Summary
Currently, all methods for converting non-neuronal cells into neurons involve injury to the brain; however, whether neuronal transdifferentiation can occur long after the period of insult remains largely unknown. Here, we use the transcription factor NEUROD1, previously shown to convert reactive glial cells to neurons in the cortex, to determine whether astrocyte-to-neuron transdifferentiation can occur under physiological conditions. We utilized adeno-associated virus 9 (AAV9), which crosses the blood-brain barrier without injury, to deliver NEUROD1 to astrocytes through an intravascular route. Interestingly, we found that a small, but significant number of non-reactive astrocytes converted to neurons in the striatum, but not the cortex. Moreover, astrocytes cultured to minimize their proliferative potential also exhibited limited neuronal transdifferentiation with NEUROD1 expression. Our results show that a single transcription factor can induce astrocyte-to-neuron conversion under physiological conditions, potentially facilitating future clinical approaches long after the acute injury phase.
Keywords: NeuroD, transdifferentiation, reprogramming, neurons, astrocytes, cortex, striatum, AAV9
Highlights
-
•
Intravascular AAV9 efficiently targets astrocytes in the cortex and striatum
-
•
NEUROD1 can convert a small, but significant number of astrocytes to neurons in vivo
-
•
NEUROD1 induces limited neuronal transdifferentiation in vitro
-
•
Injury-related factors may be important in neuronal conversion strategies
In this article, Hsieh and colleagues use AAV9 viral infection to selectively target and overexpress NEUROD1 in astrocytes in the absence of injury to the CNS. The authors show that NEUROD1 is capable of converting a small, but significant number of astrocytes to neurons in vivo in the absence of reactive gliosis.
Introduction
The regenerative capacity of the mammalian CNS is largely restricted to two areas of neurogenic potential found in the subgranular zone of the dentate gyrus and the subventricular zone (SVZ) of the lateral ventricle (Kempermann et al., 2015, Ming and Song, 2011). Neurons lost outside these areas due to injury or disease cannot be replaced, and can often have devastating consequences for affected patients. Recent studies have focused on therapies involving the transdifferentiation, or direct lineage conversion, of other resident cell types into a desired neuronal population in vivo with the hopes of being able to restore or replace lost neurons (Aravantinou-Fatorou et al., 2015, Corti et al., 2012, Guo et al., 2013, Liu et al., 2015, Niu et al., 2013, Torper et al., 2013).
During the acute phase of injury to the CNS, astrocytes become hypertrophic, resume proliferation, and upregulate expression of the intermediate filament proteins glial fibrillary acidic protein (GFAP) and vimentin in a process called reactive gliosis (Anderson et al., 2014, Bayraktar et al., 2015, Burda and Sofroniew, 2014). Many of the cellular processes associated with this phenomenon are transitive in nature, and are complete several weeks to a month after injury (Burda and Sofroniew, 2014, Robel et al., 2011). After injury to the CNS astrocytes become proliferative, and in some cases begin to express markers of neural stem/progenitor cells and neurogenic differentiation, indicating that they may be prime cellular candidates for transdifferentiation approaches (Anderson et al., 2014, Bayraktar et al., 2015, Duan et al., 2015, Magnusson et al., 2014, Nato et al., 2015). Recent work has demonstrated robust transdifferentiation of reactive cortical astrocytes into glutamatergic neurons with the overexpression of a single transcription factor, NEUROD1 (Guo et al., 2013). By direct injection of a retrovirus overexpressing NEUROD1 in the adult mouse cortex, these authors were able to successfully target and convert reactive astrocytes to neurons (Guo et al., 2013). However, what is not well understood from this study and others in the field is whether astrocyte-to-neuron conversion can still occur following the initial injury phase after reactive gliosis is resolved (Burda and Sofroniew, 2014, Guo et al., 2013, Niu et al., 2013, Torper et al., 2013). A better understanding of the neurogenic potential of non-reactive astrocytes is therefore necessary for the future design of therapeutics administered outside the window of reactive gliosis.
In this study, we seek to examine the efficacy of using adeno-associated virus 9 (AAV9) to express NEUROD1 in infected cortical and striatal astrocytes through a systemic intravascular route. Previous work suggests that intravascular introduction of AAV9 is able to specifically infect the astrocytic-perivascular endfeet that encompass approximately 99% of the vasculature in the brain, leading to widespread astrocyte targeting without breakdown of the blood-brain barrier (Foust et al., 2009). Here we make use of the previously reported transdifferentiation factor, NEUROD1, to achieve conversion of cortical and striatal astrocytes into neurons. Our work shows that NEUROD1 overexpression in astrocytes of the cortex and striatum can successfully produce mature neurons in the striatal region. However, the total number of newly converted neurons is much fewer than previously reported, indicating that future work will be necessary to enhance the clinical utility of NEUROD1 expression as an effective strategy to generate neurons in the absence of reactive gliosis.
