Abstract
Vector-borne transmitted helminthic zoonosis affects the health and economy of both developing and developed countries. The concept of episystem includes the set of biological, environmental, and epidemiological elements of these diseases in defined geographic and temporal scales. Dirofilariasis caused by different species of the genus Dirofilaria is a disease affecting domestic and wild canines and felines and man, transmitted by different species of culicid mosquitoes. This complexity is increased because Dirofilaria species harbor intracellular symbiont Wolbachia bacteriae, which play a key role in the embryogenesis and development of dirofilariae and in the inflammatory pathology of the disease. In addition, the vector transmission makes the dirofilariasis susceptible to the influence of the climate and its variations. The present review addresses the analysis of dirofilariasis from the point of view of the episystem, analyzing the complex network of interactions established between biological components, climate, and factors related to human activity, as well as the different problems they pose. The progress of knowledge on human and animal dirofilariasis is largely due to the multidisciplinary approach. Nevertheless, different aspects of the disease need to continue being investigated and cooperation between countries and specialists involved should be intensified.
1. Introduction
Vector-borne zoonotic transmitted diseases cause deaths and economic losses in human and domestic animal populations around the world, affecting seriously the social and economic development of many countries [1, 2]. Dirofilariasis is a helminthic zoonosis caused by filarial species of the genus Dirofilaria transmitted by hematophagous dipterans that primarily parasitize domestic dogs, cats, and other species of wild mammals [3]. Although some Dirofilaria species cause relatively benign processes, others such as D. immitis, responsible for cardiopulmonary dirofilariasis, pose a risk to the life of affected animals, being considered the most important parasitic disease of dogs in the USA [4]. Since many of the vector species feed indistinctly on animal reservoirs and on humans, where animal dirofilariasis exists, human infections occur [5]. Human dirofilariasis has historically been considered a minor accidental disease, but the dramatic increase in cases of some clinical variants in recent years has made it nowadays considered an emerging disease in Europe [6–8]. Additionally, its clinical importance is increasing due to the severity of some cases [9, 10]. The concept of vector-borne disease episystem includes the set of biological and environmental elements, as well as the epidemiological aspects of these diseases in defined geographic and temporal scales. Because its intrinsic nature, the episystems are in constant change, reflecting the adaptations of all its components to new situations [11]. An analysis of dirofilariasis from the point of view of the episystem allows us to understand its complexity and the consequences that this implies, encompassing in all its amplitude the need for a multidisciplinary approach.
2. The Episystem of Dirofilariasis
2.1. Biological Complexity
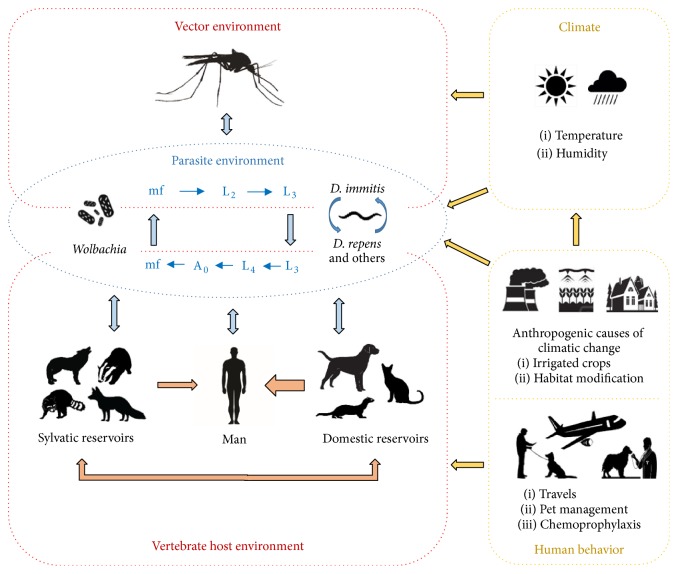
The biological component of the episystem of dirofilariasis is extremely complex as Dirofilaria spp. parasitize a wide range of vertebrate species and vectors (Figure 1), all of which have their own level of adaptation. During a blood meal, vectors deposit a hemolymph on the wound, which carries infective third-larval stage (L3) of Dirofilaria that penetrates the host's skin by their own. L3 successively molt to L4 and adults, which are located in the circulatory system and subcutaneous/ocular and many other tissues, depending on the species. In canines, female worms release microfilariae (mf) into the bloodstream, from where they are ingested by vectors during the blood meal, becoming infective after 2 additional molts. Nevertheless, some infected dogs present occult or amicrofilaremic infections. In felids and other hosts, microfilaremia is either not present or transitory and present at low levels, while in humans the worms do not usually reach maturity [6]. One or several Dirofilaria species may be present, depending on the area being considered (Figure 2), of which D. immitis and D. repens are considered the most significant owing to their wide distribution and clinical importance. On the other hand, the actual prevalence of species such as D. tenuis, D. ursi, D. subdermata, and D. striata in their natural wild hosts is not known [12, 13]. It should also be taken into account that L3, L4, adults, and mf can coexist in vertebrate hosts and can present different anatomical localizations, immune repertoires, and survival strategies; thus each infected host actually faces several organisms with different biological capabilities. Furthermore, all of the developmental stages of Dirofilaria harbor intracellular symbiotic Wolbachia bacteriae that are essential for the molting and embryogenesis of the worms [14]. Filarial death causes the release of the bacteriae, which establish a direct relationship with the host, a key fact in the progression of dirofilariasis [6].
Figure 1.
The episystem of dirofilariasis. Main interactions among organisms involved, climate, and human-derived behavior factors.
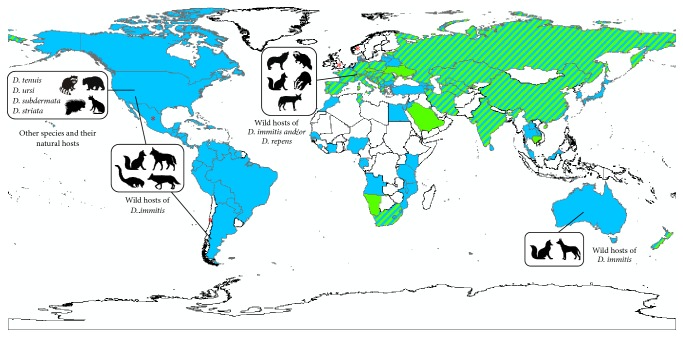
Figure 2.
Geographic distribution of the different species of Dirofilaria in the animal hosts in the world. D. immitis in pets (blue); D. repens in pets (green); D. immitis and D. repens in pets (striped); without information (white); (∗) sporadic subcutaneous infections.
Pets, such as dogs, cats, and ferrets, and a wide range of wild carnivorous species are hosts for D. immitis and/or D. repens [3, 6, 15]. The other species exclusively parasitize wild animals like raccoons, porcupines, bears, and wild felids [12]. D. immitis and D. repens show a high prevalence in pets and wild reservoirs and have a high zoonotic potential [6, 16], while those species that only infect wild reservoirs are less frequently or sporadically reported in humans [12]. Dirofilaria species are transmitted by culicid mosquitoes, except D. ursi which uses Simulium spp. as vectors. Different species of the genera Aedes, Anopheles, Culex, Culiseta, Mansonia, and Armigeres have been implicated in the transmission of D. immitis and D. repens [17].
