Summary
Background
Mass drug administration for elimination of Plasmodium falciparum malaria is recommended by WHO in some settings. We used consensus modelling to understand how to optimise the effects of mass drug administration in areas with low malaria transmission.
Methods
We collaborated with researchers doing field trials to establish a standard intervention scenario and standard transmission setting, and we input these parameters into four previously published models. We then varied the number of rounds of mass drug administration, coverage, duration, timing, importation of infection, and pre-administration transmission levels. The outcome of interest was the percentage reduction in annual mean prevalence of P falciparum parasite rate as measured by PCR in the third year after the final round of mass drug administration.
Findings
The models predicted differing magnitude of the effects of mass drug administration, but consensus answers were reached for several factors. Mass drug administration was predicted to reduce transmission over a longer timescale than accounted for by the prophylactic effect alone. Percentage reduction in transmission was predicted to be higher and last longer at lower baseline transmission levels. Reduction in transmission resulting from mass drug administration was predicted to be temporary, and in the absence of scale-up of other interventions, such as vector control, transmission would return to pre-administration levels. The proportion of the population treated in a year was a key determinant of simulated effectiveness, irrespective of whether people are treated through high coverage in a single round or new individuals are reached by implementation of several rounds. Mass drug administration was predicted to be more effective if continued over 2 years rather than 1 year, and if done at the time of year when transmission is lowest.
Interpretation
Mass drug administration has the potential to reduce transmission for a limited time, but is not an effective replacement for existing vector control. Unless elimination is achieved, mass drug administration has to be repeated regularly for sustained effect.
Funding
Bill & Melinda Gates Foundation.
Introduction
Despite the gains made towards elimination of Plasmodium falciparum malaria in the past 15 years, many countries still have endemic transmission1 and are increasingly looking to new strategies to accelerate progress. Mass drug administration (MDA) involves the time-limited distribution of drugs to a target population, irrespective of infection status. It has been used only sporadically against malaria in most settings, and cluster-randomised trials, the most robust studies of the effect of MDA on transmission, are few.2, 3 However, MDA has received renewed interest as a strategy to clear chronic asymptomatic infections (NCT01872702) and rapidly reduce transmission.4, 5
In September, 2015, WHO's Malaria Policy Advisory Committee recommended for the first time the use of MDA in specific circumstances: when transmission is close to being interrupted, vector control, effective surveillance, and access to case management are at high coverage, and importation of infection is minimal; as a component of accelerated elimination in areas of the Greater Mekong Subregion, which are under threat of multidrug resistance; or for malaria epidemics or during complex emergencies.6 National malaria control programmes and partners need to decide what role, if any, MDA should have in control and elimination strategies. To answer this question, the best operational strategies for MDA and how best to combine MDA with other interventions need to be established.
To help with the Malaria Policy Advisory Committee's decision making, an evidence review group was established to synthesise available evidence for the effect of MDA on transmission of malaria.7 This synthesis included a few prospective field trials, retrospective analyses of previous MDAs, and a mathematical-model comparison analysis. Mathematical models are a useful way of assessing the knowledge accumulated from field trials of MDA and predicting how effectiveness might vary in settings where MDA has not yet been tested. The Malaria Modelling Consortium was tasked to compare findings from four established models (OpenMalaria,8 EMOD Disease Transmission Kernel [DTK],9 Imperial,10, 11 and Mahidol Oxford Tropical Medicine Research Unit (MORU]12, 13) on the effectiveness of MDA in different settings. Model development involves deciding on model structure, parameters, and assumptions, validation against epidemiological data, and assessment of uncertainties at each stage of this process.
Research in context.
Evidence before this study
We did not do a search of published work, because three comprehensive reviews have been published in the past 4 years. A Cochrane review done in 2013 found two cluster-randomised trials, eight non-randomised controlled studies, and 22 uncontrolled before–after studies of mass drug administration. Most studies showed a substantial initial effect on parasitaemia. However, there was little evidence for effects beyond 6 months. Two further comprehensive reviews published in 2015 had wider inclusion criteria, and included published and unpublished work and studies not yet published. These reviews showed that mass drug administration was implicated in local elimination of malaria in some settings, particularly remote areas with small populations and low initial malaria transmission, but not in areas of higher transmission. Mass drug administration has been simulated in several mathematical modelling studies but, as in field studies, many factors—such as the number of doses given, number of rounds of treatment, choice of drugs, local malaria transmission intensity, and outcomes of interest—have varied greatly, all of which are likely to affect outcomes.
Added value of this study
In this study, a consortium of modelling groups investigated the degree of consensus of four established malaria transmission models in terms of the main determinants of the effect of mass drug administration and how large an effect on prevalence is likely to be achieved. In consultation with partners who are doing field trials of mass drug administration, we chose various programme options (eg, number of rounds, choice of drug) that were considered logistically feasible. We standardised many inputs and outputs of the models, such as initial slide prevalence, outcomes of interest, and implementation options. Our analysis showed—despite many differences in assumptions between the four models, for example about the underlying transmission dynamics of malaria—broad consensus between the models on how mass drug administration should be implemented to optimise effects, and the settings in which such programmes will be most effective.