Results
AAV9-GFP Labels Neocortical and Striatal Astrocytes in Postnatal Day 10 Mouse Brain
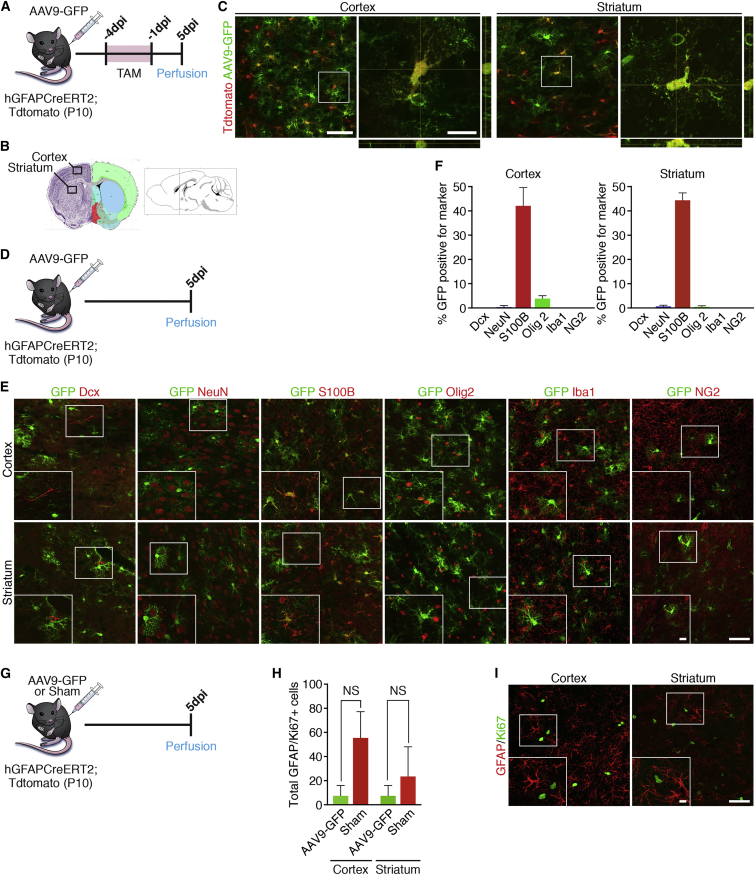
Previous work suggests that intravascular delivery of AAV9 is an ideal tool for targeting astrocytes in certain brain regions (Foust et al., 2009). To further extend these findings and validate our experimental model, we wanted to confirm the identity of AAV9 infected cells in the cortex and striatum. To investigate the identity of the newly infected cells we used an AAV9 vector that expressed GFP under the control of the chicken β-actin hybrid promoter (AAV9-GFP) (Foust et al., 2009). We used a Cre-inducible hGFAP reporter mouse to label astrocytes in the cortex and striatum irreversibly with tdTomato (Ai14) to determine the level of overlap between AAV9-GFP infected cells and GFAP-expressing astrocytes. To accomplish this we bred hGFAP-CreER male mice to Ai14 mice, and administered tamoxifen (TAM) to nursing females starting on day 7 after delivery of the pups (Figure 1A). TAM administration was repeated for 3 consecutive days to label GFAP-expressing astrocytes with tdTomato, and on the fourth day when the pups were 10 days old AAV9-GFP was injected into the jugular vein (Figure 1A). Mice were euthanized at 5 days post injection (dpi) to determine the overlap of AAV9-GFP and tdTomato. We confirmed overlap between the two markers, indicating a strong preference of AAV9 for targeting astrocytes in the cortex and striatum (Figures 1B and 1C).
Figure 1.
AAV9-GFP Labels Neocortical and Striatal Astrocytes in Postnatal Day 10 Mouse Brain
(A) Timeline showing the experimental design.
(B) Schematic of the brain showing the areas in which representative images were taken.
(C) Representative images of AAV9-GFP infection in the cortex and striatum at 5 days post injection (dpi).
(D) Timeline showing experimental design for validating identity of GFP+ cells in (E).
(E) Representative images showing co-labeling of GFP+ cells with the neuronal markers DCX and NeuN, the astrocyte marker S100b, the oligodendrocyte precursor marker OLIG2, the microglial marker IbaI, and the NG2 glial marker NG2.
(F) Quantification of overlap of GFP+ cells for respective markers in the cortex and striatum. n = 3 mice/marker. Data are shown as mean ± SEM.
(G) Timeline showing the experimental design.
(H) Quantification of GFAP/Ki67+ reactive astrocytes in the cortex and striatum 5 dpi. n = 3 mice/group. Data are shown as mean ± SEM. NS, not significant.
(I) Representative images showing sections of the cortex and striatum stained for GFAP and Ki67.
Scale bars represent 50 μm in main panels and 25 μm in insets.
Next, to provide additional confirmation that AAV9 predominantly labels astrocytes and to rule out the possibility that AAV9 infects cells that are already neuronal in identity, we repeated intravascular injections with AAV9-GFP and stained these sections with the marker doublecortin (DCX) for immature neurons and neuronal nuclei (NeuN) for mature neurons (Figures 1D and 1E). Of all the labeled cells counted throughout the cortex and striatum, only one cell, or <0.003% of GFP+ cells, were identified as double positive for DCX (Figures 1E and 1F). Likewise, only 0.79% of GFP+ cells in the cortex and 1.07% of GFP+ cells in the striatum were positive for NeuN, indicating that AAV9 does not target neuronal cells (Figures 1E and 1F). Moreover, approximately 42.46% of GFP+ cells in the cortex and 44.77% of GFP+ cells in the striatum stained with the astrocyte marker S100β, consistent with intravascular AAV9 predominantly targeting astrocytes in this region (Figures 1E and 1F). To test the possibility that AAV9 infects NG2 glia, oligodendrocyte precursor cells, or microglia, we stained these same sections for the oligodendrocyte precursor marker OLIG2, NG2 glia marker NG2, and microglial marker IbaI (Figure 1E). Quantification revealed that <5% of infected cells in the cortex and <1% of infected cells in the striatum were oligodendrocyte precursor cells, while none of the infected cells were found to co-label with the microglial marker IbaI or the NG2 glial marker NG2 (Figures 1E and 1F). These data confirm that intravascular delivery of AAV9-GFP efficiently labels astrocytes and not neurons or other glial cell types in the cortex and striatum, reinforcing this approach to evaluate NEUROD1's effects in neuronal transdifferentiation.
After confirming successful infection of astrocytes using AAV9 viral infection, we also wished to determine whether exposure to the virus, even peripherally through the circulatory system, could induce reactive gliosis. To this end we compared the total number of GFAP/Ki67+ cells in AAV9-GFP-injected postnatal day 10 pups with sham-injected pups at 5 dpi (Figure 1G). Quantification showed no significant differences in the total number of GFAP/Ki67+ cells between the animals that received viral injection and those that did not (Figures 1H and 1I). These data indicate that infection of astrocytes using intravascular AAV9 does not mediate significant levels of reactive gliosis in the pup brain.
