Abstract
Synaptic downscaling is a homeostatic mechanism that allows neurons to reduce firing rates during chronically elevated network activity. Although synaptic downscaling is important in neural circuit development and epilepsy, the underlying mechanisms are poorly described. We performed small RNA profiling in picrotoxin (PTX)‐treated hippocampal neurons, a model of synaptic downscaling. Thereby, we identified eight microRNAs (miRNAs) that were increased in response to PTX, including miR‐129‐5p, whose inhibition blocked synaptic downscaling in vitro and reduced epileptic seizure severity in vivo. Using transcriptome, proteome, and bioinformatic analysis, we identified the calcium pump Atp2b4 and doublecortin (Dcx) as miR‐129‐5p targets. Restoring Atp2b4 and Dcx expression was sufficient to prevent synaptic downscaling in PTX‐treated neurons. Furthermore, we characterized a functional crosstalk between miR‐129‐5p and the RNA‐binding protein (RBP) Rbfox1. In the absence of PTX, Rbfox1 promoted the expression of Atp2b4 and Dcx. Upon PTX treatment, Rbfox1 expression was downregulated by miR‐129‐5p, thereby allowing the repression of Atp2b4 and Dcx. We therefore identified a novel activity‐dependent miRNA/RBP crosstalk during synaptic scaling, with potential implications for neural network homeostasis and epileptogenesis.
Keywords: epilepsy, homeostatic plasticity, microRNA, RNA‐binding protein, synaptic scaling
Subject Categories: Neuroscience, RNA Biology
Introduction
Neurons possess a sophisticated repertoire of cellular mechanisms that help to maintain homeostasis in response to changes in network activity (e.g., those encountered during circuit development or upon traumatic brain injuries) that would otherwise compromise neuronal function and health. These mechanisms operate either at the level of the network (e.g., excitatory/inhibitory balance) or the neuron (intrinsic excitability/synaptic scaling). The uniform downscaling of excitatory synapses in response to chronic increases in network activity has gained significant attention due to its potential function in reducing neuronal firing rates in epilepsy (Siddoway et al, 2014; Swann & Rho, 2014) and its engagement during the activity‐dependent development of the visual system (Desai et al, 2002; Gao et al, 2010). Very recently, downscaling of excitatory synapses was also observed during sleep, suggesting an involvement in memory consolidation (Diering et al, 2017).
Despite its clinical and physiological significance, studies investigating the molecular mechanisms of synaptic downscaling are only just emerging. Many studies have focused on the role of activity‐dependent mRNA transcription of immediate early genes (e.g., Plk2, Homer1a, Arc, Narp), transcriptional repression mediated by MeCP2 and proteasome‐dependent protein degradation [e.g., SPAR, GKAP, SHANK, GluA1/2; reviewed in Siddoway et al (2014)]. In contrast to transcriptional studies, a potential contribution of mechanisms regulating de novo protein synthesis at the post‐transcriptional level, such as mRNA translation and/or stability, to synaptic downscaling has not been systematically addressed.
The 3′ untranslated regions (UTRs) of mRNAs are major hubs for the regulation of mRNA translation/stability. 3′UTR‐dependent gene regulation appears to be particularly prevalent in the brain. For example, recent bioinformatic studies described increased 3′UTR length of brain‐enriched transcripts (Miura et al, 2013) and mRNAs encoding for presynaptic proteins (Paschou et al, 2012). Two families of regulatory molecules, sequence‐specific RNA‐binding proteins (RBPs) and microRNAs (miRNAs), are the focus of studies that address the mechanisms of 3′UTR‐dependent gene regulation. When bound to 3′UTRs, RBPs can have both negative (e.g., FMRP; Li et al, 2001) and positive effects (e.g., Hu proteins, Rbfox1/3; Colombrita et al, 2013; Lee et al, 2016) on mRNA translation and stability. MiRNAs are an extensive family of small non‐coding RNAs that repress protein synthesis when bound to their targets (Bartel, 2009). In mature neurons, miRNAs have been implicated in the local control of dendritic protein synthesis and synaptic plasticity (Schratt, 2009). Recently, important roles have been attributed to a few miRNAs in homeostatic synaptic plasticity. miR‐92a (Letellier et al, 2014) and miR‐124 (Hou et al, 2015) are involved in synaptic upscaling in response to chronic activity blockade. miR‐485 regulates synaptic downscaling by a presynaptic mechanism (Cohen et al, 2011). Until now, only one miRNA (miR‐134‐5p) was shown to regulate synaptic downscaling at postsynaptic sites (Fiore et al, 2014). Functional crosstalk between miRNAs and RBPs on the same 3′UTR has been reported in non‐neuronal cells (Kedde & Agami, 2008).
In the current study, we present the first comprehensive analysis of miRNA, mRNA, and protein expression in a cellular model of synaptic downscaling. We identify a novel miRNA‐RBP crosstalk, consisting of miR‐129‐5p and Rbfox proteins, that coordinates the expression of important synaptic proteins during downscaling. We further show that this pathway is deregulated in epilepsy, suggesting an involvement in neural network homeostasis. Together, our results indicate a more widespread role of post‐transcriptional regulatory mechanisms involving miRNAs and RBPs in homeostatic plasticity than previously anticipated.
Results
A specific set of miRNAs is upregulated during synaptic downscaling in vitro
We hypothesized that miRNAs whose expression is regulated by a long‐term increase in network activity might represent strong candidates for functionally important molecules in synaptic downscaling. We used mature primary rat hippocampal neurons cultured for 20 days in vitro (20 DIV) as a cellular model of synaptic downscaling. In these cells, long‐term treatment (48 h) with the GABA‐A receptor blocker picrotoxin (PTX) induces elevated network activity followed by synaptic downscaling, as previously shown (Shepherd et al, 2006; Ibata et al, 2008). We could validate efficient synaptic downscaling by showing a PTX‐mediated downregulation of surface expression of the AMPA‐type glutamate receptor (AMPA‐R) GluA1 in these neurons (Fig 1A; Evers et al, 2010). To systematically characterize changes in miRNA expression during synaptic downscaling, we performed comparative small RNA sequencing using this PTX model. We identified a total of eight different miRNA species encoded by four different genomic miRNA loci that were differentially regulated (FDR < 0.05) in PTX‐ compared to vehicle‐treated neurons (Fig 1B; Table EV1). Intriguingly, all of these miRNAs were upregulated by PTX, consistent with a repressive function during PTX‐induced downscaling. Four miRNAs (miR‐212‐3p/5p, miR‐132‐3p/5p) are encoded by the miR‐132–212 cluster, which was previously implicated in activity‐dependent synapse development and remodeling (Wanet et al, 2012). Two miRNAs (miR‐495, miR‐543‐3p) are part of the large imprinted miR379–410 cluster, members of which have been implicated in neuronal morphogenesis (Schratt et al, 2006; Fiore et al, 2009) and homeostatic synaptic scaling (Fiore et al, 2014). The remaining two miRNAs have not been studied in the context of synapse development and homeostasis. The most PTX‐responsive miRNA, miR‐155‐5p, displays only very weak expression in nerve cells and was mostly studied in the immune system (Su et al, 2016). In contrast, miR‐129‐5p is abundantly expressed in the developing retina (Decembrini et al, 2009) and in hippocampal neurons, where it was shown to control neuronal excitability by regulating the expression of the potassium channel Kv1.1 (Sosanya et al, 2013). We therefore decided to investigate a potential involvement of miR‐129‐5p in the regulation of synaptic downscaling.
Figure 1. A specific set of miRNAs is upregulated during synaptic downscaling in vitro .
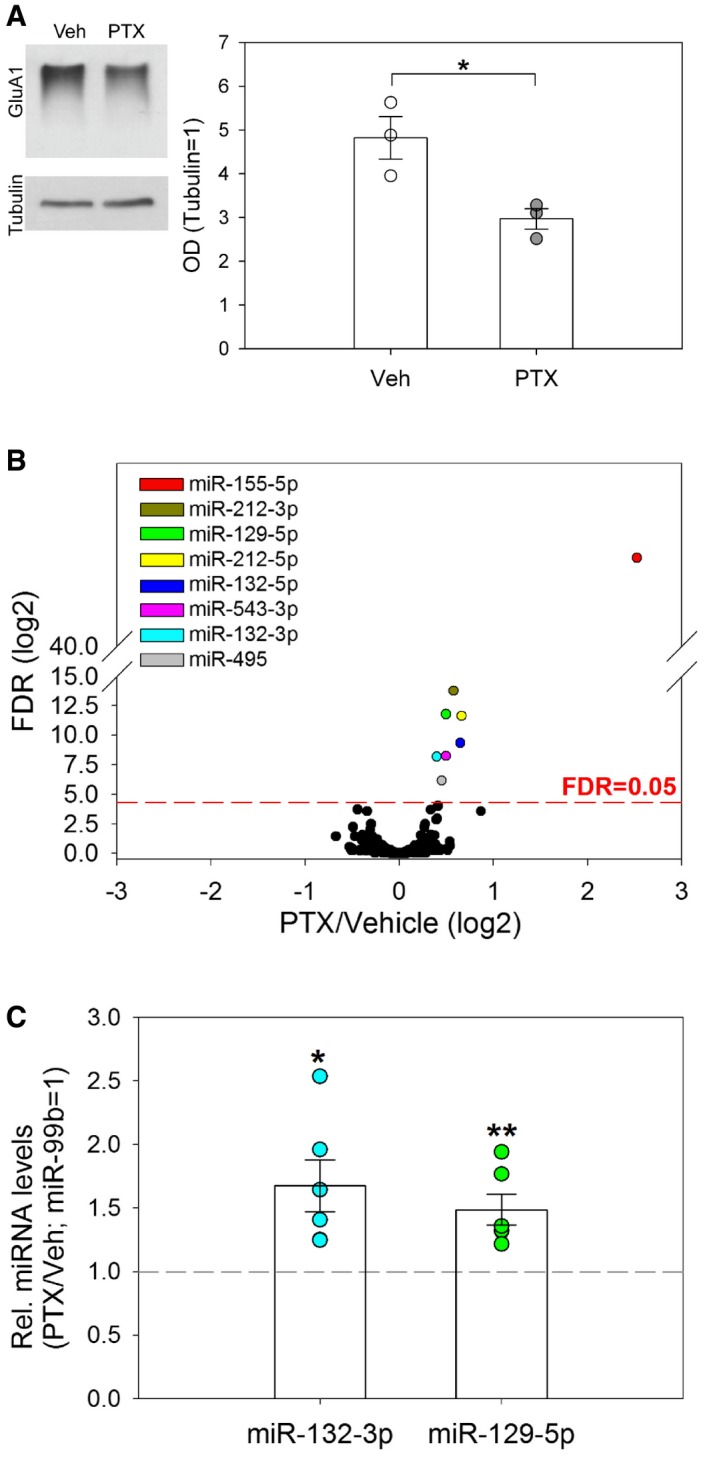
- Western blot analysis of surface GluA1 expression after cross‐linking in lysates from vehicle (Veh)‐ or picrotoxin (PTX, 48 h)‐treated hippocampal neurons. Left: Representative Western blot. Tubulin was used as a loading control. Right: Quantification of Western blot data from three independent experiments. Dot‐blot presentation with mean optical band density (OD) normalized to tubulin ± s.e.m. (independent two‐sample t‐test, two‐tailed, homoscedastic variance; *P = 0.026).
- Average changes in miRNA levels (PTX/Veh; log2) as determined by small RNA‐seq (n = 3,442 miRNAs; correction for multiple comparisons by FDR, P < 0.05). miRNAs significantly upregulated by PTX are highlighted.
- TaqMan qPCR analysis of miR‐129‐5p and miR‐132‐3p in PTX‐ and vehicle‐treated hippocampal neurons. Dot‐plot presentation with mean relative miRNA levels (PTX/Veh) normalized to miR‐99b ± s.e.m. (n = 6; independent one‐sample t‐test, two‐tailed, heteroscedastic variance; *P = 0.022, **P = 0.010).