Implications of all the available evidence
High coverage in the target population and mass drug administration in more than 1 year are important to maximise reductions in transmission. Reductions in transmission last longest in low-transmission settings but, unless elimination and prevention of reintroduction are achieved, malaria transmission will return to pre-intervention levels after the programme of mass drug administration finishes.
We report the results of the Malaria Modelling Consortium's MDA model comparison and their implications for the utility of MDA. Our aim for this analysis is to help with decisions on the relative prioritisation of MDA within wider malaria-control strategies.
Methods
Malaria-control programmes implementing MDA need to decide on several operational factors, including the number of rounds, timing, and frequency of treatment. Furthermore, the effect of MDA can be influenced by epidemiological factors in each setting, such as malaria transmission level and infection importation rates. The Malaria Modelling Consortium collaborated with partners doing field trials of MDA to identify probable scenarios for MDA deployment and common operational choices that would need to be made within realistic logistic constraints.
First, a standard MDA intervention scenario was defined to use as a basis for comparison (table 1). This scenario consisted of two rounds of treatment per year, 5 weeks apart, with a standard regimen of dihydroartemisinin–piperaquine at 70% effective coverage, for 2 years. A standard setting was chosen (table 1), specifying 5% slide P falciparum parasite rate in 2–10-year-olds (PfPR2–10) before MDA, intermediate seasonal variation in transmission with one rainy season, and no importation of infection. In further simulations, we varied operational factors that are of primary interest because they can be adjusted in an MDA programme: the number of rounds per year, the effective coverage of each round, the interval between rounds, and the duration of the MDA programme. Each model was used to do a multivariate analysis that simulated the baseline conditions with every combination of the selected parameters (table 1), producing 48 different MDA programmes. We also tested how the effect of MDA could vary depending on the local setting with respect to seasonal timing, vector control, importation of infection, and drug resistance; these analyses were done in selected models according to which were most appropriate for each setting.
Table 1.
Model input parameters for programme options and local settings for mass drug administration
| Standard scenario value | Values when varied | |
|---|---|---|
| Programmatic considerations | ||
| Rounds of mass drug administration per year | 2 | 3 |
| Effective coverage* (%) | 70% | 30%, 50%, 90% |
| Coverage correlation between rounds | 1† or 0‡ | 0 or 1 |
| Interval between rounds | 5 weeks | 4 weeks, 6 weeks |
| Duration of programme | 2 years | 1 year |
| Time of year when mass drug administration begins | Optimum (as defined by each group) in a Zambia-like seasonality | Each month of the year |
| Other interventions | Insecticide-treated bednets at 80% effective coverage and access to passive treatment with artemisinin-based combination therapy at 60% throughout the simulation | Removal of vector control, simulated by a ten-fold increase in the emergence rate of adult mosquitos starting at the beginning of the year in which mass drug administrated is implemented |
| Choice of drug | Long-lasting artemisinin-based combination therapy with properties similar to dihydroartemisinin–piperaquine | .. |
| Transmission setting characteristics | ||
| Baseline transmission intensity | 5% PfPR2–10, as measured by microscopy | 1 to 10 |
| Importation of malaria cases | None | 0·4–1·6 infections per 10 000 people per year14 |
| Population size | 10 000 | 1000 |
| Artemisinin resistance | 0% | Variable |
| Seasonality profile | Zambia-based single annual rainy season profile | Two rainy seasons per year, no seasonal variation in transmission |
The standard intervention scenario was used as a basis for comparison and values were varied as shown. PfPR2–10=Plasmodium falciparum parasite rate in children aged 2–10 years.
Defined as the percentage of the population that takes the full course of drug that clears all parasites (the product of access to intervention, adherence, and drug efficacy). The denominator corresponds to the entire population; ineligible people (eg, pregnant women) and infants younger than 6 months are not included in mass drug administrations.
The same people are treated in each round in the EMOD Disease Transmission Kernel, Imperial, and OpenMalaria models.
Random individuals are treated in each round in the Mahidol Oxford Tropical Medicine Research Unit model.
The outcome metric was the percentage reduction in annual mean all-age prevalence of P falciparum as measured by PCR (PfPRPCR) in the third year after the final round of MDA. The models assessed represent four different ways of simulating malaria transmission and MDA, and a summary of their characteristics and functionality is given in table 2.11, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29
Table 2.