2.2. Extrinsic Factors
Climate and human activity influence the biological life cycle of Dirofilaria spp. (Figure 1). Given that mosquitoes are ectothermic organisms with a life cycle linked to water, climatic factors, mainly temperature and precipitation/humidity, affect their development, population density, period of activity, and species diversity. Also, the development of L3 larvae depends on environmental temperature (extrinsic incubation), which includes a period of 8 to 20 days with temperatures ranging from 22° to 30°C. Below 14°C development arrests transiently until temperature reaches the threshold again [6]. On the other hand, urbanistic demands, the construction of irrigation systems, and water storage areas, the use, or not, of chemoprophylaxis and the transport and import of pets between endemic and nonendemic areas contribute to environmental changes and to the introduction of infected reservoirs in nonendemic areas, changing prevalence [6, 18–21]. The hunting pressure on potential wild reservoirs of Dirofilaria spp. and the anthropogenic influence on the natural environment can have epidemiological consequences, affecting the circulation of filariae between wild reservoirs, pets, and humans [15].
2.3. Interactions
The interactions established between Dirofilaria spp. and their vertebrate hosts, between developing larvae and vectors, between the different species of Dirofilaria, and between the developmental stages within the same species all contribute to the regulation of the parasite population and as a consequence to its transmission. In dogs, live D. immitis worms stimulate a permissive humoral Th2 response that has been associated with microfilaremic infections. When adults and mf die, the released Wolbachia bacteriae activate a change towards a Th1-type response, which in addition to causing inflammation and deterioration of the vascular environment is also associated with suppression of mfs [22, 23]. Moreover, in general, dogs are able to maintain the adult population at levels compatible with their own survival, eliminating a significant part of the L3 larvae acquired by reinfections [24]. Like other parasites D. immitis manipulates the immune system and various physiological processes of the host for their own benefit [25]. The elimination of significant amounts of surface antigens by the L3 larvae, the presence of proteases that lyse antibodies on the surface of the mf, the masking capacity, the variety of the antistress, detoxifying and antioxidants proteins, and antithrombotic capacity of the adult worms are mechanisms that contribute to the survival of the parasite [6, 26]. However, the cat is a less permissive host, with an intense proinflammatory immune response, which, on the one hand, can be lethal for itself and, on the other hand, impede or limit the survival of the adult worms and the production of mf [3]. Within wild reservoirs, the coyote, jackal, fox, wolf, and the raccoon dog can develop stable D. immitis and/or D. repens microfilarial infections, while other species only develop amicrofilaremic infections [15, 27, 28]. Given that the significance of a species as a reservoir is determined not only by the percentage of infected individuals but also by their survival and capacity to sustain the parasite long-term reproduction, the adaptations established between the dog and D. immitis and D. repens make this host an ideal reservoir. Among wild hosts, those that develop microfilaremic infections and show behavior that puts them in frequent contact with humans and pets environment, such as coyotes, foxes, jackals, or raccoon dogs, can be considered dangerous reservoirs [15, 29].
There are genetic differences both inter- and intraspecific that regulate the susceptibility and resistance of the mosquitoes to Dirofilaria [17, 30]. Furthermore, the invasion of the Malpighian tubules by the Dirofilaria larvae and their migration to the mouthparts is crucial for the survival of the mosquitoes. These have different structures and mechanisms that allow them to control the number of L3 larvae that complete their development. The cibarial armature, the coagulation of blood, the peritrophic membrane, the hemolymph defensins, and their melanization capacity eliminate part of developing larvae [17]. The percentage of infected vectors that survive the infection, the parasitic load that they are able to withstand, and the prevalence of infection determine the flow of L3 towards the vertebrate hosts.
D. immitis and D. repens coinfections in hosts and vectors have been described [15, 31]. However, there is little information regarding the interactions between both species coinciding within the same host. In experimental infections in dogs it was observed that when D. repens was the first species inoculated, its presence significantly decreased the number of D. immitis worms progressing to the adult stage when it was later introduced; this finding was not observed when the order of infection was reversed. This interaction, probably immune in nature, can influence the different patterns of prevalence observed [32]. Nonetheless, the fact that both species can simultaneously complete their life cycles in the same host suggests the existence of competitive exclusion, as it seems to occur in human filariae in Africa [33].
3. Epidemiology
With respect to animal dirofilariasis, a great amount of the epidemiological information refers to canine dirofilariasis, while the information regarding domestic cats and wild reservoirs is, in general, limited. Human dirofilariasis is studied from two different perspectives, which have provided complementary information: seroepidemiological studies and the retrospective review of clinical cases previously published [34]. Various seroepidemiological studies have found significant seroprevalence of anti-Dirofilaria antibodies, which suggests a high risk of infection in human populations living in endemic areas [35–38]. The retrospective review of clinical cases highlights the actual incidence of the disease. Although it is widely accepted that in many countries human dirofilariasis is underdiagnosed, a dramatic increase in the level of incidence worldwide has been confirmed and is mainly subcutaneous/ocular in nature (Figure 3(a)) [6, 29, 39].
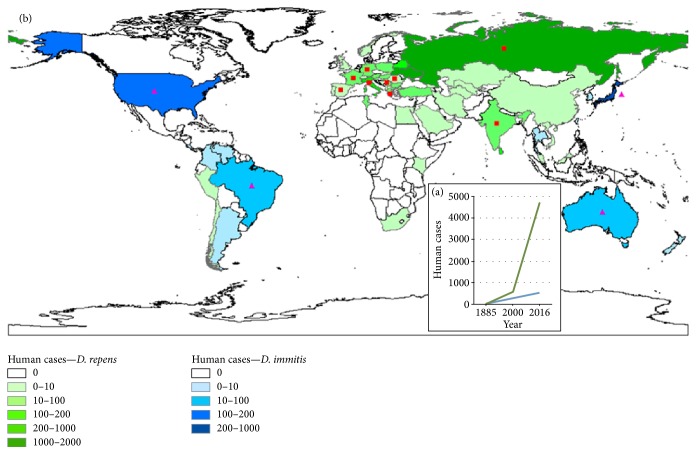
Figure 3.
Changes in the incidence of human dirofilariasis reported cases (a). Geographic distribution of human dirofilariasis (reported cases) (b). Pulmonary dirofilariasis (blue); subcutaneous/ocular dirofilariasis (green); sporadic cases of subcutaneous/ocular dirofilariasis in areas where pulmonary dirofilariasis predominates (fuchsia triangles); sporadic cases of pulmonary dirofilariasis in areas where subcutaneous/ocular dirofilariasis predominates (red squares).