Source data are available online for this figure.
miR‐129‐5p is required for homeostatic synaptic downscaling in the cellular PTX model
Using qPCR, we validated that 48‐h PTX treatment of hippocampal neurons induces mature miR‐129‐5p expression in a similar manner to the known activity‐regulated miR‐132‐3p (Remenyi et al, 2010; Fig 1C). We tested whether miR‐129‐5p is required for synaptic downscaling in hippocampal neurons. To address this, we applied specific antisense oligonucleotide inhibitors of miR‐129‐5p (anti‐miR‐129) in vehicle‐ and PTX‐treated neurons and monitored dendritic spine size by confocal fluorescence microscopy (Fig 2A). Dendritic spine size is a correlate of postsynaptic strength and uniformly reduced in 48‐h PTX‐treated neurons (Fiore et al, 2014). The efficacy and specificity of the anti‐miRs was validated in miR‐129‐5p sensor assays (Appendix Fig S1A). We found that inhibition of miR‐129‐5p completely abolished PTX‐mediated spine size reduction compared to cells transfected with GFP only or a control inhibitor (anti‐miR Control; Fig 2A), demonstrating that miR‐129‐5p is required for PTX‐dependent downscaling of dendritic spine size. Spine size was already reduced in anti‐miR‐129‐5p‐transfected neurons in the absence of PTX, suggesting that miR‐129‐5p has an additional, activity‐independent spine growth promoting effect (Appendix Fig S1B). In addition, overexpression of miR‐129‐5p in the absence of PTX decreased spine size, indicating that miR‐129‐5p is sufficient to induce spine shrinkage (Appendix Fig S1C). To examine effects on synaptic strength more directly, we performed patch‐clamp electrophysiological recordings of miniature excitatory postsynaptic currents (mEPSCs). We used the competitive GABA‐A receptor blocker bicuculline (Bic) in these experiments, since electrophysiological recordings were more stable with Bic compared to PTX. Importantly, results from different sets of experiments (Appendix Fig S1D–F; Table EV2) demonstrate that the effects of Bic and PTX on synaptic gene expression and spine morphogenesis were overall comparable. Anti‐miR‐129‐5p strongly attenuated the Bic‐mediated reduction in mean mEPSC amplitude compared to anti‐miR control (Fig 2B). Thus, miR‐129‐5p activity is required for an efficient morphological and physiological downscaling of excitatory synapses in hippocampal neurons in response to chronically elevated network activity.
Figure 2. miR‐129‐5p is required for synaptic downscaling in vitro .
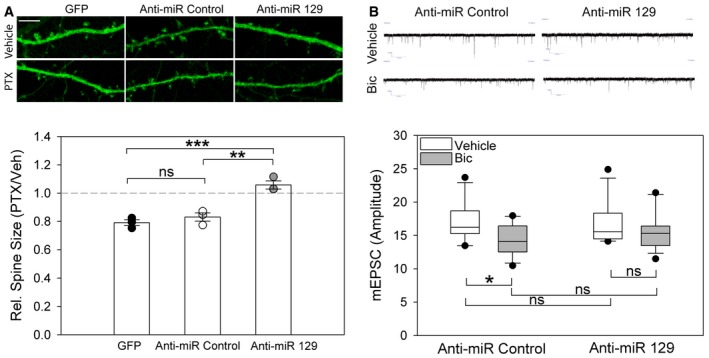
- Upper panel: Representative confocal microscopy images of dendritic segments from PTX‐ or vehicle (Veh)‐treated hippocampal neurons transfected with indicated anti‐miRs (scale bar = 5 μm). Bottom panel: Quantification of dendritic spine size from three independent experiments. Dot‐plot presentation with mean relative spine size (Ptx/Veh) ± s.e.m. (n = 3; 8 neurons, ˜200 spines/neuron per experimental condition; one‐way ANOVA; F (2,6) = 29.31, P = 0.001; groups were compared by Bonferroni post hoc test; **P = 0.0030, ***P = 0.001, ns: not significant).
- Upper panel: Representative traces of mEPSC recordings from bicuculline (Bic)‐ or vehicle‐treated neurons transfected with indicated anti‐miRs. Bottom panel: Quantification of mEPSC amplitudes from multiple neurons, data are presented as box plots (anti‐miR Control Veh/Bic n = 13/12; anti‐miR‐129 Veh/Bic n = 14/13; GLM model; activity P = 0.015; estimated means of specific experimental conditions were compared by pairwise comparison in SPSS; anti‐miR Control Veh vs. Bic *P = 0.023; anti‐miR‐129‐5p Veh vs. Bic P = 0.242; Veh anti‐miR Control vs. anti‐miR‐129‐5p P = 0.827; Bic anti‐miR Control vs. anti‐miR‐129‐5p P = 0.307; ns: not significant). The boundary of the box closest to zero indicates the 25th percentile, a line within the box marks the median, and the boundary of the box farthest from zero indicates the 75th percentile. Error bars above and below the box indicate the 90th and 10th percentiles.
miR‐129‐5p is involved in the regulation of epileptic seizures in vivo
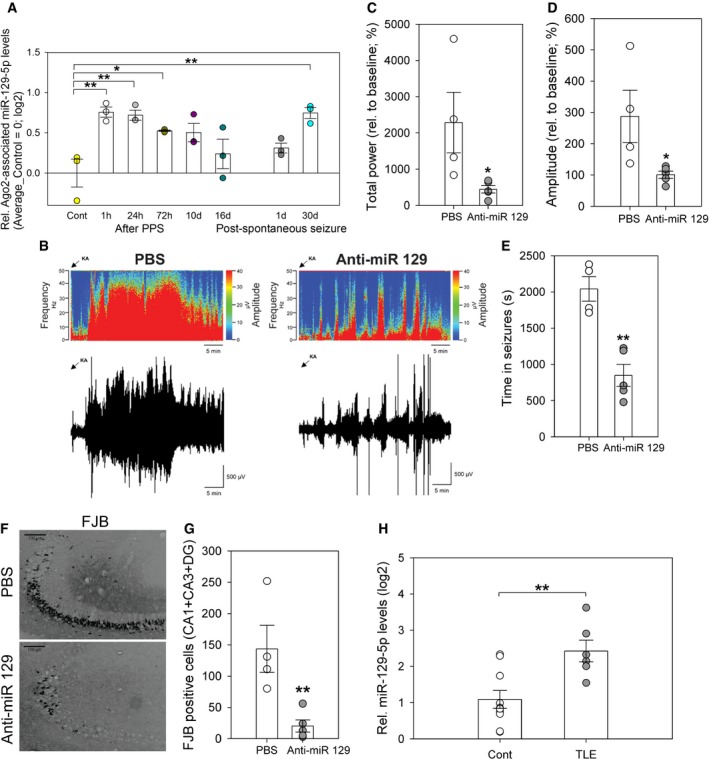
A chronic overexcitation of neural networks is observed in the epileptic brain during seizure activity. Furthermore, it was hypothesized that homeostatic plasticity mechanisms are actively engaged in the epileptic brain (Swann & Rho, 2014). We therefore decided to study a potential involvement of miR‐129‐5p in the regulation of epileptic seizures in vivo. First, we investigated miR‐129‐5p expression in rats in which mesial TLE with hippocampal sclerosis was induced by 8 h of perforant pathway stimulation (PPS; Norwood et al, 2011). This toxin‐free animal model reliably replicates essential characteristics of acquired human TLE. We found that Ago2‐associated miR‐129‐5p was significantly elevated at 1, 24, and 72 h after 8‐h PPS (Fig 3A). Thereafter (8 h + 10 days, 8 h + 16 days), miR‐129‐5p levels returned close to baseline. Intriguingly, Ago2‐associated miR‐129‐5p levels were again induced 30‐days post‐spontaneous seizure, suggesting that network hyperactivity associated with seizures could increase miR‐129‐5p Ago2 association. Ago2 association of miR‐129‐5p in the epileptic brain was specific, since expression of the corresponding miRNA* sequences (miR‐129‐1‐3p and miR‐129‐2‐3p) was not significantly altered at any time point after PPS (Appendix Fig S2A and B). We next asked whether induction of miR‐129‐5p was functionally involved in pathological brain activity in vivo. Therefore, we pretreated mice with anti‐miR‐129 or PBS (intracerebroventricular injection) 24 h before inducing status epilepticus by intra‐amygdalar injection of kainic acid (KA). We used the KA model for functional testing, since it is less time‐ and resource‐consuming and well established for the use of anti‐miRs (Jimenez‐Mateos et al, 2011, 2012, 2015; McKiernan et al, 2012). We validated that miR‐129‐5p was robustly induced by KA injection (Appendix Fig S2C). In control experiments, we found that multiple EEG parameters were not significantly different between animals injected with PBS or a scrambled oligonucleotide (anti‐miR control; Appendix Fig S3), indicating that PBS‐injected animals represent adequate controls. Compared to PBS pretreated mice, anti‐miR‐129‐5p pretreatment strongly reduced seizure severity in KA‐injected mice (Fig 3B–D; Appendix Fig S4A) and the total time mice spent in seizures (Fig 3E), as judged by EEG. Furthermore, anti‐miR‐129‐5p pretreatment strongly suppressed KA‐induced cell death in different hippocampal subfields, as judged by Fluoro‐Jade B (FJB) staining (Fig 3F and G; Appendix Fig S4B–D). Forty minutes after KA‐induced status epilepticus, all mice were injected with lorazepam to reduce mortality and morbidity. A similar seizure‐suppressive effect of anti‐miR‐129‐5p was also observed after lorazepam administration (Appendix Fig S4E–H). Successful inhibition of miR‐129‐5p in vivo was confirmed by qPCR (Appendix Fig S4I). Anti‐miR‐129‐5p did not alter EEG parameters during baseline measurement prior to KA injection (Appendix Fig S4J–L). Our data suggest that miR‐129‐5p induction in the hippocampus in vivo promotes seizure activity and associated neurodegeneration. Finally, we analyzed brain samples from human temporal lobe epilepsy (TLE) patients. Using qPCR, we found that miR‐129‐5p was significantly higher expressed in the hippocampus of TLE patients displaying hippocampal sclerosis (HS) compared to age‐matched healthy controls (Fig 3H, Table EV3). MiR‐129‐5p levels were also elevated in TLE patients without confirmed hippocampal sclerosis, although this did not reach statistical significance (Appendix Fig S5A). These results suggest that miR‐129‐5p dysregulation is conserved between the rodent and human epileptic brain.
Figure 3. miR‐129‐5p is involved in the regulation of epileptic seizures in vivo .
-
ASmall RNA‐seq analysis of Ago2‐associated miR‐129‐5p from the dentate gyrus of rats at indicated times after perforant path stimulation (PPS) or after the occurrence of the first seizure (post‐spontaneous seizure). Data are presented as fold (log2) induction over control‐treated rats in a dot plot with mean ± s.e.m. (one‐way ANOVA, correction for multiple comparisons by Benjamini–Hochberg FDR, P = 0.005; n = 3 per experimental condition, *P < 0.05, **P < 0.01). Average levels of Ago2‐associated miR‐129‐5p in control group were set to 0 (log2).
-
BRepresentative heatmap showing frequency (Hz) and amplitude (μV) of EEG recordings over time (in minutes) for a control (PBS)‐ and anti‐miR‐129‐5p‐injected mice, followed by the respective EEG traces immediately below.
-
C–EGraphs show EEG total power (C), amplitude (D), depicting % of increase from each animal's own baseline data, and time spent in seizures (E) during 40 min of observation after KA‐induced status epilepticus in mice pretreated (24 h) with PBS or anti‐miR‐129‐5p. Dot‐plot presentation with mean ± s.e.m. (n = 4–5 mice per group; independent two‐sample t‐test, two‐tailed, homoscedastic variance; total power *P = 0.0421, amplitude *P = 0.041, time in seizures **P = 0.0012).
-
FRepresentative photomicrographs from Fluoro‐Jade B (FJB) stainings of the mouse dorsal hippocampus 24 h after status epilepticus. Mice were injected with PBS or anti‐miR‐129‐5p 24 h before KA administration. Scale bar = 100 μm.
-
GQuantification of FJB‐positive cells from multiple slices performed in (F). Dot‐plot presentation with mean cell count ± s.e.m. (n = 4–5 mice per group; independent two‐sample t‐test, two‐tailed, homoscedastic variance; **P = 0.009).