Confirmation of NEUROD1 Overexpression in Neocortical and Striatal Astrocytes
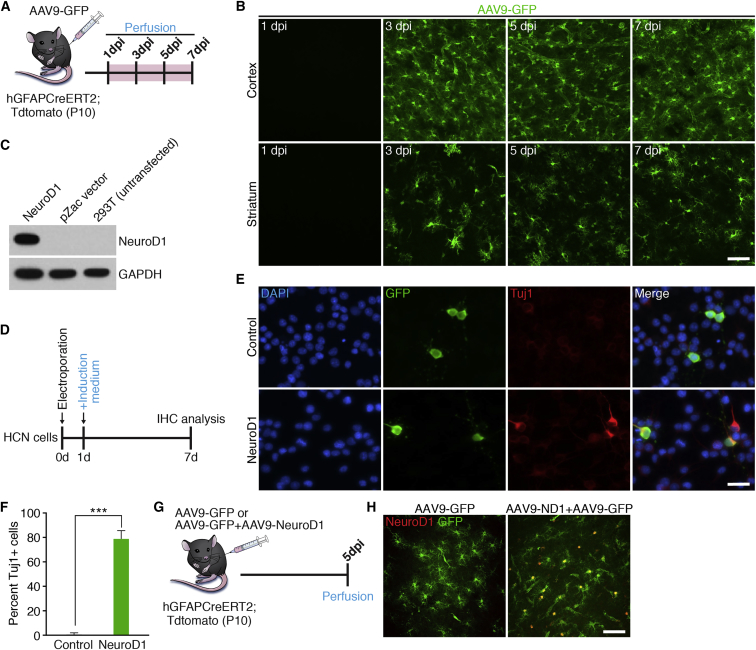
Previous work indicates that it takes approximately 3 days to 1 week after infection of reactive astrocytes with retrovirus expressing NEUROD1 to see neuronal transdifferentiation (Guo et al., 2013). Since our strategy for overexpressing NEUROD1 involved injection of AAV9 virus into the vascular system, we wanted to perform a time course to determine the kinetics of virus transduction based on expression of GFP in astrocytes. To examine this, we injected AAV9-GFP virus into 10-day-old hGFAP-CreER;Tdtomato mouse pups via the jugular vein and euthanized the mice at different time points to check GFP expression in the brain (Figure 2A). Our results showed robust GFP+ infected cells in cortex and striatum beginning around 3 dpi (Figure 2B). Therefore, in all subsequent in vivo experiments using AAV9 we decided to euthanize the mice at 10 days after infection to accommodate the time necessary for the virus to infect cells of the brain and express NEUROD1, as well as allow for successful transdifferentiation based on previous published work (Guo et al., 2013).
Figure 2.
Confirmation of NEUROD1 Overexpression in Neocortical and Striatal Astrocytes
(A) Timeline showing experimental design.
(B) Representative images taken from the cortex and striatum showing AAV9-GFP infection from 1 to 7 dpi. Scale bar, 50 μm.
(C) Western blot showing successful overexpression of NEUROD1 after cloning into the AAV9 backbone.
(D) Timeline showing experimental design.
(E) Representative in vitro images of electroporated cells, 6 days post electroporation, with either AAV9-ND1 + pllU2g-GFP or pllU2g-GFP alone. Scale bar, 50 μm.
(F) Quantification of GFP/Tuj1+ cells at 6 days post electroporation. ∗∗∗p = 0.0002. n = 3 independent experiments.
(G) Timeline showing experimental design.
(H) Representative images from the cortex showing significant overlap of infected astrocytes and NEUROD1 in mice injected with AAV9-NEUROD1, but not AAV9-GFP control, at 5 dpi. Scale bar, 50 μm.
To overexpress NEUROD1 in astrocytes of the cortex and striatum, we cloned the mouse cDNA for NEUROD1 into an AAV9 vector and confirmed NEUROD1 protein expression using western blot analysis (Figure 2C). After viral packaging we wished to confirm successful NEUROD1 expression both in vitro and in vivo. To this end, we electroporated cultured adult rat hippocampal neural stem cells (HCN cells) with either AAV9-ND1 + pllU2g-GFP or pllU2g-GFP alone (Figure 2D). Staining with the neuronal marker neuronal marker βIII-tubulin (Tuj1) 6 days after electroporation revealed that approximately 80% of electroporated cells that received AAV9-ND1 + pllU2g-GFP were neuronal in identity, consistent with NEUROD1's role in mediating neuronal differentiation in HCN cells (Figures 2E and 2F) (Hsieh et al., 2004). However, less than 1% of pllU2g-GFP electroporated cells were found to be Tuj1+, indicating that NEUROD1 overexpression in the AAV9 vector was successful (Figure 2F). To validate this same plasmid in vivo, we packaged AAV9-NEUROD1 virus and then injected AAV9-NEUROD1 + AAV9-GFP or AAV9-GFP alone into 10-day-old mice and euthanized them 5 days after injection (Figures 2G and 2H). Growth curves performed between 10 and 20 dpi confirmed that both AAV9-GFP- and AAV9-NEUROD1-injected groups were relatively healthy with no significant differences in weight, although some AAV9-injected mice at 10 dpi showed signs of mortality, which increased at longer time points presumably due to high levels of systemic AAV9 (data not shown). As expected, cortical and striatal sections stained for NEUROD1 showed high expression in GFP+ astrocytes in animals that received a mixture of AAV9-NEUROD1 and AAV9-GFP, whereas no NEUROD1 expression was noted in control animals that received only AAV9-GFP injection (Figures 2G and 2H). Taken together, these results indicate that intravascular AAV9 delivery is a suitable approach for widespread overexpression of NEUROD1 in the brain.
