Summary
Recent evidence points to the embryonic emergence of some tissue-resident innate immune cells, such as B-1a lymphocytes, prior to and independently of hematopoietic stem cells (HSCs). However, whether the full hematopoietic repertoire of embryonic HSCs initially includes these unique lineages of innate immune cells has been difficult to assess due to lack of clonal assays that identify and assess HSC precursor (pre-HSC) potential. Here, by combining index sorting of single embryonic hemogenic precursors with in vitro HSC maturation and transplantation assays, we analyze emerging pre-HSCs at the single-cell level, revealing their unique stage-specific properties and clonal lineage potential. Remarkably, clonal pre-HSCs detected between E9.5 and E11.5 contribute to the complete B cell repertoire, including B-1a lymphocytes, revealing a previously unappreciated common precursor for all B cell lineages at the pre-HSC stage and a second embryonic origin for B-1a lymphocytes.
Keywords: hematopoietic stem cell, B lymphocyte, pre-HSC, AGM, B-1a cell
Highlights
-
•
Index sorting and stromal co-culture identifies clonal embryonic pre-HSCs
-
•
Clonal pre-HSCs have both B-1a and B-2 lymphocyte potential
-
•
Clonal pre-HSCs express distinctive levels of Delta-like-4
In this article, Hadland and colleagues use index sorting of single embryonic hemogenic precursors with in vitro HSC maturation and transplantation assays to characterize pre-HSCs at the single-cell level, revealing stage-specific properties of pre-HSCs and a common origin for B-1a and B-2 lymphocytes at the emergence of HSC development.
Introduction
During embryonic development, hematopoietic cells arise from transient endothelial-like precursors known as hemogenic endothelium (HE), which are found initially in the yolk sac vascular plexus and later in various intraembryonic and extraembryonic vessels (Frame et al., 2016, Gordon-Keylock et al., 2013, Yzaguirre and Speck, 2016). An expanding number of studies have revealed multiple overlapping waves of hematopoietic progenitors generated from HE, which give rise to increasingly diverse hematopoietic lineages, including progenitors with erythroid, myeloid, and lymphoid potential detected prior to and independently of hematopoietic stem cells (HSCs) (Frame et al., 2013, Hadland et al., 2004, Kobayashi et al., 2014, Lin et al., 2014, Yoshimoto, 2015, Yoshimoto et al., 2012). Our group previously identified B-restricted progenitors that arise prior to HSCs with distinct B cell potential that includes B-1a cells, a lineage of tissue-resident B cells implicated in innate immune responses and autoimmunity (Yoshimoto et al., 2011). Further, mature B-1a cells are serially transplantable, suggesting an embryonic origin for life-long B-1a cells independent of HSCs and conventional (B-2) B cells (Kobayashi et al., 2014). Separate studies demonstrated that highly purified long-term HSCs from the adult bone marrow, transplanted to adult recipients, do not contribute significantly to the B-1a subset of B lymphocytes (Ghosn et al., 2012), further supporting the concept of a separate embryonic origin of the innate B-1a lineage. However, a recent study in which HSCs were defined functionally by long-term contribution to multilineage hematopoiesis using cellular barcoding techniques indicated contribution to both B-2 and B-1a cells by clonal fetal stage HSCs, whereas two separate studies examining whether highly purified fetal liver HSCs contribute to B-1a cells following transplantation produced conflicting results, potentially related to differences in phenotypic markers used for sorting HSCs from the fetal liver (Ghosn et al., 2016, Kristiansen et al., 2016). These studies suggest the possibility of heterogeneity within the fetal HSC pool with regard to B-1a potential and the need to determine the existence of a common precursor for HSCs and B-1a cells during early HSC development.
One of the significant challenges in understanding embryonic HSC development has been the lack of methods to distinguish clonal precursors of HSCs from temporally and phenotypically overlapping progenitors lacking HSC potential, which is necessary to identify the unique properties and lineage potential of emerging HSCs at the single-cell level. We previously described the generation of an endothelial cell (EC) niche that supports HSC development from hemogenic precursors in vitro (Hadland et al., 2015). This EC niche is derived from the embryonic aorta-gonad-mesonephros region (AGM-EC), where the first adult-engrafting HSCs are detected during development. Specifically, we showed that co-culture with AGM-ECs was sufficient to support the induction of embryonic HE and pre-HSCs isolated as early as E9.5 to bona fide HSCs, as defined by their ability to provide long-term, multilineage engraftment into adult recipients. To build upon these studies and begin to identify the unique properties and lineage potential of clonal HSC precursors, we have adapted this system to analyze the potential of individual index-sorted hemogenic precursors to give rise to hematopoietic progeny, including functional HSCs following AGM-EC co-culture. Index sorting allows the correlation of the functional output of each single-sorted cell to its precise phenotypic properties collected during fluorescence-activated cell sorting (FACS), including the distinct expression intensity of each cell surface marker included for staining (Schulte et al., 2015). By this approach, here, we identify clonal precursors from the murine embryonic para-aortic splanchnopleura (P-Sp)/AGM region from E9.5 to E11.5 capable of giving rise to multilineage-engrafting HSCs, elucidating their unique phenotypic properties and demonstrating a common capacity to contribute to all B cell lineages including B-1a cells. Importantly, these studies provide a platform for further investigation of HSC precursors at single-cell resolution during their emergence and transition to HSCs, which will provide critical insight toward the future de novo engineering of HSCs for therapeutic applications.
Results
Index Sorting Resolves Clonal E11.5 Pre-HSCs with Both B-1a and B-2 Cell Lineage Potential
To facilitate screening for HSC potential following AGM-EC co-culture, we employed a transgenic mouse expressing Zs-Green (ZsGr) in the Fgd5 locus, which was reported to enable identification and purification of adult marrow HSCs, but also marked endothelial cells (Gazit et al., 2014). In the embryonic P-Sp/AGM, Fgd5-ZsGr expression is sensitive for, but not specific to, HSC precursors as it is expressed in all VE-Cadherin+ cells (Figure S1A), which includes hemogenic precursors as well as non-hemogenic endothelial cells at this stage. Following AGM-EC co-culture of AGM-derived Fgd5-ZsGr+VE-Cadherin+ precursors cells, persistence of Fgd5-ZsGr expression in the VE-Cadherin−F4/80−Gr1− hematopoietic fraction distinguishes the population of hematopoietic cells with long-term multilineage primary and secondary engraftment potential (Figures S1B and S1C), thus making it useful in screening for generation of engrafting cells following co-culture of hemogenic precursors with AGM-ECs.
