Abstract
Background:
Depression is highly prevalent in uremic patients undergoing hemodialysis (HD). We previously found that low free-carnitine levels are associated with depression severity in male patients undergoing HD. However, whether L-carnitine supplementation improves the depression state in male patients undergoing HD remains unclear.
Methods:
Sixteen male patients undergoing HD were orally administered 900 mg L-carnitine daily or intravenously administered 1000 mg L-carnitine immediately after undergoing HD for 3 months. The depression state and various types of carnitine levels were evaluated using the self-rating depression scale (SDS) and tandem mass spectrometry, respectively, at baseline and 3 months after treatment.
Results:
L-carnitine supplementation significantly increased serum levels of free and other acylcarnitine types, associated with improved SDS scores in male patients undergoing HD. Univariate analysis revealed that low baseline butyryl- and isovaleryl-/2-methylbutyryl-carnitine levels were significantly correlated with SDS scores after treatment. Multiple regression analysis revealed that butyryl-carnitine levels were a sole independent predictor of SDS scores after treatment (r2 = 0.533).
Conclusion:
L-carnitine supplementation for 3 months improved the depression state in uremic male patients undergoing HD. Thus, low butyryl-carnitine levels may predict the clinical response to L-carnitine supplementation in male patients undergoing HD and who have mild depression.
Keywords: Carnitine, depression, hemodialysis, SDS, uremic male patients, mood disorder
1. Introduction
Depression, a mood disorder with a high personal, societal, and economic impact, is highly prevalent in patients undergoing hemodialysis (HD), possibly accounting for at least 20%–30% of patients [1]. Depression is associated with suicide risk and correlated with an increased risk for cardiovascular disease in patients with end-stage renal disease [2, 3]. Furthermore, in patients undergoing HD, depression may reduce the patient’s adherence to medication, thereby negatively affecting the quality of life [4, 5]. Although several factors such as malnutrition, inflammation, erythro- poietin resistance, environmental stress, and atherosclerotic cardiovascular disease contribute to the development and progression of depression in uremic patients, the precise underlying mechanism of depression and its therapeutic target remain unclear in patients undergoing HD [6-9].
Carnitine, a natural substance, is supplied by the intake of protein-rich foods and is synthesized by the liver, kidney, brain, epididymis, and testis in humans [10, 11]. Carnitine plays an important role in fatty acid β-oxidation and energy production by transporting long-chain fatty acids from the cytoplasm to the mitochondria as a shuttle [11]. We, along with others, reported that serum free-carnitine levels dramatically decreased in patients undergoing HD [12, 13]. In addition, we recently found that baseline low free-carnitine levels were independently correlated with the depression severity in uremic male patients, as assessed using the self-rating depression scale (SDS), which is a widely used questionnaire for evaluating depressive disorders [14]. However, whether L-carnitine supplementation improves the depression state in patients undergoing HD remains unclear. This study aimed to investigate the effects of L-carnithine supplementation on SDS scores in uremic male patients undergoing HD.
2. Materials and methods
2.1. Patients and Protocols
Sixteen uremic male patients undergoing chronic HD [mean age, 61.4 ± 10.5 years; mean duration of HD, 30.2 (1-85) months] and 16 age-matched healthy male controls (mean age, 62.1 ± 7.9 years) were enrolled. Patients were dialyzed for 4–5 h with high-flux dialyzers thrice a week. The patients were orally administered 900 mg L-carnitine daily or intravenously administered 1000 mg L-carnitine immediately after undergoing an HD session for 3 months. All the patients underwent a complete history, physical examination, and blood chemistry analysis such as total, free, and acylcarnitine levels by enzymatic methods at baseline and 3 months after the L-carnitine treatment [14]. Furthermore, acylcarnitines were profiled using tandem mass spectrometry at baseline and 3 months after the treatment, according to a previously described method [15]. Other blood chemistry parameters were measured at a commercial laboratory, as previously described (SRL., Inc. Tokyo, Japan) [16]. The depression state was assessed using SDS [14]. We were unable to repeatedly evaluate blood chemistry in one patient because of severe anemia.