NEUROD1 Mediates a Small but Significant Astrocyte-to-Neuron Conversion in Striatum
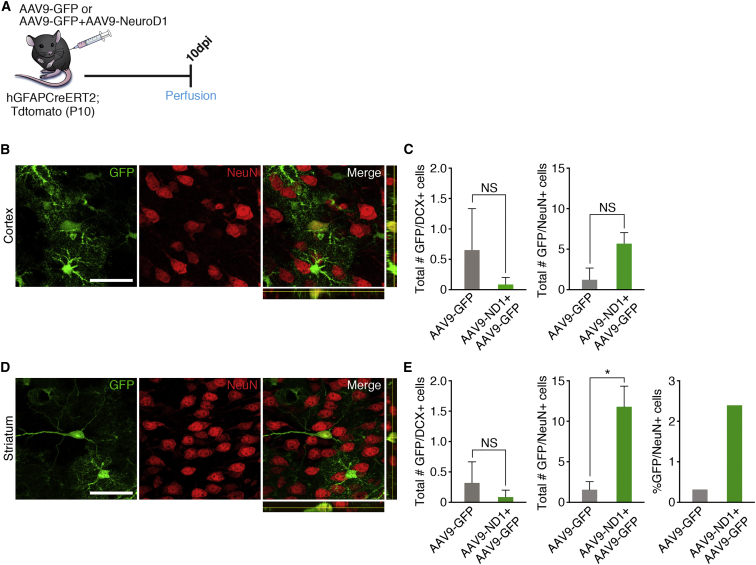
To determine whether NEUROD1 is able to convert astrocytes to neurons without direct brain injury, we injected 10-day-old mice with a mixture of AAV9-NEUROD1 and AAV9-GFP or AAV9-GFP as a control intravascularly and then euthanized the animals 10 days later (Figure 3A). Brain sections were stained for DCX and NeuN to determine the total number of GFP+ converted immature and mature neurons, respectively. Overexpression of NEUROD1 did not lead to a significant number of transdifferentiated cells expressing DCX or NeuN neuronal markers in the cortex (Figures 3B and 3C). Interestingly, a small number of GFP+/NeuN+ cells in the striatum were higher in the AAV9-NEUROD1 transduced group (2.42% of GFP+ cells) compared with the AAV9-GFP transduced group (0.34%), although there was no difference in GFP+/DCX+ cells (Figures 3D and 3E). Moreover, the observed GFP+/NeuN+ striatal cells appeared to exhibit neuron-like morphology, with longer, more elaborate processes (Figure 3D). These data suggest that NEUROD1 expression alone can mediate astrocyte-to-neuron conversion even under physiological conditions, although this was a relatively rare event.
Figure 3.
NEUROD1 Expression in Striatal Astrocytes Led to Limited Neuronal Conversion
(A) Timeline showing experimental design. Mice at 10 days old were injected with either AAV9-NEUROD1 + AAV9-GFP (experimental) or AAV9-GFP (control) and euthanized at 10 dpi to determine whether neuronal conversion had occurred.
(B) Representative images showing one of the few cells expressing NeuN in the cortex. Scale bar, 50 μm.
(C) Quantification showing the total number of GFP+ cells that co-labeled for the neuronal markers DCX and NeuN in the cortex. n = 3 mice for AAV9-GFP, n = 11 mice for AAV9-NEUROD1 + AAV9-GFP. Data are shown as mean ± SEM. NS, not significant.
(D) Representative images showing one of the few cells expressing NeuN in the striatum. Scale bar, 50 μm.
(E) Quantification showing the total number and percentage of GFP+ cells that co-labeled with the neuronal markers DCX and NeuN in the striatum. n = 3 mice for AAV9-GFP, n = 11 mice for AAV9-NEUROD1 + AAV9-GFP. Data are shown as mean ± SEM. ∗p < 0.05; NS, not significant.
NEUROD1 Expression in Cultured Astrocytes Induces Neuronal Differentiation
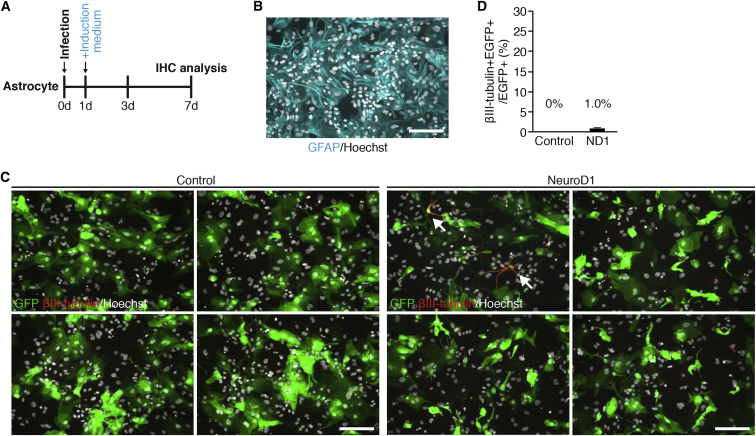
Our in vivo NEUROD1 expression results indicating limited astrocyte-to-neuron conversion suggest that reactive astrocytes induced by injury may be a vital component for robust neuronal transdifferentiation. To extend our findings, we wished to corroborate our in vivo results with infection of primary cultured astrocytes in vitro (Ellis et al., 2013). The AAV viruses are not amenable to infection of primary cultured cells in vitro, and for this reason we chose to use a lentiviral vector system to allow for maximal infection and overexpression of NEUROD1 in our in vitro cultures. Therefore, we established mouse astrocytes in vitro, treated them with AraC, and allowed them to grow without the addition of growth factors, which more closely resembles the physiologic state of non-proliferating astrocytes (Figure 4A) (Laywell et al., 2000, Leutz and Schachner, 1981, White et al., 2011). We infected cultured mouse astrocytes with lenti-CAG-NEUROD1-UbiC-GFP or lenti-CAG-UbiC-GFP as a control, switched the cultures to serum-free induction medium the following day, and stained them with markers of astrocytes (GFAP) or neurons (Tuj1) at 7 days after infection (Figure 4B). Interestingly, we observed minimal Tuj1 neuronal differentiation in NEUROD1-expressing cultured mouse astrocytes relative to control (Figures 4C and 4D). These results further corroborate the idea that NEUROD1 expression alone can convert astrocytes into neurons under physiological conditions, although the efficiency is very limited.