3.1. Geographic Distribution and Prevalence
3.1.1. Dirofilariasis in the Animal Hosts
The episystem of dirofilariasis within Europe and Asia is characterized by the presence of D. immitis and D. repens, which are sympatric in most countries, while in a few only one of them has been reported [13] (Figure 2). The highest prevalence of D. immitis was found in the Canary Islands and Madeira and in Mediterranean countries (22–40%). Prevalence of D. repens ranged from 23 to 49% in Southwestern Russia and from 25% to 38% in some central and northern European countries [6, 40–42]. In Iran, China, and India, prevalence rates between 15 and 60% for both species have been reported [6, 43–47]. The prevalence of D. immitis has increased in some areas of India [45, 48] but in Japan has decreased from 46%, in 2001, to 23%, in 2010 [49]. D. immitis has been found in feline populations in Portugal, Spain, and Italy, with prevalence rates between 3 and 27%, and there have been frequent reports in France [50–52]. Its presence has also been increasingly reported in European populations of foxes (3.7%–35%), jackals (7.7%–23.3%), and raccoon dogs (31.1%) and occasionally in wolves, while D. repens has been found in foxes, wolves, jackals, and badgers with prevalence rates that come close to 10% in some of the hosts [15, 53–55].
In the Americas D. immitis predominates, having been detected in the majority of the countries (Figure 2). The highest prevalence has been reported in the Eastern states of the USA, the Caribbean coast of Mexico, Caribbean Islands, and areas of Brazil and Argentina (20.4% to 74%) [6, 29, 56, 57]. Recent studies have described the notable increase of the prevalence in some Western states of USA [4], Mexico [58], Colombia [59], and Argentina [60]. Feline infections caused by D. immitis have been identified in canine endemic areas of the USA, Canada, Argentina, Brazil, and Venezuela. In the USA, the prevalence ranges between 3 and 19% and is higher in areas where canine prevalence is higher [61]. D. immitis is also frequently detected in coyotes, foxes, and hybrids of both species and, occasionally, in other species [3, 62]. The prevalence in coyotes widely varies between 17% in Illinois [63] and 100% in Texas [62], and a spreading of the infection in the populations of California has been detected [27]. Also, infections in the maned wolf (Chrysocyon brachyurus) in Bolivia [64] and in the coati in Argentina [65] were reported. There is no data about the prevalence of D. tenuis, D. ursi, D. subdermata, and D. striata in wild hosts. Recently, the first D. repens infection in canines was reported in Mexico [66] and also one case in Chile, where the causal agent was genetically similar, but not identical, to D. repens [67].
D. immitis also predominates in the canine populations of Africa and Australia (Figure 2). In Africa, epidemiological information lacks in many countries. Prevalence of D. immitis is between 1% and 15% [6, 68, 69] and between 3 and 6% for D. repens [6, 70–72]. D. immitis is endemic in the South Eastern Australia [73], where foxes in peri-urban areas and dingoes in areas with a low population density are the wild reservoirs [74, 75]. In New Zealand, infections caused by D. immitis and D. repens have been reported, probably being imported from Australia [76].
3.1.2. Human Dirofilariasis
Human infections caused by D. repens widely predominate in Eurasia (Figure 3(b)), where approximately 4490 cases of subcutaneous/ocular dirofilariasis have been described. Of these, 4250 have occurred in Europe, with the highest incidences occurring in Ukraine (1934 cases), Russia (1440), Italy (326), and Belorussia (131), and with only 35 pulmonary cases attributed to D. immitis [6, 39, 77]. In Asia (Figure 3(b)), Sri Lanka, with 135 cases [78, 79], and India, with at least 100 subcutaneous/ocular cases and 3 pulmonary cases [80], are the countries with the highest level of incidence for human subcutaneous/ocular dirofilariasis. In other countries very few cases have been reported [43, 81–83]. Pulmonary dirofilariasis caused by D. immitis predominates in Japan, with 280 registered cases [5, 84–86] by only 3 subcutaneous cases [87].
In the Americas 175 cases of pulmonary infection in humans have been approximately reported, located in the USA (119 cases) [6, 88, 89] and Brazil (close to 50 cases) [6, 90], with sporadic reports in Costa Rica, Colombia, Venezuela, and Argentina [6] (Figure 3(b)). Only 34 cases of subcutaneous/ocular infection have been registered, of which 30 are from the USA and Canada, caused by D. tenuis [6, 91, 92], D. ursi or D. subdermata [6, 93, 94], D. striata [95], Dirofilaria spp. [96–98], and D. immitis [99]. Sporadic cases of subcutaneous and ocular infection have been reported in Chile, Peru, and Brazil [6, 90, 100].
Sixteen human cases have been recorded in Tunisia, 15 caused by D. repens and 1 caused by D. immitis [101], and other sporadic cases documented in South Africa and Egypt [102, 103]. In Australia 20 cases of pulmonary infection caused by D. immitis have been reported [6] and 1 case in New Zealand [104] (Figure 3(b)).
3.2. Climatic Change and Spreading of Dirofilariasis: Prediction Models
Industrial activity is modifying the climate, significantly increasing the temperature and global average precipitation with respect to the figures of the preindustrial age [105]. Vector-borne diseases are among the natural systems more sensitive to climate change, such as dirofilariasis, to be affected in different ways: increase in vector density, in the duration of their annual activity period and aggressiveness, the introduction of invasive species of competent vectors in endemic areas, and the shortening of the extrinsic incubation period of the parasite. Despite serious gaps within the information concerning the impact of climate change on the distribution and emergence/reemergence of pathogens and vectors and the fact that many of the results are debatable [106, 107], there are, however, studies that reasonably show a relationship between climate change and the alteration of the epidemiological situation. The average temperature recorded in Europe, within 2002 and 2011, is 1.3°C higher than during the preindustrial age [107]. Epidemiological studies have indicated that before 2000 dirofilariasis was almost exclusively associated with the southern European countries. Nevertheless, after this date dirofilariasis extended towards colder central and northern countries, with autochthonous cases having been reported in humans until a latitude of 61°N in Russia [6, 39]. In North America, where the temperature has increased between 1.3° and 1.9°F above those registered in 1895 [108], dirofilariasis has been gradually expanding over the decades [4, 16]. One recent study showed a significant relationship between the prevalence of canine dirofilariasis and temperature and precipitation, among other factors [109]. Exotic mosquito species, such as Aedes albopictus (the tiger mosquito), introduced in Europe and America through commercial activities, have rapidly expanded in many areas where dirofilariasis is endemic. One similar situation occurred with Ae. koreicus in Italy and Switzerland [110–112]. Both are competent vectors of Dirofilaria spp. with diurnal activity, complementing the afternoon or night activity of native species [17].
