Abstract
Hypoxia occurs physiologically in the developing body, and changing oxygen tensions are known to direct tissue differentiation; however, in the context of pathology, the same hypoxia‐activated mechanisms may negatively affect tissue function. In this issue of The EMBO Journal, Esteban‐Martínez et al (2017) report that programmed mitophagy, dependent on hypoxia‐induced NIP‐3‐like protein X (BNIP3L, best known as NIX), is an essential step in differentiation of both retinal neurons and inflammatory macrophages.
Subject Categories: Autophagy & Cell Death, Development & Differentiation, Metabolism
During development, the body rapidly reshapes. At each moment, thousands of cells are formed and differentiate in synchrony, and the nature of the signals that govern this process so efficiently, yet so precisely, continues to baffle scientists. Various factors, including such offbeat mechanisms as mechanical force and electrical activity, were proposed to direct cell differentiation. The role of metabolism in this process is often overlooked, though phenotype transformation naturally requires integration of environmental and intracellular signals with the generation of energy and raw materials for new cellular structures. Mitophagy is a form of autophagy that was long considered a maintenance mechanism, whereby cells selectively degrade entire damaged mitochondria, a potential source of toxic reactive oxygen species (ROS; Ney, 2015). It is now appreciated that mitophagy can also occur as a part of cell differentiation, as for example, NIX‐dependent programmed mitophagy removes mitochondria from reticulocytes during erythrocyte maturation (Zhang et al, 2012).
In this issue of The EMBO Journal, Esteban‐Martínez et al (2017) unravel the mechanism of the prenatally occurring transition from proliferating neuroblasts to young retinal ganglion cells (RGCs)—the sole projecting neurons in the retina (Fig 1). The first critical step for RGC differentiation is hypoxia, which induces expression of NIX, a critical factor for mitophagy. Developing RGCs indeed exhibited decreased mitochondrial mass, and this was followed by a metabolic switch to glycolysis. Blocking this hypoxia‐induced pathway at any step inhibited RGC maturation (Esteban‐Martínez et al, 2017). Interestingly, differentiation and migration of olfactory bulb neurons during development also rely on autophagy signaling (Vázquez et al, 2012; Petri et al, 2017), and aerobic glycolysis (glycolysis in presence of oxygen) was proposed to occur in the human neonatal brain specifically in regions where synaptic growth rates are the highest (Goyal et al, 2014). Together, these findings suggest that brain neurons may undergo an analogous mitophagy‐dependent metabolic switch during their differentiation.
Figure 1. Hypoxia and mitophagy may direct cell differentiation in health and disease.
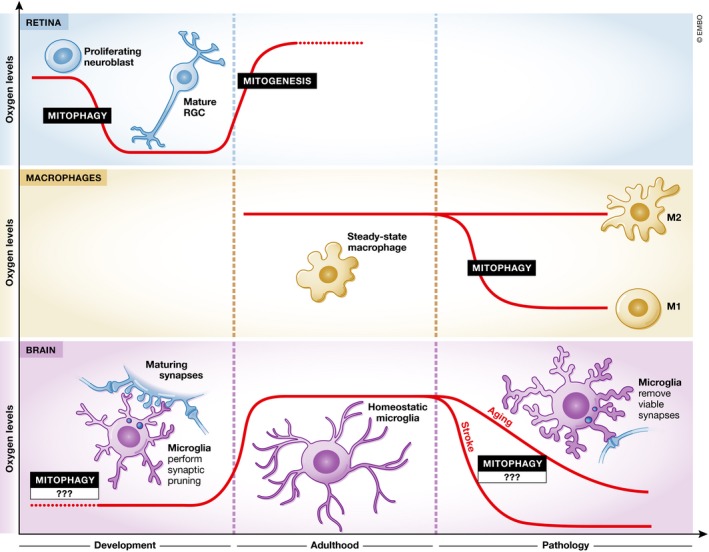
Hypoxia‐induced mitophagy directs macrophage differentiation to pro‐inflammatory M1 cells and maturation of RGCs in the retina. In the brain, hypoxic conditions temporally correlate with the periods of increased synaptic pruning activity, which shapes brain circuitry in development, but if over‐activated in pathology, may eliminate viable synapses, thereby leading to brain function decline.
Esteban‐Martínez et al (2017) further demonstrated a similar metabolic rewiring governed by response to hypoxia‐ and NIX‐dependent mitophagy in differentiation of pro‐inflammatory (M1) macrophages (Esteban‐Martínez et al, 2017; Fig 1). Inflammation and hypoxia often occur together, and there is significant cross talk between the cellular responses they elicit; thus, for example, hypoxia‐inducible factors (HIFs) and NF‐κB (an essential mediator of inflammation) have several common triggers, regulators, and targets (Biddlestone et al, 2015). Along the local immune response, inflamed tissue is initially characterized by the presence of M1 macrophages, which rely on glycolysis, while at later stages, the presence of M2 macrophages, which depend on oxidative phosphorylation, is associated with tissue regeneration (Jha et al, 2015). Here, involvement of mitophagy in M1 differentiation was only studied under in vitro conditions (Esteban‐Martínez et al, 2017); in vivo experiments will elucidate whether hypoxia and mitophagy in immune cells can modulate immune responses and restoration of homeostasis. If so, the newly discovered mechanism can shed light on pathological conditions associated with hypoxia or a hypoxia‐like response. According to the new findings, oxygen starvation and mitophagy in the brain could modulate the phenotype of both neuronal progenitors and of the brain's resident macrophages, the microglia.