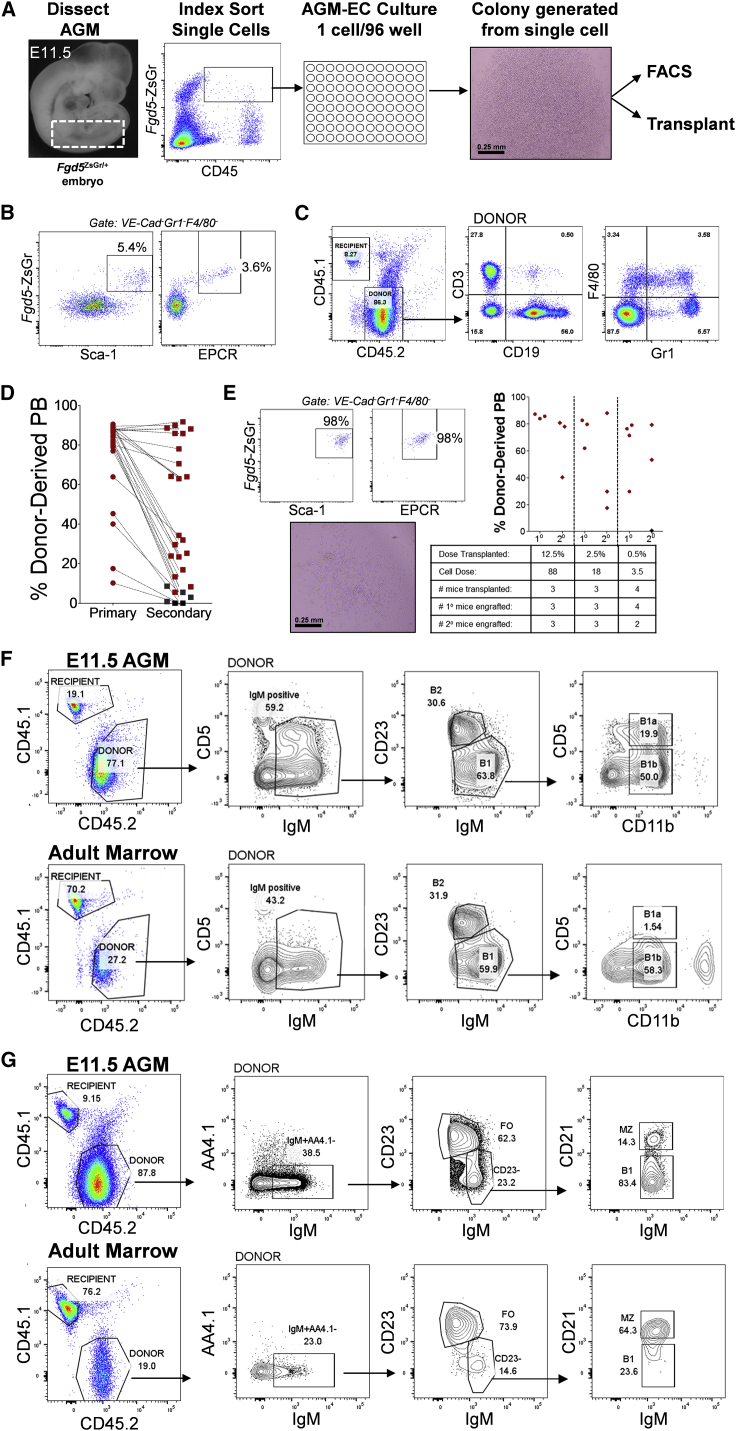
To characterize pre-HSCs from E11.5 AGM at the single-cell level, we index sorted single CD45+/loZsGr+ cells from dissected E11.5 Fgd5+/ZsGr AGM to individual wells of a 96-well plate containing an AGM-EC layer, and monitored for growth of hematopoietic colonies. Clonal frequency of colony formation ranged from 11% to 15% (n = 2). After 5 days of co-culture, cells from all wells, including those with and without hematopoietic colonies, were harvested (Figure 1A). Half of the cells from each well were assessed for expression of Fgd5-ZsGr and other phenotypic HSC markers, and half were transplanted to lethally irradiated adult Ly5.1 (CD45.1) congenic C57Bl6 wild-type mice along with 5 × 104 Ly5.1 rescue whole bone marrow cells to determine multilineage engraftment potential. By this approach, we found most hematopoietic colonies containing cells with VE-Cadherin−Gr1−F4/80−Sca1+EPCR+Fgd5-ZsGr+ phenotype (Figure 1B) were capable of long-term multilineage engraftment determined by detection of donor-lineage-positive cells in the peripheral blood (Figure 1C) of transplanted mice, and by detection of Fgd5-ZsGr+ donor cells with HSC phenotype (Lineage−c-kit+Sca-1+CD48−CD150+) in the bone marrow (Figure S1D). In contrast, neither harvested hematopoietic colonies without detectable VE-Cadherin−Gr1−F4/80−Sca1+EPCR+Fgd5-ZsGr+ cells nor cells harvested from wells without hematopoietic colonies provided detectable engraftment in transplanted mice (data not shown). In addition to assessing HSC potential following primary transplantation, we also examined secondary engraftment from individual pre-HSC clones. Most clonal E11.5 precursors capable of primary multilineage engraftment also provided multilineage secondary engraftment; however, a subset did not, suggesting heterogeneity in the ability of clonal pre-HSCs to give rise to HSCs with robust serial transplantation capacity (Figure 1D). Among engrafting pre-HSC clones, we also detected heterogeneity in colony phenotype (compare Figures 1B and 1E) and the extent of transplantation capacity. Specifically, we detected a subset of pre-HSCs that manifested extensive self-renewal behavior, generating large numbers of homogeneous phenotypic and functional HSCs with robust primary and secondary multilineage engraftment (Figure 1E), comparable with the proliferative behavior of fetal liver-stage HSCs, which eventually contribute to the more quiescent adult HSC pool (Bowie et al., 2007).
Figure 1.
Index Sorting with AGM-EC Co-culture Identifies Clonal Fgd5-ZsGr+CD45+/lo Pre-HSC with B-1a and B-2 Cell Potential
(A) Methodology for detection of clonal HSC precursors from E11.5 Fgd5ZsGr/+ embryos combining index sorting of single AGM-derived cells and AGM-EC co-culture. Dashed box indicates sample window used for index sorting Fgd5-ZsGr+CD45+/lo cells.