Figure 4.
Overexpression of NEUROD1 Resulted in Minimal Neuronal Differentiation In Vitro
(A) Timeline showing experimental design.
(B) Representative image of cultured astrocytes showing expression of the astrocyte marker GFAP. Scale bar, 50 μm.
(C) Representative images taken in vitro showing astrocytes infected with lenti-CAG-NEUROD1-UbiC-GFP or lenti-CAG-UbiC-GFP at 7 dpi co-labeled with the immature neuronal marker βIII-tubulin (Tuj1). Arrows denote GFP+/Tuj1+ cells. Scale bar, 50 μm.
(D) Graph showing the percentage of all astrocytes expressing Tuj1 7 days after infection. n = 4 independent experiments/condition. Data are expressed as mean ± SEM.
Discussion
In this work we demonstrate a method for indirect, non-invasive, and widespread targeting of astrocytes in the cortex and striatum. By utilizing overexpression of the transcription factor NEUROD1, previously reported to yield both rapid and efficient conversion of astrocytes to neurons, we present data in support of the idea that expression of this transdifferentiation factor via a non-invasive vascular route can yield newly converted neurons. While we did observe newly converted mature NeuN+ neurons in the striatum, the overall number of newly generated cells remained low compared with those reported in the previously published study (Guo et al., 2013). Future work will be necessary to not only explain why intravascular AAV9 delivery of NEUROD1 yields low numbers of newborn neurons, but also identify the types of neurons being generated through this approach. This will be particularly important when designing therapeutic strategies for patients, as introduction of non-native neuronal subtypes may lead to worsening of disease or development of other disruptions to the CNS (Buzsaki et al., 1988, Buzsaki et al., 1991, Carlsson et al., 2007, Olanow et al., 2003). For instance, grafting of fetal hippocampal tissue into the intact adult hippocampus led to the development of seizures in 30% of rats compared with rats in which the grafts failed to survive (Buzsaki et al., 1991). Similarly, transplantation of serotonin-rich neuroblast grafts resulted in worsening of behavioral dyskinesias in a rat model of Parkinson's disease (Carlsson et al., 2007). These studies highlight the need for a better understanding of the type of neurons produced when NEUROD1 is introduced under physiological conditions.
One potential explanation for why we see low numbers of astrocyte-to-neuron conversion is that fundamental differences exist between the astrocyte populations targeted by each viral approach. Indeed, direct injection of virus into the brain causes injury to the tissue, leading to a process called reactive gliosis. Reactive gliosis is typically a localized response found around the site of injury during which glial cells respond by proliferation and upregulation of certain intermediate filament proteins such as GFAP (Burda and Sofroniew, 2014, Cavanagh, 1970). Interestingly, work from others supports the idea that injury to the brain can lead to the recruitment of resident astrocytes to form neuroblast-like cells, whereas astrocytes isolated from non-injured brain fail to realize this potential (Duan et al., 2015, Magnusson et al., 2014). Previous work also shows that after injury, reactive astrocytes in the brain upregulate expression of epidermal growth factor (EGF), fibroblast growth factor 2 (FGF2), and vascular endothelial growth factor receptors, allowing them to respond to signaling pathways critical to neuroblast cell fate (Buffo et al., 2008, Burda and Sofroniew, 2014, Robel et al., 2011). Furthermore, it has been demonstrated that a higher yield of newly converted neurons can be achieved when overexpression of a neurogenic transcription factor is combined with the addition of growth factors to the lesion site, supporting the idea that fundamental environmental differences exist between the injured and non-injured brain (Grande et al., 2013). Indeed, in our work astrocytes cultured in vitro were grown in the absence of the growth factors EGF and FGF2 in contrast to previous work, lending further support to the idea that there are extrinsic signaling pathways critical to the conversion of astrocytes to neurons, perhaps by making the astrocytes more “stem-like” (Guo et al., 2013). In addition, injury to the brain has been shown to lead to disruption of the Notch1 signaling pathway in astrocytes, leading to the activation of a latent neurogenic program in these cells which might make them more primed to adopt the neuronal lineage if the correct factors are present (Magnusson et al., 2014). Previous work has shown that there exist differences in the effectiveness of astrocyte-to-neuron conversion and subsequent survival of newly generated neurons depending on the brain region in which astrocytes are initially targeted (Grande et al., 2013). Indeed, higher levels of astrocyte-to-neuron conversion were previously reported in the striatum in comparison with the cortex through overexpression of the transcription factor NEUROGENIN2 (Grande et al., 2013), consistent with our results that NEUROD1 induces higher levels of neuronal conversion in the striatum compared with the cortex. This highlights the need for a better understanding of other genetic or environmental factors that are critical in achieving higher levels of transdifferentiation in the absence of recent injury to the brain.
