Summary
Hepatic lipid accumulation has been implicated in the development of insulin resistance, but translational evidence in humans is limited. We investigated the relationship between liver fat and tissue-specific insulin sensitivity in 133 obese subjects. Although the presence of hepatic steatosis in obese subjects was associated with hepatic, adipose tissue, and peripheral insulin resistance, we found that intrahepatic triglycerides were not strictly sufficient or essential for hepatic insulin resistance. Thus, to examine the molecular mechanisms that link hepatic steatosis to hepatic insulin resistance, we comprehensively analyzed liver biopsies from a subset of 29 subjects. Here, hepatic cytosolic diacylglycerol content, but not hepatic ceramide content, was increased in subjects with hepatic insulin resistance. Moreover, cytosolic diacylglycerols were strongly associated with hepatic PKCε activation, as reflected by PKCε translocation to the plasma membrane. These results demonstrate the relevance of hepatic diacylglycerol-induced PKCε activation in the pathogenesis of NAFLD-associated hepatic insulin resistance in humans.
Keywords: insulin resistance, hepatic glucose production, hepatic steatosis, NAFLD, diacylglycerol, protein kinase Cε, glucose clamp, obesity, human
Graphical Abstract

Highlights
-
•
The presence of hepatic steatosis is associated with insulin resistance
-
•
Intrahepatic triglycerides are not sufficient for hepatic insulin resistance
-
•
Diacylglycerol in hepatic cytosol predicts insulin inhibition of glucose production
-
•
Diacylglycerol-associated insulin resistance is characterized by PKCε translocation
ter Horst et al. show that simple hepatic steatosis in the context of NAFLD does not fully explain hepatic insulin resistance in obese humans. Impaired hepatic insulin action was characterized by accumulation of diacylglycerol subspecies in the cytosol of hepatocytes and translocation of PKCε from the cytosol to the membrane.
Introduction
Insulin resistance and non-alcoholic fatty liver disease (NAFLD) are common and consequential complications of obesity (Grundy, 2000). Ectopic triglyceride accumulation in the liver has clearly been linked to non-alcoholic steatohepatitis (NASH), liver cirrhosis, and hepatocellular carcinoma (Wong et al., 2014), but it is less clear whether there is a causal relationship between intrahepatic triglyceride (IHTG) accumulation and hepatic insulin resistance (Farese et al., 2012, Shulman, 2000).
Elevated IHTG content is associated with hepatic and/or muscle insulin resistance in most, but not in all, human studies (Bugianesi et al., 2005, Deivanayagam et al., 2008, Gastaldelli et al., 2007, Korenblat et al., 2008, Kotronen et al., 2008, Petersen et al., 2002, Petersen et al., 2005, Sanyal et al., 2001, Seppälä-Lindroos et al., 2002). Moreover, in addition to triglycerides, it is now established that other (possibly more bioactive) lipid species may also accumulate in hepatocytes in the context of NAFLD. In several animal models, buildup of diacylglycerol (DAG) has been linked to hepatic insulin resistance through activation of protein kinase Cε (PKCε), which may directly interfere with hepatic insulin signaling (Jornayvaz et al., 2011, Samuel et al., 2004, Samuel et al., 2007, Samuel and Shulman, 2016, Xia et al., 2015). In this regard, PKCε has recently been shown to phosphorylate insulin receptor (INSR) threonine1160, which led to inhibition of INSR kinase activity (Petersen et al., 2016). Furthermore, mice with a threonine-to-alanine mutation at the homologous residue threonine1150 (InsrT1150A mice) were protected from high-fat-diet-induced hepatic insulin resistance and displayed increased hepatic insulin signaling, suppression of hepatic glucose production, and hepatic glycogen synthesis compared with wild-type (WT) controls during hyperinsulinemic clamp studies. These results provide a strong mechanistic link for DAG-PKCε-mediated hepatic insulin resistance in the context of NAFLD. In contrast, other studies have suggested that an increase in hepatic ceramide content is the major mediator of lipid-induced hepatic insulin resistance in the context of NAFLD (Chavez and Summers, 2012, Holland et al., 2011, Luukkonen et al., 2016), although the mechanism by which ceramides cause hepatic insulin resistance is less clear. In the few available translational studies, hepatic DAG content, but not hepatic ceramide content, correlated with indices of hepatic insulin resistance in obese humans (Kumashiro et al., 2011, Magkos et al., 2012).
There are also circumstances, in both animals and humans, in which hepatic insulin resistance is dissociated from hepatic steatosis (Amaro et al., 2010, Benhamed et al., 2012, Brown et al., 2010, Buettner et al., 2004, Monetti et al., 2007, Semple et al., 2009, Visser et al., 2011). Some, but not all, of these apparently conflicting observations may be the result of differences among studies in diagnosis and definition of fatty liver and/or insulin resistance (Farese et al., 2012). Moreover, a better understanding of the subcellular compartmentalization of hepatic DAG species and PKCε may explain some of the apparent dissociations between hepatic steatosis and hepatic insulin resistance (Samuel and Shulman, 2016). For instance, short-term high-fat feeding promotes hepatic insulin resistance, which is associated with increases in cytosolic and membrane DAG content and activation of PKCε as reflected by translocation of PKCε from the cytosol to the plasma membrane (Samuel et al., 2004, Samuel et al., 2007). In contrast, antisense oligonucleotide knockdown of hepatic CGI-58 leads to hepatic steatosis, which is associated with considerable accumulation of DAGs in lipid droplets (Cantley et al., 2013). Contrary to the high-fat-fed mice, this increase in lipid droplet DAGs is not associated with PKCε activation or hepatic insulin resistance. It is currently unknown how these paradigms translate to humans.
Therefore, we here report a clinical study in obese non-diabetic humans aimed to (1) determine the relationship between liver fat content and insulin resistance using gold-standard metabolic flux measurements and (2) determine whether the subcellular distribution of individual hepatic lipid species and PKCε in liver biopsies is relevant for hepatic insulin resistance in humans.