Brain development and various neurological conditions are largely dependent on immunological factors. In particular, microglia are involved in diverse homeostatic functions during central nervous system formation and maintenance, and their altered activity was implicated in both neurodevelopmental and neurodegenerative conditions (Schwartz & Deczkowska, 2016). For example, microglia are critical for shaping neuronal circuitry during brain development in a process of synaptic pruning, whereby microglial cells use their phagocytic potential to eliminate weak synaptic connections between neurons. Under inflammatory conditions, for example, in brain aging, stroke, or in Alzheimer's disease, microglia assume a pro‐inflammatory phenotype and their synaptic pruning machinery is re‐activated, leading to removal of viable synapses, and consequently, to cognitive loss (Stephan et al, 2012). Hypoxic conditions naturally occur in the brain during development, but brain aging was also associated with increased expression of HIF1α, probably in response to inefficient oxygen perfusion from the frail capillaries (Wang et al, 2012). Therefore, periods of intense microglia‐mediated synaptic pruning and a hypoxia response in the brain seem to be temporally correlated (Fig 1). In addition, transient ischemia (a model of ischemic stroke) leads to increased synaptic clearance by microglia (Wake et al, 2009), further suggesting that hypoxia may determine synaptic pruning intensity, and therefore, brain function, throughout life. Future studies will determine whether low oxygen tensions in these cases led to synaptic clearance due to synapse inactivity, or due to induction of microglial pro‐inflammatory phenotype upon hypoxia, and whether mitophagy has a role in this process.
Overall, changing oxygen tensions and related metabolic switches emerge as unexpected forces during both development and under pathology, especially in the brain, an organ extremely sensitive to deviations from homeostasis. Given the tremendous burden caused by brain pathologies, this new discovery may inspire future research on the possible involvement of such hypoxia‐associated pathomechanisms in neurological diseases.
See also: L Esteban-Martínez et al (June 2017)
References
- Biddlestone J, Bandarra D, Rocha S (2015) The role of hypoxia in inflammatory disease (Review). Int J Mol Med 35: 859–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban‐Martínez L, Sierra‐Filardi E, McGreal RS, Salazar‐Roa M, Mariño G, Seco E, Durand S, Enot D, Graña O, Malumbres M, Cvekl A, Cuervo AM, Kroemer G, Boya P (2017) Programmed mitophagy is essential for the glycolytic switch during cell differentiation. EMBO J 36: 1688–1706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal MS, Hawrylycz M, Miller JA, Snyder AZ, Raichle ME (2014) Aerobic glycolysis in the human brain is associated with development and neotenous gene expression. Cell Metab 19: 49–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha AK, Huang SC‐C, Sergushichev A, Lampropoulou V, Ivanova Y, Loginicheva E, Chmielewski K, Stewart KM, Ashall J, Everts B (2015) Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 42: 419–430 [DOI] [PubMed] [Google Scholar]
- Ney PA (2015) Mitochondrial autophagy: origins, significance, and role of BNIP3 and NIX. Biochimica et Biophysica Acta (BBA)‐Molecular. Cell Res 1853: 2775–2783 [DOI] [PubMed] [Google Scholar]
- Petri R, Pircs K, Jönsson ME, Åkerblom M, Brattås PL, Klussendorf T, Jakobsson J (2017) let‐7 regulates radial migration of new‐born neurons through positive regulation of autophagy. EMBO J 36: 1379–1391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M, Deczkowska A (2016) Neurological disease as a failure of brain‐immune crosstalk: the multiple faces of neuroinflammation. Trends Immunol 37: 668–679 [DOI] [PubMed] [Google Scholar]
- Stephan AH, Barres BA, Stevens B (2012) The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci 35: 369–389 [DOI] [PubMed] [Google Scholar]
- Vázquez P, Arroba AI, Cecconi F, de la Rosa EJ, Boya P, De Pablo F (2012) Atg5 and Ambra1 differentially modulate neurogenesis in neural stem cells. Autophagy 8: 187–199 [DOI] [PubMed] [Google Scholar]
- Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J (2009) Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci 29: 3974–3980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wu H, Guo H, Zhang G, Zhang R, Zhan S (2012) Increased hypoxia‐inducible factor 1alpha expression in rat brain tissues in response to aging. Neural Regen Res 7: 778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Loyd MR, Randall MS, Waddell MB, Kriwacki RW, Ney PA (2012) A short linear motif in BNIP3L (NIX) mediates mitochondrial clearance in reticulocytes. Autophagy 8: 1325–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]