One of the fundamental objectives in the study of the relationship between climate and health is to create tools that allow the prediction of change, with the aim to prevent the outcomes. The integration of information obtained from numerous sources, such as geographic information systems (GIS), global positioning (GPS), remote sensing (RS) satellite systems, and epidemiological and climatic records, as well as the improvement of analysis software, has made achieving this objective possible [113]. With respect to dirofilariasis, the majority of the models published are based on the Growing Degree Days (GDD) concept, the accumulated heat needed to complete the extrinsic incubation of the Dirofilaria larvae in the life period of the vectors [114]. These models accurately predicted that the summer temperatures of nonendemic cold areas had reached a sufficient level for the extrinsic incubation of the larvae, allowing the calculation of the number of annual generations of Dirofilaria, and the length of the transmission period, at different geographic scales [7, 16, 114–116]. Additionally, they indicated the risk of introduction of Ae. albopictus and its hypothetical period of activity in nonendemic areas such as the UK [117, 118]. Other models incorporate local geo-environmental factors like the presence of irrigation in dry climate areas that refine predictions in Spain [20]. Recently, a complex hierarchical regression model relating multiple geo-climatic, social, and biological factors, as well as the prevalence of D. immitis in canine populations in the USA, county by county, has been created [4]. The reliability of the predications should be validated using real distribution and prevalence data. In the case of the USA, prevalence is one of the factors considered, thanks to the millions of diagnoses carried out in a standardized way throughout the country. In areas or countries where the diagnostic results do not permit validation, it can become complicated due to the lack or scarcity of epidemiological data, or validation is achieved “a posteriori” as new epidemiological data appear, years after the creation of the model. Despite that modelling is not an exact science, it has been accepted that the information generated could provide very valuable guidance for the application of programs aimed at controlling dirofilariasis [105].
4. Conclusions
Dirofilariasis is an extremely complex problem, primarily veterinary, but with an undoubted impact on human health and wildlife. In addition, in each continent there are biological and epidemiological peculiarities, which give each episystem its own characteristics. The progress of knowledge and management of dirofilariasis that has occurred primarily in the USA, Europe, and Japan has been possible thanks to a multidisciplinary approach to which parasitologists, veterinarians, doctors of different specialties, molecular biologists, computer scientists, mathematicians, and meteorologists have contributed. For this reason, dirofilariasis can be considered as a paradigm of the global health approach advocated by the one health concept. However, animal dirofilariasis continues to expand in many areas and cases of human dirofilariasis are reported with increasing frequency in more and more countries, while in others the disease is virtually unknown by specialists. In addition to future technical advances that will lead to the acquisition of more data, standardization of epidemiological surveillance procedures at the global level is key for the management improvements of dirofilariasis. The proven experience of societies such as the American Heartworm Society and more recently the European Society of Dirofilariasis and Angiostrongylosis, among others, can contribute to achieving the ultimate goal of effective global disease control.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Faburay B. The case for a "one health" approach to combating vector-borne diseases. Infection Ecology and Epidemiology. 2015;5(1) doi: 10.3402/iee.v5.28132.28132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Day M. J. One health: the importance of companion animal vector-borne diseases. Parasites and Vectors. 2011;4(1, article 49) doi: 10.1186/1756-3305-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCall J. W., Genchi C., Kramer L. H., Guerrero J., Venco L. Heartworm disease in animals and humans. Advances in Parasitology. 2008;66:193–285. doi: 10.1016/S0065-308X(08)00204-2. [DOI] [PubMed] [Google Scholar]
- 4.Bowman D. D., Liu Y., McMahan C. S. Forecasting United States heartworm Dirofilaria immitis prevalence in dogs. Parasites and Vectors. 2016;9:p. 540. doi: 10.1186/s13071-016-1804-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simón F., Morchón R., González-Miguel J., Marcos-Atxutegi C., Siles-Lucas M. What is new about animal and human dirofilariosis? Trends in Parasitology. 2009;25(9):404–409. doi: 10.1016/j.pt.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 6.Simón F., Siles-Lucas M., Morchón R., et al. Human and animal dirofilariasis: the emergence of a zoonotic mosaic. Clinical Microbiology Reviews. 2012;25(3):507–544. doi: 10.1128/cmr.00012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kartashev V., Afonin A., González-Miguel J., et al. Regional warming and emerging vector-borne zoonotic dirofilariosis in the Russian Federation, Ukraine, and other post-soviet states from 1981 to 2011 and projection by 2030. BioMed Research International. 2014;2014:11. doi: 10.1155/2014/858936.858936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.EASAC [European Academies Science Advisory Council] Climate Change and Infectious Diseases in Europe. 2010. http://www.easac.eu/home/reports-and-statements/detail-view/article/climate-chan.html. [Google Scholar]
- 9.Ilyasov B., Kartashev V., Bastrikov N., Morchón R., González-Miguel J., Simón F. Delayed diagnosis of dirofilariasis and complex ocular surgery, Russia. Emerging Infectious Diseases. 2013;19(2):326–328. doi: 10.3201/eid1902.120464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ilyasov B., Kartashev V., Bastrikov N., et al. Thirty cases of human subcutaneous dirofilariasis reported in Rostov-on-Don (Southwestern Russian Federation) Enfermedades Infecciosas y Microbiologia Clinica. 2015;33(4):233–237. doi: 10.1016/j.eimc.2014.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Tabachnick W. J. Challenges in predicting climate and environmental effects on vector-borne disease episystems in a changing world. Journal of Experimental Biology. 2010;213(6):946–954. doi: 10.1242/jeb.037564. [DOI] [PubMed] [Google Scholar]