Results and Discussion
The Presence of NAFLD, but Not the Extent of Hepatic Steatosis, Is Associated with Insulin Resistance in Obese Humans
To determine whether the accumulation of IHTG in the context of NAFLD relates to abnormalities in glucose metabolism and/or tissue-specific parameters of insulin action, we measured these metabolic fluxes using the two-step hyperinsulinemic-euglycemic clamp technique in a cohort of 133 obese adults. Fifty-two subjects had normal IHTG by proton magnetic resonance spectroscopy (1H-MRS) (no steatosis, IHTG <5.56% [Szczepaniak et al., 2005]). Of the 81 subjects with 1H-MRS-defined hepatic steatosis, 41 subjects had mild steatosis (IHTG 5.56%–15%), and 40 subjects had severe steatosis (IHTG >15%). These groups did not differ in most baseline characteristics, but subjects with hepatic steatosis had higher plasma triglycerides and aminotransferases than those without steatosis (Table 1).
Table 1.
Characteristics of Study Participants According to Extent of Hepatic Steatosis, n = 133
| No Steatosis (n = 52) | Mild Steatosis (n = 41) | Severe Steatosis (n = 40) | p | |
|---|---|---|---|---|
| Female sex | 27 (51) | 14 (34) | 18 (45) | 0.229 |
| Age (years) | 49 ± 2 | 51 ± 2 | 49 ± 2 | 0.634 |
| BMI (kg/m2) | 39 ± 1 | 41 ± 1 | 41 ± 1 | 0.442 |
| Body fat (%) | 45 ± 1 | 45 ± 2 | 46 ± 1 | 0.695 |
| Fasting glucose (mmol/L) | 5.2 ± 0.1 | 5.2 ± 0.1 | 5.4 ± 0.1 | 0.301 |
| Triglycerides (mmol/L) | 1.3 ± 0.1 | 1.6 ± 0.1 | 1.7 ± 0.2a | 0.039 |
| Cholesterol (mmol/L) | 4.9 ± 0.1 | 4.9 ± 0.2 | 5.0 ± 0.2 | 0.671 |
| High-density lipoprotein (mmol/L) | 1.2 ± 0.0 | 1.1 ± 0.1 | 1.1 ± 0.0 | 0.287 |
| Low-density lipoprotein (mmol/L) | 3.0 ± 0.1 | 3.1 ± 0.2 | 3.2 ± 0.1 | 0.620 |
| Alanine aminotransferase (U/L) | 26 ± 2 | 38 ± 3b | 40 ± 3c | <0.001 |
| Aspartate aminotransferase (U/L) | 22 ± 1 | 30 ± 3 | 32 ± 3b | 0.004 |
| Gamma-glutamyl transpeptidase (U/L) | 34 ± 4 | 42 ± 7 | 42 ± 4 | 0.320 |
| C-reactive protein (mg/L) | 4.9 ± 0.9 | 6.0 ± 1.0 | 7.6 ± 1.3 | 0.197 |
| Resting energy expenditure (kcal/day) | 1,862 ± 48 | 2,000 ± 72 | 1,931 ± 54 | 0.256 |
| IHTG (%) | 2.9 ± 0.2 | 10.4 ± 0.4c | 23.3 ± 1.0,c,d | <0.001 |
Data are count (%) or mean ± SEM. No, mild, and severe steatosis were defined as intrahepatic triglycerides (IHTG) <5.56%, 5.56%–15%, or >15%, respectively.
p < 0.05 versus no steatosis.
p < 0.01 versus no steatosis.
p < 0.001 versus no steatosis.
p < 0.001 versus mild steatosis.
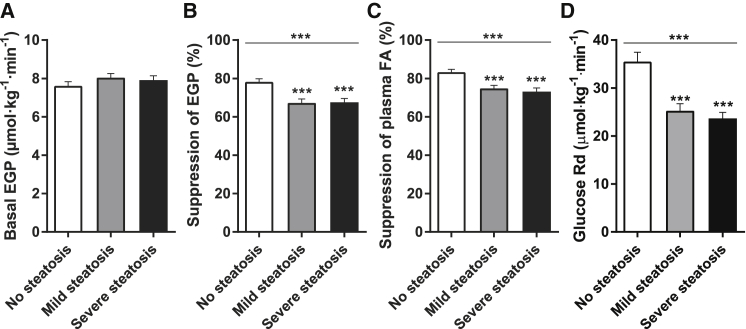
Hepatic steatosis was not associated with differences in the basal rate of endogenous glucose production (EGP) (Figure 1A). However, subjects with mild or severe hepatic steatosis had higher fasting plasma insulin levels than subjects without hepatic steatosis (Table S1). Since fasting insulin × basal EGP is an index of hepatic insulin resistance (Korenblat et al., 2008), this suggests that basal hepatic responsiveness to insulin may be impaired in obese subjects with steatosis (Figure S1A). Indeed, during the low-dose insulin step of the hyperinsulinemic-euglycemic clamp, insulin-mediated suppression of EGP was impaired in subjects with mild or severe hepatic steatosis, indicating hepatic insulin resistance (Figure 1B). In both steatosis groups, insulin suppression of plasma fatty acids (FA) and insulin stimulation of the glucose rate of disappearance (Rd) were also reduced, indicating adipose tissue and peripheral/muscle insulin resistance, respectively (Figures 1C, 1D, S1B, and S1C). Notably, there were no differences in any of these parameters between subjects with mild or severe hepatic steatosis (Figures 1B–1D). Finally, these results were not caused by differences in insulin clearance (Figures S1D and S1E) or levels of glucoregulatory hormones during the clamps (Table S1). Therefore, data from our large cohort of obese subjects show that the presence of liver steatosis is associated with impaired insulin sensitivity in the liver, adipose tissue, and muscle, but that the extent of hepatic steatosis does not predict the severity of insulin resistance.