Informed consent was obtained from all the patients, and the study protocol was approved by the Institutional Ethics Committees of Kurume University School of Medicine, Japan. This trial was registered with the University Hospital Medical Information Network clinical trials database (UMIN000010953).
3. Statistical analysis
Data are presented as means ± standard deviation. The presence or absence of diabetes mellitus and the use of renin–angiotensin system inhibitors and statins were coded as dummy variables. To compare clinical values between healthy controls and patients undergoing HD, unpaired t-test was performed. Significant differences of variables at baseline and 3 months after the L-carnitine treatment were analyzed using paired t-test. Univariate and stepwise regression analyses were also performed to determine SDS correlates after the L-carnitine treatment. Statistical significance was defined as p values of <0.05. All statistical analyses were performed using the SPSS system ver.20 (Chicago, IL, USA).
4. Results
4.1. Baseline Clinical Characteristics of Patients Undergoing HD and Healthy Controls
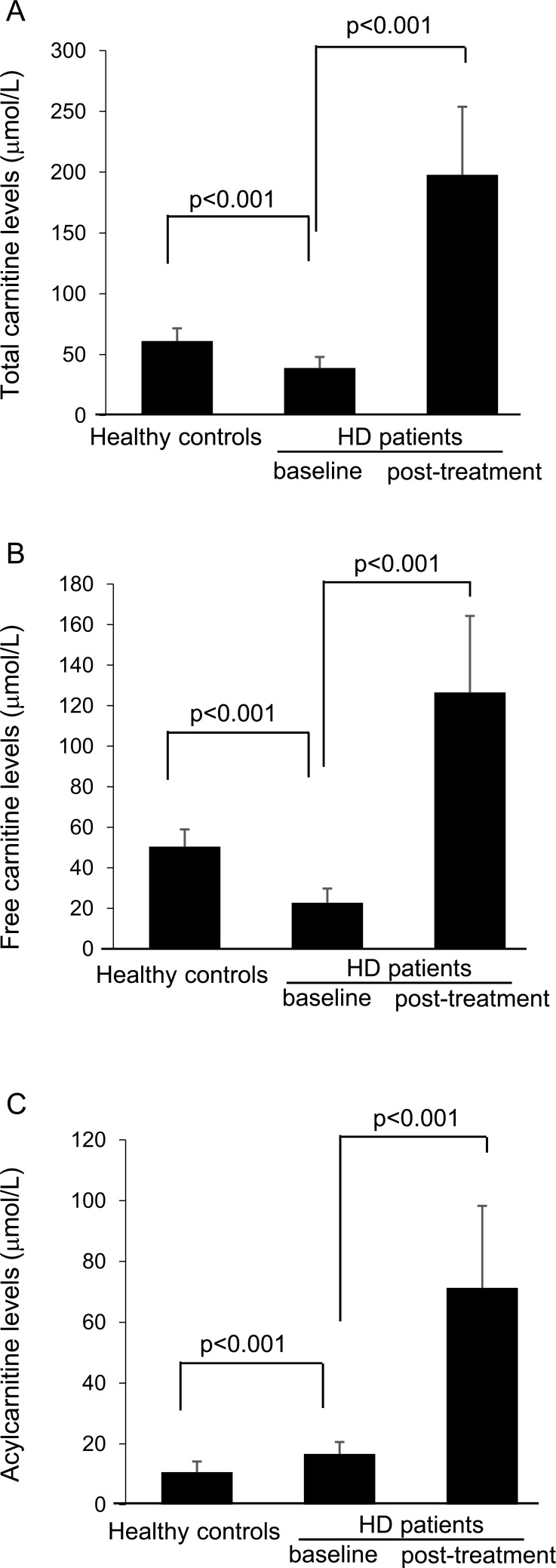
Body mass index, hemoglobin, total protein, serum albumin, alanine aminotransferase (ALT), and LDL-cholesterol levels were significantly lower and blood urea nitrogen and creatinine levels were higher in patients undergoing HD than in healthy controls (Table 1). Baseline total and free-carnitine levels just before the HD session were significantly lower and acylcarnitine levels were higher in patients undergoing HD than in healthy controls (Fig. 1A-C).