(B) Phenotypic analysis by FACS of the progeny of a single cell following 5 days co-culture, showing a population of cells co-expressing HSC markers Fgd5-ZsGr, Sca-1, and EPCR in cells gated as VE-Cadherin−Gr1−F4/80−.
(C) Representative peripheral blood engraftment in a recipient mouse transplanted with the progeny of a single cell with the “HSC” phenotype as shown in (B)
(D) Primary and secondary peripheral blood engraftment from E11.5 pre-HSC clones (primary engraftment data at 12–16 weeks post-transplant, secondary engraftment at >16 weeks post-transplant, from n = 27 clones pooled from two independent experiments; clonal HSC precursor frequency 27 of 564 total index-sorted cells). Mice with multilineage engraftment are indicated by data points in red. Mice lacking detectable peripheral blood engraftment in one or more lineages are indicated by data points in black.
(E) Representative E11.5 pre-HSC clone generating a colony of progeny with homogeneous VE-Cadherin−Gr1−F4/80−Fgd5-ZsGr+Sca1+EPCR+ “HSC” phenotype, with limit dilution transplantation of the progeny demonstrating primary (1°) and secondary (2°) engraftment. HSC frequency of the progeny calculated by limiting dilution analysis based on the fraction of mice with secondary engraftment (ELDA analysis; Hu and Smyth, 2009) is estimated at 1 in 4.5 cells (95% CI, 1 in 1.4 to 1 in 14), and total of 156 HSCs generated (95% CI, 50 to 486). Mice with multilineage engraftment at 12 weeks post-transplant in primary and16 weeks post-transplant in secondary transplants are indicated by data points in red.
(F and G) Representative engraftment of (F) B-1a, B-1b and B-2 cells in the peritoneum, and (G) B1, marginal zone (MZ), and follicular (FO) B cells in the spleen, 12 weeks following transplantation of the progeny (after AGM-EC co-culture) of clonal E11.5 AGM-derived Fgd5-ZsGr+CD45+ pre-HSCs, or adult bone marrow-derived Fgd5-ZsGr+Lin−Sca1+c-kit+CD150+CD48− HSCs. Similar results, demonstrating both B-1a and B-2 peritoneal engraftment, were obtained in all clones analyzed from E11.5 pre-HSCs (n = 27).
See Table S1.
Considering recent studies suggesting potential heterogeneity in B-1a potential of fetal liver HSCs, single-cell analysis of embryonic HSC precursors also presented the unique opportunity to evaluate clonal contribution to B-1a B cell potential from developing HSCs prior to the fetal liver stage. Thus, we examined whether E11.5 pre-HSCs, which we have shown contribute clonally to long-term multilineage hematopoiesis in the peripheral blood and marrow following AGM-EC maturation and transplantation, also contribute to tissue-resident B-1a cells in the peritoneum. We found that all clonal pre-HSCs analyzed at E11.5 contributed to the complete B cell repertoire, including B-1a, B-1b, and B-2 cells in the peritoneum (Figures 1F and Table S1), and B1, marginal zone, and follicular (B-2) B cells in the spleen (Figure 1G). To rule out the possibility that co-culture on AGM-EC stroma induced B-1a potential in cells that otherwise lacked this potential, we also tested clonal adult bone marrow-derived Fgd5-ZsGr+Lin−Sca1+c-kit+CD48−CD150+ HSCs cultured on AGM-ECs. Consistent with previous studies in which purified adult HSCs were directly transplanted (Ghosn et al., 2012), the progeny of adult HSCs cultured on AGM-ECs contributed to multilineage hematopoiesis, including marginal zone, B-2, and B-1b B cells, but did not significantly contribute to the B-1a lineage detected in the peritoneum of transplanted mice (Figures 1F and 1G). Combined with our previous studies demonstrating an HSC-independent source of B-1a cells from early E9 yolk sac and P-Sp HE (Yoshimoto et al., 2011, Kobayashi et al., 2014), these results confirm a second origin of innate B-1a cells from clonal E11.5 AGM-derived pre-HSCs that is unique to embryonic development.
Functionally Distinct E9.5 Pre-HSCs Contain B-1a and B-2 Cell Lineage Potential
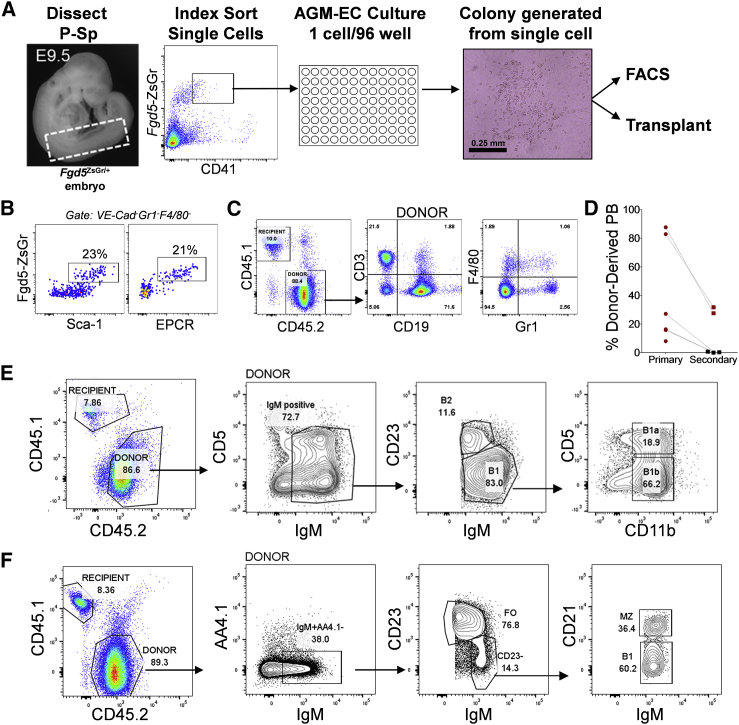
At E9.5, P-Sp cells co-expressing endothelial markers and CD41, the earliest marker of hematopoietic commitment expressed prior to CD45, represent a heterogeneous mixture of hematopoietic progenitors, including a rare population of pre-HSCs (1–2 per embryo) as determined by limiting dilution re-aggregation assays (Rybtsov et al., 2014, Rybtsov et al., 2016). To determine whether we could identify these early pre-HSCs at the single-cell level, individual cells from E9.5 Fgd5+/ZsGr embryo P-Sp were index sorted based on CD41 and Zs-Green expression to AGM-ECs in 96-well plates and monitored for growth of hematopoietic colonies (Figure 2A). Clonal frequency of colony formation ranged from 8% to 27% (18% ± 8%, n = 4). Fgd5-ZsGr+CD41lo cells giving rise to colonies with phenotypic VE-Cadherin−Gr1−F4/80−Sca1+EPCR+Fgd5-ZsGr+ cells and long-term multilineage engraftment were identified, confirming a clonal origin for HSC precursors from this earlier population (Figures 2B and 2C). Compared with pre-HSC clones identified at E11.5, clonal Fgd5-ZsGr+CD41lo pre-HSCs detected at E9.5 were rare (less than 1 per embryo equivalent), contributed less robustly to multilineage hematopoiesis in secondary transplantation assays (Figure 2D), and did not generate any homogeneous (extensively self-renewing) HSC colonies that were observed from E11.5 pre-HSC clones, suggesting distinct properties of pre-HSC clones arising during this early stage of development compared with later pre-HSCs detected at E11.5. We also examined whether E9.5 pre-HSCs contribute to B-1a cells. Like pre-HSCs at E11.5, clonal pre-HSCs analyzed from E9.5 contributed to all B cell subsets examined, including B-1a, B-1b, and B-2 cells in the peritoneum (Figures 2E and Table S1), and B1, marginal zone, and follicular (B-2) B cells in the spleen (Figure 2F). Interestingly, the relative contribution of pre-HSCs is significantly skewed toward B-1a cells from E9.5 clones and toward B-2 cells from E11.5 clones (Table S1), providing further evidence for evolution of distinct, stage-dependent properties of pre-HSCs between E9.5 and E11.5.
