5927):646–8. 10.1126/science.1168450 [DOI] [PubMed] [Google Scholar]
- 152.Giuliani NR, Mann T, Tomiyama AJ, Berkman ET. Neural systems underlying the reappraisal of personally craved foods. J Cogn Neurosci (2014) 26(7):1390–402. 10.1162/jocn_a_00563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hollmann M, Hellrung L, Pleger B, Schlögl H, Kabisch S, Stumvoll M, et al. Neural correlates of the volitional regulation of the desire for food. Int J Obes (2012) 36(5):648–55. 10.1038/ijo.2011.125 [DOI] [PubMed] [Google Scholar]
- 154.Siep N, Roefs A, Roebroeck A, Havermans R, Bonte M, Jansen A. Fighting food temptations: the modulating effects of short-term cognitive reappraisal, suppression and up-regulation on mesocorticolimbic activity related to appetitive motivation. Neuroimage (2012) 60(1):213–20. 10.1016/j.neuroimage.2011.12.067 [DOI] [PubMed] [Google Scholar]
- 155.Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage (2010) 52(4):1696–703. 10.1016/j.neuroimage.2010.05.059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hendrick OM, Luo X, Zhang S, Li C-SR. Saliency processing and obesity: a preliminary imaging study of the stop signal task. Obesity (Silver Spring) (2012) 20(9):1796–802. 10.1038/oby.2011.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.He Q, Xiao L, Xue G, Wong S, Ames SL, Schembre SM, et al. Poor ability to resist tempting calorie rich food is linked to altered balance between neural systems involved in urge and self-control. Nutr J (2014) 13:92. 10.1186/1475-2891-13-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Appelhans BM. Neurobehavioral inhibition of reward-driven feeding: implications for dieting and obesity. Obesity (Silver Spring) (2009) 17(4):640–7. 10.1038/oby.2008.638 [DOI] [PubMed] [Google Scholar]
- 159.García-García I, Horstmann A, Jurado MA, Garolera M, Chaudhry SJ, Margulies DS, et al. Reward processing in obesity, substance addiction and non-substance addiction. Obes Rev (2014) 15(11):853–69. 10.1111/obr.12221 [DOI] [PubMed] [Google Scholar]
- 160.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed Washington, DC: DSM-5 American Psychiatric Publishing; (2013). [Google Scholar]
- 161.Kessler RM, Hutson PH, Herman BK, Potenza MN. The neurobiological basis of binge-eating disorder. Neurosci Biobehav Rev (2016) 63:223–38. 10.1016/j.neubiorev.2016.01.013 [DOI] [PubMed] [Google Scholar]
- 162.Voon V. Cognitive biases in binge eating disorder: the hijacking of decision making. CNS Spectr (2015) 20(6):566–73. 10.1017/S1092852915000681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Davis C, Patte K, Curtis C, Reid C. Immediate pleasures and future consequences. A neuropsychological study of binge eating and obesity. Appetite (2010) 54(1):208–13. 10.1016/j.appet.2009.11.002 [DOI] [PubMed] [Google Scholar]
- 164.Hege MA, Stingl KT, Kullmann S, Schag K, Giel KE, Zipfel S, et al. Attentional impulsivity in binge eating disorder modulates response inhibition performance and frontal brain networks. Int J Obes (2015) 39(2):353–60. 10.1038/ijo.2014.99 [DOI] [PubMed] [Google Scholar]
- 165.Balodis IM, Molina ND, Kober H, Worhunsky PD, White MA, Sinha R, et al. Divergent neural substrates of inhibitory control in binge eating disorder relative to other manifestations of obesity. Obesity (Silver Spring) (2013) 21(2):367–77. 10.1002/oby.20068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Schulte EM, Grilo CM, Gearhardt AN. Shared and unique mechanisms underlying binge eating disorder and addictive disorders. Clin Psychol Rev (2016) 44:125–39. 10.1016/j.cpr.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gearhardt AN, White MA, Potenza MN. Binge eating disorder and food addiction. Curr Drug Abuse Rev (2011) 4(3):201–7. 10.2174/1874473711104030201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Avena NM, Rada P, Hoebel BG. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev (2008) 32(1):20–39. 10.1016/j.neubiorev.2007.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Schulte EM, Joyner MA, Potenza MN, Grilo CM, Gearhardt AN. Current considerations regarding food addiction. Curr Psychiatry Rep (2015) 17(4):563. 10.1007/s11920-015-0563-3 [DOI] [PubMed] [Google Scholar]