Table 1.
Clinical characteristics of healthy controls and HD patients.
| HD Patients | Healthy Controls | p-value | |
|---|---|---|---|
| No. of patients | 16 | 16 | |
| Age (years old) | 61.4 ± 10.5 | 62.1 ± 7.9 | 0.678 |
| HD duration (months) (range) | 30.2 (1-85) | - | - |
| HD time/day (hours) | 4.3 ± 0.6 | - | - |
| Body Mass Index (kg/m2) | 22.6 ± 3.5 | 25.5 ± 4.3 | 0.014 |
| Systolic blood pressure (mmHg) | 148 ± 25 | 139 ± 20 | 0.247 |
| Hemoglobin (g/dL) | 11.6 ± 1.1 | 14.6 ± 1.5 | <0.001 |
| Total protein (g/dL) | 6.21 ± 0.55 | 7.53 ± 0.45 | <0.001 |
| Albumin (g/dL) | 3.31 ± 0.44 | 4.56 ± 0.25 | <0.001 |
| AST (IU/L) | 19.6 ± 12.8 | 27.4 ± 10.2 | 0.065 |
| ALT (IU/L) | 15.3 ± 8.0 | 29.4 ± 18.5 | 0.009 |
| BUN (mg/dL) | 50.2 ± 12.1 | 18.6 ± 6.3 | <0.001 |
| Serum Cr (mg/dL) | 9.10 ± 3.34 | 0.81 ± 0.11 | <0.001 |
| Uric acid (mg/dL) | 6.86 ± 1.01 | 6.22 ± 1.35 | 0.203 |
| Corrected Ca (mg/dL) | 9.33 ± 0.43 | - | - |
| Phosphate (mg/dL) | 5.29 ± 1.36 | - | - |
| Total-cholesterol (mg/dL) | 159 ± 27 | 183 ± 38 | 0.057 |
| LDL-cholesterol (mg/dL) | 54 ± 22 | 109 ± 35 | 0.024 |
| Triglycerides (mg/dL) | 97 ± 46 | 99 ± 49 | 0.953 |
| Diabetes (No.) (-/+) | 8/8 | 16/0 | - |
| SDS | 43.8 ± 7.3 | - | - |
| Medication | |||
| RAS inhibitors (No.) (-/+) | 0/16 | 16/0 | - |
| Statins (No.) (-/+) | 8/8 | 16/0 | - |
Values are shown as mean±SD or range. No.=number. HD=hemodialysis; AST=aspartate aminotransaminase; ALT=alanine aminotransaminase; BUN=blood urea nitrogen; Cr=creatinine; Ca=calcium; LDL=low-density lipoprotein; SDS=self-rating depression scale; RAS=renin-angiotensin system.
Fig. (1).
Total, free, and acylcarnitines in healthy controls and patients undergoing HD.