Another potential explanation for the lack of efficient conversion in our current work could be explained by downregulation of NEUROD1 expression shortly after its overexpression. Indeed, in our current work, staining for NEUROD1 at 10 dpi showed a relative lack of NEUROD1 expression in GFP+ cells. Sustained overexpression of NEUROD1 might be necessary for proper astrocyte-to-neuron conversion, although expression of NEUROD1 in our current work appears to be silenced by an endogenous mechanism. It is possible that because NEUROD1 is a transcription factor necessary for the proper differentiation of the neuronal fate, it is ultimately silenced upon neuronal maturity. In addition, differences in the viral systems used to introduce NEUROD1 might potentially explain the differences in the total number of neurons seen in this study compared with previous work (Guo et al., 2013). Since retrovirus is known to infect only cells undergoing active division, the possibility exists that use of AAV9 in this work allowed for the infection of a separate but distinct population of astrocytes less amenable to the transdifferentiation process. The possibility also exists that because retrovirus is known to integrate into the host genome, unlike AAV9, this allowed for more sustained expression of NEUROD1 using this system, and thus a higher rate of neuronal conversion. In our current study we made use of AAV9 to target astrocytes of the cortex and striatum in a non-reactive context, while previous work has made use of retrovirus to selectively infect proliferating reactive astrocytes. In a subsequent study, we introduced retrovirus overexpressing NEUROD1 or mCherry into the cortex of adult mice and did not observe appreciable levels of retroviral infection 1 month after injection (data not shown). This finding is in contrast with those of Guo et al. (2013), who reported significant levels of infection in the cortex using retrovirus. Since we were unable to infect a population of reactive astrocytes with retrovirus, it remains an open question whether our negative data are due to the viral system or other possible differences (e.g., virus titer, route of injection). Consistent with previous work, the degree to which astrocytes proliferate following injury is highly variable in different injury contexts, which may explain in part the lack of retroviral infection in our current studies (Barreto et al., 2011, Miyake et al., 1988, Voskuhl et al., 2009).
Previous work in the field has demonstrated a wide range of cellular origins for newly transdifferentiated/reprogrammed neurons. Indeed, previous work has shown that not only astrocytes, but also NG2 glia as well as progenitor and post-mitotic neurons, are capable of undergoing neuronal conversion (Heinrich et al., 2014, Rouaux and Arlotta, 2010, Rouaux and Arlotta, 2013, Torper et al., 2015). While our results exclude significant infection of DCX+/NeuN+ neurons, OLIG2+ oligodendrocyte precursor cells, IbaI+ microglia, and NG2+ glial cells by AAV9-GFP, we cannot at this time completely rule out the possibility of other sources of neuronal conversion, such as SVZ stem cell/astrocytes, which could have migrated into the adjacent striatum and converted to neurons with NEUROD1 overexpression. It will be interesting in future studies to determine the contribution of specific astrocyte populations to newly converted neurons.
In summary, our work demonstrates that NEUROD1 is able to convert astrocytes to neurons even in the absence of reactive gliosis. Future work should focus on the identification and characterization of these newly converted cells, as well as their ability to integrate and synapse with pre-existing neurons already present in the brain. Future work should also examine strategies for increasing astrocyte-to-neuron conversion after the resolution of reactive gliosis in the hopes of translating this strategy for neuronal replacement to the clinic in patients receiving treatment outside the window of initial insult to the brain.
Experimental Procedures
Animals
All experiments were performed in compliance with the animal care guidelines issued by the NIH and by the Institutional Animal Use and Care Committee at the University of Texas Southwestern Medical Center, and the Fundamental Guidelines for Proper Conduct of Animal Experiment and Related Activities in Academic Research Institutions under the jurisdiction of the Ministry of Education, Culture, Sports, Science and Technology of Japan. All mice were bred and housed in the animal facility with a 12-hr light, 12-hr dark cycle with no more than five mice per cage, fed food (2916 Global irradiated diet, Teklad Labs) and water ad libitum. To generate the mice used in this study we crossed male hGFAP-CreER mice (originally from K.D. McCarthy's laboratory) with female Ai14;B6.Cg-Gt(ROSA)26Sortm14(CAG-tdTomato)Hze/J mice (Casper et al., 2007, Madisen et al., 2010). Mice were genotyped by PCR using genomic DNA and primers for GFAP Cre (5′-GGT CGA TGC AAC GAG TGA TGA GG-3′; 5′-GCT AAG TGC CTT CTC TAC ACC TGC G-3′) and Ai14 (5′-AAG GGA GCT GCA GTG GAG TA-3′ and 5′-CCG AAA ATC TGT GGG AAG TC-3′; 5′-GGC ATT AAA GCA GCG TAT CC-3′ and 5′-CTG TTC CTG TAC GGC ATG G-3′). hGFAP-CreER;Ai14 nursing mothers were administered TAM through oral gavage at 50 mg/kg per day for 3 days prior to intravascular injection of AAV9 into the jugular vein of the pups. TAM was dissolved in 10% EtOH/90% sunflower oil.
Intravascular Jugular Vein Injection of AAV9
Ten-day-old hGFAP-CreER;Ai14 mouse pups were anesthetized using 3%–5% isoflurane in 70% nitrous oxide and 30% oxygen. Once anesthetized pups were laid on their backs underneath a dissecting microscope and a 1-cm incision was made in the skin overlying the external jugular vein, blunt dissection was performed until the external jugular vein was visible. A 3/10-cc syringe needle was placed through the overlying muscle into the jugular vein, and a total of 130 μL of AAV9 virus was dispensed. The needle was withdrawn carefully and the vein checked for residual bleeding before the wound was closed, and pups were allowed to recover on a prewarmed heating pad. After all surgeries were complete from one litter, the surgical areas from each of the pups were cleaned with sterile water to remove residual betadine and EtOH. Pups were then rubbed with a powdered mouse chow and bedding to better facilitate acceptance back by their mother for nursing.
In Vitro Rat HCN Cell Culture and Electroporation
The adult hippocampal neural stem cell line (HCN cells) was isolated and cloned from Fisher 344 rats and characterized in accordance with previous studies (Hsieh et al., 2004, Mira et al., 2010). In brief, HCN cells were cultured in DMEM/F12 supplemented with N2, glutamate, and penicillin-streptomycin-fungizone (PSF) in the presence of FGF2 (20 ng/mL). Cells were passaged using 5% trypsin solution and electroporation was performed using an Amaxa electroporator at a ratio of 5 μg of DNA per 5 million HCN cells (NEUROD1 condition: AAV9-ND1 2.5 μg of DNA, pllU2g-GFP 2.5 μg of DNA; control condition: pllU2g-GFP 5 μg of DNA). After electroporation cells were plated and allowed to recover overnight. The following day, culture medium was switched to DMEM/F12 supplemented with N2/B27, glutamate, and PSF without the addition of FGF2. Cultures were allowed to grow for an additional 6 days before cells were fixed using 4% paraformaldehyde for immunohistochemistry analysis.