- 12.Orihel T. C., Eberhard M. L. Zoonotic filariasis. Clinical Microbiology Reviews. 1998;11:366–381. doi: 10.1128/cmr.11.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Genchi C., Guerrero J., McCall J. W., Venco L. Epidemiology and prevention of Dirofilaria infections in dogs and cats. In: Genchi C., Rinaldi L., Cringoli G., editors. Dirofilaria immitisD. repens. Naples, Italy: Rolando Editore; 2007. pp. 147–161. [Google Scholar]
- 14.Luck A. N., Evans C. C., Riggs M. D., et al. Concurrent transcriptional profiling of Dirofilaria immitis and its Wolbachia endosymbiont throughout the nematode life cycle reveals coordinated gene expression. BMC Genomics. 2014;15:p. 1041. doi: 10.1186/1471-2164-15-1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kravchenko V., Itin G., Kartashev V., et al. Dirofilaria immitis and D. repens in sylvatic reservoirs of Krasnodar Krai (Russian Federation) Veterinary Parasitology: Regional Studies and Reports. 2016;6:35–38. doi: 10.1016/j.vprsr.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 16.Genchi C., Kramer L. H., Rivasi F. Dirofilarial infections in Europe. Vector-Borne and Zoonotic Diseases. 2011;11(10):1307–1317. doi: 10.1089/vbz.2010.0247. [DOI] [PubMed] [Google Scholar]
- 17.Cancrini G., Gabrielli S. Vectors of Dirofilaria nematodes: biology, behavior and host/parasite relationships. In: Genchi C., Rinaldi L., Cringoli G., editors. Dirofilaria immitisD. repens. Naples, Italy: Rolando Editore; 2007. p. p. 211. [DOI] [Google Scholar]
- 18.Zimmerman G. L., Knapp S. E., Foreyt W. J., Erekson N. T., Mackenzie G. Heartworm infections in dogs in Northwestern United States and British Columbia, Canada. In: Soll M. D., editor. Proceedings of the Heartworm Symposium '92; 1992; Batavia, Illinois, USA. American Heartworm Society; pp. 15–20. [Google Scholar]
- 19.Levy J. K., Crawford P. C., Lappin M. R., et al. Infectious diseases of dogs and cats on Isabela Island, Galapagos. Journal of Veterinary Internal Medicine. 2008;22(1):60–65. doi: 10.1111/j.1939-1676.2007.0034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simón L., Afonin A., López-Díez L. I., et al. Geo-environmental model for the prediction of potential transmission risk of Dirofilaria in an area with dry climate and extensive irrigated crops: the case of Spain. Veterinary Parasitology. 2014;200(3-4):257–264. doi: 10.1016/j.vetpar.2013.12.027. [DOI] [PubMed] [Google Scholar]
- 21.Montoya-Alonso J. A., Carretón E., Morchón R., Silveira-Viera L., Falcón Y., Simón F. The impact of the climate on the epidemiology of Dirofilaria immitis in the pet population of the Canary Islands. Veterinary Parasitology. 2016;216:66–71. doi: 10.1016/j.vetpar.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Kramer L., Simón F., Tamarozzi F., Genchi M., Bazzocchi C. Is Wolbachia complicating the pathological effects of Dirofilaria immitis infections? Veterinary Parasitology. 2005;133(2-3):133–136. doi: 10.1016/j.vetpar.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 23.Morchón R., Bazzocchi C., López-Belmonte J., et al. iNOs expression is stimulated by the major surface protein (rWSP) from Wolbachia bacterial endosymbiont of Dirofilaria immitis following subcutaneous injection in mice. Parasitology International. 2007;56(1):71–75. doi: 10.1016/j.parint.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Simón F., Genchi C., Prieto G., Allende E. Immunity in the vertebrate hosts. In: Simón F., Genchi C., editors. Heartworm Infection in Humans and Animals. Salamanca, Spain: Ediciones Universidad de Salamanca; 2001. p. p. 218. [Google Scholar]
- 25.Poulin R., Maure F. Host manipulation by parasites: a look back before moving forward. Trends in Parasitology. 2015;31(11):563–570. doi: 10.1016/j.pt.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 26.González-Miguel J., Siles-Lucas M., Kartashev V., Morchón R., Simón F. Plasmin in parasitic chronic infections: friend or foe? Trends in Parasitology. 2016;32(4):325–335. doi: 10.1016/j.pt.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 27.Sacks B. N., Caswell-Chen E. P. Reconstructing the spread of Dirofilaria immitis in California coyotes. Journal of Parasitology. 2003;89(2):319–323. doi: 10.1645/0022-3395(2003)089[0319:RTSODI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 28.Kido N., Wada Y., Takahashi M., Kamegaya C., Omiya T., Yamamoto Y. Prevalence of Dirofilaria immitis infection in living raccoon dogs assessed by hematological examination. Journal of Veterinary Medical Science. 2011;73(6):845–847. doi: 10.1292/jvms.10-0512. [DOI] [PubMed] [Google Scholar]
- 29.Lee A. C., Montgomery S. P., Theis J. H., Blagburn B. L., Eberhard M. L. Public health issues concerning the widespread distribution of canine heartworm disease. Trends in Parasitology. 2010;26(4):168–173. doi: 10.1016/j.pt.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 30.Morchón R., Bargues M. D., Latorre J. M., et al. Haplotype H1 of Culex pipiens implicated as natural vector of Dirofilaria immitis in an endemic area of Western Spain. Vector-Borne and Zoonotic Diseases. 2007;7(4):653–658. doi: 10.1089/vbz.2007.0124. [DOI] [PubMed] [Google Scholar]
- 31.Latrofa M. S., Dantas-Torres F., Annoscia G., Genchi M., Traversa D., Otranto D. A duplex real-time polymerase chain reaction assay for the detection of and differentiation between Dirofilaria immitis and Dirofilaria repens in dogs and mosquitoes. Veterinary Parasitology. 2012;185(2-4):181–185. doi: 10.1016/j.vetpar.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 32.Genchi C., Basano F. S., Bandi C., et al. Factors influencing the spread of heartworms in Italy: interaction between Dirofilaria immitis and Dirofilaria repens. Proceedings of the Heartworm Symposium ’95; 1995; Batavia, Italy. American Heartworm Society; pp. 65–71. [Google Scholar]
- 33.Molyneux D. H., Mitre E., Bockarie M. J., Kelly-Hope L. A. Filaria zoogeography in Africa: ecology, competitive exclusion, and public health relevance. Trends in Parasitology. 2014;30(4):163–169. doi: 10.1016/j.pt.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Simón F., López-Belmonte J., Marcos-Atxutegi C., Morchón R., Martín-Pacho J. R. What is happening outside North America regarding human dirofilariasis? Veterinary Parasitology. 2005;133(2-3):181–189. doi: 10.1016/j.vetpar.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 35.Simón F., Muro A., Cordero M., Martin J. A seroepidemiologic survey of human dirofilariosis in Western Spain. Tropical Medicine and Parasitology. 1991;42:106–108. [PubMed] [Google Scholar]
- 36.Espinoza E., Cordero M., Muro A., Lorente F., Simón F. Anti-Dirofilaria immitis IgE: seroepidemiology and seasonal variation in an exposed human population. Tropical Medicine and Parasitology. 1993;44:172–176. [PubMed] [Google Scholar]
- 37.Vieira C., Vélez I. D., Montoya M. N., et al. Dirofilaria immitis in Tikuna Indians and their dogs in the Colombian Amazon. Annals of Tropical Medicine and Parasitology. 1998;92(1):123–125. doi: 10.1080/00034983.1998.11813270. [DOI] [PubMed] [Google Scholar]