Summary of models of malaria transmission
| EMOD DTK | Imperial | MORU | OpenMalaria | |
|---|---|---|---|---|
| Institutional home | Institute for Disease Modelling | Imperial College London | Mahidol Oxford Tropical Medicine Research Unit | Swiss Tropical and Public Health Institute |
| Type of model and references | Individual-based stochastic microsimulation16, 17 | Individual-based stochastic microsimulations of malaria in human beings linked to a stochastic compartmental model for mosquitoes11 | Deterministic compartmental model described by differential equations,18 including drug action on each stage of the infection | Single-location individual-based simulation of malaria in human beings14 linked to deterministic model of malaria in mosquitoes19 |
| How infections are tracked | Tracks parasite densities of different surface-antigen types | Tracks membership of categories of infection (symptomatic, asymptomatic, submicroscopic, treated) | Tracks membership of categories of infection | Tracks parasite densities corresponding to different infection events |
| Relationship between entomological innoculation rate and prevalence | Immunity is acquired through cumulative exposure to different antigenic determinants,20 with heterogeneity in individual biting rates included | Immunity is acquired through cumulative exposure to mosquito bites, with heterogeneity in individual biting rates included | Subdivides population into non-immune and immune classes | Submodels of infection of human beings14 and of blood-stage parasite densities, with main immune effects controlling parasite densities21 |
| Duration of infections | Infection duration based on malaria therapy20 and cross-sectional survey data22 | Infection duration based on fitting to asexual parasite prevalence data by age, transmission intensity, seasonality | Infection duration based on malaria therapy data and data from endemic areas | Infection duration based on malaria therapy data21 |
| Effect of mass drug administration or case management | Reduces blood-stage parasite densities according to age-specific and dose-specific pharmacokinetics and pharmacodynamics,22 with corresponding clearance and prophylactic effects | Truncates infections and has subsequent prophylactic effect based on fitting pharmacokinetic and pharmacodynamic models to field studies | Post-treatment prophylactic period derived from field studies of time to next infection | Truncates infections, and has subsequent prophylactic effect based on pharmacokinetic and pharmacodynamic studies |
| Validation against trials of mass drug administration or mass screening and treatment | Assessed against MACEPA trial of mass screening and treatment in southern Zambia23 | Assessed against a controlled trial24 of mass drug administration in Burkina Faso (model slightly optimistic about effect vs data), and the MACEPA trial of mass screening and treatment in southern Zambia (model matched data) | Fitted to a trial of mass drug administration in Cambodia25 | Fitted to the data of the Garki project (Matsari),26 and assessed against the MACEPA trial of mass screening and treatment in southern Zambia27 |
| Infectiousness to mosquitoes | A function of mature gametocyte and cytokine densities20, 22 | Related to asexual parasite dynamics and lagged to allow for development of gametocytes | Infected individuals have a constant infectiousness | Lagged function of asexual parasite density28 |
| Heterogeneity in exposure | Age-dependent biting29 and configurable distribution of household variability (the latter disabled in this analysis) | Included | Not included | Included |
| Initial state | .. | Back-calculating required mosquito density to achieve given initial prevalence at an approximate steady state in the presence of treatment and long-lasting insecticide-treated nets | Set transmission rate to achieve given initial prevalence at an approximate steady state in the presence of treatment | Back-calculating required mosquito density to achieve given initial prevalence at an approximate steady state in the presence of treatment |
| Source of seasonality pattern | Rainfall and imputed temperature29 driving larval habitat model fitted to clinical incidence patterns in Sinazongwe and Gwembe districts, Zambia | Rainfall data from Zambia combined with larval and adult mosquito model | Same entomological innoculation rate input as Imperial model | Based on pattern for southern Zambia29 |
| Age-structured model | Yes | Yes | No | Yes |
| Simulation of correlated rounds of intervention | Yes | Yes | No | Yes |
All the models are extensible to include other functionality (eg, different drugs, effects of drug resistance, effect on drug resistance, vector bionomics and details of vector control, different initial conditions, other concomitant interventions). A detailed comparison of EMOD DTK, Imperial, and OpenMalaria, including references to the data to which they are fitted, is available elsewhere.15 DTK=Disease Transmission Kernel. MORU=Mahidol Oxford Tropical Medicine Research Unit. MACEPA=Malaria Control and Elimination Partnership in Africa.
Role of the funding source
EMS and EAO are or have been employed by the study funder, and were involved in data collection, analysis, and interpretation, and writing of the Article. However, they were not involved in study design The funder had no further role in study design; data collection, analysis, or interpretation; or writing of the Article. The corresponding author had access to all study data and was responsible for the final decision to submit for publication.
Results
In all four models, simulated prevalence fell substantially immediately after MDA (figure 1) as a result of successful clearance of infection and the prophylactic effect of piperaquine, a long-acting artemisinin-combination therapy partner drug. However, in the absence of elimination, the models predicted that prevalence of infection would thereafter return to pre-intervention levels (at different rates in different models). After the prophylactic effect of the partner drug has declined, the key factors that determine local transmission intensity, such as the density of mosquitoes, have not been permanently changed. Thus, without some other long-term intervention, such as improved vector control, the effects of MDA were predicted to be transient.
Figure 1.
Sample simulated output from four different models of effect of mass drug administration on all-age PCR prevalence of Plasmodium falciparum infection
From year −1 to year 0, the models are at equilibrium. The timing of each round of mass drug administration in each model is shown by coloured arrows. The four different models show the output under the standard intervention scenario (70% coverage, 2 years of mass drug administration, two rounds per year, 5 weeks between rounds, seasonal transmission [based on Zambia], and 5% mean annual prevalence pre-intervention by microscopy in 2–10-year-olds). DTK=Disease Transmission Kernel. MORU=Mahidol Oxford Tropical Medicine Research Unit.