Author Contributions
R.B., K.N., and J.H. designed research and analyzed data; R.B. and T.M. performed research; L.Z. provided technical support; C.M. and B.K.K. provided assistance with AAV9 delivery; M.G. provided AAV9 vector backbones used in this study; R.B. and J.H. wrote the paper.
Acknowledgments
We thank Woo-ping Ge, Robert Hammer, and Derek Smith for technical assistance, Chun-Li Zhang for comments on the manuscript, and Jose Cabrera and Shradha Mukherjee for graphics support. We also thank Ken McCarthy for the hGFAP-CreER mice. This work was supported by grants from the NIH (R01NS093992, R01NS089770, R01NS081203, and K02AG041815 to J.H. and T32GM083831 to R.B.), American Heart Association (15GRNT25750034 to J.H.), Department of Defense (W81XWH-15-1-0399 to J.H.), Texas Institute of Brain Injury and Repair (to J.H.), and MEXT KAKENHI (16H06527 to K.N.).
Published: May 11, 2017
Footnotes
Supplemental Information includes Supplemental Experimental Procedures and can be found with this article online at http://dx.doi.org/10.1016/j.stemcr.2017.04.013.
Supplemental Information
References
- Anderson M.A., Ao Y., Sofroniew M.V. Heterogeneity of reactive astrocytes. Neurosci. Lett. 2014;565:23–29. doi: 10.1016/j.neulet.2013.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravantinou-Fatorou K., Ortega F., Chroni-Tzartou D., Antoniou N., Poulopoulou C., Politis P.K., Berninger B., Matsas R., Thomaidou D. CEND1 and NEUROGENIN2 reprogram mouse astrocytes and embryonic fibroblasts to induced neural precursors and differentiated neurons. Stem Cell Reports. 2015;5:405–418. doi: 10.1016/j.stemcr.2015.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreto G.E., Sun X., Xu L., Giffard R.G. Astrocyte proliferation following stroke in the mouse depends on the distance from the infarct. PLoS One. 2011;6:e27881. doi: 10.1371/journal.pone.0027881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayraktar O.A., Fuentealba L.C., Alvarez-Buylla A., Rowitch D.H. Astrocyte development and heterogeneity. Cold Spring Harb. Perspect. Biol. 2015;7:1–16. doi: 10.1101/cshperspect.a020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo A., Inmaculada R., Tripathi P., Lepier A., Colak D., Horn A., Mori T., Götz M. Origin and progeny of reactive gliosis: a source of multipotent cells in the injured brain. Proc. Natl. Acad. Sci. USA. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burda J.E., Sofroniew M.V. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 2014;81:229–248. doi: 10.1016/j.neuron.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G., Masliah E., Chen L.S., Horvath Z., Terry R., Gage F.H. Hippocampal grafts into the intact brain induce epileptic patterns. Brain Res. 1991;554:30–37. doi: 10.1016/0006-8993(91)90168-u. [DOI] [PubMed] [Google Scholar]
- Buzsaki G., Ponomareff G., Bayardo F., Shaw T., Gage F.H. Suppression and induction of epileptic activity by neuronal grafts. Proc. Natl. Acad. Sci. USA. 1988;85:9327–9330. doi: 10.1073/pnas.85.23.9327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson T., Carta M., Winkler C., Bjorklund A., Kirik D. Serotonin neuron transplants exacerbate L-DOPA induced dyskinesia in a rat model of Parkinson's disease. Neurobiol. Dis. 2007;27:8011–8022. doi: 10.1523/JNEUROSCI.2079-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casper K.B., Jones K., McCarthy K.D. Characterization of astrocyte-specific conditional knockouts. Genesis. 2007;45:292–299. doi: 10.1002/dvg.20287. [DOI] [PubMed] [Google Scholar]
- Cavanagh J.B. The proliferation of astrocytes around a needle wound in the rat brain. J. Anat. 1970;106:471–487. [PMC free article] [PubMed] [Google Scholar]
- Corti S., NIzzardo M., Simone C., Falcone M., Donadoni C., Salani S., Rizzo F., Nardini M., Riboldi G., Magri F. Direct reprogramming of human astrocytes into neural stem cells and neurons. Exp. Cell Res. 2012;318:1528–1541. doi: 10.1016/j.yexcr.2012.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C., Liu C., Shen S., Mo J.L., Yu Z., Chen X., Sun F.Y. Striatal astrocytes transdifferentiate into functional mature neurons following ischemic brain injury. Glia. 2015;63:1660–1670. doi: 10.1002/glia.22837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis B.L., Hirsch M.L., Barker J.C., Connelly J.P., Steininger R.J., Porteus M.H. A survey of ex vivo/in vitro transduction efficiency of mammalian primary cells and cell lines with nine natural adeno-associated virus (AAV1-9) and one engineered adeno-associated virus serotype. Virol. J. 2013;10:1–10. doi: 10.1186/1743-422X-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foust K.D., Nurre E., Montgomery C.L., Hernandez A., Chan C.M., Kaspar B.K. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat. Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grande A., Sumiyoshi K., Lopez-Juarez A., Howard J., Sakthivel B., Aronow B., Campbell K., Nakafuku M. Environmental impact on direct neuronal reprogramming in vivo in the adult brain. Nat. Commun. 2013;4:2373. doi: 10.1038/ncomms3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo Z., Zhang L., Wu Z., Chen Y., Wang F., Chen G. In vivo direct reprogramming of reactive glial cells into functional neurons after brain injury in an Alzheimer's disease model. Cell Stem Cell. 2013;14:188–202. doi: 10.1016/j.stem.