- 38.Tasić-Otašević S. A., Gabrielli S. V., Tasić A. V., et al. Seroreactivity to Dirofilaria antigens in people from different areas of Serbia. BMC Infectious Diseases. 2014;14(1, article 68) doi: 10.1186/1471-2334-14-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kartashev V., Tverdokhlebova T., Korzan A., et al. Human subcutaneous/ocular dirofilariasis in the Russian Federation and Belarus, 1997–2013. International Journal of Infectious Diseases. 2015;33:209–211. doi: 10.1016/j.ijid.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 40.Demiaszkiewicz A. W., Polańczyk G., Osińska B., et al. The prevalence and distribution of Dirofilaria repens in dogs in the Mazovian Province of central-eastern Poland. Annals of Agricultural and Environmental Medicine. 2014;21(4):701–704. doi: 10.5604/12321966.1129918. [DOI] [PubMed] [Google Scholar]
- 41.Bajer A., Rodo A., Mierzejewska E. J., Tołkacz K., Welc-Faleciak R. The prevalence of Dirofilaria repens in cats, healthy dogs and dogs with concurrent babesiosis in an expansion zone in central Europe. BMC Veterinary Research. 2016;12(1):p. 183. doi: 10.1186/s12917-016-0816-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miterpáková M., Iglódyová A., Čabanová V., Stloukal E., Miklisová D. Canine dirofilariosis endemic in central Europe—10 years of epidemiological study in Slovakia. Parasitology Research. 2016;115(6):2389–2395. doi: 10.1007/s00436-016-4989-2. [DOI] [PubMed] [Google Scholar]
- 43.To K. K. W., Wong S. S. Y., Poon R. W. S., et al. A novel Dirofilaria species causing human and canine infections in Hong Kong. Journal of Clinical Microbiology. 2012;50(11):3534–3541. doi: 10.1128/JCM.01590-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khedri J., Radfar M. H., Borji H., Azizzadeh M., Akhtardanesh B. Canine Heartworm in southeastern of Iran with review of disease distribution. Iranian Journal of Parasitology. 2014;9(4):560–567. [PMC free article] [PubMed] [Google Scholar]
- 45.Borthakur S. K., Deka D. K., Islam S., Sarma D. K., Sarmah P. C. Prevalence and molecular epidemiological data on Dirofilaria immitis in dogs from Northeastern States of India. Scientific World Journal. 2015;2015:7. doi: 10.1155/2015/265385.265385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leishangthem G. D., Choudhury A., Singh N. D., Bhosale S. Subcutaneous Dirofilaria repens infestation in non-descript canines. Journal of Parasitic Diseases. 2016;40(2):558–561. doi: 10.1007/s12639-014-0508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang S., Zhang N., Zhang Z., et al. Prevalence of Dirofilaria immitis infection in dogs in Henan province, central China. Parasite. 2016;23:p. 43. doi: 10.1051/parasite/2016054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Borthakur S. K., Deka D. K., Islam S., Sarmah P. C. Occult dirofilariosis in dogs of North Eastern region in India. Journal of Arthropod-Borne Diseases. 2015;10(1):92–97. [PMC free article] [PubMed] [Google Scholar]
- 49.Oi M., Yoshikawa S., Ichikawa Y., Nakagaki K., Matsumoto J., Nogami S. Prevalence of Dirofilaria immitis among shelter dogs in Tokyo, Japan, after a decade: Comparison of 1999–2001 and 2009–2011. Parasite. 2014;21:p. 10. doi: 10.1051/parasite/2014008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Traversa D., Di Cesare A., Conboy G. Canine and feline cardiopulmonary parasitic nematodes in Europe: emerging and underestimated. Parasites and Vectors. 2010;3(1, article 62) doi: 10.1186/1756-3305-3-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Montoya-Alonso J. A., Carretón E., Simón L., et al. Prevalence of Dirofilaria immitis in dogs from barcelona: validation of a geospatial prediction model. Veterinary Parasitology. 2015;212(3-4):456–459. doi: 10.1016/j.vetpar.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 52.Vieira L., Silvestre-Ferreira A. C., Fontes-Sousa A. P., et al. Seroprevalence of heartworm (Dirofilaria immitis) in feline and canine hosts from central and northern Portugal. Journal of Helminthology. 2015;89(5):625–629. doi: 10.1017/S0022149X14000352. [DOI] [PubMed] [Google Scholar]
- 53.Marconcini A., Magi M., Macchioni G., Sassetti M. Filariosis in foxes in Italy. Veterinary Research Communications. 1996;20(4):316–319. doi: 10.1007/BF00366537. [DOI] [PubMed] [Google Scholar]
- 54.Magi M., Calderini P., Gabrielli S., et al. Vulpes vulpes: a possible wild reservoir for zoonotic filariae. Vector-Borne and Zoonotic Diseases. 2008;8(2):249–252. doi: 10.1089/vbz.2007.0207. [DOI] [PubMed] [Google Scholar]
- 55.Ćirović D., Penezić A., Pavlovicv I., et al. First records of dirofilaria repens in Wild Canids from the region of central Balkan. Acta Veterinaria Hungarica. 2014;62(4):481–488. doi: 10.1556/AVet.2014.021. [DOI] [PubMed] [Google Scholar]
- 56.Little S. E., Beall M. J., Bowman D. D., Chandrashekar R., Stamaris J. Canine infection with Dirofilaria immitis, Borrelia burgdorferi, Anaplasma spp., and Ehrlichia spp. in the United States, 2010–2012. Parasites and Vectors. 2014;7(1, article 257) doi: 10.1186/1756-3305-7-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barrett A. W., Little S. E. Vector-borne infections in tornado-displaced and owner-relinquished dogs in Oklahoma, USA. Vector-Borne and Zoonotic Diseases. 2016;16(6):428–430. doi: 10.1089/vbz.2015.1923. [DOI] [PubMed] [Google Scholar]
- 58.Movilla R., García C., Siebert S., Roura X. Countrywide serological evaluation of canine prevalence for Anaplasma spp., Borrelia burgdorferi (sensu lato), Dirofilaria immitis and ehrlichia canis in Mexico. Parasites and Vectors. 2016;9(1):p. 421. doi: 10.1186/s13071-016-1686-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Labarthe N., Guerrero J. Epidemiology of heartworm: what is happening in South America and Mexico? Veterinary Parasitology. 2005;133(2-3):149–156. doi: 10.1016/j.vetpar.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 60.Cuervo P. F., Fantozzi M. C., Di Cataldo S., Cringoli G., Mera y Sierra R., Rinaldi L. Analysis of climate and extrinsic incubation of Dirofilaria immitis in southern South America. Geospatial Health. 2013;8(1):175–181. doi: 10.4081/gh.2013.64. [DOI] [PubMed] [Google Scholar]
- 61.Newcombe K. M., Ryan W. G. Prevalence of feline heartworm disease—a global review. Proceedings of the WSAVA; Granada, Spain. [Google Scholar]
- 62.Pence D. B., Custer J. W., Carley C. J. Ectoparasites of wild canids from the Gulf Coastal Prairies of Texas and Louisiana. Journal of Medical Entomology. 1981;18(5):409–412. doi: 10.1093/jmedent/18.5.409. [DOI] [PubMed] [Google Scholar]
- 63.Nelson T. A., Gregory D. G., Laursen J. R. Canine heartworms in coyotes in Illinois. Journal of Wildlife Diseases. 2003;39(3):593–599. doi: 10.7589/0090-3558-39.3.593. [DOI] [PubMed] [Google Scholar]
