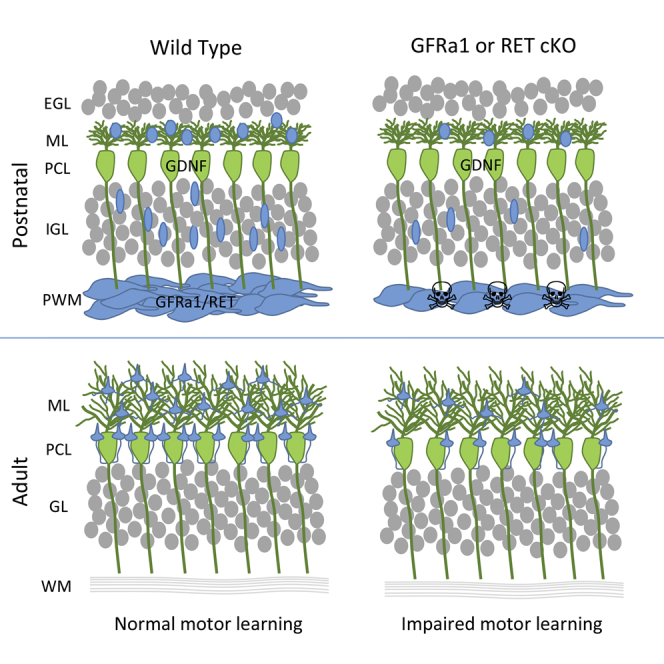
Summary
The role of neurotrophic factors as endogenous survival proteins for brain neurons remains contentious. In the cerebellum, the signals controlling survival of molecular layer interneurons (MLIs) are unknown, and direct evidence for the requirement of a full complement of MLIs for normal cerebellar function and motor learning has been lacking. Here, we show that Purkinje cells (PCs), the target of MLIs, express the neurotrophic factor GDNF during MLI development and survival of MLIs depends on GDNF receptors GFRα1 and RET. Conditional mutant mice lacking either receptor lose a quarter of their MLIs, resulting in compromised synaptic inhibition of PCs, increased PC firing frequency, and abnormal acquisition of eyeblink conditioning and vestibulo-ocular reflex performance, but not overall motor activity or coordination. These results identify an endogenous survival mechanism for MLIs and reveal the unexpected vulnerability and selective requirement of MLIs in the control of cerebellar-dependent motor learning.
Keywords: cerebellum, eyeblink conditioning, neuronal migration, Purkinje cell, synaptic inhibition, vestibulo-ocular reflex
Graphical Abstract
Highlights
-
•
The signals controlling survival of molecular layer interneurons (MLIs) are unclear
-
•
Whether MLIs are involved in normal cerebellar function was unclear
-
•
Purkinje cells express GDNF, and survival of MLIs depends on GDNF receptors GFRα1 and RET
-
•
Requirement of MLIs for cerebellar-dependent motor learning
Sergaki et al. find that conditional mutant mice lacking GDNF receptor GFRα1 or RET lose a quarter of their cerebellar molecular layer interneurons (MLIs), resulting in compromised motor learning but not overall motor coordination. These results identify an endogenous survival mechanism for MLIs and reveal their unexpected vulnerability in the control of cerebellar-dependent motor learning
Introduction
The importance of the cerebellum for motor learning has long been recognized. Cerebellar circuits adjust and fine-tune motor programs to allow movement accuracy through a trial-and-error process that is critical for normal motor learning. However, the cellular and molecular components underlying these functions remain poorly characterized. Long-term depression (LTD) of the synapse between granule cell parallel fibers and Purkinje cell (PC) dendrites was initially hypothesized to be important for cerebellar motor learning (Ito, 2000), but more recent studies have indicated that motor learning can occur without cerebellar LTD (Hirano, 2014, Schonewille et al., 2011). On the other hand, the role of cerebellar molecular layer interneurons (MLIs) in shaping the computational properties of cerebellar circuits underlying plasticity and learning has lately received more attention (Jörntell et al., 2010). By performing MLI recordings on behaving animals, a recent study uncovered a positive correlation between MLI activity and classical eyeblink conditioning, a common experimental paradigm of cerebellar motor learning (ten Brinke et al., 2015). However, the direct involvement of MLIs for the expression of cerebellar motor learning responses remains to be demonstrated.
MLIs have been subdivided in basket and stellate cells according to morphological criteria. Basket cells are located in the deeper part of the molecular layer (ML) and form perineuronal nets around the perikarya of PCs that end in a brush-shaped terminal or “pinceau” on the initial segments of PC axons, which allows them to modulate PC spike output. Stellate cells are found in the superficial part of the ML, target PC dendrites, and are thought to regulate the excitatory synaptic inputs to PCs (Sotelo, 2015). Deep and superficial MLIs also show differential expression of a subset of genes that is highly enriched among cerebellar inhibitory interneurons (Schilling and Oberdick, 2009). Despite these differences, some investigators still consider stellate and basket cells as belonging to the same population (Sotelo, 2015). In the mouse cerebellum, the different GABAergic cell types are born during different time windows of development: PCs are generated first, between embryonic day (E) 10 and E12, granule layer interneurons (GLIs; i.e., Golgi cells) between E16 and postnatal day (P) 0, and MLIs within the first postnatal week (Carletti and Rossi, 2008). MLIs originate from a single progenitor pool within the cerebellar ventricular zone (VZ) that expresses the transcription factor Ptf1a (Hoshino et al., 2005). Among MLIs, basket cells are born first, around P2, and stellate cells later, around P10 (Yamanaka et al., 2004). From the VZ, MLI progenitors migrate outward to reach the prospective white matter (WM) of the embryonic cerebellum, where they persist and continue dividing for another 2 days until they turn on expression of GAD67, a key enzyme in GABA production, and resume their migration toward the ML (Cameron et al., 2009, Yamanaka et al., 2004, Zhang and Goldman, 1996). MLI migration continues within the ML for another 2–3 days until the end of the second postnatal week (Yamanaka et al., 2004). MLIs undergo developmental programmed cell death, with the highest rates occurring between P5 (mainly in the WM) and P10 (as they migrate toward and within the ML) (Yamanaka et al., 2004). The signals that control MLI survival remain to be identified.
In the peripheral nervous system (PNS), neuron survival is controlled by neurotrophic factors that block developmental programmed cell death by binding and activating cognate cell surface receptors. Glial cell line-derived neurotrophic factor (GDNF) is the founding member of a small family of neurotrophic factors that regulate different processes during nervous system development, including neuron survival, cell migration, axon growth, and synapse formation (Paratcha and Ledda, 2008). GDNF receptors include an obligatory ligand binding subunit termed GDNF family receptor alpha 1 (GFRα1), which partners with one of two possible signaling subunits, the receptor tyrosine kinase RET or the neural cell adhesion molecule (NCAM), forming alternative receptor complexes (Ibáñez, 2013, Paratcha and Ledda, 2008). Despite the wealth of information available on this ligand-receptor system, its role in the development and function of the cerebellum is unknown.