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich C., Bergami M., Gascon S., Lepier A., Vigano F., Dimou L., Sutor B., Berninger B., Götz M. Sox2-mediated conversion of NG2 glia into induced neurons in the injured adult cerebral cortex. Stem Cell Reports. 2014;3:1000–1014. doi: 10.1016/j.stemcr.2014.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh J., Aimone J.B., Kaspar B.K., Kuwabara T., Nakashima K., Gage F.H. IGF-1 instructs multipotent adult neural progenitor cells to become oligodendrocytes. J. Cell Biol. 2004;164:111–122. doi: 10.1083/jcb.200308101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G., Song H., Gage F.H. Neurogenesis in the adult hippocampus. Cold Spring Harb. Perspect. Biol. 2015;7:a018812. doi: 10.1101/cshperspect.a018812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laywell E.D., Rakic P., Kukekov V.G., Holland E.C., Steindler D.A. Identification of a multipotent astrocytic stem cell in the immature and adult mouse brain. Proc. Natl. Acad. Sci. USA. 2000;97:13883–13888. doi: 10.1073/pnas.250471697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leutz A., Schachner M. Epidermal growth factor stimulates DNA-synthesis of astrocytes in primary cerebellar cultures. Cell Tissue Res. 1981;220:393–404. doi: 10.1007/BF00210517. [DOI] [PubMed] [Google Scholar]
- Liu Y., Miao Q., Yuan J., Han S., Zhang P., Li S., Rao Z., Zhao W., Ye Q., Geng J. Ascl1 converts dorsal midbrain astrocytes into functional neurons in vivo. J. Neurosci. 2015;35:9336–9355. doi: 10.1523/JNEUROSCI.3975-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L., Zwingman T.A., Sunkin S.M., Oh S.W., Zariwala H.A., Gu H., Ng L.L., Pamiter R.D., Hawrylycz M.J., Jones A.R. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnusson J.P., Goritz C., Tatarishvili J., Dias D.O., Smith E., Lindvall O., Kokaia Z., Frisén J. A latent neurogenic program in astrocytes regulated by Notch signaling in the mouse. Science. 2014;346:237–241. doi: 10.1126/science.346.6206.237. [DOI] [PubMed] [Google Scholar]
- Ming G.L., Song H. Adult neurogenesis in the mammalian brain: significant answers, and significant questions. Neuron. 2011;70:687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mira H., Andreu Z., Suh H., Lie D.C., Jessberger S., Consiglio A., Emeterio J., Hortiguela R., Marques-Torrejob M.A., Nakashima K. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell. 2010;7:78–89. doi: 10.1016/j.stem.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Miyake T., Hattori T., Fukuda M., Kitamura T., Fujita S. Quantitative studies on proliferation changes of reactive astrocytes in mouse cerebral cortex. Brain Res. 1988;451:133–138. doi: 10.1016/0006-8993(88)90757-3. [DOI] [PubMed] [Google Scholar]
- Nato G., Caramello A., Trova S., Avataneo V., Rolando C., Taylor V., Buffo A., Peretoo P., Luzzati F. Striatal astrocytes produce neuroblasts in an excitotoxic model of Huntington's disease. Development. 2015;142:840–845. doi: 10.1242/dev.116657. [DOI] [PubMed] [Google Scholar]
- Niu W., Zang T., Zou Y., Fang S., Smith D.K., Bachoo R., Zhang C.L. In vivo reprogramming of astrocytes to neuroblasts in the adult brain. Nat. Cell Biol. 2013;15:1164–1176. doi: 10.1038/ncb2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow C.W., Goetz C.G., Kordower J.H., Stoessl A.J., Sossi V., Brin M.F., Shannon K.M., Nauert G.M., Perl D.P., Godbold J., Freeman T.B. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson's disease. Ann. Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- Robel S., Berninger B., Götz M. The stem cell potential of glia: lesson from reactive gliosis. Nat. Rev. Neurosci. 2011;12:88–104. doi: 10.1038/nrn2978. [DOI] [PubMed] [Google Scholar]
- Rouaux C., Arlotta P. Fezf2 directs the differentiation of corticofugal neurons from striatal progenitors in vivo. Nat. Neurosci. 2010;13:1345–1347. doi: 10.1038/nn.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouaux C., Arlotta P. Direct lineage reprogramming of post-mitotic callosal neurons into corticofugal neurons in vivo. Nat. Cell Biol. 2013;15:214–221. doi: 10.1038/ncb2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torper O., Pfisterer U., Wolf D.A., Pereira M., Lau S., Jakobsson J., Bjorklund A., Grealish S., Parmar M. Generation of induced neurons via direct conversion in vivo. Proc. Natl. Acad. Sci. USA. 2013;110:7038–7043. doi: 10.1073/pnas.1303829110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torper O., Ottosson D.R., Pereira M., Lau S., Cardoso T., Grealish S., Parmar M. In vivo reprogramming of striatal NG2 glia into functional neurons that integrate into local host circuitry. Cell Rep. 2015;12:474–481. doi: 10.1016/j.celrep.2015.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskuhl R.R., Peterson R.S., Song B., Ao Y., Morales L.B.J., Tiwari-Woodruff S., Sofroniew M.V. Reactive astrocytes form scar-like perivascular barriers to leukocytes during adaptive immune inflammation of the CNS. J. Neurosci. 2009;29:11511–11522. doi: 10.1523/JNEUROSCI.1514-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R.E., Rao M., Gensel J.C., McTigue D.M., Kaspar B.K., Jakeman L.B. Transforming growth factor alpha transforms astrocytes to a growth-supportive phenotype after spinal cord injury. J. Neurosci. 2011;31:15173–15187. doi: 10.1523/JNEUROSCI.3441-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.