-
HTaqMan qPCR analysis of miR‐129‐5p levels in the hippocampus of human temporal lobe epilepsy (TLE) patients displaying hippocampal sclerosis (HS) (n = 6) or healthy controls (n = 10). Dot‐plot presentation with mean relative miR‐129‐5p levels (log2) ± s.e.m. (independent two‐sample t‐test, two‐tailed, homoscedastic variance; **P = 0.004).
Systematic miRNA target identification using a combination of RNA‐seq and quantitative proteomics
To obtain further insight into the mechanism of miR‐129‐5p‐regulated synaptic downscaling, we were interested in identifying miR‐129‐5p target mRNAs. Since we observed that miR‐129‐5p expression was upregulated by PTX, we expected that miR‐129‐5p targets should be overrepresented in PTX‐downregulated mRNAs. miRNA target site prediction in PTX‐downregulated mRNAs should then allow to enrich for direct miR‐129‐5p targets. Comparative mRNA profiling from PTX‐ and vehicle‐treated neurons using polyA RNA sequencing (Fig 4A; Table EV4) allowed us to monitor the expression of RNAs derived from roughly 9,000 genes. Of those, 957 were differentially expressed (q‐value < 0.05) between 48‐h PTX‐ and vehicle‐treated neurons based on three biological replicates. Gene ontology (GO) term analysis suggests that PTX‐decreased genes, in contrast to increased genes, are mainly associated with synaptic functions (Fig 4B), for example, glutamate receptor signaling, calcium‐mediated signaling, and synaptic plasticity. Accordingly, known regulators of synaptic scaling were mostly found among the PTX‐downregulated genes (e.g., GluA1, Ppp3ca, Akap5, Camk2a; Groth et al, 2011; Diering et al, 2014; Kim & Ziff, 2014). We could validate a PTX‐dependent reduction in mRNA levels for several candidates using qPCR (Fig 4C) and fluorescence in situ hybridization (Appendix Fig S5B–D).
Figure 4. Systematic miRNA target identification using a combination of RNA‐seq and quantitative proteomics.
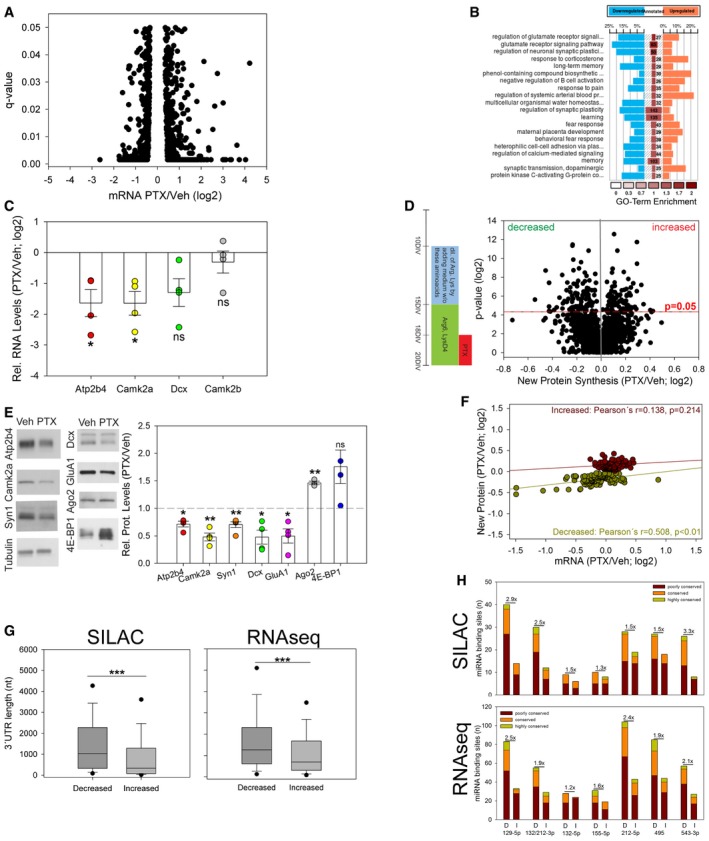
- Average changes in mRNA levels (PTX/Veh; log2) as determined by polyA RNA‐seq (n = 3; q‐values represent FDR‐corrected P‐values; q < 0.05); 495 genes were significantly upregulated and 462 genes were significantly downregulated upon PTX treatment.
- GO term analysis for 957 genes with differentially regulated mRNA levels upon 48‐h PTX treatment (Fig 4A). The color of the central column elements represents GO term enrichment in the analyzed dataset. Numbers in the central column represent total number of genes associated with the specific GO term. Percentage in the downregulated or upregulated group stands for identified number of genes associated with specific GO term in the experimental dataset.
- qPCR analysis of indicated candidate genes using polyA RNA from PTX (48 h)‐ or vehicle‐treated hippocampal neurons. Dot‐plot presentation with mean relative RNA levels (PTX/Veh (log2)) ± s.e.m. (n = 4 independent experiments; independent one‐sample t‐test, two‐tailed, heteroscedastic variance; Atp2b4 *P = 0.033, Camk2a *P = 0.023, Dcx P = 0.062, Camk2b P = 0.450; ns: not significant).
- Left panel: Timeline of pSILAC experiment in primary hippocampal neurons cultured for indicated number of days in vitro (DIV). Right panel: Average changes in new protein synthesis (PTX/Veh; log2) plotted vs. P‐value (log2) (n = 3; 1,225 proteins; independent one‐sample t‐test, two‐tailed, heteroscedastic variance). Proteins with P < 0.05 were considered as differentially synthesized (total of 182 proteins, 97↓, 85↑).
- Western blot analysis of Atp2b4, Camk2a, Syn1, Dcx, GluA1, Ago2, and 4E‐BP1 expression in lysates from vehicle (Veh)‐ and PTX (48 h)‐treated hippocampal neurons. Left: Representative Western blot. Tubulin was used as a loading control. Right: Quantification of four independent experiments except Ago2 (n = 3) and Syn1 (n = 5). Dot‐plot presentation with mean relative protein levels (PTX/Veh) ± s.e.m. (independent one‐sample t‐test, two‐tailed, heteroscedastic variance; Atp2b4 *P = 0.012, Camk2a **P = 0.005, Dcx *P = 0.026, 4E‐BP1 P = 0.09, Syn1 **P = 0.005, Ago2 **P = 0.005, GluA1 *P = 0.03; ns: not significant).
- Overlap of genes displaying differential mRNA levels (panel A) and new protein synthesis (panel D). mRNA fold changes (PTX/Veh; log2) are plotted vs. fold changes in new protein synthesis (PTX/Veh; log2). Pearson's correlation was used to compare changes at the mRNA and protein synthesis level.
- Box plot representation of the 3′UTR length of genes that are significantly regulated by PTX based on pSILAC (left; panel D) or RNA‐seq (right; panel A) (Mann–Whitney U‐test; ***P < 0.001).
- Abundance of binding sites (8mer‐1a and 7mer‐m8 seeds) for differentially regulated miRNAs (Fig 1B) in 3′UTRs of genes that are significantly regulated by PTX based on pSILAC (panel D) or RNA‐seq (panel A) according to TargetScan‐Human version 6.2. D: decreased; I: increased. Seeds were classified as poorly conserved (≤ 3 species), conserved (4–9 species), or highly conserved (≥ 10 species). Bar graphs represent total number of miRNA binding sites per group. Numbers above bars represent the ratio of total miRNA binding sites between decreased and increased genes.
Source data are available online for this figure.
Next, we investigated whether changes observed at the mRNA level translated into corresponding alterations in de novo protein synthesis. Changes in de novo protein synthesis during PTX treatment were quantified by pulsed stable isotope labeling of amino acids in culture (pSILAC) (Selbach et al, 2008) followed by mass spectrometry. Briefly, neurons were incubated with Arg6/LysD4 starting at 15 DIV, PTX was applied at 18 DIV, and proteins were extracted at 20 DIV and analyzed by mass spectrometry (Fig 4D; please refer to the Appendix Results, including Appendix Fig S5E–I and Tables EV5 and EV6, for a more detailed characterization of pSILAC experiments). In total, we were able to reproducibly detect 1225 proteins in three independent neuron preparations (Fig 4D). Of those, expression of 182 proteins was significantly (P < 0.05) different between PTX‐ and vehicle‐treated cultures (97 decreased, 85 increased; Table EV7). Similar to the analysis of differentially expressed mRNAs, GO terms for downregulated proteins identified by pSILAC were specifically enriched for synaptic functions (Appendix Fig S5J). We validated PTX regulation for five significantly downregulated (Atp2b4, Camk2a, Syn1, Dcx, GluA1) and one upregulated protein (Ago2) using Western blot (Fig 4E). Dcx protein was included in the validation experiments since it represented the top downregulated protein in two out of three pSILAC replicates. Importantly, changes in de novo protein synthesis and mRNA levels were significantly correlated for the set of PTX‐repressed, but not PTX‐induced genes (Fig 4F). This result suggests that a large fraction of PTX‐downregulated genes are coordinately regulated at the mRNA and protein level.
Interestingly, 3′UTRs corresponding to PTX‐decreased genes were on average more than twice as long as 3′UTRs corresponding to PTX‐increased genes (Fig 4G; New Protein—differentially expressed proteins; 3′UTR length calculated based on RNA‐seq data; median: 1.03 kb (decreased) vs. 0.33 kb (increased); mRNA—differentially expressed genes; ↓ mRNAs median: 1.3 kb vs. ↑ mRNAs median: 0.7 kb; Tables EV8 and EV9). In contrast, 5′UTRs are in general much shorter, and their length is not significantly different between PTX‐regulated genes (Appendix Fig S6A and B New Protein/mRNA). This result suggests an involvement of 3′UTR‐dependent post‐transcriptional mechanisms in PTX‐mediated gene repression. We next determined the frequency of miRNA binding sites in the 3′UTRs corresponding to differentially PTX‐regulated proteins and mRNAs using the TargetScan algorithm. We found that binding sites for PTX‐upregulated miRNAs were in general more abundant in the 3′UTRs of downregulated compared to upregulated genes (Fig 4H; Tables EV10 and EV11). Importantly, miR‐129‐5p binding sites were among the most strongly enriched sites in PTX‐repressed genes, consistent with an important contribution of miR‐129‐5p to the regulation of PTX‐responsive genes.
Atp2b4 and Dcx are miR‐129‐5p target genes
To narrow down on direct targets of miR‐129‐5p, we intersected the datasets obtained with proteomics, transcriptomics, and miRNA target prediction. Thereby, we identified six genes that were significantly downregulated by PTX at the mRNA and protein level and contain at least one miR‐129‐5p binding site (Fig 5A). We focused on Atp2b4 and Dcx, since both contain one highly conserved miR‐129‐5p binding site in their 3′UTR (Fig 5B). We further hypothesized that the regulation of these genes could be functionally relevant in synaptic downscaling. Atp2b4 is an ATP‐dependent calcium pump involved in the regulation of calcium homeostasis (Strehler et al, 2007), consistent with the reported central role of calcium‐dependent signaling pathways in downscaling (Turrigiano, 2012). Dcx is a microtubule‐associated protein involved in the regulation of neuronal cell migration and spine morphogenesis (Yoshihara et al, 2014).
Figure 5. Atp2b4 and Dcx are miR‐129‐5p target genes.
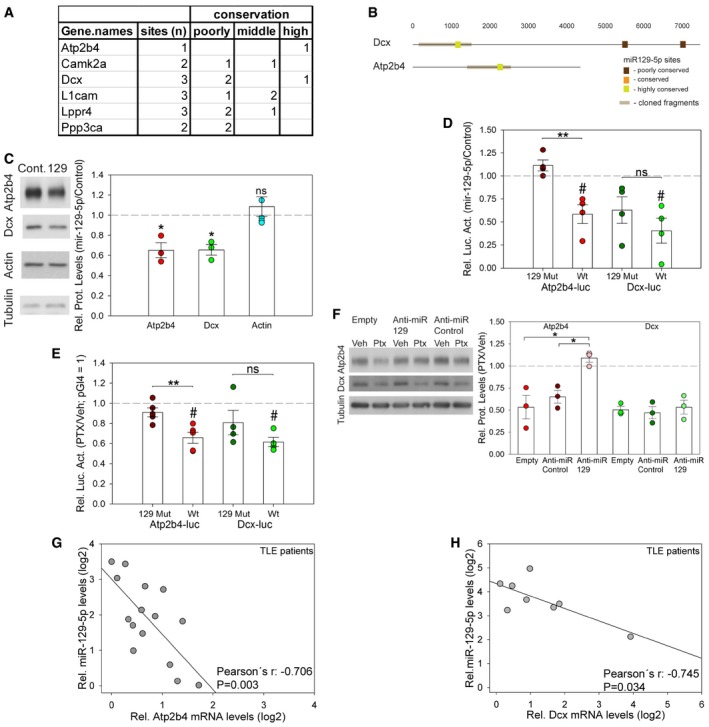
- A
-
BLocalization of mir‐129‐5p binding sites (as predicted in Fig 4H) in the 3′UTRs of Atp2b4 and Dcx. Color code indicates different degree of conservation.