In this study, we show that both GFRα1 and RET are expressed during the early stages of MLI development. Using conditional mutant mice lacking either receptor in cerebellar MLIs, we investigated the role of GFRα1/RET signaling in MLI survival and its consequences for cerebellar function and behavior.
Results
Expression of GFRα1, RET, and GDNF during Postnatal Cerebellar Development
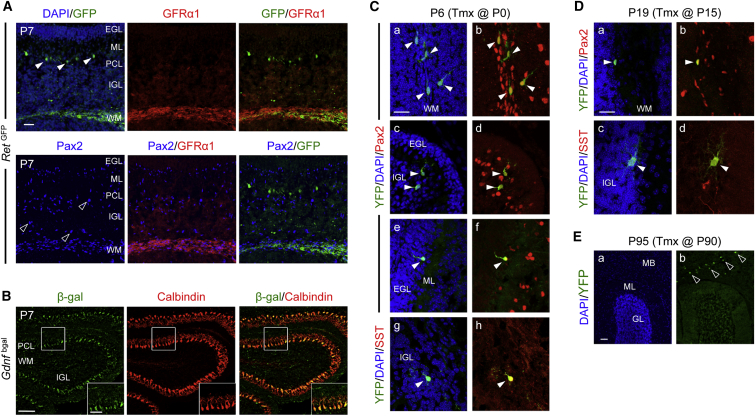
Early in situ hybridization studies indicated that Gfra1, Ret, and Gdnf mRNAs are expressed in the postnatal and adult rodent cerebellum (Golden et al., 1999, Trupp et al., 1997), but the precise cell types expressing the corresponding proteins remained unidentified. In order to define sites of RET and GFRα1 expression, we took advantage of the RetGFP mouse line that expresses GFP from the Ret locus and combined this with immunostaining for GFRα1 protein and the MLI progenitor marker Pax2 (Figure 1A). Although Pax2 marks the progenitors of both MLIs and GLIs, only basket and stellate cells, but not Golgi cells, express parvalbumin (PV) when they mature. At P7, GFP and GFRα1 signals were found to colocalize with Pax2 in the prospective WM that contains MLI progenitors (Figure 1A). During development, MLI progenitors travel through the granule and PC layers to reach the ML, after which they lose Pax2 expression (Cameron et al., 2009). RET-GFP, but not GFRα1, was also found in scattered cells in the ML (Figure 1A, solid arrowheads), which likely correspond to earlier born MLIs that have already reached the ML and turned off Pax2 expression. Neither GFRα1 nor RET was found to be expressed in Pax2+ Golgi cells of the internal granule layer (IGL), distinguished by their larger cell bodies (Geurts et al., 2001, Weisheit et al., 2006) (Figure 1A, open arrowheads). RET-GFP expression persisted at P15 and P60 in PV+ MLIs located in the deeper part of the ML, where basket cells are known to reside, but was absent elsewhere in the ML, suggesting downregulation of RET expression in mature stellate cells (Figure S1A). Processes emanating from these GFP+ cells were seen enveloping the cell bodies of PCs, labeled with calbindin, forming characteristic perineuronal nets, a feature that distinguishes basket cells (Sotelo, 2015). No immunofluorescence signal could be detected for GFRα1 at P15 or P60 (data not shown). GDNF expression was visualized by immunohistochemistry using a Gdnfbgal mouse line expressing beta-galactosidase from the Gdnf locus or by in situ hybridization. GDNF expression was found in PCs at all ages examined, from P7 to P60, and was absent in WM cells (Figures 1A, S1A, and S1C).
Figure 1.
Expression of GFRα1, RET, and GDNF during Postnatal Cerebellar Development
(A) Sagittal cerebellar sections from P7 RetGFP mice stained with antibodies against GFP, GFRα1, and Pax2. Arrowheads indicate earlier born GFP+ MLIs (solid) and Pax2+ Golgi cells in the IGL (open). EGL, external granule layer; ML, molecular layer; IGL, internal granule layer; WM, white matter. The scale bar represents 100 μm.
(B) Sagittal cerebellar sections from P7 Gdnfbgal mice stained with β-galactosidase and calbindin antibodies. PCL, Purkinje cell layer. The scale bars represent 100 and 50 μm in bottom corner insets.
(C) Sagittal cerebellar sections from P6 Gfra1CreERT2;R26YFP mice, injected with tamoxifen at birth (P0), stained with YFP (1–8), Pax2 (a–f), and somatostatin (SST) (g and h) antibodies and counterstained with DAPI (a, c, e, and f). Arrowheads indicate migrating MLIs (a–f) or Golgi cells (g and h). The scale bar represents 25 μm.
(D) Sagittal cerebellar sections from P19 Gfra1CreERT2;R26YFP mice, injected with tamoxifen at P15, stained for YFP (a–d), Pax2 (a and b), and somatostatin (c and d) antibodies and counterstained with DAPI. The scale bar represents 25 μm.
(E) Sagittal cerebellar sections from P95 Gfra1CreERT2;R26YFP mice, injected with tamoxifen at P90, stained for YFP (b), and counterstained with DAPI (a). Open arrowheads denote YFP+ cells in the midbrain (MB). The scale bar represents 50 μm.
The presence of GFRα1 in MLI progenitors at P7 and its absence at later postnatal stages suggested transient expression of GFRα1 in cerebellar MLIs. In order to investigate this further, we used a Gfra1CreERT2 mouse line expressing tamoxifen-inducible Cre recombinase (CreERT2) from the Gfra1 locus (Sergaki and Ibáñez, 2017). Gfra1CreERT2 mice were crossed with the Rosa26YFP reporter line; offspring were injected with tamoxifen at P0, P15, or P90 and analyzed at P6, P19, or P95, respectively. YFP+ cells expressing Pax2 were found in the cerebellum of P6 and P19 mice (Figures 1C and 1D) but not at P95 (Figure 1E), indicating that GFRα1 is expressed in both early- and late-born MLI progenitors but not in mature MLIs localized at the ML. At P6, YFP+ cells in the ML or close to the IGL displayed morphological features of migrating MLIs, with a typical apical dendrite (Figures 1Ca–1Cf, arrowheads). Larger YFP+ cells expressing somatostatin (SST), a marker of Golgi neurons (Geurts et al., 2001), were also found in the IGL at P6 and P19 (Figures 1Cg and 1Ch). In summary, both RET and GFRα1 are expressed in developing MLIs of the mouse cerebellum, but, whereas RET is retained in mature basket cells, GFRα1 expression is restricted to MLI progenitors. GFRα1, but not RET, is also expressed in developing Golgi neurons, while GDNF was found in PCs at all ages.