- 170.Gearhardt AN, Corbin WR, Brownell KD. Preliminary validation of the Yale Food Addiction Scale. Appetite (2009) 52(2):430–6. 10.1016/j.appet.2008.12.003 [DOI] [PubMed] [Google Scholar]
- 171.Davis C. From passive overeating to “food addiction”: a spectrum of compulsion and severity. ISRN Obes (2013) 2013:435027. 10.1155/2013/435027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Gearhardt AN, Corbin WR, Brownell KD. Development of the Yale Food Addiction Scale Version 2.0. Psychol Addict Behav (2016) 30(1):113–21. 10.1037/adb0000136 [DOI] [PubMed] [Google Scholar]
- 173.Ziauddeen H, Fletcher PC. Is food addiction a valid and useful concept? Obes Rev (2013) 14(1):19–28. 10.1111/j.1467-789X.2012.01046.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ziauddeen H, Farooqi IS, Fletcher PC. Obesity and the brain: how convincing is the addiction model? Nat Rev Neurosci (2012) 13(4):279–86. 10.1038/nrn3212 [DOI] [PubMed] [Google Scholar]
- 175.Corsica JA, Pelchat ML. Food addiction: true or false? Curr Opin Gastroenterol (2010) 26(2):165–9. 10.1097/MOG.0b013e328336528d [DOI] [PubMed] [Google Scholar]
- 176.Avena NM, Gearhardt AN, Gold MS, Wang G-J, Potenza MN. Tossing the baby out with the bathwater after a brief rinse? The potential downside of dismissing food addiction based on limited data. Nat Rev Neurosci (2012) 13(7):514; author reply 514. 10.1038/nrn3212-c1 [DOI] [PubMed] [Google Scholar]
- 177.Westwater ML, Fletcher PC, Ziauddeen H. Sugar addiction: the state of the science. Eur J Nutr (2016) 55(Suppl 2):55–69. 10.1007/s00394-016-1229-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Hebebrand J, Albayrak Ö, Adan R, Antel J, Dieguez C, de Jong J, et al. “Eating addiction”, rather than “food addiction”, better captures addictive-like eating behavior. Neurosci Biobehav Rev (2014) 47:295–306. 10.1016/j.neubiorev.2014.08.016 [DOI] [PubMed] [Google Scholar]
- 179.Pedram P, Wadden D, Amini P, Gulliver W, Randell E, Cahill F, et al. Food addiction: its prevalence and significant association with obesity in the general population. PLoS One (2013) 8(9):e74832. 10.1371/journal.pone.0074832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Long CG, Blundell JE, Finlayson G. A systematic review of the application and correlates of YFAS-diagnosed “food addiction” in humans: are eating-related “addictions” a cause for concern or empty concepts? Obes Facts (2015) 8(6):386–401. 10.1159/000442403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.De Zwaan M. Binge eating disorder and obesity. Int J Obes Relat Metab Disord (2001) 25(Suppl 1):S51–5. 10.1038/sj.ijo.0801699 [DOI] [PubMed] [Google Scholar]
- 182.Schag K, Schönleber J, Teufel M, Zipfel S, Giel KE. Food-related impulsivity in obesity and binge eating disorder – a systematic review. Obes Rev (2013) 14(6):477–95. 10.1111/obr.12017 [DOI] [PubMed] [Google Scholar]
- 183.Davis C. Attention-deficit/hyperactivity disorder: associations with overeating and obesity. Curr Psychiatry Rep (2010) 12(5):389–95. 10.1007/s11920-010-0133-7 [DOI] [PubMed] [Google Scholar]