Figure 1.
The Presence of Hepatic Steatosis, but Not Its Extent, Is Associated with Insulin Resistance in Obese Humans
(A) The basal rate of EGP was assessed after an overnight fast.
(B) Hepatic insulin sensitivity is expressed as the insulin-mediated suppression of basal EGP.
(C) Adipose tissue insulin sensitivity is expressed as the insulin-mediated suppression of circulating FA.
(D) Peripheral insulin sensitivity is expressed as the insulin-stimulated Rd of glucose.
Data are mean ± SEM (n = 125–133). ∗∗∗p ≤ 0.001.
IHTG Are Not Sufficient or Essential for the Development of Hepatic Insulin Resistance in Obese Humans
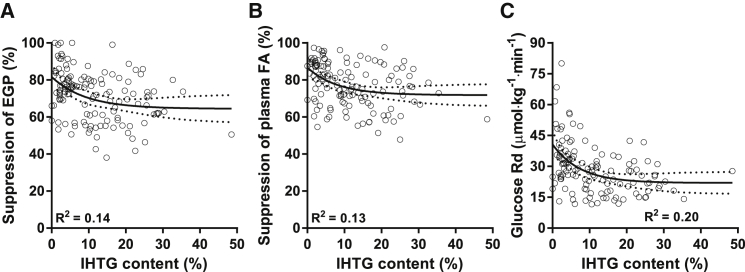
In agreement with the previous data, regression analysis confirmed that the relationship between IHTG and insulin suppression of EGP was best described by a nonlinear model: IHTG and hepatic insulin action were negatively correlated in the lower range of steatosis but dissociated in the higher range of steatosis (Figure 2A). More importantly, obese subjects with hepatic steatosis displayed nearly the same variation in hepatic insulin sensitivity as subjects without hepatic steatosis (suppression of EGP ranging from 38%–99% and 51%–100%, respectively). In fact, the distribution of both variables clearly shows that hepatic steatosis can occur without hepatic insulin resistance, and vice versa (Figure 2A). Therefore, IHTG are not strictly sufficient or necessary for hepatic insulin resistance (Farese et al., 2012), at least in the conditions of our study. We observed similar relationships between IHTG and parameters of extrahepatic insulin sensitivity (Figures 2B and 2C). These observations are consistent with previous human studies that have demonstrated IHTG accumulation without insulin resistance (Amaro et al., 2010, Visser et al., 2011) or insulin resistance in the absence of IHTG (Semple et al., 2009). Therefore, the available data do not implicate that IHTGs are a cause for hepatic insulin resistance, and this indicates that (an)other factor(s) must be responsible for NAFLD-associated hepatic insulin resistance.
Figure 2.
Relationships between IHTG Content and Tissue-Specific Measurements of Insulin Sensitivity in Obese Subjects Were Best Described by Nonlinear Models
(A) Hepatic insulin sensitivity.
(B) Adipose tissue insulin sensitivity.
(C) Peripheral insulin sensitivity.
Lines are best fit and 95% CI (n = 125–133).
Hepatic Insulin Resistance Is Associated with DAG Accumulation In Cytosol
Non-invasive measurement methods such as 1H-MRS cannot distinguish between inert lipids such as triglycerides (Yu et al., 2002) and bioactive lipids such as DAGs or ceramides. The subcellular distribution of these lipids may also be critical to their biological effects. Therefore, to determine whether these parameters are important for hepatic insulin resistance in obese humans, we obtained liver biopsies from a subset of subjects that underwent bariatric surgery <2 weeks after the clinical experiments (n = 29).
Liquid chromatography/tandem mass spectrometry (LC-MS/MS) analysis of snap-frozen liver biopsies revealed that neither hepatic ceramide (r = 0.32, p = 0.117) nor total DAG (r = 0.31, p = 0.109) content predicted hepatic insulin sensitivity in morbidly obese subjects (Figures S2A and S2B). However, DAG may be present in the cell membrane, cytosol, and lipid droplets (Cantley et al., 2013, Kumashiro et al., 2011). Using a subcellular fractionation method (Cantley et al., 2013), we separated liver biopsies into membrane and cytosolic fractions. Here, we observed that DAG accumulation in the cytosolic fraction (r = 0.43, p = 0.024), but not DAG accumulation in the membrane (r = 0.19, p = 0.342), predicted hepatic insulin resistance assessed by clamp (Figures S2C and S2D). Cytosolic DAG also strongly correlated with other parameters of hepatic insulin action including the Hepatic Insulin Sensitivity Index, HOMA-IR, and fasting plasma insulin levels (data not shown). Finally, we analyzed individual hepatic DAG species in relation to hepatic insulin sensitivity (Table S2). In the cytosolic fraction, hepatic DAGs composed of C18:1–C16:0, C16:0–C16:0, and C18:1–C18:1 were most abundant and also showed strong negative correlations with suppression of EGP. In contrast, hepatic DAG composed of C20:4–C20:5 was highly abundant in the membrane fraction and positively correlated with hepatic insulin sensitivity. Individual hepatic ceramide species were not related to hepatic insulin action (Table S3).
Hepatic DAG-Associated Insulin Resistance and PKCε Translocation
Hepatic DAGs are proposed to impair insulin signaling through activation of novel PKC (nPKC) (Farese et al., 2012, Nagle et al., 2009, Samuel and Shulman, 2016). In our prior study, we found that PKCε was the major nPKC isoform expressed in human liver, and activation of PKCε correlated with hepatic DAG content in the cytosol and HOMA-IR (Kumashiro et al., 2011). However, the association between PKCε activation and insulin suppression of hepatic glucose output has not been reported in humans. Therefore, we measured PKCε translocation to the cell membrane as an index of PKCε activation (Kumashiro et al., 2011, Schmitz-Peiffer and Biden, 2008).