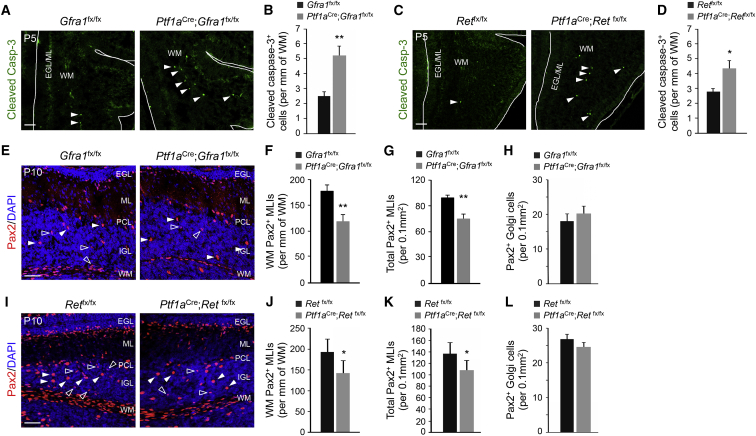
Increased Apoptosis in MLI Progenitors Lacking GFRα1 or RET
In order to investigate the function of GFRα1 and RET in MLI progenitors, we used conditional mutants for each of the receptors. Because global knockout mice lacking either protein die at birth, we eliminated GFRα1 and RET expression by crossing Gfra1fx/fx or Retfx/fx mice with the Ptf1aCre driver line. Both mutant lines (Ptf1aCre;Gfra1fx/fx and Ptf1aCre;Retfx/fx, respectively) survived to adulthood and had normal gross cerebellar morphology and architecture. Developmental cell death was investigated by immunostaining for activated caspase-3 at P5 in homozygous mutants and fx/fx controls lacking Cre. Increased apoptosis was observed among MLI progenitors in the folial WM in both mutant lines (Figures 2A–2D). At P10, the number of Pax2+ MLIs (distinguished from Golgi cells by their smaller size and morphology) was reduced in the folial WM as well as throughout the cerebellar cortex of both mutants (Figures 2E–2G and 2I–2K). On the other hand, the number of large size Pax2+ neurons corresponding to Golgi cells (Figures 2E and 2I, solid arrowheads) was not changed in the mutants (Figures 2H and 2L). Conditional deletion of either Gfra1 or Ret with the Gad67Cre driver, which turns on later than Ptf1aCre, did not affect the number of Pax2+ MLIs in the P10 cerebellum (Figures S2A–S2E), underscoring the requirement of GFRα1 and RET receptors for survival of MLI progenitors before they express GAD67. We also investigated whether the reduction in Pax2+ MLIs observed in the mutants could be due to defects in proliferation or migration. At P5, the number of proliferating cells in the folial WM, assessed 2 hr after a BrdU pulse, was not different between genotypes (Figures S3A–S3D). At this age, BrdU only marks MLI progenitors, because Golgi cells no longer proliferate. We note that some cells showing immunoreactivity for activated caspase-3 at P5 were also labeled with BrdU, indicating that both proliferating and nonproliferating cells are dying in the mutants (Figure S3E). To examine migration, we quantified the number of Pax2/BrdU double-positive cells in different layers of the cerebellar cortex at P10 (i.e., 5 days after the BrdU pulse). Again, no difference was found between genotypes (Figures S3F and S3G). Together, these data indicated that loss of GFRα1 or RET in MLI progenitors specifically compromised their survival but not proliferation or migration.
Figure 2.
Increased Apoptosis in MLI Progenitors Lacking GFRα1 or RET
(A and C) Cerebellar sections from P5 Gfra1 (A) or Ret (C) mutants and controls stained with cleaved-caspase-3 (Casp3) antibody. Arrows point to cleaved-Casp3+ cells in the WM. White lines delineate the border of the folium. EGL, external granule layer; ML, molecular layer; WM, white matter. The scale bar represents 50 μm.
(B and D) Quantification of cleaved-Casp3+ cells in the folial WM of control and Gfra1 (B) or Ret (D) mutants. Values are mean ± SEM. n = 6 mice per group.
(E and I) Cerebellar sections from P10 Gfra1 (E) or Ret (I) mutants and controls stained for Pax2 and counterstained with DAPI. Migratory MLIs (open arrows) and Golgi cells (solid arrows) are indicated. PCL, Purkinje cell layer; IGL, internal granule layer. The scale bar represents 50 μm.
(F, G, J, and K) Quantification of folial WM (F and J) or total (G and K) Pax2+ MLI progenitor densities in P10 Gfra1 (F and G) or Ret (J and K) mutants and controls. Values are mean ± SEM. n = 6 (F and G) or 5 (J and K) mice per group.
(H and L) Quantification of Pax2+ Golgi cells in the IGL of P10 Gfra1 (H) or Ret (L) mutants and controls. Values are mean ± SEM. n = 6 and 5 or 8 and 6 mice per group, respectively.