- 184.Matthews M, Nigg JT, Fair DA. Attention deficit hyperactivity disorder. In: Andersen SL, Pine DS, editors. The Neurobiology of Childhood. Berlin Heidelberg: Springer; (2013). p. 235–66. (Current Topics in Behavioral Neurosciences). Available from: http://link.springer.com/chapter/10.1007/7854_2013_249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ottosen C, Petersen L, Larsen JT, Dalsgaard S. Gender differences in associations between attention-deficit/hyperactivity disorder and substance use disorder. J Am Acad Child Adolesc Psychiatry (2016) 55(3):227.e–34.e. 10.1016/j.jaac.2015.12.010 [DOI] [PubMed] [Google Scholar]
- 186.Charach A, Yeung E, Climans T, Lillie E. Childhood attention-deficit/hyperactivity disorder and future substance use disorders: comparative meta-analyses. J Am Acad Child Adolesc Psychiatry (2011) 50(1):9–21. 10.1016/j.jaac.2010.09.019 [DOI] [PubMed] [Google Scholar]
- 187.Lee SS, Humphreys KL, Flory K, Liu R, Glass K. Prospective association of childhood attention-deficit/hyperactivity disorder (ADHD) and substance use and abuse/dependence: a meta-analytic review. Clin Psychol Rev (2011) 31(3):328–41. 10.1016/j.cpr.2011.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wilens TE, Martelon M, Joshi G, Bateman C, Fried R, Petty C, et al. Does ADHD predict substance-use disorders? A 10-year follow-up study of young adults with ADHD. J Am Acad Child Adolesc Psychiatry (2011) 50(6):543–53. 10.1016/j.jaac.2011.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nigg JT, Johnstone JM, Musser ED, Long HG, Willoughby MT, Shannon J. Attention-deficit/hyperactivity disorder (ADHD) and being overweight/obesity: new data and meta-analysis. Clin Psychol Rev (2016) 43:67–79. 10.1016/j.cpr.2015.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Cortese S, Moreira-Maia CR, St Fleur D, Morcillo-Peñalver C, Rohde LA, Faraone SV. Association between ADHD and obesity: a systematic review and meta-analysis. Am J Psychiatry (2016) 173(1):34–43. 10.1176/appi.ajp.2015.15020266 [DOI] [PubMed] [Google Scholar]
- 191.Cortese S, Ramos Olazagasti MA, Klein RG, Castellanos FX, Proal E, Mannuzza S. Obesity in men with childhood ADHD: a 33-year controlled, prospective, follow-up study. Pediatrics (2013) 131(6):e1731–8. 10.1542/peds.2012-0540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Khalife N, Kantomaa M, Glover V, Tammelin T, Laitinen J, Ebeling H, et al. Childhood attention-deficit/hyperactivity disorder symptoms are risk factors for obesity and physical inactivity in adolescence. J Am Acad Child Adolesc Psychiatry (2014) 53(4):425–36. 10.1016/j.jaac.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 193.Kaisari P, Dourish CT, Higgs S. Attention deficit hyperactivity disorder (ADHD) and disordered eating behaviour: a systematic review and a framework for future research. Clin Psychol Rev (2017) 53:109–21. 10.1016/j.cpr.2017.03.002 [DOI] [PubMed] [Google Scholar]
- 194.Duncan L, Perry JRB, Patterson N, Robinson EB, Daly MJ, Price AL, et al. An atlas of genetic correlations across human diseases and traits. Nat Genet (2015) 47(11):1236–41. 10.1038/ng.3406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cortese S, Isnard P, Frelut ML, Michel G, Quantin L, Guedeney A, et al. Association between symptoms of attention-deficit/hyperactivity disorder and bulimic behaviors in a clinical sample of severely obese adolescents. Int J Obes (Lond) (2007) 31(2):340–6. 10.1038/sj.ijo.0803400 [DOI] [PubMed] [Google Scholar]
- 196.Davis C, Levitan RD, Smith M, Tweed S, Curtis C. Associations among overeating, overweight, and attention deficit/hyperactivity disorder: a structural equation modelling approach. Eat Behav (2006) 7(3):266–74. 10.1016/j.eatbeh.2005.09.006 [DOI] [PubMed] [Google Scholar]