4.2. Effects of L-carnitine Supplementation on Clinical Variables, Carnitine Levels, and SDS Scores in Patients Undergoing HD
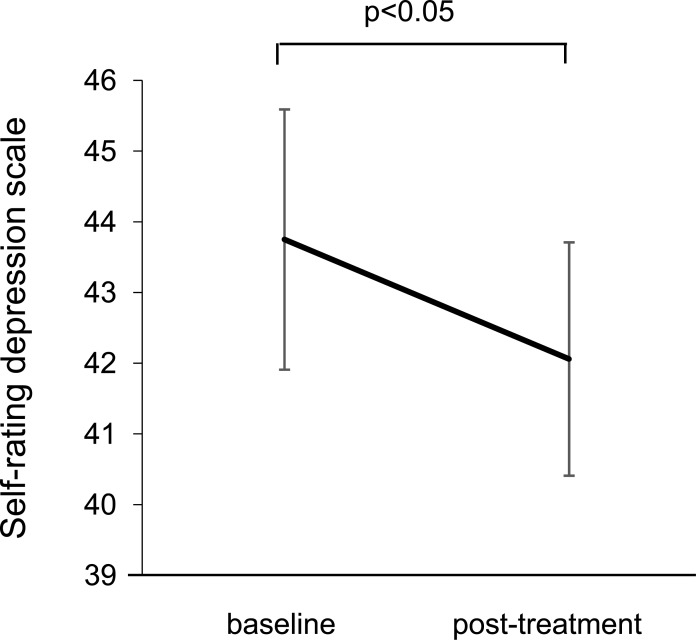
After the 3-month L-carnitine treatment, serum aspartate aminotransferase, ALT, creatinine, and uric acid levels were significantly reduced in patients undergoing HD (Table 2). Moreover, the L-carnitine treatment significantly increased total, free, and acylcarnitine levels just before the HD session, which was consistent with decreased SDS scores; i.e., the treatment improved the depression state in uremic male patients undergoing HD (Fig. 2).
Table 2.
Clinical variables at baseline and 3 months after the L-carnitine treatment.
| Variables | Baseline | Post-Treatment | p-value |
|---|---|---|---|
| Hemoglobin (g/dL) | 11.6 ± 1.1 | 11.3 ± 1.3 | 0.546 |
| Total protein (g/dL) | 6.21 ± 0.55 | 6.33 ± 0.52 | 0.125 |
| Albumin (g/dL) | 3.31 ± 0.44 | 3.31 ± 0.42 | 1.000 |
| AST (IU/L) | 19.6 ± 12.8 | 15.2 ± 7.3 | 0.024 |
| ALT (IU/L) | 15.3 ± 8.0 | 11.8 ± 4.9 | 0.024 |
| BUN (mg/dL) | 50.2 ± 12.1 | 46.3 ± 11.0 | 0.073 |
| Serum Cr (mg/dL) | 9.10 ± 3.34 | 8.24 ± 2.67 | 0.005 |
| Uric acid (mg/dL) | 6.86 ± 1.01 | 6.33 ± 0.78 | 0.007 |
| Corrected Ca (mg/dL) | 9.33 ± 0.43 | 9.09 ± 0.57 | 0.127 |
| Phosphate (mg/dL) | 5.29 ± 1.36 | 5.39 ± 1.38 | 0.818 |
| Total-cholesterol (mg/dL) | 159 ± 27 | 152 ± 26 | 0.166 |
| LDL-cholesterol (mg/dL) | 54 ± 22 | 78 ± 20 | 0.457 |
| Triglycerides (mg/dL) | 97 ± 46 | 99 ± 51 | 0.789 |
Values are shown as mean ± SD or range. HD=hemodialysis; AST=aspartate aminotransaminase; ALT=alanine aminotransaminase; BUN=blood urea nitrogen; Cr=creatinine; Ca=calcium; LDL=low-density lipoprotein.
Fig. (2).
SDS scores at baseline and 3 months after the L-carnitine treatment in patients undergoing HD.