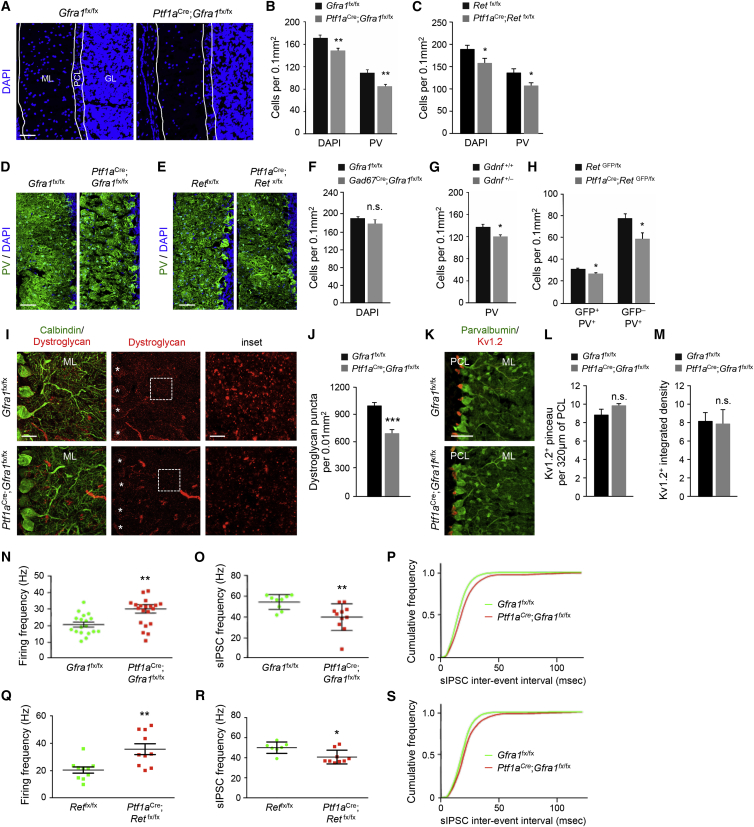
Loss of MLIs in the Cerebellum of Adult Gfra1 and Ret Conditional Mutants
At 2 months of age, the cell density and overall thickness of the ML in the cerebellum of Gfra1 and Ret conditional mutants was significantly reduced, as assessed by DAPI staining (Figures 3A–3C). This reduction could be accounted for by a specific loss of MLIs, as determined by the density of PV+ cells in this layer (Figures 3B–3E). Overall, 23% of all MLIs were missing in the cerebellum of 2-month-old Gfra1 (23.35 ± 7.03%) and Ret (22.72 ± 9.06%) conditional mutants (Figures 3B and 3C). No difference was observed after deletion of Gfra1 with the Gad67Cre driver (Figure 3F). A smaller but significant loss of PV+ MLIs was also detected in 2-month-old Gdnf heterozygote mice (Figure 3G). To determine whether basket and stellate cells were equally affected by the loss of GFRα1, we subdivided the ML in 4 strata of equal thickness and quantified cell density in each of them. Stratum #4 is adjacent to the PC layer and thus likely contains the majority of basket cells, while the remaining strata are expected to be enriched in stellate cells. However, we found no difference in cellular distribution between Gfra1 conditional mutants and controls (Figures S3A and S3B). In agreement with this, the density of both GFP+ (i.e., basket) and GFP− (i.e., stellate) subpopulations of PV+ cells were diminished in the ML of cerebella from 2-month-old Ptf1aCre;RetGFP/fx mutants compared with controls (Figure 3H). Outside the cerebellum, overlap of Ptf1a expression with GFRα1 could be detected only in the embryonic inferior olivary nucleus (Figure S3C). However, we did not observe any difference in the number of calbindin-expressing neurons in this area between adult controls and Gfra1 conditional mutants (Figures S3D and S3E).
Figure 3.
Loss of MLInterneurons Reduced GABAergic Synapses and Increased PC Firing Rate in the Cerebellum of Adult Gfra1 and Ret Conditional Mutants
(A) Cerebellar sections from 2-month-old Gfra1 mutants and controls stained with DAPI. White lines denote cerebellar layers (as identified from PV staining, not shown here). ML, molecular layer; PCL, Purkinje cell layer; GL, granule layer. The scale bar represents 50 μm.
(B and C) Quantification of DAPI+ and PV+ cells in the ML of 2-month-old Gfra1 (B) or Ret (C) mutants and controls. Values are mean ± SEM. n = 6 (B) or 5 (C) mice per group, respectively.
(D and E) Cerebellar sections from 2-month-old Gfra1 (D) or Ret (E) mutants and controls stained with parvalbumin (PV) and counterstained with DAPI. The scale bar represents 50 μm.
(F) Quantification of DAPI+ cells in the ML of 2-month-old Gad67Cre;Gfra1fx/fx mutants and controls. Values are mean ± SEM. n = 3 mice per group.
(G) Quantification of PV+ cells in the ML of 2-month-old wild-type and heterozygous Gdnf mutant mice. Values are mean ± SEM. n = 6 mice per group.
(H) Quantification of GFP+/PV+ double-positive and GFP−/PV+ cells in the ML of 2-month-old Ret mutants and controls. Values are mean ± SEM. n = 6 and 8 mice per group, respectively.
(I) Cerebellar sections from 2-month-old Gfra1 mutants and controls stained with calbindin and dystroglycan antibodies. Asterisk denotes cell bodies of PCs. The scale bars represent 25 μm (left) and 5 μm (insets).
(J) Quantification of dystroglycan+ puncta in the ML of 2-month-old Gfra1 mutants and controls. Values represent mean ± SEM. n = 6 and 7 mice per group, respectively.
(K) Cerebellar sections from 2-month-old Gfra1 mutants and controls stained with Kv1.2 and calbindin antibodies as indicated. The scale bar represents 50 μm.
(L and M) Quantification of the number of Kv1.2+ pinceau synapses (L) and Kv1.2 staining intensity (M) in the PCL of 2 month old Gfra1 mutants and controls. Values are mean ± SEM. n = 3 mice per group, respectively. n.s., not significantly different (p > 0.05).
(N and Q) PC firing frequency in cerebella of 2-month-old Gfra1 (N) or Ret (Q) mutants and controls. Each dot denotes mean from 300 s recording from individual PC. Mean and SEM are shown with parallel lines. n = 18 and 20 (N) or 5 and 7 (Q) PCs per group, respectively.
(O and R) sIPSC frequency in PCs from Gfra1 (O) or Ret (R) mutants and controls. n = 9 and 11 or 6 and 5 PCs per group, respectively.
(P and S) Cumulative frequency diagram of sIPSC inter-event interval in PCs from Gfra1 (P) or Ret (S) mutants and controls (Kolmogorov-Smirnov, p < 0.0001).
Reduced Density of Dystroglycan Puncta and Increased Firing Rate in PCs of Adult Gfra1 and Ret Conditional Mutants
MLIs provide GABAergic innervation to PCs at the dendrites and the axon initial segment. Dystroglycan is a postsynaptic marker of synapses between MLIs and PC dendrites (Briatore et al., 2010). The density of dystroglycan+ puncta in the ML of 2-month-old Gfra1 conditional mutants was significantly diminished compared with Gfra1fx/fx control mice (Figures 3I and 3J), suggesting reduced GABAergic inhibition of PC dendrites. On the other hand, we saw no change in the number of pinceau terminals on the initial segment of the PC axon, as assessed by immunostaining for the specific marker Kv1.2 (Wang et al., 1993), or in the intensity of the Kv1.2 staining (Figures 3K–3M). Because axon collaterals from five to seven basket neurons contribute to the formation of each pinceau terminal (Somogyi and Hámori, 1976), a loss of 23% of MLIs would not be expected to affect the overall number of these terminals. In agreement with a reduction in GABAergic inputs on PCs, electrophysiological recordings from the somata of PCs revealed a consistent and significant increase in the firing frequency of PCs in both Gfra1 and Ret mutants (Figures 3N, 3Q, S3F, and S3G), which was accompanied by decreased spontaneous inhibitory postsynaptic current (sIPSC) frequency (Figures 3O, 3R, S3H, and S3I) and rightward shift in the distribution of sIPSC inter-event intervals (Figures 3P and 3S). sIPSC amplitudes were not affected (Figures S3J and S3K). Taken together, these data demonstrated a marked impairment in PC inhibition, resulting in increased PC output in both receptor mutants due to reduced number of MLIs.