Figure 2.
Clonal Fgd5-ZsGr+CD41lo E9.5 Pre-HSC Have B-1a and B-2 Cell Potential
(A) Methodology for detection of clonal HSC precursors from E9.5 Fgd5ZsGr/+ embryos combining index sorting of single P-Sp-derived cells and AGM-EC co-culture. Dashed box indicates sample window used for index sorting Fgd5-ZsGr+CD41lo cells.
(B) Phenotypic analysis by FACS of progeny of a single cell following 7 day co-culture, showing a population of cells co-expressing HSC markers Fgd5-ZsGr, Sca-1, and EPCR in cells gated as VE-Cadherin−Gr1−F4/80−.
(C) Representative peripheral blood engraftment in a mouse transplanted with progeny of a single cell with “HSC” phenotype as shown in (B).
(D) Primary and secondary peripheral blood engraftment from E9.5 pre-HSC clones (primary engraftment data from n = 6 clones at 12–16 weeks post-transplant, secondary engraftment data from n = 5 clones at >16 weeks post-transplant, pooled from four independent experiments; clonal HSC precursor frequency 6 of 653 total index sorted cells). Mice with multilineage engraftment are indicated by data points in red. Mice lacking detectable peripheral blood engraftment in one or more lineages are indicated by data points in black.
(E and F) Representative engraftment of (E) B-1a, B-1b, and B-2 cells in the peritoneum, and (F) B1, marginal zone (MZ), and follicular (FO) B cells in the spleen, 12 weeks following transplantation of the progeny (after AGM-EC co-culture) of clonal E9.5 P-Sp-derived Fgd5-ZsGr+CD41lo pre-HSCs. Similar results, demonstrating both B-1a and B-2 peritoneal engraftment, were obtained in all clones analyzed from E9.5 pre-HSCs (n = 6).
See Table S1.
Clonal Pre-HSC Are Distinguished by Unique Expression of EPCR and Dll4
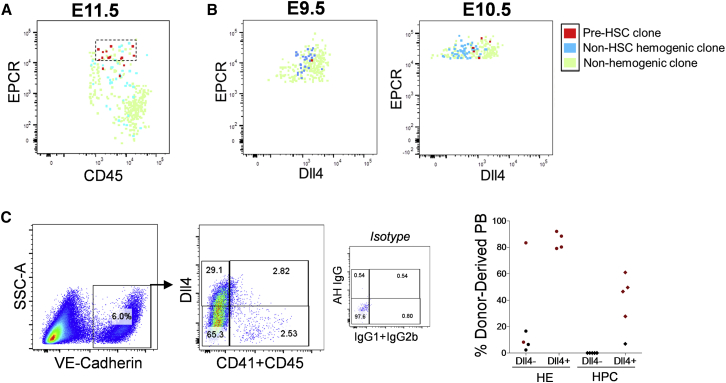
If pre-HSCs constitute a distinct population among a larger population of hemogenic precursors lacking HSC potential, we hypothesized they may be distinguished by the expression of unique surface markers. Index sorting facilitates the determination of phenotypic characteristics of different hemogenic populations either with or without HSC potential, by tracking the phenotypic parameters of each individual cell as it correlates to its hematopoietic potential and engraftment potential following induction on AGM-EC stroma. Thus, we screened for additional surface markers that could further enrich for pre-HSCs. At E11.5, we determined that the subset of Fgd5-ZsGr+CD45+/lo cells expressing high levels of EPCR, a marker previously shown to be expressed in fetal and adult long-term HSCs as well as embryonic pre-HSCs and multipotent progenitors (Balazs et al., 2006, Inlay et al., 2014, Iwasaki et al., 2010, Zhou et al., 2016), was significantly enriched in HSC potential, identifying a distinct phenotypic population with HSC potential at approximately 1 in 2 to 1 in 3 cells sorted (Figures 3A and S2A). Clonal pre-HSCs between E9.5 and E10.5 also express EPCR, although they remain significantly outnumbered by hemogenic precursors without HSC potential that are also detected in the Fgd5-ZsGr+CD41loEPCR+ population at these earlier stages; however, expression of the Notch ligand Dll4, a marker of arterial endothelium, distinguishes pre-HSCs at these stages since most EPCR+ precursors lacking HSC potential express lower levels of Dll4 (Figures 3B, S2B, and S2C). We confirmed that Dll4 expression also enriched for HSC potential at the population level in both VE-Cadherin+CD41−CD45− HE as well as VE-Cadherin+CD41+/CD45+ hematopoietic populations from E9.5 P-Sp, by sorting these populations based on Dll4 expression followed by AGM-EC co-culture and transplantation into adult mice (Figure 3C). These results demonstrate the ability to resolve distinct phenotypic populations of hemogenic precursors capable of generating HSCs from those that cannot, which favors the existence of unique populations of precursors at this stage with and without HSC potential, rather than simply reflecting inefficiencies of the co-culture assay.
Figure 3.