4.3. Effects of the L-carnitine Treatment on Serum Levels of Various Types of Acylcarnitines and their Associations with SDS Scores
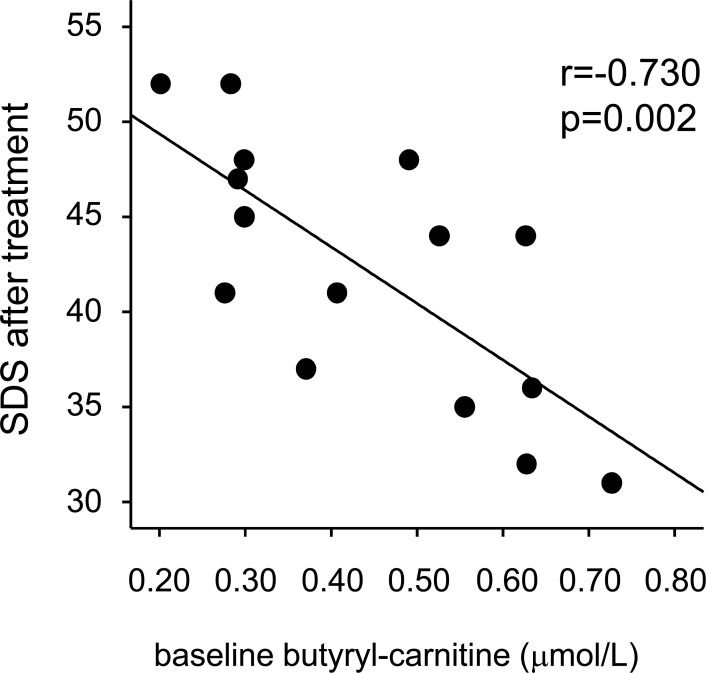
To explore which types of carnitines at baseline were correlated with SDS scores after the L-cartinine treatment, we further profiled the serum levels of carnitines using tandem mass spectrometry. Baseline free-carnitine (C0) and propionyl-carnitine (C3) levels just before the HD session were significantly lower and acylcarnitine levels of >3 carbon atoms, including butyryl-carnitine (C4), isovaleryl-/2-methylbutyryl-carnitine (C5), and 3-hydroxyoctadecenoyl-carnitine (C18:1-OH), were higher in patients undergoing HD than in healthy controls (Table 3). The L-carnitine treatment for 3 months significantly increased all carnitine levels examined, whose levels were reduced after the HD session (Table 3). Univariate regression analysis revealed that baseline butyryl-carnitine and isovaleryl-/2-methylbutyryl-carnitine levels were inversely correlated with SDS scores after the L-carnitine treatment (r=-0.730 and p = 0.002 for butyryl-carnitine and r=-0.548 and p = 0.034 for isovaleryl-/2-methylbutyryl-carnitine, respectively) (Fig. 3). Multiple regression analysis revealed that baseline butyryl-carnitine levels were a sole independent predictor of SDS scores after the L-carnitine treatment (r2 = 0.533).
Table 3.
Free and acylcarnitine levels just before and after the HD session in HD patients.
| Healthy Controls |
Before HD
Baseline |
After HD
Baseline |
Before HD
Post-Treatment |
After HD
Post-Treatment |
|
|---|---|---|---|---|---|
| C0 | 43.973±7.581 | 20.847±7.584*** | 7.566±2.720††† | 140.843±57.375&&& | 40.967±15.719### |
| C2 | 7.695±2.351 | 7.954±2.148 | 4.371±0.826††† | 57.977±36.473&&& | 18.700±9.866### |
| C3 | 0.409±0.144 | 0.216±0.124*** | 0.041±0.037††† | 2.259±1.341&&& | 0.540±0.408### |
| C4 | 0.248±0.098 | 0.440±0.167** | 0.141±0.046††† | 3.175±2.683&& | 0.753±0.690### |
| C5:1 | 0.017±0.003 | 0.039±0.010*** | 0.019±0.004††† | 0.075±0.022&&& | 0.026±0.007### |
| C5 | 0.103±0.026 | 0.103±0.034 | 0.035±0.012††† | 0.470±0.236&&& | 0.133±0.079### |
| C6 | 0.075±0.020 | 0.150±0.069** | 0.051±0.017††† | 0.869±0.772&& | 0.222±0.173## |
| C5-OH | 0.024±0.006 | 0.068±0.021*** | 0.019±0.006††† | 0.198±0.131&& | 0.049±0.037### |
| C8 | 0.136±0.061 | 0.209±0.095* | 0.057±0.018††† | 0.616±0.336&&& | 0.182±0.090### |