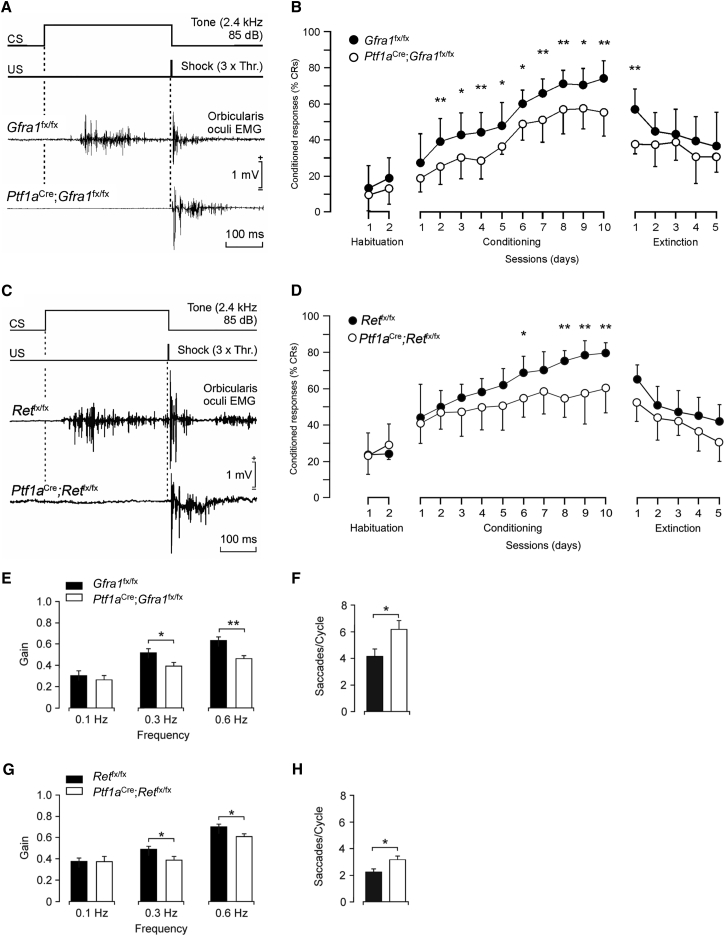
Deficient Motor Learning in Conditional Mutant Mice Lacking GFRα1 or RET in MLIs
In order to assess the behavioral consequences of the cellular and electrophysiological defects observed in the mutants, we tested the performance of Gfra1 and Ret conditional mutant mice in two paradigms that specifically evaluate cerebellum-dependent motor learning. The eyeblink is a reflex in response to corneal stimulation (Yang et al., 2015). In classical eyeblink conditioning, animals learn to associate a conditioned stimulus (CS), such as a tone, with an unconditioned stimulus (US), such as a mild electric shock to the supraorbital nerve, which evokes eyeblinks (Figure S4A). As a result of the CS-US association during training, an eyeblink conditioned response (CR) to CS presentations becomes progressively enhanced (Sánchez-Campusano et al., 2009, Thompson and Steinmetz, 2009). Figures 4A and 4C show representative electromyographic (EMG) recordings from Gfra1 and Ret mutants, respectively, obtained during the seventh conditioning session (see Experimental Procedures for details). In this example, the response of the mutants to the CS was considerably diminished, although both mutant and control mice responded equally to the US in latency (e.g., 7.6 ± 0.2 ms in Ret mutant versus 7.5 ± 0.3 ms in control) and amplitude (0.91 ± 0.12 mV in Ret mutant versus 0.85 ± 0.10 mV in control). The learning curves revealed that whereas control mice acquired classical conditioning with a progressive increase in the percentage of CRs, both Gfra1 and Ret mutants showed a markedly slower learning response throughout the conditioning sessions (Figures 4B and 4D).
Figure 4.
Deficient Motor Learning in Adult Gfra1 and Ret Conditional Mutants
(A and C) EMG recordings from the Obicularis oculi (O.O.) muscle obtained in 2-month-old Gfra1 (A) and Ret (C) mutants and controls during the seventh conditioning session. Schematics of conditioned stimulus (CS) and unconditioned stimulus (US) are shown at the top.
(B and D) Percentage of conditioned responses (CRs) during habituation, conditioning, and extinction sessions in 2-month-old Gfra1 (B) and Ret (D) mutants and their respective controls. Values are mean ± SEM. n = 8 mice per group.
(E and G) Bode plots for gain VOR reflexes recorded in a lighted room from Gfra1 (E) and Ret (G) mutants and their respective controls. Values are mean ± SEM. n = 8 mice per group. ∗p < 0.05 (two-way ANOVA).
(F and H) Mean number of compensatory saccades per cycle carried out by Gfra1 (F) and Ret (H) mutants and their respective controls. ∗p < 0.05, ∗∗p < 0.01 (two-way ANOVA).
The vestibulo-ocular reflex (VOR) helps maintain the focus on a visual image when the head turns (Figure S4B). The cerebellum plays an important role in the control of gain and phase dynamics of the VOR (Ito, 1998). VOR responses were measured in conditional Gfra1 and Ret mutants at 0.1, 0.3, and 0.6 Hz (Figures 4E–4H). The mutants displayed a slower increase in VOR gain with increasing frequency (Figures 4E and 4G) and a significant increase in the number of saccades evoked by the decrease in gain (Figures 4F and 4H). Together, these results revealed significant defects in motor learning and performance of Gfra1 and Ret conditional mice in two behavioral paradigms controlled by cerebellar circuits. Interestingly, no defects were observed in Gfra1 and Ret conditional mutants in several other motor tests, including the open field, rotarod and balance beam tests (Figures S4D–S4G), indicating a selective impairment in motor learning rather than motor coordination or activity per se.
Discussion
The prospective cerebellar WM produces different types of GABAergic interneurons at specific developmental stages from a common progenitor pool (Leto et al., 2006). The signals and molecular mechanisms that regulate this process are not well understood. Cerebellar MLIs are known to provide GABAergic innervation to PCs, but direct evidence for the necessity of a full complement of MLIs for normal cerebellar function and behavior, as well as the compensatory capacity of the MLI population, has been lacking. This study demonstrates the requirement of the GDNF/GFRα1/RET signaling pathway for the survival of cerebellar MLIs during developmental programmed cell death. Genetic disruption of this pathway resulted in the loss of approximately a quarter of cerebellar MLIs. This was sufficient to compromise synaptic inhibition of PCs and disrupt normal PC discharge and motor learning behavior. Given that no other cerebellar cell type apart from MLIs expresses RET, the effects observed in Ret conditional mutants are cell-autonomous to the MLIs. Although Golgi cells and PCs also transiently express GFRα1 during their early development, deletion of GFRα1 does not affect their number or distribution at postnatal stages (this study and Sergaki and Ibáñez, 2017). Given the nearly identical phenotypes of Gfra1 and Ret mutants, the effects observed in Gfra1 conditional mutants are also, in all likelihood, cell-autonomous to the MLIs.