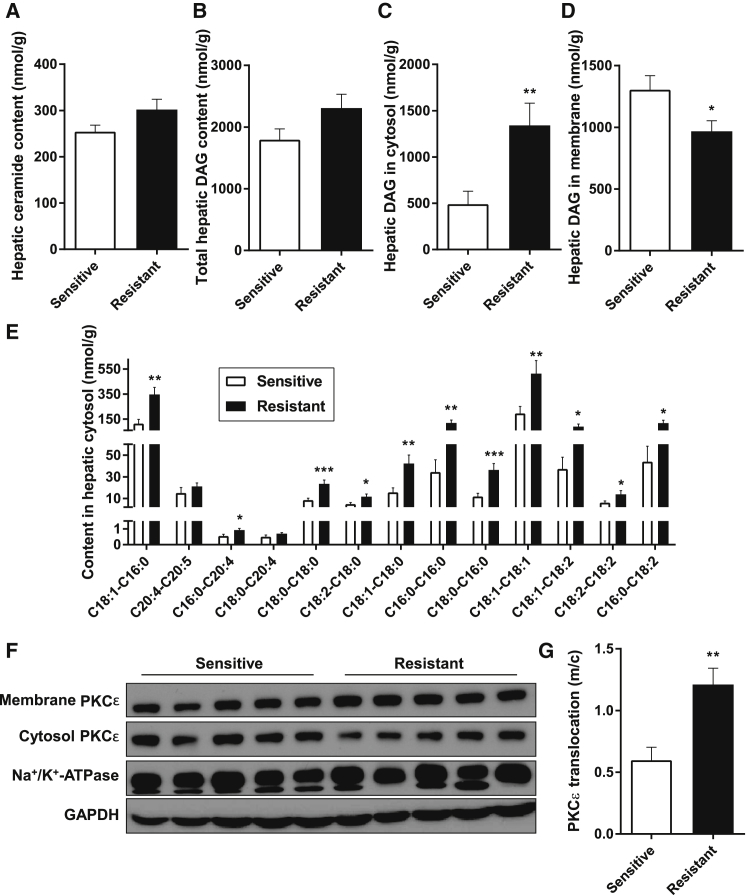
We compared liver biopsies from insulin-sensitive subjects (insulin suppression of EGP >80%, n = 10) with biopsies from insulin-resistant subjects (insulin suppression of EGP <65%, n = 8). The insulin-resistant subjects displayed both impaired fasting glucose concentrations and hyperinsulinemia (Table S4). In addition, insulin-resistant livers had a marked, 2.5-fold increase in cytosolic DAG content, but no difference in total DAG or ceramide content (Figures 3A–3D). In fact, all individual DAG species were increased in the hepatic cytosol of insulin-resistant subjects (Figure 3E). Notably, hepatic DAG-associated hepatic insulin resistance was characterized by a 2-fold increase in translocation of PKCε from the cytosol to the membrane (Figures 3F and 3G). Taken together, this suggests that hepatic DAGs in the cytosol promote PKCε translocation from the cytosol to the membrane where it can bind INSR and phosphorylate threonine1160, which, in turn, inhibits INSR kinase activity (Petersen et al., 2016).
Figure 3.
Hepatic Insulin Resistance Was Strongly Associated with Elevated DAG Content in the Cytosolic Fraction and PKCε Translocation
(A) Hepatic ceramide content in obese subjects with normal hepatic insulin sensitivity or hepatic insulin resistance.
(B) Total hepatic DAG content.
(C) Hepatic DAG content in cytosolic fraction.
(D) Hepatic DAG content in membrane fraction.
(E) Individual DAG species in hepatic cytosol. Note the segmented y axis.
(F) Representative bands show PKCε translocation from cytosol to membrane.
(G) Translocation of PKCε from cytosol (c) to membrane (m) as an index of activation.
Data are mean ± SEM (n = 18–29). ∗p < 0.05. ∗∗p < 0.01. ∗∗∗p = 0.001.
Sources for Hepatic DAG Accumulation In Obese Humans
Hepatic DAG may accumulate from a variety of sources including circulating FA derived from adipose tissue lipolysis (Donnelly et al., 2005, Finck and Hall, 2015). To explore the potential role of adipose tissue lipolysis in promoting increased hepatic DAG content, we assessed rates of lipolysis measured by tracer dilution of [1,1,2,3,3-2H5]glycerol during hyperinsulinemic conditions. Using this approach, we found that insulin-mediated suppression of the glycerol rate of appearance (Ra) and circulating FA levels during insulin infusion both strongly correlated with hepatic DAG accumulation, indicating that higher release of FA from lipolysis during hyperinsulinemic (postprandial) conditions is associated with increased cytosolic DAG content (Figures S3A and S3B). In line, hepatic lipid synthesis in rats is primarily driven by substrate (FA) delivery to the liver, which occurs in an insulin-independent manner (Vatner et al., 2015). Thus, these data are consistent with the possibility that most of the hepatic DAG derives from FA from increased lipolysis in insulin-resistant adipose tissue, underlining the importance of healthy insulin-sensitive adipose tissue in the prevention of ectopic lipid disposition and hepatic insulin resistance.
Perspective
In this study, we demonstrated that the presence of hepatic steatosis in obese subjects is associated with insulin resistance in the liver, adipose tissue, and peripheral tissues. In subjects with hepatic steatosis, however, the amount of IHTG per se did not additionally affect tissue-specific parameters of insulin action. Noteworthy, although all subjects were obese, there was a wide range in insulin sensitivity. The distribution of the data further showed that IHTG were neither sufficient nor necessary for the development of hepatic insulin resistance, indicating that another underlying mechanism must be responsible for impaired insulin suppression of EGP in the context of NAFLD. Recent animal studies showed that the major underlying mechanism for hepatic insulin resistance involves hepatic DAG-induced PKCε activation (Kumashiro et al., 2011, Petersen et al., 2016, Samuel and Shulman, 2016), but efforts to translate this paradigm to humans have been limited. Therefore, we comprehensively analyzed liver samples from subjects with normal or impaired insulin suppression of EGP and found that hepatic DAG accumulation in the cytosol was increased in obese humans with hepatic insulin resistance. Moreover, this was strongly associated with hepatic PKCε activation as reflected by its translocation to the cell membrane. In contrast, there was no relationship between hepatic ceramide content and hepatic insulin resistance in these subjects. Taken together, these results support a model for the pathogenesis of hepatic insulin resistance in NAFLD, in which DAGs in the cytosol promote translocation of PKCε to the membrane, where it inhibits hepatocellular insulin signaling. Here, we substantiate the relevance of this pathway in human metabolic disease.