Although the four different models showed similar trends in the effects of MDA with time, substantial differences were noted in both simulated pre-intervention transmission and the magnitude of the effect (Figure 1, Figure 2). Pre-intervention PfPRPCR differed between models because we standardised these initial conditions to 5% slide prevalence in 2–10-year-olds, and the models make several different assumptions, for example about the relation between slide and PCR prevalence. The EMOD DTK and MORU models predicted a reduction in PfPRPCR of 64% in the third year after MDA, OpenMalaria a reduction of 58%, and Imperial a reduction of 19%. These differences were caused by many different assumptions, including the relation between entomological inoculation rate, prevalence, and the basic case reproduction number (R0); the effect of case management; the degree of stochastic variability; and the dynamics of immunity.
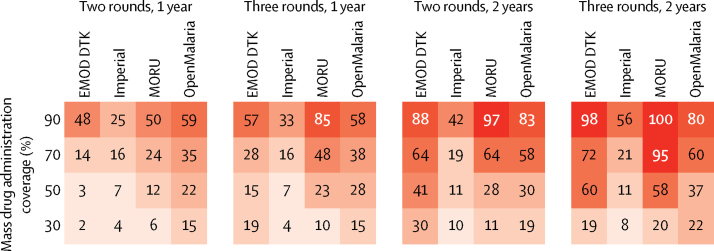
Figure 2.
Percentage reduction in mean annual all-age PCR prevalence of Plasmodium falciparum in the third year after mass drug administration
Numbers in boxes are percentage reductions. The darker the colour, the larger the reduction. DTK=Disease Transmission Kernel. MORU=Mahidol Oxford Tropical Medicine Research Unit.
Despite the differences in effect size predicted by the different models, there was generally greater agreement as to the relative effect of different operational choices. Effective coverage had a large effect on percentage reduction in PfPRPCR in all models. For example, the median percentage reduction in PfPRPCR in the standard scenario at 30% coverage was 15% (range across models 10–30); at 70% coverage, it was 61% (range 19–64; figure 2). Duration of intervention was also important in all models, with prevalence estimated to remain low for longer with 2 years of MDA than with 1 year of MDA (figure 2). When multiple rounds of MDA are done, all models showed that coverage overlap substantially affects MDA. For example, if participation was entirely random in each round, two rounds of MDA at 70% coverage would reach roughly 90% of the population with one or more treatment courses. However, if the same individuals participated in each round, then two rounds at 70% coverage would reach only 70% of the population. In reality, the situation is likely to be somewhere between the two extremes, and strategies that specifically target individuals missed in the first round are likely to be more effective as long as these strategies do not come at the expense of maximising the total number of individuals treated per year.
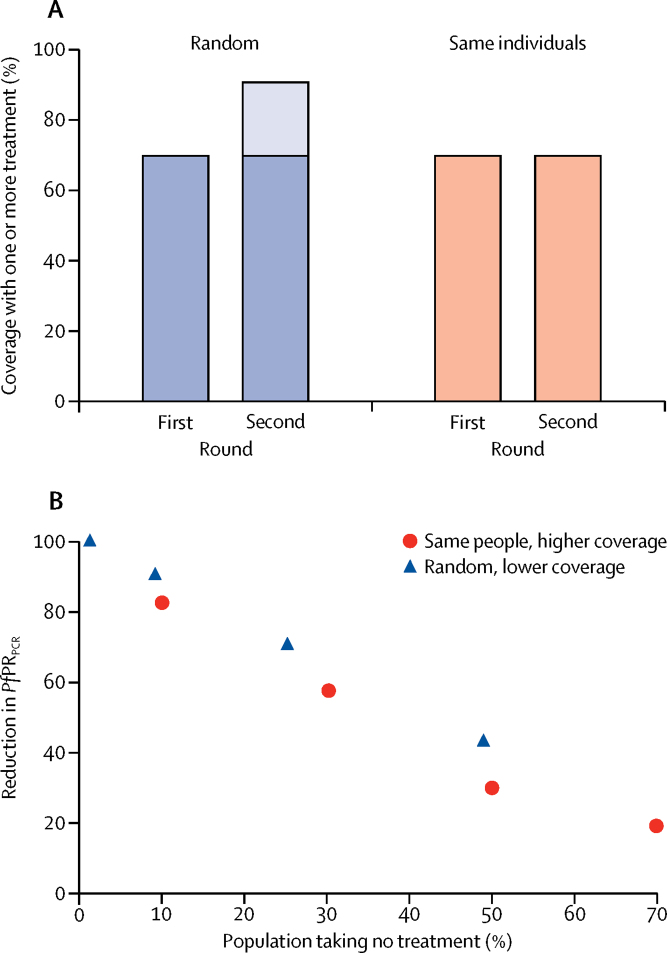
All four models suggested that, with closely spaced rounds of MDA (ie, intervals of 4–6 weeks), the most important operational factor determining effect is the proportion of the population who do not receive any treatment in any round (figure 3). Implementation of three rather than two rounds of treatment per year had negligible effects if the same individuals participated in each round at intervals of 4–6 weeks, but resulted in better outcomes if additional people were reached in the third round who had not previously received treatment that year. Spacing within-year MDA rounds 4 weeks apart rather than 6 weeks apart made little difference in terms of overall effects.