- 64.Bronson E., Emmons L. H., Murray S., Dubovi E. J., Deem S. L. Serosurvey of pathogens in domestic dogs on the border of Noël Kempff Mercado National Park, Bolivia. Journal of Zoo and Wildlife Medicine. 2008;39(1):28–36. doi: 10.1638/2006-0046.1. [DOI] [PubMed] [Google Scholar]
- 65.Vezzani D., Eiras D. F., Wisnivesky C. Dirofilariasis in Argentina: historical review and first report of Dirofilaria immitis in a natural mosquito population. Veterinary Parasitology. 2006;136(3-4):259–273. doi: 10.1016/j.vetpar.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 66.Ramos-López S., León-Galván M. F., Salas-Alatorre M., Lechuga-Arana A. A., Valencia-Posadas M., Gutiérrez-Chávez A. J. First molecular identification of Dirofilaria repens in a dog blood sample from Guanajuato, Mexico. Vector-Borne and Zoonotic Diseases. 2016;16(11):734–736. doi: 10.1089/vbz.2016.1948. [DOI] [PubMed] [Google Scholar]
- 67.López J., Valiente-Echeverría F., Carrasco M., Mercado R., Abarca Villaseca K. Morphological and molecular identification of canine filariae in a semi-rural district of the Metropolitan Region in Chile. Revista Chilena de Infectologia. 2012;29(3):248–289. doi: 10.4067/S0716-10182012000300006. [DOI] [PubMed] [Google Scholar]
- 68.Lee G. K. C., Ignace J. A. E., Robertson I. D., Irwin P. J. Canine vector-borne infections in Mauritius. Parasites and Vectors. 2015;8(1, article 174) doi: 10.1186/s13071-015-0790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alvåsen K., Johansson S. M., Höglund J., Ssuna R., Emanuelson U. A field survey on parasites and antibodies against selected pathogens in owned dogs in Lilongwe, Malawi. Journal of the South African Veterinary Association. 2016;87(1):e1–e6. doi: 10.4102/jsava.v87i1.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Noden B. H., Soni M. Vector-borne diseases of small companion animals in Namibia: literature review, knowledge gaps and opportunity for a one health approach. Journal of the South African Veterinary Association. 2015;86(1, article 1307) doi: 10.4102/jsava.v86i1.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marcos R., Pereira C., Maia J., et al. The occurrence of the filarial nematode Dirofilaria repens in canine hosts from Maio Island, Cape Verde. Journal of Helminthology. 2016;1:1–4. doi: 10.1017/S0022149X16000067. [DOI] [PubMed] [Google Scholar]
- 72.Rjeibi M. R., Rouatbi M., Mabrouk M., Tabib I., Rekik M., Gharbi M. Molecular Study of Dirofilaria immitis and Dirofilaria repens in dogs from Tunisia. Transboundary and Emerging Diseases. doi: 10.1111/tbed.12541. (in press) [DOI] [PubMed] [Google Scholar]
- 73.Nguyen C., Koh W. L., Casteriano A., et al. Mosquito-borne heartworm Dirofilaria immitis in dogs from Australia. Parasites and Vectors. 2016;9:p. 535. doi: 10.1186/s13071-016-1821-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marks C. A., Bloomfield T. E. Canine heartworm (Dirofilaria immitis) detected in red foxes (Vulpes vulpes) in urban Melbourne. Veterinary Parasitology. 1998;78(2):147–154. doi: 10.1016/S0304-4017(98)00131-9. [DOI] [PubMed] [Google Scholar]
- 75.Smout F. A., Skerratt L. F., Butler J. R. A., Johnson C. N., Congdon B. C. Dingoes (Canis dingo Meyer, 1793) continue to be an important reservoir host of Dirofilaria immitis in low density housing areas in Australia. Veterinary Parasitology. 2016;215:6–10. doi: 10.1016/j.vetpar.2015.10.020. [DOI] [PubMed] [Google Scholar]
- 76.McKenna P. B. Dirofilaria infections in New Zealand. Surveillance. 2000;27:13–14. [Google Scholar]
- 77.Sałamatin R. V., Pavlikovska T. M., Sagach O. S., et al. Human dirofilariasis due to Dirofilaria repens in Ukraine, an emergent zoonosis: epidemiological report of 1465 cases. Acta Parasitologica. 2013;58(4):592–598. doi: 10.2478/s11686-013-0187-x. [DOI] [PubMed] [Google Scholar]
- 78.Senanayake M. P., Infaq M. L., Adikaram S. G., Udagama P. V. Ocular and subcutaneous dirofilariasis in a Sri Lankan infant: an environmental hazard caused by dogs and mosquitoes. Paediatrics and International Child Health. 2013;33(2):111–112. doi: 10.1179/2046905512Y.0000000024. [DOI] [PubMed] [Google Scholar]
- 79.Jayasinghe R. D., Gunawardane S. R., Sitheeque M. A., Wickramasinghe S. A case report on oral subcutaneous Dirofilariasis. Case Reports in Infectious Diseases. 2015;2015:4. doi: 10.1155/2015/648278.648278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kini R. G., Leena J. B., Shetty P., Lyngdoh R. H., Sumanth D., George L. Human dirofilariasis: an emerging zoonosis in India. Journal of Parasitic Diseases. 2015;39(2):349–354. doi: 10.1007/s12639-013-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tavakolizadeh S., Mobedi I. Orbital dirofilariasis in Iran: a case report. Korean Journal of Parasitology. 2009;47(4):397–399. doi: 10.3347/kjp.2009.47.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Le T. A., Vi T. T., Nguyen K. L., Le T. H. A rare human case of Dirofilaria repens infection in the subcutaneous posterior thorax with molecular identification. Korean Journal of Parasitology. 2015;53(3):329–333. doi: 10.3347/kjp.2015.53.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kwok R. P. W., Chow P. P. C., Lam J. K. M., et al. Human ocular dirofilariasis in Hong Kong. Optometry and Vision Science. 2016;93(5):545–548. doi: 10.1097/OPX.0000000000000770. [DOI] [PubMed] [Google Scholar]
- 84.Akao N. Human dirofilariasis in Japan. Tropical Medicine and Health. 2011;39:65–71. doi: 10.2149/tmh.39-1-suppl_2-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Takei D., Yamaki M., Noriyuki T., et al. Pulmonary dirofilariasis. Kyobu Geka. 2015;68(1):76–79. [PubMed] [Google Scholar]
- 86.Haro A., Tamiya S., Nagashima A. A rare case of human pulmonary dirofilariasis with a growing pulmonary nodule after migrating infiltration shadows, mimicking primary lung carcinoma. International Journal of Surgery Case Reports. 2016;22:8–11. doi: 10.1016/j.ijscr.2016.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Suzuki J., Kobayashi S., Okata U., et al. Molecular analysis of Dirofilaria repens removed from a subcutaneous nodule in a Japanese woman after a tour to Europe. Parasite. 2015;22:p. 2. doi: 10.1051/parasite/2015002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Biswas A., Reilly P., Perez A., Yassin M. H. Human pulmonary dirofilariasis presenting as a solitary pulmonary nodule: a case report and a brief review of literature. Respiratory Medicine Case Reports. 2013;10:40–42. doi: 10.1016/j.rmcr.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Malik D., Amaraneni A., Singh S., Roach R. Man's best friend: how humans can develop Dirofilaria immitis infections. IDCases. 2016;4:43–45. doi: 10.1016/j.idcr.2016.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bublitz G. S., Serapião M. J., Roberge V. D., Coelho K. M., Serapião C. J. Dirofilariose humana em Joinville-SC: avaliação clinicopatológica dos primeiros casos relatados na região Sul. Jornal Brasileiro de Patologia e Medicina Laboratorial. 2012;48(5):383–389. doi: 10.1590/S1676-24442012000500012. [DOI] [Google Scholar]