Pre-HSCs Express Distinct Levels of EPCR and Delta-like-4
(A) Expression of EPCR in index-sorted E11.5 AGM-derived Fgd5-ZsGr+CD45+/lo single cells, identifying precursors with HSC potential (pre-HSC clone), hematopoietic colony formation without HSC potential (non-HSC hemogenic clone), or no hematopoietic colony formation (non-hemogenic clone) based on functional potential following AGM-EC co-culture. Dashed box contains EPCRhigh-expressing cells highly enriched for pre-HSC clones.
(B) Expression of EPCR and Delta-like-4 (Dll4) in index-sorted E9.5 (left panel) and E10.5 (right panel) P-Sp/AGM-derived Fgd5-ZsGr+CD41lo single cells (see also Figure S2 for additional index-sorting parameters).
(C) Engraftment of hematopoietic cells generated following co-culture on AGM-ECs from E9.5 P-Sp hemogenic endothelium (HE, VE-Cadherin+CD41−CD45−) and hematopoietic progenitor cells (HPC, VE-Cadherin+CD41+ and/or CD45+) sorted based on expression of Dll4. Mice with multilineage engraftment at >16 weeks post-transplant are indicated by red data points. Mice lacking detectable peripheral blood engraftment in one or more lineages are indicated by data points in black.
Discussion
To establish methods for the study of the functional and molecular properties of HSC precursors at the single-cell level, we have adapted our recently described 2D co-culture system, in which an engineered AGM-derived EC substrate provides the necessary in vitro niche for induction of functional HSCs from hemogenic precursors (Hadland et al., 2015), for clonal studies of index-sorted hemogenic precursors from embryonic development. By this approach, we have demonstrated that functionally defined, clonal pre-HSCs are phenotypically distinguishable from progenitors lacking HSC potential in the E9.5 to E11.5 embryo. Interestingly, we identified Dll4, a Notch ligand expressed on arterial endothelium, as a phenotypic marker of HSC precursor potential. Considering recent studies suggesting that the level of Notch signaling activation must be attenuated during the HE to HSC transition, it is interesting to speculate that co-expression of Dll4 with Notch1 on the surface of an HSC precursor could serve to limit Notch1 receptor activation by cis-inhibition, a well-established mechanism regulating Notch signaling in other development systems (del Álamo et al., 2011, Gama-Norton et al., 2015, Lizama et al., 2015). This could constitute a cell-autonomous mechanism to modulate the ability of pre-HSCs to receive Notch signals induced by trans activation via Notch ligands known to be expressed on adjacent endothelial cell stroma (Hadland et al., 2015), which may be essential for HSC fate, as studies including our own have demonstrated that fate determination in many developmental contexts is dependent on precise Notch signal strength (Dallas et al., 2005, Delaney et al., 2005). Additional studies will be necessary to determine the functional significance of Dll4 expression on HSC precursors in this regard, studies that may provide insight into requirements for generation of functional HSC from pluripotent stem cell-derived hemogenic precursors.
Our current study also enabled the assessment of clonal contribution to in vivo multilineage hematopoiesis from single cells at the earliest stages of HSC precursor formation, which has allowed us to identify the earliest common precursor of B-1a and B-2 cell potential in a clonal precursor to HSCs as early as E9.5. Recent reports have strengthened the concept that adult HSCs do not contribute significantly to the innate B-1a cell compartment, but suggest heterogeneity in the fetal HSC compartment with regard to B-1a cell potential (Beaudin et al., 2016, Ghosn et al., 2012, Ghosn et al., 2016, Kristiansen et al., 2016, Sawai et al., 2016). Previous studies by members of our group identified an embryonic HSC-independent B cell progenitor with B-1 but not B-2 cell potential (Kobayashi et al., 2014, Yoshimoto, 2015, Yoshimoto et al., 2011). Our ability to detect significant B-1a and B-2 cell contribution from all clonal pre-HSC tested at E9.5 and E11.5 in this study suggests a second wave of B-1a progenitors developing from a common precursor to embryonic HSCs.
A recently published study using an irreversible lineage reporter mouse model identified two distinct populations of fetal liver HSCs (Beaudin et al., 2016). Although both types provide long-term multilineage engraftment in transplantation assays, only one of these fetal HSC populations contributes to the pool of long-term HSCs in the adult bone marrow in situ, whereas the other “developmentally restricted” HSC is primed to contribute to innate immune cells, including the B-1a lineage. Our clonal analysis of emerging pre-HSCs suggests developmental asynchrony, with E9.5 pre-HSCs generating a relatively greater proportion of B-1a cells in transplantation assays and unique pre-HSC clones emerging only later at E11.5 with extensive expansion in vitro of HSCs with robust primary and secondary engraftment, consistent with the self-renewing behavior of expanding fetal liver HSCs that populate the adult marrow (Bowie et al., 2007). These results suggest that pre-HSCs, arising asynchronously between E9.5 and E11.5, generate HSCs with different functional properties and relative B-1a cell contribution that may account for the distinct populations of fetal liver HSCs described by Beaudin et al. (2016).
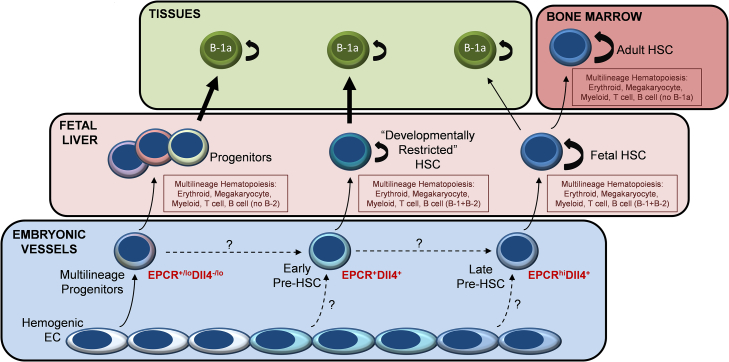
Altogether, combining the results of these recent studies with our clonal analysis of pre-HSC lineage potential presented here, we propose a refined model of developmental hematopoiesis that incorporates multiple, overlapping waves of definitive hematopoiesis, progressing through a multilineage progenitor stage generating innate immune cells including B-1a but lacking B-2 potential, early pre-HSCs giving rise to “developmentally restricted” HSCs that are biased toward generation of innate immune cells including B-1a but also generate initial B-2 cells, and late pre-HSCs with limited B-1a potential that is lost as these cells mature, self-renew, and contribute to the rapidly expanding pool of long-term fetal liver HSCs that eventually colonize the adult marrow (Figure 4). Consistent with a recently reported genetic study proposing distinct, differentially regulated waves of B-1a and B-2 cell development, this model accounts for three distinct sources of B-1a and two sources of B-2 cell potential (Montecino-Rodriguez et al., 2016). Future studies using our approach to define HSC potential at the clonal level will be required to determine whether these distinct waves of definitive hematopoiesis emerge from separate populations of HE or rather diverge following emergence of a common pool of CD41+ hematopoietic precursors. Altogether, our studies provide insight into the developmental origin of HSC heterogeneity, with critical implications for engineering HSCs from pluripotent stem cells and for understanding the ontogeny of innate immunity.