Figure 3.
Overlap in coverage between rounds of mass drug administration and effect on PfPRPCR
(A) shows the proportion of the population receiving one or more treatment courses after two rounds of mass drug administration, each at 70% coverage, with either random participation or the same individuals participating each time. (B) shows the percentage reduction in PfPRPCR 3 years after mass drug administration according to the proportion of the population not receiving treatment in any rounds in the baseline scenario. Blue triangles represent two rounds of mass drug administration randomly targeted at 30%, 50%, 70%, and 90% coverage; red dots represent the same two rounds of mass drug administration in which the same individuals are treated in each round. Results shown are from the OpenMalaria model. PfPRPCR=Plasmodium falciparum parasite rate as measured by PCR.
In settings with one rainy season a year, all models predicted greater reductions in PfPRPCR if MDA was done during the dry season or at the end of the rainy season, as previously found in similar analyses by the separate models.12, 13, 30, 31, 32 In the Imperial model, reductions in PfPRPCR were as much as 1·45 times greater if MDA was done in the dry season or at the end of the rainy season than at the beginning of the rainy season. Seasonal timing has less of an effect on PfPRPCR in the OpenMalaria and MORU models. In a setting with two rainy seasons per year, we predicted that timing of MDA had less of an effect, because transmission was more evenly spread throughout the year. At a given mean baseline slide prevalence, MDA was predicted to be marginally more effective in a seasonal setting (at the optimum time) than in a non-seasonal setting.
The introduction of MDA while vector control was simultaneously removed led to a sudden and large increase in simulated all-age prevalence, and the subsequent MDA programme did very little to reduce this shift even in the short term (OpenMalaria and Imperial models). We predicted, therefore, that an MDA programme of this type is insufficient to replace vector control, even at the highest levels of coverage.33
In a high prevalence setting (ie, 10% PfPR2–10), the percentage reduction in PfPRPCR was considerably lower in all the models compared with that in a setting with 5% prevalence. We predicted that, even with high coverage (90%), three rounds per year, and 2 years of intervention, PfPRPCR 3 years later would be reduced by only a median of 48% (range across models 19–95) from its pre-intervention level, compared with 80% (range across models 56–100) in the setting with 5% baseline prevalence. This disparity is because transmission rebounds more rapidly in a higher transmission area. However, the absolute reduction in transmission is greater in higher prevalence settings because more infections are cleared.
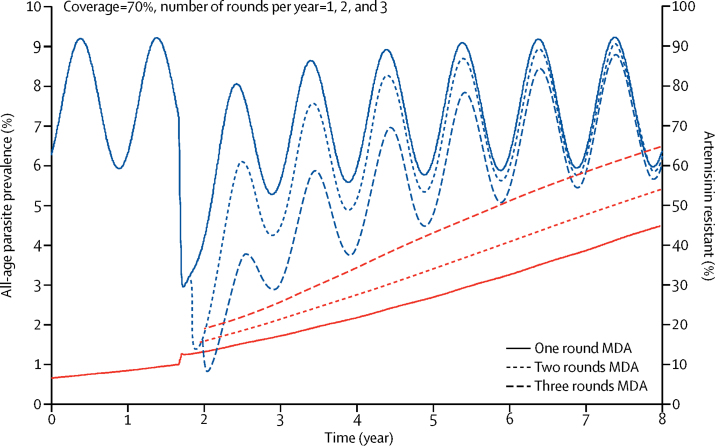
When PfPR2–10 is 5%, cases imported at a low rate (as in the WHO-recommended MDA use scenarios), based on data from Zanzibar,34 represent a very small proportion of the total infections in the population, and therefore make little difference to MDA effectiveness. However, when PfPR2–10 is lower, imported cases would be instrumental to increasing transmission. MDA more easily caused stochastic extinction in smaller than in bigger simulated populations in all the models. Finally, some evidence (only tested with the MORU model) suggested that MDA with artemisinin-combination therapies could speed up the selection of artemisinin-resistant parasite strains (figure 4). However, the size of this effect could be limited by the high selection pressure already imposed from management of symptomatic cases.
Figure 4.
Effect of MDA with artemisinin-combination therapy on malaria prevalence and the percentage of parasites that are artemisinin resistant
Results shown are from the MORU model. Blue lines show parasite prevalence, whereas red lines show the percentage artemisinin resistant. Coverage was 70% per round. MDA=mass drug administration. MORU=Mahidol Oxford Tropical Medicine Research Unit.
Discussion
Although individual models predicted different magnitudes of the effects of MDA, we found substantial consensus on which factors have the greatest influence on these effects, including both the characteristics of the programme and the setting in which MDA is applied. Percentage reductions were predicted to be highest in low-transmission settings and smaller populations, but were more transient in other settings, in line with evidence from field studies.2, 3 Infection importation rates (when transmission is not very low) and the spacing between rounds (within the 4–6 week range examined) had little effect. The proportion of the population reached by at least one round of MDA per year and the duration of the programme had a large influence on effectiveness, and our analysis suggested that these factors should be the focus of operational efforts.