| C10:1 | 0.156±0.060 | 0.272±0.114** | 0.089±0.029††† | 0.629±0.340&& | 0.230±0.119### |
| C10 | 0.255±0.120 | 0.352±0.213 | 0.096±0.043††† | 0.744±0.445&& | 0.228±0.145### |
| C4DC | 0.081±0.024 | 0.411±0.121*** | 0.111±0.030††† | 0.721±0.207&&& | 0.183±0.061### |
| C5DC | 0.119±0.044 | 0.625±0.300*** | 0.172±0.066††† | 1.717±0.841&&& | 0.453±0.254### |
| C12 | 0.067±0.029 | 0.121±0.049** | 0.051±0.019††† | 0.204±0.102& | 0.112±0.060### |
| C14:1 | 0.083±0.041 | 0.106±0.053 | 0.070±0.036† | 0.201±0.149& | 0.177±0.137 |
| C14 | 0.031±0.011 | 0.125±0.057*** | 0.047±0.017††† | 0.233±0.179& | 0.091±0.059## |
| C14-OH | 0.015±0.007 | 0.062±0.050** | 0.022±0.012†† | 0.127±0.087&& | 0.045±0.025### |
| C16 | 0.110±0.022 | 0.112±0.030 | 0.106±0.033 | 0.233±0.130&& | 0.200±0.091# |
| C16:1-OH | 0.008±0.002 | 0.012±0.004** | 0.008±0.003††† | 0.022±0.010&& | 0.016±0.008## |
| C16-OH | 0.005±0.001 | 0.013±0.009** | 0.007±0.003†† | 0.044±0.079 | 0.019±0.026 |
| C18:1 | 0.131±0.033 | 0.134±0.040 | 0.157±0.057 | 0.266±0.161&& | 0.301±0.168 |
| C18 | 0.043±0.013 | 0.040±0.010 | 0.041±0.010 | 0.078±0.037&& | 0.082±0.037 |
| C18:1-OH | 0.005±0.002 | 0.007±0.004 | 0.007±0.003 | 0.012±0.006&& | 0.012±0.007 |
| C18-OH | 0.004±0.001 | 0.005±0.002* | 0.004±0.001†† | 0.012±0.012& | 0.008±0.004 |
Data are expressed as mean±SD. HD=hemodialysis.
*p<0.05, **p<0.01, ***p<0.001 vs healthy controls; †p<0.05, ††p<0.01, †††p<0.001 vs before HD at baseline, &p<0.05, &&p<0.01, &&&p<0.001 vs before HD at baseline, #p<0.05, ##p<0.01, ###p<0.001 vs before HD at post treatment.
Fig. (3).
Correlation of baseline butyryl-carnitine levels with SDS scores after the L-carnitine treatment.
5. Discussion
Our analysis demonstrated that (1) serum total and free-carnitine levels just before the HD session significantly reduced, whereas acylcarnitine levels increased in male patients undergoing HD compared with healthy controls; (2) L-carnitine supplementation significantly increased all carnitine levels in patients undergoing HD; (3) SDS scores of the patients was significantly reduced after the L-carnitine treatment; and (4) although baseline butyryl-carnitine and isovaleryl-/2-methylbutyryl-carnitine levels were inversely correlated with SDS scores after the L-carnitine treatment, the former was a sole independent predictor of SDS scores in L-carnitine-treated patients undergoing HD.