Figure 4.
Proposed Model for Multiple Waves of Definitive Hematopoiesis Contributing to B-1a B Cells
Multiple overlapping waves of definitive hematopoiesis emerge within embryonic vessels between E9 and E11.5, giving rise initially to multilineage progenitors producing erythroid, myeloid, and innate-immune lymphoid progeny independently of HSC (Böiers et al., 2013, Godin et al., 1993, Inlay et al., 2014, Kobayashi et al., 2014, Yoshimoto et al., 2011, Yoshimoto et al., 2012), followed by emergence of the earliest clonal pre-HSCs giving rise to “developmentally restricted” HSCs with innate-immune biased lymphoid potential (Beaudin et al., 2016), and late pre-HSC clones giving rise to a pool of extensively self-renewing fetal liver HSCs that initially have limited B-1a B cell potential, which is lost as they establish the life-long HSC pool of the adult bone marrow (Ghosn et al., 2012).
Experimental Procedures
Mice
Wild-type C57BL6/J7 (Ly5.2/CD45.2) and congenic C57BL/6.SJL-Ly5.1-Pep3b (Ly5.1/CD45.1) mice were bred at the Fred Hutchinson Cancer Research Center. Transgenic Fgd5Zs-Green/+ mice in C57BL6/J7 background were generated as previously described (Gazit et al., 2014). C57BL6/J7 Ly5.2 or Fgd5Zs-Green/+ male and wild-type C57BL6/J7 Ly5.2 female mice were used for timed matings.
Embryo Dissections and Cell Sorting
Embryo P-Sp/AGM tissues were harvested as previously described (Hadland et al., 2015). Embryo age was precisely timed by counting somite pairs (sp): E9.5 (21–29sp), E10.5 (35–39sp), and E11.5 (40–47sp). Dissected AGM/P-Sp tissues were treated with 0.25% collagenase (STEMCELL Technologies) for 25 min at 37°C, pipetted to a single-cell suspension, and washed with PBS containing 10% fetal bovine serum (FBS). Cells were incubated with anti-mouse CD16/CD32 (FcγRII block) and stained with monoclonal antibodies as indicated. A detailed list of all antibodies used is shown in Table S2. DAPI staining was used to gate out dead cells. All reagents for cell staining were diluted in PBS with 10% FBS, and staining was carried out on ice or at 4°C. Cells were sorted on a BD FACSAria II equipped with BD FACSDiva Software with index-sorting capability. For index-sorted single cells, sorting was performed in single-cell mode with maximum purity mask settings to minimize contaminating cells.
Endothelial Cell Co-culture Assays
AGM-ECs were derived from ECs sorted from E10.5 to E11.5 AGM regions, infected with lentivirus containing MyrAKT construct, and cultured as previously described (Hadland et al., 2015). For co-culture experiments, AGM-ECs at passage 15 or less were plated at a density of 1 × 104 cells per well to gelatin-treated 96-well tissue culture plates for single-cell assays, or 4 × 104 cells per 24-well for bulk cultures, 24 hr prior to initiation of co-culture. Single P-Sp/AGM-derived cells were individually index sorted to each well of 96-well plates containing AGM-ECs in serum-free media consisting of X-vivo 20 (Lonza) with recombinant cytokines (Peprotech): stem cell factor and FLT3 ligand each at 100 ng/mL, and interleukin-3, and thrombopoietin each at 20 ng/mL. Cells from E9.5 embryos were cultured for 7 days, and cells from E10.5 to 11.5 embryos were cultured for 5–6 days, based on prior studies that determined the time required to detect engrafting populations following culture from precursors at different stages (Hadland et al., 2015). Following co-culture, each well was visualized for hematopoietic colony formation, and wells with more than one colony were excluded from analysis. In some experiments, wells without visible hematopoietic colonies were also harvested for phenotypic and transplantation analysis to assay for HSC generation in the absence of colony formation.
Cell Surface Analysis
Following co-culture, 50% volume of each 96-well containing a visible colony was harvested by vigorous pipetting from the EC layer and transferred to new 96-well plates for FACS. Cells were spun and re-suspended in PBS with 2% FBS, pre-incubated with anti-mouse CD16/CD32 (FcγRII block) and then stained with various combinations of monoclonal antibodies as indicated. AGM-ECs were excluded from analysis of co-cultured cells by gating based on expression of VE-Cadherin, and myeloid cells were gated by expression of Gr-1 and F4/80. DAPI was used to exclude dead cells. Flow cytometry was performed on a Becton Dickinson Canto 2 and data analyzed using FlowJo Software.
Transplantation Assays
Freshly sorted Ly5.2/CD45.2 AGM cells or cultured cells harvested by pipetting off the wells were washed with PBS with 2% FBS and re-suspended in 100 μL of PBS/2% FBS per mouse transplanted. For clonal assays, the remaining 50% volume following FACS analysis of each 96-well plate containing a visible colony was harvested individually for transplantation. For most experiments, only colonies with detectable VE-Cadherin−Gr1−F4/80−Sca1+EPCR+Fgd5-ZsGr+ cells by FACS were transplanted based on initial studies demonstrating that colonies lacking this phenotype did not provide detectable hematopoietic engraftment in transplanted mice. Freshly harvested Ly5.1/CD45.1 bone marrow cells were added at 5 × 104 cells in 100 μL of PBS/FBS per mouse to provide hematopoietic rescue. Cells were co-injected into lethally irradiated (1,000 cGy using a cesium source) Ly5.1/CD45.1 adult recipients via the tail vein. For secondary transplants, 2 × 106 whole bone marrow cells harvested from primary recipients 12–16 weeks following primary transplantation were transplanted to lethally irradiated Ly5.1/CD45.1 recipients via the tail vein. FACS analysis of peripheral blood obtained by retro-orbital bleeds was performed at 2, 6, and 12–16 weeks following transplantation, and from cells obtained from harvested bone marrow, spleen, and thymic tissues, and peritoneal fluid at the time of secondary transplant, at 12–16 weeks following primary transplant. FACS analysis of peripheral blood was performed at 16 weeks following secondary transplantation. Peritoneal cells were harvested by injecting 6 mL of PBS with a 21G needle and syringe through the peritoneal membrane into the peritoneal space and collecting back injected fluid. Lineage-specific staining from donor (CD45.2) and recipient/rescue (CD45.1) cells for peripheral blood, bone marrow, spleen, thymus, and peritoneal-derived cells was performed as previously described (Hadland et al., 2015, Kobayashi et al., 2014), using antibodies listed in Table S2. Multilineage engraftment was defined as >5% donor (CD45.2) contribution to the peripheral blood with detectable contribution of donor myeloid (Gr-1 and/or F4/80), B cells (CD19), and T cells (CD3).