We did not formally analyse which differences between the models created the variation in predicted effects of MDA. MDA can substantially affect transmission in the short term, leading to different transmission dynamics from those analysed in in-depth comparisons of models of the effects of RTS,S.15 Differences between the models in basic epidemiological quantities, including duration of untreated infections and clinical immunity, could be relevant. Different levels of within-population heterogeneity in malaria exposure are assumed, which are crucial for the stability of transmission in low-prevalence settings. Seasonal variation makes transmission less stable at a given prevalence, whereas spatial heterogeneity can make transmission more stable. We did not include spatial heterogeneity in transmission levels, but it is likely to be crucial at very low transmission levels. Simulations of small subpopulations with 5–10% prevalence, diluted by other subpopulations of unexposed individuals, might be appropriate representations of large populations with 1% average prevalence. The size of such subpopulations and their degree of interconnection could then be crucial, because stochastic extinction is much more likely in smaller than in larger populations. Consideration of spatial structure in the models will be crucial for more realistic modelling of malaria elimination. In each model, the initial prevalence for simulations was fixed, but this value could correspond to very different epidemiological patterns, for example, of the immune status of human beings or vectorial capacity.
A key next step for the modelling groups is to continue using data from forthcoming trials of MDA to further validate the models and to continue efforts to understand how and why model predictions differ, such as under the HIV, tuberculosis, and neglected tropical diseases model consortiums.35, 36 Use of modelling to understand the potential role of MDA in containing outbreaks after elimination, and to compare the predicted effects of MDA on drug resistance, will also be important.
The value of our simulations is that they show that, despite many differences in assumptions, there is a consensus between models on the relative influence of MDA operational characteristics. Many of our results accord with findings from MDA for lymphatic filariasis,37, 38, 39 although caution should be taken in extrapolating findings between the diseases in view of the generally much higher reproductive number and shorter generation time of malaria. Our results form one part of a broad evidence base, including growing evidence from malaria field trials, which should be considered when policy makers decide whether MDA is a useful strategy for their settings. This reassessment should balance the predicted benefits of MDA against equivalent investments in existing interventions, while considering other consequences such as the risk of spreading resistance. Under no circumstances did any of the models predict that MDA is an effective replacement for vector control, and indeed the overarching message from this model comparison is that, without some other sustained change, such as improved vector control, the effects of MDA are likely to be transient. When MDA is implemented, sustainability of the programme and maintenance of other interventions will be major challenges to ensure long-term reduction in malaria burden.
Acknowledgments
Acknowledgments
OJB, SIH, EMS, and EAO are members of the Malaria Modelling Consortium Secretariat and Advisors. We thank Steven Kern at the Malaria Modelling Consortium secretariat of the Bill & Melinda Gates Foundation for valuable advice on the Article. OJB is supported by the Bill & Melinda Gates Foundation (OPP1119467). HCS acknowledges support from an Imperial College London Junior Research Fellowship and the Bill & Melinda Gates Foundation. PP-R, MAP, NC, and TAS were supported by the Bill & Melinda Gates Foundation (OPP1032350), with calculations done at the sciCORE scientific computing core facility at the University of Basel (Basel, Switzerland). EW and JG are supported by the Bill & Melinda Gates Foundation through the Global Good Fund. RJM, LJW, and RA are supported by the Bill & Melinda Gates Foundation (OPP1110500) and the Wellcome Trust (106698/Z/14/Z). RJM also acknowledges additional funding from the Bill & Melinda Gates Foundation (OPP1129596 and CPT000390), the Asian Development Bank (TA-8656), and the Australian Department of Foreign Affairs and Trade (71215). ACG acknowledges funding from the Bill & Melinda Gates Foundation, the MRC under the MRC–Department for International Development Concordat agreement, and the MRC Centre for Outbreak Analysis and Modelling. SIH is funded by a Senior Research Fellowship from the Wellcome Trust (#095066) and grants from the Bill & Melinda Gates Foundation (OPP1119467, OPP1093011, OPP1106023 and OPP1132415). EAO is funded by an Intermediate Research Fellowship from the Wellcome Trust (201866/Z/16/Z). LCO acknowledges funding from a UK Royal Society Dorothy Hodgkin Fellowship, Medicines for Malaria Venture, the Bill & Melinda Gates Foundation, and a Population Health Scientist Fellowship jointly funded under the MRC–Department for International Development Concordat agreement.
Contributors
HCS, PP-R, EW, RJM, and LCO did the analyses. OJB and LCO led the writing of the first draft of the Article, with input from HCS and TAS, and coordinated the joint analyses and revisions. All authors were involved in discussions to design the study and contributed towards revising the Article and interpreting the results.
Declaration of interests
ACG declares grant funding from the UK Medical Research Council (MRC), Bill & Melinda Gates Foundation, the Wellcome Trust, the Medicines for Malaria Venture, and WHO. She has also received consultancy contracts in the past 3 years from the Medicines for Malaria Venture, Oxford Policy Management, and The Global Fund to Fight AIDS, Tuberculosis and Malaria. EMS and EAO are or have been employed by the Bill & Melinda Gates Foundation. LCO declares grant funding from WHO, the Bill & Melinda Gates Foundation, and Medicines for Malaria Venture, and has received consultancy contracts from Medicines for Malaria Venture and WHO. All other authors declare no competing interests.