A Full Complement of Cerebellar MLIs Is Required for Normal Cerebellum-Dependent Motor Learning but Not Motor Coordination
MLIs provide feedforward inhibition to PCs, thereby shaping PC activation in response to granule cell input (Dizon and Khodakhah, 2011). In a previous study, optogenetic activation of MLIs resulted in a brief suppression of spontaneous PC firing, which was sufficient to cause small orofacial movements in mice (Heiney et al., 2014). Although this result indicated a link between MLI function and movement generation, it did not provide evidence for a role of MLIs in motor learning. Moreover, as this was based on a gain-of-function strategy, the requirement of MLIs was not established in those experiments. Other studies reported that genetic ablation of the γ2 subunit of the GABA-A receptor in PCs resulted in irregular spike firing and impaired ability to adapt the phase of the VOR (Wulff et al., 2009), as well as defects in conditioned eyeblink responses (ten Brinke et al., 2015), indirectly implicating MLI-mediated inhibition in those functions. However, GABA-B receptor-mediated inhibition of PCs was unchanged in those mice (Wulff et al., 2009), and deletion of synaptic GABA-A receptors in PCs would also have disrupted any inhibition mediated by recurrent collaterals of PC axons. By compromising the survival mechanisms of MLIs, our study is the first to provide direct evidence for the requirement of these neurons for normal PC activity and cerebellar-dependent motor learning. At the circuit level, our analysis of synaptic contacts between MLIs and PCs revealed a reduction in GABAergic inputs to PC dendrites but not in pinceau terminals on the axon initial segment in Gfra1 mutant mice. This suggests that a decrease in dendritic GABAergic input may be sufficient to affect PC firing frequency. Gfra1 and Ret conditional mutants performed significantly worse than control mice in both the eyeblink conditioning and VOR paradigms, showing slower learning acquisition and failure to adjust gain responses. However, these mice performed normally in the rotarod and balance beam tests, two classical assays of cerebellum-dependent motor coordination. This indicates a selective impairment in motor learning, rather than motor coordination or activity per se, caused by a partial loss of cerebellar MLIs, and suggests distinct underlying circuitry controlling motor learning and activity. The fact that mutant mice still retained more than 75% of cerebellar MLIs underscores the importance of a full complement of cerebellar MLIs for normal cerebellum-dependent motor learning, as loss of only a quarter of all cerebellar MLIs was sufficient to cause abnormalities. In contrast, for example, the basal ganglia can withstand the loss of more than two-thirds of nigral dopaminergic neurons before Parkinsonian symptoms emerge (Dauer and Przedborski, 2003). Our results therefore reveal an unexpected vulnerability to the loss of even a minority of the MLI population.
MLIs Depend on GDNF/GFRα1/RET Signaling for Survival during Developmental Programmed Cell Death
Cerebellar MLIs undergo developmental programmed cell death between P5 and P10 (Yamanaka et al., 2004) but the mechanisms that control this process have been unknown. Unlike the situation in the PNS, the role of neurotrophic factors in the control of programmed cell death in the brain remains controversial. Although GDNF was initially discovered for its ability to promote survival of midbrain dopaminergic neurons in vitro and upon injury, mice lacking RET in dopaminergic neurons show either no defects (Jain et al., 2006) or only a marginal neuronal loss at very advanced age (Kramer et al., 2007). A report that initially claimed the absolute requirement of GDNF for survival of brain catecholaminergic neurons (Pascual et al., 2008) has more recently been challenged (Kopra et al., 2015). To date, most of the neurotrophic factors that promote survival of PNS neurons have not shown similar effects in developing neurons of the brain. This has led to the idea that other stimuli, such as neuronal activity and neurotransmitter input, play a more important role in regulating neuronal survival in the brain (Dekkers et al., 2013). The results of the present study show that signaling by GFRα1 and RET is required for the survival of cerebellar MLIs during their period of developmental programmed cell death. GDNF was expressed during the same time window by PCs (i.e., the target of MLIs). As PC axons travel through the WM to reach neurons in the deep nuclei, MLI progenitors may obtain GDNF from these axons. Together with the loss of a fraction of MLIs in Gdnf heterozygote mutant mice, these data suggest that a target-derived neurotrophic circuit operates to control the survival of this neuronal population during development. Although all MLI progenitors were seen to express GFRα1 and RET, only about a quarter of all MLIs were lost in Gfra1 and Ret mutants, suggesting heterogeneous survival requirements in these cells. The fact that MLIs were lost in equal proportion in all ML strata as well as among basket and stellate cells, argues against the loss of a particular MLI subtype in the mutants.
Conclusions
This study demonstrates the dependence of cerebellar MLIs on PC-derived GDNF for their survival during developmental programmed cell death and provides direct evidence for the requirement of a full complement of MLIs for cerebellum-dependent motor learning. The unexpected vulnerability to the loss of a fraction of MLIs reveals a previously unappreciated lack of redundancy in the cerebellar circuitry that controls motor learning.
Experimental Procedures
For detailed procedures, see Supplemental Experimental Procedures.
Ethics Statement
All animal experiments were approved by Stockholm North Ethical Committee for Animal Research (protocols N27/15, N173/15, and N26/15).
Statistical Analysis
For image analysis, Student’s t test was used for evaluation of the statistical significance of the results. For slice recordings, the two-sample Kolmogorov-Smirnov (K-S2) test was used to compare pooled cumulative frequency distributions. For behavioral studies, statistical analyses were carried out using the SPSS package (SPSS) for a statistical significance level of p < 0.05.
Histological Studies
For immunostaining, cerebellar sections were blocked for 1 hr in PBS containing 5% normal donkey serum and 0.1% Triton X-100. Incubation with primary antibodies, diluted in blocking solution, was done overnight (o/n) at 4°C. Sections were washed 3 × 10 min in PBS and then incubated with fluorescently labeled secondary antibodies (diluted in blocking solution) and 1 μg/mL DAPI (D1306; Sigma) for counterstaining for 2 hr at room temperature (RT). The slides were finally washed 3 × 10 min in PBS and mounted with DAKO fluorescent medium.
Genetic Fate Mapping and BrdU Labeling
For genetic fate mapping, Gfra1CreERT2;Rosa26YFP mice received a single subcutaneous injection of 2 mg/30 g tamoxifen (Tmx; Sigma) dissolved in corn oil (Sigma) containing 10% ethanol at P0, P15, and P90.