-
CWestern blot analysis of Atp2b4, Dcx, and actin protein expression in hippocampal neurons transfected with miR‐129‐5p or control duplex RNA. Left: Representative Western blot. Tubulin was used as a loading control. Right: Quantification of multiple experiments. Dot‐plot presentation with mean relative protein levels (miR‐129‐5p/control) ± s.e.m. (Atp2b4, Dcx n = 3; actin n = 5; independent one‐sample t‐test, two‐tailed, heteroscedastic variance; Atp2b4 *P = 0.0437, Dcx *P = 0.0236, actin P = 0.4222; ns: not significant).
-
DLuciferase reporter gene assay in miR‐129‐5p or control duplex RNA‐transfected cortical neurons co‐transfected with the indicated 3′UTR reporter constructs containing wild‐type (wt) or mutated (129mut) miR‐129‐5p binding sites. Dot‐plot presentation with mean relative luciferase activity (miR‐129‐5p/control) ± s.e.m. Effect of miR‐129‐5p was tested by one‐sample t‐test (n = 4; two‐tailed, heteroscedastic variance; Atp2b4‐luc wt # P = 0.027, Atp2b4‐luc 129mut P = 0.151, Dcx‐luc wt # P = 0.022, Dcx‐luc 129mut P = 0.082) or two‐sample t‐test (two‐tailed, homoscedastic variance; Atp2b4 wt vs. 129mut **P = 0.004; Dcx wt vs. 129mut P = 0.302).
-
ELuciferase reporter gene assay in vehicle (Veh)‐ or PTX‐treated hippocampal neurons transfected with the Atp2b4 3′UTR and Dcx 3′UTR reporter construct containing either wt or 129mut binding sites (see panel D). Dot‐plot presentation with mean relative luciferase activity (PTX/Veh) ± s.e.m. pGl4 (PTX/Veh) was set to 1. Effect of PTX was tested by one‐sample t‐test (Atp2b4‐luc n = 5; Dcx‐luc n = 4; two‐tailed, heteroscedastic variance; Atp2b4‐luc wt # P = 0.003, 129mut P = 0.117, Dcx‐luc wt # P = 0.004, 129mut P = 0.213) or two‐sample t‐test (two‐tailed, homoscedastic variance; Atp2b4‐luc wt vs. Mut **P = 0.008; Dcx‐luc wt vs. Mut P = 0.190, ns: not significant).
-
FWestern blot analysis of Atp2b4 and Dcx protein expression in PTX‐ or mock‐treated hippocampal neurons that were transfected with the indicated anti‐miRs. Left: Representative Western blot. Tubulin was used as a loading control. Right: Quantification of three independent experiments. Dot‐plot presentation with mean relative protein levels (PTX/Veh) ± s.e.m. (n = 3; one‐way ANOVA; Atp2b4: F (2,6) = 10.323, P = 0.011; groups were compared by Bonferroni post hoc test; anti‐miR‐129‐5p vs. anti‐miR Cont *P = 0.044; anti‐miR‐129‐5p vs. Empty *P = 0.015; Dcx: one‐way ANOVA; F (2,6) = 0.243; P = 0.791).
-
G, HCorrelation of miR‐129‐5p and Atp2b4 (G) or Dcx (H) mRNA expression levels in the hippocampus of human TLE patients. Data are presented as rel. RNA or miRNA levels in log2 scale (Atp2b4: n = 15, Pearson's r = −0.706; P = 0.003; Dcx: n = 8, Pearson's r = −0.745; P = 0.034).
Source data are available online for this figure.
We found that transfecting hippocampal neurons with miR‐129‐5p mimics significantly reduced endogenous protein levels of Atp2b4 and Dcx using Western blotting (Fig 5C). Thus, elevated miR‐129‐5p levels were sufficient to repress Atp2b4 and Dcx in the absence of PTX. We next investigated whether the observed miR‐129‐5p‐dependent repression of Atp2b4 and Dcx was mediated by the respective 3′UTRs. Transfection of miR‐129‐5p mimics significantly downregulated the expression of wild‐type (wt) Atp2b4 and Dcx 3′UTR luciferase reporters (Atp2b4‐luc wt, Dcx‐luc wt; Fig 5D). The repressive effect of miR‐129‐5p was largely abolished when using an Atp2b4 luciferase reporter with a mutated miR‐129‐5p binding site (129 Mut), but only attenuated when using the respective Dcx‐luc 129 Mut reporter (Fig 5D). To explore the role of the endogenous miR‐129‐5p in the regulation of Atp2b4 and Dcx, we performed luciferase assays in PTX‐treated hippocampal neurons (Fig 5E). Whereas the expression of both wt reporters was significantly decreased by PTX, mutation of the miR‐129‐5p fully abolished PTX‐mediated downregulation of Atp2b4 (Fig 5E). Similar to miR‐129‐5p overexpression experiments (Fig 5D), mutation of the miR‐129‐5p site in Dcx only slightly attenuated reporter gene repression by PTX. These results strongly suggest that miR‐129‐5p regulates Atp2b4 primarily via the conserved miR‐129‐5p binding site within its 3′UTR. In contrast, the miR‐129‐5p site within the Dcx 3′UTR only contributes to a minor extent to miR‐129‐5p‐mediated Dcx repression. In addition, transfection of anti‐miR‐129‐5p interfered with the PTX‐mediated decrease of endogenous Atp2b4, but not Dcx protein (Fig 5F), consistent with our results from reporter gene assays. We also found that the expression of miR‐129‐5p and either Atp2b4 (Fig 5G) or Dcx (Fig 5H) mRNA were inversely correlated in human TLE patients, but not healthy controls (Appendix Fig S6C and D). This finding is consistent with an inhibitory interaction between miR‐129‐5p and Atp2b4/Dcx in the human epileptic brain. Taken together, our data strongly suggest that Atp2b4 and Dcx mRNAs are targeted by miR‐129‐5p during PTX‐induced synaptic downscaling. Whereas Atp2b4 downregulation is strictly miR‐129‐5p‐dependent, the repression of Dcx likely involves additional miR‐129‐5p‐independent mechanisms.
Downregulation of miR‐129‐5p targets Atp2b4 and Dcx is required for synaptic downscaling
We next determined the functional relevance of the downregulation of Atp2b4 and Dcx in synaptic downscaling. Toward this end, we restored Atp2b4 and Dcx expression in PTX‐treated hippocampal neurons using transfection of the respective cDNAs. Using confocal microscopy, we observed a significant reduction in spine size upon 48‐h PTX treatment in neurons transfected with GFP only or an unrelated control protein (Fig 6A). In contrast, spine size was no longer reduced in PTX‐treated neurons transfected with either Atp2b4 or Dcx expression plasmids (Fig 6A). Atp2b4 or Dcx overexpression had no significant effect on spine size in the absence of PTX (Appendix Fig S6E). Thus, downregulation of either Atp2b4 or Dcx is required for the downscaling of spine size upon PTX treatment. Next, we measured mEPSC amplitudes (Fig 6B), focusing on Atp2b4‐transfected neurons. Consistent with our results from spine size analysis in both PTX‐ (Fig 6A) and Bic‐treated neurons (Appendix Fig S1D), Atp2b4 overexpression prevented the decrease in mEPSC amplitudes caused by Bic, but had no effect in vehicle‐treated neurons (Fig 6B). Taken together, our results demonstrate that downregulation of miR‐129‐5p targets is critical for morphological (Atp2b4, Dcx) and functional (Atp2b4) synaptic downscaling in hippocampal neurons.
Figure 6. Downregulation of Atp2b4 is required for synaptic downscaling in vitro .
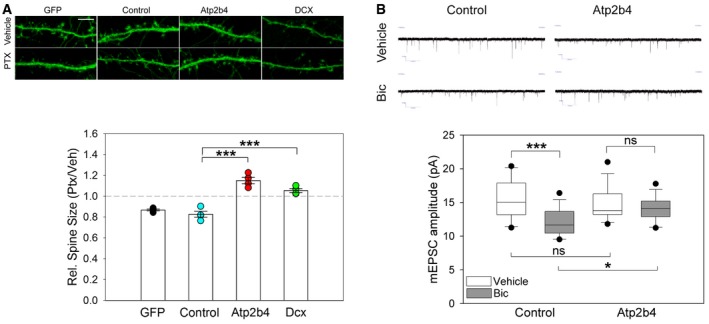
- Analysis of spine size when Atp2b4 or Dcx were overexpressed during PTX application. Upper panel: Representative confocal microscopy images of dendritic segments from PTX‐ or vehicle (Veh)‐treated hippocampal neurons transfected with indicated overexpression vectors and eGFP (control = Creb‐dMVP16, scale bar = 5 μm). Bottom panel: Quantification of dendritic spine size from four independent experiments. Dot‐plot presentation with mean relative spine size (PTX/Veh) ± s.e.m. (n = 4; 8 neurons, ˜200 spines/neuron per experimental condition; one‐way ANOVA; F (2,9) = 39.376, P < 0.001; groups were compared by Bonferroni post hoc test; Contr vs. Atp2b4 ***P < 0.001; Contr vs. Dcx ***P = 0.001).
- Upper panel: Representative traces of mEPSC recordings from bicuculline (Bic)‐ or vehicle‐treated neurons transfected with Atp2b4 or control expression vector. Bottom panel: Quantification of mEPSC amplitudes. Data are presented as box plots (control Veh/Bic n = 14/16; Atp2b4 Veh/Bic n = 13/16; GLM model; activity P = 0.001; overexpression × activity P = 0.048; estimated means of specific experimental conditions were compared by pairwise comparison in SPSS; Control protein Veh vs. Bic ***P < 0.001, Control protein Bic vs. Atp2b4 Bic *P = 0.022, Veh Control protein vs. Atp2b4 P = 0.561, Atp2b4 Veh vs. Bic P = 0.348).
Rbfox1 is a positive regulator of Dcx and Atp2b4 expression
In addition to miRNAs, RBPs have been shown to regulate post‐transcriptional gene expression by binding to conserved sequence elements within the 3′UTR of target mRNAs (Martin & Ephrussi, 2009). To narrow down on functionally important RBPs, we re‐analyzed CLIP experiments for nine different RBPs (Chi et al, 2009; Polymenidou et al, 2011; Charizanis et al, 2012; Ince‐Dunn et al, 2012; Ishigaki et al, 2012; Licatalosi et al, 2012; Wang et al, 2012; Lovci et al, 2013) that were performed in mouse brain. Thereby, we found that binding sites for Fus, Mbnl1/2, and Rbfox1/2/3 were most significantly (P < 0.001) overrepresented in the 3′UTR of PTX‐decreased genes compared to PTX‐increased genes (Fig 7A). Of those, Rbfox family members (Rbfox 1–3) were of particular interest, since Rbfox1/3 were recently shown to promote mRNA stability and translation of synaptic genes, including Camk2a (Lee et al, 2016).
Figure 7. Rbfox1 is a positive regulator of Atp2b4 and Dcx expression.