References
- 1.Bhatt S, Weiss DJ, Cameron E. The effect of malaria control on Plasmodium falciparum in Africa between 2000 and 2015. Nature. 2015;526:207–211. doi: 10.1038/nature15535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newby G, Hwang J, Koita K. Review of mass drug administration for malaria and its operational challenges. Am J Trop Med Hyg. 2015;93:125–134. doi: 10.4269/ajtmh.14-0254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poirot E, Skarbinski J, Sinclair D, Kachur SP, Slutsker L, Hwang J. Mass drug administration for malaria. Cochrane Database Syst Rev. 2013;12 doi: 10.1002/14651858.CD008846.pub2. CD008846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Von Seidlein L, Dondorp A. Fighting fire with fire: mass antimalarial drug administrations in an era of antimalarial resistance. Expert Rev Anti Infect Ther. 2015;13:715–730. doi: 10.1586/14787210.2015.1031744. [DOI] [PubMed] [Google Scholar]
- 5.Killeen GF. Characterizing, controlling and eliminating residual malaria transmission. Malar J. 2014;13:330. doi: 10.1186/1475-2875-13-330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Global Malaria Programme The role of mass drug administration, mass screening and treatment, and focal screening and treatment for malaria. http://www.who.int/malaria/publications/atoz/role-of-mda-for-malaria.pdf?ua=1 (accessed Jan 8, 2017).
- 7.WHO Mass drug administration, mass screening and treatment and focal screening and treatment for malaria. http://www.who.int/malaria/mpac/mpac-sept2015-erg-mda-report.pdf?ua=1 (accessed Jan 8, 2017).
- 8.Smith T, Ross A, Maire N. Ensemble modeling of the likely public health impact of a pre-erythrocytic malaria vaccine. PLoS Med. 2012;9:e1001157. doi: 10.1371/journal.pmed.1001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerardin J, Bever CA, Hamainza B, Miller JM, Eckhoff PA, Wenger EA. Optimal population-level infection detection strategies for malaria control and elimination in a spatial model of malaria transmission. PLoS Comput Biol. 2016;12:e1004707. doi: 10.1371/journal.pcbi.1004707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffin JT, Ferguson NM, Ghani AC. Estimates of the changing age-burden of Plasmodium falciparum malaria disease in sub-Saharan Africa. Nat Commun. 2014;5:3136. doi: 10.1038/ncomms4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Griffin JT, Bhatt S, Sinka ME. Potential for reduction of burden and local elimination of malaria by reducing Plasmodium falciparum malaria transmission: a mathematical modelling study. Lancet Infect Dis. 2016;16:465–472. doi: 10.1016/S1473-3099(15)00423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maude RJ, Socheat D, Nguon C. Optimising strategies for Plasmodium falciparum malaria elimination in Cambodia: primaquine, mass drug administration and artemisinin resistance. PLoS One. 2012;7:e37166. doi: 10.1371/journal.pone.0037166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maude RJ, Nguon C, Dondorp AM, White LJ, White NJ. The diminishing returns of atovaquone-proguanil for elimination of Plasmodium falciparum malaria: modelling mass drug administration and treatment. Malar J. 2014;13:380. doi: 10.1186/1475-2875-13-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith T, Maire N, Ross A. Towards a comprehensive simulation model of malaria epidemiology and control. Parasitology. 2008;135:1507–1516. doi: 10.1017/S0031182008000371. [DOI] [PubMed] [Google Scholar]
- 15.Penny MA, Verity R, Bever CA. Public health impact and cost-effectiveness of the RTS,S/AS01 malaria vaccine: a systematic comparison of predictions from four mathematical models. Lancet. 2016;387:367–375. doi: 10.1016/S0140-6736(15)00725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenger EA, Eckhoff PA. A mathematical model of the impact of present and future malaria vaccines. Malar J. 2013;12:126. doi: 10.1186/1475-2875-12-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckhoff P. Mathematical models of within-host and transmission dynamics to determine effects of malaria interventions in a variety of transmission settings. Am J Trop Med Hyg. 2013;88:817–827. doi: 10.4269/ajtmh.12-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maude RJ, Socheat D, Nguon C. Optimising strategies for Plasmodium falciparum malaria elimination in Cambodia: primaquine, mass drug administration and artemisinin resistance. PLoS One. 2012;7:e37166. doi: 10.1371/journal.pone.0037166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chitnis N, Hardy D, Smith T. A periodically-forced mathematical model for the seasonal dynamics of malaria in mosquitoes. Bull Math Biol. 2012;74:1098–1124. doi: 10.1007/s11538-011-9710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eckhoff P. P falciparum infection durations and infectiousness are shaped by antigenic variation and innate and adaptive host immunity in a mathematical model. PLoS One. 2012;7:e44950. doi: 10.1371/journal.pone.0044950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maire N, Smith T, Ross A, Owusu-Agyei S, Dietz K, Molineaux L. A model for natural immunity to asexual blood stages of Plasmodium falciparum malaria in endemic areas. Am J Trop Med Hyg. 2006;75(suppl):19–31. doi: 10.4269/ajtmh.2006.75.19. [DOI] [PubMed] [Google Scholar]