For BrdU labeling, pups were injected subcutaneously with 25 mg/kg BrdU (Sigma) in PBS at P5. Embryos were collected 2 hr after injection for proliferation analysis or 5 days later for migration studies. For BrdU detection, sections were incubated in 2 N HCl, 0.1% Triton X-100 at 37°C for 20 min, washed with 0.1 M sodium borate for 15 min, washed 2 × 5 min with PBS, and incubated with rat anti-BrdU antibody.
Image Analysis
All fluorescent images were captured with a Carl Zeiss LSM710 confocal microscope using ZEN 2009 software (Carl Zeiss), and cell counts were made with ImageJ software (http://imagej.nih.gov/ij/). For caspase-3 analysis, counts were made in the entire length of folial WM from six sagittal sections (14 μm thick, one section every 140 μm) per animal from medial to lateral planes. For Pax2 MLI and Golgi cell counts, two images, containing all layers of the cerebellar cortex, were obtained from each folium, approximately at the same location, and two midsagittal sections (14 μm) were analyzed per mouse. For counts in adult tissue, DAPI+, PV+ cells or dystroglycan+ puncta were analyzed in the ML and Kv1.2+ synapses on PC cell bodies.
Cerebellar Slice Recordings
Whole-cell recordings were made from PC somata primarily located in lobules 4–7 (Larsell, 1952) at near physiological temperature (34°C). The holding potential in voltage-clamp recordings was 60 mV. Recordings of sIPSCs were performed in voltage-clamp mode in the presence of 10 μM CNQX and 25 μM AP-5 to block ionotropic glutamatergic neurotransmission. All sIPSCs recordings were concluded by application of 10 μM gabazine to confirm the GABAergic nature of events by the complete loss of all synaptic currents. Recordings were performed using a Multiclamp 700B amplifier, a DigiData 1440, and pClamp10.2 software (Molecular Devices).
Behavioral Studies
Classical conditioning was achieved using a delay paradigm. The US consisted of a cathodal, square pulse applied to the supraorbital nerve (500 μs, 3 × threshold) at the end of the CS. A total of 2 habituation, 10 conditioning, and 5 extinction sessions were carried out for each animal. A conditioning session consisted of 60 CS-US presentations and lasted 30 min. For vestibular stimulation, a single animal was placed on a homemade turning-table system. Eye positions for each frequency and animal were averaged (10 complete rotations × 3 recording sessions) for offline analysis of gain and phase (de Jeu and De Zeeuw, 2012). The number of compensatory eye saccades was also quantified.
Author Contributions
M.C.S. designed and performed all experiments (except electrophysiological studies, VOR, and eyeblink conditioning) and prepared the first draft of the manuscript and figures. J.C.L.-R., A.G., and J.M.D.-G. performed the VOR and eyeblink conditioning experiments. S.S. and C.B. performed the electrophysiological studies. C.F.I. contributed to the design of experiments and interpretation of results and produced the final version of the manuscript and figures.
Acknowledgments
We thank Diana Fernández Suárez for help with calbindin staining of adult brainstem sections; Francoise Helmbacher (IBDML, Marseille, France) for providing brain tissue from Gdnfbgal mice; Mart Saarma and Jaan-Olle Andressoo (University of Helsinki, Finland) for Gfra1fx/fx mice; and Annika Andersson, José A. Santos-Naharro, and José M. González-Martín for technical assistance. Support was provided by grants from the Swedish Research Council (2016-01538), the Knut and Alice Wallenbergs Foundation (Wallenberg Scholars Program; KAW 2012.0270), and the National University of Singapore (R-185-000-227-133 and -733 to C.F.I.); from MINECO (BFU21014-56692-R to A.G. and J.M.D.-G.); and from the European Research Council (ENDOSWITCH 261286) and the Swedish Research Council (to C.B.).
Published: June 6, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and four figures and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2017.05.030.
Supplemental Information
References
- Briatore F., Patrizi A., Viltono L., Sassoè-Pognetto M., Wulff P. Quantitative organization of GABAergic synapses in the molecular layer of the mouse cerebellar cortex. PLoS ONE. 2010;5:e12119. doi: 10.1371/journal.pone.0012119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron D.B., Kasai K., Jiang Y., Hu T., Saeki Y., Komuro H. Four distinct phases of basket/stellate cell migration after entering their final destination (the molecular layer) in the developing cerebellum. Dev. Biol. 2009;332:309–324. doi: 10.1016/j.ydbio.2009.05.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carletti B., Rossi F. Neurogenesis in the cerebellum. Neuroscientist. 2008;14:91–100. doi: 10.1177/1073858407304629. [DOI] [PubMed] [Google Scholar]
- Dauer W., Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- de Jeu M., De Zeeuw C.I. Video-oculography in mice. J. Vis. Exp. 2012;65:e3971. doi: 10.3791/3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekkers M.P.J., Nikoletopoulou V., Barde Y.-A. Cell biology in neuroscience: Death of developing neurons: new insights and implications for connectivity. J. Cell Biol. 2013;203:385–393. doi: 10.1083/jcb.201306136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dizon M.J., Khodakhah K. The role of interneurons in shaping Purkinje cell responses in the cerebellar cortex. J. Neurosci. 2011;31:10463–10473. doi: 10.1523/JNEUROSCI.1350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts F.J., Timmermans J., Shigemoto R., De Schutter E. Morphological and neurochemical differentiation of large granular layer interneurons in the adult rat cerebellum. Neuroscience. 2001;104:499–512. doi: 10.1016/s0306-4522(01)00058-6. [DOI] [PubMed] [Google Scholar]
- Golden J.P., DeMaro J.A., Osborne P.A., Milbrandt J., Johnson E.M., Jr. Expression of neurturin, GDNF, and GDNF family-receptor mRNA in the developing and mature mouse. Exp. Neurol. 1999;158:504–528. doi: 10.1006/exnr.1999.7127. [DOI] [PubMed] [Google Scholar]
- Heiney S.A., Kim J., Augustine G.J., Medina J.F. Precise control of movement kinematics by optogenetic inhibition of Purkinje cell activity. J. Neurosci. 2014;34:2321–2330. doi: 10.1523/JNEUROSCI.4547-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T. Around LTD hypothesis in motor learning. Cerebellum. 2014;13:645–650. doi: 10.1007/s12311-014-0581-4. [DOI] [PubMed] [Google Scholar]