Statistics
For statistical analysis, two-tailed, unpaired Student's t test was used to calculate p values. A p value less than 0.05 was considered significant. ELDA software (Hu and Smyth, 2009) (http://bioinf.wehi.edu.au/software/elda/) was used for limit dilution transplantation analysis, calculated using the fraction of mice engrafted at each dilution of starting cells expressed per embryo equivalent, where engraftment was defined as >5% donor (CD45.2) contribution to the peripheral blood with each of donor myeloid (Gr-1 and/or F4/80), B cell (CD19) and T cell (CD3) engraftment detected in secondarily transplanted mice at 16 weeks post-transplant.
Animal Study Approval
All animal studies were conducted in accordance with the NIH guidelines for humane treatment of animals and were approved by the Institutional Animal Care and Use Committee at the Fred Hutchinson Cancer Research Center.
Author Contributions
B.K.H., B.V., I.D.B., S.R, J.M.B., M.G.P., M.C.Y., and M.Y. designed experiments. B.K.H., B.V., and M.Y. conducted experiments. P.K.M. and D.J.R. provided Fgd5Zs-Green/+ mice. B.K.H. and B.V. wrote the manuscript and I.B., M.C.Y., P.K.M., D.J.R., and M.Y. edited the manuscript.
Acknowledgments
We thank Cynthia McKay and David A. Flowers for their superb technical assistance. This work was supported by the NIH: NHLBI UO1 HL100395, Ancillary HL100395-07 Sub-award 10560, Ancillary HL099997, NIAID R56 AI110831, and NIAID R01 AI121197. Brandon Hadland is supported by the Alex’s Lemonade Stand Foundation and Hyundai Hope on Wheels Foundation
Published: May 4, 2017
Footnotes
Supplemental Information includes two figures and two tables and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2017.04.007.
Supplemental Information
References
- Balazs A.B., Fabian A.J., Esmon C.T., Mulligan R.C. Endothelial protein C receptor (CD201) explicitly identifies hematopoietic stem cells in murine bone marrow. Blood. 2006;107:2317–2321. doi: 10.1182/blood-2005-06-2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaudin A.E., Boyer S.W., Perez-Cunningham J., Hernandez G.E., Derderian S.C., Jujjavarapu C., Aaserude E., MacKenzie T., Forsberg E.C. A transient developmental hematopoietic stem cell gives rise to innate-like B and T cells. Cell Stem Cell. 2016;19:768–783. doi: 10.1016/j.stem.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowie M.B., Kent D.G., Dykstra B., McKnight K.D., McCaffrey L., Hoodless P.A., Eaves C.J. Identification of a new intrinsically timed developmental checkpoint that reprograms key hematopoietic stem cell properties. Proc. Natl. Acad. Sci. USA. 2007;104:5878–5882. doi: 10.1073/pnas.0700460104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böiers C., Carrelha J., Lutteropp M., Luc S., Green J.C., Azzoni E., Woll P.S., Mead A.J., Hultquist A., Swiers G. Lymphomyeloid contribution of an immune-restricted progenitor emerging prior to definitive hematopoietic stem cells. Cell Stem Cell. 2013;13:535–548. doi: 10.1016/j.stem.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Dallas M.H., Varnum-Finney B., Delaney C., Kato K., Bernstein I.D. Density of the Notch ligand Delta1 determines generation of B and T cell precursors from hematopoietic stem cells. J. Exp. Med. 2005;201:1361–1366. doi: 10.1084/jem.20042450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Álamo D., Rouault H., Schweisguth F. Mechanism and significance of cis-inhibition in Notch signalling. Curr. Biol. 2011;21:R40–R47. doi: 10.1016/j.cub.2010.10.034. [DOI] [PubMed] [Google Scholar]
- Delaney C., Varnum-Finney B., Aoyama K., Brashem-Stein C., Bernstein I.D. Dose-dependent effects of the Notch ligand Delta1 on ex vivo differentiation and in vivo marrow repopulating ability of cord blood cells. Blood. 2005;106:2693–2699. doi: 10.1182/blood-2005-03-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame J.M., McGrath K.E., Palis J. Erythro-myeloid progenitors: “definitive” hematopoiesis in the conceptus prior to the emergence of hematopoietic stem cells. Blood Cells Mol. Dis. 2013;51:220–225. doi: 10.1016/j.bcmd.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame J.M., Fegan K.H., Conway S.J., McGrath K.E., Palis J. Definitive hematopoiesis in the yolk sac emerges from Wnt-responsive hemogenic endothelium independently of circulation and arterial identity. Stem Cells. 2016;34:431–444. doi: 10.1002/stem.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gama-Norton L., Ferrando E., Ruiz-Herguido C., Liu Z., Guiu J., Islam A.B., Lee S.U., Yan M., Guidos C.J., López-Bigas N. Notch signal strength controls cell fate in the haemogenic endothelium. Nat. Commun. 2015;6:8510. doi: 10.1038/ncomms9510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazit R., Mandal P.K., Ebina W., Ben-Zvi A., Nombela-Arrieta C., Silberstein L.E., Rossi D.J. Fgd5 identifies hematopoietic stem cells in the murine bone marrow. J. Exp. Med. 2014;211:1315–1331. doi: 10.1084/jem.20130428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosn E.E., Yamamoto R., Hamanaka S., Yang Y., Herzenberg L.A., Nakauchi H. Distinct B-cell lineage commitment distinguishes adult bone marrow hematopoietic stem cells. Proc. Natl. Acad. Sci. USA. 2012;109:5394–5398. doi: 10.1073/pnas.1121632109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosn E.E., Waters J., Phillips M., Yamamoto R., Long B.R., Yang Y., Gerstein R., Stoddart C.A., Nakauchi H., Herzenberg L.A. Fetal hematopoietic stem cell transplantation fails to fully regenerate the B-lymphocyte compartment. Stem Cell Rep. 2016;6:137–149. doi: 10.1016/j.stemcr.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godin I.E., Garcia-Porrero J.A., Coutinho A., Dieterlen-Lièvre F., Marcos M.A. Para-aortic splanchnopleura from early mouse embryos contains B1a cell progenitors. Nature. 1993;364:67–70. doi: 10.1038/364067a0. [DOI] [PubMed] [Google Scholar]