- 22.Gerardin J, Eckhoff P, Wenger EA. Mass campaigns with antimalarial drugs: a modelling comparison of artemether-lumefantrine and DHA-piperaquine with and without primaquine as tools for malaria control and elimination. BMC Infect Dis. 2015;15:144. doi: 10.1186/s12879-015-0887-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikolov M, Bever CA, Upfill-Brown A. Malaria elimination campaigns in the Lake Kariba region of Zambia: a spatial dynamical model. PLoS Comput Biol. 2016;12:e1005192. doi: 10.1371/journal.pcbi.1005192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Escudie A, Hamon J, Schneider J. Resultats d'une chimioprophylaxie antipaludique de masse par l'association amino-4-quinoleine/amino-8-quinoleine en milieu rural africain de la region de Bobo-Dioulasso (Haute Volta) 1960. Méd Trop. 1962;22:268–290. [Google Scholar]
- 25.Song J, Socheat D, Tan B. Rapid and effective malaria control in Cambodia through mass administration of artemisinin-piperaquine. Malar J. 2010;9:57. doi: 10.1186/1475-2875-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith T, Maire N, Dietz K. Relationship between the entomologic inoculation rate and the force of infection for Plasmodium falciparum malaria. Am J Trop Med Hyg. 2006;75(suppl):11–18. doi: 10.4269/ajtmh.2006.75.2_suppl.0750011. [DOI] [PubMed] [Google Scholar]
- 27.Stuckey EM, Miller JM, Littrell M, Chitnis N, Steketee R. Operational strategies of anti-malarial drug campaigns for malaria elimination in Zambia's southern province: a simulation study. Malar J. 2016;15:148. doi: 10.1186/s12936-016-1202-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ross A, Killeen G, Smith T. Relationships between host infectivity to mosquitoes and asexual parasite density in Plasmodium falciparum. Am J Trop Med Hyg. 2006;75(suppl):32–37. doi: 10.4269/ajtmh.2006.75.32. [DOI] [PubMed] [Google Scholar]
- 29.Chabot-Couture G, Nigmatulina K, Eckhoff P. An environmental data set for vector-borne disease modeling and epidemiology. PLoS One. 2014;9:e94741. doi: 10.1371/journal.pone.0094741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker PGT, Griffin JT, Ferguson NM, Ghani AC. Estimating the most efficient allocation of interventions to achieve reductions in Plasmodium falciparum malaria burden and transmission in Africa: a modelling study. Lancet Glob Health. 2016;4:e474–e484. doi: 10.1016/S2214-109X(16)30073-0. [DOI] [PubMed] [Google Scholar]
- 31.Griffin JT. The interaction between seasonality and pulsed interventions against malaria in their effects on the reproduction number. PLoS Comput Biol. 2015;11:e1004057. doi: 10.1371/journal.pcbi.1004057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Okell LC, Griffin JT, Kleinschmidt I. The potential contribution of mass treatment to the control of Plasmodium falciparum malaria. PLoS One. 2011;6:e20179. doi: 10.1371/journal.pone.0020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yukich J, Chitnis N. When can malaria control and elimination programmes safely reduce vector control efforts? A simulation study. http://www.who.int/malaria/mpac/sep2015/en/ (accessed Jan 8, 2017).
- 34.Crowell V, Hardy D, Briet O, Chitnis N, Maire N, Smith T. Can we depend on case management to prevent re-establishment of P falciparum malaria, after local interruption of transmission? Epidemics. 2012;4:1–8. doi: 10.1016/j.epidem.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Eaton JW, Johnson LF, Salomon JA. HIV treatment as prevention: systematic comparison of mathematical models of the potential impact of antiretroviral therapy on HIV incidence in South Africa. PLoS Med. 2012;9:e1001245. doi: 10.1371/journal.pmed.1001245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stolk WA, Walker M, Coffeng LE, Basanez MG, de Vlas SJ. Required duration of mass ivermectin treatment for onchocerciasis elimination in Africa: a comparative modelling analysis. Parasit Vectors. 2015;8:552. doi: 10.1186/s13071-015-1159-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Irvine MA, Reimer LJ, Njenga SM. Modelling strategies to break transmission of lymphatic filariasis—aggregation, adherence and vector competence greatly alter elimination. Parasit Vectors. 2015;8:547. doi: 10.1186/s13071-015-1152-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kyelem D, Biswas G, Bockarie MJ. Determinants of success in national programs to eliminate lymphatic filariasis: a perspective identifying essential elements and research needs. Am J Trop Med Hyg. 2008;79:480–484. [PMC free article] [PubMed] [Google Scholar]
- 39.Kastner RJ, Stone CM, Steinmann P, Tanner M, Tediosi F. What is needed to eradicate lymphatic filariasis? A model-based assessment on the impact of scaling up mass drug administration programs. PLoS Negl Trop Dis. 2015;9:e0004147. doi: 10.1371/journal.pntd.0004147. [DOI] [PMC free article] [PubMed] [Google Scholar]