- 197.Cortese S, Castellanos FX. The relationship between ADHD and obesity: implications for therapy. Expert Rev Neurother (2014) 14(5):473–9. 10.1586/14737175.2014.904748 [DOI] [PubMed] [Google Scholar]
- 198.Sinha R, Jastreboff AM. Stress as a common risk factor for obesity and addiction. Biol Psychiatry (2013) 73(9):827–35. 10.1016/j.biopsych.2013.01.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Morris MJ, Beilharz JE, Maniam J, Reichelt AC, Westbrook RF. Why is obesity such a problem in the 21st century? The intersection of palatable food, cues and reward pathways, stress, and cognition. Neurosci Biobehav Rev (2015) 58:36–45. 10.1016/j.neubiorev.2014.12.002 [DOI] [PubMed] [Google Scholar]
- 200.Epstein DH, Willner-Reid J, Vahabzadeh M, Mezghanni M, Lin J-L, Preston KL. Real-time electronic diary reports of cue exposure and mood in the hours before cocaine and heroin craving and use. Arch Gen Psychiatry (2009) 66(1):88–94. 10.1001/archgenpsychiatry.2008.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Sinha R, Catapano D, O’Malley S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology (Berl) (1999) 142(4):343–51. 10.1007/s002130050898 [DOI] [PubMed] [Google Scholar]
- 202.Sinha R. Chronic stress, drug use, and vulnerability to addiction. Ann N Y Acad Sci (2008) 1141:105–30. 10.1196/annals.1441.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Koob GF, Le Moal M. Drug abuse: hedonic homeostatic dysregulation. Science (1997) 278(5335):52–8. 10.1126/science.278.5335.52 [DOI] [PubMed] [Google Scholar]
- 204.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol (2008) 59:29–53. 10.1146/annurev.psych.59.103006.093548 [DOI] [PubMed] [Google Scholar]
- 205.Geliebter A, Aversa A. Emotional eating in overweight, normal weight, and underweight individuals. Eat Behav (2003) 3(4):341–7. 10.1016/S1471-0153(02)00100-9 [DOI] [PubMed] [Google Scholar]
- 206.Hepworth R, Mogg K, Brignell C, Bradley BP. Negative mood increases selective attention to food cues and subjective appetite. Appetite (2010) 54(1):134–42. 10.1016/j.appet.2009.09.019 [DOI] [PubMed] [Google Scholar]
- 207.Chao A, Grilo CM, White MA, Sinha R. Food cravings mediate the relationship between chronic stress and body mass index. J Health Psychol (2015) 20(6):721–9. 10.1177/1359105315573448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Oliver G, Wardle J. Perceived effects of stress on food choice. Physiol Behav (1999) 66(3):511–5. 10.1016/S0031-9384(98)00322-9 [DOI] [PubMed] [Google Scholar]
- 209.Zellner DA, Loaiza S, Gonzalez Z, Pita J, Morales J, Pecora D, et al. Food selection changes under stress. Physiol Behav (2006) 87:789–93. 10.1016/j.physbeh.2006.01.014 [DOI] [PubMed] [Google Scholar]
- 210.Dallman MF, Pecoraro N, Akana SF, la Fleur SE, Gomez F, Houshyar H, et al. Chronic stress and obesity: a new view of “comfort food.”. Proc Natl Acad Sci U S A (2003) 100(20):11696–701. 10.1073/pnas.1934666100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Macht M, Mueller J. Immediate effects of chocolate on experimentally induced mood states. Appetite (2007) 49:667–74. 10.1016/j.appet.2007.05.004 [DOI] [PubMed] [Google Scholar]
- 212.Macht M. How emotions affect eating: a five-way model. Appetite (2008) 50(1):1–11. 10.1016/j.appet.2007.07.002 [DOI] [PubMed] [Google Scholar]
- 213.Kivimäki M, Head J, Ferrie JE, Shipley MJ, Brunner E, Vahtera J, et al. Work stress, weight gain and weight loss: evidence for bidirectional effects of job strain on body mass index in the Whitehall II study. Int J Obes (2006) 30(6):982–7. 10.1038/sj.ijo.0803229 [DOI] [PubMed] [Google Scholar]