- 91.Warthan M. L., Warthan T. L., Hearne R. H., Polk A. C., Jr., Warthan M. M. Human dirofilariasis: raccoon heartworm causing a leg nodule. Cutis. 2007;80(2):125–128. [PubMed] [Google Scholar]
- 92.Ramirez J. M., Ramirez M. A., Essilfie A., Taylor C. E., Stearns 3rd. H. C., Mollano A. Roundworm-associated median nerve compression: a case report. The Iowa Orthopaedic Journal. 2013;33:225–227. [PMC free article] [PubMed] [Google Scholar]
- 93.Beaver P. C., Wolfson J. S., Waldron M. A., Swartz M. N., Evans G. W., Adler J. Dirofilaria ursi-like parasites acquired by humans in the Northern United States and Canada: report of two cases and brief review. The American Journal of Tropical Medicine and Hygiene. 1987;37(2):357–362. doi: 10.4269/ajtmh.1987.37.357. [DOI] [PubMed] [Google Scholar]
- 94.Haldane D. J. M., Johnston B. L., Walsh N. M. G. Subcutaneous dirofilariasis in Nova Scotia. Canadian Journal of Infectious Diseases. 1996;7(1):67–69. doi: 10.1155/1996/378616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Orihel T. C., Isbey Jr E. K. Dirofilaria striata infection in a North Carolina child. The American Journal of Tropical Medicine and Hygiene. 1990;42(2):124–126. doi: 10.4269/ajtmh.1990.42.124. [DOI] [PubMed] [Google Scholar]
- 96.Udall D. N. Dirofilariasis in a 22-year-old airman deployed to the mediterranean. Military Medicine. 2011;176(9):1083–1084. doi: 10.7205/MILMED-D-10-00400. [DOI] [PubMed] [Google Scholar]
- 97.Davis R., Barsoumian A., Mauffray R., Caldwell M., Drayna P., Crosson J. Dirofilaria presenting as orbital mass. Orbit. 2015;34(1):38–40. doi: 10.3109/01676830.2014.950299. [DOI] [PubMed] [Google Scholar]
- 98.Vélez-Pérez A., Liang L., Syklawer E., Chavez V., Zhang S., Wanger A. Dirofilariasis presenting as an infiltrative mass in the right buccal space. International Journal of Surgical Pathology. 2016;24(7):660–662. doi: 10.1177/1066896916653212. [DOI] [PubMed] [Google Scholar]
- 99.Theis J. H. Public health aspects of dirofilariasis in the United States. Veterinary Parasitology. 2005;133(2-3):157–180. doi: 10.1016/j.vetpar.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 100.Beltrán M., Cancrini G., Reátegui G., Melgar R., et al. Filariosis humana en la selva peruana: reporte de tres casos. Revista Peruana de Medicina Experimental y Salud Pública. 2008;25:257–260. [Google Scholar]
- 101.Saied W., Amara K., Bouchoucha S., et al. An unusual cause of hand nodule: peri-tendon dirofilariasis. Chirurgie de la Main. 2011;30(1):66–68. doi: 10.1016/j.main.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 102.Awadalla H. N., Bayoumi D. M., Ibrahim I. R. The first case report of suspected human dirofilariasis in the eyelid of a patient from Alexandria. Journal of the Egyptian Society of Parasitology. 1998;28:941–943. [PubMed] [Google Scholar]
- 103.Moodley K., Govind C. N., Peer A. K. C., et al. First detection of human dirofilariasis in South Africa. Infectious Disease Reports. 2015;7(1, article 5726) doi: 10.4081/idr.2015.5726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jagusch M. F., Roberts R. M., Rea H. H., Priestley D. R. Human pulmonary dirofilariasis. New Zealand Medical Journal. 1984;97:556–558. [PubMed] [Google Scholar]
- 105.McMichael A. J., Woodruff R. E., Hales S. Climate change and human health: present and future risks. The Lancet. 2006;367(9513):859–869. doi: 10.1016/S0140-6736(06)68079-3. [DOI] [PubMed] [Google Scholar]
- 106.Semenza J. C., Menne B. Climate change and infectious diseases in Europe. The Lancet Infectious Diseases. 2009;9(6):365–375. doi: 10.1016/S1473-3099(09)70104-5. [DOI] [PubMed] [Google Scholar]
- 107.EEA [European Environmental Agency] Global and European Temperature. 2016. http://www.eea.europa.eu/ [Google Scholar]
- 108.Walsh J., Wuebbles D., Hayhoe K., et al. Our changing climate. In: Melillo J. M., Richmond T. C., Yohe G. W., editors. Climate Change Impacts in the United States: The Third National Climate Assessment. U.S.: Global Change Research Program; 2014. pp. 19–67. [Google Scholar]
- 109.Wang D., Bowman D. D., Brown H. E., et al. Factors influencing U.S. canine heartworm (Dirofilaria immitis) prevalence. Parasites and Vectors. 2014;7(1, article 264) doi: 10.1186/1756-3305-7-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Capelli G., Drago A., Martini S., et al. First report in italy of the exotic mosquito species Aedes (Finlaya) koreicus, a potential vector of arboviruses and filariae. Parasites and Vectors. 2011;4(1, article 188) doi: 10.1186/1756-3305-4-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bonizzoni M., Gasperi G., Chen X., James A. A. The invasive mosquito species Aedes albopictus: current knowledge and future perspectives. Trends in Parasitology. 2013;29(9):460–468. doi: 10.1016/j.pt.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Suter T., Flacio E., Fariña B. F., Engeler L., Tonolla M., Müller P. First report of the invasive mosquito species Aedes koreicus in the Swiss-Italian border region. Parasites and Vectors. 2015;8(1, article 402) doi: 10.1186/s13071-015-1010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rinaldi L., Genchi C., Musella V., Genchi M., Cringoli G. Geographical information systems as a tool in the control of heartworm infections in dogs and cats. Veterinary Parasitology. 2011;176(4):286–290. doi: 10.1016/j.vetpar.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 114.Genchi C., Rinaldi L., Cascone C., Mortarino M., Cringoli G. Is heartworm disease really spreading in Europe? Veterinary Parasitology. 2005;133(2-3):137–148. doi: 10.1016/j.vetpar.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 115.Vezzani D., Carbajo A. E. Spatial and temporal transmission risk of Dirofilaria immitis in Argentina. International Journal for Parasitology. 2006;36(14):1463–1472. doi: 10.1016/j.ijpara.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 116.Genchi C., Rinaldi L., Mortarino M., Genchi M., Cringoli G. Climate and Dirofilaria infection in Europe. Veterinary Parasitology. 2009;163(4):286–292. doi: 10.1016/j.vetpar.2009.03.026. [DOI] [PubMed] [Google Scholar]
- 117.Medlock J. M., Avenell D., Barrass I., Leach S. Analysis of the potential for survival and seasonal activity of Aedes albopictus (Diptera: Culicidae) in the United Kingdom. Journal of Vector Ecology. 2006;31(2):292–304. doi: 10.3376/1081-1710(2006)31[292:AOTPFS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 118.Medlock J. M., Snow K. R., Leach S. Possible ecology and epidemiology of medically important mosquito-borne arboviruses in Great Britain. Epidemiology and Infection. 2007;135(3):466–482. doi: 10.1017/S0950268806007047. [DOI] [PMC free article] [PubMed] [Google Scholar]