Some nuances with respect to our findings should be made. First, the above-mentioned mechanism for lipid-induced hepatic insulin resistance should be appreciated in light of interconnected defects in other organ systems. For instance, extrahepatic regulation of adipose tissue lipolysis and FA availability by insulin is another important regulator of hepatic gluconeogenesis through substrate-driven regulation of pyruvate carboxylase activity/flux by hepatic acetyl CoA, which is independent of canonical hepatic insulin signaling (Perry et al., 2015). Thus, PKCε inhibition of hepatic insulin signaling may mainly affect insulin regulation of net hepatic glycogen synthesis. Second, previous studies have suggested that increased hepatic ceramide synthesis may be causal in lipid-induced hepatic insulin resistance (Chavez and Summers, 2012, Holland et al., 2011, Luukkonen et al., 2016), but ceramides were unrelated to hepatic insulin resistance in the present study. Others have also reported such dissociations (Kumashiro et al., 2011, Magkos et al., 2012, Perry et al., 2013). Some of these conflicting results may be due to differences in experimental techniques or limited sample size/power. In one study, “metabolic NAFLD” was associated with an increase in hepatic ceramides and elevated HOMA-IR, but also with an increase in four out of five DAG species (Luukkonen et al., 2016). The authors did not perform measurements in subcellular compartments but conclude that their data do not exclude the possibility that DAGs contribute to hepatic insulin resistance in humans, indicating that results are not strictly conflicting. In addition, the I148M gene variant in PNPLA3 was associated with hepatic steatosis without insulin resistance, whereas metabolic NAFLD was associated with a harmful hepatic lipidome (Luukkonen et al., 2016). We have also reported that hepatic steatosis in the context of familial hypobetalipoproteinemia is not associated with hepatic insulin resistance (Visser et al., 2011), suggesting that genetics may, in part, explain why some people with hepatic steatosis develop insulin resistance, whereas others do not. Unfortunately, we do not have genotype data in this study, but it will be of interest to determine whether the I148M and other variants play a role in the susceptibility to lipid mediated-insulin resistance. Third, there is ample (reverse causal) evidence suggesting that extrahepatic insulin resistance contributes to the development of hepatic steatosis. In individuals with muscle and adipose tissue insulin resistance, nutrients are diverted to the liver. Accompanied by a compensatory hyperinsulinemia, these conditions provide both substrate (glucose and FA) and stimulus (insulin signaling through sterol regulatory element-binding protein 1c) for hepatic triglyceride synthesis and de novo lipogenesis (Cohen et al., 2011, Perry et al., 2014, Petersen et al., 2007, Rabøl et al., 2011, Vatner et al., 2015). Finally, an important limitation in the interpretation of these data is the cross-sectional design of the study. However, we believe that obtaining serial liver biopsies in humans would be unethical in the absence of urgent medical indications, and numerous animal studies support a causal role for hepatic DAG accumulation and PKCε activation in hepatic insulin resistance (Jornayvaz et al., 2011, Petersen et al., 2016, Samuel et al., 2007, Samuel and Shulman, 2016). These data offer further support for the DAG-PKCε hypothesis for lipid-induced hepatic insulin resistance in NAFLD and support the development of interventions that target hepatic DAG-induced PKCε activation for the prevention and/or treatment of type 2 diabetes.
Experimental Procedures
Subjects
Subjects participated in metabolic studies at the Academic Medical Center (Amsterdam, the Netherlands). Treatment-naive subjects were included in this study if they were obese (BMI ≥30 kg/m2) with stable weight (<5% weight change) for >3 months prior to examinations, and if they had undergone a two-step hyperinsulinemic-euglycemic clamp study according to standard operating procedures and liver 1H-MRS before any intervention. Subjects were excluded in case of a history of diabetes, use of alcohol (>2 units/day) or recreational drugs, use of antipsychotic or antidepressant medication, or any somatic disorder except for stable obesity-related conditions (i.e., NAFLD/NASH, dyslipidemia, hypertension, obstructive sleep apnea).
All subjects completed a medical evaluation including history, physical examination, and blood tests. Body composition was determined by bioelectrical impedance (Maltron BF-906). Resting energy expenditure was assessed by indirect calorimetry (Vmax Encore 29n; CareFusion).
All procedures were approved by the Academic Medical Center medical ethics committee, and all subjects provided written informed consent in accordance with the Declaration of Helsinki.
Liver Fat Content
We assessed IHTG content by 1H-MRS as described (van der Valk et al., 2014). This method has high diagnostic accuracy and high precision with low variability for assessment of hepatic steatosis in NAFLD (Dulai et al., 2016).