- Gordon-Keylock S., Sobiesiak M., Rybtsov S., Moore K., Medvinsky A. Mouse extraembryonic arterial vessels harbor precursors capable of maturing into definitive HSCs. Blood. 2013;122:2338–2345. doi: 10.1182/blood-2012-12-470971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadland B.K., Huppert S.S., Kanungo J., Xue Y., Jiang R., Gridley T., Conlon R.A., Cheng A.M., Kopan R., Longmore G.D. A requirement for Notch1 distinguishes 2 phases of definitive hematopoiesis during development. Blood. 2004;104:3097–3105. doi: 10.1182/blood-2004-03-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadland B.K., Varnum-Finney B., Poulos M.G., Moon R.T., Butler J.M., Rafii S., Bernstein I.D. Endothelium and NOTCH specify and amplify aorta-gonad-mesonephros-derived hematopoietic stem cells. J. Clin. Invest. 2015;125:2032–2045. doi: 10.1172/JCI80137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Smyth G.K. ELDA: extreme limiting dilution analysis for comparing depleted and enriched populations in stem cell and other assays. J. Immunol. Methods. 2009;347:70–78. doi: 10.1016/j.jim.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Inlay M.A., Serwold T., Mosley A., Fathman J.W., Dimov I.K., Seita J., Weissman I.L. Identification of multipotent progenitors that emerge prior to hematopoietic stem cells in embryonic development. Stem Cell Rep. 2014;2:457–472. doi: 10.1016/j.stemcr.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki H., Arai F., Kubota Y., Dahl M., Suda T. Endothelial protein C receptor-expressing hematopoietic stem cells reside in the perisinusoidal niche in fetal liver. Blood. 2010;116:544–553. doi: 10.1182/blood-2009-08-240903. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Shelley W.C., Seo W., Vemula S., Lin Y., Liu Y., Kapur R., Taniuchi I., Yoshimoto M. Functional B-1 progenitor cells are present in the hematopoietic stem cell-deficient embryo and depend on Cbfβ for their development. Proc. Natl. Acad. Sci. USA. 2014;111:12151–12156. doi: 10.1073/pnas.1407370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen T.A., Jaensson Gyllenbäck E., Zriwil A., Björklund T., Daniel J.A., Sitnicka E., Soneji S., Bryder D., Yuan J. Cellular barcoding links B-1a B cell potential to a fetal hematopoietic stem cell state at the single-cell level. Immunity. 2016;45:346–357. doi: 10.1016/j.immuni.2016.07.014. [DOI] [PubMed] [Google Scholar]
- Lin Y., Yoder M.C., Yoshimoto M. Lymphoid progenitor emergence in the murine embryo and yolk sac precedes stem cell detection. Stem Cells Dev. 2014;23:1168–1177. doi: 10.1089/scd.2013.0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizama C.O., Hawkins J.S., Schmitt C.E., Bos F.L., Zape J.P., Cautivo K.M., Borges Pinto H., Rhyner A.M., Yu H., Donohoe M.E. Repression of arterial genes in hemogenic endothelium is sufficient for haematopoietic fate acquisition. Nat. Commun. 2015;6:7739. doi: 10.1038/ncomms8739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montecino-Rodriguez E., Fice M., Casero D., Berent-Maoz B., Barber C.L., Dorshkind K. Distinct genetic networks orchestrate the emergence of specific waves of fetal and adult B-1 and B-2 development. Immunity. 2016;45:527–539. doi: 10.1016/j.immuni.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybtsov S., Batsivari A., Bilotkach K., Paruzina D., Senserrich J., Nerushev O., Medvinsky A. Tracing the origin of the HSC hierarchy reveals an SCF-dependent, IL-3-independent CD43(-) embryonic precursor. Stem Cell Rep. 2014;3:489–501. doi: 10.1016/j.stemcr.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybtsov S., Ivanovs A., Zhao S., Medvinsky A. Concealed expansion of immature precursors underpins acute burst of adult HSC activity in foetal liver. Development. 2016;143:1284–1289. doi: 10.1242/dev.131193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai C.M., Babovic S., Upadhaya S., Knapp D.J., Lavin Y., Lau C.M., Goloborodko A., Feng J., Fujisaki J., Ding L. Hematopoietic stem cells are the major source of multilineage hematopoiesis in adult animals. Immunity. 2016;45:597–609. doi: 10.1016/j.immuni.2016.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulte R., Wilson N.K., Prick J.C., Cossetti C., Maj M.K., Gottgens B., Kent D.G. Index sorting resolves heterogeneous murine hematopoietic stem cell populations. Exp. Hematol. 2015;43:803–811. doi: 10.1016/j.exphem.2015.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto M. The first wave of B lymphopoiesis develops independently of stem cells in the murine embryo. Ann. N. Y Acad. Sci. 2015;1362:16–22. doi: 10.1111/nyas.12612. [DOI] [PubMed] [Google Scholar]
- Yoshimoto M., Montecino-Rodriguez E., Ferkowicz M.J., Porayette P., Shelley W.C., Conway S.J., Dorshkind K., Yoder M.C. Embryonic day 9 yolk sac and intra-embryonic hemogenic endothelium independently generate a B-1 and marginal zone progenitor lacking B-2 potential. Proc. Natl. Acad. Sci. USA. 2011;108:1468–1473. doi: 10.1073/pnas.1015841108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto M., Porayette P., Glosson N.L., Conway S.J., Carlesso N., Cardoso A.A., Kaplan M.H., Yoder M.C. Autonomous murine T-cell progenitor production in the extra-embryonic yolk sac before HSC emergence. Blood. 2012;119:5706–5714. doi: 10.1182/blood-2011-12-397489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yzaguirre A.D., Speck N.A. Insights into blood cell formation from hemogenic endothelium in lesser-known anatomic sites. Dev. Dyn. 2016;245:1011–1028. doi: 10.1002/dvdy.24430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F., Li X., Wang W., Zhu P., Zhou J., He W., Ding M., Xiong F., Zheng X., Li Z. Tracing haematopoietic stem cell formation at single-cell resolution. Nature. 2016;533:487–492. doi: 10.1038/nature17997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.