Depression is one of the most serious complications in uremic patients, possibly leading to an increased risk for suicide and reducing the quality of life, even if the patients receive conventional anti-depressants [17]. Therefore, the identification of specific clinical and biochemical markers, which are associated with the depression state in patients undergoing HD, may provide us with a clue to develop a novel therapeutic strategy for the treatment and prevention of depression in uremic patients. Because we have previously found that decreased free-carnitine levels are correlated with the depression severity, as assessed using SDS, in uremic male patients undergoing HD, we specifically examined the effects of L-carnitine supplementation on the depression state in such patients. In this study, we found that oral or intravenous administration of L-carnitine for 3 months significantly increased all carnitine levels, including total, free, and acylcarnitines, with various numbers of carbon atoms and improved the depression state in patients undergoing HD. These findings have extended our previous observations showing an inverse correlation between low serum free-carnitine levels and SDS scores in uremic male patients undergoing HD [14], thus suggesting that carnitine deficiency represents a therapeutic target for preventing depression in such subjects. A previous double-blinded controlled study revealed that treatment with acetylcarnitine (C2), the most common acylcarnitine type, has beneficial effects on major depressive disorders in elderly patients [18]. Thus, L-carnitine supplementation may ameliorate the depression state in patients undergoing HD via various mechanisms, such as improvements in brain energy, phospholipid metabolism, and synaptic transmission [18].
In this study, in contrast to free (C0) and propionyl-carnitine (C3) levels, baseline serum acylcarnitine levels of >3 carbon atoms, such as butyryl-carnitine (C4) and isovaleryl-/2-methylbutyryl-carnitine (C5), were significantly increased by two- and three-fold in patients undergoing HD compared with healthy controls. Moreover, reduced butyryl-carnitine (C4) levels at baseline were found to be a sole independent predictor for the depression state, as evaluated using SDS, in L-cartinine-treated patients undergoing HD. These observations suggest that long-chain acylcarnitines of >3 carbon atoms can be excreted and/or metabolized by the kidney, possibly accounting for increased serum acylcarnitine levels in uremic male patients undergoing HD. Because the response of SDS scores to the L-carnitine treatment was low in patients undergoing HD with lower baseline butyryl-carnitine levels compared with those with higher butyryl-carnitine levels, such a small increase in butyryl-carnitine levels observed in patients undergoing HD before the L-carnitine treatment may not be enough to improve the depression state. A greater increase in serum carnitine levels, via pharmacological L-carnitine supplementation, may be needed to ameliorate the depression state in patients undergoing HD. Although we did not clarify the exact pathophysiological role of butyryl-carnitine in the depression state in uremic male patients undergoing HD, our present observations suggest that baseline butyryl-carnitine levels represent a biomarker that could predict reactivity to L-carnitine supplementation in such subjects.
conclusion
In conclusion, this study demonstrated that L-carnitine supplementation improved the depression state in uremic male patients undergoing HD, thus supporting the clinical utility of L-carnitine supplementation in the prevention and treatment of depression in such patients.
ACKNOWLEDGEMENTS
This study was supported, in part, by Grants-in-Aid for Welfare and Scientific Research (C) (no. 16k09637) (K.F) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
DISCLOSURE STATEMENT
Dr. Fukami has received honoraria as lecture fees from Otsuka (Otsuka Pharmaceutical Co., Ltd.).
References