Hyperinsulinemic-Euglycemic Clamps
Glucose kinetics and tissue-specific parameters of insulin sensitivity were assessed during a two-step hyperinsulinemic-euglycemic clamp study as described (ter Horst et al., 2015). Briefly, subjects were admitted to the metabolic unit after an overnight fast. A primed continuous infusion of the stable isotope-labeled glucose tracer [6,6-2H2]glucose (>99% enriched; Cambridge Isotopes) was started. In a subset of subjects (n = 29), we also infused the stable isotope-labeled glycerol tracer [1,1,2,3,3-2H5]glycerol (>99% enriched; Cambridge Isotopes). Basal rates of EGP and lipolysis were determined after 2 hr of tracer equilibration. Next, hepatic insulin sensitivity (expressed as suppression of basal EGP by insulin) and adipose tissue insulin sensitivity (expressed as suppression of circulating FA levels or suppression of glycerol Ra by insulin) were assessed after 2 hr of low-dose insulin infusion (Actrapid 20 mU · m−2 · min−1; Novo Nordisk Farma). Finally, peripheral insulin sensitivity (expressed as insulin-stimulated Rd of glucose) was assessed after 2 hr of high-dose insulin infusion (60 mU · m–2 · min–1). During hyperinsulinemia, plasma glucose was maintained at 5.0 mmol/L by frequent bedside monitoring and variable infusion of exogenous glucose (enriched with 1% [6,6-2H2]glucose).
Liver Biopsies
A subset of subjects (n = 29) underwent bariatric surgery shortly (<2 weeks) after the clinical assessments. Subjects were instructed to maintain stable weight by consumption of a weight-maintenance diet in the preoperative period. During surgery, an experienced surgeon obtained a wedge biopsy from the right liver lobe. Biopsies were immediately snap-frozen in liquid nitrogen and stored at −80°C.
Plasma Hormones and Metabolites
Glucose, insulin, glucagon, cortisol, and FA were measured as described (ter Horst et al., 2015). Enrichments of [6,62H2]glucose and [1,1,2,3,3-2H5]glycerol (tracer-to-tracee ratios) were measured by gas chromatography-MS (Ackermans et al., 2001).
Hepatic Lipid Metabolites
Extraction, purification, and assessment of DAGs and ceramides from liver was performed by LC-MS/MS in a blinded fashion as described (Kumashiro et al., 2011). Subcellular fractionation into membrane and cytosolic compartments was performed as reported (Cantley et al., 2013).
PKCε Translocation Assay
Membrane translocation of PKCε was assayed as described (Kumashiro et al., 2011) and expressed as the ratio of membrane protein band density over cytosol protein band density.
Calculations
Fluxes (EGP, glucose Rd, and glycerol Ra) were calculated using modified versions of the Steele equations for the steady state (basal EGP and glycerol Ra) or non-steady state (during insulin infusion) and expressed as μmol · (kg body weight)–1 · min–1 (Steele, 1959).
Statistical Analyses
Groups were compared by two-tailed t test (two groups) or one-way ANOVA with Bonferroni correction (more than two groups). Correlations between continuous variables were evaluated by Pearson’s correlation and regression analyses (linear and nonlinear). Findings were considered significant if p < 0.05. Statistical analyses were performed using IBM SPSS Statistics v.23 and GraphPad Prism v.6 (La Jolla).
Author Contributions
K.W.t.H. researched data, performed the analysis, contributed to discussions about the results, and wrote the manuscript. P.W.G. and R.I.V. researched data. M.T.A. and D.Z. were responsible for laboratory analyses. A.J.N. was responsible for 1H-MRS experiments. S.E.l.F., J.A.R., M.N., D.F.V., V.T.S., K.F.P., G.I.S., and M.J.S. contributed to discussions about the results. All authors critically reviewed and approved the final manuscript.
Acknowledgments
The authors thank Erik Endert (Academic Medical Center) for expert advice on laboratory analyses and Mario Kahn (Yale University School of Medicine) for LC-MS/MS analyses of hepatic lipid species. This study was funded, in part, by grants from the EU (FP7-EU 305707), NWO (STW 12189), and the US Public Health Service (R01 AG-023686, R24 DK-085638, P30 DK-045735). M.N. is supported by grants VIDI 2013 (016.146.327) and CVON 2012 young talent (IN-CONTROL).
Published: June 6, 2017
Footnotes
Supplemental Information includes three figures and four tables and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2017.05.035.
Supplemental Information
References
- Ackermans M.T., Pereira Arias A.M., Bisschop P.H., Endert E., Sauerwein H.P., Romijn J.A. The quantification of gluconeogenesis in healthy men by (2)H2O and [2-(13)C]glycerol yields different results: Rates of gluconeogenesis in healthy men measured with (2)H2O are higher than those measured with [2-(13)C]glycerol. J. Clin. Endocrinol. Metab. 2001;86:2220–2226. doi: 10.1210/jcem.86.5.7383. [DOI] [PubMed] [Google Scholar]
- Amaro A., Fabbrini E., Kars M., Yue P., Schechtman K., Schonfeld G., Klein S. Dissociation between intrahepatic triglyceride content and insulin resistance in familial hypobetalipoproteinemia. Gastroenterology. 2010;139:149–153. doi: 10.1053/j.gastro.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhamed F., Denechaud P.D., Lemoine M., Robichon C., Moldes M., Bertrand-Michel J., Ratziu V., Serfaty L., Housset C., Capeau J. The lipogenic transcription factor ChREBP dissociates hepatic steatosis from insulin resistance in mice and humans. J. Clin. Invest. 2012;122:2176–2194. doi: 10.1172/JCI41636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.M., Betters J.L., Lord C., Ma Y., Han X., Yang K., Alger H.M., Melchior J., Sawyer J., Shah R. CGI-58 knockdown in mice causes hepatic steatosis but prevents diet-induced obesity and glucose intolerance. J. Lipid Res. 2010;51:3306–3315. doi: 10.1194/jlr.M010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buettner R., Ottinger I., Schölmerich J., Bollheimer L.C. Preserved direct hepatic insulin action in rats with diet-induced hepatic steatosis. Am. J. Physiol. Endocrinol. Metab. 2004;286:E828–E833. doi: 10.1152/ajpendo.00453.2003. [DOI] [PubMed] [Google Scholar]