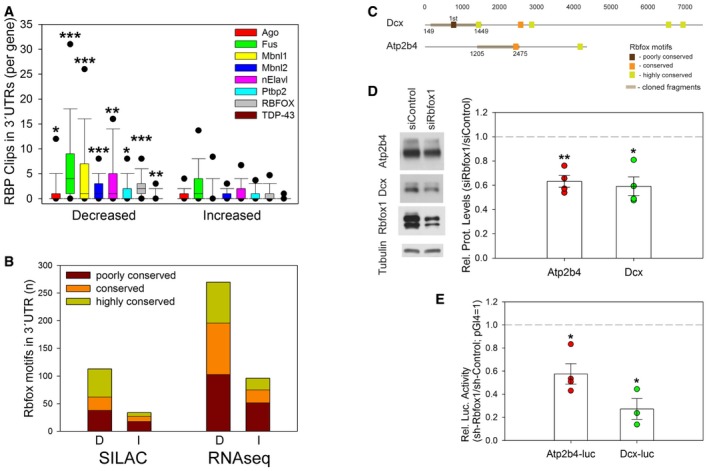
- Bioinformatic analysis of published CLIP experiments for indicated RBPs performed in mouse brains. Average number of RBP CLIP tags in 3′UTRs of genes corresponding to proteins whose synthesis is increased or decreased in PTX‐treated neurons (Mann–Whitney U‐test; *P = 0.016 (Ago); **P = 0.004 (nElavl); *P = 0.010 (Ptbp2); **P = 0.005 (TDP‐43); ***P < 0.001).
- Abundance of Rbfox binding motifs (GCAUG/UGCAUG) in 3′UTRs corresponding to genes with PTX‐decreased (D) or PTX‐increased (I) protein synthesis (Fig 4D) or mRNA levels (Fig 4A) according to TargetScan‐Human version 6.2. Seeds were classified as poorly conserved (≤ 3 species), conserved (4–9 species), or highly conserved (≥ 10 species). Bars represent total number of binding motifs per group.
- Localization of Rbfox binding motifs in the 3′UTRs of Atp2b4 and Dcx. Motifs were classified as poorly conserved, conserved, or highly conserved as described in (B).
- Western blot analysis of Atp2b4, Dcx, and Rbfox1 protein expression in hippocampal neurons transfected with siRbfox1 or siControl. Left: Representative Western blot. Tubulin was used as a loading control. Right: Quantification of four independent experiments. Dot‐plot presentation with mean relative protein levels (siRbfox1/siControl) normalized to tubulin ± s.e.m. (Atp2b4, Dcx n = 4; independent one‐sample t‐test, two‐tailed, heteroscedastic variance; Atp2b4 **P = 0.005, Dcx *P = 0.013).
- Luciferase reporter gene assay in shRNA‐Rbfox1‐ or shRNA‐Control‐transfected hippocampal neurons co‐transfected with the Dcx or Atp2b4 3′UTR reporter constructs (Fig 5D). Dot‐plot presentation with mean relative luciferase activity (shRbfox1/shControl) ± s.e.m. pGl4 (shRbfox1/shControl) was set to 1 (n = 3 for Dcx‐luc; n = 4 for Atp2b4‐luc; independent one‐sample t‐test, two‐tailed, heteroscedastic variance; Atp2b4‐luc *P = 0.017, Dcx‐luc *P = 0.015).
Source data are available online for this figure.
The Rbfox consensus binding motif ((U)GCAUG) was strongly overrepresented in 3′UTRs corresponding to PTX‐decreased genes compared to PTX‐increased proteins or mRNAs (Fig 7B; Tables EV10 and EV11). This effect was particularly pronounced for highly conserved binding sites, implying a functional importance. Atp2b4 and Dcx were also among the genes containing conserved Rbfox binding motifs in their 3′UTRs (Fig 7C), suggesting that they could be direct Rbfox targets. In support of this, Rbfox1 knockdown using a previously published Rbfox1‐specific small interfering RNA (Hu et al, 2013) significantly reduced Atp2b4 and Dcx protein expression (Fig 7D). Furthermore, shRNA‐mediated Rbfox1 knockdown significantly reduced the activity of Atp2b4‐luc and Dcx‐luc (Fig 7E), suggesting that Rbfox1 exerts its positive regulatory function via 3′UTR sequences. Taken together, these results demonstrate that Rbfox1 promotes the expression of PTX‐responsive genes, such as Atp2b4 and Dcx in conditions of normal neuronal activity.
Functional crosstalk between Rbfox1/3 and miR‐129‐5p during scaling
We next explored how the positive gene regulatory function of Rbfox can be reversed in high activity conditions that induce synaptic downscaling. Using Western blot, we found that the expression of Rbfox1 and its paralogue Rbfox3 (Fig 8A) was significantly reduced in PTX‐treated neurons compared to controls. Intriguingly, inspection of the 3′UTRs of Rbfox1 and Rbfox3 revealed the presence of multiple miR‐129‐5p binding sites, some of which with high degree of conservation (Fig 8B). This suggested that miR‐129‐5p could be involved in PTX‐dependent downregulation of Rbfox1/3. Indeed, miR‐129‐5p overexpression led to a reduction in endogenous Rbfox1/3 protein expression, suggesting that Rbfox1/3 are targets of miR‐129‐5p (Fig 8C). To test whether repression of Rbfox proteins by miR‐129‐5p is mediated by miR‐129‐5p sites in the 3′UTR, we performed luciferase assays, focusing on Rbfox1. We used reporters containing either a full‐length wt Rbfox1 3′UTR or an Rbfox1 3′UTR in which the two highly conserved miR‐129‐5p sites were mutated (129 Mut; Fig 8B). Whereas co‐transfection of miR‐129‐5p mimics significantly repressed expression of the Rbfox1‐luc wt reporter, it had no significant effect on Rbfox1‐luc 129 Mut (Fig 8D). This result indicates that miR‐129‐5p‐dependent repression of Rbfox1 is largely mediated by two highly conserved binding sites within the Rbfox1 3′UTR. We next explored the contribution of endogenous miR‐129‐5p to Rbfox1 repression. PTX treatment significantly repressed the expression of the Rbfox1‐luc wt reporter (Fig 8E), but not Rbfox1‐luc 129 Mut. These results suggest that endogenous miR‐129‐5p represses Rbfox1 during synaptic scaling via two highly conserved sites in the 3′UTR. However, mutating the two highly conserved miR‐129‐5p binding sites was not sufficient to completely prevent Rbfox1 repression by miR‐129‐5p mimics (Fig 8D) or PTX (Fig 8E), suggesting that additional miR‐129‐5p binding sites might be involved. Furthermore, anti‐miR‐129‐5p fully rescued the PTX‐dependent reduction in endogenous Rbfox1 protein (Fig 8F), confirming that miR‐129‐5p is required for Rbfox1 downregulation during scaling.
Figure 8. Rbfox1/3 proteins are downregulated by PTX and miR‐129‐5p.
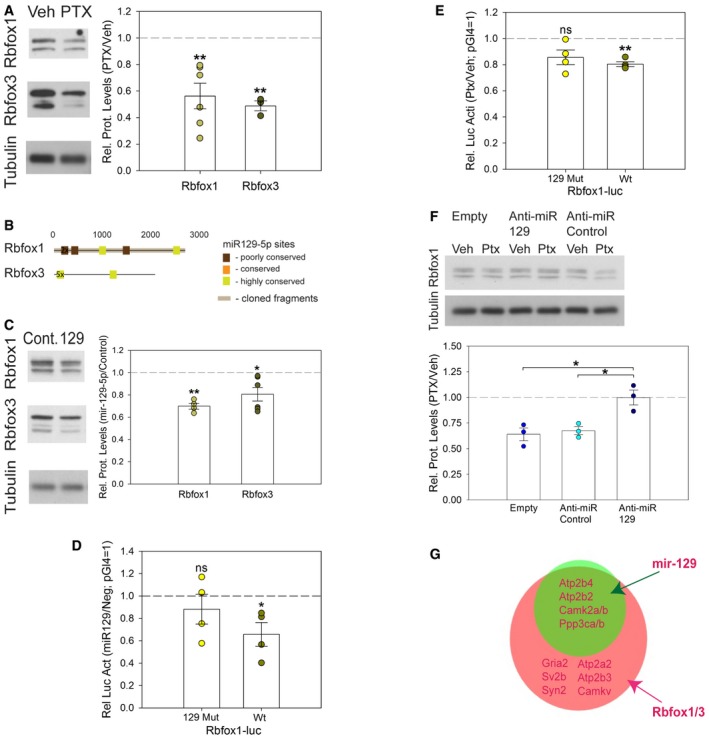
- Western blot analysis of Rbfox1 and Rbfox3 expression in lysates from vehicle (Veh)‐ and PTX (48 h)‐treated hippocampal neurons. Left: Representative Western blot. Tubulin was used as a loading control. Right: Quantification of multiple independent experiments. Dot‐plot presentation with mean relative protein levels (PTX/Veh) normalized to tubulin ± s.e.m. (Rbfox1 n = 6; Rbfox3 n = 3; independent one‐sample t‐test, two‐tailed, heteroscedastic variance; Rbfox1 **P = 0.006, Rbfox3 **P = 0.005).
- Localization of mir‐129‐5p binding sites in the 3′UTRs of Rbfox1/3 based on TargetScan‐Human version 6.0. Color code indicates different degree of conservation as described in Fig 4H.
- Western blot analysis of Rbfox1 and Rbfox3 protein expression in hippocampal neurons transfected with miR‐129‐5p or control duplex RNA. Left: Representative Western blot. Tubulin was used as a loading control. Right: Quantification of multiple independent experiments. Dot‐plot presentation with mean relative protein levels (miR‐129‐5p/control) normalized to tubulin ± s.e.m. (Rbfox1 n = 4; Rbfox3 n = 6; independent one‐sample t‐test, two‐tailed, heteroscedastic variance; Rbfox1 **P = 0.002, Rbfox3 *P = 0.025).
- Luciferase reporter gene assay in miR‐129‐5p‐ or control duplex RNA‐transfected hippocampal neurons co‐transfected with the Rbfox1 3′UTR reporter construct (Rbfox1‐luc) containing either wt or 129mut (2‐highly conserved sites) binding sites. Dot‐plot presentation with mean relative luciferase activity (miR‐129‐5p/control) normalized to pGL4 empty vector ± s.e.m. (n = 4; independent one‐sample t‐test, two‐tailed, heteroscedastic variance; Rbfox1‐luc wt *P = 0.0476, Rbfox1‐luc 129mut P = 0.4428).
- Luciferase reporter gene assay in vehicle (Veh)‐ or PTX‐treated hippocampal neurons transfected with the Rbfox1‐luc containing either wt or 129mut (2‐highly conserved sites) binding sites. Dot‐plot presentation with mean luciferase activity (PTX/Veh) ± s.e.m. pGl4 (PTX/Veh) was set to 1 (n = 4; independent one‐sample t‐test, two‐tailed, heteroscedastic variance; Rbfox1‐luc wt **P = 0.00185, Rbfox1‐luc 129mut P = 0.0812).
- Western blot analysis of Rbfox1 protein expression in PTX‐ or mock‐treated hippocampal neurons that were transfected with the indicated anti‐miRs. Upper panel: Representative Western blot. Tubulin was used as a loading control. Bottom panel: Quantification of three independent experiments. Dot‐plot presentation with mean relative protein levels (PTX/Veh) ± s.e.m. (n = 3; one‐way ANOVA; F (2,6) = 10.956, P = 0.010; groups were compared by Bonferroni post hoc test; anti‐miR‐129‐5p vs. anti‐miR Cont *P = 0.026; anti‐miR‐129‐5p vs. Empty *P = 0.016).
- Overlap between targets that contain miR‐129‐5p and Rbfox binding motifs. Selected targets are indicated.
Source data are available online for this figure.
Given the functional crosstalk between miR‐129‐5p and Rbfox1, we determined the degree of overlap between miR‐129‐5p and Rbfox binding motifs in the 3′UTR of PTX‐downregulated genes (Fig 8G). Intriguingly, almost all miR‐129‐5p targets also contain at least one potential Rbfox binding site (e.g., Atp2b4, Dcx). In addition, a large group of PTX‐downregulated genes are Rbfox targets (~28%) that lack consensus miR‐129‐5p seed matches (e.g., Gria2, Atp2b3). Therefore, by repressing Rbfox, miR‐129‐5p might increase its regulatory potential and in this way contribute to the suppression of up to 50% of PTX‐repressed proteins.