- 214.Tryon MS, Carter CS, Decant R, Laugero KD. Chronic stress exposure may affect the brain’s response to high calorie food cues and predispose to obesogenic eating habits. Physiol Behav (2013) 120:233–42. 10.1016/j.physbeh.2013.08.010 [DOI] [PubMed] [Google Scholar]
- 215.Maier SU, Makwana AB, Hare TA. Acute stress impairs self-control in goal-directed choice by altering multiple functional connections within the brain’s decision circuits. Neuron (2015) 87(3):621–31. 10.1016/j.neuron.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 216.Jastreboff AM, Sinha R, Lacadie C, Small DM, Sherwin RS, Potenza MN. Neural correlates of stress- and food cue-induced food craving in obesity: association with insulin levels. Diabetes Care (2013) 36(2):394–402. 10.2337/dc12-1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Adam TC, Epel ES. Stress, eating and the reward system. Physiol Behav (2007) 91(4):449–58. 10.1016/j.physbeh.2007.04.011 [DOI] [PubMed] [Google Scholar]
- 218.Barry D, Clarke M, Petry NM. Obesity and its relationship to addictions: is overeating a form of addictive behavior? Am J Addict (2009) 18(6):439–51. 10.3109/10550490903205579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Barry D, Petry NM. Associations between body mass index and substance use disorders differ by gender: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Addict Behav (2009) 34(1):51–60. 10.1016/j.addbeh.2008.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Grucza RA, Krueger RF, Racette SB, Norberg KE, Hipp PR, Bierut LJ. The emerging link between alcoholism risk and obesity in the United States. Arch Gen Psychiatry (2010) 67(12):1301–8. 10.1001/archgenpsychiatry.2010.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Simon GE, Von Korff M, Saunders K, Miglioretti DL, Crane PK, van Belle G, et al. Association between obesity and psychiatric disorders in the US adult population. Arch Gen Psychiatry (2006) 63(7):824–30. 10.1001/archpsyc.63.7.824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Pickering RP, Grant BF, Chou SP, Compton WM. Are overweight, obesity, and extreme obesity associated with psychopathology? Results from the national epidemiologic survey on alcohol and related conditions. J Clin Psychiatry (2007) 68(7):998–1009. 10.4088/JCP.v68n0704 [DOI] [PubMed] [Google Scholar]
- 223.Scott KM, McGee MA, Wells JE, Oakley Browne MA. Obesity and mental disorders in the adult general population. J Psychosom Res (2008) 64(1):97–105. 10.1016/j.jpsychores.2007.09.006 [DOI] [PubMed] [Google Scholar]
- 224.Sansone RA, Sansone LA. Obesity and substance misuse: is there a relationship? Innov Clin Neurosci (2013) 10(9–10):30–5. [PMC free article] [PubMed] [Google Scholar]
- 225.Green MA, Strong M, Razak F, Subramanian SV, Relton C, Bissell P. Who are the obese? A cluster analysis exploring subgroups of the obese. J Public Health (2015) 2:fdv040. 10.1093/pubmed/fdv040 [DOI] [PubMed] [Google Scholar]
- 226.King WC, Chen J-Y, Mitchell JE, Kalarchian MA, Steffen KJ, Engel SG, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA (2012) 307(23):2516–25. 10.1001/jama.2012.6147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Conason A, Teixeira J, Hsu C-H, Puma L, Knafo D, Geliebter A. Substance use following bariatric weight loss surgery. JAMA Surg (2013) 148(2):145–50. 10.1001/2013.jamasurg.265 [DOI] [PubMed] [Google Scholar]
- 228.Steffen KJ, Engel SG, Wonderlich JA, Pollert GA, Sondag C. Alcohol and other addictive disorders following bariatric surgery: prevalence, risk factors and possible etiologies. Eur Eat Disord Rev (2015) 23(6):442–50. 10.1002/erv.2399 [DOI] [PubMed] [Google Scholar]