- Hoshino M., Nakamura S., Mori K., Kawauchi T., Terao M., Nishimura Y.V., Fukuda A., Fuse T., Matsuo N., Sone M. Ptf1a, a bHLH transcriptional gene, defines GABAergic neuronal fates in cerebellum. Neuron. 2005;47:201–213. doi: 10.1016/j.neuron.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Ibáñez C.F. Structure and physiology of the RET receptor tyrosine kinase. Cold Spring Harb. Perspect. Biol. 2013;5:1–10. doi: 10.1101/cshperspect.a009134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Cerebellar learning in the vestibulo-ocular reflex. Trends Cogn. Sci. 1998;2:313–321. doi: 10.1016/s1364-6613(98)01222-4. [DOI] [PubMed] [Google Scholar]
- Ito M. Mechanisms of motor learning in the cerebellum. Brain Res. 2000;886:237–245. doi: 10.1016/s0006-8993(00)03142-5. [DOI] [PubMed] [Google Scholar]
- Jain S., Golden J.P., Wozniak D., Pehek E., Johnson E.M., Jr., Milbrandt J. RET is dispensable for maintenance of midbrain dopaminergic neurons in adult mice. J. Neurosci. 2006;26:11230–11238. doi: 10.1523/JNEUROSCI.1876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jörntell H., Bengtsson F., Schonewille M., De Zeeuw C.I. Cerebellar molecular layer interneurons - computational properties and roles in learning. Trends Neurosci. 2010;33:524–532. doi: 10.1016/j.tins.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Kopra J., Vilenius C., Grealish S., Härma M.-A., Varendi K., Lindholm J., Castrén E., Võikar V., Björklund A., Piepponen T.P. GDNF is not required for catecholaminergic neuron survival in vivo. Nat. Neurosci. 2015;18:319–322. doi: 10.1038/nn.3941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer E.R., Aron L., Ramakers G.M., Seitz S., Zhuang X., Beyer K., Smidt M.P., Klein R. Absence of Ret signaling in mice causes progressive and late degeneration of the nigrostriatal system. PLoS Biol. 2007;5:e39. doi: 10.1371/journal.pbio.0050039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsell O. The morphogenesis and adult pattern of the lobules and fissures of the cerebellum of the white rat. J. Comp. Neurol. 1952;97:281–356. doi: 10.1002/cne.900970204. [DOI] [PubMed] [Google Scholar]
- Leto K., Carletti B., Williams I.M., Magrassi L., Rossi F. Different types of cerebellar GABAergic interneurons originate from a common pool of multipotent progenitor cells. J. Neurosci. 2006;26:11682–11694. doi: 10.1523/JNEUROSCI.3656-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paratcha G., Ledda F. GDNF and GFRalpha: a versatile molecular complex for developing neurons. Trends Neurosci. 2008;31:384–391. doi: 10.1016/j.tins.2008.05.003. [DOI] [PubMed] [Google Scholar]
- Pascual A., Hidalgo-Figueroa M., Piruat J.I., Pintado C.O., Gómez-Díaz R., López-Barneo J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat. Neurosci. 2008;11:755–761. doi: 10.1038/nn.2136. [DOI] [PubMed] [Google Scholar]
- Sánchez-Campusano R., Gruart A., Delgado-García J.M. Dynamic associations in the cerebellar-motoneuron network during motor learning. J. Neurosci. 2009;29:10750–10763. doi: 10.1523/JNEUROSCI.2178-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling K., Oberdick J. The treasury of the commons: making use of public gene expression resources to better characterize the molecular diversity of inhibitory interneurons in the cerebellar cortex. Cerebellum. 2009;8:477–489. doi: 10.1007/s12311-009-0124-6. [DOI] [PubMed] [Google Scholar]
- Schonewille M., Gao Z., Boele H.-J., Veloz M.F., Amerika W.E., Šimek A.A.M., De Jeu M.T., Steinberg J.P., Takamiya K., Hoebeek F.E. Reevaluating the role of LTD in cerebellar motor learning. Neuron. 2011;70:43–50. doi: 10.1016/j.neuron.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergaki M.C., Ibáñez C.F. GFRα1 regulates Purkinje cell migration by counteracting NCAM function. Cell Rep. 2017;18:367–379. doi: 10.1016/j.celrep.2016.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P., Hámori J. A quantitative electron microscopic study of the Purkinje cell axon initial segment. Neuroscience. 1976;1:361–365. doi: 10.1016/0306-4522(76)90127-5. [DOI] [PubMed] [Google Scholar]
- Sotelo C. Molecular layer interneurons of the cerebellum: developmental and morphological aspects. Cerebellum. 2015;14:534–556. doi: 10.1007/s12311-015-0648-x. [DOI] [PubMed] [Google Scholar]
- ten Brinke M.M., Boele H.-J., Spanke J.K., Potters J.-W., Kornysheva K., Wulff P., IJpelaar A.C.H.G., Koekkoek S.K.E., De Zeeuw C.I. Evolving models of Pavlovian conditioning: cerebellar cortical dynamics in awake behaving mice. Cell Rep. 2015;13:1977–1988. doi: 10.1016/j.celrep.2015.10.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R.F., Steinmetz J.E. The role of the cerebellum in classical conditioning of discrete behavioral responses. Neuroscience. 2009;162:732–755. doi: 10.1016/j.neuroscience.2009.01.041. [DOI] [PubMed] [Google Scholar]
- Trupp M., Belluardo N., Funakoshi H., Ibáñez C.F. Complementary and overlapping expression of glial cell line-derived neurotrophic factor (GDNF), c-ret proto-oncogene, and GDNF receptor-alpha indicates multiple mechanisms of trophic actions in the adult rat CNS. J. Neurosci. 1997;17:3554–3567. doi: 10.1523/JNEUROSCI.17-10-03554.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Kunkel D.D., Martin T.M., Schwartzkroin P.A., Tempel B.L. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365:75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- Weisheit G., Gliem M., Endl E., Pfeffer P.L., Busslinger M., Schilling K. Postnatal development of the murine cerebellar cortex: formation and early dispersal of basket, stellate and Golgi neurons. Eur. J. Neurosci. 2006;24:466–478. doi: 10.1111/j.1460-9568.2006.04915.x. [DOI] [PubMed] [Google Scholar]
- Wulff P., Schonewille M., Renzi M., Viltono L., Sassoè-Pognetto M., Badura A., Gao Z., Hoebeek F.E., van Dorp S., Wisden W. Synaptic inhibition of Purkinje cells mediates consolidation of vestibulo-cerebellar motor learning. Nat. Neurosci. 2009;12:1042–1049. doi: 10.1038/nn.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka H., Yanagawa Y., Obata K. Development of stellate and basket cells and their apoptosis in mouse cerebellar cortex. Neurosci. Res. 2004;50:13–22. doi: 10.1016/j.neures.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Yang Y., Lei C., Feng H., Sui J.-F. The neural circuitry and molecular mechanisms underlying delay and trace eyeblink conditioning in mice. Behav. Brain Res. 2015;278:307–314. doi: 10.1016/j.bbr.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Zhang L., Goldman J.E. Developmental fates and migratory pathways of dividing progenitors in the postnatal rat cerebellum. J. Comp. Neurol. 1996;370:536–550. doi: 10.1002/(SICI)1096-9861(19960708)370:4<536::AID-CNE9>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.