Discussion
A miR‐129‐5p/Rbfox1 module coordinates the repression of synaptic genes during synaptic downscaling
Using a systematic analysis of small RNAs (Fig 1B), mRNAs (Fig 4A), and protein synthesis (Fig 4D) in combination with bioinformatics (Figs 4G and H, 7A and B, and 8G), we revealed extensive post‐transcriptional regulation orchestrated by miRNAs and RBPs during homeostatic downscaling. By focusing on miRNA‐dependent gene regulation, we characterized the activity‐regulated miR‐129‐5p in more detail. miR‐129‐5p is upregulated by PTX, and a high number of its seed matches are localized within the 3′UTR of downregulated genes. miR‐129‐5p likely works in conjunction with other activity‐regulated miRNAs, including miR‐134‐5p, miR‐132/212, miR‐495, and miR‐543‐3p (Figs 1B and 4H, and Table EV1). For example, a number of PTX‐downregulated synaptic genes contain miR‐134‐5p binding sites (e.g., Dcx (2x), Camk2a, Ncald). A systematic mutation of binding sites for PTX‐regulated miRNAs together with the use of anti‐miR cocktails should help to decipher the “miRNA code” involved in synaptic downscaling. In addition, we found that miR‐129‐5p inhibits Rbfox1/3 expression in a 3′UTR‐dependent manner. This suggests that miR‐129‐5p employs at least two mechanisms that work in concert to dampen the expression of synaptic genes during PTX‐induced scaling (Appendix Fig S6F). First, it directly represses mRNAs containing miR‐129‐5p seed matches in their 3′UTR, for example, Atp2b4 and, to a lesser extent, Dcx. Second, it indirectly inhibits the expression of Rbfox1/3 targets lacking canonical miR‐129‐5p binding sites (e.g., Gria2) by interfering with Rbfox1/3 synthesis. Since the vast majority of miR‐129‐5p targets also contain adjacent Rbfox motifs in their 3′UTR but not vice versa (Fig 8G), removal of the positive regulator Rbfox might be the main trigger for repression. Direct miR‐129‐5p inhibition could then be used to further strengthen repression of specific targets. In the future, genome‐wide target maps of miR‐129‐5p and Rbfox1 will be highly informative to fully understand the miR‐129‐5p/Rbfox crosstalk and its significance for synaptic scaling. The potassium channel subunit Kv1.1 was recently shown to be a direct miR‐129‐5p target (Sosanya et al, 2013). However, PTX‐dependent upregulation of miR‐129‐5p was not paralleled by reduced, but rather elevated Kv1.1 mRNA levels (Table EV4). A possible explanation could be that Kv1.1 inhibition by miR‐129‐5p in neuronal dendrites occurs specifically at the translational level and does not involve mRNA degradation, as shown for other dendritic miRNA targets (e.g., Limk1; Schratt et al, 2006). Consistent with this hypothesis, mutation of the miR‐129‐5p binding site within Kv1.1 did not lead to changes in Kv1.1 mRNA levels (Sosanya et al, 2013). Since Kv1.1 was not detectable by pSILAC (Table EV7), our study does not allow any conclusions with regard to PTX‐dependent regulation of Kv1.1 protein synthesis.
Post‐transcriptional regulation in synaptic downscaling
In addition to the identification of specific regulatory mechanisms, our study provides support for a general importance of 3′UTR‐dependent post‐transcriptional regulation in synaptic downscaling. Bioinformatic analysis provides evidence that the 3′UTRs of PTX‐downregulated genes were on average more than twice as long as those from PTX‐upregulated genes (Fig 4G and Tables EV8 and EV9) and contain more miRNA (Fig 4H) and RBP binding motifs or CLIP peaks (Fig 7A and B). Together with previous studies that showed that mRNAs encoding synaptic proteins contain extraordinarily long 3′UTRs (Miura et al, 2013), this result suggests that genes suppressed during synaptic scaling are very susceptible to regulation at the level of mRNA translation and/or stability.
Novel players in synaptic downscaling might regulate calcium homeostasis and AMPA‐R dynamics
Our comprehensive proteome and transcriptome analysis further allowed the identification of entire pathways and signaling complexes that are coordinately regulated during scaling, such as calcium‐mediated signaling and glutamate receptor signaling (Fig 4B, Appendix Fig S5J). While both processes have been previously implicated in synaptic scaling, we obtained evidence for regulation of additional players within these pathways that can be functionally tested in future studies. For example, our results point to a global reduction in the neuronal calcium extrusion system (i.e., reductions in the membrane ATPases Atp2b1‐4; Fig 4 and Tables EV4 and EV7), in agreement with an important function of calcium homeostasis in synaptic scaling (Turrigiano, 2012). In the context of glutamate receptor complexes, we observed robust PTX‐dependent downregulation of regulators of AMPA‐R dynamics, such as Mpp2/Dlg2 and Rap2b (Karnak et al, 2002; Schwenk et al, 2012). Although our studies mainly focused on postsynaptic mechanisms, we also observed a downregulation of crucial presynaptic proteins (e.g., Synj1, Ncald, Syn1/2) during scaling, which is in agreement with previous studies (Davis & Muller, 2015).
Post‐transcriptional regulation of synaptic downscaling in epileptogenesis
Concerning pathophysiological significance, intriguing links between homeostatic plasticity and epilepsy‐causing channelopathies exist (Frank, 2014). Nevertheless, it is still debated whether homeostatic plasticity is protective or maladaptive in the context of epilepsy (Swann & Rho, 2014). Our results are more consistent with a maladaptive role of homeostatic plasticity in epilepsy. First, antagonism of miR‐129‐5p and miR‐134‐5p, two miRNAs that we found to be required for synaptic scaling (Fig 2; Fiore et al, 2014), prevent the development and progression of epileptic seizures (Fig 3; Jimenez‐Mateos et al 2012). Second, loss‐of‐function mouse models for several PTX‐downregulated genes [e.g., Dcx/Dclk2 (Kerjan et al, 2009), Bsn (Altrock et al, 2003), Rbfox1 (Gehman et al, 2011)] display increased seizure susceptibility. Third human loss‐of‐function mutations in PTX‐downregulated genes (e.g., SYNGAP1, Rbfox1) have been associated with epilepsy (Carvill et al, 2013; Lal et al, 2013; Frank, 2014). By inference, antagonizing PTX‐upregulated miRNAs, such as miR‐129‐5p, might represent a promising strategy for therapeutic intervention in epilepsy.
Materials and Methods
Animal experiments
All animal experiments were performed in accordance with the European Communities Council Directive (2010/63/EU) and ARRIVE guidelines. Please see Appendix Supplementary Methods for further details.
Perforant pathway stimulation paradigms
Rats were implanted bilaterally with unipolar stainless steel recording electrodes in the dentate granule cell layer of the dorsal hippocampus and with bipolar stimulating electrodes in the angular bundles of the perforant pathway. Please see Appendix Supplementary Methods for further details.
Electrophysiological and video monitoring methods
Immediately following perforant pathway stimulation (PPS), intracranial EEG was recorded continuously (24/7) from the left dentate gyrus via the implanted EEG transmitter. In addition, continuous video recordings were performed for each rat using network cameras (Edimax Technology, Willich, Germany). The complete EEG recording was visually evaluated for seizure activity by a trained researcher using NeuroArchiver software (Open Source Instruments, Watertown, MA, USA). Each confirmed electrographic seizure was related to behavior on the time‐stamped video recordings.
Perfusion‐fixation and tissue treatment
At different time points (1, 24, 72 h, 10 and 16 days after the final PPS as well as 1 and 30 days after the first spontaneous seizure; 17 days after electrode implantation for control animals; n = 3 per group), rats were deeply anesthetized with ketamine (100 mg/kg i.p.) and xylazine (15 mg/kg i.p.) and perfused through the aorta by gravity feed with ice‐cold saline for 2 min. After perfusion, brains were quickly removed from the skull. The hippocampi were dissected on ice, immediately snap‐frozen on dry ice and stored at −80°C.
Ago2‐IP and small RNA‐seq
Rat dentate gyrus was homogenised in RNase‐free IP buffer (300 mM NaCl, 5 mM MgCl2, 0.1% NP‐40, 50 mM Tris–HCl pH 7.5, protease, and RNAse inhibitor). Cell lysate was pre‐cleared using protein A/G beads (Santa Cruz Biotechnology), and Ago‐2 antibody (Cell Signaling, Cat. #2897) was added and allowed to incubate overnight at 4°C, after which A/G agarose beads were added and allowed to incubate for 2 h at 4°C. Ago2‐immunoprecipitated RNA was purified from pelleted beads using Trizol/chloroform purification. Please see Appendix Supplementary Methods for further details.
Silencing of miR‐129‐5p in naive mice
Mice (n = 3/4 per group) underwent surgical procedure under anesthesia (isoflurane; 5% induction, 1–2% maintenance) in a mouse adapted stereotaxic frame. Body temperature was maintained within the normal physiological range with a feedback‐controlled heat pad (Harvard Apparatus, Kent, UK; Holliston, MA). After a midline scalp incision, partial craniecotomies were performed. One guide cannula (Plastic Ones Inc.) was implanted to allow intracerebroventricular (i.c.v.) injection. This cannulas was placed on the dura mater with the following coordinates from bregma: AP = +0.3 mm, L = +0.9 mm. After recovery from surgery, mice were randomly injected (i.c.v.) with 0.5 nmol/2 μl locked nucleic acid (LNA) oligonucleotide targeting miR‐129‐5p (anti‐miR‐129‐5p) or control (PBS). Twenty‐four hours later, mice were deeply anesthetized (pentobarbital) and transcardially perfused (PBS). Ipsilateral hippocampi were removed to assess the levels of miR‐129‐5p by qPCR.
Focal‐onset status epilepticus in mice
A second cohort of mice (n = 5/group) were equipped for EEG recordings under surgical anesthesia in a mouse adapted stereotaxic frame, as previously described. Animals had skull‐mounted recording electrodes placed and fixed with dental cement. Two guide cannulas (Plastic Ones Inc.) were also implanted to allow intracerebroventricular (i.c.v.) and intra‐amygdala (i.a.) injections. Please see Appendix Supplementary Methods for further details.
EEG and behavior analysis
Mouse EEG data were analyzed and quantified using LabChart 8 software (ADInstruments, Oxford, UK) by a reviewer blind to treatment. Please see Appendix Supplementary Methods for further details.
Histopathology
Irreversible neuronal injury was assessed using Fluoro‐Jade B (FJB) (Millipore), as described (Jimenez‐Mateos et al, 2012). Please see Appendix Supplementary Methods for further details.
DNA constructs
Please see Appendix Supplementary Methods for further details.
Cell culture, transfection, and stimulation
Primary cultures of Sprague Dawley rats (Charles River Laboratories, Sulzfeld, Germany) embryonic hippocampal (HC) and cortical neurons (Ctx) were done as described previously (Schratt et al, 2006). Please see Appendix Supplementary Methods for further details.
Luciferase reporter assay
Primary neurons were transfected in triplicate in 24‐well plates using for each well 100 ng of pGL4 firefly reporter constructs and equal amounts of empty Renilla reporter as transfection control, alone or with respective miRNA mimics (Ambion® Pre‐miR™ miRNA Precursor: miR‐129‐5p, Neg‐Control1). Luciferase assays (overexpression/activity experiments) were performed 4 days post‐transfection using the Dual‐Luciferase reporter assay system (Baker & Boyce, 2014) on a GloMax R96 Microplate Luminometer (Promega).
Bicistronic reporter assay
Hippocampal neurons (13 DIV) were transfected with 150 ng of bicistronic reporter constructs (pTracer‐CMV‐dsRED, 50 ng) and 50 ng of EGFP vector together with the indicated anti‐miRs (pLNAs—Exiqon). Cells were analyzed at 20 DIV. pTracer‐129‐5p‐sensor contains two copies of a perfect binding site for miR‐129‐5p in the 3′UTR of dsRED, which leads to degradation of the dsRED RNA in the presence of miR‐129‐5p. Cells in which the dsRED signal did not exceed background levels were scored as “miRNA positive”.
Image analysis
All image analysis was performed with the scientist blinded to the experimental conditions. Please see Appendix Supplementary Methods for further details.
Single molecule fluorescence in situ hybridization (smFISH)
Dissociated hippocampal neurons were fixed at 20 DIV using 4% paraformaldehyde/4% sucrose/PBS for 30 min at room temperature. FISH was performed using the QuantiGene (QG) ViewRNA kit (Affymetrix) according to the manufacturer's protocol using probes for Camk2a, Dcx, and Atp2b4. Please see Appendix Supplementary Methods for further details.