- Bugianesi E., Gastaldelli A., Vanni E., Gambino R., Cassader M., Baldi S., Ponti V., Pagano G., Ferrannini E., Rizzetto M. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: Sites and mechanisms. Diabetologia. 2005;48:634–642. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- Cantley J.L., Yoshimura T., Camporez J.P., Zhang D., Jornayvaz F.R., Kumashiro N., Guebre-Egziabher F., Jurczak M.J., Kahn M., Guigni B.A. CGI-58 knockdown sequesters diacylglycerols in lipid droplets/ER-preventing diacylglycerol-mediated hepatic insulin resistance. Proc. Natl. Acad. Sci. USA. 2013;110:1869–1874. doi: 10.1073/pnas.1219456110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez J.A., Summers S.A. A ceramide-centric view of insulin resistance. Cell Metab. 2012;15:585–594. doi: 10.1016/j.cmet.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Cohen J.C., Horton J.D., Hobbs H.H. Human fatty liver disease: Old questions and new insights. Science. 2011;332:1519–1523. doi: 10.1126/science.1204265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deivanayagam S., Mohammed B.S., Vitola B.E., Naguib G.H., Keshen T.H., Kirk E.P., Klein S. Nonalcoholic fatty liver disease is associated with hepatic and skeletal muscle insulin resistance in overweight adolescents. Am. J. Clin. Nutr. 2008;88:257–262. doi: 10.1093/ajcn/88.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly K.L., Smith C.I., Schwarzenberg S.J., Jessurun J., Boldt M.D., Parks E.J. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J. Clin. Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulai P.S., Sirlin C.B., Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: Clinical trials to clinical practice. J. Hepatol. 2016;65:1006–1016. doi: 10.1016/j.jhep.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farese R.V., Jr., Zechner R., Newgard C.B., Walther T.C. The problem of establishing relationships between hepatic steatosis and hepatic insulin resistance. Cell Metab. 2012;15:570–573. doi: 10.1016/j.cmet.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finck B.N., Hall A.M. Does diacylglycerol accumulation in fatty liver disease cause hepatic insulin resistance? BioMed Res. Int. 2015;2015:104132. doi: 10.1155/2015/104132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastaldelli A., Cusi K., Pettiti M., Hardies J., Miyazaki Y., Berria R., Buzzigoli E., Sironi A.M., Cersosimo E., Ferrannini E., Defronzo R.A. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133:496–506. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- Grundy S.M. Metabolic complications of obesity. Endocrine. 2000;13:155–165. doi: 10.1385/ENDO:13:2:155. [DOI] [PubMed] [Google Scholar]
- Holland W.L., Miller R.A., Wang Z.V., Sun K., Barth B.M., Bui H.H., Davis K.E., Bikman B.T., Halberg N., Rutkowski J.M. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat. Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jornayvaz F.R., Birkenfeld A.L., Jurczak M.J., Kanda S., Guigni B.A., Jiang D.C., Zhang D., Lee H.Y., Samuel V.T., Shulman G.I. Hepatic insulin resistance in mice with hepatic overexpression of diacylglycerol acyltransferase 2. Proc. Natl. Acad. Sci. USA. 2011;108:5748–5752. doi: 10.1073/pnas.1103451108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenblat K.M., Fabbrini E., Mohammed B.S., Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotronen A., Juurinen L., Tiikkainen M., Vehkavaara S., Yki-Järvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology. 2008;135:122–130. doi: 10.1053/j.gastro.2008.03.021. [DOI] [PubMed] [Google Scholar]
- Kumashiro N., Erion D.M., Zhang D., Kahn M., Beddow S.A., Chu X., Still C.D., Gerhard G.S., Han X., Dziura J. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA. 2011;108:16381–16385. doi: 10.1073/pnas.1113359108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luukkonen P.K., Zhou Y., Sädevirta S., Leivonen M., Arola J., Orešič M., Hyötyläinen T., Yki-Järvinen H. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J. Hepatol. 2016;64:1167–1175. doi: 10.1016/j.jhep.2016.01.002. [DOI] [PubMed] [Google Scholar]
- Magkos F., Su X., Bradley D., Fabbrini E., Conte C., Eagon J.C., Varela J.E., Brunt E.M., Patterson B.W., Klein S. Intrahepatic diacylglycerol content is associated with hepatic insulin resistance in obese subjects. Gastroenterology. 2012;142 doi: 10.1053/j.gastro.2012.03.003. 1444–6.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monetti M., Levin M.C., Watt M.J., Sajan M.P., Marmor S., Hubbard B.K., Stevens R.D., Bain J.R., Newgard C.B., Farese R.V., Sr. Dissociation of hepatic steatosis and insulin resistance in mice overexpressing DGAT in the liver. Cell Metab. 2007;6:69–78. doi: 10.1016/j.cmet.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Nagle C.A., Klett E.L., Coleman R.A. Hepatic triacylglycerol accumulation and insulin resistance. J. Lipid Res. 2009;50(Suppl):S74–S79. doi: 10.1194/jlr.R800053-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R.J., Kim T., Zhang X.M., Lee H.Y., Pesta D., Popov V.B., Zhang D., Rahimi Y., Jurczak M.J., Cline G.W. Reversal of hypertriglyceridemia, fatty liver disease, and insulin resistance by a liver-targeted mitochondrial uncoupler. Cell Metab. 2013;18:740–748. doi: 10.1016/j.cmet.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R.J., Samuel V.T., Petersen K.F., Shulman G.I. The role of hepatic lipids in hepatic insulin resistance and type 2 diabetes. Nature. 2014;510:84–91. doi: 10.1038/nature13478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R.J., Camporez J.P., Kursawe R., Titchenell P.M., Zhang D., Perry C.J., Jurczak M.J., Abudukadier A., Han M.S., Zhang X.M. Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell. 2015;160:745–758. doi: 10.1016/j.cell.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen K.F., Oral E.A., Dufour S., Befroy D., Ariyan C., Yu C., Cline G.W., DePaoli A.M., Taylor S.I., Gorden P., Shulman G.I. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J. Clin. Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen K.F., Dufour S., Befroy D., Lehrke M., Hendler R.E., Shulman G.I. Reversal of nonalcoholic hepatic steatosis, hepatic insulin resistance, and hyperglycemia by moderate weight reduction in patients with type 2 diabetes. Diabetes. 2005;54:603–608. doi: 10.2337/diabetes.54.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen K.F., Dufour S., Savage D.B., Bilz S., Solomon G., Yonemitsu S., Cline G.W., Befroy D., Zemany L., Kahn B.B. The role of skeletal muscle insulin resistance in the pathogenesis of the metabolic syndrome. Proc. Natl. Acad. Sci. USA. 2007;104:12587–12594. doi: 10.1073/pnas.0705408104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M.C., Madiraju A.K., Gassaway B.M., Marcel M., Nasiri A.R., Butrico G., Marcucci M.J., Zhang D., Abulizi A., Zhang X.M. Insulin receptor Thr1160 phosphorylation mediates lipid-induced hepatic insulin resistance. J. Clin. Invest. 2016;126:4361–4371. doi: 10.1172/JCI86013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabøl R., Petersen K.F., Dufour S., Flannery C., Shulman G.I. Reversal of muscle insulin resistance with exercise reduces postprandial hepatic de novo lipogenesis in insulin resistant individuals. Proc. Natl. Acad. Sci. USA. 2011;108:13705–13709. doi: 10.1073/pnas.1110105108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel V.T., Shulman G.I. The pathogenesis of insulin resistance: Integrating signaling pathways and substrate flux. J. Clin. Invest. 2016;126:12–22. doi: 10.1172/JCI77812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel V.T., Liu Z.X., Qu X., Elder B.D., Bilz S., Befroy D., Romanelli A.J., Shulman G.I. Mechanism of hepatic insulin resistance in non-alcoholic fatty liver disease. J. Biol. Chem. 2004;279:32345–32353. doi: 10.1074/jbc.M313478200. [DOI] [PubMed] [Google Scholar]
- Samuel V.T., Liu Z.X., Wang A., Beddow S.A., Geisler J.G., Kahn M., Zhang X.M., Monia B.P., Bhanot S., Shulman G.I. Inhibition of protein kinase Cepsilon prevents hepatic insulin resistance in nonalcoholic fatty liver disease. J. Clin. Invest. 2007;117:739–745. doi: 10.1172/JCI30400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal A.J., Campbell-Sargent C., Mirshahi F., Rizzo W.B., Contos M.J., Sterling R.K., Luketic V.A., Shiffman M.L., Clore J.N. Nonalcoholic steatohepatitis: Association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- Schmitz-Peiffer C., Biden T.J. Protein kinase C function in muscle, liver, and beta-cells and its therapeutic implications for type 2 diabetes. Diabetes. 2008;57:1774–1783. doi: 10.2337/db07-1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple R.K., Sleigh A., Murgatroyd P.R., Adams C.A., Bluck L., Jackson S., Vottero A., Kanabar D., Charlton-Menys V., Durrington P. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J. Clin. Invest. 2009;119:315–322. doi: 10.1172/JCI37432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seppälä-Lindroos A., Vehkavaara S., Häkkinen A.M., Goto T., Westerbacka J., Sovijärvi A., Halavaara J., Yki-Järvinen H. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J. Clin. Endocrinol. Metab. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- Shulman G.I. Cellular mechanisms of insulin resistance. J. Clin. Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann. N Y Acad. Sci. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Szczepaniak L.S., Nurenberg P., Leonard D., Browning J.D., Reingold J.S., Grundy S., Hobbs H.H., Dobbins R.L. Magnetic resonance spectroscopy to measure hepatic triglyceride content: Prevalence of hepatic steatosis in the general population. Am. J. Physiol. Endocrinol. Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- ter Horst K.W., Gilijamse P.W., Koopman K.E., de Weijer B.A., Brands M., Kootte R.S., Romijn J.A., Ackermans M.T., Nieuwdorp M., Soeters M.R., Serlie M.J. Insulin resistance in obesity can be reliably identified from fasting plasma insulin. Int. J. Obes. 2015;39:1703–1709. doi: 10.1038/ijo.2015.125. [DOI] [PubMed] [Google Scholar]
- van der Valk F., Hassing C., Visser M., Thakkar P., Mohanan A., Pathak K., Dutt C., Chauthaiwale V., Ackermans M., Nederveen A. The effect of a diiodothyronine mimetic on insulin sensitivity in male cardiometabolic patients: A double-blind randomized controlled trial. PLoS ONE. 2014;9:e86890. doi: 10.1371/journal.pone.0086890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vatner D.F., Majumdar S.K., Kumashiro N., Petersen M.C., Rahimi Y., Gattu A.K., Bears M., Camporez J.P., Cline G.W., Jurczak M.J. Insulin-independent regulation of hepatic triglyceride synthesis by fatty acids. Proc. Natl. Acad. Sci. USA. 2015;112:1143–1148. doi: 10.1073/pnas.1423952112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M.E., Lammers N.M., Nederveen A.J., van der Graaf M., Heerschap A., Ackermans M.T., Sauerwein H.P., Stroes E.S., Serlie M.J. Hepatic steatosis does not cause insulin resistance in people with familial hypobetalipoproteinaemia. Diabetologia. 2011;54:2113–2121. doi: 10.1007/s00125-011-2157-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong R.J., Cheung R., Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–2195. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- Xia J.Y., Holland W.L., Kusminski C.M., Sun K., Sharma A.X., Pearson M.J., Sifuentes A.J., McDonald J.G., Gordillo R., Scherer P.E. Targeted induction of ceramide degradation leads to improved systemic metabolism and reduced hepatic steatosis. Cell Metab. 2015;22:266–278. doi: 10.1016/j.cmet.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C., Chen Y., Cline G.W., Zhang D., Zong H., Wang Y., Bergeron R., Kim J.K., Cushman S.W., Cooney G.J. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J. Biol. Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.