- 1.Agganis B.T., Weiner D.E., Giang L.M. Depression and cognitive function in maintenance hemodialysis patients. Am. J. Kidney Dis. 2010;56(4):704–712. doi: 10.1053/j.ajkd.2010.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martiny C. de Oliveira e Silva, A.C.; Neto, J.P. Factors associated with risk of suicide in patients with hemodialysis. Compr. Psychiatry. 2011;52(5):465–468. doi: 10.1016/j.comppsych.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Boulware L.E., Liu Y., Fink N.E. Temporal relation among depression symptoms, cardiovascular disease events, and mortality in end-stage renal disease: contribution of reverse causality. Clin. J. Am. Soc. Nephrol. 2006;1(3):496–504. doi: 10.2215/CJN.00030505. [DOI] [PubMed] [Google Scholar]
- 4.Cukor D., Rosenthal D.S., Jindal R.M. Depression is an important contributor to low medication adherence in hemodialyzed patients and transplant recipients. Kidney Int. 2009;75(11):1223–1229. doi: 10.1038/ki.2009.51. [DOI] [PubMed] [Google Scholar]
- 5.Park H.C., Yoon H.B., Son M.J. Depression and health-related quality of life in maintenance hemodialysis patients. Clin. Nephrol. 2010;73(5):374–380. doi: 10.5414/cnp73374. [DOI] [PubMed] [Google Scholar]
- 6.Cano N.J., Heng A.E., Pison C. Multimodal approach to malnutrition in malnourished maintenance hemodialysis patients. J. Ren. Nutr. 2011;21(1):23–26. doi: 10.1053/j.jrn.2010.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Iglesias P., Carrero J.J., Díez J.J. Gonadal dysfunction in men with chronic kidney disease: clinical features, prognostic implications and therapeutic options. J. Nephrol. 2012;25(1):31–42. doi: 10.5301/JN.2011.8481. [DOI] [PubMed] [Google Scholar]
- 8.Afsar B. The relation between Internet and social media use and the demographic and clinical parameters, quality of life, depression, cognitive function and sleep quality in hemodialysis patients: Social media and hemodialysis. Gen. Hosp. Psychiatry. 2013 doi: 10.1016/j.genhosppsych.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Afsar B. The relationship between depressive symptoms and erythropoietin resistance in stable hemodialysis patients with adequate iron stores. Int. J. Artif. Organs. 2013;36(5):314–319. doi: 10.5301/ijao.5000184. [DOI] [PubMed] [Google Scholar]
- 10.Bøhmer T., Hoel P., Purvis K. Carnitine levels in human accessory sex organs. Arch. Androl. 1978;1(1):53–59. doi: 10.3109/01485017808988318. [DOI] [PubMed] [Google Scholar]
- 11.Evans A.M., Fornasini G. Pharmacokinetics of L-carnitine. Clin. Pharmacokinet. 2003;42(11):941–967. doi: 10.2165/00003088-200342110-00002. [DOI] [PubMed] [Google Scholar]
- 12.Evans A. Dialysis-related carnitine disorder and levocarnitine pharmacology. Am. J. Kidney Dis. 2003;41(Suppl. 4):13–26. doi: 10.1016/s0272-6386(03)00113-6. [DOI] [PubMed] [Google Scholar]
- 13.Adachi T., Fukami K., Yamagishi S. Decreased serum carnitine is independently correlated with increased tissue accumulation levels of advanced glycation end products in haemodialysis patients. Nephrology (Carlton) 2012;17(8):689–694. doi: 10.1111/j.1440-1797.2012.01642.x. [DOI] [PubMed] [Google Scholar]
- 14.Fukami K., Yamagishi S., Sakai K. Carnitine deficiency is associated with late-onset hypogonadism and depression in uremic men with hemodialysis. Aging Male. 2014;17(4):238–242. doi: 10.3109/13685538.2014.888053. [DOI] [PubMed] [Google Scholar]
- 15.Shigematsu Y., Hirano S., Hata I. Newborn mass screening and selective screening using electrospray tandem mass spectrometry in Japan. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2002;776(1):39–48. doi: 10.1016/s1570-0232(02)00077-6. [DOI] [PubMed] [Google Scholar]
- 16.Nagano M., Fukami K., Yamagishi S. Tissue level of advanced glycation end products is an independent determinant of high-sensitivity C-reactive protein levels in haemodialysis patients. Nephrology (Carlton) 2011;16(3):299–303. doi: 10.1111/j.1440-1797.2010.01419.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen C.K., Tsai Y.C., Hsu H.J. Depression and suicide risk in hemodialysis patients with chronic renal failure. Psychosomatics. 2010;51(6):528–528. doi: 10.1176/appi.psy.51.6.528. [DOI] [PubMed] [Google Scholar]
- 18.Pettegrew J.W., Levine J., McClure R.J. Acetyl-L-carnitine physical-chemical, metabolic, and therapeutic properties: Relevance for its mode of action in Alzheimer’s disease and geriatric depression. Mol. Psychiatry. 2000;5(6):616–632. doi: 10.1038/sj.mp.4000805. [DOI] [PubMed] [Google Scholar]