Preparation of protein extracts and Western blot
A total of 2.7 million hippocampal cells were plated on one 6‐well plate and treated with fluorodeoxyuridine (FUDR; Sigma; F‐0503) + uridine (Sigma; F‐303) (final concentration 10 μM) from 3 DIV to stop proliferation of non‐neuronal cells. At 20 DIV, neuronal enriched cultures were washed once with warm DPBS (Invitrogen). Afterward, modified RIPA buffer (50 mM Tris–HCl, 150 mM NaCl, 0.5% NP‐40, 0.1% SDS, pH 7.4) containing Complete Protease Inhibitor Cocktail EDTA‐free (Roche) was added. Please see Appendix Supplementary Methods for further details.
Cross‐linking assay for measuring GluA1 cell surface expression
To be able to differentiate between surface and intracellularly expressed GluA1 by SDS–PAGE and immunoblotting, we used a modified protein cross‐linking assay developed by Boudreau et al (2012). Please see Appendix Supplementary Methods for further details.
Electrophysiology
Miniature excitatory postsynaptic currents (mEPSCs) were recorded in whole‐cell voltage‐clamp mode using an EPC‐10 patch‐clamp amplifier and PATCHMASTER software (HEKA Elektronik, Lambrecht, Germany). Dissociated hippocampal neurons were transfected at 12 DIV with corresponding plasmids or anti‐miRs (pLNAs; Exiqon). Bicuculline (20 μM) was applied on 18–19 DIV for 48 h before cells were transferred to the bath solution on 20–21 DIV. Please see Appendix Supplementary Methods for further details.
Pulsed SILAC labeling of primary hippocampal neuronal cells
Analysis of arginine‐6 and lysine‐D4 incorporation rates
At 10 DIV, Neurobasal medium without arginine and lysine was added to FUDR‐treated HC (1:1 dilution with “old” medium). Media containing arginine‐6/lysine‐D4 (final conc. 200 μM/400 μM; + proline 1.72 mM) was added to the cells at 12, 13, 14, 15, 16, and 17 DIV. Non‐labeled proline was used to prevent arginine to proline conversion. At 18 DIV, proteins were extracted with modified RIPA buffer (150 mM NaCl, 50 mM Tris–HCl pH 7.4, 0.5% NP‐40, 0.1% SDS), measured, and processed as described in Nolte et al (2015). Please see Appendix Supplementary Methods for further details.
SILAC labeling for protein quantification
Arginine‐10 (200 μM), lysine‐8 (400 μM), and non‐labeled proline (1.72 mM) were applied to neuronal cells 4 h after plating for 20–27 days. Almost 90% SILAC labeling was achieved after 20 days (Table EV5), and those labeled proteins were used as SILAC spike‐in to accurately quantify protein abundances of non‐labeled (pre‐existing) and arginine‐6/lysine‐D4‐labeled (newly synthesized) proteins in primary neuronal cells (Geiger et al, 2010).
PTX experiment
Neuronal enriched cultures were fed without Arg/Lys from 9 DIV (to dilute the non‐labeled arginine and lysine). Decrease in non‐labeled arginine and lysine concentration in the medium did not have obvious effect on cell morphology and viability. At 15 DIV, Arg6/LysD4 (200 μM/400 μM; + Pro at 1.72 mM) was added to the medium. PTX was applied at 18 DIV for 48 h. At 20 DIV, proteins were extracted and concentration measured as described earlier. Samples were mixed 1:1 with neuronal spike‐in standard and separated by 1D SDS–PAGE gel electrophoresis (Bio‐Rad). Please see Appendix Supplementary Methods for further details.
RNA extraction
RNA was extracted from FUDR‐treated HC using peqGOLD Isolation Systems TriFast™ (Peqlab) following the manufacturer's protocol. To remove potential DNA contamination, RNA samples were treated with TURBO™ DNase (Ambion) and RNA was re‐extracted as described before. Samples were stored at −80°C until further use. For in vivo experiments with anti‐miR‐129‐5p application, total RNA was isolated from brain samples using Trizol (Invitrogen), as described previously (Jimenez‐Mateos et al, 2012).
Quantitative real‐time PCR
RNA was reverse‐transcribed with SuperScript® III Reverse Transcriptase (Invitrogen) using random hexamers (total RNA) or oligo dT20 primers (poly‐RNA) according to manufacturer's instructions. Quantitative real‐time PCR was performed with the StepOnePlus Real‐Time PCR System (Applied Biosystems), using iTaq SYBR Green Supermix with ROX (Bio‐Rad) for detection of mRNA and TaqMan MicroRNA Assay kits (Applied Biosystems) for detection of mature miRNAs (miR‐132‐3p, miR‐129‐5p). Please see Appendix Supplementary Methods for further details.
Small RNA‐seq
Small RNA libraries were constructed and sequenced by the EMBL genomic core facility (Heidelberg, Germany). Please see Appendix Supplementary Methods for further details.
PolyA RNA‐seq
PolyA RNA sequencing was performed by EMBL genomic core facility (Heidelberg, Germany).
Bioinformatic analysis
Please see Appendix Supplementary Methods for a detailed description of bioinformatic approaches (mapping; differential gene expression; 3′UTR length estimation; miRNA and Rbfox motif analysis; GO term enrichment; CLIP analysis).
Statistics
Unless otherwise stated, three independent experiments were performed for each data set. Detailed information about statistics for each figure is in Table EV12. Please see Appendix Supplementary Methods for further details.
Data access
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository (Vizcaino et al, 2016) with the dataset identifier PXD004150. Data from RNA‐seq experiments were submitted to the GEO repository with the dataset identifier GSE81437.
Author contributions
MR designed and performed most of the experiments, analyzed the data, and wrote the manuscript. FM and CD analyzed data from RNA‐seq experiments and performed integrative bioinformatic analyses. RF performed and analyzed spine experiments. SK analyzed data from small RNA sequencing. AA‐A performed and analyzed patch‐clamp recordings. CRR performed in vivo rescue experiments with anti‐miR‐129. SBi performed and analyzed FISH experiments. SBa, BN, and FR performed rat PPS. MTV and JK performed Ago2‐IP and analyzed small RNA‐seq. MK and TB supervised mass spectrometry analysis. RR and GB processed the human samples and ran the qPCR. ND performed patient recruitment. MF performed tissue preparation and hippocampal sclerosis grading. DO performed operations of human TLE patients. DH supervised the human epilepsy study. GS wrote the manuscript and coordinated the entire project.
Conflict of interest
The authors declare that they have no conflict of interest.
Supporting information
Appendix
Table EV1
Table EV2
Table EV3
Table EV4
Table EV5
Table EV6
Table EV7
Table EV8
Table EV9
Table EV10
Table EV11
Table EV12
Review Process File
Source Data for Figure 1
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 7
Source Data for Figure 8
Acknowledgements
We thank J.D. Richter and M.E. Greenberg for providing reagents. We thank R. Fiore and A. Antoniou for comments on the manuscript. We thank E. Becker, R. Gondrum, U. Beck and B. Kowalski for excellent technical assistance. This work was supported by grants from the EU (ERC Starting Grant “Neuromir”; FP7 “EpimiRNA” No. 602130) and the DFG (SPP1738; FOR2107; SCHR 1136/8‐1) to G.S. as well as DFG FI 2157/2‐1 to R.F. S.Bi. is a recipient of a grant from the Von Behring‐Röntgen‐Stiftung (62‐0004).
The EMBO Journal (2017) 36: 1770–1787
References
- Altrock WD, tom Dieck S, Sokolov M, Meyer AC, Sigler A, Brakebusch C, Fassler R, Richter K, Boeckers TM, Potschka H, Brandt C, Loscher W, Grimberg D, Dresbach T, Hempelmann A, Hassan H, Balschun D, Frey JU, Brandstatter JH, Garner CC et al (2003) Functional inactivation of a fraction of excitatory synapses in mice deficient for the active zone protein bassoon. Neuron 37: 787–800 [DOI] [PubMed] [Google Scholar]
- Baker JM, Boyce FM (2014) High‐throughput functional screening using a homemade dual‐glow luciferase assay. J Vis Exp 88: e50282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136: 215–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Milovanovic M, Conrad KL, Nelson C, Ferrario CR, Wolf ME (2012) A protein cross‐linking assay for measuring cell surface expression of glutamate receptor subunits in the rodent brain after in vivo treatments. Curr Protoc Neurosci Chapter 5: Unit 5.30.31‐19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvill GL, Heavin SB, Yendle SC, McMahon JM, O'Roak BJ, Cook J, Khan A, Dorschner MO, Weaver M, Calvert S, Malone S, Wallace G, Stanley T, Bye AM, Bleasel A, Howell KB, Kivity S, Mackay MT, Rodriguez‐Casero V, Webster R et al (2013) Targeted resequencing in epileptic encephalopathies identifies de novo mutations in CHD2 and SYNGAP1. Nat Genet 45: 825–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charizanis K, Lee KY, Batra R, Goodwin M, Zhang C, Yuan Y, Shiue L, Cline M, Scotti MM, Xia G, Kumar A, Ashizawa T, Clark HB, Kimura T, Takahashi MP, Fujimura H, Jinnai K, Yoshikawa H, Gomes‐Pereira M, Gourdon G et al (2012) Muscleblind‐like 2‐mediated alternative splicing in the developing brain and dysregulation in myotonic dystrophy. Neuron 75: 437–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi SW, Zang JB, Mele A, Darnell RB (2009) Argonaute HITS‐CLIP decodes microRNA‐mRNA interaction maps. Nature 460: 479–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JE, Lee PR, Chen S, Li W, Fields RD (2011) MicroRNA regulation of homeostatic synaptic plasticity. Proc Natl Acad Sci USA 108: 11650–11655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombrita C, Silani V, Ratti A (2013) ELAV proteins along evolution: back to the nucleus? Mol Cell Neurosci 56: 447–455 [DOI] [PubMed] [Google Scholar]
- Davis GW, Muller M (2015) Homeostatic control of presynaptic neurotransmitter release. Annu Rev Physiol 77: 251–270 [DOI] [PubMed] [Google Scholar]
- Decembrini S, Bressan D, Vignali R, Pitto L, Mariotti S, Rainaldi G, Wang X, Evangelista M, Barsacchi G, Cremisi F (2009) MicroRNAs couple cell fate and developmental timing in retina. Proc Natl Acad Sci USA 106: 21179–21184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai NS, Cudmore RH, Nelson SB, Turrigiano GG (2002) Critical periods for experience‐dependent synaptic scaling in visual cortex. Nat Neurosci 5: 783–789 [DOI] [PubMed] [Google Scholar]
- Diering GH, Gustina AS, Huganir RL (2014) PKA‐GluA1 coupling via AKAP5 controls AMPA receptor phosphorylation and cell‐surface targeting during bidirectional homeostatic plasticity. Neuron 84: 790–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diering GH, Nirujogi RS, Roth RH, Worley PF, Pandey A, Huganir RL (2017) Homer1a drives homeostatic scaling‐down of excitatory synapses during sleep. Science 355: 511–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evers DM, Matta JA, Hoe HS, Zarkowsky D, Lee SH, Isaac JT, Pak DT (2010) Plk2 attachment to NSF induces homeostatic removal of GluA2 during chronic overexcitation. Nat Neurosci 13: 1199–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore R, Khudayberdiev S, Christensen M, Siegel G, Flavell SW, Kim TK, Greenberg ME, Schratt G (2009) Mef2‐mediated transcription of the miR379‐410 cluster regulates activity‐dependent dendritogenesis by fine‐tuning Pumilio2 protein levels. EMBO J 28: 697–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore R, Rajman M, Schwale C, Bicker S, Antoniou A, Bruehl C, Draguhn A, Schratt G (2014) MiR‐134‐dependent regulation of Pumilio‐2 is necessary for homeostatic synaptic depression. EMBO J 33: 2231–2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank CA (2014) How voltage‐gated calcium channels gate forms of homeostatic synaptic plasticity. Front Cell Neurosci 8: 40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao M, Sossa K, Song L, Errington L, Cummings L, Hwang H, Kuhl D, Worley P, Lee HK (2010) A specific requirement of Arc/Arg3.1 for visual experience‐induced homeostatic synaptic plasticity in mouse primary visual cortex. J Neurosci 30: 7168–7178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehman LT, Stoilov P, Maguire J, Damianov A, Lin CH, Shiue L, Ares M Jr, Mody I, Black DL (2011) The splicing regulator Rbfox1 (A2BP1) controls neuronal excitation in the mammalian brain. Nat Genet 43: 706–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger T, Cox J, Ostasiewicz P, Wisniewski JR, Mann M (2010) Super‐SILAC mix for quantitative proteomics of human tumor tissue. Nat Methods 7: 383–385 [DOI] [PubMed] [Google Scholar]
- Groth RD, Lindskog M, Thiagarajan TC, Li L, Tsien RW (2011) Beta Ca2 + /CaM‐dependent kinase type II triggers upregulation of GluA1 to coordinate adaptation to synaptic inactivity in hippocampal neurons. Proc Natl Acad Sci USA 108: 828–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Q, Ruan H, Gilbert J, Wang G, Ma Q, Yao WD, Man HY (2015) MicroRNA miR124 is required for the expression of homeostatic synaptic plasticity. Nat Commun 6: 10045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Ho AL, Yuan L, Hu B, Hua S, Hwang SS, Zhang J, Hu T, Zheng H, Gan B, Wu G, Wang YA, Chin L, DePinho RA (2013) From the Cover: neutralization of terminal differentiation in gliomagenesis. Proc Natl Acad Sci USA 110: 14520–14527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibata K, Sun Q, Turrigiano GG (2008) Rapid synaptic scaling induced by changes in postsynaptic firing. Neuron 57: 819–826 [DOI] [PubMed] [Google Scholar]
- Ince‐Dunn G, Okano HJ, Jensen KB, Park WY, Zhong R, Ule J, Mele A, Fak JJ, Yang C, Zhang C, Yoo J, Herre M, Okano H, Noebels JL, Darnell RB (2012) Neuronal Elav‐like (Hu) proteins regulate RNA splicing and abundance to control glutamate levels and neuronal excitability. Neuron 75: 1067–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigaki S, Masuda A, Fujioka Y, Iguchi Y, Katsuno M, Shibata A, Urano F, Sobue G, Ohno K (2012) Position‐dependent FUS‐RNA interactions regulate alternative splicing events and transcriptions. Sci Rep 2: 529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez‐Mateos EM, Bray I, Sanz‐Rodriguez A, Engel T, McKiernan RC, Mouri G, Tanaka K, Sano T, Saugstad JA, Simon RP, Stallings RL, Henshall DC (2011) miRNA Expression profile after status epilepticus and hippocampal neuroprotection by targeting miR‐132. Am J Pathol 179: 2519–2532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez‐Mateos EM, Engel T, Merino‐Serrais P, McKiernan RC, Tanaka K, Mouri G, Sano T, O'Tuathaigh C, Waddington JL, Prenter S, Delanty N, Farrell MA, O'Brien DF, Conroy RM, Stallings RL, DeFelipe J, Henshall DC (2012) Silencing microRNA‐134 produces neuroprotective and prolonged seizure‐suppressive effects. Nat Med 18: 1087–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez‐Mateos EM, Engel T, Merino‐Serrais P, Fernaud‐Espinosa I, Rodriguez‐Alvarez N, Reynolds J, Reschke CR, Conroy RM, McKiernan RC, deFelipe J, Henshall DC (2015) Antagomirs targeting microRNA‐134 increase hippocampal pyramidal neuron spine volume in vivo and protect against pilocarpine‐induced status epilepticus. Brain Struct Funct 220: 2387–2399 [DOI] [PubMed] [Google Scholar]
- Karnak D, Lee S, Margolis B (2002) Identification of multiple binding partners for the amino‐terminal domain of synapse‐associated protein 97. J Biol Chem 277: 46730–46735 [DOI] [PubMed] [Google Scholar]
- Kedde M, Agami R (2008) Interplay between microRNAs and RNA‐binding proteins determines developmental processes. Cell Cycle 7: 899–903 [DOI] [PubMed] [Google Scholar]
- Kerjan G, Koizumi H, Han EB, Dube CM, Djakovic SN, Patrick GN, Baram TZ, Heinemann SF, Gleeson JG (2009) Mice lacking doublecortin and doublecortin‐like kinase 2 display altered hippocampal neuronal maturation and spontaneous seizures. Proc Natl Acad Sci USA 106: 6766–6771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Ziff EB (2014) Calcineurin mediates synaptic scaling via synaptic trafficking of Ca2+‐permeable AMPA receptors. PLoS Biol 12: e1001900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal D, Trucks H, Moller RS, Hjalgrim H, Koeleman BP, de Kovel CG, Visscher F, Weber YG, Lerche H, Becker F, Schankin CJ, Neubauer BA, Surges R, Kunz WS, Zimprich F, Franke A, Illig T, Ried JS, Leu C, Nurnberg P et al (2013) Rare exonic deletions of the RBFOX1 gene increase risk of idiopathic generalized epilepsy. Epilepsia 54: 265–271 [DOI] [PubMed] [Google Scholar]
- Lee JA, Damianov A, Lin CH, Fontes M, Parikshak NN, Anderson ES, Geschwind DH, Black DL, Martin KC (2016) Cytoplasmic Rbfox1 regulates the expression of synaptic and autism‐related genes. Neuron 89: 113–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letellier M, Elramah S, Mondin M, Soula A, Penn A, Choquet D, Landry M, Thoumine O, Favereaux A (2014) miR‐92a regulates expression of synaptic GluA1‐containing AMPA receptors during homeostatic scaling. Nat Neurosci 17: 1040–1042 [DOI] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Ku L, Wilkinson KD, Warren ST, Feng Y (2001) The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Res 29: 2276–2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licatalosi DD, Yano M, Fak JJ, Mele A, Grabinski SE, Zhang C, Darnell RB (2012) Ptbp2 represses adult‐specific splicing to regulate the generation of neuronal precursors in the embryonic brain. Genes Dev 26: 1626–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovci MT, Ghanem D, Marr H, Arnold J, Gee S, Parra M, Liang TY, Stark TJ, Gehman LT, Hoon S, Massirer KB, Pratt GA, Black DL, Gray JW, Conboy JG, Yeo GW (2013) Rbfox proteins regulate alternative mRNA splicing through evolutionarily conserved RNA bridges. Nat Struct Mol Biol 20: 1434–1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KC, Ephrussi A (2009) mRNA localization: gene expression in the spatial dimension. Cell 136: 719–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKiernan RC, Jimenez‐Mateos EM, Sano T, Bray I, Stallings RL, Simon RP, Henshall DC (2012) Expression profiling the microRNA response to epileptic preconditioning identifies miR‐184 as a modulator of seizure‐induced neuronal death. Exp Neurol 237: 346–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miura P, Shenker S, Andreu‐Agullo C, Westholm JO, Lai EC (2013) Widespread and extensive lengthening of 3′ UTRs in the mammalian brain. Genome Res 23: 812–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte H, Holper S, Housley MP, Islam S, Piller T, Konzer A, Stainier DY, Braun T, Kruger M (2015) Dynamics of zebrafish fin regeneration using a pulsed SILAC approach. Proteomics 15: 739–751 [DOI] [PubMed] [Google Scholar]
- Norwood BA, Bauer S, Wegner S, Hamer HM, Oertel WH, Sloviter RS, Rosenow F (2011) Electrical stimulation‐induced seizures in rats: a “dose‐response” study on resultant neurodegeneration. Epilepsia 52: e109–e112 [DOI] [PubMed] [Google Scholar]
- Paschou M, Paraskevopoulou MD, Vlachos IS, Koukouraki P, Hatzigeorgiou AG, Doxakis E (2012) miRNA regulons associated with synaptic function. PLoS One 7: e46189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polymenidou M, Lagier‐Tourenne C, Hutt KR, Huelga SC, Moran J, Liang TY, Ling SC, Sun E, Wancewicz E, Mazur C, Kordasiewicz H, Sedaghat Y, Donohue JP, Shiue L, Bennett CF, Yeo GW, Cleveland DW (2011) Long pre‐mRNA depletion and RNA missplicing contribute to neuronal vulnerability from loss of TDP‐43. Nat Neurosci 14: 459–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remenyi J, Hunter CJ, Cole C, Ando H, Impey S, Monk CE, Martin KJ, Barton GJ, Hutvagner G, Arthur JS (2010) Regulation of the miR‐212/132 locus by MSK1 and CREB in response to neurotrophins. Biochem J 428: 281–291 [DOI] [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, Greenberg ME (2006) A brain‐specific microRNA regulates dendritic spine development. Nature 439: 283–289 [DOI] [PubMed] [Google Scholar]
- Schratt G (2009) microRNAs at the synapse. Nat Rev Neurosci 10: 842–849 [DOI] [PubMed] [Google Scholar]
- Schwenk J, Harmel N, Brechet A, Zolles G, Berkefeld H, Muller CS, Bildl W, Baehrens D, Huber B, Kulik A, Klocker N, Schulte U, Fakler B (2012) High‐resolution proteomics unravel architecture and molecular diversity of native AMPA receptor complexes. Neuron 74: 621–633 [DOI] [PubMed] [Google Scholar]
- Selbach M, Schwanhausser B, Thierfelder N, Fang Z, Khanin R, Rajewsky N (2008) Widespread changes in protein synthesis induced by microRNAs. Nature 455: 58‐63 [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF (2006) Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron 52: 475–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddoway B, Hou H, Xia H (2014) Molecular mechanisms of homeostatic synaptic downscaling. Neuropharmacology 78: 38–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosanya NM, Huang PP, Cacheaux LP, Chen CJ, Nguyen K, Perrone‐Bizzozero NI, Raab‐Graham KF (2013) Degradation of high affinity HuD targets releases Kv1.1 mRNA from miR‐129 repression by mTORC1. J Cell Biol 202: 53–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strehler EE, Filoteo AG, Penniston JT, Caride AJ (2007) Plasma‐membrane Ca(2+) pumps: structural diversity as the basis for functional versatility. Biochem Soc Trans 35: 919–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su W, Aloi MS, Garden GA (2016) MicroRNAs mediating CNS inflammation: small regulators with powerful potential. Brain Behav Immun 52: 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swann JW, Rho JM (2014) How is homeostatic plasticity important in epilepsy? Adv Exp Med Biol 813: 123–131 [DOI] [PubMed] [Google Scholar]
- Turrigiano G (2012) Homeostatic synaptic plasticity: local and global mechanisms for stabilizing neuronal function. Cold Spring Harb Perspect Biol 4: a005736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizcaino JA, Csordas A, del‐Toro N, Dianes JA, Griss J, Lavidas I, Mayer G, Perez‐Riverol Y, Reisinger F, Ternent T, Xu QW, Wang R, Hermjakob H (2016) 2016 update of the PRIDE database and its related tools. Nucleic Acids Res 44: D447–D456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanet A, Tacheny A, Arnould T, Renard P (2012) miR‐212/132 expression and functions: within and beyond the neuronal compartment. Nucleic Acids Res 40: 4742–4753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ET, Cody NA, Jog S, Biancolella M, Wang TT, Treacy DJ, Luo S, Schroth GP, Housman DE, Reddy S, Lecuyer E, Burge CB (2012) Transcriptome‐wide regulation of pre‐mRNA splicing and mRNA localization by muscleblind proteins. Cell 150: 710–724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara S, Takahashi H, Nishimura N, Kinoshita M, Asahina R, Kitsuki M, Tatsumi K, Furukawa‐Hibi Y, Hirai H, Nagai T, Yamada K, Tsuboi A (2014) Npas4 regulates Mdm2 and thus Dcx in experience‐dependent dendritic spine development of newborn olfactory bulb interneurons. Cell Rep 8: 843–857 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix
Table EV1
Table EV2
Table EV3
Table EV4
Table EV5
Table EV6
Table EV7
Table EV8
Table EV9
Table EV10
Table EV11
Table EV12
Review Process File
Source Data for Figure 1
Source Data for Figure 4
Source Data for Figure 5
Source Data for Figure 7
Source Data for Figure 8


